Abstract
Cerebral microinfarcts are small lesions that are presumed to be ischaemic. Despite the small size of these lesions, affected individuals can have hundreds to thousands of cerebral microinfarcts, which cause measurable disruption to structural brain connections, and are associated with dementia that is independent of Alzheimer’s disease pathology or larger infarcts (ie, lacunar infarcts, and large cortical and non-lacunar subcortical infarcts). Substantial progress has been made with regard to understanding risk factors and functional consequences of cerebral microinfarcts, partly driven by new in-vivo detection methods and the development of animal models that closely mimic multiple aspects of cerebral microinfarcts in human beings. Evidence from these advances suggests that cerebral microinfarcts can be manifestations of both small vessel and large vessel disease, that cerebral microinfarcts are independently associated with cognitive impairment, and that these lesions are likely to cause damage to brain structure and function that extends beyond their actual lesion boundaries. Criteria for the identification of cerebral microinfarcts with in-vivo MRI are provided to support further studies of the association between these lesions and cerebrovascular disease and dementia.
Introduction
Cerebral microinfarcts are small lesions presumed to be of ischaemic origin. Although the lesions are often not visible to the naked eye on autopsy, definitions of specific size cutoffs vary and depend on different detection modalities. Cerebral microinfarcts are a common finding at brain autopsy, particularly in individuals with dementia and in individuals with other manifestations of cerebrovascular disease.1,2 A systematic review2 of neuropathological studies reported a cerebral microinfarct prevalence of 62% in patients with a diagnosis of vascular dementia, 43% in Alzheimer’s disease, and 24% in individuals aged around 75 years or older without a diagnosis of dementia before autopsy. Affected individuals are estimated to have hundreds to thousands of cerebral microinfarcts.3,4 A 2012 review1 highlighted the importance of these lesions, which are considered to be the most widespread form of brain infarction, with effects that extend beyond the tissue injury identified at autopsy. Cerebral microinfarcts cause measurable disruption to structural brain connections, and are associated with dementia that is independent of Alzheimer’s disease pathology. These findings suggest that cerebral microinfarcts represent an important mechanistic link between cerebrovascular disease and dementia.5
In 2012, cerebral microinfarcts were considered to be so-called invisible lesions,1 but high-resolution structural MRI has enabled the largest cerebral microinfarcts to be visualised in vivo. Additionally, animal models have been established to study the effect of cerebral microinfarcts on the brain with unprecedented temporal and spatial resolution. This Review describes these recent developments and provides an update on the frequency, risk factors, and functional consequences of cerebral microinfarcts. We propose criteria for identification of cerebral microinfarcts with MRI and provide a framework to translate candidate cerebral microinfarct mechanisms and outcome markers from animal models to MRI-guided clinical trials.
Detection of microinfarcts
Three different modalities are available for the detection of cerebral microinfarcts: neuropathological examination, diffusion-weighted imaging, and high-resolution structural MRI. An important consideration is that none of these three modalities capture the entire cerebral microinfarct burden in the brain (table). It is therefore important to note that neuropathological assessment—currently considered the reference standard—is not directly translatable to neuroimaging definitions. Furthermore, whether the different subtypes of cerebral microinfarcts—detected by different modalities—have the same causes and functional implications remains unclear. Further cross-modal studies are warranted to investigate this.
Table.
Comparison of the three modalities available for the detection of cerebral microinfarcts
| Neuropathological examination |
Diffusion-weighted MRI | High-resolution structural MRI |
|
|---|---|---|---|
| Spatial resolution | High | Moderate | Moderate |
| Microinfarct size | 100 µm–few mm | >1 mm | >1 mm |
| Temporal resolution | High | Low | High |
| Microinfarct stage | Hyperacute to chronic | Hyperacute (<2 weeks) | Acute to chronic (within hours on T2, within days on T1) |
| Brain coverage | Low | High | Partial |
| Volume assessed | <0·01%, few sections | Whole brain | Cortical grey matter |
Neuropathological examination
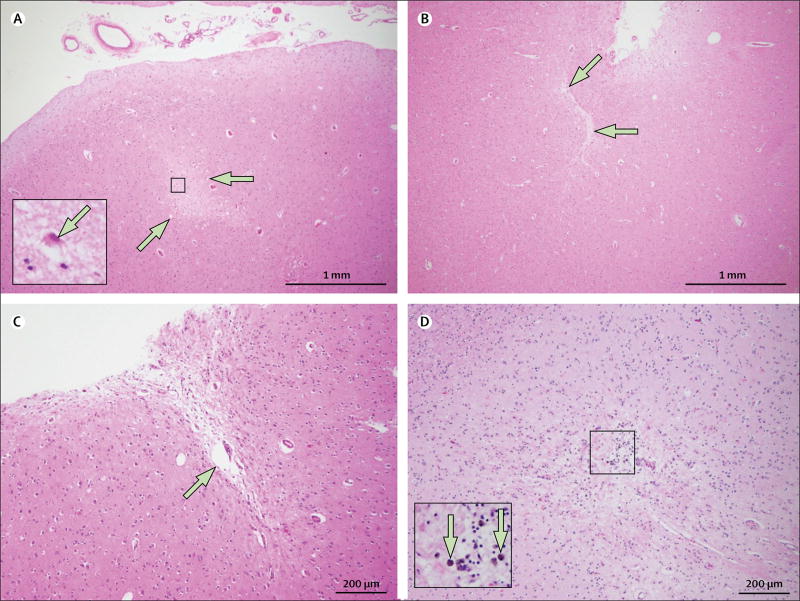
Neuropathological examination can detect the smallest acute (<24 h-old) and subacute to chronic (>24 h-old) cerebral microinfarcts. However, detection by most neuropathological methods has restricted brain coverage. In a standard autopsy of the brain, only a few samples (usually ≤20) are taken for processing into paraffin sections (approximately 4–6 µm-thick) for histopathological analysis; thus, less than 0·01% of the entire brain is sampled. Therefore, the detection of one or several cerebral microinfarcts at routine brain autopsy might indicate the presence of hundreds to thousands of cerebral microinfarcts throughout the brain that remain undetected.3 On microscopic examination of standard histological haematoxylin and eosin-stained sections, typical older (chronic) cerebral microinfarcts can be discriminated as focal lesions of tissue cavitation with evidence of gliosis and often a few remaining macrophages—a histological signature suggestive of ischaemia. Less extensive chronic lesions might show only neuronal cell loss with or without macrophages or substantial gliosis, with tissue pallor or puckering (figure 1). Evidence of previous haemorrhage in the form of haemosiderin or haematoidin might also be present, but unlike microhaemorrhages, the presence of such blood-breakdown products is not the primary feature of these lesions. Recent (acute) cerebral microinfarcts have well defined foci of cell injury (red neurons in the grey matter on haematoxylin and eosin staining) without cavitation or cell loss (figure 1). Most neuropathological studies focus on chronic cerebral microinfarcts because these lesions are most readily associated with cognitive impairment, often observed in the months before death. Acute cerebral microinfarcts might also be associated with peri-mortem events rather than factors relevant during life. Some studies report immunohistochemical approaches for the enhanced detection of cerebral microinfarcts, such as glial fibrillary acidic protein for gliosis, CD68 for macrophages, human leucocyte antigen markers for microglia,2 and calcineurin.6 However, the sensitivity and specificity of these approaches for the detection of cerebral microinfarcts is not clear.
Figure 1. Haematoxylin and eosin-stained sections of cerebral microinfarcts on neuropathological examination.
(A) An acute microinfarct (arrows) in the midfrontal cortex and a red hypoxic neuron (inset; arrow). (B) A chronic slit-like microinfarct with puckering in the inferior parietal cortex (arrows). (C) A chronic microinfarct with cavitation (arrow) in the inferior parietal cortex. (D) A chronic microinfarct with haemosiderin deposits in the inferior parietal cortex (inset; arrows).
The differences between pathological subtypes of cerebral microinfarcts (cavitated, slit-like, haemorrhagic, or recent; figure 1) have not yet been linked to specific pathogenic mechanisms, but do affect the appearance on MRI and the detectability of the lesions (panel 1). Conventionally, cerebral microinfarcts are only considered microinfarcts (as opposed to larger infarcts), when they are not visible to the naked eye on gross pathology. The reported sizes of microinfarcts vary between 100 µm and a few mm.2 Of note, standard neuropathological evaluation might underestimate the actual sizes of cerebral microinfarcts, because often only part of the lesion is included in a single thin paraffin section.
Panel 1. Proposed detection and visual rating criteria for cerebral microinfarcts on in-vivo MRI.
Acute cerebral microinfarcts on diffusion-weighted imaging (ie, small incidental diffusion-weighted imaging lesions) should meet all of the following criteria
Hyperintense on diffusion-weighted imaging
Apparent diffusion coefficient should be isointense or hypointense at the same location (to rule out so-called T2-shine-through of high-T2 signal)
Isointense or hyperintense on T2*-weighted MRI or blood-sensitive scans (eg, gradient echo or susceptibility-weighted imaging)
Any brain parenchymal location
Operationally defined as less than 5 mm in greatest dimension
Durable cortical cerebral microinfarcts on structural MRI should meet all of the following criteria
Hyperintense on T2-weighted MRI (ie, fluid-attenuated inversion recovery, T2), with or without cavitation on fluid-attenuated inversion recovery
Hypointense on T1-weighted MRI
Isointense on T2*-weighted MRI or blood-sensitive scans (eg, gradient echo or susceptibility-weighted imaging)
Operationally defined as strictly intracortical and less than 4 mm in greatest dimension
Distinct from enlarged perivascular spaces
Visible in at least two planes (eg, sagittal, transversal, coronal)
Important mimics for cortical cerebral microinfarcts are enlarged perivascular spaces in the immediate underlying juxtacortical areas that might extend into the cortical ribbon, leptomeningeal vessels (especially in the temporal lobes), anatomical variations (eg, gyral curvatures), and cortical cerebral microbleeds (signal on blood-sensitive scans should always be verified). We suggest that cerebral microinfarcts in close proximity (ie, <1 cm in the same gyrus) to larger strokes should not be considered as independent cerebral microinfarcts.
Diffusion-weighted imaging
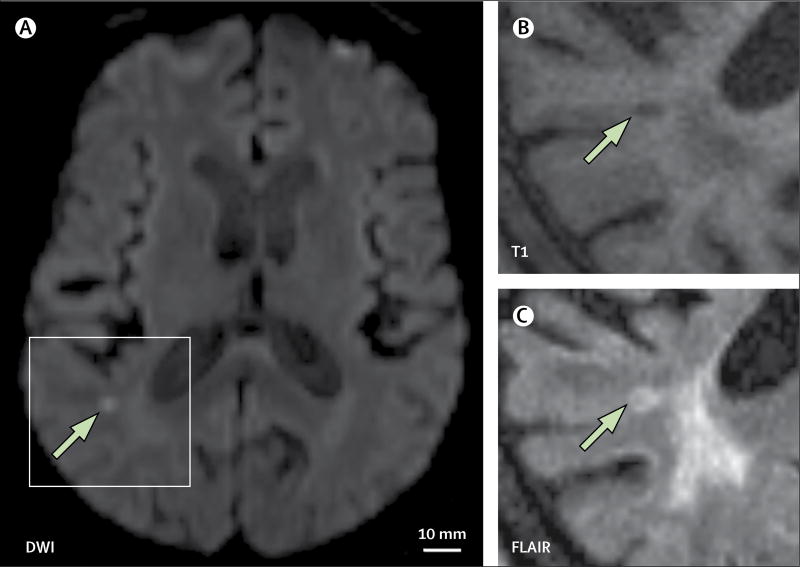
Diffusion-weighted imaging detects recent brain infarction with high sensitivity (figure 2).7 Because of the signal generated by diffusion-weighted imaging, even very small infarcts can be detected, including those 1–2 mm in diameter, which represent the upper limit of lesions that would be pathologically defined as cerebral microinfarcts. However, a disadvantage of diffusion-weighted imaging is that the signal fades over 2 weeks.8 Therefore, although diffusion-weighted imaging offers full brain coverage, temporal resolution for microinfarct detection is poor. A study published in 20154 used mathematical models to estimate the annual incidence of new cerebral microinfarcts according to the presence of incidental diffusion-weighted imaging lesions. The estimations indicated that the detection of one or two incidental small lesions on diffusion-weighted imaging—presumed to be acute cerebral microinfarcts large enough to produce a diffusion-weighted imaging signal—could reflect an annual incidence of hundreds of new cerebral microinfarcts of any size.4
Figure 2. Incidental small diffusion-weighted imaging lesion.
(A) DWI 1·5T MRI shows a hyperintense subcortical lesion (arrow). Follow-up imaging at 3 months shows that the lesion (arrows) is still visible, appearing hypointense on T1 (B) and hyperintense on FLAIR (C).
DWI=diffusion-weighted imaging. FLAIR=fluid-attenuated inversion recovery. T1=T1-weighted MRI.
Incidental small diffusion-weighted imaging lesions have been reported in 23–41% of patients with a recent intracerebral haemorrhage (<3 months) in whom they are associated with MRI manifestations of small vessel disease.9–12 These lesions have also been identified in 15% of patients with cerebral amyloid angiopathy and recent intracerebral haemorrhage,13 6% of patients with ischaemic stroke,14 14% of patients after carotid endarterectomy,15 and 1–4% of patients with cognitive impairment or dementia.16,17
On the basis of these prevalence data, the average patient with cerebral amyloid angiopathy is estimated to develop as many as eight asymptomatic diffusion-weighted imaging lesions per year.13 By contrast, the prevalence of incidental small diffusion-weighted imaging lesions in the general population appears much lower. In a population-based study of 793 participants aged 40–75 years, no incidental diffusion-weighted imaging lesions were found (95% confidence upper limit 0·5%),18 whereas in a community-based study of 623 older individuals (mean age 71 years) with more vascular risk factors, the prevalence was 1%.17
Most studies of incidental small diffusion-weighted imaging lesions have been cross-sectional. A notable exception was a small study19 of five patients with extensive white matter hyperintensities who had weekly MRI scans over 16 weeks, during which three patients had a total of nine incidental subcortical diffusion-weighted imaging lesions. Another longitudinal study11 found incidental diffusion-weighted imaging lesions in 30 (27%) of 113 patients with intracerebral haemorrhage scanned 30 days after admission.
The long-term outcomes of incidental small diffusion-weighted imaging lesions (ie, their MRI signature after weeks or months) are largely unknown. Preliminary data suggest that these lesions can evolve into a small cavity, a T2 hyperintensity without cavitation (figure 2), or become radiologically inapparent.20 In some patients, acute small diffusion-weighted imaging lesions resolved into cavities less than 3 mm in diameter, which in their chronic stage would not be classifiable as infarcts according to STRIVE criteria.21
Most studies of incidental small diffusion-weighted imaging lesions do not report their actual size, and instead use a cutoff (eg, <5 mm in diameter). However, representative figures from previous publications9,10,13,20 suggest that most small diffusion-weighted imaging lesions are only a few mm in size and potentially consistent with cerebral microinfarcts. Whether upper size cutoffs for diffusion-weighted imaging lesions compatible with acute cerebral microinfarcts can distinguish them from acute lacunar infarcts or large acute cortical infarcts remains unclear. The STRIVE criteria21 for recent small subcortical infarcts include an upper size cutoff of 20 mm, a criterion designed to identify lacunar infarcts in their acute stage, without providing a lower size cutoff and without criteria for classification of cortical small infarcts. Another issue is that size of diffusion-weighted imaging lesions depends on field strength and the parameters of the diffusion-weighted imaging sequence.
On the basis of the available evidence, we postulate that diffusion-weighted imaging lesions with an axial diameter of 5 mm or more are unlikely to evolve into what would be considered a cerebral microinfarct on structural brain imaging or neuropathological examination, and should not be described as cerebral microinfarct. Hence, we propose a size criterion of less than 5 mm for acute cerebral microinfarcts (panel 1). Notably, this cutoff was operationally defined and might need to be refined in the future as more data about the long-term MRI signature of incidental small diffusion-weighted imaging lesions and the association between radiological and pathological findings become available.
High-resolution structural MRI
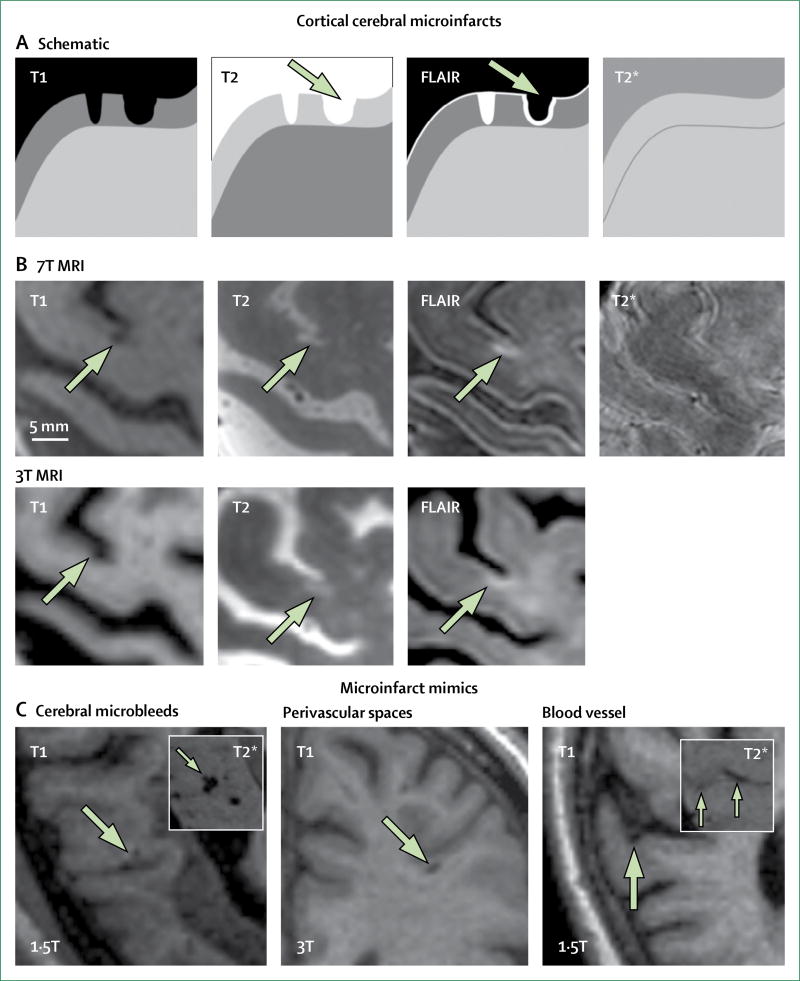
Detection of cerebral microinfarcts on structural MRI provides a major step forward in determining the causes and consequences of these small ischaemic lesions. Evidence from radiological–histopathological correlation studies22–24 using high-field strength 7T MRI suggested that durable (ie, not just visible in the subacute or acute stage) cerebral microinfarcts of at least 1–2 mm in size in the cortical grey matter can be discerned on structural MRI scans (figure 3, panel 1). These post-mortem verification studies22–24 indicated high specificity (26 of 27 lesions on post-mortem MRI were verified as cerebral microinfarcts on the corresponding histological sections). These initial studies used a pragmatic approach to specifically focus on the detection of cerebral microinfarcts in cortical areas of the brain because in the white matter it is difficult to distinguish cerebral microinfarcts from other lesions such as white matter hyperintensities, lacunar infarcts, and enlarged perivascular spaces. Indeed, in juxtacortical areas (directly adjacent to, rather than within, cortical grey matter), lesions that had an appearance similar to cortical cerebral microinfarcts were enlarged perivascular spaces on histological examination.23 Hence, we consider punctate subcortical lesions on structural MRI—without previous information about diffusion-weighted imaging positivity—currently unclassifiable as cerebral microinfarcts.
Figure 3. Durable cortical cerebral microinfarcts and mimics on structural MRI.
(A) Schematic representation of a cortical cerebral microinfarct (left schematic lesion), which is hypointense on T1, hyperintense on T2 and FLAIR, and isointense on T2*-weighted images. The right schematic lesion (arrow) represents a cortical cerebral microinfarct with cavitation, which appears hypointense on FLAIR with a hyperintense rim. (B) Examples of a cortical cerebral microinfarct (arrows) without cavitation imaged in the same individual on the same day using 7T and 3T MRI. (C) Examples of common cerebral microinfarct mimics, such as cerebral microbleeds (arrows), which are distinct from cerebral microinfarcts because they appear hypointense on T2*-weighted MRI (inset); enlarged perivascular spaces (arrow), which are distinct from cortical cerebral microinfarcts because they are located in juxtacortical areas; and blood vessels (arrow), which are distinct from cortical cerebral microinfarcts because they appear hypointense on T2*-weighted MRI and can be traced over several slices (inset; arrows). FLAIR=fluid-attenuated inversion recovery. T1=T1-weighted MRI. T2=T2-weighted MRI. T2*=T2*-weighted MRI.
The first studies22,25–27 to detect cortical cerebral microinfarcts in vivo used 7T MRI. A later 7T MRI study28 of 23 patients showed that 27% of the lesions were also visible on 3T MRI scans done on the same day. Subsequently, cortical cerebral microinfarcts were examined in several studies14,28–30 using 3T MRI. Cerebral microinfarcts have also occasionally been observed using 1·5T MRI (appendix), so this possibility should not be overlooked. In addition to field strength, the sensitivity of detection depends on the scan protocol and level of scrutiny used during examination.
Proposed rating criteria for the detection of durable cerebral microinfarcts on in-vivo MRI and representative examples are provided in panel 1, figure 3, and the appendix. The shape of durable cortical cerebral microinfarcts on in-vivo structural MRI generally matches the perfusion territory of small, single penetrating cortical arterioles (ie, a cylindrical shape perpendicular to the cortical surface extending towards the border with the white matter). Importantly, such cerebral microinfarcts can be detected using existing datasets from large cohort studies, which provides an important opportunity to advance this field. For reliable detection of cortical cerebral microinfarcts on structural MRI, datasets should ideally include high-resolution (≤1 × 1 × 1 mm voxel size) three-dimensional (3D) T1-weighted and 3D T2-weighted (ie, fluid-attenuated inversion recovery or T2) MRI, and blood-sensitive scans to rule out cerebral microinfarct mimics, such as cerebral microbleeds. Because most existing datasets from 1·5–3T MRI studies include only one 3D scan—often the T1—detection of cortical cerebral microinfarcts in those datasets on T1 is recommended, aided by two-dimensional (2D) T2-weighted images, to rule out possible microinfarct mimics (ie, a lesion that is compatible with a microinfarct on 3D T1-weighted images but cannot be detected as a hyperintense lesion on 2D T2-weighted imaging, because of lesion orientation or slice thickness, can be accepted as a microinfarct if the T2* image is not hypointense at the same site). Two 3T MRI studies measured the actual size of such lesions smaller than 5 mm: one study28 reported a median lesion size of 3 mm in 75 patients, with only 8% of lesions larger than 3 mm. Similarly, the other study31 of 209 patients reported a mean lesion size of 2·8 mm (SD 0·8; 95% CI 1·1–4·5). We therefore propose a size criterion for durable cortical cerebral microinfarcts of less than 4 mm (panel 1).
Additional methodological details and examples of cerebral microinfarcts are available,32 which includes a video instruction to guide detection of cortical cerebral microinfarcts on both 7T and 3T in-vivo structural MRI. Currently, visual rating of cortical cerebral microinfarcts is time intensive (approximately 30 min per patient), and inter-rater agreement is modest (two studies reported intraclass correlation coefficients of 0·3933 and 0·76,26 and another study30 reported a kappa of 0·83). Development of semi-automated detection techniques is ongoing26,34 and would greatly improve observer reliability and the efficiency of evaluation of existing 3T MRI datasets from large cohort studies.
Causes and risk factors of microinfarcts
Cerebral microinfarcts have multiple underlying causes, which can coexist in a single patient. The three main causes are cerebral small vessel disease (eg, cerebral amyloid angiopathy, arteriolosclerosis), microemboli, and hypoperfusion. Neuropathological studies have shown that cerebral microinfarcts are common in the presence of severe cerebral amyloid angiopathy at autopsy.35–37 Subsequently, cerebral microinfarcts have been assessed with regard to manifestations of cerebral amyloid angiopathy in living patients where durable cortical cerebral microinfarcts were associated with lobar cerebral microbleeds on 7T MRI25,26 and 3T MRI.28,38,39 A study40 that assessed cortical cerebral microinfarcts on in-vivo 7T MRI in patients with hereditary cerebral amyloid angiopathy showed that cerebral microinfarcts were one of the earliest markers of the disease, compared with other clinical and MRI manifestations.
Neuropathological studies have further explored the association between cerebral microinfarcts and cerebral amyloid angiopathy, atherosclerosis, and arteriolosclerosis. In one study,41 which examined the brains of approximately 1000 community-dwelling individuals at autopsy, cortical cerebral microinfarcts were associated with cerebral amyloid angiopathy, whereas subcortical cerebral microinfarcts were associated with atherosclerosis and arteriolosclerosis. In a smaller study of 80 patients at autopsy,42 all of whom had multiple cerebral microinfarcts on routine pathological examination, cerebral microinfarcts in the occipital cortex were associated with local cerebral amyloid angiopathy pathology, whereas cerebral microinfarcts in the frontal cortex and hippocampus were not.
In addition to cerebral amyloid angiopathy, cerebral microinfarcts are also associated with other manifestations of small vessel disease on MRI. Both in patients who attended a memory clinic28 and in population-based cohorts,29,30 cerebral microinfarcts co-occur with lacunar infarcts28,30 and the presence of cerebral microinfarcts is associated with a higher volume of white matter hyperintensities28–30 and cerebral atrophy.28 The presence of cerebral microinfarcts has been linked to a history of stroke in population-based cohorts,29,30 but also among patients admitted to hospital for stroke14,31,43 and in patients attending a memory clinic with various diagnoses, including subjective cognitive complaints, Alzheimer’s disease, and vascular dementia.28 This association between cerebral microinfarcts and stroke might also be linked to large vessel disease, because large cortical infarcts on MRI and cerebral microinfarcts have been found to co-occur.28,30 Notably, cerebral microinfarcts have been associated with intracranial stenosis in studies of patients recruited from a memory clinic28 and in patients with ischaemic stroke,31,43 and commonly occur ipsilateral to the stenosis.28,43 A 2017 study15 reported that the incidence of acute cerebral microinfarcts was 14% on diffusion-weighted imaging in patients who had carotid endarterectomy. Atrial fibrillation has also been identified as an important risk factor for cortical cerebral microinfarcts.14,28,44 Moreover, biomarkers of cardiac disease, particularly N-terminal pro-brain natriuretic peptide and high-sensitivity cardiac troponin T, were associated with cortical cerebral microinfarcts on 3T MRI in patients attending a memory clinic.44 Whether these links between cerebral microinfarcts and both large vessel atherosclerosis and cardiac disease reflect microemboli as a causative mechanism or even hypoperfusion—which has been proposed as an alternative mechanism of cerebral microinfarct formation—remains unclear.45,46
Literature has emerged about the association between vascular risk factors and cerebral microinfarcts on MRI. A 7T MRI study33 that assessed cortical cerebral microinfarcts in 48 patients with type 2 diabetes mellitus and 49 control participants found no difference in occurrence or number of cortical cerebral microinfarcts between patients with diabetes and controls. This observation is consistent with two neuropathological studies47,48 that assessed 1228 and 2365 patient autopsies, which showed an association between diabetes and large infarcts, but not between diabetes and cerebral microinfarcts. In a 3T MRI study28 of 238 patients who attended a memory clinic, cerebral microinfarcts were not associated with diabetes or hypertension, but were significantly associated with hyperlipidaemia. By contrast, in a study14 of 231 patients with ischaemic stroke or transient ischaemic attack, none of these risk factors were associated with the presence of cerebral microinfarcts, whereas in a cohort30 of 861 participants from the general population, cerebral microinfarcts were associated with hypertension. These inconsistent results indicate that identification of risk factor patterns for cerebral microinfarcts may be influenced by the setting in which they are studied, and this is an area that warrants further investigation.
Taken together, these studies clearly show that the cause of cerebral microinfarcts is heterogeneous, and is linked to different manifestations of small vessel disease, large vessel disease, and cardiac disease. In most settings, the presence of cerebral microinfarcts cannot be regarded as a marker of specific causes. However, in specific patient subgroups with a high burden of one particular pathology—eg, patients diagnosed with probable cerebral amyloid angiopathy—presence of cerebral microinfarcts might be regarded as a likely marker of that pathology. Whether particular cerebral microinfarct features, such as location, size, or shape might be indicative of specific causes requires investigation. Autopsy studies do indeed suggest that a link might exist between cerebral microinfarct location and different subtypes of small vessel diseases, but such studies are limited by restricted brain coverage. MRI data26,28,31 from cohorts of patients who attended a memory clinic or had a stroke suggest a predisposition for cerebral microinfarcts to occur in parietal and frontal areas (appendix). However, MRI studies are restricted by the fact that durable subcortical lesions and lesions smaller than 1–2 mm cannot be detected with current techniques.
With the development of semi-automated cerebral microinfarct detection techniques, a wealth of MRI data from existing, large population, and clinic-based cohorts with detailed information about risk factors and comorbidities is expected to become available. Additionally, studies that combine post-mortem MRI and histopathological examination are ongoing. These techniques and studies might provide more unbiased data about risk factors and causes of cerebral microinfarcts.
Functional effect of microinfarcts on the brain
The presence of cerebral microinfarcts on brain autopsy has been associated with ante-mortem cognitive dysfunction.1 Neuropathological studies49–54 have confirmed the link between cerebral microinfarcts on autopsy and ante-mortem dementia and cognitive impairment, although the trajectories of cognitive decline later in life with regard to cerebral microinfarcts and other co-occurring pathologies are complex and might require further investigation. Studies suggest a strong association between cerebral microinfarcts and age,51,52 race,53 and additive effects with comorbid pathologies.53
Advances in cerebral microinfarct detection during life greatly facilitate studies of their functional effect. In one of the first studies25 that investigated the detection of cerebral microinfarcts using in-vivo 7T MRI, a higher number of cerebral microinfarcts was found in 14 patients with Alzheimer’s disease compared with 18 healthy controls, although the proportion of participants with cerebral microinfarcts did not differ between the groups (86 vs 72%). Similarly, another 7T MRI study26 found no difference in cerebral microinfarct occurrence between 29 patients with early Alzheimer’s disease and 22 healthy controls (55 vs 45%), but did not confirm differences in cerebral microinfarct numbers between groups. Both of these initial studies were limited by small numbers of participants, which is inherent to most in-vivo 7T MRI studies. Moreover, one of the studies25 also considered juxtacortical lesions, which might include cerebral microinfarct mimics, particularly enlarged perivascular spaces.23 Cerebral microinfarcts can also be detected on 3T MRI scans, which allows assessment of the association between these lesions and cognition in larger cohorts, with findings that are likely to have greater generalisability than findings from small cohorts. In a cohort28 which included 238 patients who had attended a memory clinic, cerebral microinfarcts were associated with dementia, most strongly with vascular dementia (cerebral microinfarcts occurred in 12 [55%] of 22 patients with vascular dementia). In particular, cerebral microinfarcts were associated with worse global cognition and poorer performance on tasks associated with visuoconstructive abilities and language. In a sample of patients14 with ischaemic stroke or transient ischaemic attack both cortical cerebral microinfarcts and acute cerebral microinfarcts at baseline predicted worse cognitive performance at 2 year follow-up than in patients without cerebral microinfarcts, especially in the domain of visuospatial functioning. In an Asian study30 of 861 participants at risk of developing cognitive impairment, the presence of cortical cerebral microinfarcts on in-vivo 3T MRI was independently associated with dementia. Furthermore, cortical cerebral microinfarcts were significantly associated with worse global cognition and poorer performance on tasks in the domains of executive function, visual memory, and verbal memory.30 Notably, these studies considered confounding effects of other markers of vascular damage and neurodegeneration, such as atrophy.
One neuropathology study55 of 850 patients at autopsy found that cerebral microinfarcts were associated with impaired motor function before death. The effect of cerebral microinfarcts on motor function has not yet been investigated using in-vivo MRI.
Taken together, these initial in-vivo MRI studies support neuropathological observations that cerebral microinfarcts are associated with worse cognition, independent of other age-associated pathologies. However, whether such associations are causal remains unclear. It is possible that, as a result of the high numbers and widespread distribution of cerebral microinfarcts throughout the brain, these lesions might cause sufficient disruption resulting in functional impairment, which has been supported by animal studies. Alternatively, cerebral microinfarcts might be a marker of other underlying vascular pathologies that are even more widespread than the lesions themselves, and thus also affect the brain without visible focal injury.
Animal models of microinfarcts
Mechanisms
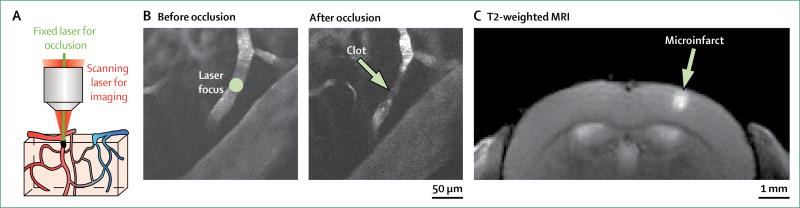
Cerebral microinfarcts have been successfully modelled in the brains of rodents by occluding penetrating arterioles that form bottlenecks in microvascular perfusion.56–62 Penetrating arterioles have emerged as a key locus for occlusion, because unlike the interconnected pial and capillary systems, blood flow through a penetrating arteriole cannot be efficiently re-routed around a localised clot.63 Cerebral microinfarcts induced in rodents show remarkable similarity to human cerebral microinfarcts, with respect to their range of shapes, size, and location, and their temporal evolution (figure 4). For example, the core of a cerebral microinfarct in an animal model becomes packed with microglia and is surrounded by reactive astrocytes, which is also observed in cerebral microinfarcts in human beings.56
Figure 4. Modelling microinfarcts in rodent cortex by targeted photothrombotic occlusion of individual penetrating arterioles.
(A) Under the guidance of multiphoton imaging, a single penetrating arteriole is visualised with a scanning laser and occluded with a second fixed green laser after intravenous injection of rose bengal (a photosensitising agent). (B) In-vivo multiphoton images of a single penetrating arteriole, before and after occlusion.
(C) A cortical microinfarct visualised using in-vivo T2-weighted MRI. Despite a large difference in brain size, rodent microinfarcts are similar in absolute size to human microinfarcts because microvascular topology is relatively well conserved between species.64,65
Two distinct but complementary strategies have been used to model cerebral microinfarcts. One method involves the intra-arterial injection of occlusive microbeads,57 cholesterol crystals,58,59 or microthrombi.60 This sudden influx of microemboli produces cerebral microinfarcts that are broadly distributed, with shapes ranging from wedge or column-like lesions in the cortex to smaller, circumscribed cerebral microinfarcts in both cortical and subcortical tissues. A second, more recently developed model of cortical microinfarction enables the location and size of cerebral microinfarcts to be targeted reproducibly in rodent cortex. Typically coupled with in-vivo multiphoton imaging, single penetrating arterioles are selectively occluded by inducing clots with precise laser irradiation, with and without intravenous photosensitising agents.61,62,66–68
Cerebral microinfarcts that occur spontaneously have also been identified in mouse models. When subjected to forebrain hypoperfusion with bilateral carotid stenosis, the Tg-SwDI mouse model of cerebral amyloid angiopathy develops cortical and subcortical cerebral microinfarcts between the age of 18 and 32 weeks.45 Application of carotid stenosis to non-transgenic C57BL/6 mice also led to spontaneous cerebral microinfarct formation and microhaemorrhages, but with a lower incidence than observed in Tg-SwDI mice.69 Mice deficient in endothelial nitric oxide synthase have cerebral hypoperfusion and also develop both cortical and subcortical cerebral microinfarcts.70 These models can provide important clues about the cause of cerebral microinfarcts in human beings, and the vascular changes responsible for cerebral microinfarct formation require thorough investigation.
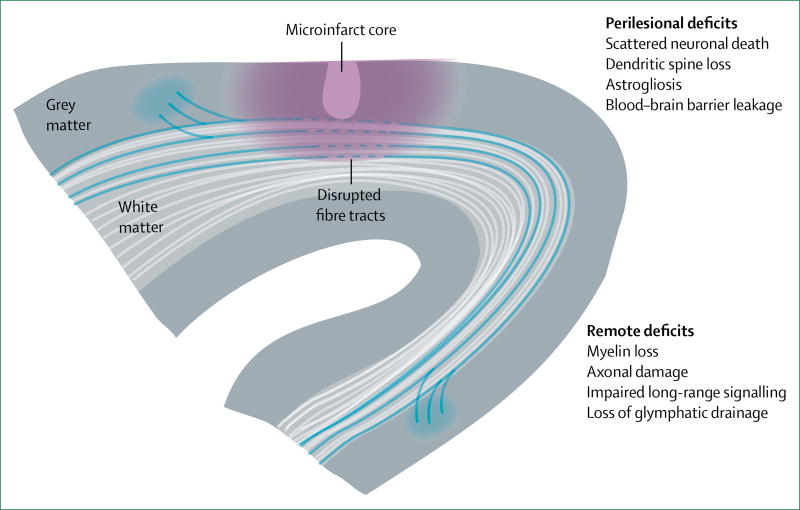
Perilesional and remote effects of microinfarcts
Animal studies have provided evidence that the physiological effect of cerebral microinfarcts extends beyond the non-viable lesion core observed by MRI or in neuropathological studies (figure 5). By strategically targeting cerebral microinfarcts in the mouse sensory or motor cortices, in-vivo imaging studies71 have reported deficits in both neural and haemodynamic function in tissue surrounding the cerebral microinfarct that can last for weeks. These deficits involved persistent alterations in neuronal circuitry, observed by displacement and disorganisation of sensorimotor maps.72 A study published in 201756 estimated that a single cerebral microinfarct, with a mean lesion core of 0·2 mm3 in volume (approximately 0·5 mm in diameter), could impair neuronal function over cortical regions at least 12 times larger in size than the lesion core. However, this calculation is likely to be an underestimate because cerebral microinfarcts in both grey and white matter can also disrupt neighbouring fibres of passage, with demyelination and damage of axonal structure.57–59 Consistent with cerebral microinfarcts having a considerable effect on brain function, rodent studies using unilateral58,59 or repeated bilateral cholesterol crystal injections73 have reported deficits in cognitive tasks, despite the actual cerebral microinfarct load being small compared with overall brain size. Two of these studies59,74 also showed brain-wide impairment of glymphatic pathways, a perivascular network for clearance of amyloid-β and other cytotoxic metabolites from the brain.
Figure 5. Functional deficits in tissues beyond the non-viable microinfarct core.
Perilesional deficits are caused by secondary effects of ischaemic injury, such as spreading depression, blood–brain barrier disruption, and neuroinflammation. Remote deficits arise when microinfarcts damage white matter fibres, or occur in areas that are connected to, and are crucial for, the function of other brain regions
Examination of the perilesional areas of experimentally induced cerebral microinfarcts has revealed numerous pathological changes that are likely to be substrates for observed functional and behavioural deficits. Evidence supports delayed, selective neuronal loss (5–10% decrease),58 thinning of dendritic processes, and reduced dendritic spine density.56,59 Non-neuronal changes include widespread astrogliosis, reduced expression of aquaporin 4, and myelin loss.56,58,59,61,75 Blood–brain barrier disruption is also evident on the basis of extravasation of plasma proteins and nitrosative stress at the capillary walls.61,73 One striking manifestation of this pathology is the coalescence of isolated cerebral microinfarcts into larger contiguous infarcts when their perilesional zones begin to overlap.61 This finding emphasises the susceptibility of tissues in perilesional areas, and suggests that injury can be shifted towards further exacerbation, or rescued by therapeutic agents.
The perilesional and remote effects of cerebral microinfarcts are likely to involve the same pathophysiological processes that occur with larger strokes, but on a smaller scale. For example, cerebral microinfarcts can evoke waves of ischaemic spreading depression that appear several mm from the cerebral microinfarct core, which might contribute to neuronal damage and hypoperfusion.61 Neuronal dysfunction might also involve diaschisis, whereby death of neurons within the cerebral microinfarct core disables the cortical and subcortical circuits to which they were previously integrated. Maladaptive increases of inhibitory tone in perilesional tissues might also depress neuronal excitation.76 Additionally, cerebral microinfarcts located within or in close proximity to white matter tracts are likely to disrupt communication between distant brain regions by damaging axonal structure. Impairment of glymphatic drainage might elevate toxic metabolites across the whole brain.59,74 These potential mechanisms of cerebral microinfarct-induced pathology need to be studied in greater detail using animal models, because the relative importance of each mechanism remains poorly understood.
Conclusions and future directions
The course of cerebral microinfarct research has largely depended on the relatively complex problem of how to assess their total burden in the brain. Accurate quantification of cerebral microinfarcts is crucial for the identification of their specific effect on brain function, and the pathogenic processes involved in their development. It is thus exciting to note that substantial progress has been made in recent years regarding cerebral microinfarct detection, particularly the publication of pathologically validated structural MRI methods and proposed rating criteria for cortical cerebral microinfarcts (panel 1). Further development of these methods is likely, including the possibility of semi-automated detection methods for cortical cerebral microinfarcts that could be used to analyse large, population-derived 3T MRI datasets. However, current methods can detect only a small fraction of cerebral microinfarcts and therefore a bias exists towards lesions with a particular set of spatial, temporal, or anatomical characteristics (table). In practice, these limitations imply that all studies of the effects of cerebral microinfarcts have considerable built-in error as a result of miscounting and biased counting of lesions. Moreover, the reliability of visual and emerging computational assessments remains to be assessed (panel 2).
Panel 2. Directions for future research.
Identification and definition of microinfarcts
Refine operational rating criteria for the detection of acute cerebral microinfarcts on diffusion-weighted MRI (eg, size cutoff)
Verify the ischaemic nature of these small incidental diffusion-weighted imaging lesions by comparing MRI scans with histopathological examinations
Establish sensitivity, specificity, and rater reliability for proposed criteria for detection of cerebral microinfarcts on MRI accounting for differences in field strength and other imaging parameters
Establish MRI detection methods for durable subcortical cerebral microinfarcts
Investigate topographical distribution of cerebral microinfarcts (eg, lobar distribution, grey matter vs white matter, and underlying small vessel diseases)
Do crossmodal studies that investigate different pathological subtypes of cerebral microinfarcts (eg, cavitated, haemorrhagic, slit-like), lesion sizes, and MRI appearance77
Causes, risk factors, and functional effects
Do longitudinal MRI studies in different populations, including the general population and specific patient cohorts, with rich phenotyping of risk factors, causes, and functional outcomes
Identify pathophysiological mechanisms underlying cerebral microinfarct formation in different patient populations focusing on the roles of vascular risk factors, small vessel diseases, microemboli, and hypoperfusion
Establish whether features such as size, distribution, and appearance of cerebral microinfarcts reflect different pathogenic mechanisms
Study the remote effects of cerebral microinfarcts on brain structure and function, and translate insights from animal models to human beings
Use animal models that spontaneously develop cerebral microinfarcts to better understand vascular factors that lead to cerebral microinfarcts
Treatment
Establish whether cerebral microinfarcts can be used as reliable and informative biomarkers in multicentre clinical trials
Study the involvement of aggressive blood pressure reduction and other vascular interventions in the formation of cerebral microinfarcts
Another area of both notable progress and substantial remaining uncertainty is the extent to which cerebral microinfarcts disrupt the brain tissue in their immediate surrounding, as well as the brain’s network structure and function as a whole. Such potential widespread effects of cerebral microinfarcts might allow these numerous, spatially distributed lesions to exert a greater effect on brain function than predicted by their small aggregate volume (which is also likely to be true for other brain lesions in the context of small vessel disease). Because the strongest evidence for widespread effects of cerebral microinfarcts is derived from animal model studies (figure 5), future work needs to translate imaging markers from animals to human beings (panel 2).
Future improvements in cerebral microinfarct detection and measurement of their widespread effects will establish the role of these lesions, not just as markers of microvascular injury and cognitive impairment, but as direct contributors to cognitive decline. Identification of a major causative role for cerebral microinfarcts would lead to the inclusion of these lesions as candidate outcome markers for trials aimed at preventing vascular cognitive impairment. Evidence that suggests the accrual of cerebral microinfarcts has a major causal role in progressive cognitive impairment is needed to bring renewed focus on neuroprotection in ischaemic stroke—a field that has been extensively studied, but has not yielded clinically effective treatments.78 The potential impact of improvements in the cerebral microinfarct field thus extends beyond simply providing another imaging biomarker of cerebral small vessel disease to the larger goal of guiding future therapeutic approaches.
Supplementary Material
Search strategy and selection criteria.
We searched PubMed to identify articles for this Review, focusing on papers published in English between Jan 1, 2012, and April 10, 2017. The search terms (and synonyms) ‘microinfarct*’, in combination with ‘brain or cerebral’, and ‘autopsy’, ‘neuropathology’, ‘MRI’, ‘imaging’, or ‘diffusion’ were used. To identify relevant animal studies, we used the search terms ‘microinfarct*’, ‘microemboli’, ‘ministroke’, or ‘infarct’ and ‘brain or cerebral’, and ‘rodent or mouse’, and ‘penetrating vessel’. SJvV and GJB selected papers identified through these searches, supplemented with additional papers from personal records, with feedback from the coauthors. Original papers and, where appropriate, high impact reviews were included. The final reference list was generated on the basis of relevance and originality with regard to the topics covered in this Review.
Acknowledgments
Declaration of interests
SJvV reports a grant from the Netherlands Organisation for Scientific Research (019.153LW.014). AYS reports grants from the National Institute of Neurological Disorders and Stroke (NS085402, NS096997), the Dana Foundation, The National Science Foundation (1539034), South Carolina Clinical and Translational Institute (UL1TR000062), Alzheimer’s Association (New Investigator Research Grant), and the US National Institute of General Medical Sciences (P20GM109040); and has received the Charleston Conference on Alzheimer’s Disease New Vision Award. EES reports grants from the University of Calgary, Alberta Innovates–Health Solutions, the Canadian Institutes of Health Research, Brain Canada, the Heart and Stroke Foundation of Canada, the Canadian Partnership Against Cancer, and the European Union Joint Programme-Neurodegenerative Disease Research. CC reports grants from the National Medical Research Council Singapore (NMRC/CIRG/1446/2016, NMRC/CG/013/2013). JAS serves as a consultant for Navidea Biopharmaceuticals, Eli Lilly Inc, Genentech, and the Michael J Fox Foundation; and reports a grant from the US National Institute of Health (UH2 NS100599-01). JMW reports grants from Fondation Leducq (CVD-05), the European Union Horizon 2020 (666881), the Medical Research Council (MR/K026992/1), Age UK, Alzheimer Society (ASPG-14-033), the Wellcome Trust (088134/Z/09), Innovate UK, the British Heart Foundation, and the Chief Scientist Office of the Scottish Executive. SMG reports grants from the US National Institute of Health (R01 AG26484, R01 NS096730). GJB reports grants from the Dutch Heart Association (2010T073), ZonMw (91711384, 91816616), the Netherlands Organisation for Health Research and Development, and European Union Horizon 2020 (666881).
Footnotes
Contributors
SJvV and GJB coordinated the overall process of manuscript production. SJvV, GJB, and SMG were involved in study design. SJvV, AYS, EES, JAS, and JMW performed literature searches, and SJvV and GJB selected relevant papers. SJvV, AYS, EES, SMG, and GJB wrote the manuscript. SJvV and AYS prepared the figures. All authors were involved in critically reading and editing the manuscript. All authors gave final approval for submission.
Contributor Information
Susanne J van Veluw, Department of Neurology, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht, Netherlands; Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Andy Y Shih, Department of Neuroscience, Medical University of South Carolina, Charleston, SC, USA.
Eric E Smith, Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada.
Christopher Chen, Memory Ageing and Cognition Centre, National University Health System, Singapore.
Prof Julie A Schneider, Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, IL, USA.
Prof Joanna M Wardlaw, Centre for Clinical Brain Sciences and Centre for Cognitive Ageing and Cognitive Epidemiology, University of Edinburgh, Edinburgh, UK.
Prof Steven M Greenberg, Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Prof Geert Jan Biessels, Department of Neurology, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht, Netherlands.
References
- 1.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012;11:272–82. doi: 10.1016/S1474-4422(11)70307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brundel M, De Bresser J, Van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab. 2012;32:425–36. doi: 10.1038/jcbfm.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westover MB, Bianchi MT, Yang C, Schneider JA, Greenberg SM. Estimating cerebral microinfarct burden from autopsy samples. Neurology. 2013;13:1–5. doi: 10.1212/WNL.0b013e31828c2f52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auriel E, Westover MB, Bianchi MT, et al. Estimating total cerebral microinfarct burden from diffusion-weighted imaging. Stroke. 2015;46:2129–35. doi: 10.1161/STROKEAHA.115.009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 6.Pleiss MM, Sompol P, Kraner SD, et al. Calcineurin proteolysis in astrocytes: implications for impaired synaptic function. Biochim Biophys Acta. 2016;1862:1521–32. doi: 10.1016/j.bbadis.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keir SL, Wardlaw JM. Systematic review of diffusion and perfusion imaging in acute ischemic stroke. Stroke. 2000;31:2723–31. doi: 10.1161/01.str.31.11.2723. [DOI] [PubMed] [Google Scholar]
- 8.Lansberg MG, Thijs VN, Brien MW, et al. Evolution of apparent diffusion coefficient, diffusion-weighted, and T2-weighted signal intensity of acute stroke. Stroke. 2001;22:637–44. [PMC free article] [PubMed] [Google Scholar]
- 9.Prabhakaran S, Gupta R, Ouyang B, et al. Acute brain infarcts after spontaneous intracerebral hemorrhage: a diffusion-weighted imaging study. Stroke. 2010;41:89–94. doi: 10.1161/STROKEAHA.109.566257. [DOI] [PubMed] [Google Scholar]
- 10.Gregoire SM, Charidimou A, Gadapa N, et al. Acute ischaemic brain lesions in intracerebral haemorrhage: multicentre cross-sectional magnetic resonance imaging study. Brain. 2011;134:2376–86. doi: 10.1093/brain/awr172. [DOI] [PubMed] [Google Scholar]
- 11.Menon RS, Burgess RE, Wing JJ, et al. Predictors of highly prevalent brain ischemia in intracerebral hemorrhage. Ann Neurol. 2012;71:199–205. doi: 10.1002/ana.22668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg RK, Liebling SM, Maas MB, Nemeth AJ, Russell EJ, Naidech AM. Blood pressure reduction, decreased diffusion on MRI, and outcomes after intracerebral hemorrhage. Stroke. 2012;43:67–71. doi: 10.1161/STROKEAHA.111.629493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimberly WT, Gilson A, Rost NS, et al. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009;72:1230–35. doi: 10.1212/01.wnl.0000345666.83318.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Van Veluw SJ, Wong A, et al. Risk factors and cognitive relevance of cortical cerebral microinfarcts in patients with ischemic stroke or transient ischemic attack. Stroke. 2016;47:2450–55. doi: 10.1161/STROKEAHA.115.012278. [DOI] [PubMed] [Google Scholar]
- 15.Gwon JG, Kwon TW, Cho YP, Kang DW, Han Y, Noh M. Analysis of risk factors for cerebral microinfarcts after carotid endarterectomy and the relevance of delayed cerebral infarction. J Clin Neurol. 2017;13:32–37. doi: 10.3988/jcn.2017.13.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saini M, Ikram K, Hilal S, Qiu A, Venketasubramanian N, Chen C. Silent stroke: not listened to rather than silent. Stroke. 2012;43:3102–04. doi: 10.1161/STROKEAHA.112.666461. [DOI] [PubMed] [Google Scholar]
- 17.Saini M, Suministrado MS, Hilal S, et al. Prevalence and risk factors of acute incidental infarcts. Stroke. 2015;46:2722–27. doi: 10.1161/STROKEAHA.115.009963. [DOI] [PubMed] [Google Scholar]
- 18.Batool S, O’Donnell M, Sharma M, et al. Incidental magnetic resonance diffusion-weighted imaging-positive lesions are rare in neurologically asymptomatic community-dwelling adults. Stroke. 2014;45:2115–17. doi: 10.1161/STROKEAHA.114.005782. [DOI] [PubMed] [Google Scholar]
- 19.Conklin J, Silver FL, Mikulis DJ, Mell DM. Are acute infarcts the cause of leukoaraiosis? Brain mapping for 16 consecutive weeks. Ann Neurol. 2014;76:899–904. doi: 10.1002/ana.24285. [DOI] [PubMed] [Google Scholar]
- 20.Auriel E, Edlow BL, Reijmer YD, et al. Microinfarct disruption of white matter structure: a longitudinal diffusion tensor analysis. Neurology. 2014;83:182–88. doi: 10.1212/WNL.0000000000000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Veluw SJ, Zwanenburg JJ, Engelen-Lee J, et al. In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J Cereb Blood Flow Metab. 2013;33:322–29. doi: 10.1038/jcbfm.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Veluw SJ, Zwanenburg JJ, Rozemuller AJ, Luijten PR, Spliet WG, Biessels GJ. The spectrum of MR detectable cortical microinfarcts: a classification study with 7-tesla postmortem MRI and histopathology. J Cereb Blood Flow Metab. 2015;35:676–83. doi: 10.1038/jcbfm.2014.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Veluw SJ, Charidimou A, Van der Kouwe AJ, et al. Microbleed and microinfarct detection in amyloid angiopathy: a high-resolution MRI-histopathology study. Brain. 2016;139:3151–62. doi: 10.1093/brain/aww229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Rooden S, Goos JD, Van Opstal AM, et al. Increased number of microinfarcts in Alzheimer disease at 7-T MR imaging. Radiology. 2014;270:205–11. doi: 10.1148/radiol.13130743. [DOI] [PubMed] [Google Scholar]
- 26.Van Veluw SJ, Heringa SM, Kuijf HJ, Koek HL, Luijten PR, Biessels GJ. Cerebral cortical microinfarcts at 7Tesla MRI in patients with early Alzheimer’s disease. J Alzheimers Dis. 2014;39:163–67. doi: 10.3233/JAD-131040. [DOI] [PubMed] [Google Scholar]
- 27.Van Veluw SJ, Jolink WM, Hendrikse J, et al. Cortical microinfarcts on 7T MRI in patients with spontaneous intracerebral hemorrhage. J Cereb Blood Flow Metab. 2014;34:1104–06. doi: 10.1038/jcbfm.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Veluw SJ, Hilal S, Kuijf HJ, et al. Cortical microinfarcts on 3T MRI: clinical correlates in memory-clinic patients. Alzheimers Dement. 2015;11:1500–09. doi: 10.1016/j.jalz.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Van Dalen JW, Scuric EE, Van Veluw SJ, et al. Cortical microinfarcts detected in vivo on 3 tesla MRI: clinical and radiological correlates. Stroke. 2015;46:255–57. doi: 10.1161/STROKEAHA.114.007568. [DOI] [PubMed] [Google Scholar]
- 30.Hilal S, Sikking E, Shaik MA, et al. Cortical cerebral microinfarcts on 3T MRI: a novel marker of cerebrovascular disease. Neurology. 2016;87:1583–90. doi: 10.1212/WNL.0000000000003110. [DOI] [PubMed] [Google Scholar]
- 31.Fu R, Wang Y, Wang Y, et al. The development of cortical microinfarcts is associated with intracranial atherosclerosis: data from the Chinese Intracranial Atherosclerosis Study. J Stroke Cerebrovasc Dis. 2015;24:2447–54. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Van Veluw SJ, Biessels GJ, Luijten PR, Zwanenburg JJ. Assessing cortical cerebral microinfarcts on high resolution MR images. J Vis Exp. 2015;105:1–8. doi: 10.3791/53125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brundel M, Reijmer YD, Van Veluw SJ, et al. Cerebral microvascular lesions on high-resolution 7-tesla MRI in patients with type 2 diabetes. Diabetes. 2014;63:3523–29. doi: 10.2337/db14-0122. [DOI] [PubMed] [Google Scholar]
- 34.Kuijf HJ, Zijlstra F, Van Veluw SJ, Viergever MA, Vincken KL, Biessels GJ. Detecting cortical cerebral microinfarcts in 7.0 T MR images. Proc IEEE Int Symp Biomed Imaging. 2013;2:970–73. [Google Scholar]
- 35.Lauer A, Van Veluw SJ, William CM, et al. Microbleeds on MRI are associated with microinfarcts on autopsy in cerebral amyloid angiopathy. Neurology. 2016;87:1488–92. doi: 10.1212/WNL.0000000000003184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ringman JM, Sachs MC, Zhou Y, Monsell SE, Saver JL, Vinters HV. Clinical predictors of severe cerebral amyloid angiopathy and influence of APOE genotype in persons with pathologically verified Alzheimer disease. JAMA Neurol. 2014;71:878–83. doi: 10.1001/jamaneurol.2014.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kövari E, Herrmann FR, Hof PR, Bouras C. The relationship between cerebral amyloid angiopathy and cortical microinfarcts in brain ageing and Alzheimer’s disease. Neuropathol Appl Neurobiol. 2013;39:498–509. doi: 10.1111/nan.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ii Y, Maeda M, Kida H, et al. In vivo detection of cortical microinfarcts on ultrahigh-field MRI. J Neuroimaging. 2012;23:28–32. doi: 10.1111/j.1552-6569.2012.00722.x. [DOI] [PubMed] [Google Scholar]
- 39.Ueda Y, Satoh M, Tabei KI, et al. Neuropsychological features of microbleeds and cortical microinfarct detected by high resolution magnetic resonance imaging. J Alzheimers Dis. 2016;53:315–25. doi: 10.3233/JAD-151008. [DOI] [PubMed] [Google Scholar]
- 40.Van Rooden S, Van Opstal AM, Labadie G, et al. Early magnetic resonance imaging and cognitive markers of hereditary cerebral amyloid angiopathy. Stroke. 2016;47:3041–44. doi: 10.1161/STROKEAHA.116.014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arvanitakis Z, Capuano AW, Leurgans SE, Buchman AS, Bennett DA, Schneider JA. The relationship of cerebral vessel pathology to brain microinfarcts. Brain Pathol. 2016;27:77–85. doi: 10.1111/bpa.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kövari E, Herrmann FR, Gold G, Hof PR, Charidimou A. Association of cortical microinfarcts and cerebral small vessel pathology in the ageing brain. Neuropathol Appl Neurobiol. 2016 doi: 10.1111/nan.12366. published online Oct 26. [DOI] [PubMed] [Google Scholar]
- 43.Dieleman N, Van der Kolk AG, Zwanenburg JJ, et al. Relations between location and type of intracranial atherosclerosis and parenchymal damage. J Cereb Blood Flow Metab. 2016;36:1271–80. doi: 10.1177/0271678X15616401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilal S, Chai YL, Van Veluw SJ, et al. Association between subclinical cardiac biomarkers and clinically manifest cardiac diseases with cortical cerebral microinfarcts. JAMA Neurol. 2017;74:403–10. doi: 10.1001/jamaneurol.2016.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamoto Y, Yamamoto T, Kalaria RN, et al. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol. 2012;123:381–94. doi: 10.1007/s00401-011-0925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suter OC, Sunthorn T, Kraftsik R, et al. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002;33:1986–92. doi: 10.1161/01.str.0000024523.82311.77. [DOI] [PubMed] [Google Scholar]
- 47.Pruzin JJ, Schneider JA, Capuano AW, et al. Diabetes, hemoglobin A1C, and regional Alzheimer disease and infarct pathology. Alzheimer Dis Assoc Disord. 2017;31:41–47. doi: 10.1097/WAD.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abner EL, Nelson PT, Kryscio RJ, et al. Diabetes is associated with cerebrovascular but not Alzheimer neuropathology. Alzheimers Dement. 2016;12:1–8. doi: 10.1016/j.jalz.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ince PG, Minett T, Forster G, et al. Microinfarcts in an older population-representative brain donor cohort (MRC CFAS): prevalence, relation to dementia and mobility, and implications for the evaluation of cerebral small vessel disease. Neuropathol Appl Neurobiol. 2016 doi: 10.1111/nan.12363. published online Sept 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyle PA, Yu L, Wilson RS, Schneider JA, Bennett DA. Relation of neuropathology with cognitive decline among older persons without dementia. Front Aging Neurosci. 2013;5:1–8. doi: 10.3389/fnagi.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corrada MM, Sonnen JA, Kim RC, Kawas CH. Microinfarcts are common and strongly related to dementia in the oldest-old: The 90+ study. Alzheimer’s Dement. 2016;12:900–08. doi: 10.1016/j.jalz.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: the 90+ Study. Neurology. 2015;85:535–42. doi: 10.1212/WNL.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White LR, Edland SD, Hemmy LS, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology. 2016;86:1000–08. doi: 10.1212/WNL.0000000000002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyle PA, Yang J, Yu L, et al. Varied effects of age-related neuropathologies on the trajectory of late life cognitive decline. Brain. 2017;140:804–12. doi: 10.1093/brain/aww341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buchman AS, Yu L, Boyle PA, et al. Microvascular brain pathology and late-life motor impairment. Neurology. 2013;80:712–18. doi: 10.1212/WNL.0b013e3182825116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Summers PM, Hartmann DA, Hui ES, et al. Functional deficits induced by cortical microinfarcts. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X16685573. published online Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silasi G, She J, Boyd JD, Xue S, Murphy TH. A mouse model of small-vessel disease that produces brain-wide-identified microocclusions and regionally selective neuronal injury. J Cereb Blood Flow Metab. 2015;35:734–38. doi: 10.1038/jcbfm.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang M, Iliff JJ, Liao Y, et al. Cognitive deficits and delayed neuronal loss in a mouse model of multiple microinfarcts. J Neurosci. 2012;32:17948–60. doi: 10.1523/JNEUROSCI.1860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venkat P, Chopp M, Zacharek A, et al. White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol Aging. 2017;50:96–106. doi: 10.1016/j.neurobiolaging.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rapp JH, Pan XM, Yu B, et al. Cerebral ischemia and infarction from atheroemboli <100 micrometer in size. Stroke. 2003;34:1976–80. doi: 10.1161/01.STR.0000083400.80296.38. [DOI] [PubMed] [Google Scholar]
- 61.Shih AY, Blinder P, Tsai PS, et al. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat Neurosci. 2012;16:55–63. doi: 10.1038/nn.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci. 2007;104:365–70. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blinder P, Shih AY, Rafie C, Kleinfeld D. Topological basis for the robust distribution of blood to rodent neocortex. Proc Natl Acad Sci. 2010;107:12670–75. doi: 10.1073/pnas.1007239107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blinder P, Tsai PS, Kaufhold JP, Knutsen PM, Suhl H, Kleinfeld D. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci. 2013;16:889–97. doi: 10.1038/nn.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duvernoy HM, Delon S, Vannson JL. Cortical blood vessels of the human brain. Brain Res Bull. 1981;7:519–79. doi: 10.1016/0361-9230(81)90007-1. [DOI] [PubMed] [Google Scholar]
- 66.Taylor ZJ, Hui ES, Watson AN, et al. Microvascular basis for growth of small infarcts following occlusion of single penetrating arterioles in mouse cortex. J Cereb Blood Flow Metab. 2016;36:1357–73. doi: 10.1177/0271678X15608388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishimura N, Rosidi NL, Iadecola C, Schaffer CB. Limitations of collateral flow after occlusion of a single cortical penetrating arteriole. J Cereb Blood Flow Metab. 2010;30:1914–27. doi: 10.1038/jcbfm.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Q, Lan Y, He XF, et al. Allopurinol protects against ischemic insults in a mouse model of cortical microinfarction. Brain Res. 2015;1622:361–67. doi: 10.1016/j.brainres.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 69.Holland PR, Searcy JL, Salvadores N, et al. Gliovascular disruption and cognitive deficits in a mouse model with features of small vessel disease. J Cereb Blood Flow Metab. 2015;35:1005–14. doi: 10.1038/jcbfm.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan XL, Xue YQ, Ma T, et al. Partial eNOS deficiency causes spontaneous thrombotic cerebral infarction, amyloid angiopathy and cognitive impairment. Mol Neurodegener. 2015;10:24. doi: 10.1186/s13024-015-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anenberg E, Arstikaitis P, Niitsu Y, et al. Ministrokes in channelrhodopsin-2 transgenic mice reveal widespread deficits in motor output despite maintenance of cortical neuronal excitability. J Neurosci. 2014;34:1094–104. doi: 10.1523/JNEUROSCI.1442-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harrison TC, Silasi G, Boyd JD, Murphy TH. Displacement of sensory maps and disorganization of motor cortex after targeted stroke in mice. Stroke. 2013;44:2300–06. doi: 10.1161/STROKEAHA.113.001272. [DOI] [PubMed] [Google Scholar]
- 73.Rapp JH, Pan XM, Neumann M, Hong M, Hollenbeck K, Liu J. Microemboli composed of cholesterol crystals disrupt the blood-brain barrier and reduce cognition. Stroke. 2008;39:2354–61. doi: 10.1161/STROKEAHA.107.496737. [DOI] [PubMed] [Google Scholar]
- 74.Wang M, Ding F, Deng S, et al. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J Neurosci. 2017;37:2870–77. doi: 10.1523/JNEUROSCI.2112-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng W, Watts LT, Holstein DM, et al. Purinergic receptor stimulation reduces cytotoxic edema and brain infarcts in mouse induced by photothrombosis by energizing glial mitochondria. PLoS One. 2010;5:e14401. doi: 10.1371/journal.pone.0014401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–09. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Reuck J, Deramecourt V, Auger F, et al. Post-mortem 7.0-tesla magnetic resonance study of cortical microinfarcts in neurodegenerative diseases and vascular dementia with neuropathological correlates. J Neurol Sci. 2014;346:85–89. doi: 10.1016/j.jns.2014.07.061. [DOI] [PubMed] [Google Scholar]
- 78.Sutherland BA, Minnerup J, Balami JS, Arba F, Buchan AM, Kleinschnitz C. Neuroprotection for ischaemic stroke: Translation from the bench to the bedside. Int J Stroke. 2012;7:407–18. doi: 10.1111/j.1747-4949.2012.00770.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.