Abstract
Amplification of EGFR and its active mutant EGFRvIII occur frequently in GBM. While EGFR and EGFRvIII play critical roles in pathogenesis, targeted therapy with EGFR-tyrosine kinase inhibitors (TKIs) or antibodies has only shown limited efficacy in patients. Here, we discuss signaling pathways mediated by EGFR/EGFRvIII, current therapeutics, and novel strategies to target EGFR/EGFRvIII-amplified GBM.
Keywords: GBM, glioblastoma, epidermal growth factor, EGFR, EGFRvIII, tyrosine kinase inhibitor, TKI
Introduction
GBM is the most common malignant brain tumor in adults, and among the most lethal of all cancers.1 Current treatment: surgery, radiotherapy and chemotherapy, results in a median survival of only 12–15 months.1 Understanding the molecular principles and signaling pathways involved in GBM is critical for development of more effective and targeted therapies for this devastating disease.
GBM, WHO grade IV gliomas,2 are classified into primary tumors that arise de novo (>90% of all cases) and secondary tumors that progress from low-grade gliomas.3 According to differences in expression patterns, GBMs are divided into four subtypes: classical, proneural, mesenchymal and neural, with intratumoral heterogeneity observed in single-cell RNA-sequencing studies.4, 5 EGFR amplification is enriched in the classical subtype.4 Amplification of the EGFR gene occurs in 57.4% of primary GBM patients compared to 8% of secondary GBM patients and is associated with high levels of EGFR protein.6, 7
EGFR, also known as HER1 or ERBB1, is a transmembrane receptor tyrosine kinase of the ERBB family.8 This is a family of four receptors (ERBB1-4 or HER1-4) with EGFR the best characterized.8 Upon binding to ligands which include epidermal growth factor (EGF), Transforming growth factor alpha (TGF-α), amphiregulin, betacellulin, heparin-binding EGF (HB-EGF), epigen or epiregulin, EGFR forms homodimers or heterodimers with other ERBB family members.9 Dimerization of EGFR leads to transphosphorylation (autophosphorylation) of its C-terminal tail, which serves as the docking site for SRC homology 2 (SH2) domain-containing signaling proteins including growth factor receptor-bound protein 2 (GRB2), phosphoinositide 3-kinase (PI3K), SRC homology 2 domain-containing transforming protein 1 (SHC1) and signal transducer and activator of transcription (STAT) proteins.10, 11 These signaling proteins regulate downstream physiological and pathological processes.
Activating mutations in the EGFR kinase domain are frequently detected in non-small cell lung cancer. These mutations, L858R in exon 21 and in-frame deletion in exon 19, are rare in GBM.12 In contrast, a separate group of EGFR deletions and point mutations is found frequently in GBM. EGFR deletions in GBM include EGFRvI (N-terminal deletion), vII (deletion of exons 14–15), vIII (deletion of exons 2–7), vIV (deletion of exons 25–27), vV (deletion of exons 25–28), among which vII and vIII are oncogenic.12 In addition, point mutations in the extracellular region of EGFR such as R108K, A289V/D/T, G598D and other extracellular domain mutations are identified in 24% GBM samples 6. These point mutations keep EGFR in an active conformation.12 Among EGFR mutants found in GBM, EGFRvIII occurs most commonly;6 and is felt to represent a late event, occurring after amplification of EGFRWT.13 Compared to EGFRWT, EGFRvIII lacks amino acids 6–273, and deletion of those 268 amino acids creates a junction site with a new glycine residue between amino acids 5 and 274.14
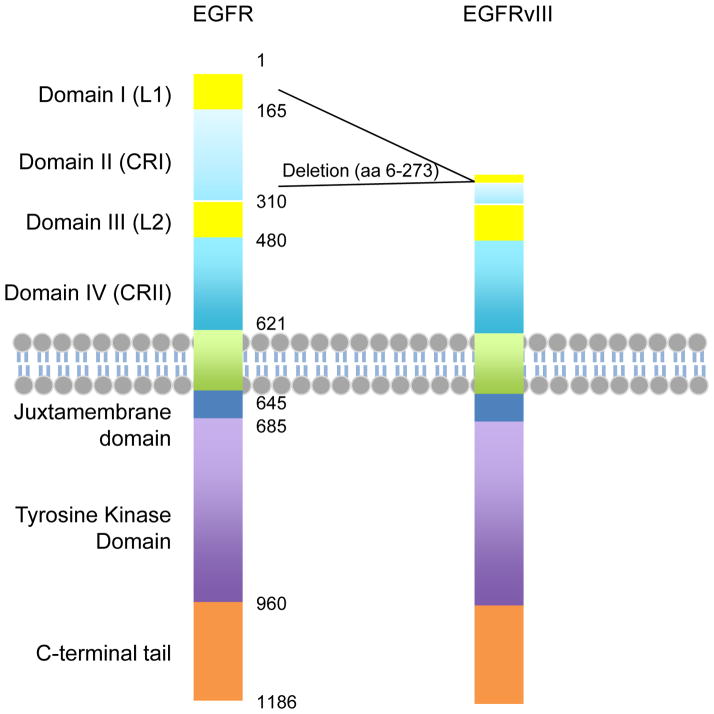
Functional domains of EGFR and EGFRvIII are illustrated in Figure 1. EGFRvIII does not contain a ligand-binding domain, and is constitutively active.15, 16 Our data suggests that EGFRvIII is a substrate for EGFR,17 and that the kinase activity of EGFRvIII is dispensable for some of the augmented signaling observed, when EGFRWT and EGFRvIII are co-expressed. The kinase activity of EGFRvIII is much weaker than that for ligand-activated full-length EGFR, and this weak constitutive kinase activity has been reported as sufficient to confer growth advantage to tumors.18, 19 However, most mouse and human cells express some EGFRWT. It is therefore possible that some of the growth advantage observed in EGFRvIII transduced cells results from EGFRWT-directed phosphorylation of EGFRvIII, rather than from EGFRvIII alone.
Figure 1. Functional domains of EGFR and EGFRvIII.
EGFR is a transmembrane tyrosine kinase receptor. The extracellular region includes four domains, L1, CR1, L2 and CR2. L1 and L2 are Leucine-rich domains that directly bind ligands. EGFRvIII is with the deletion of almost the entire L1 and CR1 domains, resulting in deficiency in ligand binding. The transmembrane and intracellular regions of EGFR and EGFRvIII are identical.
EGFRvIII forms molecular clusters on the cell membrane, which may be important for its pro-tumorigenic function,20 although intracellular localization of EGFRvIII has also been proposed to contribute to activity.21 Expression of EGFRvIII promotes cell proliferation, angiogenesis, and invasion in different model systems.22–25 EGFRvIII expression has been found only in tumors and not in normal tissue, suggesting it as a good candidate for targeted therapy.26 However, recent observations that EGFRvIII amplicons can hide during therapy, only to re-emerge; combined with the recent failure of a phase III clinical trial (ACT IV) testing an anti-EGFRvIII vaccine on newly diagnosed EGFRvIII-positive patients, indicates that targeting EGFRvIII will be challenging.27, 28 In this review, we discuss signaling pathways mediated by EGFR and EGFRvIII, current therapeutics targeting EGFR and/or EGFRvIII, and future directions in treating EGFR/EGFRvIII-amplified GBM.
Signaling mediated by EGFR
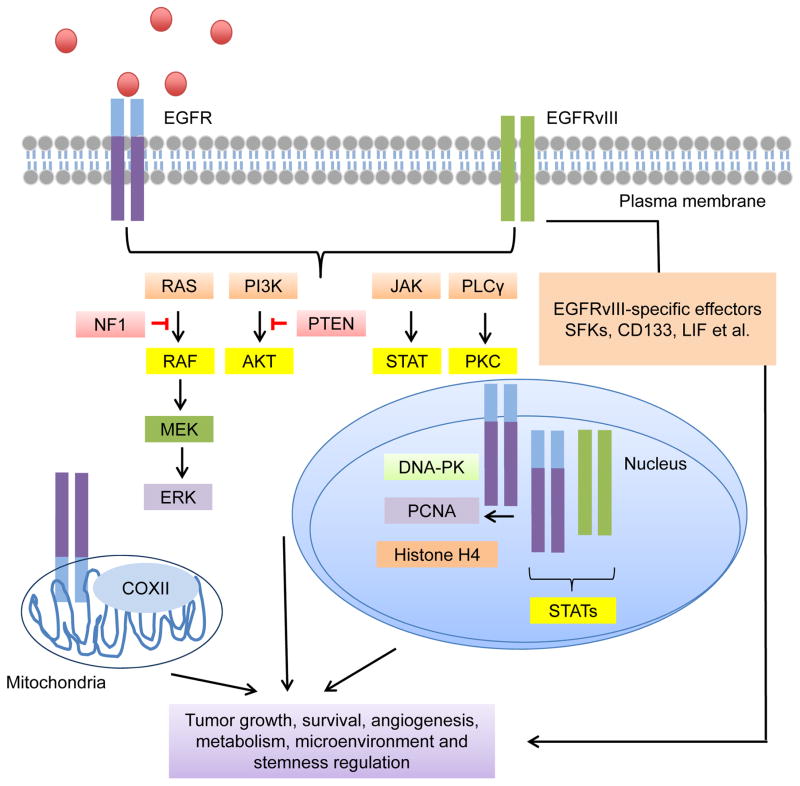
Signaling pathways mediated by EGFR are summarized in Figure 2. EGFR signaling can be divided based on EGFR localization. The first group of pathways are mediated by EGFR signaling from the plasma membrane, including the RAS/mitogen activated protein kinase (MAPK)/extracellular signal–regulated kinase (ERK) pathway, PI3K/protein kinase B (PKB/AKT) pathway, Janus Kinase (JAK)/STAT pathway, and protein kinase C (PKC) pathway. The second group includes signaling driven by localization of EGFR in non-plasma membrane compartments, including nuclear- (signaling molecules include DNA-PK, PCNA, Histone H4 and STAT proteins) and mitochondrial (signaling molecules include COXII)-localized EGFR. How EGFR signals to these targets is more poorly characterized but is attracting increasing attention. EGFRvIII shares signaling pathways with EGFR but also displays unique features, as presented below. Some signaling molecules downstream of EGFR/EGFRvIII are mutated frequently in GBM, emphasizing the importance of EGFR/EGFRvIII signaling in GBM. The most frequent mutations of EGFR/EGFRvIII-regulated molecules are summarized in Table 1.
Figure 2. Signaling pathways mediated by EGFR/EGFRvIII.
EGFR and EGFRvIII are able to transduce signals via classic RTK pathways including the RAS/RAF/MEK/ERK pathway, the PI3K/AKT pathway, the JAK/STAT pathway and the PKC pathway. The function of mitochondrial EGFR was also reported. Mitochondrial EGFR effector includes COXII. EGFR and EGFRvIII can also localize to the nucleus to activate a group of transcription factors and proteins involved in DNA damage responses, such as DNA-PK, PCNA, histone H4 and STATs. EGFRvIII has some unique signaling effectors. Activation of these signaling pathways and effector molecules together promote the fitness of the tumors.
Table 1.
Mutations of EGFR and related pathways in GBM
“Percentage” indicates frequencies of mutations in GBM patients.
| GENE Symbol | Chromosomal location | Alteration | Percentage |
|---|---|---|---|
| EGFR | Ch 7p | Amplification/mutation | 57.4 |
| 11.8 with EGFRvIII | |||
| p110α | Ch 3q | Mutation | 18.3 |
| p85α | Ch 5q | Mutation | 6.8 |
| PTEN | Ch 10q | Mutation/deletion | 34.3 |
| NF1 | Ch 17q | Mutation/deletion | 10 |
Signaling mediated by the membrane-bound RTK function of EGFR
The RAS/MAPK/ERK pathway
RAS proteins are small GTPases.29 The GTP to GDP switch is regulated by guanine nucleotide exchange factors (GEFs) or GTPase activating proteins (GAPs), and controls activation/inactivation of RAS proteins.29 RAS-GEFs include son of sevenless (SOS), while RAS-GAPs include the tumor suppressor neurofibromin 1 (NF1).29
Upon activation, EGFR recruits the SH2 domain-containing protein GRB2 in a preformed complex with RAS GEF SOS, facilitating activation of RAS.30 Activated RAS drives the RAF-MAPK/ERK kinase (MEK)-ERK1/2 signaling cascade. Activated ERK1/2 in turn phosphorylate a large number of downstream substrates, and translocate to the nucleus to modulate the activity of a range of proteins and transcription factors.31 ERK1/2 play critical roles role in regulating proliferation, survival and metabolism. ERK1/2 can also function in tumor suppressing pathways and this function is dependent on activation strength.31
The three human RAS genes (K-RAS, H-RAS and N-RAS) are mutated in 20–30% of all human cancers.32 Although mutation of RAS is rare in GBM (only 2%), high RAS activity in the tumor is frequently observed.33, 34 Additionally, the RAS-GAP NF1 is mutated or deleted in 18% of GBM patients. Tumors with NF1 mutation/deletion show activation of RAS, measured by p-ERK and p-MEK.6, 33 These results indicate that the EGFR/RAS/MEK/ERK pathway plays an important role in pathogenesis. A recent study indicates that oncogenic KRAS and EGFR mutation in lung adenocarcinoma leads to synthetic lethality.35 It will be interesting to know if oncogenic RAS coupled with EGFR/EGFRvIII amplification similarly promotes synthetic lethality in GBM, which may provide insight into the low frequency of RAS mutations in GBM.
The PI3K/AKT pathway
The PI3Ks are kinases that phosphorylate cellular lipids.36 Based on differences in structure and substrate specificity, PI3Ks can be classified into three different classes, among which Class IA PI3Ks play major roles in cancers. The functions and signaling pathways of PI3Ks have been reviewed recently.11, 36
Class IA PI3Ks contain catalytic p110 and regulatory p85 subunits.11 Active EGFR associates with p85 through dimerization with human epidermal growth factor receptor 3 (HER3), or through the docking protein GRB2-associated binder 1 (GAB1), and relieves the inhibitory effect of p85.37 P110 is able to phosphorylate phosphotidylinositol 4,5-Bisphosphate (PIP2) to phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which serves as a membrane-docking site for AKT.36 Phosphoinositide-dependent kinase 1 (PDK1) then partially activates AKT by phosphorylating it at T308, and mammalian target of rapamycin complex 2 (mTORC2) fully activates it by phosphorylating S473.36 Phosphatase and Tensin Homolog (PTEN), a tumor suppressor frequently mutated in GBM, dephosphorylates PIP3 to PIP2 and negatively regulates the PI3K/AKT signaling.36 A recent study indicates that loss of chromosome 10q (including PTEN) is an early event that precedes EGFR amplification.38
AKT substrates, proteins critical in regulating cell proliferation and survival, include tuberous sclerosis complex (TSC),39 BCL2 associated death protein (BAD),40 Beclin 1,41 Caspase-9,42 inhibitor of nuclear factor kappa-B (NFκB) kinase subunit alpha (IKKα),43 transcription factors cAMP response element-binding protein (CREB)44 and forkhead homeobox type O (FOXO).45 AKT also promotes metabolism by facilitating membrane localization and expression of glucose transporters,46 phosphorylating critical enzymes in metabolism such as 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase,47 and phosphorylating ATP-citrate lyase (ACLY), enabling production of acetyl Co-A production.48 ACLY regulates histone acetylation, which correlates with levels of p-AKT.48
The JAK/STAT pathway
JAKs are receptor tyrosine kinases that interact with cytokine receptors. The interaction of JAK2 with EGFR confers resistance to EGFR inhibitors.49 Upon cytokine binding, JAK is activated and phosphorylates STAT proteins, which dimerize and translocate to the nucleus.50 Nuclear STAT proteins can activate or repress transcription of target genes to facilitate transformation, inflammation-associated tumorigenesis, stemness and migration. STAT3 can also be directly phosphorylated and activated by EGFR.51 Phosphorylation at Y705 by EGFR induces dimerization of STAT3.51, 52 AKT phosphorylation of EZH2, which mediates DNA methylation, could further activate STAT3.53 As AKT is also downstream of EGFR, EGFR can activate STAT3 through multiple downstream effectors.
The role of STAT3 in GBM tumorigenesis depends on the mutational status of other genes.54 For example, STAT3 suppresses PTEN loss-induced malignant transformation of astrocytes.54 However, when EGFRvIII is expressed, STAT3 forms a complex with EGFRvIII and drives malignant transformation.17, 54 STAT3 also initiates mesenchymal transformation in high-grade gliomas.55 Activation of STAT3 promotes tumor growth and survival by suppressing the antitumor response of the immune system, and also by inducing tumor stemness and angiogenesis.56–58 Inhibition of STAT using small molecule inhibitors of JAK2 sensitizes U87.EGFR and U87.EGFRvIII cells to the EGFR inhibitor gefitinib in vitro.59
The phospholipase C (PLC)/PKC pathway
Active EGFR is able to recruit and activate PLC, which catalyzes the cleavage of PIP2 to inositol 1,4,5-trisphosphate (IP3) and diacyl glycerol (DAG).60 Activated PLC activates PKC. PKCs are a large family of phospholipid-dependent serine/threonine kinases classified into three families based on structure and activation mechanisms:61 classic PKCs (PKCα, βI, βII, and γ), nonclassic PKCs (δ, ε, η, and θ) and atypical PKCs [PKCζ and ι(λ)]. PKCs may be tumor suppressors or oncogenes depending on context.61–63 PKCs are able to activate a large group of effector molecules to regulate tumor proliferation, angiogenesis, infiltration, and survival. Downstream effectors of PKC isoenzymes include cell cycle regulators p53 and p21, cell growth and proliferation regulators RAS-RAF1 and glycogen synthase kinase 3 (GSK3), cell motility regulators integrins, cell survival regulators BCL2 and BAD, and the inflammation regulator NFκB.61
Different types of tissues usually show expression of different PKC isoenzymes. In GBM, PKC isoenzymes including PKCα, PKCη and PKCδ showed pro-tumorigenic functions.64–67 High levels of PLCγ are correlated inversely with survival.68 EGFR transduces signaling to mTOR in a PKC-dependent manner.64 Inhibition of PKC decreased viability of GBM cells, indicating that PKC is a critical signaling node.64
The function of non-plasma-membrane-bound EGFR
EGFR also plays important roles in intracellular compartments in the mitochondria and the nucleus (topology shown in Fig. 2). Phosphorylation of Y845 of EGFR by SRC stimulates the mitochondrial trafficking of EGFR.69 Mitochondrial EGFR associates with and phosphorylates a subunit of Complex IV, cytochrome oxidase subunit II (COXII) at the inner mitochondrial membrane, leading to decrease in COXII activity and respiratory inhibition.69
EGFR also localizes to the nucleus,70 where it functions as a cofactor alongside transcription factors such as STAT3/5 to enhance transcription of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX2), MYC, Aurora-A kinase (AURKA) and other genes critical in cell growth and survival.70 Nuclear EGFR (nEGFR) can also phosphorylate DNA-dependent protein kinase (DNA-PK), proliferating cell nuclear antigen (PCNA) and histone H4 to function in DNA repair and replication.71–73 Nuclear translocation of EGFR is regulated by SRC Family Kinases (SFKs), AKT, Phosphatidylinositol 3-phosphate 5-kinase (PIKfyve), AXL and PKCε.74–76 EGFR can phosphorylate EGFRvIII to activate STAT3/5 in the nucleus, and facilitate tumor progression.17 nEGFR/nEGFRvIII also play critical roles in GBM growth and survival.77, 78
Signaling mediated by EGFRvIII
EGFRvIII confers a growth advantage to GBM. Patients with EGFRvIII have significantly shortened survival.79 The pro-tumorigenic function of EGFRvIII results from its ability to promote tumor growth, survival, invasion, stemness, metabolism and angiogenesis.23, 24, 80–82 EGFRvIII signaling is different from EGFR signaling both quantitatively (difference in signal strength) and qualitatively (difference in signaling molecules). EGFRvIII has lower-level constitutive kinase activity, which is partially due to impaired endocytosis and degradation.83 As a constitutively active mutant of EGFR, EGFRvIII is able to transduce signals via traditional EGFR pathways controlled by RAS/MAPK, PI3K/AKT and JAK/STAT. The pro-tumorigenic function of EGFRvIII is dependent on its kinase activity, as kinase dead EGFRvIII in some contexts fails to promote tumor formation and growth.16 Epigenomic and transcriptomic analyses indicate that EGFRvIII specifically activates more than 2000 enhancers and regulates GBM sensitivity to the small-molecule bromodomain ligand JQ1 by modulating transcription.84 Below we discuss some examples of EGFRvIII-regulated pathways.
EGFRvIII phosphorylates other kinases and receptors
EGFRvIII interacts with and phosphorylates both kinases and receptor molecules. For example, EGFRvIII phosphorylates SFKs, which further activate dedicator of cytokinesis 1 (DOCK1).85 DOCK1 plays critical roles in mediating cell growth and migration, contributing to the pro-tumorigenic function of EGFRvIII.23, 85 EGFRvIII can also increase phosphorylation of DOCK1 through PKA.23 EGFRvIII forms a complex with the cytokine receptor OSMR, which regulates the EGFRvIII-STAT3 signaling axis.86 Additionally, SFK activation promotes mitochondrial localization of EGFRvIII, and increases cell survival under low glucose conditions.87 Moreover, EGFRvIII activates hepatocyte growth factor receptor (MET), which in-turn drives STAT3.88, 89 EGFRvIII also activates c-SRC, which promotes secretion of vascular endothelial growth factor (VEGF) and angiogenesis.13 Phosphorylation of these kinases further activates downstream signaling pathways and contributes to tumor progression.
EGFRvIII modulates gene and protein expression
EGFRvIII is able to remodel global transcription.84 EGFRvIII increases expression of proteins critical in apoptosis, invasion, stemness, metabolism and angiogenesis including BCL-xL, extracellular matrix (ECM) proteins, abnormal spindle-like microcephaly-associated protein (ASPM), glucose transporter protein type 1 (GLUT1), leukemia inhibitory factor (LIF) and heterogeneous nuclear ribonucleoprotein A1 splicing factor (hnRNPA1).24, 78, 82, 90–92 These molecules contribute to proliferation and survival.
EGFR/EGFRvIII-related mouse models of glioma
Mice carrying targeted deletions in the Ink4a/arf locus displayed glioma-like lesions in the setting of an active mutant of EGFR.93 The EGFR homologue v-erbB expressed under the S100β promoter led to low-grade gliomas, which were higher grade and more aggressive after crossing to mice with p53 or Ink4a/arf mutations.94 These observations suggested that other mutations cooperated with v-erbB to promote high-grade glioma. Neither EGFR nor EGFRvIII expressed under the GFAP promoter generated tumors.95 However, EGFRvIII was able to accelerate tumors driven by HRasG12V, changing the histopathology of these gliomas from astrocytoma to oligodendroglioma or mixed oligoastrocytoma.95
Crosstalk among EGFR, EGFRvIII and other RTKs
EGFRvIII regulates EGFR activity by inducing expression of EGFR ligands such as TGF-α and HB-EGF.96 EGFRvIII can also form transient dimers with EGFR, ERBB family members, and self-dimerize.97–99 Forced-dimerization of EGFRvIII increased its capacity to promote tumor growth.100 We and others have shown that EGFRvIII is a substrate of EGFR, but EGFRvIII is unable to phosphorylate EGFR.17, 98 A previous publication indicated that EGFRvIII was able to phosphorylate wild-type EGFR in a murine cell line Ba/F3 that did not express endogenous EGFR.99 Whether different experimental systems in these studies led to distinct results awaits further evaluation.
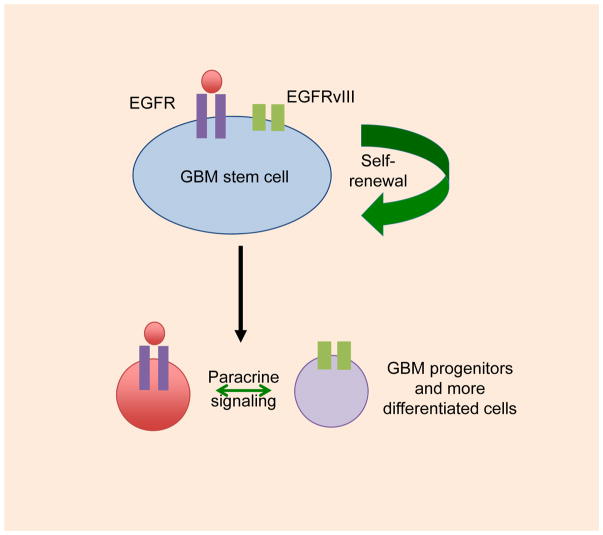
While rare cells in GBM co-express EGFR and EGFRvIII,17 the majority of tumor cells express predominantly EGFR or EGFRvIII. These observations suggest a hierarchical model, with EGFR and EGFRvIII potentially signaling in cancer stem cell compartment, and segregating separately to daughter cells in the tumor (Figure 3). In cells where EGFR and EGFRvIII are expressed in separate cells, EGFRvIII promotes the growth of cells expressing wild-type EGFR via a paracrine interleukin 6 (IL-6) and/or LIF-dependent manner. These cytokines activate EGFR via glycoprotein 130 (GP130).92
Figure 3. Hierarchical model for EGFR and EGFRvIII signaling.
Rare cells in human GBM tumors that co-amplify EGFR and EGFRvIII show co-expression, with co-expression of EGFR and EGFRvIII driving malignancy more robustly, as compared to cells expressing EGFR or EGFRvIII alone. Most cells in human GBM tumors that co-amplify EGFR and EGFRvIII express predominantly EGFR or EGFRvIII, and cells expressing EGFRvIII can signal in a paracrine manner to cells expressing EGFR. A possible model incorporating these features is shown. See text for details.
The EGFRvIII RNA and protein can also be transferred to other cells via microvesicles to increase the fitness of the tumor.101, 102 Importantly, single-cell sequencing verified that EGFRvIII only exists in a subset of tumor cells. Different cells in the same tumor can have distinct EGFR mutations,103 and, distinct tumor cell subpopulations can amplify different RTKs.104 For example, in platelet derived growth factor receptor alpha (PDGFRA)-amplified GBM tumors, amplification of MET or EGFR is also observed frequently.105 Inhibition of a single RTK can lead to upregulation of other RTKs. For example, inhibition of EGFR upregulated transcription of PDGFRβ and promoted resistance to EGFR TKI treatment.106 Tumor cell subpopulations harboring distinct EGFR mutations or RTK amplifications add to the complexity of GBM and contribute to resistance to EGFR/EGFRvIII-directed therapies.103, 105 As compensatory activation of ERBB family receptors also occurs in the setting of EGFR inhibition,107 co-inhibition of EGFR and other ERBBs may cooperate to inhibit GBM growth.
Targeting EGFR/EGFRvIII for therapy in GBM
Current anti-EGFR/EGFRvIII therapeutics are summarized in Table 2. They include, but are not limited to small molecule TKIs, antibodies, vaccines, chimeric antigen receptor (CAR) T cells and RNA-based therapies.
Table 2.
EGFR/EGFRvIII-targeted therapies
| EGFR/EGFRvIII targeted therapy | Effects in GBM | References | ||
|---|---|---|---|---|
| Inhibitors | 1st generation | Gefitinib | Not very effective in GBM clinical trials. | 108–109 |
| Erlotinib | ||||
| Lapatinib | ||||
| 2nd generation | Afatinib | Good safety but limited single-reagent activity on GBM patients. Promising in combination with TMZ in a case report. | 115 | |
| Dacomitinib | Promising in pre-clinical models. | 116 | ||
| 3rd generation | Rociletinib | Effects on GBM to be evaluated. | ||
| AZD9291 | In phase I/II clinical trial | |||
| Antibodies | Targeting the L2 domain of EGFR | Cetuximab | Not very effective in GBM clinical trials. | 119–122 |
| Panitumumab | ||||
| Nimotuzumab | ||||
| Targeting the EGFRvIII-specific sequence | mAB806 | Good safety. Anti-GBM effects to be evaluated. | 123 | |
| Toxin or radioactive isotope conjugated | 125I mAB425 | When used singly or in combination with TMZ, significantly prolonged patient survival. | 124 | |
| BisAbs | bscEGFRvIIIxCD3 | Promising in pre-clinical models. | 126 | |
| Vaccines | Rindopepimut (CDX110) | Significantly prolonged patient survival when co-administrated with TMZ. Failed phase III trial. | 130–133 | |
| CARs | CAR-T cells targeting EGFRvIII | Promising in pre-clinical models. Currently under phase I clinical trial. | 134–136 | |
| RNA-based therapies | Antisense oligonucleotides, RNAi, ribozymes and adjuvant miRNA based therapy | Feasibility to be further evaluated in pre-clinical models. | 137–138 | |
Small molecule inhibitors of EGFR/EGFRvIII
First generation EGFR inhibitors were designed to orthosterically block the ATP/substrate-binding pocket of EGFR in the tyrosine kinase domain. FDA-approved first-generation EGFR inhibitors include gefitinib (ZD1839; Iressa), erlotinib (OSI-774; Tarceva) and lapatinib (GW572016; Tykerb/Tyverb). Although these inhibitors showed promising results in inhibiting growth and improving survival in preclinical models, they have had limited activity in patients.108, 109 Erlotinib and gefitinib are known to affect EGFR activity in patients with lung cancer, whose activating mutations typically lie in exons 19 and 21 of the tyrosine kinase domain.110 These mutations do not exist in GBM, potentially contributing to the lack of survival benefit in patients treated with erlotinib or gefitinib.
The blood brain barrier represents a real barrier to effective use of EGFR TKIs. Melanoma patients treated with vemurafenib required a dose that blocked over 80% of downstream p-ERK to achieve a clinical response.111 If pathway inhibition of >80% is required for clinical responses more generally, then the blood brain barrier represents a clear obstacle. While the blood brain barrier in GBM is compromised, this magnitude of this compromise is likely inadequate to enable such efficient inhibition. Indeed, for patients treated with lapatinib (which binds EGFR better than erlotinib or gefitinib112), only modest inhibition of p-EGFR was achieved in biopsied post-treatment tumors.112 In addition, erlotinib has lower affinity for EGFRvIII compared to EGFR mutants in non-small-cell lung cancer (NSCLC), and releases more rapidly than from EGFRWT.112, 113 The blood-brain barrier clearly limits drug concentration in tumors, and is an important factor in the resistance of GBM to EGFR inhibitors.114
Second generation EGFR-TKIs are designed to bind irreversibly to the tyrosine kinase domain of EGFR and other ERBB family members. Afatinib and dacomitinib are FDA-approved. In a phase I/randomized phase II study, afatinib showed good safety but limited single-reagent activity in GBM patients.115 In preclinical studies, dacomitinib showed efficacy in GBM.116 Whether these inhibitors can achieve therapeutic dose in the tumors of GBM patients awaits more studies.
Third generation EGFR inhibitors such as rociletinib and AZD9291 are currently being tested pre-clinically in GBM. These new drugs efficiently overcome resistance caused by the EGFR T790M gatekeeper mutation in non-small cell lung cancer, improving survival.117, 118 In preclinical models, AZD9291 demonstrated lower potency in inhibiting EGFRvIII compared to afatinib.117 Although the T790M mutation is infrequent in GBM patients, AZD9291 can efficiently cross the blood-brain barrier, making it an attractive candidate for inhibiting EGFR in GBM. AZD9291 is now in a phase I/II clinical trial in GBM (NCATS 1-UH2-TR001370-01)
EGFR/EGFRvIII Antibodies
FDA-approved anti-EGFR antibodies include cetuximab (C225; Erbitux), panitumumab (ABX-EGF; Vectibix), and nimotuzumab. All of these antibodies bind to the L2 domain, preventing ligand binding and/or dimerization of EGFR.119 Cetuximab binds to EGFRvIII and induces its internalization and mitochondrial localization. However, It fails to inhibit the activity of EGFRvIII.120 Nimotuzumab inhibits growth of U87MG.EGFRvIII cells in vitro.121 So far, these antibodies have not shown promise in treating GBM patients.122
Compared to EGFRWT, EGFRvIII is not responsive to antibodies targeting the L2 domain because of the deletion mutation in the ligand-binding domain. mAb806 (ABT-806), a monoclonal antibody which only targets overexpressed-EGFR or co-expression of EGFR and EGFRvIII due to its binding affinity properties, was safe in GBM patients in a phase I clinical trial.123 This antibody did not show significant binding to wild-type EGFR in normal human tissues.123
Antibodies can be conjugated to toxins or radioactive isotopes to enhance tumor killing. 125I mAb 425, either singly used or in combination with TMZ, was well-tolerated in patients and prolonged survival in a phase II clinical trial.124 Bispecific antibodies (bisAbs) contain two different binding specificities fused into one molecule.125 They can be engineered to bispecific T-cell engagers (BiTEs), which bind to the CD3 T-cell coreceptor to recruit cytotoxic T cells. A BiTE named bscEGFRvIIIxCD3, designed to redirect T-cells to tumors expressing EGFRvIII, showed potent killing of EGFRvIII-expressing GBM in vitro and in mice.126 Injection of bscEGFRvIIIxCD3 intravenously achieved complete cure in up to 75% in NSG mice with U87.EGFRvIII intracranial xenografts.126 Whether this BiTE can be used in patients awaits further study.
An important question for using antibodies to treat GBM is whether they can be effectively delivered to the tumors. Antibodies typically neither cross the blood-brain barrier effectively nor achieve adequate penetration depth inside the tumor,127 although invasive surgery can disrupt the blood-brain barrier temporarily. Recently, studies show that the blood-brain barrier can be opened using non-invasive methods.128 Advancement in modulating the permeability of the blood-brain barrier and new drug delivery technology will be helpful to solve those problems.
Anti- EGFRvIII vaccines
Vaccines can activate the host immune system, provide durable responses and therefore hold promise for therapy. Anti-GBM vaccines, such as GlioVac, a mixture of inactivated tumor cells, are under clinical evaluation.129 As EGFRvIII is tumor-specific, a targeted vaccine of EGFRvIII is appealing. Rindopepimut (CDX-110) consists of a 14-mer peptide that spans the mutation site of EGFRvIII conjugated to the immune adjuvant keyhole limpet hemocyanin (KLH). CDX-110 specifically eliminates tumor cells that express EGFRvIII.130 This vaccine was proven to be safe, immunogenic, and tumor-specific in a phase I clinical trial.131 In a multicenter phase II trial, together with TMZ, CDX-110 treatment of a relatively healthy group of GBM patients significantly improved overall survival of EGFRvIII+ GBM compared to less healthy historical controls.132 However, CDX-110 failed in a double-blind randomized phase III trial (“ACT IV”) that had an active control arm.27, 132, 133
Chimeric antigen receptors (CARs)
CARs are engineered receptors containing a single-chain variable fragment (scFv) derived from monoclonal antibodies coupled to the transmembrane and the intracellular activation domains of the surface receptors of immune cells, such as T cells and NK cells. Cells expressing these CARs recognize and kill target cells that express the antibody-targeted molecule. EGFRvIII-specific CAR-T cells, when administered systemically, suppressed tumor growth and enhanced survival of mice in preclinical GBM models.134 Currently, CAR-T cells targeting EGFRvIII are under phase I study to determine safety and feasibility for EGFRvIII positive GBM patients (NCT02209376).134 Recently, CAR-modified NK cells also showed potent killing of GBM cells.135 CAR modified immune cells are able to cross the blood-brain barrier in patients and preclinical models, which shows promise in GBM therapy.136
RNA-based therapies
Current RNA-based therapies targeting EGFR/EGFRvIII include antisense oligonucleotides, RNA interference (RNAi), ribozymes and adjuvant microRNA (miRNA) based therapies. The principle is to reduce mRNA levels of EGFR/EGFRvIII to inhibit tumor growth. Although robust decreases in EGFR/EGFRvIII expression and tumor proliferation were observed in several studies in vitro, in vivo efficacy of RNA-based therapies still needs to be improved.137, 138 The blood-brain-barrier, inefficient tumor-targeted delivery, and off-target effects represent obstacles that need to be overcome.
Factors contributing to resistance to EGFR/EGFRvIII inhibition in GBM
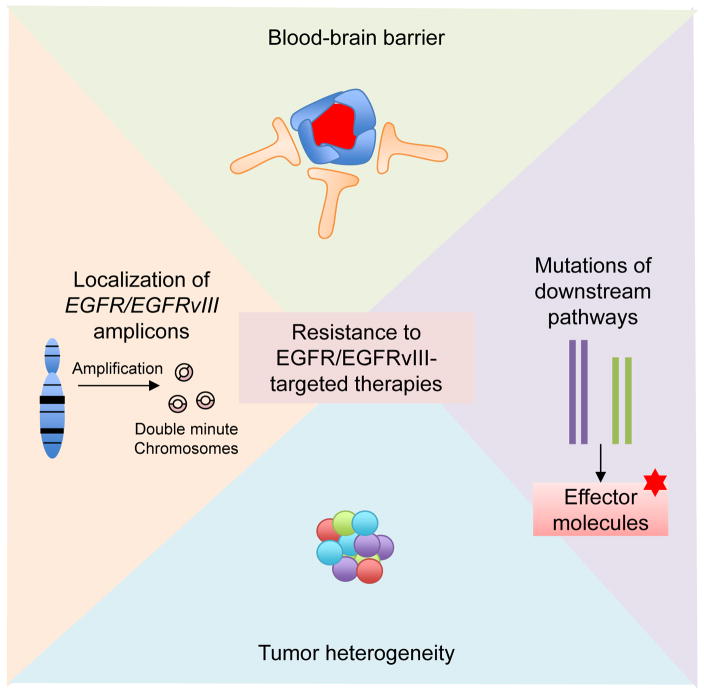
Many chemicals or antibodies targeting EGFR/EGFRvIII are not efficient at crossing the blood-brain barrier, which limits their efficacy. Additionally, EGFR/EGFRvIII are tyrosine kinases at the upstream end of signal transduction pathways (Figure 4). Mutations or deregulation of downstream molecules and upregulation of redundant receptor tyrosine kinases may bypass EGFR/EGFRvIII inhibition. PTEN negatively regulates PI3K signaling downstream of EGFR.139, 140 Patients with EGFR amplification and with an intact PTEN gene showed a radiographic response (but ultimately did not show improved survival) when treated with EGFR inhibitors. Similar patients whose tumors showed loss of PTEN did not respond radiographically to EGFR inhibitors.140 SFK and FGFR-dependent phosphorylation of PTEN at Y240 also confers resistance.141 Moreover, EGFR inhibition by erlotinib increased nuclear expression of the promyelocytic leukemia (PML) tumor suppressor, prevented cell death and promoted tumor cell survival.142 As RTKs usually activate similar downstream pathways, in GBM, upregulation or activation of receptors such as the IGF-1 receptor (IGF-1R), MET, and PDGFRβ is also a mechanism contributing to resistance to EGFR/EGFRvIII inhibition.106, 143–145
Figure 4. Factors contributing to resistance to EGFR/EGFRvIII-targeted therapies.
Factors contributing to resistance to EGFR/EGFRvIII inhibition include blood-brain barrier penetrance (i.e. many antibodies and chemicals cannot across the blood-brain barrier), mutations of signaling molecules downstream of EGFR/EGFRvIII (i.e. PTEN mutation and NF1 mutation, which maintain activation of downstream pathways despite upstream inhibition), tumor heterogeneity (distinct tumor cells can harbor different mutations or receptor kinase amplification, interaction between tumor cells and stromal cells in the tumor microenvironment) and extrachromosomal localization of EGFR and EGFRvIII amplicons (facilitating the cells’ ability to evade EGFR inhibitors).
GBMs are highly heterogeneous. They usually contain a mixture of cells with EGFR, MET, or PDGFA amplification.105, 146 Therefore, targeting a single RTK may not be sufficient to inhibit GBM.104 In addition, RTK amplification is usually located at extrachromosomal double minute structures, which confers another layer of resistance by increasing tumor heterogeneity and drug resistance.28, 147 In response to EGFR TKI treatment, EGFRvIII from extrachromosomal DNA was eliminated. However, after drug withdrawal, extrachromosomal DNA with EGFRvIII reemerged.28 A better understanding of the dynamics of EGFR/EGFRvIII containing extrachromosomal DNA promises to provide insights into EGFR/EGFRvIII-targeted therapies. The heterogeneity in GBM also extends to tumor-infiltrating cells, among which microglia/macrophages make up the largest population, contributing at least one third of the total tumor mass.148 Despite the fact that almost all EGFRvIII+ GBM tumors co-amplify EGFR, and that co-expression of those two molecules dramatically promotes tumor growth, how EGFR and EGFRvIII cooperate to regulate infiltration of immune cells in the tumor microenvironment remains largely unknown. A recent study performed parallel RNAi screens in vitro and in vivo in GBM. Hits in-vitro identified genes regulating cell metabolism, whereas the in-vivo screen identified genes that promoted survival of GBM cells in the tumor microenvironment, reinforcing the importance of targeting tumor-stroma cross talk.149
Conclusions and future directions
EGFR and EGFRvIII amplification are frequently observed in GBMs, contributing to tumorigenesis and progression. However, therapies directed against EGFR and EGFRvIII have not yet shown clear benefit clinically. Inefficient blood brain barrier penetration, as well as intratumoral heterogeneity, compensatory signaling pathways and secondary mutations all contribute to resistance. Immunotherapy targeting EGFRvIII may effectively kill EGFRvIII-positive tumor cells, but leaves behind residual tumor cells that retain EGFR-dependence, independently of EGFRvIII. Combination therapies are needed to improve outcome. A better understanding of GBM heterogeneity, crosstalk among tumor cells and stromal cells, along with improved understanding of the EGFR/EGFRvIII signaling network, its interplay with other pathways, better drugs, improved brain penetration and clarifying mechanisms of resistance will improve our ability to target this pathway.
Acknowledgments
Z.A. is supported by the Alex’s Lemonade Stand Foundation and by Program for Breakthrough Biomedical Research, which is partially funded by the Sandler Foundation. The Weiss lab is supported by NIH grants CA148699, NS091620, NS0883555, NS089868, CA183692, CA176287, and CA82103; the Alex’s Lemonade Stand, Children’s Tumor, Katie Dougherty, Pediatric Brain Tumor, Ross K. MacNeill, St. Baldrick, and Samuel G. Waxman Foundations; and the Evelyn and Mattie Anderson Chair.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma: from molecular pathology to targeted treatment. Annual review of pathology. 2014;9:1–25. doi: 10.1146/annurev-pathol-011110-130324. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 3.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:764–772. doi: 10.1158/1078-0432.CCR-12-3002. [DOI] [PubMed] [Google Scholar]
- 4.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jawhari S, Ratinaud MH, Verdier M. Glioblastoma, hypoxia and autophagy: a survival-prone ‘menage-a-trois’. Cell death & disease. 2016;7:e2434. doi: 10.1038/cddis.2016.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer cell. 2014;25:282–303. doi: 10.1016/j.ccr.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh B, Coffey RJ. Trafficking of epidermal growth factor receptor ligands in polarized epithelial cells. Annual review of physiology. 2014;76:275–300. doi: 10.1146/annurev-physiol-021113-170406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer BJ. The discovery of modular binding domains: building blocks of cell signalling. Nature reviews Molecular cell biology. 2015;16:691–698. doi: 10.1038/nrm4068. [DOI] [PubMed] [Google Scholar]
- 11.Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nature reviews Cancer. 2015;15:7–24. doi: 10.1038/nrc3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furnari FB, Cloughesy TF, Cavenee WK, Mischel PS. Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nature reviews Cancer. 2015;15:302–310. doi: 10.1038/nrc3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskilsson E, Rosland GV, Talasila KM, Knappskog S, Keunen O, Sottoriva A, et al. EGFRvIII mutations can emerge as late and heterogenous events in glioblastoma development and promote angiogenesis through Src activation. Neuro-oncology. 2016;18:1644–1655. doi: 10.1093/neuonc/now113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugawa N, Ekstrand AJ, James CD, Collins VP. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:8602–8606. doi: 10.1073/pnas.87.21.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo G, Gong K, Wohlfeld B, Hatanpaa KJ, Zhao D, Habib AA. Ligand-Independent EGFR Signaling. Cancer research. 2015;75:3436–3441. doi: 10.1158/0008-5472.CAN-15-0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD, et al. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. The Journal of biological chemistry. 1997;272:2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- 17.Fan QW, Cheng CK, Gustafson WC, Charron E, Zipper P, Wong RA, et al. EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer cell. 2013;24:438–449. doi: 10.1016/j.ccr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batra SK, Castelino-Prabhu S, Wikstrand CJ, Zhu X, Humphrey PA, Friedman HS, et al. Epidermal growth factor ligand-independent, unregulated, cell-transforming potential of a naturally occurring human mutant EGFRvIII gene. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1995;6:1251–1259. [PubMed] [Google Scholar]
- 19.Moscatello DK, Montgomery RB, Sundareshan P, McDanel H, Wong MY, Wong AJ. Transformational and altered signal transduction by a naturally occurring mutant EGF receptor. Oncogene. 1996;13:85–96. [PubMed] [Google Scholar]
- 20.Boyd PS, Struve N, Bach M, Eberle JP, Gote M, Schock F, et al. Clustered localization of EGFRvIII in glioblastoma cells as detected by high precision localization microscopy. Nanoscale. 2016;8:20037–20047. doi: 10.1039/c6nr05880a. [DOI] [PubMed] [Google Scholar]
- 21.Ekstrand AJ, Liu L, He J, Hamid ML, Longo N, Collins VP, et al. Altered subcellular location of an activated and tumour-associated epidermal growth factor receptor. Oncogene. 1995;10:1455–1460. [PubMed] [Google Scholar]
- 22.Pedersen MW, Tkach V, Pedersen N, Berezin V, Poulsen HS. Expression of a naturally occurring constitutively active variant of the epidermal growth factor receptor in mouse fibroblasts increases motility. International journal of cancer Journal international du cancer. 2004;108:643–653. doi: 10.1002/ijc.11566. [DOI] [PubMed] [Google Scholar]
- 23.Feng H, Hu B, Vuori K, Sarkaria JN, Furnari FB, Cavenee WK, et al. EGFRvIII stimulates glioma growth and invasion through PKA-dependent serine phosphorylation of Dock180. Oncogene. 2014;33:2504–2512. doi: 10.1038/onc.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonavia R, Inda MM, Vandenberg S, Cheng SY, Nagane M, Hadwiger P, et al. EGFRvIII promotes glioma angiogenesis and growth through the NF-kappaB, interleukin-8 pathway. Oncogene. 2012;31:4054–4066. doi: 10.1038/onc.2011.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katanasaka Y, Kodera Y, Kitamura Y, Morimoto T, Tamura T, Koizumi F. Epidermal growth factor receptor variant type III markedly accelerates angiogenesis and tumor growth via inducing c-myc mediated angiopoietin-like 4 expression in malignant glioma. Molecular cancer. 2013;12:31. doi: 10.1186/1476-4598-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Congdon KL, Gedeon PC, Suryadevara CM, Caruso HG, Cooper LJ, Heimberger AB, et al. Epidermal growth factor receptor and variant III targeted immunotherapy. Neuro-oncology. 2014;16(Suppl 8):viii20–25. doi: 10.1093/neuonc/nou236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malkki H. Trial Watch: Glioblastoma vaccine therapy disappointment in Phase III trial. Nature reviews Neurology. 2016;12:190. doi: 10.1038/nrneurol.2016.38. [DOI] [PubMed] [Google Scholar]
- 28.Nathanson DA, Gini B, Mottahedeh J, Visnyei K, Koga T, Gomez G, et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science. 2014;343:72–76. doi: 10.1126/science.1241328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papke B, Der CJ. Drugging RAS: Know the enemy. Science. 2017;355:1158–1163. doi: 10.1126/science.aam7622. [DOI] [PubMed] [Google Scholar]
- 30.Kolch W, Halasz M, Granovskaya M, Kholodenko BN. The dynamic control of signal transduction networks in cancer cells. Nature reviews Cancer. 2015;15:515–527. doi: 10.1038/nrc3983. [DOI] [PubMed] [Google Scholar]
- 31.Deschenes-Simard X, Kottakis F, Meloche S, Ferbeyre G. ERKs in cancer: friends or foes? Cancer research. 2014;74:412–419. doi: 10.1158/0008-5472.CAN-13-2381. [DOI] [PubMed] [Google Scholar]
- 32.Spiegel J, Cromm PM, Zimmermann G, Grossmann TN, Waldmann H. Small-molecule modulation of Ras signaling. Nature chemical biology. 2014;10:613–622. doi: 10.1038/nchembio.1560. [DOI] [PubMed] [Google Scholar]
- 33.Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nature genetics. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 35.Unni AM, Lockwood WW, Zejnullahu K, Lee-Lin SQ, Varmus H. Evidence that synthetic lethality underlies the mutual exclusivity of oncogenic KRAS and EGFR mutations in lung adenocarcinoma. eLife. 2015;4:e06907. doi: 10.7554/eLife.06907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klempner SJ, Myers AP, Cantley LC. What a tangled web we weave: emerging resistance mechanisms to inhibition of the phosphoinositide 3-kinase pathway. Cancer discovery. 2013;3:1345–1354. doi: 10.1158/2159-8290.CD-13-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattoon DR, Lamothe B, Lax I, Schlessinger J. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC biology. 2004;2:24. doi: 10.1186/1741-7007-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozawa T, Riester M, Cheng YK, Huse JT, Squatrito M, Helmy K, et al. Most human non-GCIMP glioblastoma subtypes evolve from a common proneural-like precursor glioma. Cancer cell. 2014;26:288–300. doi: 10.1016/j.ccr.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nature cell biology. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 40.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 41.Wang RC, Wei Y, An Z, Zou Z, Xiao G, Bhagat G, et al. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science. 2012;338:956–959. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 43.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 44.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. The Journal of biological chemistry. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 45.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 46.Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Molecular and cellular biology. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Novellasdemunt L, Tato I, Navarro-Sabate A, Ruiz-Meana M, Mendez-Lucas A, Perales JC, et al. Akt-dependent activation of the heart 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB2) isoenzyme by amino acids. The Journal of biological chemistry. 2013;288:10640–10651. doi: 10.1074/jbc.M113.455998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JV, Carrer A, Shah S, Snyder NW, Wei S, Venneti S, et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell metabolism. 2014;20:306–319. doi: 10.1016/j.cmet.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He K, Qi Q, Chan CB, Xiao G, Liu X, Tucker-Burden C, et al. Blockade of glioma proliferation through allosteric inhibition of JAK2. Science signaling. 2013;6:ra55. doi: 10.1126/scisignal.2003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annual review of medicine. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park OK, Schaefer TS, Nathans D. In vitro activation of Stat3 by epidermal growth factor receptor kinase. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13704–13708. doi: 10.1073/pnas.93.24.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 53.Kim E, Kim M, Woo DH, Shin Y, Shin J, Chang N, et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer cell. 2013;23:839–852. doi: 10.1016/j.ccr.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de la Iglesia N, Konopka G, Puram SV, Chan JA, Bachoo RM, You MJ, et al. Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway. Genes & development. 2008;22:449–462. doi: 10.1101/gad.1606508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.See AP, Han JE, Phallen J, Binder Z, Gallia G, Pan F, et al. The role of STAT3 activation in modulating the immune microenvironment of GBM. Journal of neuro-oncology. 2012;110:359–368. doi: 10.1007/s11060-012-0981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong AH, Wei P, Zhang S, Yao J, Yuan Y, Zhou AD, et al. FoxM1 Drives a Feed-Forward STAT3-Activation Signaling Loop That Promotes the Self-Renewal and Tumorigenicity of Glioblastoma Stem-like Cells. Cancer research. 2015;75:2337–2348. doi: 10.1158/0008-5472.CAN-14-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garner JM, Fan M, Yang CH, Du Z, Sims M, Davidoff AM, et al. Constitutive activation of signal transducer and activator of transcription 3 (STAT3) and nuclear factor kappaB signaling in glioblastoma cancer stem cells regulates the Notch pathway. The Journal of biological chemistry. 2013;288:26167–26176. doi: 10.1074/jbc.M113.477950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lo HW, Cao X, Zhu H, Ali-Osman F. Constitutively activated STAT3 frequently coexpresses with epidermal growth factor receptor in high-grade gliomas and targeting STAT3 sensitizes them to Iressa and alkylators. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:6042–6054. doi: 10.1158/1078-0432.CCR-07-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kadamur G, Ross EM. Mammalian phospholipase C. Annual review of physiology. 2013;75:127–154. doi: 10.1146/annurev-physiol-030212-183750. [DOI] [PubMed] [Google Scholar]
- 61.Garg R, Benedetti LG, Abera MB, Wang H, Abba M, Kazanietz MG. Protein kinase C and cancer: what we know and what we do not. Oncogene. 2014;33:5225–5237. doi: 10.1038/onc.2013.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antal CE, Hudson AM, Kang E, Zanca C, Wirth C, Stephenson NL, et al. Cancer-associated protein kinase C mutations reveal kinase’s role as tumor suppressor. Cell. 2015;160:489–502. doi: 10.1016/j.cell.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma L, Tao Y, Duran A, Llado V, Galvez A, Barger JF, et al. Control of nutrient stress-induced metabolic reprogramming by PKCzeta in tumorigenesis. Cell. 2013;152:599–611. doi: 10.1016/j.cell.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fan QW, Cheng C, Knight ZA, Haas-Kogan D, Stokoe D, James CD, et al. EGFR signals to mTOR through PKC and independently of Akt in glioma. Science signaling. 2009;2:ra4. doi: 10.1126/scisignal.2000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uht RM, Amos S, Martin PM, Riggan AE, Hussaini IM. The protein kinase C-eta isoform induces proliferation in glioblastoma cell lines through an ERK/Elk-1 pathway. Oncogene. 2007;26:2885–2893. doi: 10.1038/sj.onc.1210090. [DOI] [PubMed] [Google Scholar]
- 66.Aeder SE, Martin PM, Soh JW, Hussaini IM. PKC-eta mediates glioblastoma cell proliferation through the Akt and mTOR signaling pathways. Oncogene. 2004;23:9062–9069. doi: 10.1038/sj.onc.1208093. [DOI] [PubMed] [Google Scholar]
- 67.Amos S, Martin PM, Polar GA, Parsons SJ, Hussaini IM. Phorbol 12-myristate 13-acetate induces epidermal growth factor receptor transactivation via protein kinase Cdelta/c-Src pathways in glioblastoma cells. The Journal of biological chemistry. 2005;280:7729–7738. doi: 10.1074/jbc.M409056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mawrin C, Diete S, Treuheit T, Kropf S, Vorwerk CK, Boltze C, et al. Prognostic relevance of MAPK expression in glioblastoma multiforme. International journal of oncology. 2003;23:641–648. [PubMed] [Google Scholar]
- 69.Boerner JL, Demory ML, Silva C, Parsons SJ. Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Molecular and cellular biology. 2004;24:7059–7071. doi: 10.1128/MCB.24.16.7059-7071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang SC, Hung MC. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:6484–6489. doi: 10.1158/1078-0432.CCR-08-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liccardi G, Hartley JA, Hochhauser D. EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer research. 2011;71:1103–1114. doi: 10.1158/0008-5472.CAN-10-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang SC, Nakajima Y, Yu YL, Xia W, Chen CT, Yang CC, et al. Tyrosine phosphorylation controls PCNA function through protein stability. Nature cell biology. 2006;8:1359–1368. doi: 10.1038/ncb1501. [DOI] [PubMed] [Google Scholar]
- 73.Chou RH, Wang YN, Hsieh YH, Li LY, Xia W, Chang WC, et al. EGFR modulates DNA synthesis and repair through Tyr phosphorylation of histone H4. Developmental cell. 2014;30:224–237. doi: 10.1016/j.devcel.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Er EE, Mendoza MC, Mackey AM, Rameh LE, Blenis J. AKT facilitates EGFR trafficking and degradation by phosphorylating and activating PIKfyve. Science signaling. 2013;6:ra45. doi: 10.1126/scisignal.2004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim J, Jahng WJ, Di Vizio D, Lee JS, Jhaveri R, Rubin MA, et al. The phosphoinositide kinase PIKfyve mediates epidermal growth factor receptor trafficking to the nucleus. Cancer research. 2007;67:9229–9237. doi: 10.1158/0008-5472.CAN-07-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brand TM, Iida M, Corrigan KL, Braverman CM, Coan JP, Flanigan BG, et al. The receptor tyrosine kinase AXL mediates nuclear translocation of the epidermal growth factor receptor. Science signaling. 2017:10. doi: 10.1126/scisignal.aag1064. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Lo HW, Cao X, Zhu H, Ali-Osman F. Cyclooxygenase-2 is a novel transcriptional target of the nuclear EGFR-STAT3 and EGFRvIII-STAT3 signaling axes. Molecular cancer research : MCR. 2010;8:232–245. doi: 10.1158/1541-7786.MCR-09-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Latha K, Li M, Chumbalkar V, Gururaj A, Hwang Y, Dakeng S, et al. Nuclear EGFRvIII-STAT5b complex contributes to glioblastoma cell survival by direct activation of the Bcl-XL promoter. International journal of cancer Journal international du cancer. 2013;132:509–520. doi: 10.1002/ijc.27690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer research. 2003;63:6962–6970. [PubMed] [Google Scholar]
- 80.Nagane M, Coufal F, Lin H, Bogler O, Cavenee WK, Huang HJ. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer research. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 81.Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Babic I, Anderson ES, Tanaka K, Guo D, Masui K, Li B, et al. EGFR mutation-induced alternative splicing of Max contributes to growth of glycolytic tumors in brain cancer. Cell metabolism. 2013;17:1000–1008. doi: 10.1016/j.cmet.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grandal MV, Zandi R, Pedersen MW, Willumsen BM, van Deurs B, Poulsen HS. EGFRvIII escapes down-regulation due to impaired internalization and sorting to lysosomes. Carcinogenesis. 2007;28:1408–1417. doi: 10.1093/carcin/bgm058. [DOI] [PubMed] [Google Scholar]
- 84.Liu F, Hon GC, Villa GR, Turner KM, Ikegami S, Yang H, et al. EGFR Mutation Promotes Glioblastoma through Epigenome and Transcription Factor Network Remodeling. Molecular cell. 2015;60:307–318. doi: 10.1016/j.molcel.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feng H, Hu B, Jarzynka MJ, Li Y, Keezer S, Johns TG, et al. Phosphorylation of dedicator of cytokinesis 1 (Dock180) at tyrosine residue Y722 by Src family kinases mediates EGFRvIII-driven glioblastoma tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3018–3023. doi: 10.1073/pnas.1121457109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jahani-Asl A, Yin H, Soleimani VD, Haque T, Luchman HA, Chang NC, et al. Control of glioblastoma tumorigenesis by feed-forward cytokine signaling. Nature neuroscience. 2016;19:798–806. doi: 10.1038/nn.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cvrljevic AN, Akhavan D, Wu M, Martinello P, Furnari FB, Johnston AJ, et al. Activation of Src induces mitochondrial localisation of de2-7EGFR (EGFRvIII) in glioma cells: implications for glucose metabolism. Journal of cell science. 2011;124:2938–2950. doi: 10.1242/jcs.083295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greenall SA, Donoghue JF, Van Sinderen M, Dubljevic V, Budiman S, Devlin M, et al. EGFRvIII-mediated transactivation of receptor tyrosine kinases in glioma: mechanism and therapeutic implications. Oncogene. 2015;34:5277–5287. doi: 10.1038/onc.2014.448. [DOI] [PubMed] [Google Scholar]
- 89.Garnett J, Chumbalkar V, Vaillant B, Gururaj AE, Hill KS, Latha K, et al. Regulation of HGF expression by DeltaEGFR-mediated c-Met activation in glioblastoma cells. Neoplasia. 2013;15:73–84. doi: 10.1593/neo.121536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Horvath S, Zhang B, Carlson M, Lu KV, Zhu S, Felciano RM, et al. Analysis of oncogenic signaling networks in glioblastoma identifies ASPM as a molecular target. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17402–17407. doi: 10.1073/pnas.0608396103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lal A, Glazer CA, Martinson HM, Friedman HS, Archer GE, Sampson JH, et al. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer research. 2002;62:3335–3339. [PubMed] [Google Scholar]
- 92.Inda MM, Bonavia R, Mukasa A, Narita Y, Sah DW, Vandenberg S, et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes & development. 2010;24:1731–1745. doi: 10.1101/gad.1890510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes & development. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weiss WA, Burns MJ, Hackett C, Aldape K, Hill JR, Kuriyama H, et al. Genetic determinants of malignancy in a mouse model for oligodendroglioma. Cancer research. 2003;63:1589–1595. [PubMed] [Google Scholar]
- 95.Ding H, Shannon P, Lau N, Wu X, Roncari L, Baldwin RL, et al. Oligodendrogliomas result from the expression of an activated mutant epidermal growth factor receptor in a RAS transgenic mouse astrocytoma model. Cancer research. 2003;63:1106–1113. [PubMed] [Google Scholar]
- 96.Ramnarain DB, Park S, Lee DY, Hatanpaa KJ, Scoggin SO, Otu H, et al. Differential gene expression analysis reveals generation of an autocrine loop by a mutant epidermal growth factor receptor in glioma cells. Cancer research. 2006;66:867–874. doi: 10.1158/0008-5472.CAN-05-2753. [DOI] [PubMed] [Google Scholar]
- 97.Kancha RK, von Bubnoff N, Duyster J. Asymmetric kinase dimer formation is crucial for the activation of oncogenic EGFRvIII but not for ERBB3 phosphorylation. Cell communication and signaling : CCS. 2013;11:39. doi: 10.1186/1478-811X-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li L, Chakraborty S, Yang CR, Hatanpaa KJ, Cipher DJ, Puliyappadamba VT, et al. An EGFR wild type-EGFRvIII-HB-EGF feed-forward loop regulates the activation of EGFRvIII. Oncogene. 2014;33:4253–4264. doi: 10.1038/onc.2013.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luwor RB, Zhu HJ, Walker F, Vitali AA, Perera RM, Burgess AW, et al. The tumor-specific de2-7 epidermal growth factor receptor (EGFR) promotes cells survival and heterodimerizes with the wild-type EGFR. Oncogene. 2004;23:6095–6104. doi: 10.1038/sj.onc.1207870. [DOI] [PubMed] [Google Scholar]
- 100.Hwang Y, Chumbalkar V, Latha K, Bogler O. Forced dimerization increases the activity of DeltaEGFR/EGFRvIII and enhances its oncogenicity. Molecular cancer research : MCR. 2011;9:1199–1208. doi: 10.1158/1541-7786.MCR-11-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nature cell biology. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 102.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature cell biology. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Francis JM, Zhang CZ, Maire CL, Jung J, Manzo VE, Adalsteinsson VA, et al. EGFR variant heterogeneity in glioblastoma resolved through single-nucleus sequencing. Cancer discovery. 2014;4:956–971. doi: 10.1158/2159-8290.CD-13-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 105.Szerlip NJ, Pedraza A, Chakravarty D, Azim M, McGuire J, Fang Y, et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3041–3046. doi: 10.1073/pnas.1114033109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Akhavan D, Pourzia AL, Nourian AA, Williams KJ, Nathanson D, Babic I, et al. De-repression of PDGFRbeta transcription promotes acquired resistance to EGFR tyrosine kinase inhibitors in glioblastoma patients. Cancer discovery. 2013;3:534–547. doi: 10.1158/2159-8290.CD-12-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Clark PA, Iida M, Treisman DM, Kalluri H, Ezhilan S, Zorniak M, et al. Activation of multiple ERBB family receptors mediates glioblastoma cancer stem-like cell resistance to EGFR-targeted inhibition. Neoplasia. 2012;14:420–428. doi: 10.1596/neo.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Halatsch ME, Gehrke EE, Vougioukas VI, Botefur IC, FAB, Efferth T, et al. Inverse correlation of epidermal growth factor receptor messenger RNA induction and suppression of anchorage-independent growth by OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in glioblastoma multiforme cell lines. Journal of neurosurgery. 2004;100:523–533. doi: 10.3171/jns.2004.100.3.0523. [DOI] [PubMed] [Google Scholar]
- 109.Reardon DA, Groves MD, Wen PY, Nabors L, Mikkelsen T, Rosenfeld S, et al. A phase I/II trial of pazopanib in combination with lapatinib in adult patients with relapsed malignant glioma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:900–908. doi: 10.1158/1078-0432.CCR-12-1707. [DOI] [PubMed] [Google Scholar]
- 110.Rosell R, Taron M, Reguart N, Isla D, Moran T. Epidermal growth factor receptor activation: how exon 19 and 21 mutations changed our understanding of the pathway. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:7222–7231. doi: 10.1158/1078-0432.CCR-06-0627. [DOI] [PubMed] [Google Scholar]
- 111.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vivanco I, Robins HI, Rohle D, Campos C, Grommes C, Nghiemphu PL, et al. Differential sensitivity of glioma-versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer discovery. 2012;2:458–471. doi: 10.1158/2159-8290.CD-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barkovich KJ, Hariono S, Garske AL, Zhang J, Blair JA, Fan QW, et al. Kinetics of inhibitor cycling underlie therapeutic disparities between EGFR-driven lung and brain cancers. Cancer discovery. 2012;2:450–457. doi: 10.1158/2159-8290.CD-11-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roth P, Weller M. Challenges to targeting epidermal growth factor receptor in glioblastoma: escape mechanisms and combinatorial treatment strategies. Neuro-oncology. 2014;16(Suppl 8):viii14–19. doi: 10.1093/neuonc/nou222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reardon DA, Nabors LB, Mason WP, Perry JR, Shapiro W, Kavan P, et al. Phase I/randomized phase II study of afatinib, an irreversible ErbB family blocker, with or without protracted temozolomide in adults with recurrent glioblastoma. Neuro-oncology. 2015;17:430–439. doi: 10.1093/neuonc/nou160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zahonero C, Aguilera P, Ramirez-Castillejo C, Pajares M, Bolos MV, Cantero D, et al. Preclinical Test of Dacomitinib, an Irreversible EGFR Inhibitor, Confirms Its Effectiveness for Glioblastoma. Molecular cancer therapeutics. 2015;14:1548–1558. doi: 10.1158/1535-7163.MCT-14-0736. [DOI] [PubMed] [Google Scholar]
- 117.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer discovery. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tan CS, Gilligan D, Pacey S. Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. The Lancet Oncology. 2015;16:e447–459. doi: 10.1016/S1470-2045(15)00246-6. [DOI] [PubMed] [Google Scholar]
- 119.Gan HK, Burgess AW, Clayton AH, Scott AM. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer research. 2012;72:2924–2930. doi: 10.1158/0008-5472.CAN-11-3898. [DOI] [PubMed] [Google Scholar]
- 120.Dreier A, Barth S, Goswami A, Weis J. Cetuximab induces mitochondrial translocalization of EGFRvIII, but not EGFR: involvement of mitochondria in tumor drug resistance? Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2012;33:85–94. doi: 10.1007/s13277-011-0248-4. [DOI] [PubMed] [Google Scholar]
- 121.Chong DQ, Toh XY, Ho IA, Sia KC, Newman JP, Yulyana Y, et al. Combined treatment of Nimotuzumab and rapamycin is effective against temozolomide-resistant human gliomas regardless of the EGFR mutation status. BMC cancer. 2015;15:255. doi: 10.1186/s12885-015-1191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Neyns B, Sadones J, Joosens E, Bouttens F, Verbeke L, Baurain JF, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2009;20:1596–1603. doi: 10.1093/annonc/mdp032. [DOI] [PubMed] [Google Scholar]
- 123.Cleary JM, Reardon DA, Azad N, Gandhi L, Shapiro GI, Chaves J, et al. A phase 1 study of ABT-806 in subjects with advanced solid tumors. Investigational new drugs. 2015;33:671–678. doi: 10.1007/s10637-015-0234-6. [DOI] [PubMed] [Google Scholar]
- 124.Li L, Quang TS, Gracely EJ, Kim JH, Emrich JG, Yaeger TE, et al. A Phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. Journal of neurosurgery. 2010;113:192–198. doi: 10.3171/2010.2.JNS091211. [DOI] [PubMed] [Google Scholar]
- 125.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nature biotechnology. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 126.Choi BD, Kuan CT, Cai M, Archer GE, Mitchell DA, Gedeon PC, et al. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:270–275. doi: 10.1073/pnas.1219817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fousek K, Ahmed N. The Evolution of T-cell Therapies for Solid Malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:3384–3392. doi: 10.1158/1078-0432.CCR-14-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Appelboom G, Detappe A, LoPresti M, Kunjachan S, Mitrasinovic S, Goldman S, et al. Stereotactic modulation of blood-brain barrier permeability to enhance drug delivery. Neuro-oncology. 2016;18:1601–1609. doi: 10.1093/neuonc/now137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schijns VE, Pretto C, Devillers L, Pierre D, Hofman FM, Chen TC, et al. First clinical results of a personalized immunotherapeutic vaccine against recurrent, incompletely resected, treatment-resistant glioblastoma multiforme (GBM) tumors, based on combined allo- and auto-immune tumor reactivity. Vaccine. 2015;33:2690–2696. doi: 10.1016/j.vaccine.2015.03.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Swartz AM, Li QJ, Sampson JH. Rindopepimut: a promising immunotherapeutic for the treatment of glioblastoma multiforme. Immunotherapy. 2014;6:679–690. doi: 10.2217/imt.14.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE, 2nd, Lally-Goss D, et al. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Molecular cancer therapeutics. 2009;8:2773–2779. doi: 10.1158/1535-7163.MCT-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schuster J, Lai RK, Recht LD, Reardon DA, Paleologos NA, Groves MD, et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro-oncology. 2015;17:854–861. doi: 10.1093/neuonc/nou348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zussman BM, Engh JA. Outcomes of the ACT III Study: Rindopepimut (CDX-110) Therapy for Glioblastoma. Neurosurgery. 2015;76:N17. doi: 10.1227/01.neu.0000465855.63458.0c. [DOI] [PubMed] [Google Scholar]
- 134.Johnson LA, Scholler J, Ohkuri T, Kosaka A, Patel PR, McGettigan SE, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Science translational medicine. 2015;7:275ra222. doi: 10.1126/scitranslmed.aaa4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Han J, Chu J, Keung Chan W, Zhang J, Wang Y, Cohen JB, et al. CAR-Engineered NK Cells Targeting Wild-Type EGFR and EGFRvIII Enhance Killing of Glioblastoma and Patient-Derived Glioblastoma Stem Cells. Scientific reports. 2015;5:11483. doi: 10.1038/srep11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chow KH, Gottschalk S. Cellular immunotherapy for high-grade glioma. Immunotherapy. 2011;3:423–434. doi: 10.2217/imt.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shir A, Levitzki A. Inhibition of glioma growth by tumor-specific activation of double-stranded RNA-dependent protein kinase PKR. Nature biotechnology. 2002;20:895–900. doi: 10.1038/nbt730. [DOI] [PubMed] [Google Scholar]
- 138.Halatsch ME, Schmidt U, Botefur IC, Holland JF, Ohnuma T. Marked inhibition of glioblastoma target cell tumorigenicity in vitro by retrovirus-mediated transfer of a hairpin ribozyme against deletion-mutant epidermal growth factor receptor messenger RNA. Journal of neurosurgery. 2000;92:297–305. doi: 10.3171/jns.2000.92.2.0297. [DOI] [PubMed] [Google Scholar]
- 139.Bianco R, Shin I, Ritter CA, Yakes FM, Basso A, Rosen N, et al. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene. 2003;22:2812–2822. doi: 10.1038/sj.onc.1206388. [DOI] [PubMed] [Google Scholar]
- 140.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. The New England journal of medicine. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 141.Fenton TR, Nathanson D, Ponte de Albuquerque C, Kuga D, Iwanami A, Dang J, et al. Resistance to EGF receptor inhibitors in glioblastoma mediated by phosphorylation of the PTEN tumor suppressor at tyrosine 240. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14164–14169. doi: 10.1073/pnas.1211962109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Iwanami A, Gini B, Zanca C, Matsutani T, Assuncao A, Nael A, et al. PML mediates glioblastoma resistance to mammalian target of rapamycin (mTOR)-targeted therapies. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4339–4344. doi: 10.1073/pnas.1217602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer research. 2002;62:200–207. [PubMed] [Google Scholar]
- 144.Jun HJ, Bronson RT, Charest A. Inhibition of EGFR induces a c-MET-driven stem cell population in glioblastoma. Stem cells. 2014;32:338–348. doi: 10.1002/stem.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li L, Puliyappadamba VT, Chakraborty S, Rehman A, Vemireddy V, Saha D, et al. EGFR wild type antagonizes EGFRvIII-mediated activation of Met in glioblastoma. Oncogene. 2015;34:129–134. doi: 10.1038/onc.2013.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Snuderl M, Fazlollahi L, Le LP, Nitta M, Zhelyazkova BH, Davidson CJ, et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer cell. 2011;20:810–817. doi: 10.1016/j.ccr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 147.Turner KM, Deshpande V, Beyter D, Koga T, Rusert J, Lee C, et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature. 2017;543:122–125. doi: 10.1038/nature21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro-oncology. 2012;14:958–978. doi: 10.1093/neuonc/nos116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miller TE, Liau BB, Wallace LC, Morton AR, Xie Q, Dixit D, et al. Transcription elongation factors represent in vivo cancer dependencies in glioblastoma. Nature. 2017 doi: 10.1038/nature23000. [DOI] [PMC free article] [PubMed] [Google Scholar]