Abstract
BACKGROUND
The association of Cardiovascular Health (CVH; defined by the American Heart Association by assigning points for health-related behavioral and clinical factors) with endothelial and erectile dysfunction has not been reported, although endothelial and erectile dysfunction have been associated with components of CVH.
METHODS
Data were collected in 1,136 men in the Multi-Ethnic Study of Atherosclerosis at baseline and erectile dysfunction status (measured by survey or medication use) at exam 5. CVH was determined with 7 health metrics. Endothelial function was measured with brachial artery flow-mediated dilation (FMD). Poisson regression was used to determine associations between CVH and erectile dysfunction across categories of CVH (low, moderate, and high).
RESULTS
Age and proportion of Black or Latino participants decreased while proportion of Chinese-American participants increased with higher CVH category. FMD was higher in men without erectile dysfunction and higher in men with high vs. low CVH. Erectile dysfunction prevalence was lower with better CVH; 58% in men with low CVH, 41% with moderate CVH, and 33% with high CVH (P < 0.001). CVH was associated with erectile dysfunction; prevalence ratio = 0.75 (95% confidence interval (CI) = 0.66, 0.84) with moderate CVH and 0.68 (95% CI = 0.49, 0.94) with high CVH (vs. men with low CVH) and 0.93 (95% CI = 0.91, 0.96) for every 1-point higher CVH score in a fully adjusted model, including FMD, age, education, depression score, use of antidepressant or beta-blocker medications, chronic disease, heavy drinking, and race.
CONCLUSION
CVH is associated with future erectile dysfunction, even after adjustment for baseline FMD. Maintaining high CVH may improve quality of life for men.
Keywords: blood pressure, cardiovascular health, erectile dysfunction, flow-mediated dilation, hypertension.
In 2010, the American Heart Association (AHA) began a focused campaign to promote Cardiovascular Health (CVH) in all Americans. The AHA identified 7 health and behavioral factors, including blood pressure (BP), total cholesterol level, blood glucose, body mass index, physical activity, healthy diet, and smoking status, that can be combined to generate an overall CVH score.1 CVH scores are associated with heart failure, cardiovascular events, left ventricular characteristics, and many other health outcomes and quality of life measures in a variety of populations.2–5
Several of the individual components of CVH have been associated with endothelial function and erectile dysfunction, but whether or not the composite CVH score is associated with future erectile dysfunction has not been investigated.6–10 Endothelial dysfunction, which can be identified as reduced flow-mediated dilation (FMD) of the brachial artery, is an early manifestation of cardiovascular disease (CVD) and often exists before overt disease is manifest.11 Similarly, erectile dysfunction may also represent widespread vascular dysfunction,12 and both erectile and endothelial dysfunction are associated with CVD.12–14 Suboptimal FMD is related to erectile dysfunction,12 and it has been suggested that improving endothelial function may result in improvements in erectile dysfunction symptoms and reduce overall CVD risk.13 However, it is unknown whether the composite CVH metric is associated with endothelial function or later-life erectile dysfunction.
Our aim was to determine whether CVH status among middle-aged and older men is associated with endothelial function and the prevalence of erectile dysfunction 9 years later among middle-aged and older men. We hypothesized that CVH at baseline would be associated with endothelial function at baseline and with erectile dysfunction 9 years later in participants of the Multi-Ethnic Study of Atherosclerosis (MESA). We further hypothesized that the strength of the CVH-erectile dysfunction association would be attenuated or absent after adjusting for endothelial function, suggesting that the maintenance of CVH may protect against erectile dysfunction, at least in part, by preserving endothelial function.
METHODS
Study population
The MESA was designed to study determinants and progression of subclinical CVD in middle-aged and older adults (from 45 to 84 years at baseline exam). Between 2000 and 2002, participants without overt CVD were recruited from 6 field centers for the baseline exam (Baltimore City and County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; New York, New York; St. Paul, Minnesota; and Los Angeles County, California). Random population samples were identified at each field center from lists of area residents; additional details have been published.15 After screening, 59.8% of those deemed eligible participated in the study. At each site, institutional review board approval was obtained, and participants signed a written informed consent document. Four follow-up examinations have been conducted since the initial exam: exam 2 (2002–2004), 3 (2004–2005), 4 (2005–2007), and 5 (2010–2012). This study used data from the baseline exam and exam 5.
Participants
Our sample included male MESA participants (n = 3,212) and excluded participants who did not complete the erectile dysfunction survey at exam 5 (n = 1,078), answered “I don’t know” to the question about erectile function (n = 138), or were missing erectile dysfunction drug use information (n = 9). For our main analysis, we also excluded men who did not undergo the FMD test at exam 1 (n = 821) and any subjects missing any information needed to calculate the CVH score at exam 1 (n = 30). Our final sample consisted of 1,136 men. Characteristics of men in the study compared to men who did not complete exam 5 are shown in Supplementary Table 1; those who did not complete exam 5 (when erectile dysfunction was ascertained) tended to be older, less educated and have a somewhat lower CVH score, and were somewhat more likely African American and have a history of prostate cancer.
Cardiovascular health
CVH was scored as previously described.16 Briefly, participants received 0 (poor level), 1 (intermediate), or 2 (ideal) points for each health behavior (diet, physical activity, body mass index, and nonsmoking) and health factor (fasting glucose, BP, and total cholesterol). A composite score was calculated, ranging from 0 - 14, representing lowest to highest CVH. This continuous score was then stratified into low (0–7 points), moderate (8–11 points), and high (12–14 points) categories, as in prior studies.17 Smoking history was determined by self-report. A food frequency questionnaire was used to assess diet, and a detailed, self-report survey was used to measure physical activity. Body mass index was calculated from height and weight. BP was measured in the seated position in triplicate, with an average of the final 2 readings included in analysis. Total cholesterol, low-density lipoprotein and high-density lipoprotein cholesterol, triglycerides, and glucose were measured in plasma following a 12-hour fast. For a more detailed description of data collection, please visit the MESA website: https://www.mesa-nhlbi.org/.
Brachial artery FMD
Brachial artery FMD was obtained using ultrasonography at baseline. Participants fasted for at least 6 hours before undergoing measurement in the supine position following 15-minute rest. BP and pulse rate were measured in the left arm at 5-minute intervals throughout the examination with an automated sphygmomanometer (Dinamap). A standard BP cuff was positioned 2 inches below the antecubital fossa of the right arm. The artery was imaged 5 to 9 cm superior to the antecubital fossa using a linear-array transducer at 9 MHz (GE Logiq 700 Device). Baseline images were collected (for 20 seconds prior to cuff inflation and 10 seconds after cuff inflation), and then the cuff was inflated to 50 mm Hg above the participant’s systolic BP for 5 minutes and then rapidly released. The digitized images of the brachial artery were captured again for 10 seconds prior to cuff release and for 90 seconds after cuff release (105 seconds in total) to ensure the maximum vasodilator response was captured. More details regarding the scanning and reading protocol are found on the MESA web site (www.mesa-nhlbi.org).
FMD was calculated as [(maximum diameter − baseline diameter)/baseline diameter] × 100%. We included %FMD because it is predictive of other cardiovascular events.18
Reproducibility was tested by performing a blinded, quality control re-read of images from 40 MESA subjects. The intraclass correlation coefficients were 0.99 for baseline diameter, 0.99 for maximum diameter, and 0.93 for % FMD. Intra-subject variability was determined by comparing FMD between repeated examinations of 19 subjects on 2 separate visits, 1 week apart. The intraclass correlation coefficients were 0.90 for baseline diameter, 0.90 for maximum diameter, and 0.54 for % FMD. Technical error for baseline and maximum diameters and %FMD were 1.39%, 1.47%, and 28.4%, respectively.
Determination of erectile dysfunction
Erectile dysfunction was defined as a response of “Never Able” or “Sometimes Able” to the question “Ability to get and keep an erection for satisfactory intercourse,” or the use of erectile dysfunction medication. This method of obtaining erectile dysfunction status has been validated and has been used in other large, national cohort studies.19,20
Covariable measurements
Education, chronic disease diagnosis, and medication use were determined by self-report. The center for epidemiologic studies depression score (CES-D) was used to quantify depressive symptoms.21 Heavy drinking status was defined as >14 drinks per week and included because alcohol intake has been associated with erectile dysfunction.22
Statistical analysis
Differences in subject characteristics and prevalence of erectile dysfunction across categorical CVH categories were evaluated using an analysis of variance, Kruskal–Wallis, or chi-square test, as appropriate. We used Poisson regression (with the robust option to control for mild violations of underlying assumptions) to determine prevalence ratios and estimate associations of continuous and categorically defined CVH with erectile dysfunction in an unadjusted model, model 1, after adjustment for FMD, Model 2, and after fully adjusting for covariables: study center, age, race, educational attainment, diagnosis of chronic disease (cancer-including prostate cancer, kidney or liver disease, emphysema), use of antidepressant or beta-blocker medications, CES-D score, and heavy drinking, Model 3. We tested for FMD interactions by race in model 3, based on the observation that African-Americans have impaired resistance vessel function compared to Caucasians.23 We compared prevalence ratios and beta coefficients for CVH before and after the addition of FMD to the model to determine whether associations of CVH with erectile dysfunction were reduced or absent once FMD was added to the model. We then stratified our cohort into 4 age groups: 45–54, 55–64, 65–74, and 75–84 years at baseline, and tested whether CVH was associated with erectile dysfunction in each age group. Finally, we used an analysis of variance with post-hoc Scheffe correction to test for differences in proportion of men with erectile dysfunction between CVH categories in the 4 age groups to determine whether youngest men with low CVH experience erectile dysfunction at the same rate as the oldest men with high CVH. STATA version 14 (College Station, TX) was used.
RESULTS
Study sample and baseline characteristics
In our sample, participants with high CVH tended to be younger and completed more levels of education compared to those with low CVH. Rates of heavy drinking and prostate cancer were similar across groups (Table 1). Antidepressant use at the time of erectile dysfunction report (exam 5) was the same (P = 0.57) across categories: 7% in men in the low category, 6% in the moderate category, and 4% in the high category.
Table 1.
Subject characteristics at baseline by CVH category
| CVH category | Low, <8 | Moderate, 8–11 | High, ≥12 |
|---|---|---|---|
| n = 387 | n = 670 | n = 79 | |
| CVH score* | 5.9 ± 0.1 | 9.3 ± 0.1 | 12.3 ± 0.1 |
| Age, years* | 60 ± 1 | 59 ± 1 | 57 ± 1 |
| Study center, n (%)* | |||
| Chicago, IL | 71 (18) | 161 (24) | 27 (34) |
| Forsyth, NC | 94 (24) | 126 (19) | 9 (11) |
| New York, NY | 66 (17) | 132 (20) | 17 (22) |
| Los Angeles, CA | 78 (20) | 136 (20) | 20 (25) |
| St. Paul, MN | 78 (20) | 115 (17) | 6 (8) |
| Race/ethnicity, n (%)* | |||
| Caucasian | 132 (34) | 254 (38) | 32 (40) |
| Chinese-American | 31 (8) | 125 (19) | 25 (32) |
| African-American | 109 (28) | 125 (19) | 14 (18) |
| Latino | 115 (30) | 166 (24) | 8 (10) |
| Education, n (%)* | |||
| Less than high school | 68 (18) | 77 (11) | 5 (6) |
| High school | 79 (20) | 85 (13) | 4 (5) |
| Post-secondary school | 130 (34) | 161 (24) | 14 (18) |
| Bachelor’s degree | 56 (14) | 148 (22) | 22 (28) |
| Graduate or professional degree | 54 (14) | 199 (30) | 34 (43) |
| Heavy drinkers, n (%) | 161 (42) | 261 (39) | 29 (37) |
| Prostate cancer, n (%) | 11 (5) | 10 (1) | 0 (0) |
Heavy drinking is defined as >14 drinks/week for males, *indicates a difference between categories, P < 0.05.
Flow-mediated dilation
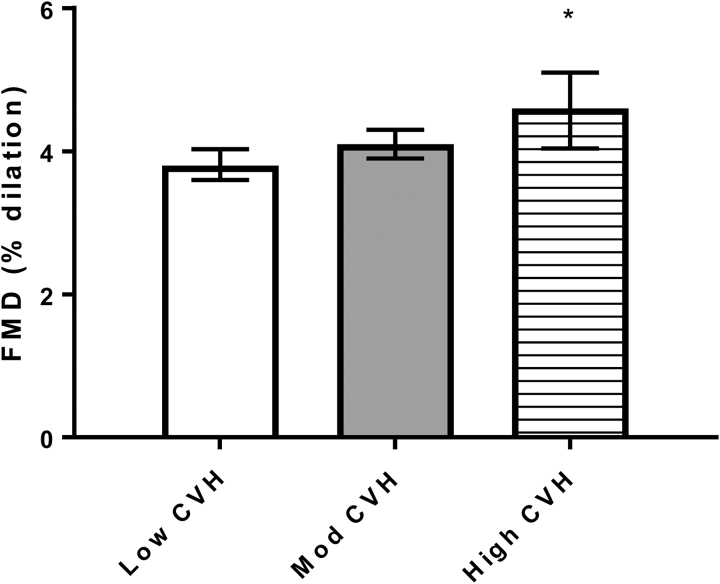
Participants with high CVH had higher FMD compared to those with low CVH (Figure 1), indicating better endothelial function in the high compared to low CVH group. FMD at baseline was also associated with erectile dysfunction 9 years later. Participants without erectile dysfunction at exam 5 had higher FMD at baseline than participants with erectile dysfunction (4.3 ± 0.1% vs. 3.7 ± 0.1%, P < 0.0001).
Figure 1.
FMD (%) by CVH Category (mean, 95% CI). Abbreviations: CI, confidence interval; CVH, cardiovascular health; FMD, flow-mediated dilation.
Baseline CVH and erectile dysfunction at follow up
Erectile dysfunction was prevalent in 526 of our 1,136 participants (46%) by exam 5, 9 years after baseline. Prevalence of erectile dysfunction was lower with higher baseline CVH: 233/387 (58%) in men with low CVH, 277/670 (41%) in men with medium CVH, and 26/79 (33%) in men with high CVH, P < 0.001 for trend.
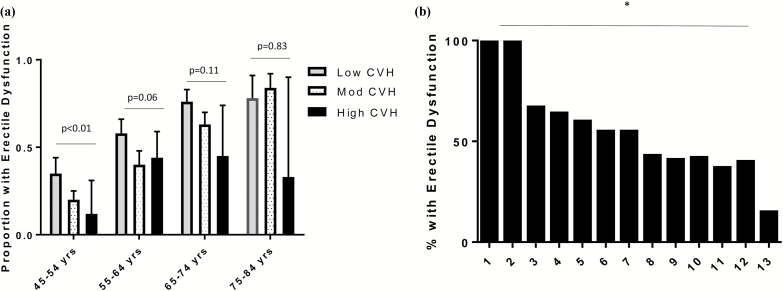
When we stratified the men into 4 groups according to baseline age, the unadjusted association between CVH category and erectile dysfunction was significant in the youngest men (Figure 2a), with similar trends but lower power among older men.
Figure 2.
Prevalence of erectile dysfunction by CVH category and score. (a) Proportion of men with erectile dysfunction by CVH category and age group (mean, 95% CI). (b) Percentage of men with erectile dysfunction by total CVH point total. Abbreviations: CI, confidence interval; CVH, cardiovascular health.
The percentage of men with erectile dysfunction at exam 5 was lower with greater CVH total at baseline, from 100% of men with a total score of 1 or 2 to 15% of men with a total score of 13, P < 0.001 for trend (Figure 2b).
Multivariable analyses
In unadjusted models (model 1 and 2), both CVH (represented either by category or point score) and FMD (b = −0.11 ± 0.03, P < 0.001 when included in a model with CVH category and b = −0.11 ± 0.03, P < 0.001 in the model with CVH point total) were associated with subsequent erectile dysfunction (Table 2).
Table 2.
Prevalence ratios for erectile dysfunction by CVH score at baseline
| Low CVH | Moderate CVH | High CVH | Per 1-point higher CVH score | |
|---|---|---|---|---|
| PR | PR (95% CI) | PR (95% CI) | PR (95% CI) | |
| Unadjusted | 1 (reference group) | 0.72 (0.63, 0.81)* | 0.57 (0.41, 0.79)* | 0.92 (0.89, 0.94)* |
| Model 2 | 1 (reference group) | 0.72 (0.64, 0.82)* | 0.58 (0.42, 0.81)* | 0.92 (0.90, 0.95)* |
| Model 3 | 1 (reference group) | 0.75 (0.66, 0.84)* | 0.68 (0.49, 0.94)* | 0.93 (0.91, 0.96)* |
Model 2 includes FMD score. Model 3 includes FMD score, age, study center, education, heavy drinking, diagnosis of chronic illness: kidney or liver disease, emphysema, cancer (including prostate cancer), CES-D score, use of antidepressant medication, and use of beta-blocker medication. Abbreviations: CES-D, center for epidemiologic studies depression score; CI, confidence interval; CVH, cardiovascular health; FMD, flow-mediated dilation; PR, prevalence ratio. *P ≤ 0.01.
However, in a model adjusted for demographics and other risk factors (model 3), CVH (category and total score), CES-D score, and age, but not FMD or race, were associated with erectile dysfunction (Table 3). Antidepressant medication use was only independently associated with erectile dysfunction in the model with CVH category.
Table 3.
Association between CVH and covariables of interest with erectile dysfunction in a fully adjusted model (Model 3)
| b (95% CI) | |
|---|---|
| Per 1-point higher CVH score | −0.07 (−0.11, −0.03)* |
| FMD | −0.00 (−0.09, 0.08) |
| Age | 0.05 (0.04, 0.06)* |
| Race | 0.01 (−0.13. 0.15) |
| Antidepressant medication use | 0.20 (−0.12, 0.53) |
| CES-D score | 0.01 (0.00, 0.03) |
| Beta-blocker use | 0.11 (−0.11, 0.32) |
Model 3 includes FMD score, age, study center, education, heavy drinking, diagnosis of chronic illness: kidney or liver disease, emphysema, cancer (including prostate cancer), CES-D score, use of antidepressant medication, and use of beta-blocker medication. Prevalence ratios for erectile dysfunction by CVH category or 1-unit increase in total CVH point total. Abbreviations: b, beta-coefficient; CES-D, center for epidemiologic studies depression score; CI, confidence interval; CVH, cardiovascular health; FMD, flow-mediated dilation; PR, prevalence ratio. *P ≤ 0.01.
The association between CVH and erectile dysfunction persisted when we included all men with the information needed to calculate CVH at exam 1 and erectile dysfunction at exam 5, including those without FMD data, n = 1,844 (Supplementary Table 2).
Sensitivity analysis
When we stratified by 4 age groups (45–54, 55–64, 65–74, and 75–84 years at baseline), the prevalence of erectile dysfunction was similar between the youngest men with low CVH and the oldest men with high CVH, P > 0.05 for difference between groups, Figure 2a.
DISCUSSION
The main finding of this study is that CVH status is associated with future erectile dysfunction. This association persisted even after adjustment for age and other clinical and demographic factors. Men with high CVH had better endothelial function than men with low CVH at baseline, and baseline endothelial function was better in men without (vs. with) future erectile dysfunction. The CVH-erectile dysfunction association also persisted even after adjustment for endothelial function measured at baseline and adjustment for other potential confounders. In contrast, the endothelial function-erectile dysfunction association was abolished in our fully adjusted model. This suggests that CVH is associated with future erectile dysfunction, independent of FMD.
This is the first study to demonstrate that CVH is associated with both endothelial function and future erectile dysfunction. These findings may be anticipated as the differences in endothelial function between men with and without erectile dysfunction are in line with existing literature reporting that endothelial and erectile dysfunction are linked.12,13,24 Both endothelial and erectile dysfunction have also been associated with future CVD.13 Although we did find a difference in endothelial function between men with the low vs. high CVH, the actual mean difference was only 0.6%, and the clinical significance of a difference of this magnitude is not known.
An earlier study in MESA participants reported that men with lower FMD scores, and those with scores below the 25th percentile were more likely to have erectile dysfunction.25 Similar to our findings, low FMD score did not significantly associate with erectile dysfunction in a fully adjusted model.25 The authors speculated that this could be owing to the technically difficult nature of the FMD measurement.25 We also believe that adjusting for age may have negated the endothelial function-erectile dysfunction association because age is so consistently associated with endothelial dysfunction26 and was strongly (P < 0.001) and independently associated with erectile dysfunction in our study. It is possible that the reduction in endothelial function with age contributes to the higher prevalence of erectile dysfunction among the older men.
Associations between CVH and erectile dysfunction, independent of the presumed intermediary (endothelial function), is somewhat unexpected but not unprecedented. Although residual confounding due to FMD measurement error is possible, Xanthakis et al. also found a persistent association between CVH and incident CVD events after adjustment for factors presumed to be in the casual pathway between CVH and CVD.27 In our study, it seems that although the effect of some of the components of CVH (i.e., smoking) on erectile dysfunction may by mediated by endothelial function, the composite CVH score is a broader measure that can be obtained through different combinations of factors, some of which are independent of endothelial function. The results from Xanthakis, et al., and our study suggest that the value of the composite CVH score may provide greater information than a simple sum of the individual components.5,27
Associations between CVH across the lifespan and other forms of future subclinical and clinical CVD have been previously demonstrated.5,28 The CVH score has also been associated with incident cancer in older adults.29 This study adds to the body of literature that links CVH score not only to subclinical and clinical manifestation of CVD, but also to other diseases related to lifestyle (cancer) and conditions that affect overall quality of life for men (erectile dysfunction). Maintaining optimal CVH appears to be a viable strategy for avoiding a variety of adverse health outcomes.
Both behavioral and clinical components of CVH have been linked to erectile dysfunction in earlier studies. Bacon et al. found that alcohol consumption, physical activity, and body mass index were associated with erectile function in men over 50 years after excluding men with prostate surgery.30 Physical activity has been shown to effectively prevent or treat erectile dysfunction in clinical populations.8,9,31 Another study linked dietary factors, such as: alcohol, nut and vegetable consumption, to erectile dysfunction.22 These and our studies provide strong evidence for addressing multiple potentially causative pathways in both the lifestyle and clinical domains to combat erectile dysfunction prevalence and severity and reduce CVD risk.
Our study is limited by the single time point measurement of erectile dysfunction, so we are unable to determine if differences at baseline or changes in CVH over time associate with incident erectile dysfunction or changes in prevalence of erectile dysfunction. We used a brief, validated survey to determine self-reported erectile dysfunction status rather than an objective measurement of penile blood flow or use of a multidimensional measure of erectile dysfunction (International Index of Erectile Function (IIEF) Questionnaire). There were also few men (especially older men) in the highest category of CVH compared to number of men in the moderate and low categories. The intraclass correlation coefficient for FMD in this study is also very low in MESA, which may have resulted in sizeable misclassification; this could have biased our FMD-erectile dysfunction results toward the null, leading to underestimation of the association. Thus, we cannot rule out a residual association of FMD with future erectile dysfunction in MESA men. A strength of our study is the large, well-characterized sample that includes participants from 4 different race groups in various cities across the United States.
We observed that having higher CVH is associated with better vascular endothelial function concurrently and reduced prevalence of erectile dysfunction after 9 years of follow up. CVH is significantly associated with future erectile dysfunction independent of endothelial function. Younger men (age 45–54 at baseline) with low CVH have a prevalence of erectile dysfunction that is similar to older men (age 75–84 at baseline) with high CVH. Maintaining high CVH in middle and older age may not only reduce CVD risk and vascular aging but also improve quality of life for older men.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
The research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-RR-024156 and UL1-RR-025005 from NCRR. The authors thank the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. Dr Lane-Cordova is supported by the American Heart Association’s Strategically Focused Research Network in Prevention.
REFERENCES
- 1. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010; 121:586–613. [DOI] [PubMed] [Google Scholar]
- 2. Allen NB, Badon S, Greenlund KJ, Huffman M, Hong Y, Lloyd-Jones DM. The association between cardiovascular health and health-related quality of life and health status measures among U.S. adults: a cross-sectional study of the National Health and Nutrition Examination Surveys, 2001-2010. Health Qual Life Outcomes 2015; 13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foraker RE, Abdel-Rasoul M, Kuller LH, Jackson RD, Van Horn L, Seguin RA, Safford MM, Wallace RB, Kucharska-Newton AM, Robinson JG, Martin LW, Agha G, Hou L, Allen NB, Tindle HA. Cardiovascular Health and Incident Cardiovascular Disease and Cancer: The Women’s Health Initiative. Am J Prev Med 2016; 50:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nayor M, Enserro DM, Vasan RS, Xanthakis V. Cardiovascular Health Status and Incidence of Heart Failure in the Framingham Offspring Study. Circ Heart Fail 2016; 9:e002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lloyd-Jones DM. Cardiovascular health and protection against CVD: more than the sum of the parts? Circulation 2014; 130:1671–1673. [DOI] [PubMed] [Google Scholar]
- 6. Jackson G. The metabolic syndrome and erectile dysfunction: multiple vascular risk factors and hypogonadism. Eur Urol 2006; 50:426–427. [DOI] [PubMed] [Google Scholar]
- 7. Joris PJ, Zeegers MP, Mensink RP. Weight loss improves fasting flow-mediated vasodilation in adults: a meta-analysis of intervention studies. Atherosclerosis 2015; 239:21–30. [DOI] [PubMed] [Google Scholar]
- 8. La Vignera S, Condorelli R, Vicari E, D’Agata R, Calogero A. Aerobic physical activity improves endothelial function in the middle-aged patients with erectile dysfunction. Aging Male 2011; 14:265–272. [DOI] [PubMed] [Google Scholar]
- 9. Leoni LA, Fukushima AR, Rocha LY, Maifrino LB, Rodrigues B. Physical activity on endothelial and erectile dysfunction: a literature review. Aging Male 2014; 17:125–130. [DOI] [PubMed] [Google Scholar]
- 10. Ryan JG, Gajraj J. Erectile dysfunction and its association with metabolic syndrome and endothelial function among patients with type 2 diabetes mellitus. J Diabetes Complications 2012; 26:141–147. [DOI] [PubMed] [Google Scholar]
- 11. Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol 2013; 168:344–351. [DOI] [PubMed] [Google Scholar]
- 12. Uslu N, Gorgulu S, Alper AT, Eren M, Nurkalem Z, Yildirim A, Ozer O. Erectile dysfunction as a generalized vascular dysfunction. J Am Soc Echocardiogr 2006; 19:341–346. [DOI] [PubMed] [Google Scholar]
- 13. Vlachopoulos C, Ioakeimidis N, Terentes-Printzios D, Stefanadis C. The triad: erectile dysfunction–endothelial dysfunction–cardiovascular disease. Curr Pharm Des 2008; 14:3700–3714. [DOI] [PubMed] [Google Scholar]
- 14. Vlachopoulos C, Aznaouridis K, Ioakeimidis N, Rokkas K, Tsekoura D, Vasiliadou C, Stefanadi E, Askitis A, Stefanadis C. Arterial function and intima-media thickness in hypertensive patients with erectile dysfunction. J Hypertens 2008; 26:1829–1836. [DOI] [PubMed] [Google Scholar]
- 15. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002; 156:871–881. [DOI] [PubMed] [Google Scholar]
- 16. Hernandez R, Kershaw KN, Siddique J, Boehm JK, Kubzansky LD, Diez-Roux A, Ning H, Lloyd-Jones DM. Optimism and cardiovascular health: Multi-Ethnic Study of Atherosclerosis (MESA). Health Behav Policy Rev 2015; 2:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; ARIC Study Investigators . Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011; 57:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the Multi-Ethnic Study of Atherosclerosis. Circulation 2009; 120:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loprinzi PD, Nooe A. Erectile dysfunction and mortality in a national prospective cohort study. J Sex Med 2015; 12:2130–2133. [DOI] [PubMed] [Google Scholar]
- 20. O’Donnell AB, Araujo AB, Goldstein I, McKinlay JB. The validity of a single-question self-report of erectile dysfunction. Results from the Massachusetts Male Aging Study. J Gen Intern Med 2005; 20:515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Measurement 1977; 1:385–401. [Google Scholar]
- 22. Ramírez R, Pedro-Botet J, García M, Corbella E, Merino J, Zambón D, Corbella X, Pintó X; Xarxa de Unitats de Lípids i Arteriosclerosi (XULA) Investigators Group . Erectile dysfunction and cardiovascular risk factors in a Mediterranean diet cohort. Intern Med J 2016; 46:52–56. [DOI] [PubMed] [Google Scholar]
- 23. Kahn DF, Duffy SJ, Tomasian D, Holbrook M, Rescorl L, Russell J, Gokce N, Loscalzo J, Vita JA. Effects of Black race on forearm resistance vessel function. Hypertension 2002; 40:195–201. [DOI] [PubMed] [Google Scholar]
- 24. Castela Â, Costa C. Molecular mechanisms associated with diabetic endothelial-erectile dysfunction. Nat Rev Urol 2016; 13:266–274. [DOI] [PubMed] [Google Scholar]
- 25. Feldman DI, Cainzos-Achirica M, Billups KL, DeFilippis AP, Chitaley K, Greenland P, Stein JH, Budoff MJ, Dardari Z, Miner M, Blumenthal RS, Nasir K, Blaha MJ. Subclinical vascular disease and subsequent erectile dysfunction: the Multiethnic Study of Atherosclerosis (MESA). Clin Cardiol 2016; 39:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011; 120:357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, Wang TJ, Tofler G, Vasan RS. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation 2014; 130:1676–1683. [DOI] [PubMed] [Google Scholar]
- 28. Laitinen TT, Pahkala K, Magnussen CG, Viikari JS, Oikonen M, Taittonen L, Mikkilä V, Jokinen E, Hutri-Kähönen N, Laitinen T, Kähönen M, Lehtimäki T, Raitakari OT, Juonala M. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation 2012; 125:1971–1978. [DOI] [PubMed] [Google Scholar]
- 29. Rasmussen-Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation 2013; 127:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med 2003; 139:161–168. [DOI] [PubMed] [Google Scholar]
- 31. Begot I, Peixoto TC, Gonzaga LR, Bolzan DW, Papa V, Carvalho AC, Arena R, Gomes WJ, Guizilini S. A home-based walking program improves erectile dysfunction in men with an acute myocardial infarction. Am J Cardiol 2015; 115:571–575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.