Abstract
Tumor-reactive T lymphocytes can promote the regression of established tumors. However, their efficacy is often limited by immunosuppressive mechanisms that block T cell accumulation or function. ACT provides the opportunity to ameliorate immune suppression prior to transfer of tumor-reactive T cells to improve the therapeutic benefit. We evaluated the combination of lymphodepleting whole body irradiation (WBI) and agonist anti-CD40 (αCD40) antibody on control of established autochthonous murine neuroendocrine pancreatic tumors following the transfer of naïve tumor-specific CD8 T cells. Sublethal WBI had little impact on disease outcome but did promote T cell persistence in the lymphoid organs. Host conditioning with αCD40, an approach known to enhance APC function and T cell expansion, transiently increased donor T cell accumulation in the lymphoid organs and pancreas, but failed to control tumor progression. In contrast, combined WBI and αCD40 prolonged T cell proliferation and dramatically enhanced accumulation of donor T cells in both the lymphoid organs and pancreas. This dual conditioning approach also promoted high levels of inflammation in the pancreas and tumor, induced histological regression of established tumors, and extended the lifespan of treated mice. Prolonged survival was entirely dependent upon adoptive transfer, but only partially dependent upon IFNγ production by donor T cells. Our results identify the novel combination of two clinically relevant host conditioning approaches that synergize to overcome immune suppression and drive strong tumor-specific T cell accumulation within well-established tumors.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2115-2) contains supplementary material, which is available to authorized users.
Keywords: CD8 T cell, CD40 agonist antibody, Immunotherapy, Transgenic mice, Pancreatic neuroendocrine cancer
Introduction
The presence of tumor-infiltrating T cells is associated with a positive prognosis in several cancers and correlates with the success of some immune-based therapies [1, 2]. However, tumor-reactive T cells are susceptible to mechanisms that suppress their function in the TME, and in the case of non-inflamed tumors, are excluded from the TME entirely [3]. ACT provides the opportunity to modify the immunosuppressive host environment prior to introduction of tumor-reactive T cells and can generate durable regression of established cancer in both experimental mouse models and cancer patients [4]. Host conditioning regimens that modify immune suppression are closely linked to successful ACT. In particular, nonmyeloablative lymphodepletion dramatically improves the success of ACT-based therapies, most often consisting of chemotherapy in patients [5, 6] and effectively reproduced by WBI in mouse models [5]. Additional approaches to target immune suppression during ACT include administration of antibodies that enhance immune function or block immune checkpoint inhibitory pathways [7–11], as well as the use of adjuvants and immunization to improve T cell expansion [12, 13].
For solid tumors, ACT with in vitro expanded tumor-infiltrating T lymphocytes in combination with lymphodepletion has a high success rate in metastatic melanoma patients [14], but a gap remains in our understanding of the efficacy of this approach for other tumor types. In mice, host conditioning with WBI prior to ACT induces lymphodepletion, which can increase donor cell access to survival cytokines and eliminate regulatory T cells [5]. WBI can also promote normalization of the tumor vasculature [15, 16], enhance host APC function [17, 18], and increase type I IFN production and innate sensing of tumors [19, 20]. Our group previously demonstrated that WBI increased the magnitude and duration of the donor CD8 T cell response, resulting in regression and durable control of established autochthonous SV40 T antigen (T Ag)-induced brain tumors [7, 21]. This approach also was beneficial in the immunosuppressive TRansgenic Adenocarcinoma of the Mouse Prostate (TRAMP) model, resulting in regression of established lesions and CD8 T cell persistence [22]. However, combining the beneficial effects of host lymphodepletion with other activators of T cell immunity may provide a more potent anti-tumor response that could broaden the success of T cell-based therapies.
We previously demonstrated that agonist αCD40 administration dramatically enhanced initial CD8 T cell priming against established T Ag-induced tumors, and resulted in either prolonged tumor control or tumor regression [7, 8, 23]. Increased T cell accumulation in vivo is consistent with the proposed mechanism of αCD40-enhanced antigen presentation and delivery of co-stimulatory and cytokine signals by professional APCs [24–26]. In addition, αCD40 reverses the accumulation of suppressive myeloid cells, facilitating T cell-based anti-tumor immunity [27, 28]. Whether host lymphodepletion can be combined with agonist αCD40 to mediate more effective control of established tumors is unknown.
To address this question, we utilized the Rip1-Tag4 (RT4) model of neuroendocrine pancreatic cancer that undergoes multistage carcinogenesis as a result of T Ag expression from the rat insulin II promoter [29]. T Ag expression in the β cells beginning at 5 weeks of age [30] leads to widespread islet hyperplasia and progression to insulinomas by 3 and 6 months of age, respectively [29]. All mice succumb to tumor progression at an average of 263 days of age [30]. Once T Ag is expressed in the pancreas, peripheral CD8 T cell tolerance manifests and abrogates the ability of immunization to block tumor progression [30, 31]. Adoptively transferred naïve tumor-specific T cells are efficiently activated in tumor-bearing RT4 mice, but are rapidly deleted and fail to alter tumor progression [31]. Administration of agonist αCD40 antibody can enhance the accumulation of T Ag-specific donor T cells within both the peripheral lymphoid organs and tumors of RT4 mice. However, these T cells are rapidly eliminated, and treatment had only a transient impact on tumor progression [32]. Thus, this model provides a challenging setting to test the hypothesis that the novel combination of host lymphodepletion and agonist αCD40 can improve ACT-mediated immunotherapy by promoting the expansion and accumulation of functional tumor-specific donor T cells.
Materials and methods
Mice
RT4 mice [30] on the C57BL/6J background were maintained in specific pathogen-free barrier housing in the Penn State College of Medicine animal vivarium. Both male and female hemizygous mice were used for all experiments. Therapy was initiated at 6 months of age, when mice have established neoplasia [32]. TCR-I mice on the C57BL/6J background have been described previously [8], and are available from the Jackson Laboratory [B6.Cg-Tg(TcraY1,TcrbY1)416Tev/J]. TCR-I mice were used between the ages of 8–12 weeks.
Cell lines and reagents
B6/WT-19 cells that express full-length, wild-type SV40 T Ag were originally derived by Tevethia, and have been described previously [33]. Processing and short-term culture of single-cell lymphocyte suspensions from mice was performed in RPMI 1640 medium supplemented with 2% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM l-glutamine, 10 mM HEPES, and 50 µM 2-ME.
Adoptive immunotherapy
6-month old RT4 mice were exposed to 4 Gray (Gy) whole body γ-irradiation using a 60Co-source GammaCell 220 irradiator (Nordion International) 1 day before adoptive transfer or injected intraperitoneally with 100 µg αCD40 agonist monoclonal antibody (clone FGK45; BioXCell) 1 day before and 1 day following adoptive transfer. Alternatively, the two conditioning approaches were combined with mice receiving WBI 6–8 h prior to the first dose of αCD40. RT4 mice were intravenously injected with 1 × 106 TCR-I cells in PBS. TCR-I cell purity was determined by flow cytometric detection with Site I-specific MHC tetramer, and ranged from 30 to 45% of the bulk spleen and LN single-cell suspension. For proliferation experiments, 5 µM CFSE in 0.1% bovine serum albumin in PBS was used to label cells prior to transfer [31]. Blood glucose levels were measured every 7–10 days in peripheral blood from the tail vein using a TRUE2go glucose meter (NIPRO Diagnostics).
Processing, staining and flow cytometry analyses
Red blood cell-depleted single-cell suspensions from spleens and LNs were obtained as previously described [34]. Pancreata were dissected from the mouse, and enzymatically digested with a master mix of 1% FBS, 1 mg/ml collagenase (Life Technologies) and 50 U/ml DNase I (Roche) in RPMI 1640 complete media with rocking at 37 °C for 15–30 min. Large particulates were removed by passing the cell suspension through wire mesh.
Aliquots of 2 × 105 pancreatic or LN cells or 2 × 106 splenocytes were stained with commercially available antibodies and PE-labeled H-2Db/I tetramer (TetI) [35] prior to analysis on a FACSCanto II, LSRII or LSR Fortessa (BD Biosciences) in the Penn State Hershey Flow Cytometry Core Facility. Samples were fixed with 2% paraformaldehyde or stained with 7-aminoactinomycin D (7-AAD) prior to analysis. Data were analyzed using FlowJo software (FlowJo, LLC). Antibodies used include: CD45.2-V500 (clone 104), CD8-V450 (clone 53-6.7), CD8-BV786 (clone 53-6.7), CD8-PE (clone 53-6.7), CD90.1-APC (clone HIS51), CD90.1-PerCP Cy5.5 (clone HIS51), CD44-FITC (clone IM7), CD44-Alexa fluor700 (clone IM7), CD62L-APCe780 (clone MEL-14), CD62L-APC-Cy7 (clone MEL-14), KLRG1-PE Cy7 (clone 2F1), PD-1-FITC (clone J43), CD127-V450 (clone SB/199), Ki67-PE (clone SolA15). Antibodies were purchased from BD Biosciences or eBioscience. Donor T cells were identified as 7-AADneg CD45.2 + CD8 + and then CD90.1 + and/or TetI+.
Intracellular cytokine staining
2 × 106 cells from single-cell suspensions were incubated at 37 °C in RPMI 1640 complete media containing 2% FBS, 1 µg/ml brefeldin A and 1µM Site I peptide (206SAINNYAQKL215) or H-2Kb/HSV glycoprotein B (gB) peptide (498SSIEFARL505). Following a 4-h incubation, cells were stained for CD8 and CD90.1. The Cytofix/Cytoperm kit (BD Pharmingen) was used to permeabilize and intracellularly stain cells with IFNγ-FITC (clone XMG1.2) and TNFα-PE (clone MP6-XT22) antibodies per the manufacturer’s instructions. Site I-reactive T cells was calculated by subtracting the proportion of gB peptide responders in parallel samples.
Histology
Mice were euthanized by CO2 asphyxiation and perfused with 10 ml of PBS followed by 10 ml of 10% neutral buffered formalin. The pancreas was dissected from the mouse, and immersion fixed in 10% neutral buffered formalin for 24 h followed by transfer into 70% ethanol for at least 24 h. Pancreata were embedded in paraffin, and sections stained with H&E or Masson’s trichrome for blinded scoring by a board-certified veterinary pathologist. Images were collected using an Olympus BX51 microscope fitted with an Olympus DP71 digital camera and cellSens Standard 1.12 imaging software (Olympus). Briefly, severity of pancreatic lesions (pancreatitis) was quantified as previously described [36]: 0, no pathological changes; 1, minimal infiltration of periductal tissue with leukocytes but no parenchymal destruction; 2, moderate periductal infiltration with leukocytes associated with beginning parenchymal destruction; 3, severe periductal inflammation and/or more extended parenchymal destruction; 4, diffuse leukocyte infiltrates, destruction of acini and (partial) replacement by adipose tissue. Inflammation in the pancreatic islets (isletitis) was scored as follows: 0, no significant inflammation; 1, scattered low numbers of mononuclear leukocytes; 2, multifocal moderate numbers of mononuclear leukocytes; 3, diffuse and large numbers of mononuclear leukocytes. Hyperplastic islets were identified as described [29, 37].
Statistical analyses
Two-sided Student’s t test, one-way ANOVA or two-way ANOVA were used to compare the outcomes of different treatment groups. In all figures, graphical data represent the mean ± SEM. Kaplan–Meier survival curves and log-rank were used to determine differences in survival outcome. All statistical analyses were performed with the significance level set to 0.05 using GraphPad Prism software (v5.0f or higher, San Diego, CA).
Results
Dual conditioning of RT4 mice with WBI and anti-CD40 delays tumor progression following ACT
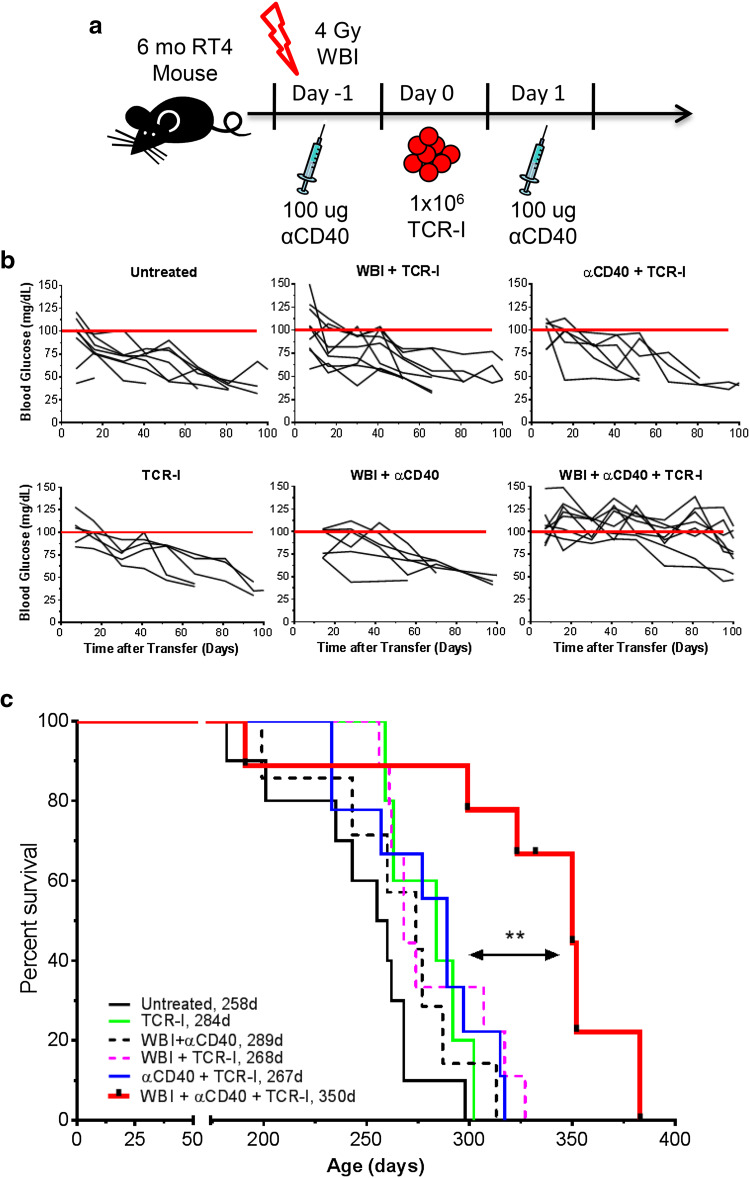
To evaluate the role of combined lymphodepletion and αCD40 (dual conditioning) on ACT-mediated control of tumor progression, groups of 6-month old RT4 mice were administered WBI and/or agonist αCD40 with transfer of naïve TCR-I T cells (Fig. 1a), specific for the H-2Db-restricted T Ag site I determinant. Tumor progression was monitored by the measurement of blood glucose which decreases due to increased insulin production by insulinomas [32]. Neither TCR-I cell transfer alone nor in combination with either αCD40 or WBI conditioning was able to delay progression of established tumors (Fig. 1b). However, dual conditioning with ACT (WBI + αCD40 + TCR-I) delayed tumor progression, with blood glucose levels remaining near normal for approximately 80 days post treatment in most animals. This effect required donor TCR-I cells.
Fig. 1.
Dual conditioning plus TCR-I transfer delays tumor progression and increases the survival of RT4 mice. a Treatment schema. b Peripheral blood glucose levels of individual mice (black lines). The red line indicates 100 dl/ml (normal). c Kaplan–Meier survival analysis. Median survival (days) is listed beside each group in the key. b, c N = 5–10/group; WBI + αCD40 + TCR-I vs. all other treatment groups; ***p < 0.001 by log-rank test in c
Control of tumor progression in dual-conditioned RT4 mice receiving ACT resulted in a highly significant extension of life span compared to all other groups (Fig. 1c; median of 350 days). No other treatment had a significant impact on survival. Three mice from the dual-conditioned + ACT group were killed at 332 days and observed to contain advanced insulinomas, consistent with tumor progression at this late time point (unpublished observations). These data demonstrate that αCD40 and WBI conditioning synergize to improve ACT-mediated control of pancreatic tumors, while the individual approaches had no impact.
Dual conditioning with ACT reduces overall tumor burden
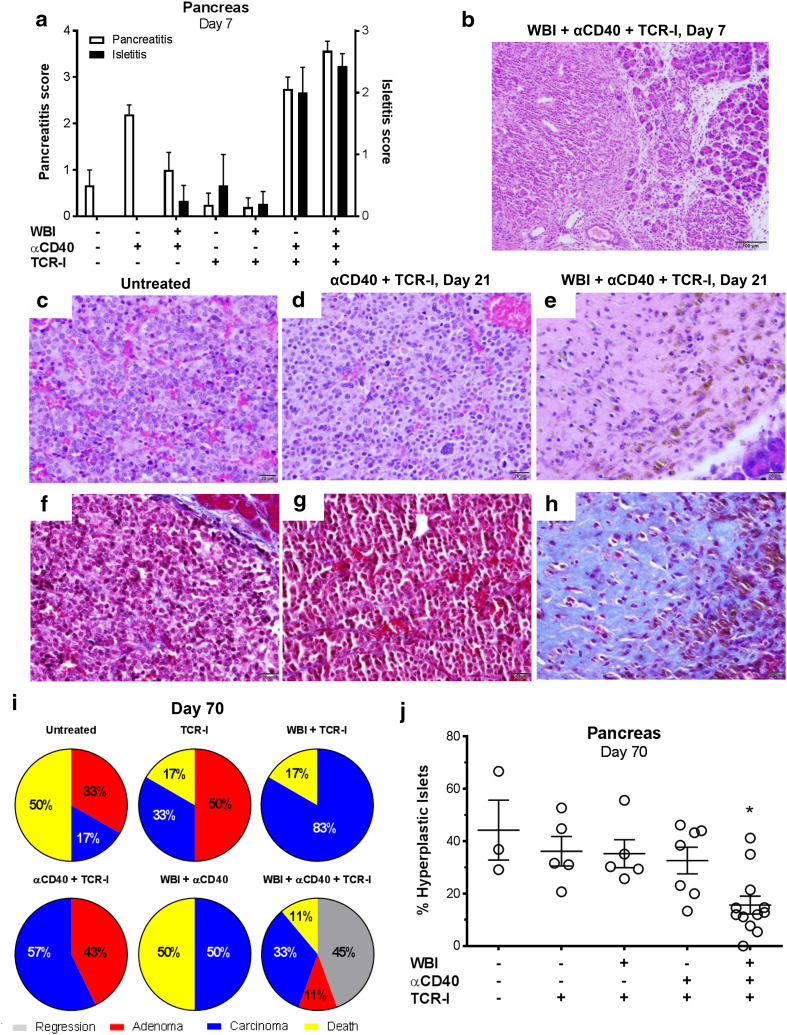
We evaluated the impact of each conditioning regimen on inflammation within the pancreas 7 days post ACT. Blinded scoring of H&E-stained pancreas sections revealed that WBI + ACT failed to induce inflammation in the periductal region and acini of the pancreas (pancreatitis) or within the pancreatic islets (isletitis) (Fig. 2a). αCD40 alone induced general pancreatitis that was reduced when mice also received WBI, suggesting that irradiation suppressed or reduced the endogenous inflammatory cells (Fig. 2a; Supplementary Fig. 1c, d). In contrast, administration of αCD40 + ACT promoted both pancreatitis and isletitis (Fig. 2a; Supplementary Fig. 1a) with the pancreatitis mainly observed peritumorally and adjacent to the LNs (unpublished observations). Mice receiving dual conditioning with ACT displayed a similar high degree of pancreatitis and isletitis (Fig. 2a; Supplementary Fig. 1b). In this case, however, inflammation was observed both throughout the pancreas and within the tumor (Fig. 2b), indicating a more uniform level of inflammation than observed in αCD40 + ACT treated mice. These results demonstrate that αCD40 conditioning triggers inflammation within the pancreas but donor T cells are required for inflammation within the islets and tumors, while WBI + αCD40 + TCR-I produced more uniform inflammation than αCD40 + ACT.
Fig. 2.
Dual conditioning with ACT reduces disease burden in neoplastic islets. a Inflammation scores in the periductal and acinar regions (pancreatitis) and islets (isletitis) from H&E-stained sections of whole pancreata harvested 7 days after the indicated treatments. N = 4–6/group. b Representative H&E-stained section showing inflammation within a pancreatic carcinoma and adjacent pancreatic acinar compartment. c–h Representative H&E (c–e) and Masson’s trichrome-stained (f–h) tumors harvested from untreated (c, f) or RT4 mice that received anti-CD40 + TCR-I cells (d, g) or dual conditioning + TCR-I cells (e, h). i Highest disease stage observed 70 days after treatment, N = 6–9/group. j Percentage hyperplastic islets in whole pancreas sections from mice shown in i that survived to day 70 post treatment. Each point represents an individual mouse. N = 5–12/group; WBI + αCD40 + TCR-I vs. all other treatment groups, *p < 0.05 by one-way ANOVA with Dunnett’s post-test
By 21 days post ACT, insulinomas in dual-conditioned mice showed significant loss of cellularity by H&E staining, consistent with tumor regression (Fig. 2e). Regressed tumors were replaced with coarse fibrosis as indicated by positive (blue) staining in Masson’s trichrome-stained sections (Fig. 2h). No other treatment produced tumor regression and H&E stained sections revealed well-developed insulinomas (Fig. 2c, d, f, g and unpublished observations). Only dual-conditioned mice receiving ACT exhibited a subset of tumor-free mice 70 days post treatment (Fig. 2i) while at least one tumor was identified in all surviving mice in other treatment groups. In addition, only WBI + αCD40 + TCR-I led to a significantly reduced percentage of hyperplastic islets (Fig. 2j). These results indicate that dual conditioning, but neither single conditioning regimen, significantly reduced the tumor burden following ACT.
Dual conditioning increases donor T cell accumulation in the lymphoid organs and tumor-bearing pancreas
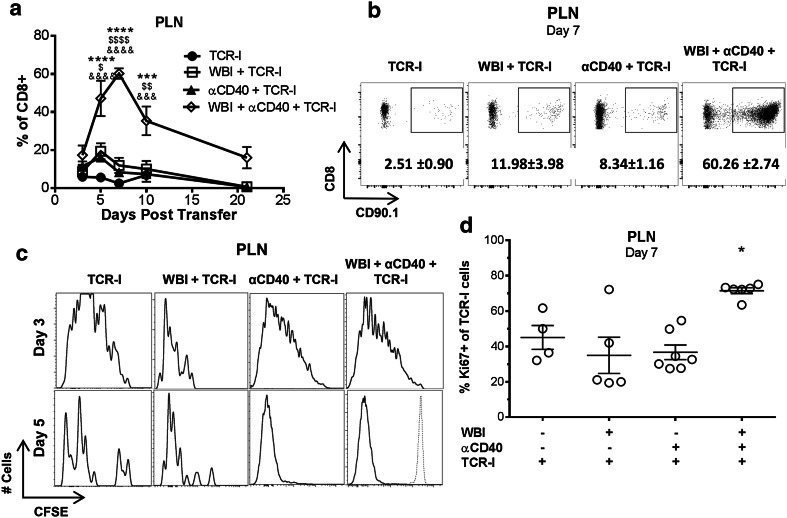
Donor cell accumulation in the tumor-draining pancreaticoduodenal LN (PLN) was assessed to define the impact of each conditioning regimen on initial T cell priming and subsequent accumulation. Individually, WBI and αCD40 each had a small impact on the frequency of donor TCR-I cells in the PLN at day 5, followed by contraction at day 7 (Fig. 3a, b). In contrast, dual conditioning dramatically increased the frequency of TCR-I cells, achieving peak levels at day 7. This increase was statistically significant compared to all other groups at days 5, 7 and 10, and donor T cells remained readily detectable in dual-conditioned mice at day 21. In contrast, total CD8 T cells were only significantly increased at day 7 in dual-conditioned mice (Supplementary Fig. 2).
Fig. 3.
Dual conditioning prolongs donor T cell proliferation and increases accumulation in the PLN. a Percentage of CD90.1 + or TetI + TCR-I cells accumulating in the PLN over time. N = 6–18/group (days 3–10), 2–3/group (days 14, 21): WBI + αCD40 + TCR-I vs. TCR-I (asterisks), vs. WBI + TCR-I (dollar symbol), vs. αCD40 + TCR-I (ampersand); 1 digit p < 0.05, 2 digits p < 0.01, 3 digits p < 0.001, 4 digits p < 0.0001 by two-way ANOVA with Bonferroni corrections. b Representative flow cytometry panels from a. Numbers indicate the mean percentage +/− SEM of CD8+ cells. c CFSE staining of CD90.1 + TCR-I cells in the PLN 3 and 5 days post treatment. d Ki67 staining of CD90.1 + TCR-I cells in the PLN 7 days post treatment. N = 4–6/group; WBI + αCD40 + TCR-I vs. all other groups, **p < 0.01 by one-way ANOVA with Dunnett’s post-test
We evaluated donor T cell proliferation using both CFSE dilution and Ki67 staining. By day 3 post transfer, almost all CFSE-labeled donor T cells in the PLN had undergone multiple rounds of cell division regardless of host conditioning (Fig. 3c). CFSE signal was almost completely eliminated by day 5 in all treatment groups (Fig. 3c), despite differences in T cell accumulation at this early time point (Fig. 3a). To evaluate donor T cell proliferation beyond day 5, recovered donor cells were stained for Ki67. T cells from dual-conditioned mice maintained a significantly higher proportion of proliferating cells 7 days after transfer (Fig. 3d). Sustained proliferation corresponded with higher accumulation of donor T cells at days 7 and 10 in the dual-conditioned group (Fig. 3a). These results indicate that sufficient antigen is available to prime tumor-specific T cells in the PLN independent of host conditioning, while dual conditioning promotes continued proliferation and accumulation of donor T cells.
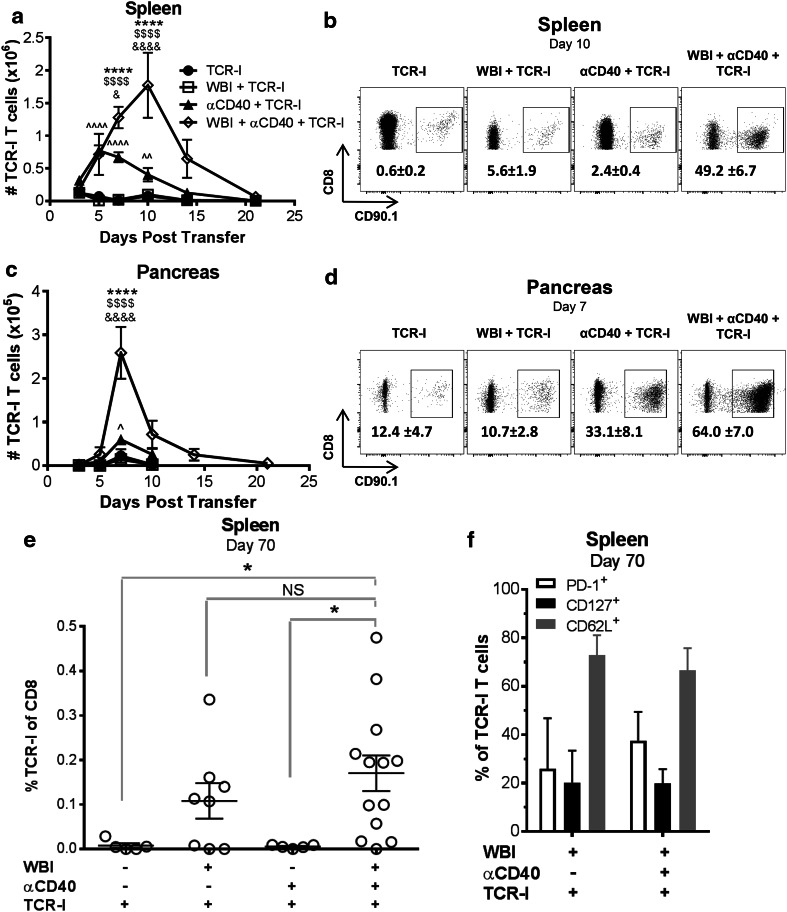
We also quantified T cells in the spleen as an indication of systemic immunity. Donor T cell accumulation was not improved by WBI, but was initially increased with αCD40 (Fig. 4a). TCR-I cells in αCD40 treated mice exhibited peak accumulation at days 5–7, and contracted to baseline levels within 2 weeks. In contrast, accumulation of TCR-I cells in dual-conditioned mice peaked 10 days post ACT, and achieved significantly higher total cells at days 7 and 10 compared to all other treatments. This increase in total donor T cell accumulation was mirrored by a proportional increase among splenic CD8 T cells (Fig. 4b). T cells from dual-conditioned mice contracted approximately threefold by day 14, but remained above the levels observed with other treatments (Fig. 4a). These results indicate that systemic accumulation of donor T cells is most effectively increased by dual conditioning.
Fig. 4.
Dual conditioning enhances accumulation of TCR-I cells in the spleen and pancreas. a, b Absolute numbers of CD90.1 + or TetI + TCR-I cells in the spleen (a) and pancreas (c) were determined by flow cytometry. N = 6–18 mice/group (days 3–10), 2–10/group (days 14, 21). WBI + αCD40 + TCR-I vs. TCR-I (asterisk), vs. WBI + TCR-I (dollar symbol), vs. αCD40 + TCR-I (ampersand); αCD40 + TCR-I vs. TCR-I alone (hat symbol). 1 digit p < 0.05, 2 digits p < 0.01, 4 digits p < 0.0001 by two-way ANOVA with Bonferroni corrections. b, d representative flow cytometry plots for TCR-I T cells recovered from spleen (b) and pancreas (d). Numbers inside the dot plots represent the mean percentage of each group +/− SE of CD8 + cells identified as TCR-I cells. e TCR-I T cells recovered from the spleen 70 days post treatment. N = 5–13/ group. WBI + αCD40 + TCR-I vs. indicated group, *p < 0.05 by one-way ANOVA with Dunnett’s post-test, NS not significant. f Proportion of donor TCR-I T cells expressing the indicated surface markers 70 days post treatment. N = 2–7/group. No significant differences by Student’s t test
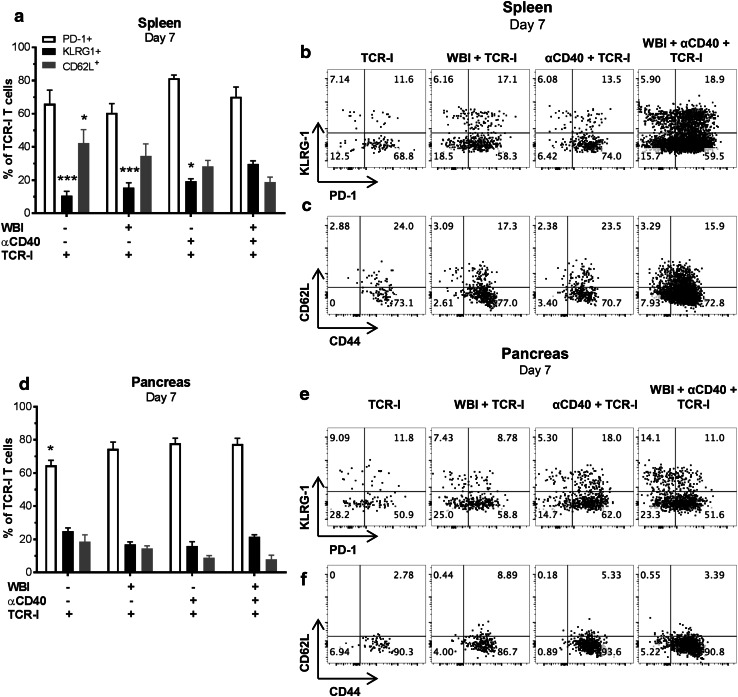
As found in the lymphoid organs, the number of TCR-I cells infiltrating the pancreas was not significantly enhanced by WBI, but αCD40 produced a small, transient donor cell accumulation 7 days after transfer (Fig. 4c, d). Importantly, dual conditioning increased peak accumulation of TCR-I T cells in the pancreas at day 7 above that achieved in all other groups (Fig. 4c). TCR-I T cells contracted after day 7 with kinetics similar to that observed in the PLN rather than continuing to accumulate as observed in the spleen. Donor T cells recovered from spleen (Fig. 5a–c) and pancreas (Fig. 5d–f) on day 7 did not significantly vary in expression of phenotypic surface markers including CD62L and PD-1. A high, yet similar proportion of donor T cells recovered from the spleen and pancreas of treated mice expressed PD-1, regardless of the type of treatment. Splenic T cells recovered from dual-conditioned mice showed a minor but statistically significant increase in the proportion of KLRG1 + cells (Fig. 5a). Thus, no major phenotypic differences were observed despite the dramatic increase in T cell accumulation following dual conditioning.
Fig. 5.
Phenotype of donor T cells recovered from RT4 mice. a, d expression of surface markers on CD8 + CD90.1 + donor cells 7 days post treatment. N = 7–12/group. WBI + αCD40 + TCR-I vs. indicated group; *digit p < 0.05, ***digits p < 0.001 by one-way ANOVA with Dunnett’s post-test. b, c, e, f representative flow cytometry plots for TCR-I T cells recovered from the spleen (b, c) and pancreas (e, f)
Donor T cells in the pancreas remained at low but detectable numbers up to 21 days post ACT in dual-conditioned mice (Fig. 4c: average 5595 cells/pancreas). While TCR-I T cells were not detected at later time points in the pancreas (unpublished observations), donor T cells persisted at low levels in the spleen up to 70 days post ACT in a subset of both dual- and WBI-conditioned mice, but not in αCD40- or ACT only mice (Fig. 4e). Persisting T cells showed some features consistent with memory T cell formation such as CD62L expression but few cells expressed CD127 or PD-1 (Fig. 4f). These results indicate that αCD40 modestly improved donor T cell accumulation in the spleen and pancreas of tumor-bearing RT4 mice while WBI promoted residual T cell persistence. Dual conditioning prolonged T cell expansion and dramatically increased acute T cell accumulation.
IFNγ production by donor T cells modestly impacts survival
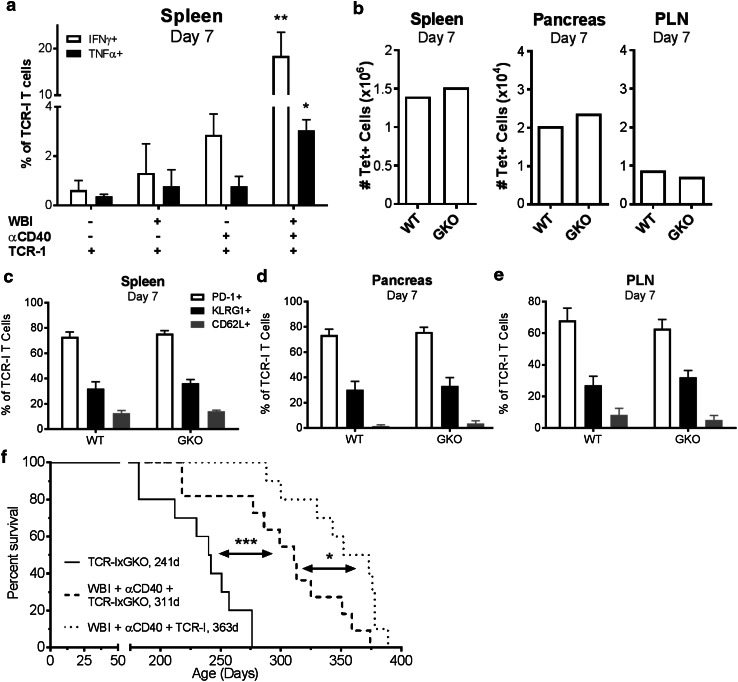
We next evaluated T cell functions that may be associated with control of tumor progression including production of IFNγ and TNFα. Low proportions of donor T cells from mice treated with ACT alone or ACT with αCD40 or WBI produced these cytokines, while a significantly increased proportion of donor cells produced IFNγ and TNFα in dual-conditioned mice (Fig. 6a). Previous investigations have demonstrated that donor T cells control tumor progression through IFNγ-dependent tumor cell killing and cell cycle arrest [21, 38–41]. To assess the role of IFNγ in the anti-tumor response in this system, we transferred naive IFNγ-deficient TCR-I donor cells (TCR-IxGKO) into dual-conditioned RT4 mice. TCR-IxGKO T cells accumulated to similar frequencies as wild-type TCR-I T cells in both the lymphoid organs and pancreas of dual-conditioned mice (Fig. 6b). TCR-IxGKO donor T cells recovered at day 7 were predominantly PD-1+ and CD62Llo but did not vary significantly in phenotype from wild-type TCR-I T cells (Fig. 6c-e). Thus, loss of IFNγ did not negatively impact T cell accumulation or alter differentiation in dual-conditioned RT4 mice.
Fig. 6.
IFNγ-producing donor cells are required to achieve maximum control of tumor progression. a IFNγ- and TNFα-production by TCR-I donor cells measured by intracellular cytokine staining. N = 3/group. WBI + αCD40 + TCR-I vs. all other groups; *p < 0.05, **p < 0.01 by one-way ANOVA with Dunnett’s post-test. b–f RT4 mice received dual conditioning and ACT of wild-type TCR-I or TCR-IxGKO cells. b Total TCR-I donor cells 7 days post treatment. N = 6–7/group. No significant differences by Student’s t test. c–e Surface marker expression on TCR-I cells recovered from the spleen (c), pancreas (d) and PLN (e). N = 3–7/group. No significant differences by Student’s t test. f Survival analysis with median survival (days) indicated. N = 10/group. *p < 0.05, ***p < 0.001 by log-rank test
In addition, TCR-IxGKO donor T cells mediated a highly significant increase in survival of RT4 mice following dual conditioning compared to mice that received TCR-IxGKO ACT alone (Fig. 6f). However, this increase was significantly less than achieved with wild-type TCR-I cells. These data indicate that IFNγ production by donor T cells plays a modest role in control of tumor progression, but suggest that alternative donor T cell effector mechanisms are likely to promote anti-tumor immunity in this system.
Discussion
Approaches that promote T cell accumulation within tumors are likely to improve the response rate to immune-based therapies, particularly for tumors that effectively exclude T cells [3]. We demonstrate that combining two distinct conditioning approaches, WBI and agonist αCD40, improves ACT. These regimens function synergistically to produce a more potent anti-tumor response than either achieves alone. Our results show that αCD40 promotes T cell accumulation in the lymphoid organs and pancreas, although this inflammatory effect was not sufficient to control tumor progression. WBI dramatically improved acute accumulation of tumor-specific T cells within the pancreas when combined with αCD40, consistent with the more potent impact of this combined regimen on tumor progression.
Agonist αCD40 has been shown to license APCs to effectively initiate cytotoxic CD8 T cell responses in tolerizing environments [24, 42]. While increased αCD40-induced donor T cell accumulation could be explained by improved tumor antigen presentation, we did not directly measure the quantitative effects of αCD40 on antigen presentation. However, our results do show that sufficient tumor antigen was constitutively presented to activate almost all of the donor T cells within the draining LNs regardless of treatment (Fig. 3c). Thus, improved T cell accumulation within the lymphoid tissues of αCD40-treated mice may be explained by a qualitatively better signal, such as improved costimulatory signals or altered cytokines provided during T cell differentiation [43, 44]. Addition of WBI to αCD40 conditioning may prolong the period of effective tumor antigen presentation resulting in extended donor T cell proliferation in the PLN (Fig. 3d). These effects could be mediated through radiation-induced immunogenic tumor cell death [45] or further licensing of APCs [18, 19].
A second role for αCD40 may be to induce a pro-inflammatory state that promotes immune cell infiltration into the pancreas. Clinically, αCD40 administration is associated with cytokine release syndrome [46] and triggering of CD40 on human endothelial cells promotes increased levels of adhesion molecules and production of pro-inflammatory cytokines [47]. In mice, intraperitoneal delivery of αCD40 promotes an acute hepatitis and accumulation of myeloid cells in tumor-bearing and tumor-free hosts [48]. Thus, αCD40 may play a key role in facilitating T cell entry into the pancreas, which is further augmented by WBI. In the related Rip1-Tag5 model, irradiation transformed the irregular insulinoma vasculature into a phenotypically normal capillary network to facilitate T cell infiltration [15]. Radiation up-regulated expression of adhesion molecules on tumor blood vessels, correlating with T cell extravasation into the tumor [15, 49]. These changes appear to be dependent on the development of an inflammatory microenvironment, and may be mediated through M1-skewing of tumor-associated macrophages [16]. Here we found that WBI + ACT did not promote T cell accumulation or pancreatic inflammation, while the combination of αCD40 + WBI + ACT promoted extensive T cell accumulation and inflammation throughout the pancreas and tumors. A similar effect was recently found in mice with pancreatic ductal adenocarcinomas, where the combination of chemotherapy and αCD40 dramatically improved T cell-dependent control of tumor progression [50]. Intriguingly, that response was dependent on the presence of Batf3 + DCs, critical for antigen cross-presentation, and was associated with decreased regulatory T cells and improved T cell effector function in the TME. Indeed the presence of DCs in the TME may be essential for recruitment of donor T cells [51]. The nature of the inflammatory cells recruited into the pancreas by either αCD40 alone or dual conditioning and their role in T cell recruitment in RT4 mice remains to be determined.
We also demonstrate that WBI extended the persistence of a small population of donor T cells (Fig. 4e). However, the level of T cell persistence was not improved by dual conditioning suggesting that the increased magnitude of acute T cell accumulation did not increase long-term T cell persistence in this tumor model. Whether additional interventions prior to T cell contraction might improve T cell persistence remains to be determined. Nonetheless, WBI prevented the complete loss of donor T cells observed with αCD40 conditioning [7], offsetting a major limitation to αCD40 conditioning in the setting of ACT. This combination may represent a general approach to prolong donor T cell persistence. Our previous studies in other models also demonstrated the capacity for WBI conditioning to extend survival of donor T cells [21, 22]. The lymphodepleting effect of both WBI and chemotherapy is associated with creating sufficient space and access to survival cytokines in tumor-bearing recipients to promote differentiation and persistence of memory-like T cells [52, 53]. WBI was sufficient to promote donor T cell persistence in RT4 mice, but the addition of αCD40 was required to produce a population of donor T cells capable of exerting a measurable therapeutic effect. In addition, only dual conditioning promoted an increase in the proportion of IFNγ and TNFα-producing donor T cells. Taken together, these data suggest that effective tumor control required high-level acute donor T cell accumulation and effector T cell differentiation that was only effectively accomplished in the setting of dual conditioning.
Our findings are consistent with previous studies in other tumor models showing that IFNγ is important for elimination of tumor antigen-expressing cells and the tumor stroma [38, 39, 54]. IFNγ produced by T cells in the TME slows tumor growth by arresting tumor cells in the G1/G0 phase of the cell cycle [40, 41]. However, we demonstrate that IFNγ-deficient TCR-I T cells maintained a significant capacity to control tumor progression, suggesting that additional effector mechanisms (e.g., Fas-FasL function, cytotoxicity) may contribute to the anti-tumor response observed in dual-conditioned RT4 mice. Indeed, sublethal irradiation can increase Fas expression on tumor cells [55], potentially rendering insulinomas in dual-conditioned mice more sensitive to Fas-mediated apoptosis than tumors in αCD40-only conditioned mice. Our studies do not rule out a role for host-cell derived IFNγ in the observed anti-tumor effect, although our data clearly demonstrate that ACT is required for dual conditioning to control tumor progression. Further studies are needed to determine the specific contributions of IFNγ-dependent and -independent components of donor T cell activity involved in RT4 tumor regression and stasis.
These results have the potential to be applied clinically using patient TILs and autologous T cells reprogrammed with chimeric antigen receptors or cloned TCRs. The individual conditioning approaches have previously been applied in patients; WBI administered with ACT in the setting of metastatic melanoma [4], and αCD40 administered without ACT in the settings of pancreatic ductal adenocarcinoma and metastatic melanoma [27]. Combining these two approaches with ACT in cancer patients could have potential toxicities as αCD40-induced acute hepatitis in mice has been observed. How WBI may impact the αCD40-induced inflammatory response remains unknown [48]. While we observed no toxicity in our studies either in the target organ or at other sites, more in-depth studies are required to assess potential toxicities. In summary, we found that combining distinct host conditioning regimens with ACT produced immune-enhancing effects beyond those predicted using each individual approach, allowing for effective therapy against established tumors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Jeremy Haley for outstanding technical assistance and the staff of the Penn State Hershey Flow Cytometry Core Facility for expert assistance.
Abbreviations
- αCD40
Anti-CD40
- gB
Glycoprotein B
- GKO
Interferon gamma knockout
- Gy
Gray
- PLN
Pancreaticoduodenal lymph node
- RT4
Rip1-Tag4
- T Ag
T antigen
- TetI
H-2Db/I tetramer
- TRAMP
Transgenic adenocarcinoma of the mouse prostate
- WBI
Whole body irradiation
Author contributions
Conception and design: Lindsay K. Ward-Kavanagh, Kathleen M. Kokolus, Todd D. Schell. Development of methodology: Lindsay K. Ward-Kavanagh, Timothy K. Cooper, Todd D. Schell. Acquisition of data (performed experiments, provided mice, collected images, etc.): Lindsay K. Ward-Kavanagh, Kathleen M. Kokolus, Aron E. Lukacher, Timothy K. Cooper, Todd D. Schell. Analysis and interpretation of data (computational and statistical analysis): Lindsay K. Ward-Kavanagh, Kathleen M. Kokolus, Todd D. Schell. Writing, review and/or revision of the manuscript: Lindsay K. Ward-Kavanagh, Kathleen M. Kokolus, Aron E. Lukacher, Timothy K. Cooper, Todd D. Schell. Study supervision: Todd D. Schell.
Funding
This work was supported by Research Grants R01 CA025000 from the National Cancer Institute, National Institutes of Health (to Todd D. Schell) and R01 AI102543 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (to Aron E. Lukacher). Lindsay K. Ward-Kavanagh and Kathleen M. Kokolus were supported by Training Grant T32 CA060395 from the National Cancer Institute, National Institutes of Health.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal studies were approved by the Penn State Hershey Institutional Animal Care and Use Committee (Protocol #47088) and were performed in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals.
Animal source
Mice were bred in specific pathogen-free barrier housing in the Penn State College of Medicine animal vivarium. RT4 mice on the C57BL/6J background were maintained as a homozygous line and bred with C57BL/6J mice to produce hemizygous RT4 mice for experiments. Hemizygous TCR-I mice were bred to homozygous B6.PL-Thy1a/CyJ females (the Jackson Laboratory) to generate CD90.1+ donor T cells. TCR-I mice on the IFNγ-knockout background (TCR-IxGKO) were derived by backcrossing TCR-I mice to homozygous B6.129S7-Ifngtm1Ts/J mice from the Jackson Laboratory.
Footnotes
Lindsay K. Ward-Kavanagh and Kathleen M. Kokolus contributed equally to this manuscript.
References
- 1.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 2.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013;25:268276. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev. 2014;257:56–71. doi: 10.1111/imr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goff SL, Dudley ME, Citrin DE, Somerville RP, Wunderlich JR, Danforth DN, Zlott DA, Yang JC, Sherry RM, Kammula US, Klebanoff CA, Hughes MS, Restifo NP, Langhan MM, Shelton TE, Lu L, Kwong ML, Ilyas S, Klemen ND, Payabyab EC, Morton KE, Toomey MA, Steinberg SM, White DE, Rosenberg SA. Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol. 2016;34:2389–2397. doi: 10.1200/JCO.2016.66.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cozza EM, Cooper TK, Budgeon LR, Christensen ND, Schell TD. Protection from tumor recurrence following adoptive immunotherapy varies with host conditioning regimen despite initial regression of autochthonous murine brain tumors. Cancer Immunol Immunother. 2015;64:325336. doi: 10.1007/s00262-014-1635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staveley-O’Carroll K, Schell TD, Jimenez M, Mylin LM, Tevethia MJ, Schoenberger SP, Tevethia SS. In vivo ligation of CD40 enhances priming against the endogenous tumor antigen and promotes CD8 + T cell effector function in SV40 T antigen transgenic mice. J Immunol. 2003;171:697–707. doi: 10.4049/jimmunol.171.2.697. [DOI] [PubMed] [Google Scholar]
- 9.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, Yagita H, Overwijk WW, Lizee G, Radvanyi L, Hwu P. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res. 2012;72:5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8 + T cell tolerance to tumor by a CD4 + T cell- and IL-2-dependent mechanism. Immunity. 1999;11:483493. doi: 10.1016/S1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 11.John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH, Darcy PK. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 12.Nobuoka D, Yoshikawa T, Takahashi M, Iwama T, Horie K, Shimomura M, Suzuki S, Sakemura N, Nakatsugawa M, Sadamori H, Yagi T, Fujiwara T, Nakatsura T. Intratumoral peptide injection enhances tumor cell antigenicity recognized by cytotoxic T lymphocytes: a potential option for improvement in antigen-specific cancer immunotherapy. Cancer Immunol Immunother. 2013;62:639652. doi: 10.1007/s00262-012-1366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, Borman ZA, Kerkar SP, Scott CD, Finkelstein SE, Rosenberg SA, Restifo NP. Determinants of successful CD8 + T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17:5343–5352. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duong CP, Yong CS, Kershaw MH, Slaney CY, Darcy PK. Cancer immunotherapy utilizing gene-modified T cells: from the bench to the clinic. Mol Immunol. 2015;67:46–57. doi: 10.1016/j.molimm.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–1470. [PubMed] [Google Scholar]
- 16.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schakel K, Garbi N, Jager D, Weitz J, Schmitz-Winnenthal H, Hammerling GJ, Beckhove P. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, Schwendener R, Pruschy M, Knuth A, van den Broek M. Radiotherapy promotes tumor-specific effector CD8 + T cells via dendritic cell activation. J Immunol. 2012;189:558566. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 18.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, Gattinoni L, Antony PA, Rosenberg SA, Restifo NP. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8 + T cells via TLR4 signaling. J Clin Invest. 2007;117:21972204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim JY, Gerber SA, Murphy SP, Lord EM. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer Immunol Immunother. 2014;63:259271. doi: 10.1007/s00262-013-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tatum AM, Mylin LM, Bender SJ, Fischer MA, Vigliotti BA, Tevethia MJ, Tevethia SS, Schell TD. CD8 + T cells targeting a single immunodominant epitope are sufficient for elimination of established SV40 T antigen-induced brain tumors. J Immunol. 2008;181:4406–4417. doi: 10.4049/jimmunol.181.6.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward-Kavanagh LK, Zhu J, Cooper TK, Schell TD. Whole-body irradiation increases the magnitude and persistence of adoptively transferred T cells associated with tumor regression in a mouse model of prostate cancer. Cancer Immunol Res. 2014;2:777788. doi: 10.1158/2326-6066.CIR-13-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan CM, Staveley-O’Carroll K, Schell TD. Combined anti-CD40 conditioning and well-timed immunization prolongs CD8 + T cell accumulation and control of established brain tumors. J Immunother. 2008;31:906920. doi: 10.1097/CJI.0b013e318189f155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 25.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, Clendenin C, Gladney WL, Knoblock DM, Guirnalda PD, Vonderheide RH. Exclusion of T cells from pancreatic carcinomas in mice is regulated by Ly6C(low) F4/80(+) extratumoral macrophages. Gastroenterology. 2015;149:201210. doi: 10.1053/j.gastro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 30.Ye X, McCarrick J, Jewett L, Knowles BB. Timely immunization subverts the development of peripheral nonresponsiveness and suppresses tumor development in simian virus 40 tumor antigen-transgenic mice. Proc Natl Acad Sci USA. 1994;91:3916–3920. doi: 10.1073/pnas.91.9.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otahal P, Schell TD, Hutchinson SC, Knowles BB, Tevethia SS. Early immunization induces persistent tumor-infiltrating CD8 + T cells against an immunodominant epitope and promotes lifelong control of pancreatic tumor progression in SV40 tumor antigen transgenic mice. J Immunol. 2006;177:3089–3099. doi: 10.4049/jimmunol.177.5.3089. [DOI] [PubMed] [Google Scholar]
- 32.Otahal P, Knowles BB, Tevethia SS, Schell TD. Anti-CD40 conditioning enhances the T(CD8) response to a highly tolerogenic epitope and subsequent immunotherapy of simian virus 40 T antigen-induced pancreatic tumors. J Immunol. 2007;179:6686–6695. doi: 10.4049/jimmunol.179.10.6686. [DOI] [PubMed] [Google Scholar]
- 33.Tevethia SS, Greenfield RS, Flyer DC, Tevethia MJ. SV40 transplantation antigen: relationship to SV40-specific proteins. Cold Spring Harb Symp Quant Biol. 1980;44 Pt 1:235242. doi: 10.1101/sqb.1980.044.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Schell TD, Mylin LM, Georgoff I, Teresky AK, Levine AJ, Tevethia SS. Cytotoxic T-lymphocyte epitope immunodominance in the control of choroid plexus tumors in simian virus 40 large T antigen transgenic mice. J Virol. 1999;73:5981–5993. doi: 10.1128/jvi.73.7.5981-5993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mylin LM, Schell TD, Roberts D, Epler M, Boesteanu A, Collins EJ, Frelinger JA, Joyce S, Tevethia SS. Quantitation of CD8(+) T-lymphocyte responses to multiple epitopes from simian virus 40 (SV40) large T antigen in C57BL/6 mice immunized with SV40, SV40 T-antigen-transformed cells, or vaccinia virus recombinants expressing full-length T antigen or epitope minigenes. J Virol. 2000;74:6922–6934. doi: 10.1128/JVI.74.15.6922-6934.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asghari F, Fitzner B, Holzhuter SA, Nizze H, de Castro Marques A, Muller S, Moller S, Ibrahim SM, Jaster R. Identification of quantitative trait loci for murine autoimmune pancreatitis. J Med Genet. 2011;48:557562. doi: 10.1136/jmg.2011.089730. [DOI] [PubMed] [Google Scholar]
- 37.Ryschich E, Schmidt J, Hammerling GJ, Klar E, Ganss R. Transformation of the microvascular system during multistage tumorigenesis. Int J Cancer. 2002;97:719725. doi: 10.1002/ijc.10074. [DOI] [PubMed] [Google Scholar]
- 38.Zhang B, Karrison T, Rowley DA, Schreiber H. IFN-gamma- and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers. J Clin Invest. 2008;118:13981404. doi: 10.1172/JCI33522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollenbaugh JA, Dutton RW. IFN-gamma regulates donor CD8 T cell expansion, migration, and leads to apoptosis of cells of a solid tumor. J Immunol. 2006;177:3004–3011. doi: 10.4049/jimmunol.177.5.3004. [DOI] [PubMed] [Google Scholar]
- 40.Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, Ranta F, Ullrich S, Mocikat R, Braungart K, Mehra T, Fehrenbacher B, Berdel J, Niessner H, Meier F, van den Broek M, Haring HU, Handgretinger R, Quintanilla-Martinez L, Fend F, Pesic M, Bauer J, Zender L, Schaller M, Schulze-Osthoff K, Rocken M. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 41.Matsushita H, Hosoi A, Ueha S, Abe J, Fujieda N, Tomura M, Maekawa R, Matsushima K, Ohara O, Kakimi K. Cytotoxic T lymphocytes block tumor growth both by lytic activity and IFNgamma-dependent cell-cycle arrest. Cancer Immunol Res. 2015;3:26–36. doi: 10.1158/2326-6066.CIR-14-0098. [DOI] [PubMed] [Google Scholar]
- 42.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Ju W, Yao Z, Yu P, Wei BR, Simpson RM, Waitz R, Fasso M, Allison JP, Waldmann TA. Augmented IL-15Ralpha expression by CD40 activation is critical in synergistic CD8 T cell-mediated antitumor activity of anti-CD40 antibody with IL-15 in TRAMP-C2 tumors in mice. J Immunol. 2012;188:6156–6164. doi: 10.4049/jimmunol.1102604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C, Lewis CM, Lou Y, Xu C, Peng W, Yang Y, Gelbard AH, Lizee G, Zhou D, Overwijk WW, Hwu P. Agonistic antibody to CD40 boosts the antitumor activity of adoptively transferred T cells in vivo. J Immunother. 2012;35:276282. doi: 10.1097/CJI.0b013e31824e7f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, O’Dwyer PJ. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286–6295. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dechanet J, Grosset C, Taupin JL, Merville P, Banchereau J, Ripoche J, Moreau JF. CD40 ligand stimulates proinflammatory cytokine production by human endothelial cells. J Immunol. 1997;159:5640–5647. [PubMed] [Google Scholar]
- 48.Medina-Echeverz J, Ma C, Duffy AG, Eggert T, Hawk N, Kleiner DE, Korangy F, Greten TF. Systemic agonistic anti-CD40 treatment of tumor-bearing mice modulates hepatic myeloid-suppressive cells and causes immune-mediated liver damage. Cancer Immunol Res–. 2015;3:557566. doi: 10.1158/2326-6066.CIR-14-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao ZA, Daniel D, Hanahan D. Sub-lethal radiation enhances anti-tumor immunotherapy in a transgenic mouse model of pancreatic cancer. BMC Cancer. 2002;2:11. doi: 10.1186/1471-2407-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Byrne KT, Vonderheide RH. CD40 stimulation obviates innate sensors and drives T cell immunity in cancer. Cell Rep. 2016;15:2719–2732. doi: 10.1016/j.celrep.2016.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spranger S, Dai D, Horton B, Gajewski TF. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell. 2017;31:711723e4. doi: 10.1016/j.ccell.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8 + T cells. J Exp Med. 2005;202:907912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barth RJ, Jr, Mule JJ, Spiess PJ, Rosenberg SA. Interferon gamma and tumor necrosis factor have a role in tumor regressions mediated by murine CD8 + tumor-infiltrating lymphocytes. J Exp Med. 1991;173:647658. doi: 10.1084/jem.173.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.