Abstract
Biological aging measures have been proposed as proxies for extension of healthy life span in trials of geroprotective therapies that aim to slow aging. Several methods to measure biological aging show promise but it is not known if these methods are sensitive to changes caused by geroprotective therapy. We conducted analysis of two proposed methods to quantify biological aging using data from a recently concluded trial of an established geroprotector, caloric restriction. We obtained data from the National Institute on Aging CALERIE randomized trial through its public-access biobank (https://calerie.duke.edu/). The CALERIE trial randomized N = 220 nonobese adults to 25% caloric restriction (n = 145; 11.7% caloric restriction was achieved, on average) or to maintain current diet (n = 75) for 2 years. We analyzed biomarker data collected at baseline, 12-, and 24-month follow-up assessments. We applied published biomarker algorithms to these data to calculate two biological age measures, Klemera–Doubal Method Biological Age and homeostatic dysregulation. Intent-to-treat analysis using mixed-effects growth models of within-person change over time tested if caloric restriction slowed increase in measures of biological aging across follow-up. Analyses of both measures indicated caloric restriction slowed biological aging. Weight loss did not account for the observed effects. Results suggest future directions for testing of geroprotective therapies in humans.
Keywords: Caloric restriction, Biological age, Geroscience, Geroprotector
Population aging threatens a rising tide of disease and disability (1). The new field of geroscience aims to respond to this challenge by devising therapies to extend healthy life years (2,3). Several such “geroprotective” therapies are poised for proof-of-concept testing in humans (4). But there is not yet consensus about how proof-of-concept tests should be conducted (5). Extension of healthy life span is a challenging outcome metric for human trials because of cost and other impracticalities of extended follow-up. Proposed measures of so-called “biological aging” may provide a surrogate—a measure that can be tracked over relatively short duration of follow-up and whose response to geroprotective therapy can serve as a proxy for extension of healthy life span.
Biological aging refers to the gradual and progressive decline in the integrity of the body’s systems occurring with advancing chronological age (6,7). Rather than any specific disease process, this decline in system integrity is thought to reflect biological changes having their origins in aging itself (3,8). Whereas chronological age increases at the same rate for everyone, biological age can increase faster for some and slower for others. To the extent that geroprotective therapies modify basic biological processes of aging, their effects should be reflected in a slowed rate of decline in system integrity—slowed biological aging.
Recently, several methods have been proposed to quantify biological aging using algorithms that combine information from multiple biomarkers (9–13). These algorithms integrate information from multiple organ systems in the body to produce a single composite measure of system integrity. The underlying hypothesis is that, among individuals of the same chronological age, a difference in measured biological aging reflects differences in risk for age-related disease, disability, and death. In observational studies of biological aging measures, people with “slower” biological aging have lower risk for morbidity, disability, and mortality (14–18). However, it is not known if measured biological aging can be modified by geroprotective therapy.
Caloric restriction is among the oldest and most effective geroprotective interventions in worms, flies, and mice (19). Growing evidence suggests caloric restriction also benefits life span and healthspan in primates and humans (20–22). A unique resource to study effects of caloric restriction in humans is the National Institute on Aging 2-year randomized controlled trial of caloric restriction in young, non-obese healthy humans, Comprehensive Assessment of the Long-term Effects of Reducing Intake of Energy (CALERIE) (23). CALERIE data are now publicly available (https://calerie.duke.edu/). These data provide a resource to characterize potential geroprotective effects of caloric restriction (24). They also provide the opportunity to develop and refine methods that can be generalized to tests of other geroprotective interventions.
We analyzed CALERIE Biobank data to test whether recently proposed methods to quantify biological aging would prove sensitive to geroprotective effects of caloric restriction over the relatively short, 2-year span of the human trial. The hypothesis was that caloric restriction would slow the rate of change in measured biological age. We conducted intent-to-treat analysis using two different measures of biological aging implementable with data already existing within the CALERIE Biobank: the Klemera–Doubal method (KDM) Biological Age (25,26) and homeostatic dysregulation (11,15). Algorithms were applied to measures of serum albumin, alkaline phosphatase, C-reactive protein, total cholesterol, creatinine, glycated hemoglobin (estimated from serum glucose), systolic blood pressure, urea nitrogen, uric acid, and white blood cell count. KDM Biological age and homeostatic dysregulation algorithms have not been studied before in the CALERIE trial, although some data used to compute the algorithms were reported previously (23). The analysis we report below evaluates effects of the CALERIE intervention on composite measures of aging-related changes in organ-system function and provides a proof-of-concept test for using biological aging algorithms as outcome measures in geroprotector trials.
Method
CALERIE
The CALERIE trial is described in detail elsewhere (23,27,28). Briefly, N = 220 normal-weight (22.0 ≤ BMI < 28 kg/m2) participants (70% female, 77% White) aged 21–50 years at baseline were randomized to caloric restriction or ad libitum conditions with a 2:1 ratio (n = 145 to caloric restriction, n = 75 to ad libitum). “Ad libitum” (normal) caloric intake was determined from two consecutive 14-day assessments of total daily energy expenditure using doubly labeled water (29). Average percent caloric restriction over 6-month intervals was retrospectively calculated by the intake-balance method with simultaneous measurements of total daily energy expenditure using doubly labeled water and changes in body composition (30,31). Over the course of the trial, participants in the caloric-restriction arm averaged 12% reduction in caloric intake (about half the prescribed reduction). Participants in the ad libitum condition reduced caloric intake by <2% (23). Additional details about CALERIE are reported in the Supplementary Materials.
Biobank Data
We obtained publicly available biomarker data from the CALERIE Biobank website (https://calerie.duke.edu/apply-samples-and-data-analysis). Of the N = 220 CALERIE participants with baseline biomarker data to quantify biological aging, 200 and 191 also provided necessary data at the 12- and 24-month follow-up time points, respectively; 92% of ad libitum-arm participants and 83% of caloric-restriction-arm participants provided necessary data at all three time points.
Measures of Biological Age
Calculating human biological age is a relatively recent enterprise (32) and there is disagreement about methods (33). Our goal was to borrow and implement validated methods using available data. A recent comparison of five different biological age algorithms as predictors of mortality across 20 years of follow-up identified the Klemera–Doubal method (25) as performing the best: consistent with results from other studies (25,34), it predicted mortality; did so better than chronological age; and accounted for the association between chronological age and mortality (14). Although this method is imperfect (26), it can be implemented in young, healthy adults, and is associated with functional outcomes in this younger population (12,35). The Klemera–Doubal method has been criticized for including chronological age information (36). Therefore, to evaluate the robustness of findings, we also measured biological age using the homeostatic dysregulation method (11,15), which does not include information about chronological age.
Klemera–Doubal Method Biological Age Algorithm
Following the approach described by Levine (10), we calculated CALERIE participants’ biological ages using the Klemera–Doubal equation (25). The equation takes information from m number of regression lines of chronological age regressed on m biomarkers:
where x is the value of biomarker j measured for an individual in the CALERIE trial. To ensure independence of the Klemera–Doubal Biological Age algorithm from the CALERIE database, we estimated all algorithm parameters from a reference data set. We used the 2007–2008 and 2009–2010 panels of the U.S. National Health and Nutrition Examination Survey to match the assessment period of the CALERIE trial. For each biomarker j, the parameters k, q, and s were estimated from a regression of chronological age on the biomarker in the reference data set. k, q, and s, are the regression intercept, slope, and root mean squared error, respectively. sBA is a scaling factor equal to the square root of the variance in chronological age explained by the biomarker panel in the reference database. CA is chronological age. The biomarkers from the CALERIE database were: serum albumin, alkaline phosphatase, C-reactive protein, total cholesterol, creatinine, glycated hemoglobin (estimated from serum glucose), systolic blood pressure, urea nitrogen, uric acid, and white blood cell count. This biomarker set departed in two cases from previous analysis (10,12,14,37). The CALERIE protocol did not measure lung function or cytomegalovirus antibody. Instead, we included white blood cell count and uric acid plasma concentration, which are related to immune and kidney function, show prospective relationships with mortality (38,39), and were measured in both CALERIE and the NHANES. We report sensitivity analyses in the supplement in which algorithms are computed excluding these additional markers. Following Levine (10), regressions of chronological age on biomarker values included all NHANES participants aged 30–75 years, not pregnant, and with complete biomarker data (n = 7,694). Biomarkers with skewed distributions were log-transformed for analysis. Models were estimated separately for men and women. For each biomarker, model R-squared, root-mean-squared-error, intercept, and age-coefficient were saved (Supplementary Table 1, Panel A). These parameters were used to form the Klemera–Doubal Biological Age algorithm in our study.
Homeostatic dysregulation algorithm
We calculated CALERIE participants’ homeostatic dysregulation using the approach described by Cohen and colleagues (15,40). Homeostatic dysregulation quantifies deviation of a person’s physiology from a reference norm based on biomarker Mahalanobis distance (41). We computed parameters for the homeostatic dysregulation algorithm based on the same ten biomarkers used to calculate the Klemera–Doubal Biological Age algorithm. We formed the reference norm from young, healthy NHANES participants: NHANES 2007–2008 and 2009–2010 participants aged 20–30 years who were not obese and whose biomarker values fell within the age- and sex-specific normal range (42) (N = 689). We standardized all biomarkers to have mean = 0, SD = 1 within sex and computed the biomarker variance-covariance matrix (Supplementary Table 1, Panel B). This matrix, together with the Mahalanobis distance equation (41) formed the homeostatic dysregulation algorithm.
Biological aging measures in NHANES
We conducted analysis of the two biological age algorithms in independent NHANES data not used for algorithm development (Supplementary Information). Both algorithms were (a) correlated with chronological age (r = 0.92 for KDM Biological Age, r = 0.41 for homeostatic dysregulation); (b) associated with physical limitations independent of chronological age (r = 0.34 for KDM Biological Age, r = 0.16 for homeostatic dysregulation); and (c) indicated accelerated aging in a socially disadvantaged population (low educational attainment predicted a b = 1.56 95% CI [95% CI 1.09, 2.03] “year” acceleration of KDM Biological Age and b = 0.31 [95% CI 0.25, 0.37] standard deviation increase in homeostatic dysregulation independent of chronological age). Details of analyses are reported in the Supplementary Information.
Calculation of biological aging measures in CALERIE
We applied KDM Biological Age and homeostatic dysregulation algorithms to CALERIE participants’ biomarker data. Biomarker data came from blood collected at baseline, 12-month, and 24-month follow-up visits (baseline n = 220, 12-month n = 200, 24-month n = 191). Details on CALERIE Biobank data are reported in Supplementary Table 2. Glycated hemoglobin (%) was estimated from serum glucose using the equation HbA1C = (glucose+46.7)/28.7 (43). Alkaline phosphatase, blood urea nitrogen, creatinine, high sensitivity C-reactive protein, HbA1C, and uric acid were log transformed for analysis. KDM Biological age was computed directly from the biomarker values. To compute homeostatic dysregulation, biomarker values were standardized to have mean and standard deviation equal to one based on sex-specific distributions in the NHANES reference sample.
Missing data
Of a total 6,110 possible biomarker observations (10 biomarkers × 611 observations), 6,084 (>99%) were available in the CALERIE Biobank database. For individuals who participated in baseline, 12- and 24-month assessments, missing biomarker values from a single assessment were imputed as linear combinations of observed values from the other two assessments. In the case of six observations, missing biomarker values could not be imputed. KDM biological age values for these observations were pro-rated to account for missing data. Homeostatic dysregulation values could not be calculated, and values for these six observations were coded as missing.
Analysis
We conducted intent-to-treat analysis to test the hypothesis that caloric restriction would slow the rate of biological aging. We tested if randomization to the caloric-restriction arm of the CALERIE trial was associated with slowed biological aging using a mixed-effects growth model (44). The model included main effect terms for follow-up time and treatment arm, an interaction term testing differential effect of follow-up time by treatment arm, and covariates for chronological age at baseline and sex. The model took the form
where ΔBio Ageit is change in the biological age measure from baseline for individual “I” at time “t,” γ1 estimates annual change in biological age for ad libitum-arm participants, γ2 estimates any baseline difference in biological age between participants in ad libitum and caloric-restriction arms of the trial, γ3 estimates the difference in annual change in biological age between ad libitum-arm and caloric restriction-arm participants, χ is a vector of covariates, and μ0i and μ1i are the “random” intercepts and slopes estimated for each individual “i.” The coefficient γ3 tests the hypothesis that biological aging was slowed for participants randomized to the caloric-restriction arm of the trial. All models included sex and baseline age as covariates. Growth-model analysis included 611 observations of 220 individuals.
We also evaluated whether participants who achieved a higher “dose” of caloric restriction exhibited a stronger “response” in terms of more pronounced slowing in their rate of biological aging. CALERIE participants were not blind to their intervention condition and were active participants in their own “dosing;” i.e., participants’ behavior influenced their degree of caloric restriction. For this reason, even though treatment condition (caloric-restriction vs. ad libitum) was randomly assigned, the dose of caloric restriction achieved by participants in the treatment arm could vary systematically with their rate of biological aging prior to the start of the trial. We compared biological aging in CALERIE participants in the ad libitum condition to two subgroups of caloric-restriction-arm participants: those who achieved less than 10% caloric restriction on average across the 12- and 24-month follow-up periods and those who achieved 10% or more caloric restriction during these periods. Subjects were not randomized to <10/≥10% adherence and thus may differ across a range of values possibly related to both level of CR attained and biological aging. The analysis of dose–response effects shown are therefore purely exploratory in nature and intended to provide hypothesis generation rather than confirmatory evidence.
Results
Baseline
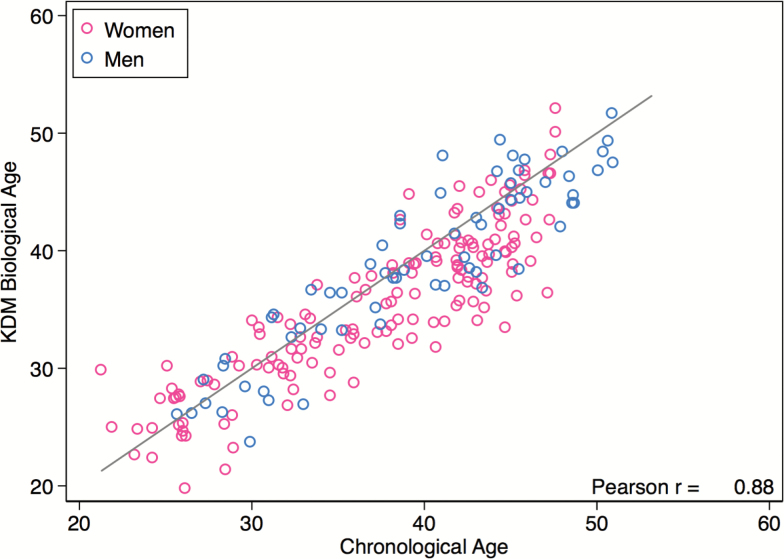
CALERIE participants’ chronological and Klemera–Doubal method (KDM) Biological Ages were correlated (r = 0.88, Figure 1). At baseline, there was no difference in KDM Biological Age between caloric restriction and ad libitum arms of the CALERIE trial (p value for difference = .777). Participants’ biological ages were slightly younger than their chronological ages. Mean chronological age at baseline among CALERIE participants was 38 years (SD = 7). Mean KDM Biological Age was 37 years (SD = 7). This difference may reflect: the sampling frames used for CALERIE and the NHANES; volunteer bias; and that CALERIE participants were selected to be in good health, whereas the NHANES sample used to estimate KDM Biological Age parameters represented the general population.
Figure 1.
CALERIE participants’ KDM Biological Ages were highly correlated with their chronological ages at the time of baseline assessment but tended to be slightly younger. The plotted line shows a one-to-one correspondence between biological age and chronological age. The larger number of plotted points below the line as compared to above the line indicates that CALERIE participants’ KDM Biological Ages tended to be slightly younger than their chronological ages.
Longitudinal Change
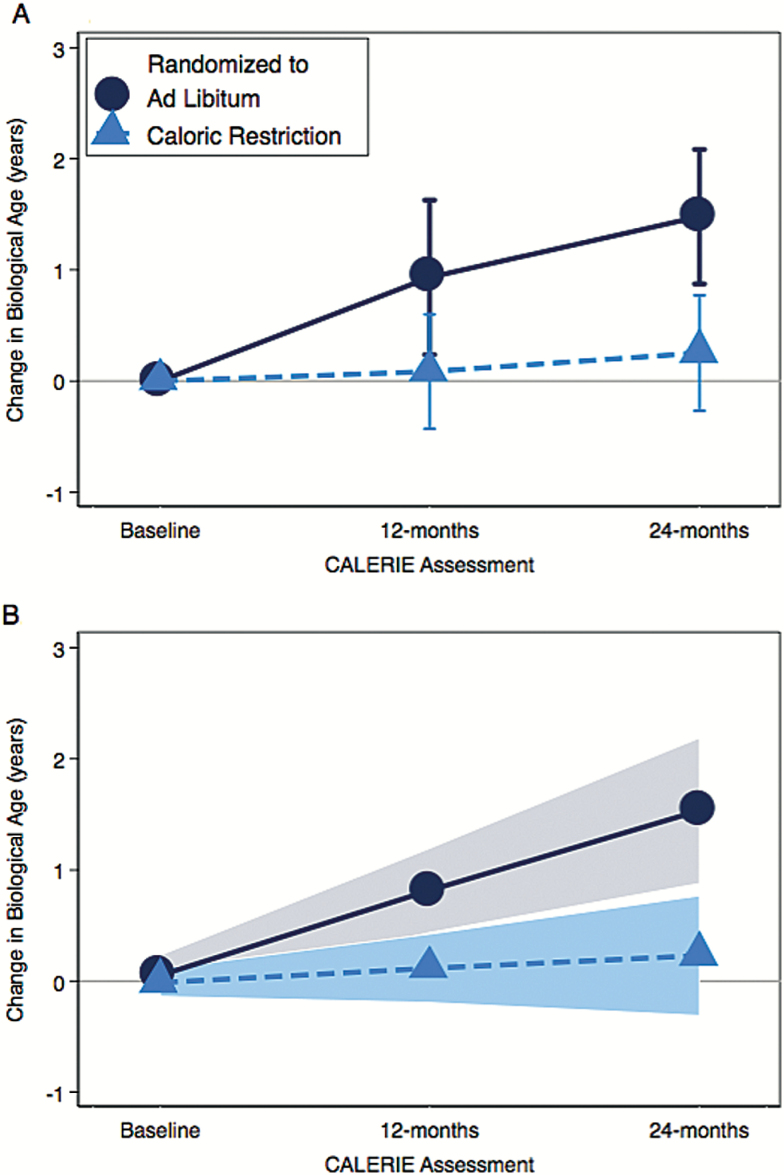
Across follow-up, CALERIE participants randomized to the trial’s caloric restriction arm experienced slower biological aging as compared to participants randomized to the ad libitum arm (treatment-by-time interaction: b = −0.60 (95% CI −0.99, −0.21), p = .003). Average change from baseline in KDM Biological Age is plotted for ad libitum- and caloric-restriction-arm participants in Figure 2. CALERIE participants randomized to the ad libitum arm of the trial experienced average increase in KDM Biological Age of 0.71 “years” per 12-month follow-up (95% CI 0.41, 1.01). For participants randomized to caloric restriction, KDM Biological Age increased more slowly, by an average of 0.11 (95% CI −0.13, 0.36) “years” per 12-month follow-up interval. This change was not statistically different from zero (p = .353), indicating slowed biological aging in caloric-restriction-arm participants. Growth model coefficients are reported in Table 1 and Supplementary Table 3.
Figure 2.
Change in KDM Biological Age from Baseline to 12- and 24-month follow-ups in the ad libitum (black dots) and caloric-restriction (blue dots) arms of the CALERIE trial. Panel A shows mean values with 95% confidence intervals calculated for each follow-up. Panel B shows slopes estimated from the growth model with the shaded areas indicating 95% confidence intervals.
Table 1.
Estimated Annual Change in Klemera–Doubal (KDM) Biological Age From Baseline Through 24-Month Follow-up in Ad libitum- and Caloric Restriction-arm Participants in the CALERIE Randomized Trial
| b | [95% CI] | p Value | N | |
|---|---|---|---|---|
| Estimated Annual Change in KDM Biological Age from Baseline through 24-month follow-up | ||||
| Ad Libitum-Arm Participants | 0.71 | [0.41, 1.01] | 2.97E−06 | 75 |
| Caloric Restriction-Arm Participants | 0.11 | [−0.13, 0.36] | .353 | 145 |
| Test of Interaction | −0.60 | [−0.99, −0.21] | .003 | 220 |
Note: The regression model included sex and age at baseline as covariates. KDM Biological Age increased in ad libitum-arm participants. Change in KDM Biological Age was not different from zero in caloric-restriction-arm participants. The difference between treatment arms in rate of change in KDM Biological Age was statistically significant (test of interaction p value = .003), indicating slowed biological aging in caloric-restriction-arm participants as compared to ad libitum-arm participants. CI = Confidence interval.
Association With Weight Loss
The slowed pace of biological aging among CALERIE participants randomized to the caloric-restriction arm was not accounted for by weight loss during the intervention. CALERIE participants in the caloric-restriction arm of the trial experienced significant weight loss relative to those in the ad libitum arm (23). We tested if the slower pace of biological aging observed among participants in the caloric-restriction arm could be attributed to this weight loss. We repeated growth-model analysis, including weight as a time-varying covariate. Independent of weight loss, caloric-restriction arm participants experienced slower biological aging as compared to ad libitum-arm participants (weight-loss adjusted treatment-by-time interaction b = −0.55 (95% CI −0.95, −0.16), p = .006).
Dose Response
Slowed pace of biological aging among CALERIE participants randomized to the caloric-restriction arm was greater (i.e., slowed by a larger degree) for participants who achieved more caloric restriction. The degree of caloric restriction varied across participants (Supplementary Figure 2). We divided the n = 130 caloric restriction-arm participants who attended follow-up assessments into those who failed to achieve an average 10% caloric restriction across follow-ups (n = 66) and those who achieved 10% or greater caloric restriction (n = 62). Visit-specific caloric restriction information was unavailable for n = 2 participants. We then repeated our analysis. Both groups of caloric restriction arm participants experienced slowed biological aging relative to ad libitum-arm participants (for the <10% group, b = −0.49 [95% CI −0.95, −0.04]; for the ≥10% group, b = −0.72 [95% CI −1.19, −0.25]; Supplementary Figure 3). However, although the pattern of effect-sizes was consistent with a dose–response relationship, the difference in effect between the <10% and ≥10% groups was not statistically significant (p = .361). Growth model coefficients are reported in Supplementary Table 5.
Replication With Homeostatic Dysregulation
We repeated analysis, this time using homeostatic dysregulation to index biological aging. Replication of analysis using homeostatic dysregulation in place of KDM Biological Age yielded similar results. At baseline, homeostatic dysregulation had mean (SD) of 3.44 (0.42), was positively correlated with CALERIE participants’ chronological ages (r = 0.12), and was not different between treatment and ad libitum arms of the trial (p = .815). Across follow-up, caloric-restriction arm participants experienced slower biological aging compared to ad libitum-arm participants as measured by homeostatic dysregulation (treatment-by-time interaction b = -0.07 [95% CI −0.12, −0.01], p = .014). Homeostatic dysregulation was unchanged across follow-up among ad libitum-arm CALERIE participants (b = 0.01 units per 12-month follow-up [95% CI −0.03, 0.06], p = .644). In contrast, homeostatic dysregulation declined by −0.05 (95% CI −0.08, −0.02) units among caloric restriction-arm participants (p < .001). Slower biological aging in caloric restriction-arm participants compared to ad libitum-arm participants as measured by homeostatic dysregulation was not accounted for by weight-loss (weight loss adjusted treatment-by-follow-up-time interaction b = −0.07 (95% CI −0.12, −0.01), p = .020). Within the caloric restriction arm, there were no dose effects observed (p = .470). Homeostatic dysregulation growth model coefficients are reported in Supplementary Tables 4 and 5.
Discussion
We conducted intent-to-treat analysis of the CALERIE randomized trial to test if caloric restriction slowed biological aging over the course of a 2-year intervention. Tests using two different methods to quantify biological aging (Klemera–Doubal method Biological Age and homeostatic dysregulation) produced a consistent result: participants in the caloric-restriction arm of the trial experienced slowed biological aging as compared to participants in the ad libitum arm. Sensitivity analysis showed that slowed biological aging in the caloric restriction arm of the trial was not accounted for by weight loss during the intervention phase.
The main contribution of this study is to provide initial evidence that methods to quantify biological aging are sensitive enough to detect effects of geroprotective therapy delivered to middle-aged adults in a small randomized trial. This evidence adds to findings from observational studies that accelerated biological aging measured using the same methods predicts functional decline, morbidity, and mortality in older adults (10,11,14,15,37,40). Together, this evidence argues for using methods to quantify biological aging as outcomes in trials of geroprotective therapies. For example, it is thought that pharmacotherapy may be able to replicate the geroprotective effects of caloric restriction (45). Methods for assessing biological aging used in this study provide benchmarks against which such pharmacotherapy might be evaluated.
Second, we illustrate here how methods originally designed for cross-sectional analysis of biological age can be implemented within a longitudinal design. To our knowledge, this is the first longitudinal analysis of KDM Biological Age and homeostatic dysregulation. The aging process involves changes occurring across time within individuals. To date, with some exceptions (46–50), most analysis of biological aging in humans continues to focus on cross-sectional differences between younger and older persons. Several large databases allow for repeated measures analysis of indices of biological aging. An important next step in translating these indices for use in geroprotector trials is to develop better understanding of rates of change over time in the general population and relevant subgroups.
Finally, this study provides evidence that biological aging measures developed in mixed-age samples and tested for predicting outcomes primarily in older adults are sensitive to changes produced by geroprotective intervention in the physiologies of relatively young individuals. Trajectories of aging begin to diverge as early as young adulthood (12), suggesting geroprotective therapies may be most effective when administered early, before age-related disease onsets (51). Younger persons may also be better able to sustain toxicity associated with some geroprotectors (52). Tried and tested metrics of biological aging like physical frailty (53) and accumulation of deficits (54) are mostly invariant in young and midlife adults. Indices of biological aging may provide an alternative for geroprotector trials testing therapies in younger populations.
Limitations
Quantification of biological aging remains a work in progress. We applied two previously developed methods to quantify biological aging and obtained similar results. But there are many other proposed methods to quantify biological aging. For example, several proposed methods utilize genomic and other molecular data not yet available in the CALERIE Biobank (9,55–60). As new types of data are added to the CALERIE database, analysis of other biological aging measures will become possible. The methods we used were imperfect. For example, the Klemera–Doubal method is based on linear models relating biomarker levels to chronological age. But many biomarkers show nonlinear trajectories in aging, and biomarker relationships with mortality risk may also change with advancing age (61). As methods are refined, analyses can be repeated. CALERIE participants were almost exclusively white (95%). CALERIE participants were not blinded to intervention condition. Slowed biological aging in the caloric restriction-arm participants may partly reflect a placebo effect. Replication in other populations is needed. Both the Klemera–Doubal Biological Age and homeostatic dysregulation methods have similar validity in white and non-White populations (15,37). But it is unknown if caloric restriction may have ethnicity-specific geroprotective effects. Follow-up was right censored. The CALERIE trial was comparatively short (2 years). Whether changes to the rate of biological aging can be sustained over longer intervals is unknown. Also unknown is whether slowed biological aging observed in this study is sustained beyond the term of the intervention.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This investigation was made possible through use of the CALERIE data repository and was supported in part by the CALERIE Research Network grant to W.E.K. and C.F.P. (U24AG047121). D.W.B. is supported by NIA grants P30AG028716 and R01AG032282, and an Early-Career Fellowship from the Jacobs Foundation. The CALERIE data repository is supported by the CALERIE Research Network and contains an array of clinical, physiologic, and molecular data. These data as well as blood and tissue samples are available to researchers for investigations of caloric restriction effects on cardiometabolic risks, aging, and other health-related issues. More information about repository contents and how to request data and samples can be found at the following website: https://calerie.duke.edu/.
Supplementary Material
References
- 1. Harper S. Economic and social implications of aging societies. Science. 2014;346:587–591. doi:10.1126/science.1254405. [DOI] [PubMed] [Google Scholar]
- 2. Burch JB, Augustine AD, Frieden LA et al. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S1–S3. doi:10.1093/gerona/glu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kennedy BK, Berger SL, Brunet A et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi:10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fontana L, Kennedy BK, Longo VD, Seals D, Melov S. Medical research: treat ageing. Nature. 2014;511:405–407. doi:10.1038/511405a. [DOI] [PubMed] [Google Scholar]
- 5. Moskalev A, Chernyagina E, Tsvetkov V et al. Developing criteria for evaluation of geroprotectors as a key stage toward translation to the clinic. Aging Cell. 2016;15:407–415. doi:10.1111/acel.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaeberlein M. Longevity and aging. F1000Prime Rep. 2013;5:5. doi:10.12703/P5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi:10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 8. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi:10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi:10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013;68:667–674. doi:10.1093/gerona/gls233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen AA, Milot E, Yong J et al. A novel statistical approach shows evidence for multi-system physiological dysregulation during aging. Mech Ageing Dev. 2013;134:110–117. doi:10.1016/j.mad.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belsky DW, Caspi A, Houts R et al. Quantification of biological aging in young adults. Proc Natl Acad Sci USA 2015;112:E4104–E4110. doi:10.1073/pnas.1506264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sebastiani P, Thyagarajan B, Sun F et al. Biomarker signatures of aging. Aging Cell. 2017;16:329–338. doi:10.1111/acel.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levine ME, Crimmins EM. A comparison of methods for assessing mortality risk. Am J Hum Biol. 2014;26:768–776. doi:10.1002/ajhb.22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Q, Wang S, Milot E et al. Homeostatic dysregulation proceeds in parallel in multiple physiological systems. Aging Cell. 2015;14:1103–1112. doi:10.1111/acel.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marioni RE, Shah S, McRae AF et al. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int J Epidemiol. 2015;44:1388–1396. doi:10.1093/ije/dyu277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Breitling LP, Saum KU, Perna L, Schöttker B, Holleczek B, Brenner H. Frailty is associated with the epigenetic clock but not with telomere length in a German cohort. Clin Epigenetics. 2016;8:21. doi:10.1186/s13148-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen BH, Marioni RE, Colicino E et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY). 2016;8:1844–1865. doi:10.18632/aging.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weindruch R, Sohal RS. Caloric intake and aging. N Engl J Med. 1997;337:986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, Madeo F. The search for antiaging interventions: from elixirs to fasting regimens. Cell. 2014;157:1515–1526. doi:10.1016/j.cell.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi:10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee C, Longo V. Dietary restriction with and without caloric restriction for healthy aging. F1000Research. 2016;5. doi:10.12688/f1000research.7136.1. eCollection 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ravussin E, Redman LM, Rochon J et al. ; CALERIE Study Group A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci. 2015;70:1097–1104. doi:10.1093/gerona/glv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fontana L, Villareal DT, Das SK et al. ; CALERIE Study Group Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell. 2016;15:22–27. doi:10.1111/acel.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127:240–248. doi:10.1016/j.mad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 26. Levine ME. Response to Dr. Mitnitski’s and Dr. Rockwood’s Letter to the Editor: Biological age revisited. J Gerontol A Biol Sci Med Sci. 2013;3:297-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stewart TM, Bhapkar M, Das S et al. ; CALERIE Study Group Comprehensive assessment of long-term effects of reducing intake of energy phase 2 (CALERIE Phase 2) screening and recruitment: methods and results. Contemp Clin Trials. 2013;34:10–20. doi:10.1016/j.cct.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rochon J, Bales CW, Ravussin E et al. ; CALERIE Study Group Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol A Biol Sci Med Sci. 2011;66:97–108. doi:10.1093/gerona/glq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Redman LM, Kraus WE, Bhapkar M et al. ; CALERIE Study Group Energy requirements in nonobese men and women: results from CALERIE. Am J Clin Nutr. 2014;99:71–78. doi:10.3945/ajcn.113.065631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Racette SB, Das SK, Bhapkar M et al. ; CALERIE Study Group Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. Am J Physiol Endocrinol Metab. 2012;302:E441–E448. doi:10.1152/ajpendo.00290.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong WW, Roberts SB, Racette SB et al. The doubly labeled water method produces highly reproducible longitudinal results in nutrition studies. J Nutr. 2014;144:777–783. doi:10.3945/jn.113.187823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jackson SH, Weale MR, Weale RA. Biological age–what is it and can it be measured? Arch Gerontol Geriatr. 2003;36:103–115. [DOI] [PubMed] [Google Scholar]
- 33. Mitnitski A, Howlett SE, Rockwood K. Heterogeneity of human aging and its assessment. J Gerontol A Biol Sci Med Sci. 2017;72:877–884. doi:10.1093/gerona/glw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cho IH, Park KS, Lim CJ. An empirical comparative study on biological age estimation algorithms with an application of Work Ability Index (WAI). Mech Ageing Dev. 2010;131:69–78. doi:10.1016/j.mad.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 35. Schaefer JD, Caspi A, Belsky DW et al. Early-life intelligence predicts midlife biological age. J Gerontol B Psychol Sci Soc Sci. 2016;71:968–977. doi:10.1093/geronb/gbv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mitnitski A, Rockwood K. Biological age revisited. J Gerontol A Biol Sci Med Sci. 2014;69:295–296. doi:10.1093/gerona/glt137. [DOI] [PubMed] [Google Scholar]
- 37. Levine ME, Crimmins EM. Evidence of accelerated aging among African Americans and its implications for mortality. Soc Sci Med. 2014;118:27–32. doi:10.1016/j.socscimed.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruggiero C, Metter EJ, Cherubini A et al. White blood cell count and mortality in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2007;49:1841–1850. doi:10.1016/j.jacc.2007.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meisinger C, Koenig W, Baumert J, Döring A. Uric acid levels are associated with all-cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population: the MONICA/KORA cohort study. Arterioscler Thromb Vasc Biol. 2008;28:1186–1192. doi:10.1161/ATVBAHA.107.160184. [DOI] [PubMed] [Google Scholar]
- 40. Cohen AA, Li Q, Milot E et al. Statistical distance as a measure of physiological dysregulation is largely robust to variation in its biomarker composition. PLoS One. 2015;10:e0122541. doi:10.1371/journal.pone.0122541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mahalanobis P. Mahalanobis distance. Proc Natl Acad Sci India 49:234–256. [Google Scholar]
- 42. Mayo Clinic Medical Laboratories Test Catalog. http://www.mayomedicallaboratories.com/test-catalog/ (Accessed February 21, 2017).
- 43. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c-Derived Average Glucose Study Group Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–1478. doi:10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Singer JD, Willett JB. 2003. Applied Longitudinal Data Analysis. New York: Oxford University Press. [Google Scholar]
- 45. Ingram DK, Zhu M, Mamczarz J et al. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108. doi:10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 46. Mitnitski A, Rockwood K. The rate of aging: the rate of deficit accumulation does not change over the adult life span. Biogerontology. 2016;17:199–204. doi:10.1007/s10522-015-9583-y. [DOI] [PubMed] [Google Scholar]
- 47. Armstrong JJ, Godin J, Launer LJ et al. Changes in frailty predict changes in cognition in older men: the Honolulu-Asia Aging Study. J Alzheimers Dis. 2016;53:1003–1013. doi:10.3233/JAD-151172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buchman AS, Wilson RS, Bienias JL, Bennett DA. Change in frailty and risk of death in older persons. Exp Aging Res. 2009;35:61–82. doi:10.1080/03610730802545051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Christiansen L, Lenart A, Tan Q et al. DNA methylation age is associated with mortality in a longitudinal Danish twin study. Aging Cell. 2016;15:149–154. doi:10.1111/acel.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arbeev KG, Cohen AA, Arbeeva LS et al. Optimal versus realized trajectories of physiological dysregulation in aging and their relation to sex-specific mortality risk. Front Public Health. 2016;4:3. doi:10.3389/fpubh.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moffitt TE, Belsky DW, Danese A, Poulton R, Caspi A. The longitudinal study of aging in human young adults: knowledge gaps and research agenda. J Gerontol A Biol Sci Med Sci. 2017;72:210–215. doi:10.1093/gerona/glw191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Su DW, Mita M, Mita AC. The clinical pharmacology and toxicity profile of rapalogs. mTOR Inhibition for Cancer Therapy: Past, Present and Future. Mita M, Mita A, Rowinsky EK (Eds.). Springer Paris; 2016:161–189. [Google Scholar]
- 53. Fried LP, Tangen CM, Walston J et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 54. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi:10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hannum G, Guinney J, Zhao L et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. doi:10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weidner CI, Lin Q, Koch CM et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. 2014;15:R24. doi:10.1186/gb-2014-15-2-r24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sood S, Gallagher IJ, Lunnon K et al. A novel multi-tissue RNA diagnostic of healthy ageing relates to cognitive health status. Genome Biol. 2015;16:185. doi:10.1186/s13059-015-0750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peters MJ, Joehanes R, Pilling LC et al. ; NABEC/UKBEC Consortium The transcriptional landscape of age in human peripheral blood. Nat Commun. 2015;6:8570. doi:10.1038/ncomms9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–1198. doi:10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 60. Putin E, Mamoshina P, Aliper A et al. Deep biomarkers of human aging: Application of deep neural networks to biomarker development. Aging (Albany NY). 2016;8:1021–1033. doi:10.18632/aging.100968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yashin AI, Arbeev KG, Wu D et al. How lifespan associated genes modulate aging changes: lessons from analysis of longitudinal data. Front Genet. 2013;4:3. doi:10.3389/fgene.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.