Abstract
BACKGROUND
Intact red blood cells (RBCs) appear to support thrombin generation in in vitro models of blood coagulation. During storage of RBC units, biochemical, structural and physiological changes occur including alterations to RBC membranes and release of microparticles, which are collectively known as storage lesion. The clinical consequences of microparticle formation in RBC units are unclear. This study was performed to assess thrombin generation via the prothrombinase complex by washed RBCs and RBC-derived microparticles as a function of RBC unit age.
METHODS
Well-characterized kinetic and flow cytometric assays were used to quantify and characterize microparticles isolated from leukocyte-reduced RBC units during storage for 42 days under standard blood banking conditions.
RESULTS
Stored RBCs exhibited known features of storage lesion including decreasing pH, cell lysis and release of microparticles demonstrated by scanning electron microscopy. The rate of thrombin formation by RBC units linearly increased during storage, with the microparticle fraction accounting for ~70% of the prothrombinase activity after 35 days. High resolution flow cytometric analyses of microparticle isolates identified phosphatidylserine positive RBC-derived microparticles; however, their numbers over time did not correlate with thrombin formation in that fraction.
CONCLUSIONS
RBC-derived microparticles capable of supporting prothrombinase function accumulate during storage suggesting an increased potential of transfused units as they age to interact in unplanned ways with ongoing hemostatic processes in injured individuals, especially given the standard blood bank practice of using the oldest units available.
Keywords: cell-derived microparticles, flow cytometry, red blood cell transfusion, scanning electron microscopy, thrombin
INTRODUCTION
RBC units are transfused into surgical, trauma or critically ill patients with anemia to improve oxygen delivery. In standard blood banking practice, RBC units can be stored for up to 42 days at 4°C in the presence of phosphate, adenine and other preservatives (1). This storage limit is based primarily on the degree of hemolysis (<1%) at the end of storage, as well as the percent transfused RBCs (≥75%) remaining in the recipient after 24 hrs (2), rather than clinical evidence. Removal of ~25% of transfused RBCs by the reticuloendothelial system of the recipient within 24 hrs of transfusion is a consequence of the damage caused by biochemical, structural and physiological changes that occur and accumulate during storage (3). These changes, referred to collectively as RBC storage lesion, include loss of viability, depletion of adenosine triphosphate (ATP), decreased 2,3-diphosphoglycerate, oxidative damage to lipids and proteins and dramatic membrane changes leading to loss of deformability, exposure of phosphatidylserine (PS) and release of microparticles (3, 4). It is standard blood banking practice to utilize the oldest units first to minimize waste and increase RBC availability (5). In the United States, the mean age of RBC units at the time of transfusion was 17.9 days (6). It is common for blood banks to transfer units that are nearing expiration from community hospitals to larger medical centers (5). As a consequence of this practice, patients with more severe illness tend to be transfused with older units than those that are less acutely ill. Several studies suggest that the metabolic and biochemical changes that RBC undergo as they age during storage affect patient safety and outcomes; however, the results of large clinical studies (randomized controlled trials, observational studies and meta-analyses) comparing morbidity and mortality following transfusion of old vs. young RBCs are, at best, conflicting. Remy and Spinella (5) performed a comprehensive review of these studies and concluded that studies that randomize based on RBC age may not accurately reflect RBC quality. Similarly, Antonelou and Seghatchian concluded that storage time is not predictive of RBC quality (7). In addition, the effect of storage on RBCs is highly variable and donor-dependent (8). Thus, the need for additional studies assessing the biochemical and physiological changes that occur in RBC units and the effects of transfusion on health outcomes is evident.
Previous studies have demonstrated the potential of intact RBCs (9–11) and RBC-derived microparticles (11–13) to support coagulation during storage. In in vitro models of blood coagulation, a small subpopulation of RBCs (0.5–0.6%) expresses PS on its membrane surface (14, 15) that supports prothrombinase complex assembly (i.e. factor Va/factor Xa) and contributes to thrombin generation (15). The goal of the current study was to determine the ability of stored RBCS and RBC-derived microparticles generated during storage to support thrombin generation via prothrombinase complex assembly and function.
METHODS
Materials
Spectrozyme TH was purchased from Sekisui Diagnostics (Lexington MA, USA). Bovine lactadherin and active site-blocked human factor Xa conjugated to fluorescein isothiocyanate (FITC) were purchased from Haematologic Technologies, Inc (Essex Junction VT, USA). Mouse anti-human glycophorin A antibody and mouse IgG conjugated to phycoerythrin (PE) were obtained from Merck Millipore (Billerica MA, USA). Prothrombin and factor X were isolated from fresh frozen human plasma using a modification of the procedure first described by Bajaj et al. (16), and purged of all traces of active proteases as described (17). Factor X was activated to factor Xa with the factor X activator purified from Russell's viper venom (18). Factor V was purified from fresh frozen human plasma (19). Synthetic phospholipid vesicles [75% phosphatidylcholine (PC) and 25% phosphatidylserine (PS); PCPS] were prepared as described (20) using reagents obtained from Avanti Polar Lipids (Alabaster AL, USA). Leukocyte-reduced RBC units (CPD-AS-1) were obtained from the University of Vermont Medical Center (UVMMC). Measurements of pH, annexin V binding, and prothrombinase complex assembly and function over time were performed contemporaneously using the same five RBC units. In contrast, measures of microparticle release were performed at a later time and used either eight different day 42 RBC units or three different RBC units from days 7 to 42.
RBC Washing and Isolation of Microparticles
On days 7, 14, 21, 28, 35, and 42 of storage, RBC samples were removed, diluted 1:2 with RBC wash buffer (0.0047 M KCl, 0.140 M NaCl, 0.021 M Hepes, 0.0111 M dextrose, 0.1% polyethylene glycol 8000, pH 7.4), and subjected to five rounds of centrifugation (200 g, 5 min) followed by resuspension in RBC wash buffer. The final RBC pellets were resuspended in RBC wash buffer to their original volume and labeled “washed RBCs”. RBC concentrations were determined before and after washing using the pocH-100i automated hematology analyzer (Sysmex, Mundelein IL, USA). The RBC concentration did not vary over time, leukocytes were undetectable (<0 × 103/µL) and the platelet concentrations ranged from <0 – 4 × 103/µL, which is well below physiological platelet concentrations, in these RBC units.
After each centrifugation step, the supernatants were removed and pooled. The total supernatant pool was subjected to sequential, differential centrifugation at 800 g (10 min, ambient temperature), 1,600 g (10 min, ambient temperature), and 40,000 g (60 min, 4°C). Following the final centrifugation step at 40,000 g, the supernatant was collected and the pellet was resuspended with 1 mL RBC wash buffer and labeled “microparticles”.
Measurements of RBC Physical Characteristics
pH
The pH of the storage medium of five RBC units was determined on days 7, 14, 21, 28, 35 and 42 by using both an OPTI CCA-TS blood gas analyzer (OPTI Medical Systems Inc., Roswell GA, USA) and Ag/AgCl pH electrode (Thermo Scientific, Waltham MA, USA).
RBC lysis
RBC lysis in five RBC units was quantitated via spectrophotometric determination of soluble hemoglobin on days 7, 14, 21, 28, 35, and 42. Washed cells (500–600 × 106 cells) were lysed with dH2O. Cell debris was removed via centrifugation at 3,000 g for 5 min and then 14,000 g for 1 min. A series of dilutions yielding RBC equivalents from 0.5–6 × 106 cells/mL were made and the absorbance between 350 nm and 500 nm was measured. A plot of the maximum absorbance (~415 nm), reflecting the presence of hemoglobin, versus RBC equivalents was made. To estimate the % lysis in RBC units across the 42 day storage period, aliquots from each RBC unit were removed and the RBC concentration determined. RBCs were then centrifuged at 800 g for 10 min and the supernatant was removed and centrifuged at 14,000 g for 1 min to remove debris. Absorbance values at 415 nm in each supernatant were converted to lysed cell equivalents by reference to the standard curve, and this number was expressed relative to the number of intact RBCs present in each unit.
Scanning Electron Microscopy
Samples were layered on 13 mm Thermonox coverslips (Thermo Scientific, Waltham, MA, USA) and subjected to fixation in Karnovsky’s fixative (2.5% glutaraldehyde, 1% para-formaldehyde, 0.1 M cacodylate buffer, pH 7.4) for 30 min at ambient temperature. The samples were subsequently rinsed three times in 0.1 M cacodylate buffer (5 min each rinse), and incubated for 30 min at 4°C in 0.1 M cacodylate buffer containing 1% OsO4. Following dehydration using a standard ethanol protocol, the samples were subjected to critical point drying with CO2, mounted with colloidal graphite onto aluminum specimen stubs and coated with gold and palladium in a sputter coater (3 min, 24 mA). The samples were imaged with a JSM 6060 scanning electron microscope (Jeol USA, Inc, Peabody, MA, USA) at 15 – 20 kV.
Measurements of Thrombin Generation
Quality control of enzyme assays
Given that the experimental design involved conducting prothrombinase assays over a six week period with the aim of directly comparing these measurements, rigorous control of assay reagents was essential. The same stocks of factor Xa, prothrombin, and factor V were used over the six week period. PCPS vesicle preparations are replaced every three weeks, so two lots were needed to span the time frame. Extensive testing of reagents was conducted each week prior to assays of experimental materials. The stability of the factor Xa stock was assessed weekly by measuring the rate of hydrolysis of Spectrozyme Xa (200 µM) using a Molecular Devices (Sunnyvale CA, USA) THERMOmax microplate reader. Control prothrombinase reactions contained 20 nM factor Va, 10 pM factor Xa, 1.4 µM prothrombin and 2 or 20 µM PCPS in 20 mM Hepes, 0.15 M NaCl, pH 7.4 (HBS)/0.1% polyethylene glycol (PEG) 8000/5 mM CaCl2. Factor Va was made fresh prior to all experiments. Factor V (1 µM) was reacted with 10 nM thrombin at 37°C for 20 min after which the reaction was put on ice and 12 nM recombinant hirudin (American Diagnostica CT, USA) added. The activity of the resulting factor Va was then assessed with a Diagnostica Stago STart 4 Hemostasis Analyzer (Diagnostica Stago, Parsippany, NJ, USA) using factor V deficient plasma prepared in house (19) and TriniCLOT PT Excel S reagent (Tcoag Ireland Ltd, Ireland). PCPS vesicles were preincubated with factor Va and factor Xa for 3 min at 37°C. Reactions were initiated by the addition of prothrombin and aliquots were removed (0.5, 1.5, 2.5, 3.5, 4.5, 5.5, 6.5 and 7.5 min) and diluted eight-fold into HBS/0.1% PEG 8000/20 mM EDTA (quench buffer). Thrombin concentrations in quenched samples were assessed by measuring the rate of hydrolysis of SpectrozymeTH (200 µM) using a Molecular Devices THERMOmax microplate reader. Rates of substrate hydrolysis at each time point were converted to thrombin concentration using a standard curve prepared by various dilutions of thrombin. Secondary plots of thrombin concentration versus time were constructed with the slope of the best fitting line reported as the rate of thrombin formation by prothrombinase.
Prothrombinase on RBCs and microparticle isolates
Assessments of prothrombinase function were done each week on RBCs, washed RBC and microparticle isolates from five units. Assays (200 µL) contained 20 nM factor Va, 50 pM factor Xa, 1.4 µM prothrombin, in HBS/0.1% PEG 8000/5 mM CaCl2, and a phospholipid membrane provided by either RBCs, washed RBCs, or microparticle isolate. For RBCs and washed RBCs a final concentration of 0.5×109 RBCs/mL was used (generally 16–18 µL). Since microparticle isolates were resuspended in volumes equivalent to the initial volume of RBCs from which they were separated, the volume of microparticle isolate added to the reaction was the same as the volume of RBCs needed to yield 0.5×109 RBCs/mL. RBCs, washed RBCs or microparticle isolates were preincubated with factor Va and factor Xa for 3 min at 37°C. Reactions were initiated by addition of prothrombin. Timed (0.5–7.5 min) aliquots were removed and diluted eight-fold into quench buffer. Cells were removed from quenched reactions via centrifugation at 2,000 g for 25 sec at room temperature and the supernatants assayed for thrombin by measuring the rate of hydrolysis of SpectrozymeTH as described above.
Flow Cytometric Analyses
Intact washed RBCs
The ability of washed RBCs from five units to express PS and bind prothrombinase was assessed by flow cytometry. As these studies were to be performed over a six week period with the aim of directly comparing these measurements, rigorous quality control of the assay reagents was performed. On each day of assay, washed RBCs (10×106/mL) were treated with 10 mM N-ethylmaleimide (NEM) and 10 µM calcium ionophore A23187 (10 min, ambient temperature) to elicit RBC activation and maximal PS exposure. RBCs (10×106/mL), with or without treatment with NEM and A23187, were diluted 1:10 in RBC wash buffer (+ 5 mM CaCl2) and incubated with 20 nM factor Va plus 2 nM FITC-conjugated active site-blocked factor Xa, or 20 nM FITC-conjugated bovine lactadherin. RBCs were identified by positive immunostaining with PE-conjugated anti-glycophorin A antibody relative to an isotype-matched mouse IgG conjugated to PE used at the same concentration and fluorophore-to-protein ratio. Following a 15 min incubation (ambient temperature), 10,000 cells were analyzed on an FC500 flow cytometer (Beckman Coulter, Brea, CA, USA). Analyses regions were set such that ≤ 2% RBCs incubated with FITC-conjugated active site-blocked factor Xa alone or 20 nM BSA-FITC were positive. Following activation with NEM and A23187, ≥99% of the RBCs bound bovine lactadherin. The factor Va-dependent binding of factor Xa was more variable (47.3±7.3%) but consistent with previous observations (15).
Microparticles
Microparticles and the 40,000 g supernatant were prepared for and analyzed by flow cytometric analyses as follows. Aliquots of each fraction were incubated with PE-conjugated anti-human glycophorin A antibody (5 µL per reaction) (15 min, ambient temperature) followed by 20 nM FITC-conjugated bovine lactadherin (15 min, ambient temperature). Following fixation with 4% para-formaldehyde, 200 µL of each reaction was analyzed using a MACSQuant® VYB flow cytometer (Miltenyi Biotech, Bergisch Gladbach, Germany), which utilizes spectrally-matched photomultiplier tubes for all channels allowing for amplification of weak intensity photon emissions. Microparticles were identified based on their FSC and SSC relative to 0.88 µm FITC-conjugated SPHERO™ polystyrene beads (Spherotech, Inc, Lake Forest IL, USA). Positive immunostaining with PE-conjugated anti-glycophorin A antibody was defined relative to mouse IgG-PE. The positive binding of FITC-conjugated bovine lactadherin was defined relative to 20 nM BSA-FITC. Analyses regions were set such that ≤2% of the microparticles incubated with mouse IgG-PE or BSA-FITC were positive. The data were analyzed using the FlowJo V10 software package (FlowJo LLC, Ashland OR, USA). Absolute microparticle counts were determined volumetrically to quantify the numbers of microparticles per RBC unit.
Statistical Analysis
The data are presented as the mean ± SD. Comparisons across time were made using a repeated measures ANOVA, with a post-hoc test for a linear contrast if significant. p≤0.05 was considered significant.
RESULTS
The RBC units used in this study exhibited well-defined characteristics of RBC storage lesion. From day 7 to day 42 of storage, a statistically significant, linear increase in the % RBC lysis from 0.024 ± 0.22% to 1.33 ± 0.47% was observed (P=0.002) (data not shown). Over this same time period, a statistically significant, linear decrease in the extracellular pH of the RBC units from 6.75 ± 0.04 to 6.50 ± 0.08 was also observed (P=0.002) (data not shown). These parameters and their changes over time are consistent with previous studies (21).
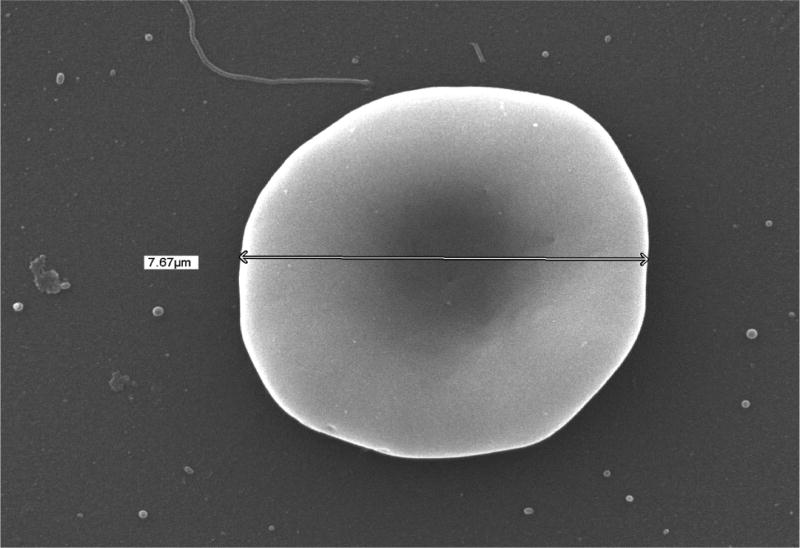
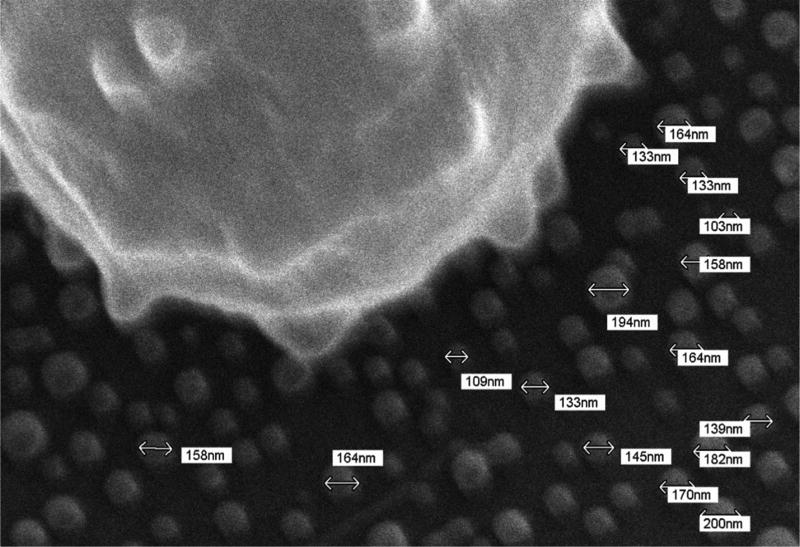
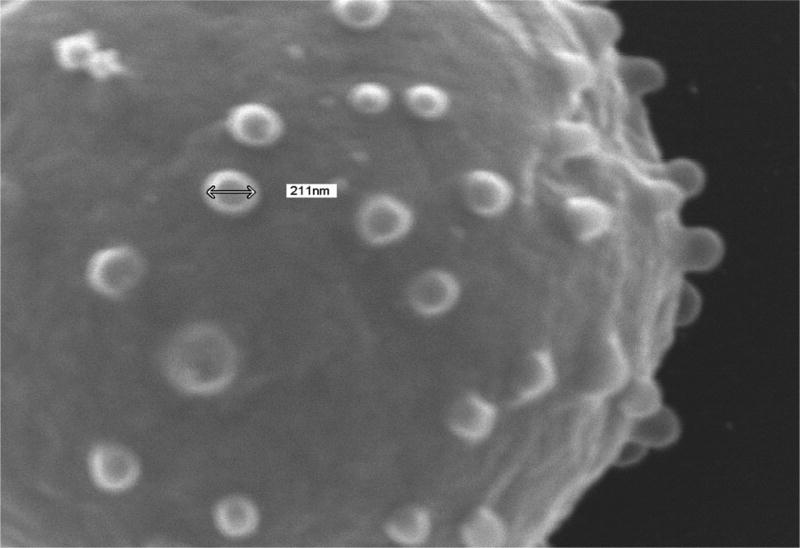
The effects of RBC storage on thrombin generation via assembly and function of the membrane-dependent prothrombinase complex were assessed using washed RBCs and RBC-derived microparticles isolated from 42 day old RBC units by centrifugation. The presence of intact RBCs and microparticles in the washed RBC and microparticle fractions was verified by scanning electron microscopy (SEM). In Fig 1A is an SEM image of an intact RBC (diameter = 7.67 µm) in the washed RBC isolate. SEM of a microparticle isolate revealed the presence of microparticle-sized cellular vesicles 153.1±27.5 nm in diameter (Fig 1B). This observation is consistent with measurements of extracellular vesicles isolated from outdated RBCs by various methods including electron microscopy and nanoparticle tracking (22). Similar-sized vesicles (211 nm) extruding from echinocytic spines on the surface of RBCs were observed in the washed RBC isolate (Fig 1C).
Fig. 1. Scanning electron microscopy of red blood cell units.
Samples were prepared for scanning electron microscopy from day 42 RBCs as described in the Methods and imaged with a JSM 6060 scanning electron microscope at 15–20 kV. The diameters of the intact cells and microparticles were estimated using the microscope software. (A) Washed RBC. (B) Microparticle fraction. (C) Close up of a washed RBC demonstrating release of microparticles.
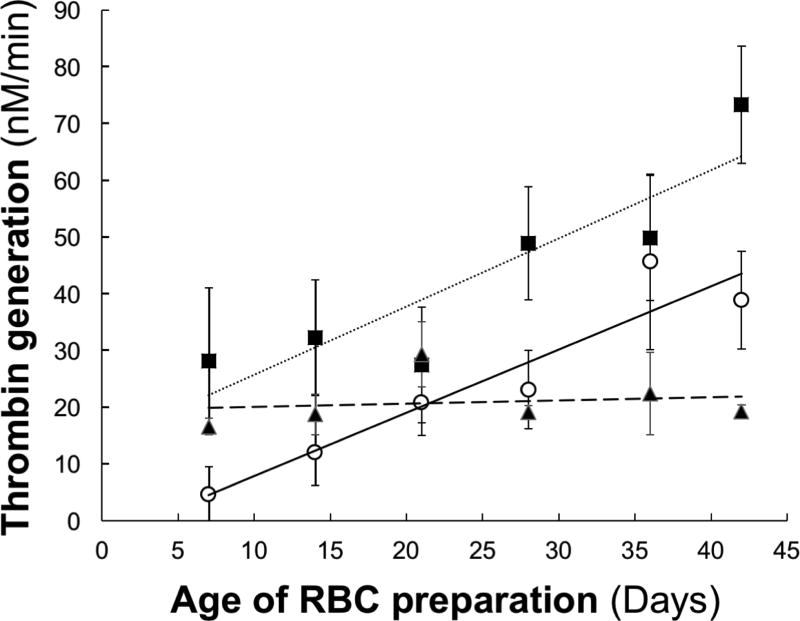
Kinetic analyses indicated that the rate of thrombin generation by the RBC preparations increased linearly from day 7 to day 42 of storage (P<0.001) (Fig 2, closed squares). At day 7, ~80% of the total prothrombinase activity was associated with the washed RBC fraction and 20% with the microparticle fraction. There was a slight, but statistically significant increase (P=0.024) in the level of prothrombinase activity of the washed RBC fraction over time of storage (Fig 2, closed triangles). Consistent with this observation, the binding of fluorescently-labeled factor Xa in the presence of factor Va to the intact, washed RBCs isolated from these preparations, while variable between donors, increased slightly and approached statistical significance (P=0.073) (Table 1). The binding of fluorescently-labeled bovine lactadherin (Table 2) did not change over the 35 day storage period (P=0.38) despite a modest increase at day 42. Interestingly, analysis of lactadherin binding from days 7 and 35 of storage demonstrates a statistically significant decrease (P=0.015) in binding; however, without an additional data point beyond day 42, it is impossible to know whether this increase is an aberration or the start of an upward trend. In contrast, a substantial increase in the prothrombinase activity supported by microparticle isolates was observed over storage (Fig 2, open circles). Thrombin generation increased linearly ~8 fold from day 7 until day 42 of RBC storage (P<0.001), and accounted for ~70% of the total prothrombinase activity at day 42.
Fig. 2. Effects of storage time on thrombin generation in red blood cell units.
The ability of RBCs (-■-), washed RBCS (-▲-) and microparticles (-○-) to support thrombin generation via the prothrombinase complex was assessed as described in the Methods. Reaction mixtures contained cells (0.5×109/mL) or microparticles (equivalent volume), 20 nM factor Va, 50 pM factor Xa, and 1.4 µM prothrombin. The data are presented as the mean±SD from five RBC units.
Table 1.
Effects of storage time on factor Va-dependent FITC-conjugated factor Xa binding to washed RBCs.*
| Storage Age | Unit 1 | Unit 2 | Unit 3 | Unit 4 | Unit 5 | Mean±SD |
|---|---|---|---|---|---|---|
| Day 7 | 0 | 0.035 | 0.145 | 0.065 | 0.2 | 0.059±0.11 |
| Day 14 | 0 | 0.11 | 0.15 | 0.24 | 0.14 | 0.13±0.09 |
| Day 21 | 0.01 | 0.08 | 0.18 | 0.18 | 0.18 | 0.13±0.09 |
| Day 28 | 0 | 0.16 | 0.35 | 0.29 | 0.37 | 0.23±0.15 |
| Day 35 | 0.06 | 0.17 | 0.34 | 0.2 | 0.4 | 0.23±0.13 |
| Day 42 | 0 | 0.29 | 0.34 | 0.35 | 0 | 0.20±0.18 |
Washed RBCs were incubated with FITC-conjugated active site-blocked factor Xa (2 nM) in the presence of 20 nM factor Va. RBCs were identified by their FSC and SSC and immunostaining with anti-human glycophorin A-PE. The data are presented as the average of duplicate samples and as the mean±SD of the five RBC units (R2=0.11 over time).
Table 2.
Effects of storage time on binding of FITC-conjugated bovine lactadherin to washed RBCs.*
| Storage Age | Unit 1 | Unit 2 | Unit 3 | Unit 4 | Unit 5 | Mean±SD |
|---|---|---|---|---|---|---|
| Day 7 | 0.50 | 1.49 | 0.63 | 1.66 | 1.47 | 1.14± 0.54 |
| Day 14 | 0.81 | 2.39 | 0.93 | 2.15 | 1.68 | 1.59±0.72 |
| Day 21 | 0.38 | 1.56 | 0.47 | 0.63 | 0.63 | 0.73±0.47 |
| Day 28 | 0.47 | 1.66 | 0.35 | 0.76 | 0.41 | 0.73±0.54 |
| Day 35 | 0.31 | 0.82 | 0.24 | 0.68 | 1.33 | 0.68±0.45 |
| Day 42 | 1.18 | 3.26 | 1.13 | 2.82 | 2.06 | 2.09±0.96 |
Washed RBCs were incubated with FITC-conjugated bovine lactadherin (2 nM). RBCs were identified by FSC and SSC and immunostaining with anti-human glycophorin A-PE. The data are presented as the average of duplicate samples and as the mean±SD of the five RBC units (R2=0.04 over time).
High resolution flow cytometric analyses were used to characterize and quantify microparticles isolated from eight different RBC units (42 days old). FITC-conjugated SPHERO™ polystyrene beads were used to define appropriate flow cytometer settings for the detection of microparticles isolated from RBC units and were resolved based on fluorescence (FITC) and SSC, as well as FSC and SSC. Particles with a FSC and SSC lower than the 0.88 µm beads were identified as microparticles (see Figure, Supplemental Digital Content 1A, which illustrates the FSC and SSC of microparticles relative to 0.88 µm beads). Binding of fluorescently-labeled anti-glycophorin A and bovine lactadherin was determined relative to appropriate fluorescently-labeled controls (PE-labeled isotype control or FITC-conjugated-BSA) to determine the distributions of glycophorin A, an RBC integral membrane protein, and PS on the isolated microparticles’ outer membrane surfaces (see Figure, Supplemental Digital Content 1B, which shows the position of the positive analysis regions relative to the controls). The microparticles were heterogeneous in number and membrane composition (Table 3). Quantitation of the numbers of microparticles that expressed these markers demonstrated that 50–60% of the particles expressed glycophorin A or PS. Nearly 100% of the PS positive microparticles also expressed glycophorin A. In contrast, ~25% of the glycophorin A positive microparticles were PS negative. A similar distribution of glycophorin A and PS on microparticle-sized fragments retained in the 40,000g supernatant was observed (data not shown).
Table 3.
Quantification of glycophorin A-positive and PS-positive RBC-derived microparticles isolated from 42 day old RBC units using flow cytometric analyses.*
| PS+ | PS− | Total | |
|---|---|---|---|
| Glycophorin A+ | 9357±4593† | 3370±6237 | 12727±4219 |
| Glycophorin A− | 328±6245 | 9003±11822 | 9331±10038 |
| Total | 9685±4231 | 12373±10043 | 22058±9108 |
Microparticle isolates (n=8) were analyzed by flow cytometry as described in Fig 3.
The data are presented as the concentration of microparticles per mL of original RBC unit (mean±SD).
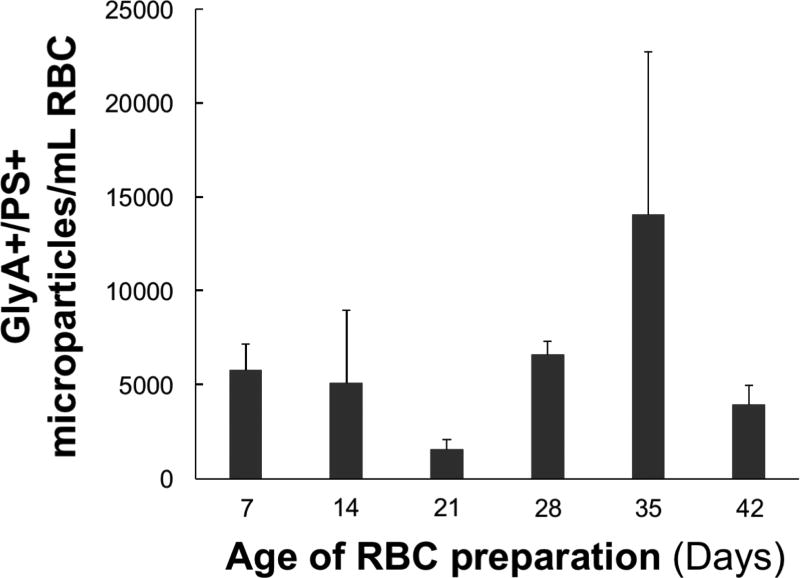
Identical flow cytometric analyses were used to quantify microparticle formation over time of storage and to determine the relative distributions of glycophorin A and PS in three new RBC units. From day 7 to day 42 of storage, the concentration of glycophorin A positive PS positive microparticles varied over time and between individuals (Fig 3).
Fig. 3. Longitudinal analysis tracking the development of microparticle subpopulations in red blood cell units.
Microparticle subpopulations in the microparticle fraction isolated from RBCs (n=3) on days 7, 14, 21, 28, 35, and 42 of storage were identified and quantified as described in the Methods. The numbers of glycophorin A positive, PS positive (GlyA+/PS+) microparticles per mL RBC are shown. The data are expressed as the mean±SD of three RBC units.
DISCUSSION
RBC storage lesion is characterized by a substantial depletion of ATP stores over storage (< 50% normal levels by day 42 (3)) and a loss of cellular viability as the pyruvate formed via anaerobic glycolysis cannot be further used for ATP production. The excess pyruvate is reduced to lactate to recycle the NADH that is produced during glycolysis and released into the storage media leading to an accumulation of [H+] and a time-dependent decrease in the pH of the storage medium (6). During storage, RBCs also exhibit progressive, drastic membrane alterations including changes in the proportion of membrane lipids (cholesterol, phospholipids) (23) and exposure of PS (24), oxidative damage to membrane proteins and lipids (4), increased cellular rigidity (24) and fragility (7), and decreased deformability (3, 23). Despite these ongoing, unfavorable membrane changes, the results of the current study demonstrate that during storage the ability of intact, washed RBCs isolated from RBC units to support thrombin generation via membrane-dependent prothrombinase complex assembly was unaffected. In a study by Dinkla and colleagues, PS expression by stored RBCs was highly variable between donors and the number of PS-positive RBCs never exceeded 1% (25) suggesting a limited ability to support thrombin generation. In contrast, Lu and colleagues demonstrated a dramatic increase in PS-dependent prothrombinase activity in long-term stored RBCs, which was reported to be dependent in part on a 90-fold increase in cells expressing PS (0.2–18.4%) (9). In these latter studies, the RBCs were not washed so it is likely that the time-dependent increase procoagulant activity was due, at least in part, to the presence of microparticles.
Release of microparticles is a well-known feature of RBC storage lesion (4). They are released from the tips of echinocytic spines and can vary greatly in terms of size, structure, protein composition and phospholipid exposure. The negatively charged membrane surface provided by PS-positive microparticles can support the assembly and function of the coagulation complexes involved in thrombin formation (26). However, it is important to note that more recent evidence suggests that not all microparticles expose PS on their surface (26). In addition, microparticle release can be affected by the RBC storage conditions including the type of storage media used and leukoreduction, with the latter diminishing microparticle-mediated procoagulant levels (4). The numbers of microparticles isolated from RBC units are variable (13), increase over time of storage (13, 24), and support thrombin generation in a factor XI-dependent manner in plasma-based assays (11–13). These observations are also supported by other studies demonstrating that microparticles generated in vitro by treatment with calcium ionophore (27) or via high pressure extrusion (28) support thrombin generation, and RBC microparticles isolated from individuals with sickle cell disease promote thrombin generation in a factor XI-dependent manner (29). The current study demonstrates that the majority of the thrombin generation observed in RBC units is mediated by RBC-derived microparticles, and increases with age. PS-positive RBC-derived microparticle formation over storage was highly variable and did not correlate with increases in thrombin generation. A similar observation was made by Owen and colleagues who demonstrated that microparticle number did not correlate with procoagulant activity (30). We hypothesize that while PS expression on the RBC-derived microparticle surface is necessary for assembly and function of the prothrombinase complex, activity of the complex is regulated by the membrane environment. This hypothesis is supported by several observations. Microparticles in RBC units become more heterogeneous over storage with a gradual increase in size and protein content (31, 32) and a decrease in PS exposure (32). Previous studies using synthetic phospholipid vesicles of defined content have demonstrated that in the presence of low concentrations (1–5%) of PS, phosphatidylethanolamine (PE) increases thrombin generation (33) by decreasing the Kmapp for prothrombin as well as the Kdapp for formation of the prothrombinase complex (34). Therefore, increases in prothrombinase activity over storage may be a result of exposure of PE coupled to decreased PS expression. This is supported by the observation that PE is exposed on the surface of microparticles released from various cell types (35) and duramycin, which binds PE, inhibits thrombin generation (35, 36). Co-isolation of unknown molecules or changes in microparticle protein composition may also lead to a more procoagulant phenotype despite decreases in PS expressing microparticles as studies using activated platelets have long supported the existence of specific receptors for factors Va and Xa [reviewed in (37)].
Between January and March 2014, ~95,000 RBC units were distributed from the New York Blood Center. Around 70% of them were greater than 30 days old (Dr. Mohandas Narla, personal communication). A similar analyses of same timeframe, indicates that ~80% of the RBC units used at UVMMC were greater than 30 days old. In Denver, a cross-sectional analysis of all leukocyte-reduced RBC transfusions administered to critically ill patients from January to June 2009 at a 20 bed academic surgical ICU indicated that that 80% of the RBCs transfused were greater than 14 days old and averaged 26 days old (38). Approximately 75% of the RBC-derived microparticles expressed PS at day 42 and therefore have the potential to support the binding of coagulation proteins including the components of the prothrombinase complex. Thus, during ongoing hemostasis in acutely bleeding patients, transfused RBC-derived microparticles have the potential to influence clot formation by delivering coagulation proteins to sites of clot formation. In support of this concept are the results of a retrospective cohort study demonstrating that in trauma patients receiving ≥ five units of RBC, transfusion of RBCs ≥ 28 days old results in an increased incidence of deep vein thrombosis (39). On the other hand, transfused RBC-derived microparticles may influence clot formation by sequestration of coagulation proteins to dampen the hemostatic response; however there are no data in support of this.
RBC-derived microparticles could have several other roles in vivo including transport and transfer of bioactive molecules, modulation of inflammatory and immunological responses and cell activation (40–42). RBC-derived microparticles may also serve an anticoagulant function, which would exacerbate any ongoing bleeding. Prothrombin activation on the surface of RBCs proceeds through the meizothrombin intermediate (15). Meizothrombin is an active enzyme and can support the anticoagulant function of thrombin through activation of protein C (43). Although formation of meizothrombin on the microparticle surface has yet to be determined, prothrombin activation occurs through this intermediate on the surface of synthetic phospholipid vesicles (44). RBC-derived microparticles also bind activated protein C and protein S and can mediate inactivation of factors Va and VIIIa (27).
Limitations
While many different RBC additive solutions have been developed and are used worldwide (SAGM, AS-1, AS-3, AS-5, AS-7 MAP and PAGGSM), only three, AS-1, AS-3 and AS-5, are approved by the Food and Drug Administration and hence licensed for use in the United States (45). While these solutions have similar compositions, they vary in their presence or absence of mannitol, NaH2PO4, citric acid and sodium citrate. A limitation of this study is our use exclusively of RBC units stored in additive solution AS-1. Since ~90,000,000 RBC units are transfused worldwide each year (1), the results of this study underscore the need for more studies to define the potential impact of transfusion of RBC-derived microparticles and to fully appreciate their relevance in mediating adverse clinical events. Indeed, thrombin generation and microparticle release by RBC stored in different additive solutions may yield markedly different results. Studies comparing microparticle formation and thrombin generation by RBC units stored in AS-3 vs. other additive solutions are particularly of interest as storage of RBCs in AS-3 has been shown to be superior by several investigators (46).
Supplementary Material
Acknowledgments
K.G.M. is Chairman of the Board of Directors for Haematologic Technolgies, Inc. and a consultant for Stago, Novo Nordisk, Shire and Vascular Solutions.
Funding
This study was supported by UM1 HL120877 from the National Institutes of Health (to K.G.M.).
The studies would not have been possible without the assistance of Lynn Griswold (University of Vermont Medical Center) in obtaining RBC units. We are also grateful to Dr David Schneider (Department of Medicine, The Larner College of Medicine at the University of Vermont) for the use of his FC500 flow cytometer and to Heidi Taatjes for instructing us in its use; Roxana del Rio PhD (Director, The Larner College of Medicine at the University of Vermont Flow Cytometry and Cell Sorting Facility) for assistance in flow cytometric analyses of RBC-derived microparticles; Michelle von Turkovich (The Larner College of Medicine at the University of Vermont Microscopy Imaging Center) for assistance in scanning electron microscopy of RBCs and RBC-derived microparticles; and Dana Bourne for technical assistance.
Footnotes
Conflicts of Interest
The other authors have no conflicts to disclose.
Meeting Presentation
Portions of this work were presented at the XXVth Congress of the International Society of Thrombosis & Haemostasis, Toronto, Ontario, June 20–25 2015.
AUTHOR CONTRIBUTIONS
B.A.B. designed experiments, collected, analyzed and interpreted data, and wrote the manuscript. T.O. conceived of the research, designed experiments, interpreted data, and wrote the manuscript. H.N.K., E.M.L. and M.G. collected and analyzed data, and reviewed the manuscript. M.G. provided statistical analysis. M.F. provided data on RBC usage at the University of Vermont Medical Center, provided access to RBC units and reviewed the manuscript. K.G.M. conceived of the research and reviewed the manuscript.
References
- 1.Flegel WA, Natanson C, Klein HG. Does prolonged storage of red blood cells cause harm? Br J Hematol. 2014;165(1):3–16. doi: 10.1111/bjh.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogman CF, Meryman HT. Red blood cells intended for transfusion: quality criteria revisted. Transfus. 2006;46:137–42. doi: 10.1111/j.1537-2995.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida T, Shevkoplyas SS. Anaerobic storage of red blood cells. Blood Transfus. 2010;8:220–36. doi: 10.2450/2010.0022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Alessandro A, Kriebardis AG, Rinalducci S, Antonelou MH, Hansen KC, Papassideri IS, Zolla L. An update on red blood cell storage lesion, as gleaned throgh biochemistry and omics technologies. Transfus. 2015;55:205–19. doi: 10.1111/trf.12804. [DOI] [PubMed] [Google Scholar]
- 5.Remy KE, Spinella PC. Red blood cell storage age - what we know from clinical trials. Expert Rev Hematol. 2016;9(11):1011–3. doi: 10.1080/17474086.2016.1243051. [DOI] [PubMed] [Google Scholar]
- 6.Qu L, Triulzi DJ. Clinical effects of red blood cell storage. Cancer Control. 2015;22:26–37. doi: 10.1177/107327481502200105. [DOI] [PubMed] [Google Scholar]
- 7.Antonelou MH, Seghatchian J. Insights into red blood cell storage: Toward a new appreciation. Transfus Apher Sci. 2016;55:296–301. doi: 10.1016/j.transci.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Tzounakas VL, Georgatzakou HT, Kriebardis AG, Voulgaridou AI, Stamoulis KE, Foudoulaki-Paparizos LE, Antonelou MH, Papassideri IS. Donor variation effect on red blood cell storage lesion: a multivariable, yet consistent, story. Transfus. 2016;56:1274–86. doi: 10.1111/trf.13582. [DOI] [PubMed] [Google Scholar]
- 9.Lu C, Shi J, Yu H, Hou J, Zhou J. Procoagulant activity of long-term stored red blood cells due to phosphatidylserine exposure. Transfus Med. 2011;21:150–7. doi: 10.1111/j.1365-3148.2010.01063.x. [DOI] [PubMed] [Google Scholar]
- 10.Keating FK, Butenas S, Fung MK, Schneider DJ. Platelet-white blood cell (WBC) interaction, WBC apoptosis, and procoagulant activity in stored red blood cells. Transfus. 2011;51:1086–95. doi: 10.1111/j.1537-2995.2010.02950.x. [DOI] [PubMed] [Google Scholar]
- 11.Aleshnick M, Foley JH, Keating FK, Butenas S. Procoagulant activity in stored units of red blood cells. Biochem Biophys Res Comm. 2016;474:680–5. doi: 10.1016/j.bbrc.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Rubin O, Delobel J, Prudent M, Lion N, Kohl K, Tucker EI, Tissot J-D, Angelillo-Scherrer A. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfus. 2013;53:1744–54. doi: 10.1111/trf.12008. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Lv L, Liu S, Ma G, Su T. Elevated levels of thrombin-generating microparticles in stored red blood cells. Vox Sang. 2103;105:11–7. doi: 10.1111/vox.12014. [DOI] [PubMed] [Google Scholar]
- 14.Peyrou V, Lormeau JC, Herault JP, Gaich C, Pfliegger AM, Herbert JM. Contribution of erythrocytes to thrombin generation in whole blood. Thromb Haemost. 1999;81(3):400–6. [PubMed] [Google Scholar]
- 15.Whelihan MF, Zachary V, Orfeo T, Mann KG. Prothrombin activation in blood coagulation: the erythrocyte contribution to thrombin generation. Blood. 2012;120(18):3837–45. doi: 10.1182/blood-2012-05-427856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajaj SP, Rapaport SI, Prodanos C. A simplified procedure for purification of human prothrombin, factor IX and factor X. Prep Biochem. 1981;11(4):397–412. doi: 10.1080/00327488108065531. [DOI] [PubMed] [Google Scholar]
- 17.van 't Veer C, Mann KG. Regulation of tissue factor initiated thrombin generation by the stoichiometric inhibitors tissue factor pathway inhibitor, antithrombin-III, and heparin cofactor-II. J Biol Chem. 1997;272(7):4367–77. doi: 10.1074/jbc.272.7.4367. [DOI] [PubMed] [Google Scholar]
- 18.Jesty J, Nemerson Y. The activation of bovine coagulation factor X. Method Enzymol. 1976;45:95–107. doi: 10.1016/s0076-6879(76)45014-0. [DOI] [PubMed] [Google Scholar]
- 19.Katzmann JA, Nesheim ME, Hibbard LS, Mann KG. Isolation of functional human coagulation factor V by using a hybridoma antibody. Proc Natl Acad Sci U S A. 1981;78(1):162–6. doi: 10.1073/pnas.78.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins DL, Mann KG. The interaction of bovine factor V and factor V-derived peptides with phospholipid vesicles. J Biol Chem. 1983;258(10):6503–8. [PubMed] [Google Scholar]
- 21.Holme S, Dean Elfath M, Whitley P. Evaluation of in vivo and in vitro quality of apheresis-collected RBC stored for 42 days. Vox Sang. 1998;75:212–7. [PubMed] [Google Scholar]
- 22.Varga Z, Yuana Y, Grootemaat AE, van der Pol E, Gollwitzer C, Krumrey M, Nieuwland R. Towards traceable size determination of extracellular vesicles. J Extracell Vesicles. 2014;3:23298. doi: 10.3402/jev.v3.23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almizraq R, Tchir JDR, Holovati JL, Acker JP. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfus. 2013;53:2258–67. doi: 10.1111/trf.12080. [DOI] [PubMed] [Google Scholar]
- 24.Rubin O, Crettaz D, Canellini G, Tissot J-D, Lion N. Microparticles in stored red bloos cells: an approach using low cytometry and proteomics tools. Vox Sang. 2008;95:288–97. doi: 10.1111/j.1423-0410.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 25.Dinkla S, Peppelman M, van der Raadt J, Atsma F, Novotny VMJ, van Kraaij MGJ, Joosten I, Bosman GJCGM. Phosphatidylserine exposure on stored red blood cells as a parametr for donor-dependent variation in product quality. Blood Transfus. 2014;12:204–9. doi: 10.2450/2013.0106-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mooberry MJ, Key NS. Microparticle analysis in disorders of hemostasis and thrombosis. Cytometry A. 2016;89A:111–22. doi: 10.1002/cyto.a.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koshiar RL, Somajo S, Norstrom E, Dahlback B. Erythrocyte-derived microparticles supporting activated protein C-mediated regulation of blood coagulatioon. PLOS One. 2014;9:e104200. doi: 10.1371/journal.pone.0104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jy W, Johansen ME, Bidot C, Jr, Horstma LL, Ahn YS. Red cell-derived microparticles (RMP) as haemostatic agent. Thromb Haemosta. 2013;110(4):751–60. doi: 10.1160/TH12-12-0941. [DOI] [PubMed] [Google Scholar]
- 29.van Beers EJ, Schaap MCL, Berckmans RJ, Nieuwl R, Sturk A, van Doormaal FF, Meijers JCM, Biemond BJ. Circulating eythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94:1513–9. doi: 10.3324/haematol.2009.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen BAL, Xue A, Heit JA, Owen WG. Procoagulant activity, but not number, of microparticles increases with age in individuals after a single venous thromboembolism. Thromb Res. 2011;127:39–46. doi: 10.1016/j.thromres.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation, and signaling components. Transfus. 2008;48:1943–53. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 32.Bosman GJCGM, Lasonder E, Groenen-Dopp YAM, Willekens FLA, Werre JM, Novotny VMJ. Comparative proteomics o erythrocyte aging in vivo and in vitro. J Proteomics. 2010;73:396–402. doi: 10.1016/j.jprot.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Smeets EF, Comfurius P, Bevers EM, Zwaal RFA. Contribution of diferent phospholipid classes to the prothrombin converting capacity of sonicated lipid vesicles. Thromb Res. 1996;81:419–26. doi: 10.1016/0049-3848(96)00014-x. [DOI] [PubMed] [Google Scholar]
- 34.Smirnov MD, Ford DA, Esmon CT, Esmon NL. The effect of membrane composition on the hemostatic balance. Biochemistry. 1999;38:3591–8. doi: 10.1021/bi982538b. [DOI] [PubMed] [Google Scholar]
- 35.Larson MC, Woodliff JE, Hillery CA, Kearl TJ, Zhao M. Phosphatidylethanolamine is externalized at the surface of microparticles. Biochim Biophys Acta. 2012;1821(12):1501–7. doi: 10.1016/j.bbalip.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yates KR, Welsh J, Echrish HH, Greenman J, Maraveyas A, Madden LA. Pancreatic cancer cell and microparticle procoagulant surface characterization: involvement of membrane-expressed tissue factor, phosphatidylserine and phosphatidylethanolamine. Blood Coagul Fibrinolysis. 2011;22:680–7. doi: 10.1097/MBC.0b013e32834ad7bc. [DOI] [PubMed] [Google Scholar]
- 37.Bouchard BA, Silveira JR, Tracy PB. Interactions between platelets and the coagulation system. In: Michelson AD, editor. Platelets. Third. San Diego, CA: Academic Press; 2013. pp. 425–51. [Google Scholar]
- 38.Pieracci FM, Moore EM, Chin T, Townsens N, Gonzalez E, Burlew CC, Barnett CC., Jr The age of transfused blood predicts hematocrit response among critically ill surgical patients. Am J Surg. 2012;204(3):269–73. doi: 10.1016/j.amjsurg.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spinella PC, Carroll CL, Staff I, Gross R, Mc Quay J, Keibel L, Wade CE, Holcomb JB. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burnouf T, Chou M-L, Goubran H, Cognasse F, Garraud O, Seghatchian J. An overview of the role o microparticles/microvesicles in blood components: Are clinically beneficial or harmful? Transfus Apher Sci. 2015;53:137–45. doi: 10.1016/j.transci.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Chang AL, Kim Y, Seitz AP, Schuster RM, Lentsch AB, Pritts TA. Erythrocyte derived microparticles activate pulmonary endothelial cells in a murine model of transfusion. Shock. 2017;47(5):632–7. doi: 10.1097/SHK.0000000000000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Straat M, Boing AN, Tuip-De Boer A, Nieuwland R, Juffermans NP. Extracellular vesicles from red blood cell products induce a strong pro-inlammatory host response, dependent on both numbers and storage duration. Transfus Med Hemother. 2016;43:302–5. doi: 10.1159/000442681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hackeng TM, Tans G, Koppelman SJ, de Groot PG, Rosing J, Buoma BN. Protein C activation on endothelial cells by prothrombin activation products generated in situ: meizothrombin is a better protein C activator than alpha-thrombin. Biochem J. 1996;319:399–405. doi: 10.1042/bj3190399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood JP, Silveira JR, Maille NM, Haynes LM, Tracy PB. Prothrombin activation on the activated platelet surface optimizes expression of procoagulant activity. Blood. 2011;117(5):1710–8. doi: 10.1182/blood-2010-09-311035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sparrow RL. Time to revisit red blood cell additive solutions and storage conditions: a role for "omics" analyses. Blood Transfus. 2012;10(Suppl 2):s7–s11. doi: 10.2450/2012.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D'Alessandro A, Nemkov T, Kelher M, West BF, Schwindt RK, Banerjee A, Moore EE, Silliman CC, Hansen KC. Routine storage of red blood cell units in additive solution-3: a comprehensive investigation of the RBC metabolome. Transfus. 2015;55:1155–68. doi: 10.1111/trf.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.