Abstract
Calorie restriction without malnutrition increases longevity and delays the onset of age-associated disorders in multiple species. Recently, greater emphasis has been placed on healthy life span and preventing frailty than on longevity. Here, we show the beneficial effect of long-term calorie restriction on frailty in later life in a nonhuman primate. Frail phenotypes were evaluated using metabolic and physical activity data and defined using the Fried index. Shrinking was defined as unintentional weight loss of greater than 5% of body weight. Weakness was indicated by decline in high intensity spontaneous physical activity. Poor endurance or exhaustion was indicated by a reduction in energy efficiency of movements. Slowness was indicated by physical activity counts in the morning. Low physical activity level was measured by total energy expenditure using doubly labeled water divided by sleeping metabolic rate. Weakness, poor endurance, slowness, and low physical activity level were significantly higher in control compared with calorie restriction (p < .05) as was total incidence of frailty (p < .001). In conclusion, we established a novel set of measurable criteria of frailty in nonhuman primates, and using these criteria, showed that calorie restriction reduces the incidence of frailty and increases healthy life span in nonhuman primates.
Keywords: Frailty, Monkey, Caloric restriction
Frailty is a state of high vulnerability for adverse health outcomes; a higher risk of disability, falls, hospitalization, and mortality (1). The prevalence of frailty increases with increasing age (2). Based on projections by the United Nations, the world population of older people has just exceeded that of children younger than 5 years of age, and more people are living longer and to more extreme ages than ever before (3). Thus, the total worldwide population of frail elderly adults is predicted to dramatically increase (4).
Physical function is known to affect quality of life. Given the overall aging of the population, increasing healthy life span without frailty and/or trying to decrease the gap between whole life span and healthy life span are emerging scientific objectives. As a result, there is now greater emphasis placed on measurement of physical function during intervention studies in both animal models and humans (5). In light of this, recent rodent studies have been establishing and applying measurements of frailty (6–11). There are two common assessments of frailty that have been successfully adapted to aging studies in mice, the Fried frailty phenotype and the Rockwood-based frailty index (9). The Fried frailty phenotype (12) conceptualizes frailty functionally with five standard measurable outcome variables (unintentional weight loss, exhaustion, weakness, slow walking speed, low physical activity). Using this index, frailty is defined as a clinical condition when three or more of the functional criteria are present. The Rockwood-based frailty index (13) conceptualizes frailty as a multidimensional clinical syndrome taking into account a wide array of outcome variables. It is defined as the number of deficits in an individual divided by the total number of deficits measured and as such is an index of deficit accumulation.
Longitudinal human studies (14,15) have revealed individual variability in the relationship between chronological age and physical capacity, or biological age. This variability is likely due to subtle or pathologic physiological changes experienced throughout the life span. Therefore, it would be informative to examine the effects of long-term lifestyle interventions initiated in adulthood on later-life physical capacity. Such studies in humans would be technically and ethically challenging if not impossible. Therefore, insight into these questions from animal models is necessary. The Wisconsin National Primate Research Center (WNPRC) “Caloric Restriction and Aging in Rhesus Monkeys” Study (16–20) gives us the unique opportunity to understand the effects and mechanisms of long-term diet intervention in a nonhuman primate.
Caloric restriction (CR) without malnutrition increases longevity and delays the onset of age-associated disorders in a variety of laboratory organism, including yeasts, worms, flies, and rodents (21). Although fewer controlled studies have been performed in nonhuman primates and humans, outcomes in terms of body composition and circulating lipoprotein and metabolite profiles are similar (16,18,22,23), suggesting conservation of beneficial effects among primate species. In rhesus monkeys, CR has been shown to reduce or delay the onset of diverse age-related diseases and conditions such as diabetes (24), immune senescence (25), hypertension, cancer, bone demineralization, and brain atrophy (16,26) in rhesus monkeys. We have also shown that CR attenuated sarcopenia and age-related decline in physical activity in rhesus monkeys (17,27).
In the field of human clinical investigations, the concept of frailty has been an area of much research interest (28). With regard to animal models, Arum and colleagues (6) and Kane and colleagues (8,9). reported that CR reduced frailty in mice. However, measures of frailty have yet to be fully defined in nonhuman primates and the effect of long-term CR on frailty in nonhuman primates has not been assessed. Thus, we developed a novel objective index of metabolic and physical activity data that we propose to be similar to measures of physical performance based on the Fried model and used these parameters to examine the effect of CR on frailty. We hypothesized that long-term, adult-onset CR would prevent frailty in later life in a rhesus monkey model.
Methods
Animals
The overall design of the CR study at WNPRC has been previously described (20,29,30), as have the detailed protocols used to measure metabolic and physical activity data (27). Briefly, the study includes three groups of adult rhesus monkeys (Macaca mulatta of Indian origin); 30 male monkeys entered in the study in 1989 (Group 1), and 30 females (Group 2) and an additional 16 males (Group 3) were introduced in 1994. All groups averaged ~10 years of age at the onset of CR. Within each group, animals were evenly randomized based on body weight to either the control (C) or CR group. For this analysis, energy expenditure and physical activity data collected from 2007 to 2008 was used as later-life data. At the end of the 2008 data collection period, 18 of 38 C animals and 24 of 38 CR were still alive. These 42 surviving animals were used for this analysis. Mean age was 24.6 ± 2.8 years, and intervention duration was over 18 years for Group 1 and over 13 years for Groups 2 and 3. The overall median life span of rhesus monkeys at WNPRC is 27 years, and the maximum life span is 40 years (26,31).
The animals were housed in individual cages (89-cm wide × 86-cm deep × 86-cm high) to minimize aggressive encounters and to control and quantify food intake. All animals had extensive visual and auditory contact with other study animals. The animals were allowed continuous access to water, and the rooms were maintained at 21−26°C with ~50%−65% relative humidity. Artificial room lighting was automatically controlled to provide 12-hour light and dark periods. The protocol was approved by the Animal Care and Use Committee of the Graduate School of the University of Wisconsin–Madison, an AAALAC-accredited program.
Diet and Weight Loss
At the outset of the study, ad libitum food intake was assessed to define an individual baseline intake for each animal. Thereafter, the C monkeys were allowed ad libitum access to food for ~6–8 h/d, whereas CR monkeys underwent individualized caloric restriction of ~30% from the baseline assessment amount. The C monkeys were fed a defined, pelleted diet (no. 85387, Teklad, Madison, WI) containing 15% lactalbumin, 10% corn oil, and approximately 65% carbohydrate, as previously described (29). CR animals were fed a similar diet (no. 93131, Teklad), except that the mineral and vitamin mix were increased by about 30% to minimize differences in total daily mineral and vitamin intake between groups. Food was provided in the morning and removed 6–8 hours later. At this time, weight of spilled food was estimated, and food remaining in the trays was weighed to calculate daily food intake, and the animals were given a piece of fresh fruit. Weight change from 1999–2000 to 2007–2008 period was calculated and unintentional weight loss of greater than 5% of body weight was defined as shrinking within the index of frail phenotype (frailty criterion #1).
Calorimetry and Weakness
Twenty-four-hour energy expenditure (24hEE) was measured in a standard cage enclosed within a transparent metabolic chamber with dimensions of 75-cm wide × 75-cm deep × 80-cm high as previously described (32,33). Chamber temperature was maintained at 21°C to maintain the animals at thermoneutrality. The animals were housed in these chambers 1 day before the start of measurements for acclimatization. Respiratory gas exchange was measured for two consecutive days. The chamber was located in a room where other animals were housed to provide a familiar social environment.
Filtered air was drawn into the chamber, and the flow rate, temperature, and humidity were measured. A portion of the exhaust air from the chamber was dried and analyzed for oxygen (S-3A O2 analyzer; Ametek, Pittsburgh, PA) and CO2 (CD-3 CO2 analyzer; Ametek) contents. The 24hEE was calculated on the basis of the oxygen consumption and CO2 release rates using the modified Weir equation. Ethanol was burned from a lamp and the %recovery of O2 and CO2 was used to calibrate the chambers. Sleeping metabolic rate was defined as the lowest continuous 3-hour period recorded during the night with confirmation of no physical activity by accelerometer based activity count. Metabolic equivalent intensity were expressed as measured metabolic rate divided by sleeping metabolic rate as calculated every 5 minutes. Duration of high-intensity activities, greater than 1.8 metabolic equivalent intensity, was calculated and engaging in these activities for less than 60 min/d was defined as weakness within the index of frail phenotype (frailty criterion #2).
Doubly Labeled Water Procedures and Low Physical Activity
Total energy expenditure was determined during a 5-day period by the two-point double-labeled water methodology described previously (34). After anesthesia (ketamine HCl, 15 mg/kg im), a baseline blood sample was collected, and a premixed 1.51 g/kg estimated total body water dose of double-labeled water was given intravenously. The dose was composed of 0.3 g/kg estimated total body water of 94% H218O (Rotem Industries, Beer Sheva, Israel) and 0.17 g/kg estimated total body water of 99.9% 2H2O (Cambridge Isotope Laboratories, Andover, MA) and was diluted with 3% NaCl to physiological osmolarity. Blood samples were collected at 2 hours and 1 and 5 days after dosing. Immediately after collection, blood was centrifuged for 10 minutes for serum separation. Serum was stored at −20°C in cryogenically stable tubes until analysis by isotope ratio mass spectrometry.
Water from serum samples was extracted by centrifugation (4°C, 1 hour, RCF 12,000g) on regenerated cellulose filters (YM-50, Centricon, Bedford, MA). Deuterium and oxygen-18 isotopic enrichments were determined as previously described (34). CO2 production was calculated according to the equation of Schoeller and colleagues (35).
where N represents the average isotope dilution space of deuterium and 18O calculated from Coward and colleagues (36) by the plateau method using the 2-hour postdose sample and corrected for isotope exchange by the factors 1.041 and 1.007, respectively. The isotope dilution space ratio was 1.041 ± 0.009 (mean ± SD). ko and kd represent the isotope elimination rates calculated by linear regression of the natural logarithm of isotope enrichment as a function of elapsed time from Day 1 samples. Total energy expenditure was calculated using the modified Weir equation and a food quotient of 0.93, which was estimated from the animal’s diet. Day 1 samples were chosen for calculation of ko and kd to avoid the potential artifacts (hypometabolism, hypoactivity, etc.) introduced in the total energy expenditure estimates by anesthesia. Physical activity level was calculated by total energy expenditure/sleeping metabolic rate, and physical activity level of less than 1.3 was defined as low physical activity within the index of frail phenotype (frailty criterion #3).
Accelerometer and Slowness
Physical activity data were collected using a commercial accelerometer (Actiwatch AW-64, Resprionics/Mini-Mitter, Bend, OR) designed to quantify physical motion. The accelerometer was attached on a collar fastened around the animal’s neck. Annual accelerometry measures were taken over a 3- to 4-week period each year. The first ~5 days of activity were considered to be the adaptation period and those data were not included in the analysis. Accelerometer data were collected for all CR animals but only 13 of 18 C because at the time of measurement the collars were not well tolerated by 5 of the C animals. The accelerometer sampled activity counts every 1 minute and the log-transformed activity counts during the morning (0600–1200 hours) of less than eight was defined as slowness within the index of frail phenotype (frailty criterion #4).
Metabolic Cost for Movements and Poor Energy and Exhaustion
The relationship between metabolic rate and activity count is approximately linear over a broad range of activity counts (37–39), and cost of activity can be calculated as the slope of the regression of metabolic rate (the dependent variable) on activity counts because the regressions of activity counts on metabolic rates return significant intercepts (40,41). We plotted metabolic rates against activity counts for every hour in the respiratory chamber to estimate cost of activity for each individual. Using least squares linear regressions, we estimated the slope (ie, incremental cost of activity) and intercept for each individual. An increase of cost of activity higher than 0.0045 was defined as poor endurance or exhaustion with the index of frail phenotype (frailty phenotype #5).
Frailty Thresholds
Because frailty indices have not been defined for rhesus monkeys, the thresholds for each frailty phenotype were based on an evaluation of data from this cohort. In humans aged 65 years and older, reported prevalence of frailty in the community varies enormously (range 4.0%–59.1%) (42). We assumed that the prevalence in lean rhesus monkeys (CR group) older than the average life span of this species in the wild and with limited living space and mobility is about 20%–30%, and set each threshold to reflect this level in the current study. The parameters were then tested using incremental thresholds on either side of this cut off, and in each case, the difference between C and CR groups was significant. Final specifications for each index are outlined earlier in Methods section.
Statistical Analysis
Results were presented as the mean ± standard deviation (SD). Age, body weight, energy intake and the degree of presence of each frail phenotype were compared between the C and CR groups by unpaired student’s t-tests. Morning activity count data were log transformed (ln) to improve normality. The two-way analysis of covariance with diet (C vs CR) and sex (male vs female) as between-subject factors, and age as a covariate in the model was applied because the animals of all three groups varied in age. Because no significant interaction of diet treatment × sex was found for any frail phenotype, combined male and female data were also shown. The prevalence of nonfrail, prefrail, and frail between the C and CR groups was compared by chi-square test. All analyses were performed using SPSS 22.0 for Windows, and results were considered significant at p < .05.
Results
We utilized metabolic and physical activity data to assess frailty in animals that had been subjected to long-term, adult-onset CR and their age-matched C counterparts. At the time of the 2007–2008 assessments, both the C and CR animals were ~24 years of age, which is close to the 50% mortality for this species at ~26 years of age. By design, CR monkeys had lower body weight and lower energy intake compared with C (p < .05, Table 1). Our definition of thresholds for each frailty phenotype can be found in Frailty Thresholds (Methods section).
Table 1.
Physical Characteristics of the Subjects
| Control | CR | Significance | |
|---|---|---|---|
| N | 18 | 24 | |
| Male/female | 10/8 | 14/10 | |
| Age (y) | 24.3 ± 2.7 | 24.9 ± 2.9 | p = .46 |
| Weight (kg) | 11.6 ± 3.3 | 9.0 ± 1.7 | p < .05 |
| Energy intake (MJ/d) | 2.41 ± 0.52 | 2.03 ± 0.52 | p < .01 |
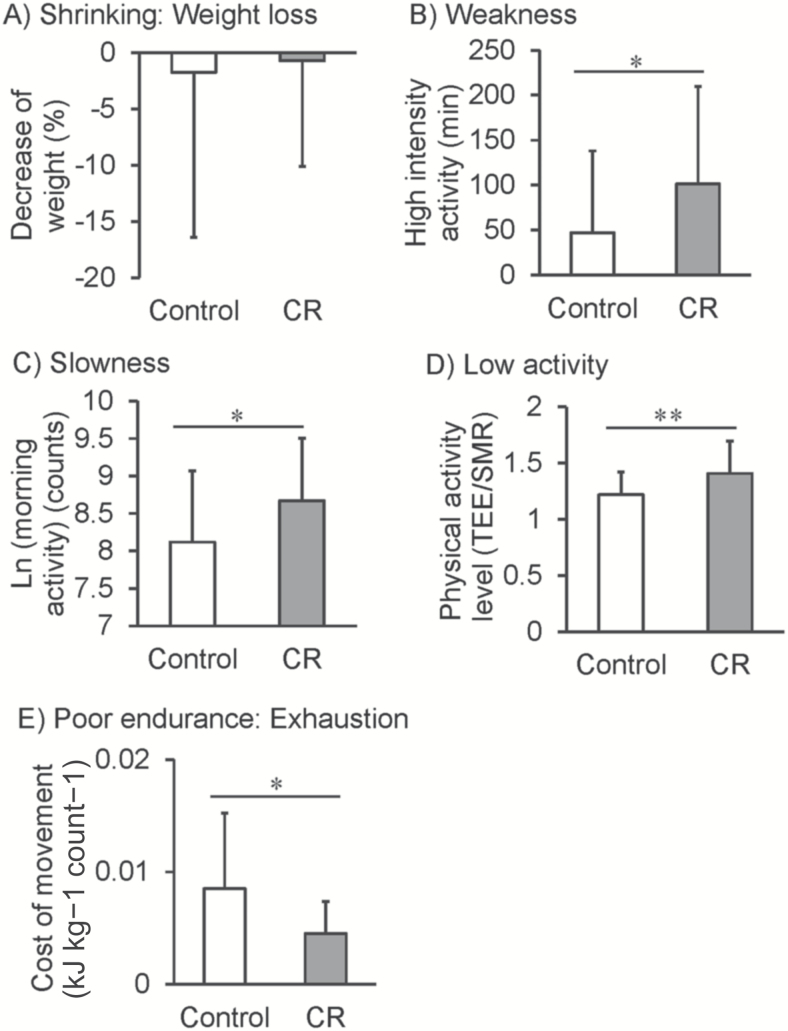
Figure 1 shows group comparisons for each component of the Fried frail phenotype as we defined them in our model system using metabolic and physical activity data. Weight loss, as an indictor of shrinking, did not differ between groups (Figure 1A); however, other frail phenotypes were different between C and CR groups. Metabolic chamber data were used to index weakness. C animals had lower levels of high-intensity activity (metabolic equivalent intensities) compared with CR (Figure 1B). Accelerometer measures of voluntary physical activity were conducted while the animals were in the metabolic chambers. Morning activity (counts) was used as an index of slowness and were lower in C animals compared with CR (Figure 1C). Total activity was also assessed by double-labeled water measurements. Physical activity levels were used as an index of low activity and were lower in C compared with CR animals (Figure 1D). Metabolic cost of movement analysis was conducted for each animal in the cohort to index poor endurance or exhaustion. C animals had a higher cost of movement compared with CR animals consistent with previous reports (Figure 1E) (27).
Figure 1.
Group comparisons for each component of the Fried phenotype. Open bars represent C animals; closed bars represent CR animals. Data are shown as mean ± standard deviation (SD) Analysis was conducted using unpaired student’s t-tests *p < .05, **p < .01.
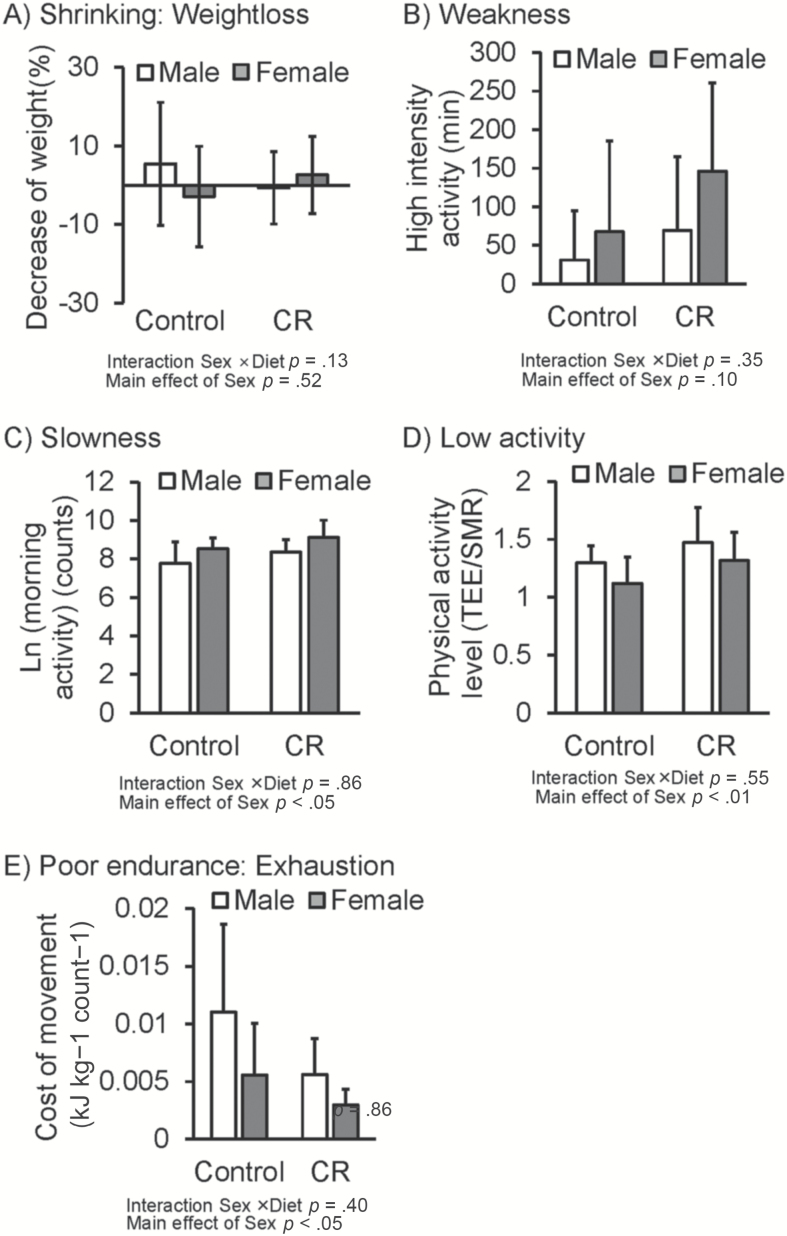
Figure 2 shows group and sex comparisons for each component of the Fried frail phenotype. No significant interaction of diet treatment × sex was found for any of the frail phenotypes (p = .13–.55). There was no significant difference between male and female in weight loss and weakness. Cost of movement was significantly better and morning activity counts was greater in female than male (p < .05). In contrast, total physical activity level was higher in male than female (p < .01).
Figure 2.
Group and sex comparisons for each component of the Fried phenotype. Open bars represent male animals; closed bars represent female animals. Data were analyzed using two-way analysis of covariance (ANCOVA) with diet (C vs CR) and sex (male vs female) as between-subject factors.
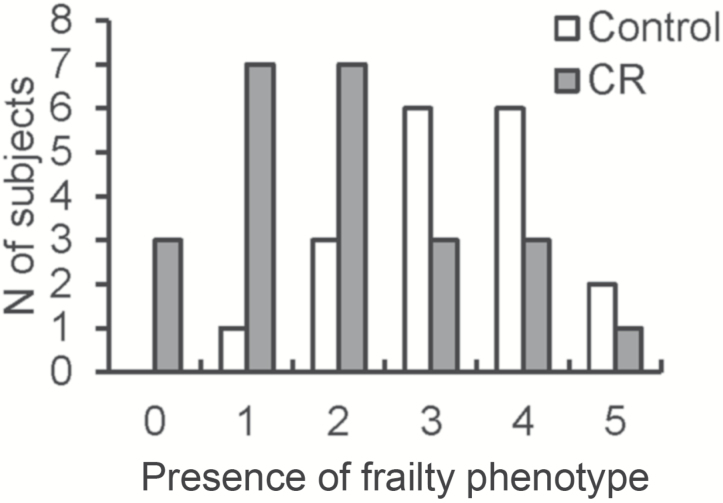
Taking each parameter into consideration, the five indices were summed to derive incidence and severity of frailty for the C and CR groups. There was a higher incidence of frailty phenotype in C compared with CR monkeys (p < .01, Figure 3) as indicated by the shift in distribution of summed frailty phenotypes. Table 2 shows the cross-tabulation table of dietary treatment and frailty. Overall frailty was defined as the assignment of frail for 3 of the 5 individual frailty indices. Although 78% of C were categorized as frail (14/18), only 29% of CR monkeys were categorized as frail (7/24; p < .01).
Figure 3.
The presence of Fried frailty phenotype in C and CR groups. Open bars represent C animals, closed bars represent CR animals. Bars indicate the number of subjects per group presenting with the number of phenotypes identified on the x-axis. Animals with no frailty phenotypes are defined as not frail, 1–2 phenotypes are prefrail, and 3–5 phenotypes are frail. The prevalence of nonfrail, prefrail, and frail between the C and CR groups were compared by chi-square test.
Table 2.
Cross-tabulation Table of Dietary Treatment and Frailty Incidence, N (%)
| Group | Not Frail | Prefrail | Frail | Total | Significance |
|---|---|---|---|---|---|
| Control | 0 (0%) | 4 (22%) | 14 (78%) | 18 | p < .01 |
| CR | 3 (12.5%) | 14 (58.3%) | 7 (29.2%) | 24 |
Discussion
We have previously shown that CR in rhesus monkeys leads to improved health span and life span (16,18). Here, we further define this improvement in health span by evaluating frailty in the same population. We found that animals subjected to long-term, adult-onset CR had less frailty than their age-matched C counterparts for both males and females.
Chronological age, or the number of years an individual has lived, and biological age, or a description of an individual’s stage of development, frequently differ. This difference can be defined as an individual’s degree of fitness or frailty. Frailty is increasingly recognized as a clinically relevant syndrome defining increased vulnerability that may be addressed therapeutically. Any translation of potential frailty treatments from laboratory to clinical practice requires the development and validation of measurable outcomes, as well as sensitive and specific biomarkers of healthy aging that have been validated in experimental animal models of aging.
One such highly relevant animal model of aging is the rhesus monkey. Rhesus monkeys share ~93% sequence identity with the human genome (43,44), and this similarity extends to numerous aspects of their anatomy, physiology, neurology, endocrinology, immunology, and behavior (31). Unlike rodents, rhesus monkeys display patterns of eating and sleeping behavior that mirror those of humans, have a life span measured in decades, and develop and age in similar ways to humans. Unlike in human studies, environment, dietary intake, and medical history can be fully controlled, and studies can be designed to assure comprehensive subject monitoring and strict protocol adherence.
Little work has been done on surrogate outcomes for frailty, and to our knowledge, this is the first study in which frailty in a highly relevant nonhuman primate model of aging has been evaluated. We adapted the Fried frailty phenotype, which allowed us to operationalize frailty as a syndrome. We chose to adapt the Fried phenotype for several reasons. Given the established design of the larger CR and aging study, we had well-controlled, relevant, and consistently measured data to use for each of the five defined components of the Fried phenotype. In contrast, the unlimited nature of the Rockwood index relies heavily on consistent evaluation of all possible outcomes over time. Given the length of this study, the life span of our species of choice and changes in the type, quantity, and quality of clinical evaluation over the course of the study, we could not be fully confident that each animal would have had an equally thorough evaluation.
We found that the CR animals had healthier values for four of the five components that define the Fried phenotype. The only phenotype for which we found no difference was shrinking. Our operational definition of shrinking was an unintentional weight loss of greater than 5% over an approximately 8-year period. Although the C animals did lose more weight than did the CR animals, this failed to reach significance likely due to the high variability in this measurement. For the remaining four measurements; weakness, slowness, low activity, and poor endurance or exhaustion, differences between the C and CR group were in the expected direction. Furthermore, when we looked at the overall presence of frailty phenotypes in our population, we found that 78% of C animals exhibited three or more of the frail phenotypes and were thus considered frail compared with only 29% of CR animals. These analyses combine to show that the CR paradigm delays or prevents frailty in rhesus monkeys.
We found no significant interaction between diet condition and sex for any frailty phenotype, although there were significant sex differences in three of five components of the frailty phenotype. In humans at any age, women have been shown to accumulate more deficits then men (45). However, the effect of long-term CR treatment on healthy life span is positive for both sexes in monkeys and a sex difference is not detected in the impact of CR on measures of mortality or morbidity (16,18).
There are limitations of our study design that should be considered. Given that the CR animals weighed less, but their diet was supplemented by 30% in vitamins and minerals, CR animals received more vitamins and minerals per bodyweight than C animals. This difference might imply a healthier diet in CR animals and may therefore be more likely to prevent frailty. On the other hand, protein and energy intake, known to be inversely correlated with frailty, was lower in the CR compared with C animals. It is important to appreciate that although activity levels are a large component of the phenotypes we evaluated, our animals are cage-living with limited space for physical activity. However, all animals, both C and CR, live in the same caging conditions and therefore have the same limitations for physical activity. Furthermore, these animals have been housed in similar environments for most of their lives and are well adapted to these circumstances. Ideally, this frailty phenotype would be further evaluated in a larger study of nonhuman primates living in large social groupings with access to abundant space for physical activity. In reality, however, the likelihood of accomplishing this is extremely limited, particularly within the context of long-term CR.
As a highly translational model for aging research, the development of an easy to apply and well-validated indicator of overall frailty in rhesus monkeys will facilitate evaluation of aging interventions and potentially move new therapeutic options from laboratory to clinical practice more quickly and efficiently. The demonstration that CR positively impacts indices contributing to the frailty index further supports the utility of this model, as health and survival benefits of the CR paradigm have already been established in this primate species.
Funding
This work was supported by the National Institutes of Health (R01AG07831, P01AG011915, R01AG040178, R01AG047358, P51RR000167, and P51OD011106) and the Japan Society for the Promotion of Science (23-333 and 15H05363 to Y.Y.). This publication was made possible in part by NCRR/ORIP grants P51RR000167/P51OD011106 to the Wisconsin National Primate Research Center, University of Wisconsin-Madison and the use of resources and facilities at the William S. Middleton Memorial Veterans Hospital, Madison, WI.
Acknowledgments
Thanks to animal care, veterinary, and research staff at the WNPRC including Scott Baum and Julie Adriansjach.
References
- 1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi:10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamada M, Arai H. Predictive value of frailty scores for healthy life expectancy in community-dwelling older Japanese adults. J Am Med Dir Assoc. 2015;16:1002.e7–1002.11. doi:10.1016/j.jamda.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 3. Global Health and Aging. National Institute on Aging, National Institutes of Health; 2011. NIH Publication no. 11-7737.
- 4. World Report on Ageing and Health. Luxemborg: World Health Organization. www.who.int. Published 2015. [Google Scholar]
- 5. Richardson A, Fischer KE, Speakman JR, et al. Measures of healthspan as indices of aging in mice—a recommendation. J Gerontol A Biol Sci Med Sci. 2016;71:427–430. doi:10.1093/gerona/glv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arum O, Rasche ZA, Rickman DJ, Bartke A. Prevention of neuromusculoskeletal frailty in slow-aging Ames dwarf mice: longitudinal investigation of interaction of longevity genes and caloric restriction. PLoS One. 2013;8:e72255. doi:10.1371/journal.pone.0072255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feridooni HA, Sun MH, Rockwood K, Howlett SE. Reliability of a frailty index based on the clinical assessment of health deficits in male C57BL/6J mice. J Gerontol A Biol Sci Med Sci. 2015;70:686–693. doi:10.1093/gerona/glu161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kane AE, Hilmer SN, Boyer D, et al. Impact of longevity interventions on a validated mouse clinical frailty index. J Gerontol A Biol Sci Med Sci. 2016;71:333–339. doi:10.1093/gerona/glu315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kane AE, Hilmer SN, Mach J, Mitchell SJ, de Cabo R, Howlett SE. Animal models of frailty: current applications in clinical research. Clin Interv Aging. 2016;11:1519–1529. doi:10.2147/CIA.S105714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu H, Graber TG, Ferguson-Stegall L, Thompson LV. Clinically relevant frailty index for mice. J Gerontol A Biol Sci Med Sci. 2014;69:1485–1491. doi:10.1093/gerona/glt188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whitehead JC, Hildebrand BA, Sun M, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69:621–632. doi:10.1093/gerona/glt136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 13. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi:10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Granic A, Davies K, Jagger C, Kirkwood TB, Syddall HE, Sayer AA. Grip strength decline and its determinants in the very old: longitudinal findings from the Newcastle 85+ Study. PLoS One. 2016;11:e0163183. doi:10.1371/journal.pone.0163183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakamura E, Miyao K. A method for identifying biomarkers of aging and constructing an index of biological age in humans. J Gerontol A Biol Sci Med Sci. 2007;62:1096–1105. [DOI] [PubMed] [Google Scholar]
- 16. Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi:10.1126/science.1173635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci Med Sci. 2008;63:556–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi:10.1038/ncomms4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colman RJ, Ramsey JJ, Roecker EB, Havighurst T, Hudson JC, Kemnitz JW. Body fat distribution with long-term dietary restriction in adult male rhesus macaques. J Gerontol A Biol Sci Med Sci. 1999;54:B283–B290. [DOI] [PubMed] [Google Scholar]
- 20. Kemnitz JW, Weindruch R, Roecker EB, Crawford K, Kaufman PL, Ershler WB. Dietary restriction of adult male rhesus monkeys: design, methodology, and preliminary findings from the first year of study. J Gerontol. 1993;48:B17–B26. [DOI] [PubMed] [Google Scholar]
- 21. Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161:106–118. doi:10.1016/j.cell.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ravussin E, Redman LM, Rochon J, et al. ; CALERIE Study Group A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci. 2015;70:1097–1104. doi:10.1093/gerona/glv057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi:10.1056/NEJM199710023371407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gresl TA, Colman RJ, Roecker EB, et al. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab. 2001;281:E757–E765. [DOI] [PubMed] [Google Scholar]
- 25. Messaoudi I, Warner J, Fischer M, et al. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci USA. 2006;103:19448–19453. doi:10.1073/pnas.0606661103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Colman RJ, Anderson RM. Nonhuman primate calorie restriction. Antioxid Redox Signal. 2011;14:229–239. doi:10.1089/ars.2010.3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamada Y, Colman RJ, Kemnitz JW, et al. Long-term calorie restriction decreases metabolic cost of movement and prevents decrease of physical activity during aging in rhesus monkeys. Exp Gerontol. 2013;48:1226–1235. doi:10.1016/j.exger.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Angulo J, El Assar M, Rodríguez-Mañas L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol Aspects Med. 2016;50:1–32. doi:10.1016/j.mam.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 29. Ramsey JJ, Colman RJ, Binkley NC, et al. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35:1131–1149. [DOI] [PubMed] [Google Scholar]
- 30. Ramsey JJ, Roecker EB, Weindruch R, Kemnitz JW. Energy expenditure of adult male rhesus monkeys during the first 30 mo of dietary restriction. Am J Physiol. 1997;272(5 Pt 1):E901–E907. [DOI] [PubMed] [Google Scholar]
- 31. Colman RJ, Kemnitz JW. Aging experiments using nonhuman primates. In: Yu, BP, ed. Methods in Aging Research. Boca Raton, FL: CRC Press; 1998:249–267. [Google Scholar]
- 32. Raman A, Baum ST, Colman RJ, Kemnitz JW, Weindruch R, Schoeller DA. Metabolizable energy intake during long-term calorie restriction in rhesus monkeys. Exp Gerontol. 2007;42:988–994. doi:10.1016/j.exger.2007.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raman A, Ramsey JJ, Kemnitz JW, et al. Influences of calorie restriction and age on energy expenditure in the rhesus monkey. Am J Physiol Endocrinol Metab. 2007;292:E101–E106. doi:10.1152/ajpendo.00127.2006 [DOI] [PubMed] [Google Scholar]
- 34. Blanc S, Schoeller D, Kemnitz J, et al. Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J Clin Endocrinol Metab. 2003;88:16–23. doi:10.1210/jc.2002-020405 [DOI] [PubMed] [Google Scholar]
- 35. Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jéquier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol. 1986;250(5 Pt 2):R823–R830. [DOI] [PubMed] [Google Scholar]
- 36. Coward WA, Prentice AM. Isotope method for the measurement of carbon dioxide production rate in man. Am J Clin Nutr. 1985;41:659–663. [DOI] [PubMed] [Google Scholar]
- 37. Puyau MR, Adolph AL, Vohra FA, Zakeri I, Butte NF. Prediction of activity energy expenditure using accelerometers in children. Med Sci Sports Exerc. 2004;36:1625–1631. [PubMed] [Google Scholar]
- 38. Schutz Y, Bessard T, Jéquier E. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am J Clin Nutr. 1984;40:542–552. [DOI] [PubMed] [Google Scholar]
- 39. Usui C, Ando T, Ohkawara K, et al. Validity and reproducibility of a novel method for time-course evaluation of diet-induced thermogenesis in a respiratory chamber. Physiol Rep. 2015;3:1–13. doi:10.14814/phy2.12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ravussin E, Burnand B, Schutz Y, Jéquier E. Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. Am J Clin Nutr. 1982;35:566–573. [DOI] [PubMed] [Google Scholar]
- 41. Rezende EL, Kelly SA, Gomes FR, Chappell MA, Garland T., Jr Effects of size, sex, and voluntary running speeds on costs of locomotion in lines of laboratory mice selectively bred for high wheel-running activity. Physiol Biochem Zool. 2006;79:83–99. doi:10.1086/498187 [DOI] [PubMed] [Google Scholar]
- 42. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi:10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 43. Rhesus Macaque Genome Sequencing and Analysis Consortium , Analysis C, Gibbs RA, Rogers J, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi:10.1126/science.1139247 [DOI] [PubMed] [Google Scholar]
- 44. Zimin AV, Cornish AS, Maudhoo MD, et al. A new rhesus macaque assembly and annotation for next-generation sequencing analyses. Biol Direct. 2014;9:20. doi:10.1186/1745-6150-9-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi:10.1111/j.1532-5415.2005.00506.x [DOI] [PubMed] [Google Scholar]