Abstract
The proliferative response of non-β islet endocrine cells in response to type 1 diabetes (T1D) remains undefined. We quantified islet endocrine cell proliferation in a large collection of nondiabetic control and T1D human pancreata across a wide range of ages. Surprisingly, islet endocrine cells with abundant proliferation were present in many adolescent and young-adult T1D pancreata. But the proliferative islet endocrine cells were also present in similar abundance within control samples. We queried the proliferating islet cells with antisera against various islet hormones. Although pancreatic polypeptide, somatostatin, and ghrelin cells did not exhibit frequent proliferation, glucagon-expressing α-cells were highly proliferative in many adolescent and young-adult samples. Notably, α-cells only comprised a fraction (∼1/3) of the proliferative islet cells within those samples; most proliferative cells did not express islet hormones. The proliferative hormone-negative cells uniformly contained immunoreactivity for ARX (indicating α-cell fate) and cytoplasmic Sox9 (Sox9Cyt). These hormone-negative cells represented the majority of islet endocrine Ki67+ nuclei and were conserved from infancy through young adulthood. Our studies reveal a novel population of highly proliferative ARX+ Sox9Cyt hormone-negative cells and suggest the possibility of previously unrecognized islet development and/or lineage plasticity within adolescent and adult human pancreata.
Introduction
Type 1 diabetes (T1D) is characterized by a considerable loss of β-cells and subsequent insulin deficiency (1–7). Although β-cells have been reported to persist in T1D pancreata for several years after diagnosis, we recently found that T1D pancreata do not exhibit evidence of increased β-cell proliferation or evidence of β-cell neogenesis or transdifferentiation (1). However, the impact of T1D on non–β-cells has not been studied. Thus, the regenerative response of islet endocrine cells to T1D remains poorly understood.
Lineage-tracing studies in mice suggest that α-cells may have unappreciated plasticity. α-Cells appear to transdifferentiate into β-cells in mice under some circumstances (2–4). These results imply that α-cells might be a potential source for β-cell neogenesis as a novel therapy for diabetes. Indeed, insulin-glucagon–coexpressing cells have been reported within pancreata of human patients with acute pancreatitis (5). However, potential compensatory responses from non-β-cell sources in human pancreata with long-standing T1D remain poorly understood, as only a few studies have been performed. Increased Ki67+ islet cells have been observed in both α- and β-cells of pancreata from individuals with recent-onset T1D (6). Ki67+ ductal cells have also been described in transplanted pancreas of patients with T1D (7). Increased cell proliferation has also been reported in pancreatic duct glands of T1D pancreata (8). Taken together, these observations hint at a role for non–β-cell sources in T1D pathophysiology or compensation.
Given the lack of consensus, we considered the possibility that other islet endocrine cells could participate or respond to autoimmunity with attempted regeneration. We surveyed human islet proliferation in nondiabetic control and T1D pancreata from the JDRF Network for Pancreatic Organ Donors with Diabetes (nPOD) collection, applying high-throughput imaging and analysis using techniques similar to those used in our previous study (1). We find that islet proliferation did not increase in response to T1D. But islet cell proliferation was sharply increased in many adolescent and young-adult pancreata of individuals with and without T1D. We identify a novel population of highly proliferative, α-related cells within many adolescent and young-adult pancreata.
Research Design and Methods
Human Pancreatic Samples
Paraffin-embedded pancreas tissue sections were obtained from the JDRF nPOD after a waiver from our institutional review board. Pancreata were studied based on availability. Tissues were processed by nPOD by standardized operating procedures (http://www.jdrfnpod.org/for-investigators/standard-operating-procedures/). Paraffin-embedded tissues were fixed in 10% neutral buffered formalin for 24 h and up to 40 h for pancreata with high fat content (1).
Sample Population
Fifty-nine control subjects without diabetes and 47 subjects with T1D were studied, selected to include various ages—infants (age 0–1.4 years), children (1.5–13.9 years), adolescents (14–20.9 years), young adults (21–39 years), and older adults (≥40 years)—as previously described (1). Recent-onset T1D was defined as disease duration ≤10 years. See Supplementary Tables 1 and 2 for further information.
Immunohistochemistry
Paraffin sections were incubated with primary antisera (Supplementary Table 3), followed by the appropriate secondary antisera conjugated to aminomethylcoumarin (AMCA), Cy2, Cy3, or Cy5 (Jackson ImmunoResearch) and DAPI (Molecular Probes, Eugene, OR) as previously described (1). Primary antisera were as follows: 1:100, ARX (AF7068; R&D Systems), β3 tubulin (NB100-1612; Novus Biologicals), CD3 (PA1-37282; Thermo Fisher Scientific), CD31 (ab28364; Abcam), chromagranin A (ab8204; Abcam), ghrelin (H-031-77; Phoenix Pharmaceuticals), GLUT1 (07-1401; Millipore), ISL1&2 (39.4D5; DSHB), INSM1 (sc-271408; Santa Cruz Biotechnology), NeuN (MAB377; Millipore), Nkx2.2 (ab191077; Abcam), Nkx6.1 (F55A12; DSHB), pancreatic polypeptide (PP) (18-0043; Invitrogen), PCNA (2586S; Cell Signaling Technology), PC1/3 (AB10553; Millipore), Pdx1 (NBP2-38865; Novus Biologicals), phospho-histone H3 (9701S; Cell Signaling Technology), proinsulin (GN-ID4; DSHB), SNAP25 (MAB331; Millipore), somatostatin (SS) (18-0078; Invitrogen), synaptotagmin 1A (ab133856; Abcam), Sox9 (AB5535; Millipore), Sox9 (pS181) (ab59252; Abcam), and synaptophysin (18-0130, Thermo Fisher Scientific, and AB6245, Abcam); 1:250, glucagon (ab8055 and ab10988; Abcam) and Ki67 (550609; BD Biosciences); and 1:1,500, insulin (A0564; Dako).
Islet Morphometry
Islet endocrine and α-cell morphometry were assessed with Volocity 6.1.1 (PerkinElmer) as previously described (9). Zeiss AxioImager M1 (Carl Zeiss Microscopy) with automated X-Y stage and Orca ER camera (Hamamatsu) acquired images of tens of thousands of individual nuclei/sample (Supplementary Table 4).
Proliferation Analysis
Ki67+ islet endocrine (synaptophysin), α-cell (glucagon), PP, SS, ghrelin, and cytoplasmic Sox9 (Sox9Cyt) proliferation were calculated as % total cells. A subset of high proliferators was quantified as % intra-islet Ki67+ cells for insulin and all other markers. High proliferation was defined as having an islet endocrine cell replication rate >0.71%, corresponding to z score of 0.5.
TUNEL
Apoptosis analysis was performed in a subset of available control and T1D samples as previously described (1). Total terminal deoxynucleotide TUNEL-positive islet endocrine cells were assessed in >85,000 islet cells/condition. Total TUNEL+ Sox9Cyt cells were assessed in 993 islet cells/condition. In every sample, TUNEL+ pancreatic ducts were imaged to ensure adequate TUNEL staining.
Results
Increased Islet Endocrine Cell Proliferation in Some Adolescents and Young Adults Irrespective of T1D Status
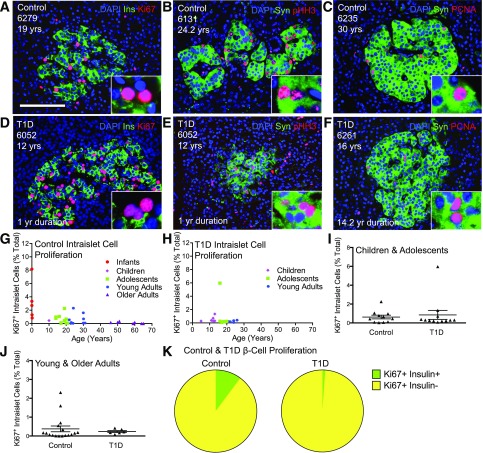
We previously quantified β-cell proliferation in control and T1D nPOD pancreas organ donor samples and found no evidence for a β-cell regenerative response to T1D in any age-group (1). During our studies, we noted that some samples had islets with abundant Ki67+ cells, indicating islet proliferation (Fig. 1A–C). Samples with highly proliferative islets represented only a portion of pancreatic donors, largely from adolescents and young adults. Proliferative islet cells were present in control and T1D samples; T1D did not influence the proliferative islet cell phenotype (Fig. 1D–F and Supplementary Table 5). Cell cycle entry within islets, as measured by the percentage of Ki67+ islet cells among total islet nuclei, was equivalent between control and T1D for age-matched cohorts of children and adolescents (0.63 vs. 0.81%) or adults (0.38 vs. 0.24%), with proliferation more variable in control subjects (Fig. 1G–J and Supplementary Table 5). Most proliferative islet cells did not express insulin, suggesting they were not β-cells (Fig. 1A and B). We quantified Ki67+ β-cells as a fraction of the total Ki67+ islet cells among the highly proliferative pancreatic samples. The vast majority of Ki67+ islet cells did not contain insulin; 89.8% of Ki67+ intra-islet cells of control subjects and 98.86% of Ki67+ intra-islet cells of T1D subjects did not express insulin (Fig. 1K and Supplementary Table 6). Moreover, Ki67+ islet cells were not CD3+ or CD31+, thereby excluding lymphocytes or endothelial cells, respectively (Supplementary Fig. 1). We therefore considered the possibility of proliferative non-β islet endocrine cells within our samples.
Figure 1.
High levels of intra-islet proliferation within some adolescents and young adults but not within β-cells. Islet images for control (A–C) and T1D (D–F) pancreata stained for DAPI (blue), insulin (Ins)/synaptophysin (Syn) (green), and Ki67/pHH3/PCNA (red). Scale bar: 100 μm. G and H: Quantification of intra-islet cell proliferation in control (G) and T1D (H) represented by age (years) demonstrates high intra-islet proliferation in both control and T1D islets. Data points represent the mean value for each of 33 control and 18 T1D pancreata. I and J: Ki67+ intra-islet cells (% total) in T1D were very similar to control child and adolescent pancreata (I) and young- and older-adult pancreata (J). Results expressed as mean ± SEM for 11 control and 12 T1D children and adolescent subjects, and 17 control and 6 T1D young- and older-adult subjects. K: Ki67+ β-cells represent a small fraction of the total intra-islet Ki67+ cells in a subset of highly proliferating control (n = 12) and T1D (n = 6) pancreata, identified as described in research design and methods. yr, year; yrs, years.
Increased Islet Endocrine Cell Proliferation in Pancreata From Some Adolescents and Young Adults Irrespective of T1D Status
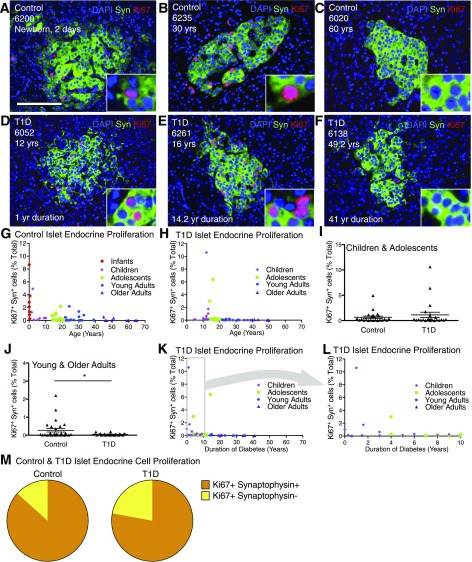
We imaged and quantified total islet endocrine cell proliferation using Ki67 and synaptophysin (marker of islet endocrine cells) in 59 control and 47 T1D pancreata to determine the identity of the proliferative islet cells and to test for a proliferative response to T1D. Similar to previous findings of β-cell proliferation (1,10,11), islet endocrine cell proliferation was greatest in pancreatic samples from infants and children without diabetes (up to 8.68%) and declined with age (Fig. 2A–H and Supplementary Tables 7 and 8). Islet endocrine cell proliferation was comparable between control and T1D samples from age-matched cohorts of children (0.94 ± 0.59 vs. 1.50 ± 1.03%; P = 0.66) (Fig. 2I and J and Supplementary Tables 7 and 8). However, a subset of both control and T1D adolescent and young-adult subjects exhibited many Ki67+ islet cells (0.48–6.38% [hereby referred to as “high proliferators”]) (Supplementary Tables 5–8). Islet cell proliferation was not associated with duration of diabetes and was minimal in the other samples from control and T1D adolescent and young-adult subjects (Fig. 2K and L and Supplementary Tables 7 and 8). Pancreatic samples from control and T1D older adult subjects had very few Ki67+ cells and minimal islet endocrine cell proliferation (Fig. 2G and H and Supplementary Tables 7 and 8). Synaptophysin+ Ki67+ cells represented the vast majority of intra-islet Ki67+ cells in both control and T1D samples (Fig. 2M and Supplementary Table 9). Islet endocrine cell proliferation was verified with other markers of cell cycle entry. Abundant synaptophysin+ phospho-histone H3+ (pHH3) and PCNA+ nuclei were observed, confirming increased cell cycle entry (Supplementary Fig. 2).
Figure 2.
Islet endocrine cell proliferation is greatly increased in some adolescent and young-adult pancreata. Analysis of islet endocrine cells in control and T1D pancreata. Islet images for control (A–C) and T1D (D–F) stained for DAPI (blue), synaptophysin (Syn) (green), and Ki67 (red). Scale bar: 100 μm. G and H: Quantification of islet endocrine cell proliferation in control (G) and T1D (H) represented by age (years). Data points represent the mean value for each of 59 control and 46 T1D pancreata. Ki67+ synaptophysin+ cells (% total) in control and T1D versus age (years) demonstrates that islet endocrine cell proliferation generally declines with age with notable proliferation in adolescents and young adults. I and J: Ki67+ synaptophysin+ (% total) in T1D was very similar to control child and adolescent pancreata (I) but reduced compared with control young- and older-adult pancreata (J). Results expressed as mean ± SEM for 23 control and 23 T1D children and adolescent subjects and 28 control and 23 young- and older-adult subjects. *P < 0.05. K and L: Islet endocrine cell proliferation vs. T1D duration (years) reveals that most pancreata from T1D individuals exhibited low rates of islet endocrine cell proliferation. M: Ki67+ islet endocrine cells represent a small fraction of the total intra-islet Ki67+ cells in a subset of highly proliferating control (n = 12) and T1D (n = 6) pancreata. yr, year; yrs, years.
To test for regional variation of the islet cell proliferative phenotype, we studied samples from the opposite region of the pancreas (e.g., head/body when tail had been previously measured) from four adolescent and young-adult case subjects and compared synaptophysin+ Ki67+. Reassuringly, there was minimal intrasample variation of islet cell proliferation (Supplementary Fig. 3 and Supplementary Table 10). Thus, the proliferative islet endocrine cell population did not substantially vary within pancreatic region. Taken together, these studies indicate that proliferative islet endocrine cells are present within a significant population of pancreata from adolescent and young-adult donors.
α-Cell Proliferation Declines With Age and Is Greatly Increased in Some Adolescent and Young-Adult Pancreata
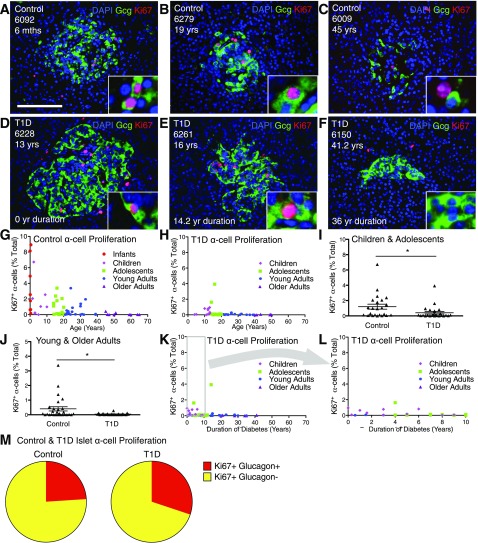
We considered the possibility that α-cells could represent the proliferating islet endocrine cells of pancreata from adolescents and young adults. We measured α-cell proliferation in control samples, quantifying glucagon+ Ki67+ nuclei (Fig. 3A–F and Supplementary Table 7). Infants exhibited very high (up to 8.93%) and older adults very little (0–0.42%) α-cell proliferation. (Fig. 3G and Supplementary Table 7). However, α-cell proliferation was quite elevated (up to 3.4% of α-cells were Ki67+) in samples from a fraction (∼1 of 3) of adolescents and young adults. Thus, α-cell proliferation gradually decreased with age, consistent with previous findings in islets (above), α-cells (12), and β-cells (1,10,11). Notably, many pancreata from adolescents and young adults with increased islet endocrine cell proliferation also had abundant α-cell proliferation (Supplementary Table 8).
Figure 3.
α-Cell proliferation is greatly increased in some adolescent and young-adult pancreata; no compensatory β-cell proliferation in T1D. Analysis of α-cells in control and T1D pancreata. Islet images for control (A–C) and T1D (D–F) stained for DAPI (blue), glucagon (Gcg) (green), and Ki67 (red). Scale bar: 100 μm. G and H: Quantification of α-cell proliferation in control (G) and T1D (H) represented by age (years). Data points represent the mean value for each of 59 control and 47 T1D pancreata. Ki67+ α-cells (% total) in control and T1D vs. age (years) demonstrates that α-cell proliferation declines with age with notable proliferation in adolescents and young adults. I and J: Ki67+ α-cells (% total) in T1D were reduced compared with in control child and adolescent pancreata (I) and young- and older-adult pancreata (J). Results expressed as mean ± SEM for 23 control children and adolescent subjects and 24 children and adolescents with T1D and for 28 young- and older-adult control subjects and 23 young and older adults with T1D. *P < 0.05. K and L: α-Cell proliferation vs. T1D duration (years) demonstrates that most pancreata from T1D individuals exhibited low rates of α-cell proliferation. M: Ki67+ α-cells represent a fraction of the total intra-islet Ki67+ cells in a subset of highly proliferating control (n = 12) and T1D (n = 6) pancreata. mths, months; yr, year; yrs, years.
We tested for a proliferative response of α-cells to T1D. Glucagon+ Ki67+ cells were present in some T1D samples of children, adolescents, and young adults (Fig. 3D–F). Abundant α-cell proliferation was present in a fraction of pancreata from children, adolescents, and young adults (Fig. 3H and Supplementary Table 8). Similar to findings in control subjects, α-cell proliferation declined as a function of age to very low levels in older T1D adults (0–0.07%). Notably, α-cell proliferation was decreased in T1D samples from children/adolescents and young/older adults (Fig. 3I and J and Supplementary Tables 7 and 8). Similarly, Ki67+ α-cells per islet were decreased in T1D pancreata from children, adolescents, and young adults (Supplementary Table 11). Individuals with T1D with the most α-cell proliferation were mainly children and adolescents with short disease duration (Fig. 3K and L and Supplementary Table 8). α-Cell proliferation was present in the same subset of control and T1D adolescent and young-adult samples that exhibited islet endocrine cell proliferation (Supplementary Tables 7 and 8).
Increased α-Cell Proliferation Represents a Fraction of Total Islet Cell Proliferation
Given parallel trends of proliferation between islet endocrine cells and α-cells, we considered the possibility that α-cells could represent the majority of proliferative islet cells. However, glucagon+ Ki67+ cells only accounted for 24 and 30% of total intra-islet cell proliferation in the highly proliferative control and T1D samples, respectively (Fig. 3M and Supplementary Table 12). Since β-cell proliferation comprised only 10.2 and 1.1% of total intra-islet cell proliferation in control and T1D pancreata (Fig. 1G and Supplementary Table 6), most islet endocrine proliferation could not be accounted for within α- and β-cells. Thus, our studies revealed the presence of another population of highly proliferative islet endocrine cells within adolescents and young adults.
PP, SS, and Ghrelin Cells Exhibit Minimal Proliferation
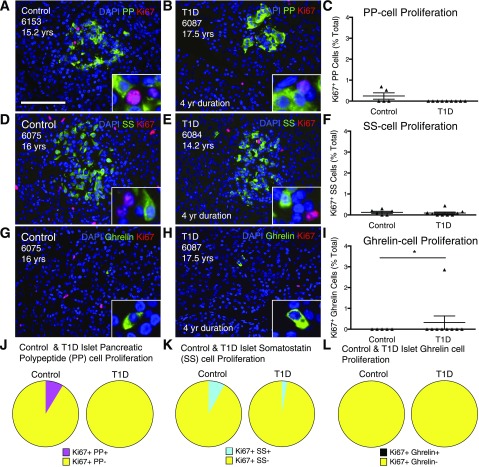
In a further attempt to identify the highly proliferative islet endocrine cells, we separately quantified Ki67 staining with PP, SS, and ghrelin. However, there were very few proliferating PP, SS, and ghrelin cells within a random sampling of adolescent and young-adult pancreata (Fig. 4A–I and Supplementary Table 13). No difference was noted between control and T1D pancreata for any cell populations. A separate analysis quantified PP, SS, and ghrelin proliferation as a percentage of intra-islet proliferation (Fig. 4J–L) with use of the subset of donors with or without T1D identified with high levels of proliferation. PP, SS, and ghrelin cell proliferation accounted for very little of the total intra-islet proliferation within control and T1D pancreata (Fig. 4J–L and Supplementary Table 14). Thus, most of the islet cells entering the cell cycle within the proliferative samples did not contain any of the five known islet endocrine hormones, although some Ki67+ cells were glucagon+. Our studies therefore reveal the presence of highly proliferative islet endocrine cells that do not express any of the known islet hormones.
Figure 4.
PP, SS, and ghrelin cells exhibit minimal proliferation in control and T1D subjects. Control (A, D, and G) and T1D (B, E, and H) islets stained for DAPI (blue) and Ki67 (red) with an islet endocrine hormone (green): PP (A and B), SS (D and E), or ghrelin (G and H). Scale bar: 100 μm. C, F, and I: Quantification of PP, SS, and ghrelin cell proliferation for adolescent control and T1D pancreata. Results expressed as mean ± SEM for five control (PP/ghrelin), six control (SS), and nine T1D. *P < 0.05. PP (J), SS (K), and ghrelin (L) cells represent a small fraction of the total intra-islet Ki67+ cells in a subset of highly proliferating control (n = 3) and T1D (n = 3) pancreata. yr, year; yrs, years.
No Association of Islet Endocrine Cell Proliferation With Pancreas Harvest Conditions
Since increased islet endocrine cell proliferation was only present within a fraction of pancreata from adolescents and young adults in the control (9 of 34) and T1D (2 of 31) groups, we considered the possibility that islet cell cycle entry might be influenced by the postmortem state or conditions of organ harvest. We compared synaptophysin+ Ki67+ cell content of pancreatic samples versus donor clinical parameters. Reassuringly, there was no association between duration of intensive care unit (ICU) stay and islet cell proliferation in control or T1D subjects (Supplementary Fig. 4A and B and Supplementary Table 15). Similarly, there was no association between α-cell proliferation and ICU stay (Supplementary Fig. 4C and D and Supplementary Table 15).
Concerns have been raised regarding the potential impact of warm or cold ischemia to reduce Ki67 immunoreactivity and thus confound measurement of human islet cell proliferation (13). Although pancreatic transit time varied dramatically, there was no correlation with islet cell proliferation or α-cell proliferation (Supplementary Fig. 4E–H and Supplementary Table 15). Thus, impaired sample quality did not seem to explain the variability of islet endocrine proliferation in our population.
Sox9Cyt in Proliferative Islet Cells
We considered the possibility that highly proliferative islet endocrine cells could express markers of islet endocrine progenitors. Ngn3, a key islet endocrine cell progenitor, was not detected within either control or T1D pancreata (data not shown). We then tested for Sox9 expression, which influences development of pancreatic progenitor cells and adult exocrine cells, among other organs (14–16). Surprisingly, most Ki67 or PCNA proliferative cells showed Sox9 immunoreactivity in the cytoplasm (Sox9Cyt)—in sharp contrast to pancreatic ducts (Fig. 5A and B and Supplementary Fig. 5A–D). To further test Sox9Cyt immunoreactivity, we stained pancreata with phospho-specific antisera against serine 181 of Sox9 (p-Sox9). p-Sox9 detection was consistent with earlier Sox9 studies (17–23). p-Sox9 antisera yielded cytoplasmic immunoreactivity in scattered islet endocrine cells that were uniformly ARX+ (Supplementary Fig. 6A–D). Many p-Sox9+ ARX+ cells also expressed glucagon in variable amounts. (Supplementary Fig. 6C and D). In contrast to the above Sox9 studies, p-Sox9+ antisera did not stain nuclear antigens in pancreatic acini and only weakly detected nuclei within duct structures (Supplementary Fig. 6E and F). Collectively, these studies with p-Sox9+ antisera support the possibility of Sox9Cyt immunoreactivity within the highly proliferative ARX+ glucagon variable islet endocrine cells.
Figure 5.
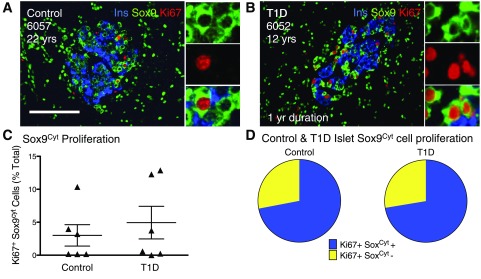
Proliferative Sox9Cyt cells in adolescent and young-adult samples represent the majority of proliferating islet cells. A and B: Islet images for control (A) and T1D (B) subjects stained for insulin (Ins) (blue), Sox9 (green), and Ki67 (red). Insets indicate Ki67+ Sox9Cyt+ cells. Scale bar: 100 μm. C: Quantification of Sox9Cyt cell proliferation in a random sampling of adolescent control (n = 6) and T1D (n = 6) pancreata, represented as % total Sox9Cyt cells. Results expressed as mean ± SEM for control (n = 6) and T1D pancreata (n = 6). D: Ki67+ Sox9Cyt+ cells comprise the majority of proliferating intra-islet cells measured in a subset of highly proliferative control (n = 3) and T1D pancreata (n = 3). yr, year; yrs, years.
Quantification revealed very high amounts of Sox9Cyt Ki67+ cells (up to ∼13%) in some adolescent control and T1D samples (Fig. 5C and Supplementary Table 16). Indeed, Sox9Cyt immunoreactive cell proliferation accounted for ∼72% of total intra-islet proliferation in the subset of control and T1D samples with high endocrine cell proliferation (Fig. 5D and Supplementary Table 16). Thus, Sox9Cyt Ki67+ cells represented the proliferative hormone negative intra-islet cells, which were also detected by p-Sox9 antisera (Supplementary Fig. 6G and H). Notably, Sox9Cyt Ki67+ cells were specific to islets and not primarily associated with pancreatic ducts or pancreatic parenchyma, as detected by ductal structures or cytokeratin-19 (Supplementary Fig. 5E–G and Supplementary Table 17).
Absence of Neuronal Markers in Sox9Cyt Immunoreactive Cells
Synaptophysin is a neuroendocrine marker and could also detect neurons in the pancreas or islet. Hence, we used additional neuronal markers to test whether Sox9Cyt cells represented dedifferentiated neurons. Sox9Cyt cells did not express β3-tubulin or NeuN, which detect peripheral neurons (Supplementary Fig. 6). Thus, proliferative intra-islet cells did not express neuronal related markers.
Absence of β-Cell–Specific Markers in Proliferative Islet Cells
We used additional β-cell and endocrine differentiation markers to test whether proliferating intra-islet cells represented dedifferentiated β-cells. The Ki67+ islet cells did not express Pdx1 (Supplementary Fig. 8A), which defines mature β-cells and a few SS islet cells. Islet Sox9Cyt cells also did not express the mature β-cell marker, Nkx6.1, which was always found within insulin+ cells and absent from glucagon+ cells (Supplementary Fig. 8B) (1). Similarly, Ki67+ cells did not express MafA, GAD65, PC1/3, or GLUT1 (Supplementary Fig. 8C–F). These studies indicate that the proliferative intra-islet cells did not express β-cell related markers.
Proliferative Islet Endocrine Sox9cyt Cells Express α-Cell Markers (ARX and Variable Glucagon)
Further analysis of islet Sox9Cyt cells demonstrated that virtually all Sox9Cyt coexpressed synaptophysin (control group = 99.9% and T1D group = 99.6%) (Fig. 6A and B and Supplementary Table 18), which is consistent with our synaptophysin-Ki67 studies. Having excluded β-cell–related markers, we considered the possibility that the proliferative islet endocrine Sox9Cyt cells might be related to α-cells. We looked for α-cell progenitors after finding increased α-cell proliferation, given that proliferative α-cells did not represent the majority of proliferative islet cells. We stained pancreata with antisera against ARX, a marker of α-cell–related fate. Indeed, virtually all Sox9Cyt cells were also ARX+ (control = 99.3% and T1D = 98.4%) (Fig. 6C and D and Supplementary Table 19). ARX+ Sox9Cyt cells variably expressed glucagon: some ARX+ Sox9Cyt cells contained glucagon, while others were glucagon negative (Fig. 6C and D). Sox9Cyt cells also variably expressed GLP1, and many GLP1+ cells coexpressed glucagon (Supplementary Fig. 9). Notably, many ARX+ glucagon negative cells were Ki67+ (Fig. 6E and F and Supplementary Table 20). These studies of ARX+ Sox9Cyt Ki67+ glucagon cells reveal that many of the proliferative islet endocrine cells have an α-cell–related phenotype.
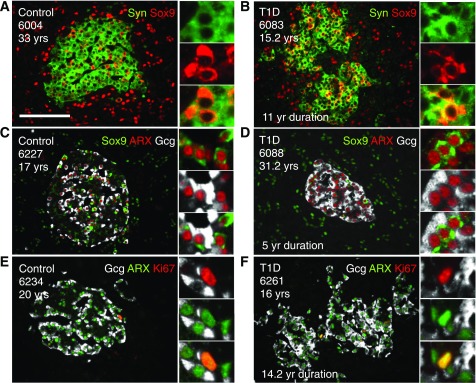
Figure 6.
Highly proliferative Sox9Cyt cells are islet endocrine cells, expressing ARX and variable glucagon (Gcg). Control (A, C, and E) and T1D (B, D, and F) islets stained for endocrine, Sox9, Ki67, and α-cell markers. A and B: Islets stained for synaptophysin (Syn) (green) and Sox9 (red) show synaptophysin-Sox9Cyt copositive cells. C and D: Islets stained for Sox9 (green), ARX (red), and glucagon (white) indicate some Sox9Cyt-ARX-glucagon triple-positive cells (inset of C) and some Sox9Cyt ARX+ cells that are clearly glucagon negative, as visible in the inset of D. E and F: Islets stained for glucagon (white), ARX (green), and Ki67 (red) indicate ARX-Ki67 copositive cells that do not express glucagon. Scale bar: 100 μm. yr, year; yrs, years.
We tested for other islet endocrine markers of ARX+ Sox9Cyt cells. We first looked for INSM1, a transcription factor present within insulinomas (24) and in murine α-, β-, SS, and PP cells (25). INSM1 expression was consistently observed in β- and α-cells of young-adult control and TID samples and also in ARX+ Sox9Cyt cells (Supplementary Fig. 10A–D). Similarly, Isl1 and -2 were readily detected in ARX+ Sox9Cyt cells (Supplementary Fig. 10E). Additionally, Nkx2.2 marked both α- and β-cells and was observed in highly proliferative Ki67+ insulin-negative islet cells (Supplementary Fig. 10F and G). Thus, ARX+ Sox9Cyt Ki67+ cells seem to express many markers common to islet endocrine cells.
To further characterize the endocrine properties of the proliferative cells, we tested for chromogranin A and other vesicular or exocytosis-related proteins. ARX+ Sox9Cyt cells expressed chromagranin A, as well as exocytotic machinery components synaptotagmin 1a and SNAP25 (Supplementary Fig. 11A–F). Interestingly, secretogranin III, which is typically contained within islet endocrine cells including α- and β-cells (26,27), was absent from Ki67+ cells or some ARX+ cells (Supplementary Fig. 11G and H). Thus, the ARX+ Sox9Cyt islet endocrine cells exhibit many (but not all) of the common features of hormone-secreting α-cells.
ARX+ Sox9Cyt Islet Endocrine Cells Are Conserved in Infant and Child Pancreata
To determine whether the proliferative islet endocrine cells are unique to adolescents and young adults, we studied additional pancreata from infant and young children control subjects. Sox9Cyt synaptophysin+ cells were abundant in islets from infants and young children (Fig. 7A and B). ARX+ Sox9Cyt cells were readily detected in islets from infants and young children, with glucagon present in some but not all cells (Fig. 7C and D). Similar to adolescents and young adults, most of the ARX+ Ki67+ cells in younger samples did not express glucagon (Fig. 7E and F). ARX+ Sox9Cyt cells consistently expressed INSM1 (Fig. 7G and H). These studies reveal that ARX+ Sox9Cyt islet endocrine cells are developmentally conserved and readily detected within pancreata across a wide range of ages.
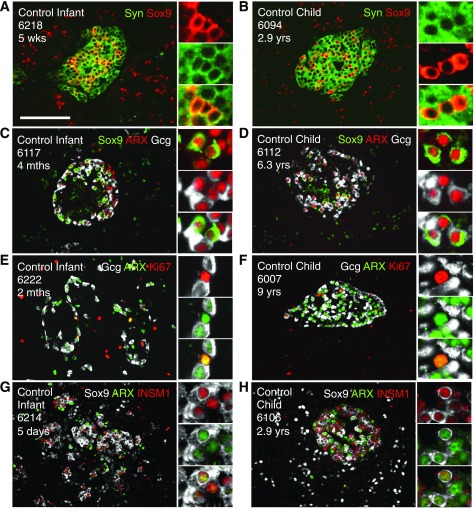
Figure 7.
Highly proliferative Sox9Cyt cells that express ARX, INSM1, and variable glucagon are also present in infant and child pancreata. Control islets of infants (A, C, E, and G) and children (B, D, F, and H) stained for islet endocrine, Sox9, Ki67, and α-cell markers. A and B: Islets stained for synaptophysin (Syn) (green) and Sox9 (red). Insets indicate synaptophysin-Sox9Cyt copositive cells. C and D: Islets stained for Sox9 (green), ARX (red), and glucagon (Gcg) (white). Insets indicate Sox9Cyt-ARX-glucagon triple-positive cells, with a Sox9Cyt ARX+ cell that is clearly glucagon negative visible in the inset of D. E and F: Islets stained for glucagon (white), ARX (green), and Ki67 (red). Insets indicate ARX-Ki67 copositive cells that do not express glucagon. G and H: Islets stained for Sox9 (white), ARX (green), and INSM1 (red). Insets indicate Sox9Cyt ARX+ cells expressing INSM1. Scale bar: 100 μm. mths, months; wks, weeks; yrs, years.
Cell Death Within Proliferative Islet Cells
We hypothesized that vast proliferation of ARX+ Sox9Cyt islet endocrine cells might be counterbalanced by increased cell death. We quantified islet endocrine cell death via TUNEL assay. Some control and T1D adolescent and young-adult individuals exhibited intra-islet TUNEL nuclei (Supplementary Fig. 12A–C, F, and G and Supplementary Table 21). However, Sox9Cyt cells were not routinely TUNEL+ (Supplementary Fig. 12D and E and Supplementary Table 22). Thus, the ARX+ Sox9Cyt islet endocrine cells seem to be a stable population that does not immediately undergo cell death. However, overall islet endocrine cell death seemed to be dramatically increased within some samples, especially those from adolescents and young adults.
Increased cell death could limit the net expansion of islet endocrine cells. To directly test the impact of increased islet endocrine cell proliferation, we quantified islet endocrine cell mass using synaptophysin as a pan-endocrine islet marker. While islet area and mass were decreased in T1D samples compared with control subjects, islet endocrine area and mass did not correlate with age in either cohort (Supplementary Fig. 13 and Supplementary Table 23). Thus, the proliferative islet endocrine cells do not appear to result in accumulation of islet endocrine mass.
Discussion
We analyzed human islet cell proliferation and found high amounts of islet endocrine and α-cell proliferation in some control and T1D pancreata from adolescents and young adults. The proliferative cells appear to be related to α-cells and express ARX, Sox9Cyt, and various markers of islet endocrine cells including INMS1, Isl1 and -2, and Nkx2.2 (Table 1). Sox9Cyt cells comprised the majority of islet endocrine cell proliferation (up to ∼72%). ARX+ Sox9Cyt cells expressed markers of secretory vesicles and machinery, suggesting some capacity for hormone secretion. ARX+ Sox9Cyt cells were abundant in nonproliferative individuals in a wide age range. Our studies reveal a novel population of highly proliferative α-related cells and suggest previously unappreciated replicative capacity and plasticity within islet populations of human pancreata in adolescents and young adults.
Table 1.
Adolescent and young-adult pancreata contain a population of highly proliferative α-related cells that are mostly glucagon negative: a summary of markers found to be detected or absent in α-, highly proliferative α-related cell, β-, γ-, δ-, and ε-cells
| α-Cell | Highly proliferative α-related cell | β-Cell | γ-Cell | δ-Cell | ε-Cell | |
|---|---|---|---|---|---|---|
| Detected | Glucagon | ARX | Insulin | PP | SS | Ghrelin |
| GLP1 | Sox9Cyt | Nkx6.1 | ||||
| ARX | Nkx2.2 | MafA | ||||
| Sox9Cyt | Insm1 | GAD65 | ||||
| Nkx2.2 | Isl1 and -2 | Insm1 | ||||
| Insm1 | Synaptophysin | Isl1 and -2 | ||||
| Isl1 and -2 | CgA | Nkx2.2 | ||||
| Synaptophysin | SCG3 | Synaptophysin | ||||
| SCG3 | SNAP25 | CgA | ||||
| SNAP25 | SYT1 | SCG3 | ||||
| SYT1 | Ki67 | SNAP25 | ||||
| CgA | PCNA | SYT1 | ||||
| Ki67 | pHH3 | |||||
| Absent | Insulin | Glucagon | Glucagon | Ki67 | Ki67 | Ki67 |
| Nkx6.1 | GLP1 | |||||
| MafA | Insulin | ARX | ||||
| GAD65 | Nkx6.1 | Sox9Cyt | ||||
| MafA | Ki67 | |||||
| GAD65 | ||||||
| Pdx1 | ||||||
| SCG3 |
Although β-cell proliferation decreases dramatically after infancy (1,10,11), we found that islet endocrine and α-cell proliferation was high in a subset of adolescents and young adults. Interestingly, α-cell proliferation was decreased in T1D compared with control subjects, suggesting that islet proliferation in adolescents and young adults may be impaired by diabetes. Furthermore, we observed an increase in hormone-negative α-related cells with substantial proliferation, suggesting that adolescent and young-adult pancreata may harbor cells with retained plasticity and replicative capacity. These α-related cells were relatively abundant and comprised the majority of islet Ki67+ nuclei within donors with highly proliferative islets. They did not exhibit mature β-cell markers: Pdx1, Nkx6.1, MafA, and GAD65. Notably, islet cell proliferation in adolescent T1D pancreata was equivalent to that in control nondiabetic pancreata. Although a regenerative response to T1D was not observed, few samples were obtained from very recent T1D onset. In contrast, Willcox et al. (6) demonstrated that individuals with <18 months’ disease duration may have compensatory α- and β-cell proliferation. However, the high rates of islet and α-cell proliferation observed in some T1D adolescents do not appear to be attempted β-cell regeneration from T1D-associated β-cell deficiency. Rather, these highly proliferative cells seem to represent previously unrealized developmental plasticity within islet endocrine lineages, unrelated to T1D. While most islet proliferation occurs during the perinatal period (28), islet cell proliferation and apoptosis were comparable in our study, further suggesting adolescence and young adulthood as a unique period of high proliferation.
Sox9Cyt cells, which express many islet and α-cell markers, might be in a transition state. However, whether they are transitioning to or from α-cells remains unclear. Sox9 is an important transcription factor in developing pancreas for islet endocrine differentiation and development (29). In contrast to typical nuclear Sox9 localization (30), we find highly proliferative cells with Sox9Cyt immunoreactivity. Interestingly, Sox9 activity has been described to change with its localization in the cytoplasm and nucleus within gonad, chondrocytes, breast, and gastric tissues (17–23). In cultured cells, nuclear translocation of Sox9 has been linked to β-catenin degradation and thus inhibition of Wnt signaling (22). In mature islets, future studies will determine whether Sox9Cyt plays a role in cell proliferation or differentiation.
Our study has notable strengths but also limitations inherent to autopsy specimens. We studied a large number of control and T1D samples across a wide age range and quantified vast numbers of cells within several different cell types. Thus, our findings of highly proliferative islet endocrine cells seem to represent a genuine phenomenon, and these Ki67+ cells can be readily detected. However, studies of autopsy tissues are complicated by potential confounding variables, including the cause and conditions of death of the patient, tissue transport conditions, and other preexisting medical conditions prior to the patient’s demise. For example, hypoxemia or other conditions of ICU stay could alter pancreas physiology and possibly influence islet endocrine cell proliferation. Thus, it will be difficult to resolve the highly proliferative α-related cells in vivo given the challenges of pancreatic biopsy from T1D individuals, which include pancreatitis (31). Although samples can be obtained from patients who require pancreatic resection for cancer or tumors (32), such lesions typically occur in the middle-aged and elderly. Consequently, it might be difficult to obtain pancreatic samples from healthy adults. Therefore, nPOD autopsy specimens represent a unique window into human physiology, albeit with limitations.
In conclusion, we find no compensatory islet proliferation in T1D. However, we find a highly proliferative population of α-related islet endocrine cells in adolescents and young adults. The presence of proliferative ARX+ Sox9Cyt cells across a range of ages (infants to young adults) demonstrates developmental conservation of this α-related cell population and hints that α-cells may play an essential role in islet function and growth.
Supplementary Material
Article Information
Acknowledgments. The authors thank the pancreas donors and their families. This research was performed with the support of nPOD, a collaborative T1D research project sponsored by the JDRF. Organ procurement organizations, partnering with nPOD to provide research resources, are listed at www.jdrfnpod.org/our-partners.php. The authors thank M. Yang, I. Kusmartseva, A. Pugliese, C. Wasserfall, M. Campbell-Thompson, and M. Atikinson from the JDRF nPOD organization for generous and essential contributions to this work. Additionally, the authors thank A. Myers, M. Beery, and E. Verney from the JDRF nPOD organization for helping with acquisition of samples for this study.
Funding. This study was supported by funding from the Robert and Janice McNair Foundation, National Institute for Health Research (1R01AG040110), and Diabetes Research Center of the Baylor College of Medicine (DRC - P30-DK-079638).
Duality of Interest. J.A.K. serves on the scientific advisory board for Lexicon and Know Foods. M.M.R. is a current employee of Janssen Pharmaceuticals of Johnson & Johnson. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.J.L., A.R.C., D.R.J., M.M.R., and J.A.K. conceived and designed the experiments. C.J.L., A.R.C., D.R.J., and M.M.R. performed the experiments. C.J.L., A.R.C., D.R.J., M.M.R., and J.A.K. analyzed data. C.J.L., A.R.C., and J.A.K. wrote the manuscript. J.A.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 2nd Joint Meeting of the European Association for the Study of Diabetes Islet Study Group and the Beta Cell Workshop, Dresden, Germany, 7–10 May 2017, and the JDRF nPOD 8th Annual Scientific Meeting, Miami, FL, 22–25 February 2016.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-1114/-/DC1.
References
- 1.Lam CJ, Jacobson DR, Rankin MM, Cox AR, Kushner JA. β cells persist in T1D pancreata without evidence of ongoing β-cell turnover or neogenesis. J Clin Endocrinol Metab 2017;102:2647–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorel F, Népote V, Avril I, et al. . Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010;464:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Meulen T, Mawla AM, DiGruccio MR, et al. . Virgin beta cells persist throughout life at a Neogenic Niche within pancreatic islets. Cell Metab 2017;25:911–926.e916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Othman N, Vieira A, Courtney M, et al. . Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell 2017;168:73–85.e11 [DOI] [PubMed] [Google Scholar]
- 5.Chung CH, Hao E, Piran R, Keinan E, Levine F. Pancreatic β-cell neogenesis by direct conversion from mature α-cells. Stem Cells 2010;28:1630–1638 [DOI] [PubMed] [Google Scholar]
- 6.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Evidence of increased islet cell proliferation in patients with recent-onset type 1 diabetes. Diabetologia 2010;53:2020–2028 [DOI] [PubMed] [Google Scholar]
- 7.Martin-Pagola A, Sisino G, Allende G, et al. . Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with type 1 diabetes and recurrence of autoimmunity. Diabetologia 2008;51:1803–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moin AS, Butler PC, Butler AE. Increased proliferation of the pancreatic duct gland compartment in type 1 diabetes. J Clin Endocrinol Metab 2017;102:200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007;12:817–826 [DOI] [PubMed] [Google Scholar]
- 10.Meier JJ, Butler AE, Saisho Y, et al. . Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cnop M, Hughes SJ, Igoillo-Esteve M, et al. . The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia 2010;53:321–330 [DOI] [PubMed] [Google Scholar]
- 12.Cnop M, Igoillo-Esteve M, Hughes SJ, Walker JN, Cnop I, Clark A. Longevity of human islet α- and β-cells. Diabetes Obes Metab 2011;13(Suppl. 1):39–46 [DOI] [PubMed] [Google Scholar]
- 13.Sullivan BA, Hollister-Lock J, Bonner-Weir S, Weir GC. Reduced Ki67 staining in the postmortem state calls into question past conclusions about the lack of turnover of adult human β-cells. Diabetes 2015;64:1698–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seymour PA, Freude KK, Dubois CL, Shih HP, Patel NA, Sander M. A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation. Dev Biol 2008;323:19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald E, Li J, Krishnamurthy M, Fellows GF, Goodyer CG, Wang R. SOX9 regulates endocrine cell differentiation during human fetal pancreas development. Int J Biochem Cell Biol 2012;44:72–83 [DOI] [PubMed] [Google Scholar]
- 16.Furuyama K, Kawaguchi Y, Akiyama H, et al. . Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 2011;43:34–41 [DOI] [PubMed] [Google Scholar]
- 17.Gasca S, Canizares J, De Santa Barbara P, et al. . A nuclear export signal within the high mobility group domain regulates the nucleocytoplasmic translocation of SOX9 during sexual determination. Proc Natl Acad Sci U S A 2002;99:11199–11204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakravarty G, Moroz K, Makridakis NM, et al. . Prognostic significance of cytoplasmic SOX9 in invasive ductal carcinoma and metastatic breast cancer. Exp Biol Med (Maywood) 2011;236:145–155 [DOI] [PubMed] [Google Scholar]
- 19.Chakravarty G, Rider B, Mondal D. Cytoplasmic compartmentalization of SOX9 abrogates the growth arrest response of breast cancer cells that can be rescued by trichostatin A treatment. Cancer Biol Ther 2011;11:71–83 [DOI] [PubMed] [Google Scholar]
- 20.Malki S, Nef S, Notarnicola C, et al. . Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation. EMBO J 2005;24:1798–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moniot B, Farhat A, Aritake K, et al. . Hematopoietic prostaglandin D synthase (H-Pgds) is expressed in the early embryonic gonad and participates to the initial nuclear translocation of the SOX9 protein. Dev Dyn 2011;240:2335–2343 [DOI] [PubMed] [Google Scholar]
- 22.Topol L, Chen W, Song H, Day TF, Yang Y. Sox9 inhibits Wnt signaling by promoting beta-catenin phosphorylation in the nucleus. J Biol Chem 2009;284:3323–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung VY, Gao B, Leung KK, et al. . SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet 2011;7:e1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang T, Chen C, Breslin MB, Song K, Lan MS. Extra-nuclear activity of INSM1 transcription factor enhances insulin receptor signaling pathway and Nkx6.1 expression through RACK1 interaction. Cell Signal 2014;26:740–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia S, Ivanov A, Blasevic D, et al. . Insm1 cooperates with Neurod1 and Foxa2 to maintain mature pancreatic β-cell function. EMBO J 2015;34:1417–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakai Y, Hosaka M, Yoshinaga A, Hira Y, Harumi T, Watanabe T. Immunocytochemical localization of secretogranin III in the endocrine pancreas of male rats. Arch Histol Cytol 2004;67:57–64 [DOI] [PubMed] [Google Scholar]
- 27.Stridsberg M, Grimelius L, Portela-Gomes GM. Immunohistochemical staining of human islet cells with region-specific antibodies against secretogranins II and III. J Anat 2008;212:229–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 2000;49:1325–1333 [DOI] [PubMed] [Google Scholar]
- 29.Seymour PA, Freude KK, Tran MN, et al. . SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A 2007;104:1865–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp JL, Dubois CL, Schaffer AE, et al. . Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 2011;138:653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krogvold L, Edwin B, Buanes T, et al. . Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia 2014;57:841–843 [DOI] [PubMed] [Google Scholar]
- 32.Inaishi J, Saisho Y, Sato S, et al. . Effects of obesity and diabetes on α- and β-cell mass in surgically resected human pancreas. J Clin Endocrinol Metab 2016;101:2874–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.