Highlights
-
•
LINAC-based frameless radiosurgery shows favorable local control.
-
•
18–20 Gy were delivered as single fraction.
-
•
No treatment related side effects ≥grade 2 were observed.
-
•
SRS and deferred WBRT remain salvage therapies for distant intracranial relapse.
-
•
Extracranial stable disease and GTV ≤ 2.5 cm3 were significant predictors of OS.
Keywords: Brain metastases, Radiosurgery, LINAC
Abbreviations: LINAC, Linear Accelerator; SRS, Stereotactic radiosurgery; IGRT, Image-Guided Radiotherapy; PFS, progression-free survival; LC, Local Control; OS, Overall Survival; GTV, Gross Tumor Volume; PTV, planning target volume; CTCAE, Common Terminology Criteria for Adverse Events v4.0; WBRT, Whole Brain Radiotherapy; DC, distant intracranial tumor control; CT, computed tomography; CBCT, cone-beam CT; DRR, digitally reconstructed radiographs; RPA, recursive partitioning analysis
Abstract
Introduction
Stereotactic radiosurgery (SRS) has been increasingly advocated for 1–3 small brain metastases. The goal of this study was to evaluate the clinical results in patients with brain metastases treated with LINAC-based SRS using a thermoplastic mask (non-invasive fixation system) and Image-Guided Radiotherapy (IGRT).
Material and Methods
In this single-institution study 48 patients with 77 brain metastases were treated between February 2012 and January 2014. The prescribed dose was 20 Gy or 18 Gy as a single fraction. SRS was performed with a True Beam STX Novalis Radiosurgery LINAC (Varian Medical Systems). The verification of positioning was done using the BrainLAB ExacTrac ® X-ray 6D system and cone-beam CT.
Results
In 69 of 77 treated brain metastases (90%) the follow-up was documented on MR imaging performed every 3 months. Mean follow-up time was 10.86 months. Estimated 1-year local control was 83%, using the Kaplan-Meier method. In 7/69 brain metastases (10%) local failure (LF) was diagnosed. Median progression free survival (PFS) was 3.73 months, largely due to distant brain relapse. A GTV of ≤2.0 cm3 was significantly associated with a better PFS than a GTV >2.0 cm3. Extracranial stable disease and GTV ≤2.5 cm³ were significant predictors of OS.
We observed 2 cases of radiation necrosis diagnosed by histology after surgical resection. No other cases of severe side effects (CTACE ≥ 3) were observed.
Conclusion
LINAC-based frameless SRS with the BrainLAB Mask using the BrainLAB ExacTrac ® X-ray 6D system for patient positioning is well tolerated, safe and leads to favorable crude local control of 90%. In our experience, local control after frameless (ringless) SRS is as good as ring-based SRS reported in literature. Without invasive head fixation, radiotherapy is more comfortable for patients.
Introduction
Brain metastases have a high incidence of up to 65% during the disease trajectory, dependent on primary tumor type, and can cause significant morbidity and mortality [1], [2]. Stereotactic radiosurgery (SRS) has become a standard of care over the years in patients with 1–3 small brain metastases [3], [4], [5], [6]. It is effective regarding in-field control and patients who are treated with it, will develop less side effects compared with patients who are treated with whole brain radiotherapy (WBRT), especially concerning neurocognition [7]. It can also be used to salvage patients who underwent WBRT as primary therapy [8], [9], [10]. Local control after SRS ranges between 70% to 90% [11], [12], [13], [14].
In patients treated with linear accelerator (LINAC)-based SRS the head immobilization was generally realized by invasive head fixation using a stereotactic ring, at least during the first decades. Due to the development of LINACs with image-guided radiotherapy (IGRT) capability, the head positioning can now be accomplished with a thermoplastic mask. The advantage of the mask is that it is more convenient for the patients, is not invasive and the treatment planning can be done one or more days before the delivery of the treatment.
In this retrospective single-institutional study we investigated local control (LC) as a primary endpoint in patients undergoing SRS for brain metastases. Intracranial progression-free survival (PFS), distant intracranial tumor control (DC), overall survival (OS) and side effects were secondary endpoints.
Material and Methods
Patient characteristics
In this single-institution study 48 patients harboring 77 brain metastases were treated by SRS between February 2012 and January 2014. The patient characteristics are summarized in Table 1.
Table 1.
Patients’ characteristics (others: bladder carcinoma, hypopharynx carcinoma, oropharynx carcinoma, colon carcinoma, unknown primary).
| Number of Patients | 48 | |
| Number of treated Brain metastases | 77 | |
| Number of brain metastases at the first SRS | ||
| n = 1 | 10 | 20.8% |
| n = 2 | 11 | 22.9% |
| n = 3 | 6 | 12.5% |
| n = 4 | 8 | 16.7% |
| n ≥ 5 | 13 | 27.1% |
| Local Failures | 7/69 | 10% |
| Age | ||
| Median | 58 | |
| Range | 22–79 | |
| Gender | ||
| Male | 25 | |
| Female | 23 | |
| Primary tumor site | ||
| NSCLC | 27 | 56.3% |
| Melanoma | 9 | 18.8% |
| Breast cancer | 7 | 14.6% |
| Others | 5 | 10.4% |
| Extracranial Metastases | ||
| Yes | 36 | 75.0% |
| No | 12 | 25.0% |
| Extracranial stable disease | ||
| Yes | 18 | 37.5% |
| No | 30 | 62.5% |
| RPA classification | ||
| I | 2 | 4.3% |
| II | 45 | 93.8% |
| III | 1 | 2.1% |
Median age was 58 years (range 22 to 79 years), 25 (52%) men and 23 (48%) women were included. The most common primary tumors were non-small-cell lung cancer, malignant melanoma and breast cancer (Table 1). Extracerebral metastases were found in 36/48 patients (75%). Twelve of 48 patients (25%) had only brain metastases. Eighteen of 48 patients (37.5%) had stable extracranial disease and 30/48 patients (62.5%) were staged as having progressive disease.
The present work complies with the principles laid down in the Declaration of Helsinki and was approved by the appropriate ethical committees in the institution in which it was performed.
Radiation therapy
Radiotherapy planning was based on computed tomography (CT) with 2 mm slice thickness in the early phase of the study and 1 mm afterwards, i.e. when most patients were treated. Patients were immobilized in a thermoplastic mask (BrainLAB, Feldkirchen, Germany), as shown in Fig. 1.
Fig. 1.
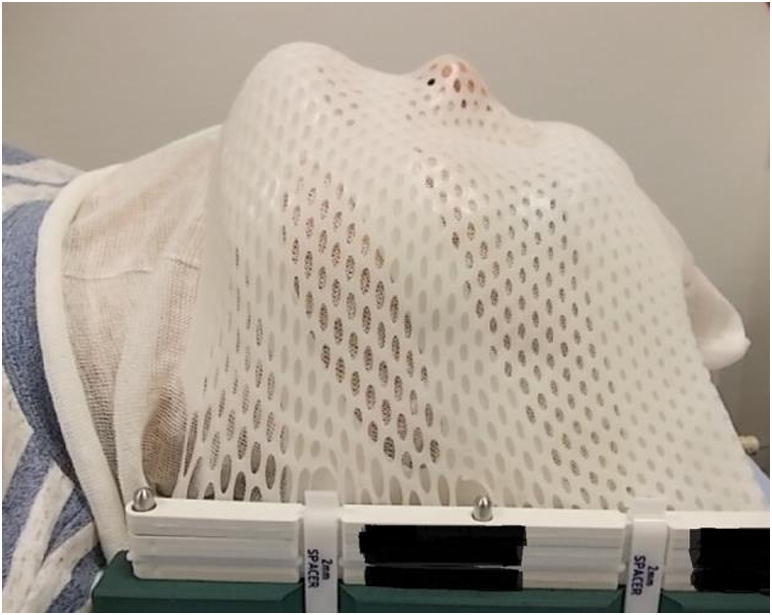
BrainLAB mask for patient positioning.
The gross tumor volume (GTV) for each brain metastasis was defined on gadolinium enhanced magnetic resonance imaging (GdT1-MRI, Siemens ESPREE, Siemens Medical Systems, Erlangen, Germany) with 1 mm slice thickness (MP-Rage sequence) [15]. For each patient a planning CT and an MRI were performed at the same day. The radiosurgery was performed two days after the planning CT/MRI. Treatment planning was performed with iPlan RT Image 4.1.1 (BrainLab, Feldkirchen, Germany). The planning target volume (PTV) was defined as GTV plus 1 mm. Irradiation dose was prescribed to ensure coverage of at least 80% of the PTV with the prescription dose. The target volume covered 100% of the prescribed dose; the allowed maximum dose in the PTV was 125%. The prescribed dose was 20 Gy or 18 Gy (80% isodose) as a single dose. The dose of 18 Gy was used when the brain metastases were located within or next to the brain stem. SRS was performed with a True Beam STX Novalis Radiosurgery LINAC with 6 MeV photons. Patient positioning and positioning verification was done using the BrainLAB ExacTrac® X-ray 6D system and subsequent cone-beam CT (CBCT). Using infrared markers, which are detected by a camera system, the patient was moved automatically into treatment position. First, two radiographs were taken registered to digitally reconstructed radiographs (DRR) calculated from the CT used for treatment planning. From this information the deviation of the patient’s actual position from his/her intended value was computed and the corresponding shift was applied. To test the correctness of the positioning procedure a cone-beam CT scan was performed and the resulting dataset was registered with the planning CT.
Follow-up
Patient follow-up after SRS included MRI and physical examination at 6–8 weeks. Subsequent follow-up was every 3 months. Local failure was defined as an increase of the maximum diameter of the treated brain metastasis. RANO criteria were used. Distant intracranial failure was defined as new brain metastases or leptomeningeal enhancement outside the previously irradiated volume. The primary endpoint for this study was local control (LC). LC was evaluated for each treated brain metastasis. Secondary endpoints included distant intracranial tumor control (DC), intracranial progression-free survival (PFS), overall survival (OS) and side effects after SRS. PFS was defined as time to local failure, intracranial progression or death.
Statistical methods
LC, PFS and OS were measured using the Kaplan–Meier method. All analyses were performed with SPSS 22.0.0.0 (IBM, New York, USA) and R-Statistics [16]. Cox regression and Log Rank test were used to analyze subgroups of patients concerning LC, DC and OS. A competing risk analysis was performed with R-Statistics.
Several variables (recursive partitioning analysis (RPA) classification, GTV, gender, extracerebral metastases) were evaluated as possible predictors of outcome. GTV size was dichotomized not only by median, but also by other cut-offs in order to identify the most relevant definition of small or large, i.e. the cut-off with biggest separation of the Kaplan-Meier curves.
Theory/calculation
As brain metastases can lead to significant morbidity and mortality efficient and safe treatment becomes more and more important. In this work we wanted to present our data in a single center with LINAC-based frameless radiosurgery. We included all patients treated by radiosurgery from February 2012 to January 2014 and performed a retrospective analysis.
Results
Patient characteristics
Patient characteristics are described in Materials and Methods and listed in Table 1. At the time of the first SRS only 10 patients (21%) had a single brain metastasis. Thirty-eight (79%) patients had two or more brain metastases. Thirteen (27%) patients had n ≥ 5 brain metastases.
Treatment parameters
Treatment parameters are listed in Table 2.
Table 2.
Treatment Parameters.
| (n) | % | |
|---|---|---|
| Patients | 47 | |
| Treated brain metastases | 77 | |
| Dose | ||
| 20 Gy | 75 | 97.4 |
| 18 Gy | 2 | 2.6 |
| Median GTV | 0.4 cm2 | |
| Range 0.1 cm2 to 6.9 cm2 | ||
| ≤2.0 cm2 | 63 | 81.8 |
| >2 cm2 | 14 | 18.2 |
| WBRT before SRS | 9 | 18.8 |
| WBRT after SRS | 12 | 24 |
Most of the brain metastases were treated with a single dose of 20 Gy (75 brain metastases, 97.4%). Two of 77 brain metastases (3%) were treated with a dose of 18 Gy. The median GTV was 0.4 cm3 (average 1.06 cm3; range 0.1 cm3 to 6.8 cm3). Sixty-three of 77 brain metastases (82%) were ≤ 2.0 cm3, 14/77 metastases (18.2%) had a size between 2.1 cm3 and 6.8 cm3.
Nine of 47 patients (19%) had WBRT before SRS. The median time between WBRT and SRS was 17 months (range 8–33 months). In 12/47 (24%) patients we performed WBRT as a salvage therapy in case of intracranial progression after SRS. The median time to salvage therapy was 6 months (range 1–12 months).
Local and distant control
Mean follow-up time was 10.9 months (range 1–43 months). Local control was estimated for every single treated brain metastasis of each patient. To evaluate local control, patients were censored when they died. Because some patients were treated with more than one SRS (competing risks), in case of death all brain metastases of the respective patient were censored in order to analyze every treated metastasis separately. A competing risk analysis was performed (s. Fig. 2), and the risk of death was higher than the risk of local progression.
Fig. 2.

Competing Risks: Patients have a higher risk to die than to develop local recurrence.
Estimated 1-year local control was 83%, using the Kaplan-Meier method (Fig. 3). In 7/69 brain metastases (10%) local failure was detected on MRI. Median time to local failure was 4.8 months (range 1–14 months). In 4 cases of local recurrence on MRI the diagnosis was established by imaging follow-up. Two brain metastases with local failure were resected. The third patient was treated with SRS after biopsy confirmed local recurrence.
Fig. 3.
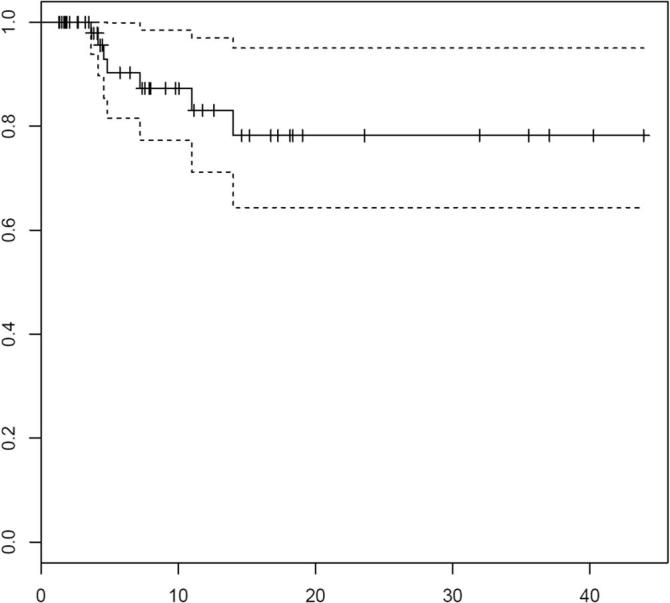
LC, Local control for 69 metastases treated with SRS (for 8 metastases no imaging follow-up was available.) The dash lines represent 95% confidence intervals. Tick marks represent censoring times/events.
Women (41.4 vs. 24.5 months in men, p = .011) and patients with a GTV ≤ 2.0 cm3 (38.4 vs. 8.9 months, p = .001) had a longer time without local failure. In the multivariate analysis only small (≤2.0 cm3) GTV remained significant (p = .037).
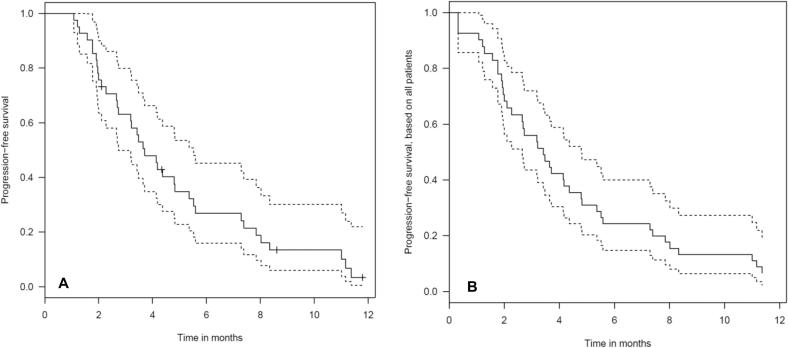
Progression-free survival
Median intracranial progression-free survival (PFS) was 3.73 months (range 1–17 months, 95% CI 3.35–5.28 months), Fig. 4. A histology of breast cancer was associated with significantly worse PFS (p = .004, median PFS 2.27 vs. 4.37 months). We observed a trend towards worse PFS in patients with lung cancer (p = .069). A GTV of ≤ 2.0 cm3 was significantly associated with a better PFS than a GTV > 2.0 cm3 (median PFS 4.38 vs. 3.19 months, p = .038).
Fig. 4.
A. Progression-free intracranial survival for 41 patients treated with SRS (considering the first treated metastasis, seven patients had no imaging follow-up). The dash lines represent 95% confidence intervals. Tick marks represent censoring times/events. B Progression-free intracranial survival for all 48 patients treated with SRS (Interval censored). The dash lines represent 95% confidence intervals. Tick marks represent censoring times/events.
In the multivariate Cox regression test GTV > 2.0 cm3 (p = .009) and breast cancer (p = .001) were associated with a worse PFS.
Overall survival
Thirty-three of 48 patients died during follow-up. The median survival was 8 months (95% CI 2.82–13.11; range 0.66 months to 45 months). The estimated 1-year survival rate was 35.6% using the Kaplan-Meier method (Fig. 5). Fig. 6
Fig. 5.
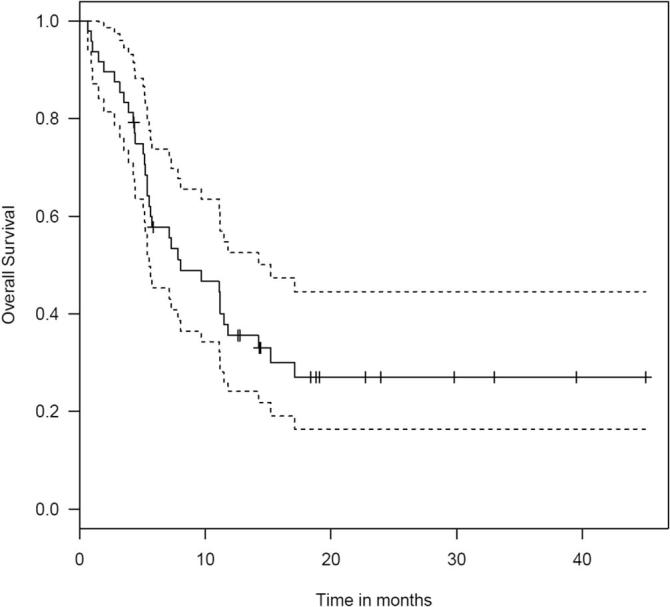
OS, Overall survival in 48 patients treated with frameless SRS. The dash lines represent 95% confidence intervals. Tick marks represent censoring times/events.
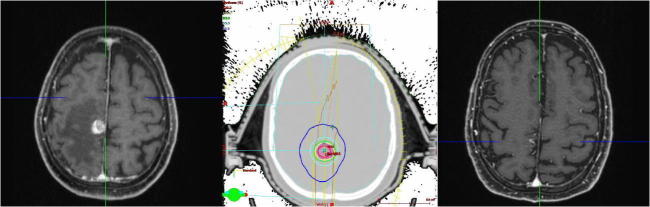
Fig. 6.
One patient treated by SRS, left: MRI before treatment, large metastasis with edema, the patient had a motoric weakness, right: MRI after treatment, there is only a small residuum of the metastasis left, the patient‘s weakness was much better, middle: conformal SRS treatment plan.
In the univariate analysis the following variables were associated with a better survival: age ≤ 58 years (median OS 15.2 vs. 7.2 months, p = .031), GTV ≤ 2.5 cm3 (median OS 11.2 months vs. 5.1 months, p = .005) and stable disease outside the brain (median OS 27 vs. 8.6 months, p = .006). For those variables a multivariate analysis was performed. Extracranial stable disease (p = .015) and GTV ≤ 2.5 cm3 (p = .012) were still significant.
The general practitioners of the patients were contacted in order to obtain the cause of death. Three patients (9%) died because of their cerebral metastases and 3 further patients (9%) died most likely because of their cerebral metastases. In eighteen patients (55%) the cause of death were not the brain metastases, in 3 patients the general practitioners did not know the reason for the patients’ death and in 6 cases we did not receive any reply.
In 46 patients we had information about the extracerebral follow-up. Forty (87%) had progressive disease, 5 patients (11%) were stable and one patient had a complete remission.
Side effects
During follow-up side effects were reported using the Common Terminology Criteria for Adverse Events v4.0 (CTCAE).
All patients tolerated frameless SRS well with no severe side effects. Only fatigue was reported as acute side effect. We observed two confirmed cases of radionecrosis. In one additional case there was the suspicion of a radionecrosis on MRI follow-up. Even in patients who underwent WBRT before radiosurgery was performed or in patients who underwent WBRT during follow-up as salvage therapy after SRS no severe side effects were seen.
Discussion
In our experience local control after frameless SRS was as good as the previous strategy of frame-based SRS reported in literature. In a study by Ramakrishna et al. [17] an overall system accuracy similar to the invasive frame-based SRS was reported. An advantage of frameless SRS is that the thermoplastic mask is tolerated better by the patients.
In a study by Becker et al. local control rates for brain metastases at 6 and 12 months of 74% and 61% were reported after frame-based LINAC radiosurgery [18]. In the randomized trial reported by Kocher et al. 90 patients were treated with radiosurgery alone and 61 of them had single brain metastases [19]. Local control rates were approximately 75% at 12 months (estimated from the published Kaplan-Meier curves) and 69% at 24 months. In a meta-analysis that also included the Kocher et al. data 186 patients had received radiosurgery, of whom 60% had single brain metastases [20]. Local control was reported in 80%. Local control in our study was 90%. Estimated 1-year local control was 83% using the Kaplan-Meier method. An intrinsic selection bias is possible as we performed a retrospective single institution study but we had similar results as reported in literature. In the study by Breneman et al. local control with a frameless system was 80% at one year [11]. In other institutions reporting their clinical experience with frameless radiosurgery crude rate for local control was 88% and 90% [21], [22] (s. Table 3).
Table 3.
Literature overview on LINAC based radiosurgery.
| Author | Year | Patients (n) | Dose (Gy) | Number of Fractions | 1-year Local Control (%) | Crude Local Rate (%) | 1-year OS (%) | Median OS (months) |
|---|---|---|---|---|---|---|---|---|
| Breneman | 2009 | 53 | 12–22 | 1 | 80 | Not available | 44 | Not available |
| Bilger | 2016 | 47 | 18–20 | 1 | 83.1 | 90 | 36 | 8 |
| Chen | 2009 | 54 | 14–20 | 1 | 80 | 90 | 28 | 8.6 |
| Kamath | 2005 | 17 | 12.5–20 | 1 | Not available | 86 | Not available | 7.1 |
| Nath | 2010 | 65 | 14–22 | 1 | 76 | 88 | 40 | Not available |
| Minniti | 2011 | 102 | 16–20 | 1 | 91 | 91 | 58% | 15.5 |
Compared with radiosurgery performed with Cyberknife we found similar outcomes concerning local control [23], [24]. With the new Gammaknife icon no invasive head fixation is needed anymore [25]. An advantage of the LINAC-based technique is that the duration of treatment is shorter. For many, especially smaller, departments, the increased complexity with equipment from several different vendors is not desirable. The Gammaknife uses a cobalt radiation source and for the patient positioning a stereotactic frame is used. In our study SRS was performed with a True Beam STX Novalis Radiosurgery LINAC with 6 MeV photons. The accuracy of the BrainLab Mask was reported before [26]. Using infrared markers, which are detected by a camera system, the patient is moved automatically into treatment position. During our early experience we tested the correctness of the positioning procedure with a cone-beam CT scan and registered the resulting dataset with the planning CT. The accuracy results of the ExacTrac® X-ray 6D suggest that no additional CBCT is needed in the future. The radiosurgery was performed 2 days after planning CT/MRI. Even complex plans can be calculated and realized by the physicists in this period. An invasive fixation would not be tolerated for 3 days and would lead to a higher infection risk.
In our analysis it might be possible that local control is overestimated because of the statistical problem of competing risks, which means that death from extracranial progression resulted in censoring in the Kaplan-Meier curve. In a competing risk analysis patients had a higher risk to die than to have local recurrence. In case of local recurrence there was no histology obtained. Only in 2 patients with suspected radionecrosis we obtained a histology which confirmed radionecrosis. Another patient had the suspicion of radionecrosis on MRI but we did not perform an operation to confirm radionecrosis. It is possible that occasional patients with “local failures” had a radionecrosis instead because we did not always obtain histology.
In our data the histology of breast cancer was associated with significantly worse PFS, but no effect on overall survival was seen. This might be a selection bias as we had a small sample size. In literature patients with the histology of breast cancer have better survival [27].
In 9/47 patients (19%) WBRT had been performed before SRS. In these cases radiosurgery was a salvage strategy for symptomatic or new brain lesions. This explains that 13 patients (27%) had n ≥ 5 brain metastases when the first SRS was performed.
Diagnosis of new brain metastases was common. Deferred WBRT remains a possible salvage therapy. In 24% of our patients salvage WBRT was performed. No additional serious side effects were seen in case of salvage treatment.
Median OS was 8 months. According to the RPA classification median OS was between 2.3 and 7.1 months for patients largely treated with primary WBRT [28]. In our analysis most patients were in RPA class II (94%). GTV ≤ 2.5 cm3 and stable disease outside the brain were significant predictors of better OS. Also in the literature extracranial disease status is a well-known prognostic factor for survival [29], [30].
Radiosurgery is a well-tolerated and short treatment [31]. No systemic treatment break is needed in case of extracranial progressive disease. This might increase the patients’ chance of extracranial response.
Conclusion
LINAC-based frameless SRS with the BrainLAB Mask using the BrainLAB ExacTrac® X-ray 6D system for patient positioning is well tolerated, patient-friendly, safe and leads to favorable local control (90%, crude local control).
In some patients SRS was performed as salvage treatment after WBRT or WBRT was performed during follow-up after SRS in case of progression. In a large number of patients after the first radiosurgical treatment repeated SRS in case of progression was performed. No additional side effects were seen.
Compared to other technologies, little changes of departmental complexity, workflow and expertise are necessary. Failures in non-treated brain regions are a generally known drawback of any SRS approach. However, they can be salvaged, e.g., by WBRT or repeat SRS.
Ethics approval and consent to participate
The present work complies with the principles laid down in the Declaration of Helsinki and was approved by the appropriate ethical committees in the institution in which it was performed. All patients were enrolled after giving informed consent.
Consent for publication
Not applicable. Consent for publication has been obtained from the respective person concerning Fig.1.
Availability of data and Materials
Data available on request from the authors.
Competing interests
The authors declare that they have no competing interests.
Funding
No.
Authors’ contributions
AB and FF acquired and analyzed data. AB wrote the manuscript. CN, DM and OO helped to draft the manuscript. RW and VP provided technical assistance and ALG provided clinical supervision. ALG defined the study design. All authors revised the manuscript and then approved the final manuscript.
Disclosure
The authors state that they have not published or submitted the manuscript elsewhere.
Acknowledgements
The authors thank Dr. Gerta Rücker for statistical advice. The present study was supported by the German Cancer Consortium (DKTK).
Contributor Information
Angelika Bilger, Email: angelika.bilger@uniklinik-freiburg.de.
Florian Frenzel, Email: florian.frenzel1988@t-online.de.
Oliver Oehlke, Email: oliver.oehlke@uniklinik-freiburg.de.
Rolf Wiehle, Email: rolf.wiehle@uniklinik-freiburg.de.
Dusan Milanovic, Email: dusan.milanovic@ngh.nhs.uk.
Vesna Prokic, Email: prokic@hs-koblenz.de.
Carsten Nieder, Email: carsten.nieder@nordlandssykehuset.no.
Anca-Ligia Grosu, Email: anca.grosu@uniklinik-freiburg.de.
References
- 1.Tsao M.N., Lloyd N., Wong R.K.S., Chow E., Rakovitch E., Laperriere N. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2012;(4):CD003869. doi: 10.1002/14651858.CD003869.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin. 2003 21(1):1–23, vii. [DOI] [PubMed]
- 3.Ahluwalia MS, Vogelbaum MV, Chao ST, Mehta MM. Brain metastasis and treatment. F1000prime Rep. 2014;6:114. [DOI] [PMC free article] [PubMed]
- 4.Wen PY, Loeffler JS. Management of brain metastases. Oncol Williston Park N. 1999; 13(7):941–54, 957–961–962, 9. [PubMed]
- 5.Ueki K., Matsutani M., Nakamura O., Tanaka Y. Comparison of whole brain radiation therapy and locally limited radiation therapy in the treatment of solitary brain metastases from non-small cell lung cancer. Neurol Med Chir (Tokyo). 1996 Jun;36(6):364–369. doi: 10.2176/nmc.36.364. [DOI] [PubMed] [Google Scholar]
- 6.Rades D., Huttenlocher S., Hornung D., Blanck O., Schild S.E. Radiosurgery alone versus radiosurgery plus whole-brain irradiation for very few cerebral metastases from lung cancer. BMC Cancer. 2014;14:931. doi: 10.1186/1471-2407-14-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linskey M.E., Andrews D.W., Asher A.L., Burri S.H., Kondziolka D., Robinson P.D. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010 Jan;96(1):45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurtz G., Zadeh G., Gingras-Hill G., Millar B.-A., Laperriere N.J., Bernstein M. Salvage radiosurgery for brain metastases: prognostic factors to consider in patient selection. Int J Radiat Oncol Biol Phys. 2014 Jan 1;88(1):137–142. doi: 10.1016/j.ijrobp.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Yomo S., Hayashi M. The efficacy and limitations of stereotactic radiosurgery as a salvage treatment after failed whole brain radiotherapy for brain metastases. J Neurooncol. 2013 Jul;113(3):459–465. doi: 10.1007/s11060-013-1138-y. [DOI] [PubMed] [Google Scholar]
- 10.Kocher M, Wittig A, Piroth MD, Treuer H, Seegenschmiedt H, Ruge M, et al. Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol Organ Dtsch Röntgenges Al. 2014; 190(6):521–32. [DOI] [PubMed]
- 11.Breneman J.C., Steinmetz R., Smith A., Lamba M., Warnick R.E. Frameless image-guided intracranial stereotactic radiosurgery: clinical outcomes for brain metastases. Int J Radiat Oncol Biol Phys. 2009 Jul 1;74(3):702–706. doi: 10.1016/j.ijrobp.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Varlotto J.M., Flickinger J.C., Niranjan A., Bhatnagar A.K., Kondziolka D., Lunsford L.D. Analysis of tumor control and toxicity in patients who have survived at least one year after radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2003 Oct 1;57(2):452–464. doi: 10.1016/s0360-3016(03)00568-6. [DOI] [PubMed] [Google Scholar]
- 13.Pirzkall A., Debus J., Lohr F., Fuss M., Rhein B., Engenhart-Cabillic R. Radiosurgery alone or in combination with whole-brain radiotherapy for brain metastases. J Clin Oncol Off J Am Soc Clin Oncol. 1998 Nov;16(11):3563–3569. doi: 10.1200/JCO.1998.16.11.3563. [DOI] [PubMed] [Google Scholar]
- 14.Bhatnagar A.K., Flickinger J.C., Kondziolka D., Lunsford L.D. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006 Mar 1;64(3):898–903. doi: 10.1016/j.ijrobp.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Brant-Zawadzki M., Gillan G.D., Nitz W.R. MP RAGE: a three-dimensional, T1-weighted, gradient-echo sequence–initial experience in the brain. Radiology. 1992 Mar 1;182(3):769–775. doi: 10.1148/radiology.182.3.1535892. [DOI] [PubMed] [Google Scholar]
- 16.R: The R Project for Statistical Computing [Internet]. [cited 2017 May 17]. Available from: https://www.r-project.org/.
- 17.Ramakrishna N., Rosca F., Friesen S., Tezcanli E., Zygmanszki P., Hacker F. A clinical comparison of patient setup and intra-fraction motion using frame-based radiosurgery versus a frameless image-guided radiosurgery system for intracranial lesions. Radiother Oncol. 2010 Apr;95(1):109–115. doi: 10.1016/j.radonc.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Becker G., Jeremic B., Engel C., Buchgeister M., Paulsen F., Duffner F. Radiosurgery for brain metastases: the Tuebingen experience. Radiother Oncol. 2002 Feb;62(2):233–237. doi: 10.1016/s0167-8140(01)00496-0. [DOI] [PubMed] [Google Scholar]
- 19.Kocher M., Soffietti R., Abacioglu U., Villà S., Fauchon F., Baumert B.G. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahgal A., Aoyama H., Kocher M., Neupane B., Collette S., Tago M. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol. 2015;91(4):710–717. doi: 10.1016/j.ijrobp.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Kamath R., Ryken T.C., Meeks S.L., Pennington E.C., Ritchie J., Buatti J.M. Initial clinical experience with frameless radiosurgery for patients with intracranial metastases. Int J Radiat Oncol Biol Phys. 2005 Apr 1;61(5):1467–1472. doi: 10.1016/j.ijrobp.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Chen J.C.T., Bugoci D.M., Girvigian M.R., Miller M.J., Arellano A., Rahimian J. Control of brain metastases using frameless image-guided radiosurgery. Neurosurg Focus. 2009 Dec;27(6):E6. doi: 10.3171/2009.8.FOCUS09131. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z., Yuan Z., Zhang W., You J., Wang P. Brain metastasis treated with Cyberknife. Chin Med J (Engl). 2009 Aug 20;122(16):1847–1850. [PubMed] [Google Scholar]
- 24.Alongi F., Fiorentino A., Mancosu P., Navarria P., Levra N.G., Mazzola R. Stereotactic radiosurgery for intracranial metastases: linac-based and gamma-dedicated unit approach. Expert Rev Anticancer Ther. 2016 Jul 2;16(7):731–740. doi: 10.1080/14737140.2016.1190648. [DOI] [PubMed] [Google Scholar]
- 25.Wowra B., Muacevic A., Tonn J.-C. CyberKnife radiosurgery for brain metastases. 2012;25:201–209. doi: 10.1159/000331193. [DOI] [PubMed] [Google Scholar]
- 26.Minniti G., Scaringi C., Clarke E., Valeriani M., Osti M., Enrici R.M. Frameless linac-based stereotactic radiosurgery (SRS) for brain metastases: analysis of patient repositioning using a mask fixation system and clinical outcomes. Radiat Oncol Lond Engl. 2011;6:158. doi: 10.1186/1748-717X-6-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cagney D.N., Martin A.M., Catalano P.J., Redig A.J., Lin N.U., Lee E.Q. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro-Oncol. 2017 doi: 10.1093/neuonc/nox077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaspar L., Scott C., Rotman M., Asbell S., Phillips T., Wasserman T. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997 Mar 1;37(4):745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 29.Park Y.H., Kim T.H., Jung S.-Y., Kim Y.-E., Bae J.-M., Kim Y.-J. Combined primary tumor and extracranial metastasis status as constituent factor of prognostic indices for predicting the overall survival in patients with brain metastases. J Korean Med Sci. 2013 Feb;28(2):205–212. doi: 10.3346/jkms.2013.28.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieder C., Hintz M., Grosu A.L. Predicted survival in patients with brain metastases from colorectal cancer: is a current nomogram helpful? Clin Neurol Neurosurg. 2016 Apr;143:107–110. doi: 10.1016/j.clineuro.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Nieder C., Grosu A.L., Gaspar L.E. Stereotactic radiosurgery (SRS) for brain metastases: a systematic review. Radiat Oncol. 2014 Jul 12;9(1):155. doi: 10.1186/1748-717X-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.