Abstract
Objective
Using a simple simulation, we illustrate why associations estimated from studies restricted to preterm births cannot be interpreted causally.
Design, Setting, and Population
Data simulation involving a hypothetical cohort of fetuses who may be healthy or have one or more of four pathological factors (termed A through D, increasing in severity) with known effects on gestational length and risk of mortality. We focus on babies born ≤32 weeks of gestation.
Methods
We visually represent the simulated population and compare the association between A (which may represent preeclampsia) and neonatal death. We then repeat the exercise with D (standing in for chorioamnionitis) as the exposure of interest.
Main Outcome Measures
Odds ratios of neonatal death in the simulated data.
Results
In most weeks, and for both A and D, the calculated odds ratios are substantially biased and underestimate the true risk of neonatal death associated with each pathology. For example, factor A has a true causal odds ratio of 1.50, yet it appears protective among births ≤32 weeks (estimated crude odds ratio = 0.39; gestational age-adjusted odds ratio = 0.71).
Conclusions
Among very preterm births, virtually all babies are born with pathologies that increase risk of adverse outcomes. Thus, babies exposed to one factor (e.g., preeclampsia) are compared with babies who have a mix of other pathologies. Such selection bias affects studies carried out among very preterm births (e.g., where preeclampsia appears to reduce risk of neonatal outcomes).
Keywords: Causal inference, neonatal networks, perinatal epidemiology, preterm birth
Introduction
Preterm birth etiology is complex and multi-factorial,1 and a large proportion of preterm births have no known cause.2 Neonatal networks systematically collect information on infants born very preterm or with a very low birth weight according to a standardized protocol common to all participating hospitals.3, 4 Despite the undisputed value of such data, estimating associations in such highly selected populations also complicates interpretation. Estimates of the association between a given exposure (e.g., preeclampsia) and outcome (e.g., retinopathy of prematurity (ROP)5–8) in very preterm births have prognostic value, e.g., for assessing prospective risk of a newborn developing a specific outcome, but they do not reflect the causal effects. The problem we set out to clarify is that many such associations estimated among very preterm births and/or very low birthweight infants (e.g., apparent protective effects of preeclampsia on ROP, cerebral palsy,9, 10 intraventricular hemorrhage,11, 12 and a beneficial effect on childhood neurodevelopment13) may be explained either partly or entirely by bias, so clinicians and researchers should exercise caution in interpreting them. While many authors are aware of these limitations,7, 14 findings from these studies are sometimes interpreted causally.5, 8
Within very preterm infants, the vast majority of “unexposed” babies (i.e., those born before 33 weeks without the factor under study, e.g., preeclampsia) will have a mix of other pathologies that caused early delivery and may affect risk of the outcome (e.g., chorioamnionitis, placental abruption, etc.).15, 16 Thus, if preeclampsia is the exposure of interest, its “effect” on the outcome will depend on the mix and severity of the other conditions in the “unexposed” infants with whom they are compared. In this paper, we present a simple demonstration of these concepts, aimed at practicing clinicians and clinician-scientists who are well-versed in perinatal/neonatal content and have basic familiarity with epidemiologic concepts and terminology.
We build on prior work17, 18 to demonstrate in an intuitive manner the consequences of assessing the association between a prenatal/intrapartum factor (e.g., preeclampsia) and a neonatal outcome (e.g., neonatal death) in a sample of very preterm births. Working within a simple simulated universe where we know all the relevant causal effects, we show why the estimated association between a specific factor and mortality in infants born up to 32 weeks of gestation does not represent the true causal effect in the presence of unknown or unmeasured causes of preterm birth that also affect mortality.
Methods
Simulated data
The simulation, expanded from a previous version,17, 18 represents a highly simplified universe with only four factors increasing risk of both preterm birth and mortality. Two causes, termed A and B, each have a prevalence of 1.5% and result in the same moderately high reduction of gestation (mean 20 days), and B increases odds of death at a magnitude twice that of A. In contrast, the more harmful causes termed C and D are rarer than A and B (with prevalence of 0.5%), shorten gestation by a larger amount, and increase odds of neonatal death in a manner that compounds with each passing week. For this example, A may stand in for preeclampsia, D for chorioamnionitis, and B and C for unmeasured (or unknown) factors. In the simulation, the expected length of gestation for “healthy” babies is determined by a normal distribution. However, in affected babies, the four pathologies, acting either singly or in combination (no interaction), will shorten duration of gestation. There is also a small random risk of early birth (i.e., not due to pathology), and a small background risk of fetal and neonatal death (“frailty”), which is increased by factors A-D, as well as by prematurity itself. The characteristics of the normal distribution of births and each factor appear in Table 1, and technical details of the simulation appear in the appendix (Appendix S1; Figures S1–S2).
Table 1.
Model parameters of the simulation for the population of births and causes of preterm birth and neonatal mortality
| Baseline model parameters | Parameters for pathological factors (i.e., the 4 factors that affect risk of both preterm birth and neonatal death) |
|||||
|---|---|---|---|---|---|---|
| Gestation at birth, weeks, Mean (SD) | Risk (events per million) |
Frequency (percent) |
Gestation at birth, weeks Mean (SD) | Effect om frailty (Odds ratio, OR) | Weekly incremental increase of frailty OR | |
| Healthy births | 40 (1.64) | |||||
| Baseline stillbirth risk, per week (“frailty”) | 80 | |||||
| Baseline risk of birth, per week | 4 | |||||
| Births with A | 1.5 | 37 (2.4) | 1.5 | – | ||
| Births with B | 1.5 | 37 (2.4) | 3.0 | – | ||
| Births with C | 0.5 | 32 (3.8) | 2.0 | 20% | ||
| Births with D | 0.5 | 31 (3.8) | 2.0 | 20% | ||
Abbreviations: OR, odds ratio; SD, standard deviation
Analysis of simulated data
We show the distribution of births at each week of gestation by category (i.e., no factor, factor A-D, and more than 1 factor). We then focus only on babies born alive between 22 and 32 weeks, to reflect data comparable to those from a neonatal network. We first examine A (i.e., preeclampsia) and then D (i.e., chorioamnionitis) as the index cause (i.e., the exposure of interest). We compare preterm infants with the given factor to those without by showing the composition of live births with and without Factor A and D at each week, taking advantage of our knowledge of all the causes. We also present week-specific odds ratios (ORs) of neonatal death, and the percent bias comparing the observed OR and the true OR.19
Results
In the simulated population (shown in Figure 1), 6.2% of babies are born before 37 weeks and 3.95% of all babies have one or more pathological factor (Table S1). At term, most infants are born healthy (i.e., no pathological factor), while most preterm births have at least one pathological factor (Figure 1).
Figure 1.
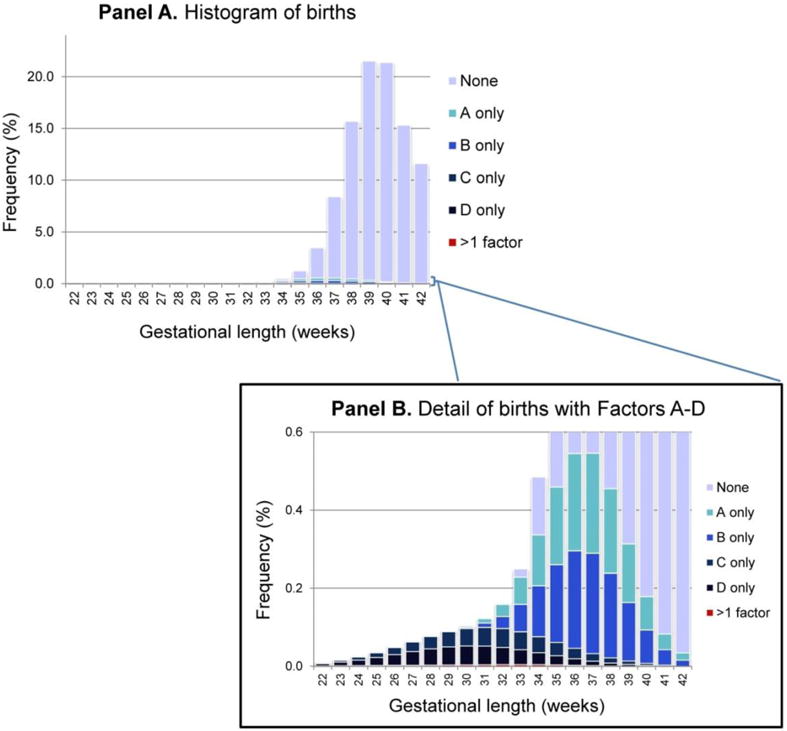
Histogram of births by week of gestation, demonstrating the week-specific proportion of births with an underlying pathology (i.e., Factors A-D) and with no Factor
Panel A is the overall histogram of births by week. The inset (Panel B) is a magnification of the bottom portion of Panel A (0 – 0.6%), to enable visualization of the proportion of births at each week affected by one or more of Factors A-D.
Among babies born ≤ 32 weeks, those with A/preeclampsia have lower odds of neonatal death than those without (crude OR: 0.39; OR adjusted for gestational age at birth: 0.71). Figure 2 shows that nearly all babies have at least one pathology, with the specific mix varying by gestational age at birth. In babies with A, the proportion that also have C or D decreases sharply around week 28, such that after week 29 most have only A or A&B (i.e., the most benign factors; Figure 2, Panel A). Therefore these infants, despite having A, are better off than their peers without A, who have a higher frequency of the more severe pathologies (i.e., C and D). This situation is reflected in the pattern of the weekly ORs of mortality due to A (Figure 3, panel A): up to week 26, the OR is very close to 1.5 (i.e., the “truth”). The OR then starts to decrease, as fewer of the infants with A have additional pathologies, and appears protective from week 29 onward. The percent bias is correspondingly high at these weeks of gestation.
Figure 2.
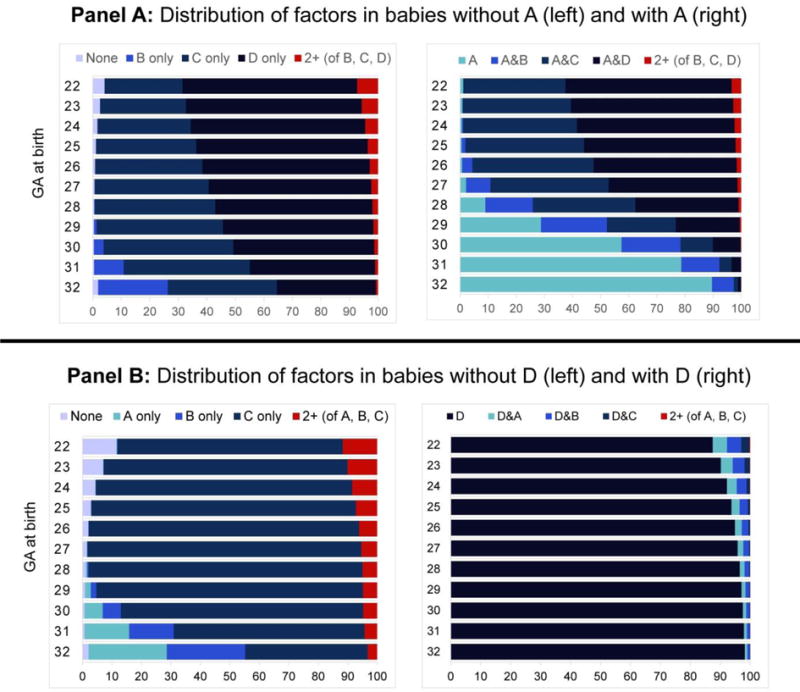
Gestation-stratified distribution of birth categories ≤32 weeks’ gestation, among babies with Factor A and without Factor A (Panel A) and with Factor D and without Factor D (Panel B)
Figure 3.
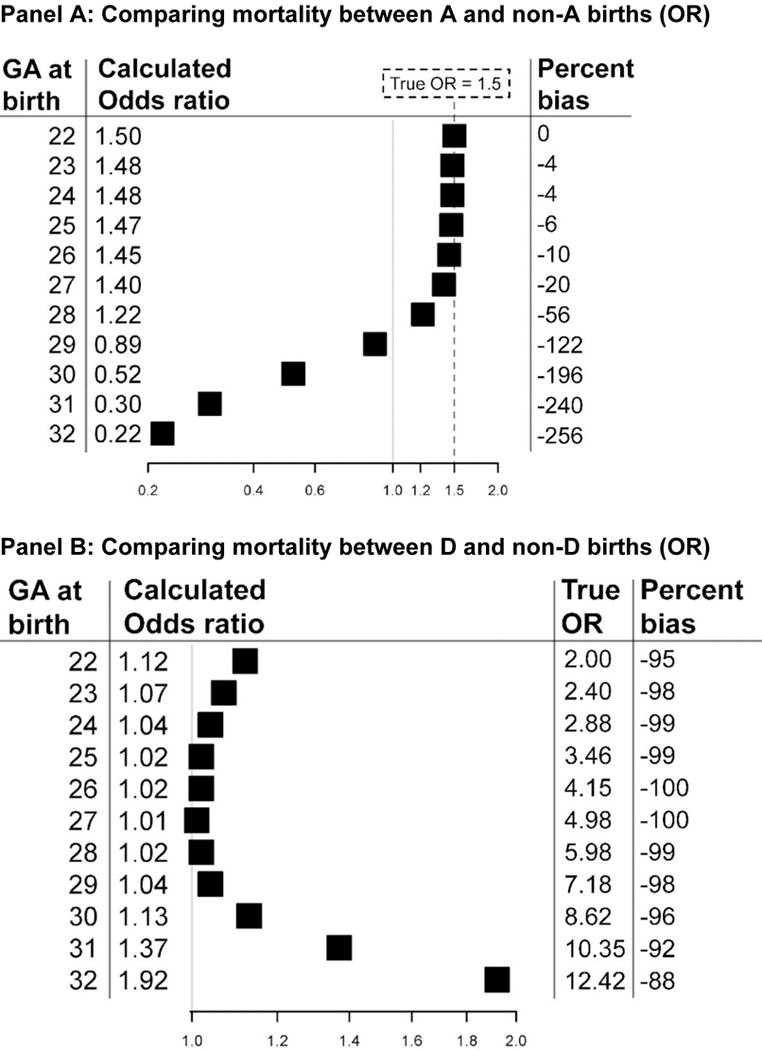
Gestation-specific odds ratios (calculated, true, and percent bias*) for births ≤32 weeks, comparing mortality in A to non-A births (panel A) and comparing D to non-D births (Panel B)
* Percent bias = [(ORcalculated − ORtrue)/(ORtrue − 1)] * 10030
When D/chorioamnionitis is the index factor (Figures 2 and 3, panel B), the calculated OR is never <1, but it still greatly underestimates the true OR, which increases with advancing gestation. For example, at 30 weeks the calculated OR is 1.13, whereas the true OR is 8.62 (−96% bias; Figure 3, panel B). Gestation-specific odds ratios for the other two factors (B and C) are shown in Table S2.
Discussion
Main findings
This paper provides a simple demonstration of why studies restricted to preterm births that examine a specific cause of preterm birth (such as preeclampsia) in relation to a given outcome (e.g., neonatal death) cannot generally be interpreted causally. If most early births are due to pathologies that themselves affect the outcome of interest, there is no true unexposed group. Indeed, infants in the reference category may have a comparatively higher risk than those having the factors under study (as was the case in our simulation when the relatively benign factor A was examined as the exposure).
Strengths and limitations
Although our demonstration rests on a simplified – and fictional – version of reality, the underlying principles plausibly reflect the more complex mechanisms operating in the real world. That said, the factors we postulated do not mimic specific complications, and our simulation omits other nuances, such as the potentially protective physiologic effects of some preterm birth precursors (e.g., preeclampsia) on survival immediately after very preterm birth (e.g., through a cortisol response).20–24 However, these limitations do not negate our main point that the estimates obtained in such a selected population cannot be interpreted causally. In essence, very preterm birth selectively samples pathological fetuses from the total fetal population and transfers them into the population of live births, resulting in a highly selected sample consisting of a varying mix of mostly pathological infants.
Interpretation (in light of other evidence)
The bias demonstrated in our scenario is a form of selection bias25–27 known as “collider-stratification bias” in this context.28, 29 This bias is introduced when gestation-stratified/adjusted associations are calculated (i.e., gestational length is “conditioned on”) when there is uncontrolled mediator-outcome confounding, regardless of whether gestational length is restricted on (as here), or adjusted for in a model. In our example, the crude OR of neonatal death associated with A in births ≤32 weeks was 0.39, and the OR adjusted for gestational age at birth was 0.71. Had we included all infants, regardless of timing of birth, the crude estimated OR would have been 2.55 (reflecting the effect of both A and preterm birth), whereas the OR adjusted for gestational age would have been 0.63, i.e., suggesting a protective effect of A. Thus, inappropriately controlling for gestational length reverses the direction of the association, another example of the complexities involved in calculating gestation-stratified or gestation-adjusted associations between prenatal exposures and postnatal endpoints.
These topics have received substantial attention in the epidemiology literature, including in the context of preterm birth and low birthweight.17, 18, 30–35 In our example, preterm birth is the collider (i.e., a common effect of both the exposure and other factors, as represented in causal diagrams36 in Figure S3). The effect of preeclampsia on neonatal death among very preterm births (or, the direct effect of preeclampsia on neonatal death unmediated by preterm birth) could only be estimated if “healthy” preterm births (i.e., those born for reasons that do not affect the outcome) exist and could be identified. However, this would require (1) knowledge of all common causes of preterm birth and neonatal death (or at least the ability to identify babies whose cause of preterm birth does not affect the outcome) and (2) accurate recording of such factors. As both these conditions are unlikely to be achieved, we posit that, for practical purposes, causal inference is out of reach in samples of very preterm births.
Recent years have seen the development and dissemination of methods to address selection bias and enable causal interpretation even in its presence (e.g., inverse-probability of censoring weights to estimate adjusted causal associations;37, 38 simulation-based bias analysis to quantify the magnitude of likely selection bias39). Although these approaches hold promise, the absence of a true “unexposed” group (or, the inability to identify it in practice, per our two precepts above) likely limits the utility of these methods in this instance. Therefore, we encourage researchers in this field to either select questions with a clearly defined comparison group (e.g., outcomes among preterm infants born after preeclampsia versus those born after chorioamnionitis6), or in the absence of a clearly defined comparison group, to limit interpretation of findings to prognostic and descriptive associations rather than causal ones. In other words, very preterm infants born after preeclampsia may be less likely to die than their counterparts that were not exposed to preeclampsia, but we cannot state that having preeclampsia is beneficial as opposed to not having it.
Conclusion
While studies of very preterm infants provide valuable information on the conditions of neonates at the highest risk of severe complications, inferring causal effects among such populations is not possible at present (and may never be). Research should continue to explore approaches to estimate causal effects, and in the meantime, clinicians and researchers should interpret findings from such studies appropriately (i.e., for descriptive or prognostic purposes, not as causal associations).
Supplementary Material
Acknowledgments
We acknowledge Mekhala Dissanayake1 for her assistance in preparing this manuscript. 1. Department of Obstetrics and Gynecology; Oregon and Health Science University
Funding: JMS is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, United States National Institutes of Health (grant number R00 HD079658-03).
Footnotes
Disclosure of interests: The authors report no conflicts of interest. The ICMJE disclosure forms are available as online supporting information.
Ethics approval statement: This study did not involve human subjects so institutional ethics approval was not required.
Contribution to authorship:
JMS contributed to the design and analysis of the study, and to the interpretation of the simulation results. He drafted the initial manuscript, and generated tables and figures.
OB generated the study concept, created the simulation, carried out initial analyses, reviewed and revised the manuscript, and approved the final version as submitted.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008 Jan 5;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Gravett MG, Iams J, Papageorghiou AT, Waller SA, Kramer M, et al. The preterm birth syndrome: issues to consider in creating a classification system. American journal of obstetrics and gynecology. 2012 Feb;206(2):113–8. doi: 10.1016/j.ajog.2011.10.865. [DOI] [PubMed] [Google Scholar]
- 3.Gould JB. The role of regional collaboratives: the California Perinatal Quality Care Collaborative model. Clin Perinatol. 2010 Mar;37(1):71–86. doi: 10.1016/j.clp.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Horbar JD. The Vermont Oxford Network: evidence-based quality improvement for neonatology. Pediatrics. 1999 Jan;103(1 Suppl E):350–9. [PubMed] [Google Scholar]
- 5.Yu XD, Branch DW, Karumanchi SA, Zhang J. Preeclampsia and retinopathy of prematurity in preterm births. Pediatrics. 2012 Jul;130(1):e101–7. doi: 10.1542/peds.2011-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gagliardi L, Rusconi F, Bellu R, Zanini R, Italian Neonatal N Association of maternal hypertension and chorioamnionitis with preterm outcomes. Pediatrics. 2014 Jul;134(1):e154–61. doi: 10.1542/peds.2013-3898. [DOI] [PubMed] [Google Scholar]
- 7.Gemmell L, Martin L, Murphy KE, Modi N, Hakansson S, Reichman B, et al. Hypertensive disorders of pregnancy and outcomes of preterm infants of 24 to 28 weeks’ gestation. Journal of perinatology : official journal of the California Perinatal Association. 2016 Sep 01; doi: 10.1038/jp.2016.133. [DOI] [PubMed] [Google Scholar]
- 8.Fortes Filho JB, Costa MC, Eckert GU, Santos PG, Silveira RC, Procianoy RS. Maternal preeclampsia protects preterm infants against severe retinopathy of prematurity. J Pediatr. 2011 Mar;158(3):372–6. doi: 10.1016/j.jpeds.2010.08.051. [DOI] [PubMed] [Google Scholar]
- 9.Murphy DJ, Sellers S, MacKenzie IZ, Yudkin PL, Johnson AM. Case-control study of antenatal and intrapartum risk factors for cerebral palsy in very preterm singleton babies. Lancet. 1995 Dec 02;346(8988):1449–54. doi: 10.1016/s0140-6736(95)92471-x. [DOI] [PubMed] [Google Scholar]
- 10.Wilson-Costello D. Risk factors for neurologic impairment among very low-birth-weight infants. Seminars in pediatric neurology. 2001 Jun;8(2):120–6. doi: 10.1053/spen.2001.25228. [DOI] [PubMed] [Google Scholar]
- 11.Perlman JM, Risser RC, Gee JB. Pregnancy-induced hypertension and reduced intraventricular hemorrhage in preterm infants. Pediatric neurology. 1997 Jul;17(1):29–33. doi: 10.1016/s0887-8994(97)00073-8. [DOI] [PubMed] [Google Scholar]
- 12.Shankaran S, Bauer CR, Bain R, Wright LL, Zachary J. Prenatal and perinatal risk and protective factors for neonatal intracranial hemorrhage. National Institute of Child Health and Human Development Neonatal Research Network. Archives of pediatrics & adolescent medicine. 1996 May;150(5):491–7. doi: 10.1001/archpedi.1996.02170300045009. [DOI] [PubMed] [Google Scholar]
- 13.Silveira RC, Procianoy RS, Koch MS, Benjamin AC, Schlindwein CF. Growth and neurodevelopment outcome of very low birth weight infants delivered by preeclamptic mothers. Acta paediatrica. 2007 Dec;96(12):1738–42. doi: 10.1111/j.1651-2227.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 14.Brookfield KF, Osmundson SS, Caughey AB, Snowden JM. Does Infection During Pregnancy Outside of the Time of Delivery Increase the Risk of Cerebral Palsy? American journal of perinatology. 2016 Jul 11; doi: 10.1055/s-0036-1585411. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood C, Yudkin P, Sellers S, Impey L, Doyle P. Why is there a modifying effect of gestational age on risk factors for cerebral palsy? Archives of disease in childhood Fetal and neonatal edition. 2005 Mar;90(2):F141–6. doi: 10.1136/adc.2004.052860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagliardi L, Rusconi F, Da Fre M, Mello G, Carnielli V, Di Lallo D, et al. Pregnancy disorders leading to very preterm birth influence neonatal outcomes: results of the population-based ACTION cohort study. Pediatric research. 2013 Jun;73(6):794–801. doi: 10.1038/pr.2013.52. [DOI] [PubMed] [Google Scholar]
- 17.Basso O, Wilcox AJ. Might rare factors account for most of the mortality of preterm babies? Epidemiology. 2011 May;22(3):320–7. doi: 10.1097/EDE.0b013e31821266c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. American journal of epidemiology. 2011 Nov 1;174(9):1062–8. doi: 10.1093/aje/kwr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiology and drug safety. 2006 May;15(5):291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 20.Schoof E, Girstl M, Frobenius W, Kirschbaum M, Dorr HG, Rascher W, et al. Decreased gene expression of 11beta-hydroxysteroid dehydrogenase type 2 and 15-hydroxyprostaglandin dehydrogenase in human placenta of patients with preeclampsia. The Journal of clinical endocrinology and metabolism. 2001 Mar;86(3):1313–7. doi: 10.1210/jcem.86.3.7311. [DOI] [PubMed] [Google Scholar]
- 21.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. The Journal of physiology. 2006 Apr 01;572(Pt 1):31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aufdenblatten M, Baumann M, Raio L, Dick B, Frey BM, Schneider H, et al. Prematurity is related to high placental cortisol in preeclampsia. Pediatric research. 2009 Feb;65(2):198–202. doi: 10.1203/PDR.0b013e31818d6c24. [DOI] [PubMed] [Google Scholar]
- 23.Braun T, Challis JR, Newnham JP, Sloboda DM. Early-life glucocorticoid exposure: the hypothalamic-pituitary-adrenal axis, placental function, and long-term disease risk. Endocrine reviews. 2013 Dec;34(6):885–916. doi: 10.1210/er.2013-1012. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Basso O, Kramer MS. Association between unintentional injury during pregnancy and excess risk of preterm birth and its neonatal sequelae. American journal of epidemiology. 2015 Nov 1;182(9):750–8. doi: 10.1093/aje/kwv165. [DOI] [PubMed] [Google Scholar]
- 25.Berkson J. Limitations of the application of fourfold table analysis to hospital data. Biometrics. 1946 Jun;2(3):47–53. [PubMed] [Google Scholar]
- 26.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004 Sep;15(5):615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 27.Haneuse S. Distinguishing Selection Bias and Confounding Bias in Comparative Effectiveness Research. Medical care. 2016 Apr;54(4):e23–9. doi: 10.1097/MLR.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology. 2003 May;14(3):300–6. [PubMed] [Google Scholar]
- 29.Cole SR, Platt RW, Schisterman EF, Chu H, Westreich D, Richardson D, et al. Illustrating bias due to conditioning on a collider. International journal of epidemiology. 2010 Apr;39(2):417–20. doi: 10.1093/ije/dyp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basso O, Wilcox AJ, Weinberg CR. Birth weight and mortality: causality or confounding? American journal of epidemiology. 2006 Aug 15;164(4):303–11. doi: 10.1093/aje/kwj237. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez-Diaz S, Schisterman EF, Hernan MA. The birth weight “paradox” uncovered? American journal of epidemiology. 2006 Dec 1;164(11):1115–20. doi: 10.1093/aje/kwj275. [DOI] [PubMed] [Google Scholar]
- 32.Whitcomb BW, Schisterman EF, Perkins NJ, Platt RW. Quantification of collider-stratification bias and the birthweight paradox. Paediatric and perinatal epidemiology. 2009 Sep;23(5):394–402. doi: 10.1111/j.1365-3016.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basso O, Wilcox AJ. Intersecting birth weight-specific mortality curves: solving the riddle. American journal of epidemiology. 2009 Apr 01;169(7):787–97. doi: 10.1093/aje/kwp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VanderWeele TJ, Hernandez-Diaz S. Is there a direct effect of pre-eclampsia on cerebral palsy not through preterm birth? Paediatric and perinatal epidemiology. 2011 Mar;25(2):111–5. doi: 10.1111/j.1365-3016.2010.01175.x. [DOI] [PubMed] [Google Scholar]
- 35.Ananth CV, Schisterman EF. Confounding, Causality and Confusion: The Role of Intermediate Variables in Interpreting Observational Studies in Obstetrics. American journal of obstetrics and gynecology. 2017 Apr 17; doi: 10.1016/j.ajog.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999 Jan;10(1):37–48. [PubMed] [Google Scholar]
- 37.Howe CJ, Cole SR, Chmiel JS, Munoz A. Limitation of inverse probability-of-censoring weights in estimating survival in the presence of strong selection bias. American journal of epidemiology. 2011 Mar 01;173(5):569–77. doi: 10.1093/aje/kwq385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012 Jan;23(1):119–28. doi: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayeda ER, Tchetgen Tchetgen EJ, Power MC, Weuve J, Jacqmin-Gadda H, Marden JR, et al. A Simulation Platform for Quantifying Survival Bias: An Application to Research on Determinants of Cognitive Decline. American journal of epidemiology. 2016 Sep 01;184(5):378–87. doi: 10.1093/aje/kwv451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


