Abstract
The history of H2S - as an environmental toxin - dates back to 1700, to the observations of the Italian physician Bernardino Ramazzini, whose book “De Morbis Artificum Diatriba” described the painful eye irritation and inflammation of “sewer gas” in sewer workers. The gas has subsequently been identified as hydrogen sulfide (H2S), and opened three centuries of research into the biological roles of H2S. The current article highlights the key discoveries in the field of H2S research, including (a) the toxicological studies, which characterized H2S as an environmental toxin, and identified some of its modes of action, including the inhibition of mitochondrial respiration; (b) work in the field of bacteriology, which, starting in the early 1900’s, identified H2S as a bacterial product - with subsequently defined roles in the regulation of periodontal disease (oral bacterial flora), intestinal epithelial cell function (enteral bacterial flora) as well as in the regulation of bacterial resistance to antibiotics; and (c), work in diverse fields of mammalian biology, which, starting in the 1940’s, identified H2S as an endogenous mammalian enzymatic product, the functions of which - among others, in the cardiovascular and nervous system - have become subjects of intensive investigation for the last decade. The current review not only enumerates the key discoveries related to H2S made over the last three centuries, but also compiles the most frequently cited papers in the field which have been published over the last decade and highlights some of the current ‘hot topics’ in the field of H2S biology.
Keywords: gasotransmitters, nitric oxide, cystathionine-β-synthase, cystathionine gamma lyase, 3-mercaptopyruvate sulfurtransferase
Graphical Abstract
“Those who cannot learn from history are doomed to repeat it.”
(George Santayana)
1. H2S as an environmental toxin
The history of H2S - as an environmental toxin - dates back to 1700, to the observations of the Italian physician Bernardino Ramazzini (1633-1714), whose book “De Morbis Artificum Diatriba” [1] is the reason why he is widely considered the founding father of the discipline of occupational medicine1 [2]. The book did not gain a wide audience until it was translated to English and published in 1940 [3]. In a chapter entitled “Diseases of Cleaners of Privies and Cesspits” he described the painful irritation and inflammation of “sewer gas” on the eye in sewer workers. He noted that this inflammation can lead to subsequent bacterial infections, and that it can result in very severe health effects including blindness. Although Ramazzini was not aware of the chemical nature of the species responsible, with remarkable insight, Ramazzini hypothesized that when the cleaners disturbed the excrement during their work, an unknown volatile acid was produced, which was the cause of the adverse ocular effects. He also, correctly, predicted that this gas may also damage the lungs,2 although in his book he did not report any pulmonary pathologies in the workers he examined. He also surmised that this species may have been also responsible for the pungent smell of the excrement. Ramazzini also noted that copper coins in the workers’ pockets turn black on their surfaces, and hypothesized that this effect is due to the same, acidic, gaseous substance that is also causing the adverse effects on the eye. With this amazing insight, Ramazzini can be credited as the first scientist not only to propose H2S/copper chemistry in specific,3 but also to be a pioneer of three centuries of subsequent H2S research (Table 1) in general.
Table 1. Chronology of hydrogen sulfide research.
See the text for further context and literature citations.
| 1700 | Recognition of the adverse health effects of “sewer gas” |
| 1775–1776 | Discovery and elucidation of the chemical composition of H2S |
| 1785 | Recognizing the role of H2S in the intoxication of sewer workers in Paris |
| 1803–present | Animal experiments start: testing the toxicity of H2S (inhaled or injected, high doses) |
| 1862 | Literary description of sewer workers’ diseases (V. Hugo - Les Miserables) |
| 1863 | Sulfhemoglobin formation in response to H2S |
| 1865 | Suggestion that H2S induces “oxygen hunger” (chemical hypoxia) |
| 1877–1884 | Early experiments demonstrating that bacteria produce H2S |
| 1917–1960’s | H2S production by multiple bacterial species, role of cysteine, partial characterization of the enzymes involved |
| 1940 | Demonstration that H2S inhibits cytochrome C oxidase activity |
| 1942 | Demonstration of H2S production in mammalian tissues (during transsulfuration reactions) |
| 1950–1970’s | H2S production in mammalian cells and tissues, role of cysteine, partial characterization of the enzymes involved |
| 1948 | Evidence for the chemical formation of hybrid S/N species |
| 1959 | Enzymatic formation of polysulfides from 3-mercaptopyruvate |
| 1965 | Bacterial co-culture experiments suggesting the role of bacterial H2S as a factor that confers resistance against antimicrobials |
| 1966–1970 | Protective effects of H2S administration (balneotherapy) on cardiac and vascular function in animals |
| 1967 | Suggestion that H2S, produced by oral bacteria, contributes to halitosis |
| 1968 | Demonstration of H2S production in plants |
| 1970 | Description of a widespread H2S system in marine sediments |
| 1975 | Purification and biochemical characterization of CBS |
| 1977 | Discovery of deep-sea hydrothermal vents and associated biological communities |
| 1980-present | Characterization of H2S-based biological environments in deep-sea hydrothermal vents |
| 1982 | Detailed biochemical characterization of CBS and CSE-mediated H2S formation |
| 1983 | Detailed biochemical characterization of 3-MST-mediated H2S formation |
| 1989–90 | Evidence of detectable levels of (endogenous) H2S in brain samples from animals and humans |
| 1986 | H2S oxidation is coupled to oxidative phosphorylation in mitochondria of Solemya reidi |
| 1996 | Proof of H2S-producing enzymes in the brain; effects of low concentrations of H2S on neuronal function |
| 1997 | Proof of H2S-producing enzymes in blood vessels; effects of low concentrations of H2S on vessel function (in synergy with NO) |
| 1998 | Suggestions linking enteral H2S production to colonic inflammation |
| 2001 | Implication of KATP channels in the vascular effects of H2S |
| 2003 | Endogenous H2S is overproduced in Down Syndrome |
| 2004 | Demonstration of free radical/oxidant scavenging effects of H2S |
| 2004-present | Antioxidant/cytoprotective effects of H2S |
| 2005 | Demonstration of H2S-induced ‘hibernation’ in mice |
| 2005 | Demonstration of the pathogenic role of H2S overproduction in sepsis |
| 2005 | Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti- inflammatory nonsteroidal drugs |
| 2006 | Demonstration of DNA damage induced by H2S (high concentrations) |
| 2006 | Hydrogen sulfide suppresses leukocyte-mediated inflammation |
| 2006 | Formation of a nitrosothiol from the reaction of NO and H2S |
| 2007 | Demonstration of the effect of H2S on lifespan in C Elegans |
| 2007 | H2S generation from garlic-derived polysulfides |
| 2007 | Proangiogenic effect of H2S donation |
| 2007-present | Beneficial effect of H2S donation in cardiovascular, renal and inflammatory diseases and cancer |
| 2007-present | Multiple lines of studies investigating the mode of H2S’s cytoprotective, anti-inflammatory and preconditioning effects |
| 2007 | Cardioprotective effect of H2S in coronary ischemia-reperfusion |
| 2007 | Sulfide as an inorganic substrate of the mitochondrial electron transport in human cells |
| 2008 | GYY4137: synthesis and characterization of a slow-release H2S donor |
| 2008-present | Synthesis and characterization of multiple classes of H2S donors and combined donors |
| 2009 | Proangiogenic effect of endogenous H2S |
| 2009 | Sulfhydration: a posttranslational process triggered by H2S |
| 2010 | Human clinical trials with IK-1001 (sodium sulfide for i.v. injection) |
| 2011 | Proving the role of bacterial H2S as a factor that confers resistance against antimicrobials |
| 2013 | Polysulfides as signaling molecules in the CNS |
| 2013 | Demonstration of the pathogenic role of H2S overproduction in cancer and therapeutic potential of CBS inhibition |
| 2014 | First reports on the synthesis and characterization of mitochondrially targeted H2S donors |
| 2014–2016 | Demonstration that H2S facilitates DNA repair |
| 2015 | Demonstration that H2S production is required for dietary restriction benefits |
| 2016 | Phase II clinical trials with ATB-346, a H2S-releasing nonsteroidal anti-inflammatory drug development candidate |
In the late 1770’s Parisians experienced many accidents due to a gas emanating from its antiquated sewer system. A commission was appointed to study the causes of the accidents. Their report, published in 1785, described two types of poisonings: a mild form (affecting the eye, in line with Ramazzini’s observations) which they called “mitte” and a severe form of rapid, severe asphyxia which they designated as “plomb”. Although the initial report did not make the connection to H2S gas, subsequent reports did, and the concept of H2S-associated intoxication was born. It is likely that these public observations inspired the, vivid description of the ill effect of sewer gas (produced in the ancient sewer systems of Paris) of Victor Hugo in his classic book Les Miserables (1862). Hugo referred to the Parisian sewers as “the intestine of the Leviathan.” The presence of H2S gas in sewers was subsequently directly confirmed by Dupuytren in 1806 [6].
As a pure chemical species, the discovery of H2S (1775) is credited to Swedish-German chemist Carl Wilhelm Scheele (1742-1786), who produced it through the reaction of acid with metal (poly)sulfides or by heating sulfur in hydrogen gas.4 In 1776, the chemical composition of the gas was determined by the French chemist Claude Louis Berthollet (1789) who also noted its acidic chemical nature [7].
The first biological experiment (of which the records are available) that directly investigated the effect of pure H2S in animals was published in 1803 by French anatomist Francois Chaussier (1746-1828) [8]. He described the lethal effects of H2S gas, when the gas was absorbed through the skin, while the horse was allowed to breathe fresh air. He also documented adverse effects of H2S-containing solutions when administered directly into the stomach or into the rectum. Subsequent studies used injections of H2S-saturated solutions, and reported respiratory effects (excitation, deep breathing), convulsive movements, and, at high doses, asphyxia and death. Other studies, after injection of H2S-containing solutions into the venous system of animals, reported that some of the gas is exiting the animal through exhalation via the lung. Some of these early studies have also noted a change in blood color, an effect, which was subsequently attributed to the reaction of H2S with hemoglobin, producing sulfhemoglobinemia (the latter term was coined by Hoppe-Seyler in 1863, who also characterized the absorption spectrum of hemoglobin) [reviewed in: 7].
Over the subsequent 150 years, generations of toxicological researchers have investigated the toxicological effects of H2S in various species including human. The results of these studies are now summarized in comprehensive monographs, including limits and methods of its detection, occupational exposure limits, and the multiple biological effects of H2S on various cells and organs. [reviewed in: 7,9–15]. Among the more recent studies, the work of Attene-Ramos should be mentioned, who demonstrated the genotoxic effect of high doses of H2S [16]. Nicholson [17], Khan [18] and later Dorman and colleagues [19] have directly demonstrated the inhibition of cytochrome c oxidase activity ex vivo in tissues after H2S exposure of experimental animals, and implicating these effects in the disruption of respiratory and mitochondrial functions in the mammalian brain (and other tissues) after H2S exposure in vivo. Dorman and colleagues have also conducted comprehensive, state-of-the-art toxicology evaluation of H2S inhalation in various animal species, as well as provided the first microarray analysis demonstrating that H2S has wide-ranging effects on gene expression (associated with a variety of biological processes including cell cycle regulation, cellular division, DNA metabolism and repair, protein kinase regulation, cytoskeletal organization and biogenesis). [20,21].
As far as the mode of H2S’ toxic action, the initial theory focusing on the formation of sulfhemoglobin in blood, was subsequently put into question, when it was noted that patients with H2S poisoning do not exhibit sulfhemoglobinemia; later on, even the formation of sulfhemoglobin was called into question [reviewed in: 7]. It is currently accepted that H2S exerts its toxicological actions on the molecular level primarily through the inhibition of mitochondrial respiration, via inhibition of mitochondrial Complex IV (cytochrome c oxidase). Via this action, the consumption of O2 is inhibited and mitochondrial electron transport and ATP generation is blocked. Nervous and cardiac tissues, which have the highest oxygen demand, are especially sensitive to the disruption of oxidative metabolism [22]. The first precursor of this theory can be attributed to Kaufmann and Rosenthal in 1865, who proposed that H2S poisoning induces “oxygen hunger” and that H2S poisoning has similarities to suffocation [23]. The fact that H2S is an inhibitor of cytochrome c oxidase was already known in the 1940’s [24]; it has been further characterized by Chance in 1965 [25] and subsequently thoroughly characterized through three decades of work by Nichols, starting in 1975 [26,27]. However - as demonstrated through the work of dozens of investigators over the last five decades - the toxicological mode of H2S’ action is definitely more complex, as H2S is capable of interacting with multiple intra- and extracellular proteins, via a variety of actions (including sulfhydration reactions, see below); these actions are likely to mediate (or modulate) the toxicological action of this gas.
Although the toxic/metabolism-suppressing effects of H2S has many aspects, we would like to emphasize one particular discovery that generated a lot of attention and contributed to the expansion and further revitalization of the field of H2S research over the last decade. The subject relates to the concept of “H2S-induced, on-demand suspended animation” (or hibernation). In a short article in Science, in 2005, Roth and colleagues from the Fred Hutchinson Cancer Center in Seattle reported that “H2S induces a suspended animation-like state in mice” [28]. In this study, mice, subjected to H2S inhalation were shown to develop a “hibernation-like state”. When placed in an atmosphere of 20–80 ppm H2S gas, mice exhibited dose-dependent reductions in core body temperature and metabolic rate. Over the course of several hours of H2S exposure, the animals’ output (down to 10% of metabolic rate continued to decrease, as measured by their CO2 baseline). When the chamber of the animals was cooled, body temperature reached as low as 15 °C. These effects were found reversible after resuscitation at room air and warming of the chambers. The original “hibernation” studies were subsequently reproduced by other groups and it was suggested that some of the H2S-induced cardiovascular responses (e.g. decreased heart rate) may be consistent with the physiology of hibernation [29,30]. Although subsequent studies have indicated that (a) the metabolic effects are only minimal or nonexistent in larger animals and (b) a decreased physical activity of the mice (and the consequently decreased skeletal muscle-related energy consumption) is a significant contributor to the hibernation-like effects of H2S inhalation in conscious mice [31], the study by Roth and colleagues has attracted significant attention to multiple facets of H2S biology and has stimulated multiple lines of present-day work aimed to explore the potential beneficial and therapeutic effects of H2S donation (see also below in more detail).
2. H2S as an endogenous product in bacteria, plants and other non-mammalian species
The history of H2S - as a bacterial product - dates back to 1877 [32], to the work of French microbiologist Ulysse Gayon (1845–1919) who noticed the production of H2S by microbes in spoiled chicken eggs and described the use of paper strips impregnated with lead acetate for detecting the presence of this gaseous byproduct.5 In subsequent studies, Beijerinck [35], Schardinger [36] and Durham [37] described the production of H2S by putrefactive organisms associated with soil and feces. For example, in 1894, Schardinger observed that when water polluted with fecal material was added to peptone solution and incubated, it produces a characteristic odor, and that it blackens a strip of lead acetate paper which was suspended over the liquid [36].
In the subsequent 100 years, the formation of H2S has been confirmed in a large number of bacterial species - e.g. [38–51]; various substrates that stimulate H2S production have been identified, as well as the biochemical pathways involved in H2S production have been partially characterized. L-cysteine was found a common substrate in many bacterial species - e.g. [45,46]. In addition, thiosulfate has been shown to be a stimulator of bacterial H2S production via the action of bacterial thiosulfate reductase [47].6 It is now clear that the mammalian enzymes responsible for H2S production (cystathionine-beta-synthase, CBS; cystathionine-gamma-lyase, CSE and 3-mercaptopyruvate sulfurtransferase, 3-MST) have bacterial homologs with similar function.7 For example, in Klebsiella pneumoniae, cystathionine β-synthase is the main source of H2S, and it is encoded by mtcB [48], while in E. coli, 3-MST is the main source of H2S, and it is encoded by mstA [49,50].
For many decades, the actual function of the bacterially produced H2S remained largely unclear; it was considered a source of foul smell in wastewaters, perhaps as an environmental hazard, or, at best, an indicator of bacterial overgrowth in spoiled dairy or meat products [44].
Starting with Rizzo’s work in 1967, who demonstrated the production of H2S in periodontal pockets of patients [54], a distinct line of studies focused on H2S production by components of the oral microbiota, largely as a source of oral halitosis [45,54–58] or perhaps as a contributor to enamel damage associated with periodontal inflammation [59]. The severity of oral halitosis (and the efficacy of antibacterial mouth rinses) is commonly quantified using the Halimeter, a device that was designed as a H2S gas sensor for dentists [57,58].8,9
Another line of research focused on the production of H2S by bacteria of the intestinal microbiota, initially mainly as a component of flatus [62], but subsequently also as a potential regulator of intestinal epithelial cell function (proliferation, energetics) and colon function (mucus formation and inflammatory and carcinogenic responses) - as one of the many “effector” molecules produced by the intestinal microbiome) [63–67] as well as a potential source of circulating H2S for the host [67,68].
A recent line of studies, conducted in a range of bacteria (B. anthracis, P aeruginosa, S aureus, and E coli) by Nudler and colleagues (New York University School of Medicine) placed bacterial H2S production into a new light: multiple lines of data suggest that bacterial H2S acts as an endogenous, bacterial-derived protective factor, which renders these pathogens highly resistant to a multitude of antibiotics [49,50]. Similarly, bacterially produced H2S confers resistance to elimination by the immune system in vitro and in vivo [69]. These studies then identify the bacterial H2S-producing enzymes as potential targets for antimicrobial intervention. Interestingly, the idea that bacterial H2S confers bacterial resistance has a precursor study from the mid-60’s: Schutzenberger and Bennett (University of Houston, Texas), working with S. aureus/E. coli co-cultures noticed that the presence of E. coli (which was the more prominent H2S producer of the two bacteria) markedly reduced the sensitivity of the co-cultured S. aureus to mercury-based antibiotics and suggested that H2S (as a secreted, diffusible mediator) is responsible for this effect [43].
Not only bacteria, but a variety of non-mammalian species produce H2S, and utilize it for various biological processes. Plant-derived H2S production was first observed by De Cormis in 1968 [70]. It is now well established various plants produce H2S (primarily from sulfate), and it functions not only as a way to contribute to sulfur elimination, but also as a protective (e.g. fungo-toxic) and signaling molecule [71].
Marine biology has several interesting H2S-related aspects, one of which being the description [72] by Fenchel and Riedel the “sulfide system” as a new biotic community present underneath marine sediments worldwide. The next major discovery came after the discovery by Jack Corliss, in 1977, the existence of deep-sea hydrothermal vents, and their surrounding rich biological environments. The extensive sulfur chemistry/biology in deep sea hydrothermal vents - first described in the early 80’s - represents an example of ancient, life-supporting functions of H2S, with important evolutionary implications [73–75]. H2S is sometimes called “the sunlight of the deep ocean”, because, in this dark environment, H2S, emitted from the deep-sea volcanic vents is a vital source of metabolic energy for the primary producers of living matter - reviewed in [75,76]. In this environment, many sulfo-oxidant bacteria derive their metabolic energy from driving electrons from sulfide to oxygen. In addition, multicellular organisms (such as tubeworms) living in these deep-sea-vent “biological oases” utilize H2S as a primary source of energy. In this system, the worm takes up HS− from its environment, and, in turn, delivers sulfide to its internal bacterial symbionts, which, in turn, utilize it. According to one line of evolutionary theory, billions of years ago, life evolved near deep sea vents, and was therefore based on sulfide chemistry (rather than oxygen chemistry, as, at this time oxygen did not yet exist on the planet in significant amounts) [75–79].
From the multitude of non-mammalian life forms, the H2S-related aspects of the model organism C. elegans is also worth noting, because this organism is commonly used to address fundamental questions related to stress, lifespan and longevity. The first study to examine the effect of exogenously applied H2S on the life span of C. elegans was published by Miller and Roth in 2007 [80]. Several of the subsequent studies have focused on endogenously produced H2S in this organism. Similar to mammals and bacteria, three H2S-synthesizing enzymes are present in C. elegans, namely CSE, CBS and 3-MST, and regulate cell signaling, signal transduction, bioenergetics and lifespan [81–86].
4. H2S as an endogenous mammalian mediator
The history of H2S - as a mammalian product - dates back to the work of the American biochemist Vincent Du Vigneaud (1901–1978). “If one views the totality of du Vigneaud’s contributions to science, one recognizes a thread of continuity connecting sulfur-containing, biologically important compounds. This thread extends from insulin to cysteine, homocysteine, methionine, cystathionine, biotin, penicillin, oxytocin. and vasopressin” [87]. His work on oxytocin in the 1950’s (which included the delineation of its sequence, and its successful chemical synthesis) was recognized with the Nobel Prize in 1955.
However, a decade prior to his work on oxytocin, in the 1930’s and 1940’s, first at Washington University, and subsequently at Cornell University, Du Vigneaud started working on the oxidation of sulfur-containing amino acids in tissues and in whole animals [88]. discovered and named the transsulfuration pathway, a metabolic pathway involving the interconversion of cysteine and homocysteine, through the intermediate cystathionine. Du Vigneaud described this pathway in various mammalian tissues, including liver homogenates and originally simply termed in “transulfuration” [88].10 To prove the importance of the transsulfuration pathway, Du Vigneaud synthesized L-cystathionine, and demonstrated that this compound sustained growth of rats on a cysteine-deficient diet [89]. It was during the course of a series of transsulfuration-related studies, in a paper published by Francis Binkley and Vincent Du Vigneaud in 1942, the production of cysteine from homocysteine and serine was measured in liver homogenates, and data were presented to show the “production of H2S” [90]. The method for the H2S quantification was not disclosed in the paper, and the amount of H2S was noted with (++ or +++ symbols only). Surprisingly - other than a side note claiming that cyanide was able to inhibit H2S formation from the homogenates - the authors did not make any speculation with respect to any of the potential biological role of the H2S formed, even though, at the time of this paper, bacterial H2S production was already a known phenomenon (see previous section). 11 After 1942, to our knowledge, Du Vigneaud no longer studied mammalian H2S production, nor did he mention it his later papers. We must conclude that he must have failed to recognize its potential biological significance.
After Du Vigneaud’s early observations, for several decades, no published work can be found on the potential endogenous formation of H2S in mammalian cells or tissues. In the 60’s and 70’s, several groups of investigators have conducted increasingly more detailed and sophisticated characterizations of the biochemical properties of the enzymes of the transsulfuration pathway, including cystathionine-beta-synthase (CBS) and cystathionine-gamma-lyase (CSE), and in several of these reports, the H2S produced by purified enzymes or tissue extracts (commonly, rat liver homogenates) was also determined (typically quantified as nmoles of H2S/min per mg of protein) [91–99].12 These early reports include a study from Hylin and Wood showing that 3-MST results in polysulfide formation [99].13More detailed reports, published in the early 80’s, came from Stipanuk’s group, who have demonstrated that CBS and CSE (from L-cysteine and homocysteine) [101] as well as 3-MST (from 3-mercaptopyruvate) [102] can stimulate H2S generation in various mammalian tissues. However, these studies, as far as H2S was concerned, remained at the level of biochemical description, and there were no suggestions (much less experimental studies) to presume or to test any potential biological effect or relevance of this gas. In other words, until the early 90’s, the mammalian production of H2S, although already known on a biochemical level, was merely viewed as a biochemical phenomenon (of some interest), rather than a (potential) biological regulatory mechanism.
In 1989/1990, three independent groups - Warenycia and co-workers14 [103], Goodwin and co-workers [104], and Savage and Gould [105] reported their findings on the concentrations of H2S in brain tissues of animals [103] or human subjects [104,105] exposed to toxic doses of H2S. Although the original motivation of the work was to conduct toxicological/toxicokinetic analysis, to improve the area of environmental toxicology, in these papers, the surprising finding was also noted that normal brain tissues - i.e. in the absence of any external H2S exposure - contain significant amounts of H2S. For instance, Warenycia and co-workers quantified basal brain H2S levels as 1.2–1.7 μg/g tissue [103].15 In this report, the first speculation appears with respect to biological production and its potential physiological or pathophysiological role.16
Inspired by the above reports, Abe and Kimura (at the time, working at the Salk Institute in San Diego) decided to conduct direct experiments to test the potential role of H2S as an endogenous mediator in the CNS. The results of the resulting paper, published in 1996 [106] were two-fold. First, the study showed that CBS is highly expressed in the brain, it produces H2S and this can be blocked by prototypical CBS inhibitors (aminooxyacetate, hydroxylamine). Second, it showed that low concentrations of H2S (concentrations, that were considered physiological by the authors of the study at the time) affect neuronal function (e.g. enhance NMDA-mediated responses and facilitate long-term potentiation). Although the studies outlined in the paper did not definitely prove the functional role of endogenously produced H2S in the brain (e.g. it did not include studies examining the effect of inhibition of endogenous H2S production on neuronal functions), based on the data, Kimura suggested that endogenous H2S plays a functional role in the regulation of neuronal function. The paper became one of the highest-cited original paper in the field of H2S biology (receiving over 1,500 citations up till now) and it is widely considered a starting point for follow-up studies testing the functional role of endogenously produced H2S in various mammalian systems. After the paper was finally published in the Journal of Neuroscience in 1996, the preeminent neuroscientist/pharmacologist Solomon Snyder wrote an enthusiastic commentary in Science News [107], in which he stated: “They have very impressive evidence that H2S is a potential neurotransmitter. It’s an exciting paper that should stimulate a lot of people’s interest.”17
However, it took some time until this interest became apparent. Nevertheless, Kimura and his group continued his pioneering efforts in the field. In a separate line of studies, published in 1997, the Kimura group focused on smooth muscle function, and demonstrated the presence of H2S-producing enzymes in vascular tissue, and showed the smooth muscle relaxant effect of H2S, alone and in synergy with nitric oxide [108]. Once again, the functional effect of inhibition of endogenously produced H2S was not studied; nevertheless, the totality of the data suggested the emergence of a new endogenous regulatory factor (gasotransmitter) in the neuronal and cardiovascular system.
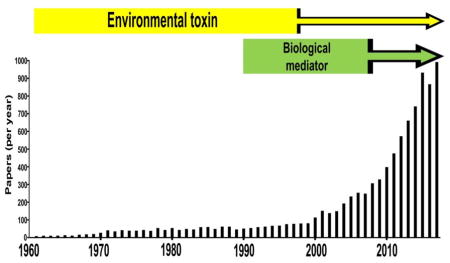
Rui Wang (at the time: University of Saskatchewan, Saskatoon, Canada) became interested in further investigating the cardiovascular regulatory roles of H2S. In his 2001 paper in the EMBO Journal [109], he set out to address the following questions: “Does H2S affect the cardiovascular function in vivo of whole animal or in vitro at the cellular level? Do the cardio- vascular effects of H2S have physiological significance?” In a series of elegant studies, his group has found answers to some of these questions, and demonstrated, among others, the importance of KATP channels in the vascular relaxant effect of H2S, and proved the role of endogenous H2S in the regulation of vascular tone and blood pressure [109–114]. He also proposed that H2S is the “third” biological gas (or “gasotransmitter”); the first and second ones being nitric oxide (NO) and carbon monoxide (CO), respectively [115–117]. Thus, although it might have taken several centuries, H2S was promoted from a toxic substance and environmental hazard into an important endogenous mammalian biological messenger. Some of the key observations along the way are reiterated in Table. 1.
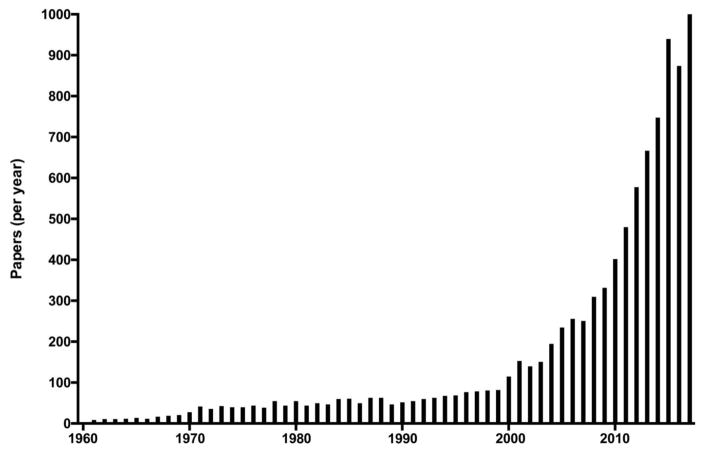
Over the last decade, a large number of groups have entered the field of H2S biology, and the field exploded in terms of quality and quantity of data (Fig. 1, Tables 2–4) [118–153]. Table 2 lists the most highly cited publications in the field of H2S biology, primarily over the last decade. Many of these highly cited papers can be grouped into several (in part, overlapping) categories: neuroscience [106,119,132,135,140,148], cardiovascular [34,107–113,114,118,121,123,127,128,130,133,136,139,141,145,152,153], inflammatory/cell death signaling [122,124–126,138,142,143] and metabolism [28,85,141,147,150]. Moreover - following the early observation of the formation of a nitrosothiol from the reaction of NO and H2S [154] - additional studies have investigated the various chemical and biological interactions between the NO and the H2S pathway [145,146,152,153,155]. The current “output” is approximately 1,000 published publications per year (according to PubMed) (Fig. 1). For the last decade, the exchange of information and the collaborations in the field of H2S biology have been significantly fostered by regular scientific meetings focusing on the biology and medicine of H2S (Table 3).
Figure 1. Number of published articles on H2S per year.
The figures are based on PubMed literature search and do not distinguish between biology or physics or chemistry related papers. The number of 2017 publications is a projection assuming that the publication rate seen in the first 8 months of the year continues at the same rate.
Table 2. Highly cited papers in the field of hydrogen sulfide biology.
Original research articles with at least 30 citations per year (based on Google Scholar) are compiled; review articles and “methods” papers were excluded from the analysis.
| Authors | Title | Reference | Citations | Reference |
|---|---|---|---|---|
| 1996 | ||||
| Abe K, Kimura H. | The possible role of H2S as an endogenous neuromodulator. | J Neurosci. 16:1066–71, 1996. | 1496 (71/yr) | 106 |
| 1997 | ||||
| Hosoki R, Matsuki N, Kimura H. | The possible role of H2S as an endogenous smooth muscle relaxant in synergy with nitric oxide. | Biochem Biophys Res Commun. 237:527–31, 1997. | 1091 (55/yr) | 107 |
| 2001 | ||||
| Zhao W, Zhang J, Lu Y, Wang R. | The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. | EMBO J. 2001 Nov 1;20(21):6008–16 | 1726 (108/yr) | 109 |
| 2002 | ||||
| Zhao W, Wang R. | H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. | Am J Physiol Heart Circ Physiol. 283:H474–80, 2002. | 543 (36/yr) | 110 |
| 2004 | ||||
| Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, Du J, Tang C. | Endogenous H2S regulation of myocardial injury induced by isoproterenol. | Biochem Biophys Res Commun. 318:756–63, 2004. | 462 (36/yr) | 118 |
| Kimura Y, Kimura H. | H2S protects neurons from oxidative stress. | FASEB J. 18:1165–7, 2004. | 726 (55/yr) | 119 |
| Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK. | The novel neuromodulator H2S: an endogenous peroxynitrite ‘scavenger’? | J Neurochem. 90:765–8, 2004. | 536 (41/yr) | 120 |
| Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. | H2S-induced relaxation of resistance mesenteric artery beds of rats. | Am J Physiol Heart Circ Physiol. 287:H2316–23, 2004. | 438 (34/yr) | 121 |
| 2005 | ||||
| Blackstone E, Morrison M, Roth MB. | H2S induces a suspended animation-like state in mice. | Science. 308:518, 2005. | 657 (55/yr) | 28 |
| Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. | H2S is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. | FASEB J. 19:1196–8, 2005. | 636 (53/yr) | 122 |
| Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V, Morelli A. | The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. | Hepatology. 42:539–48, 2005. | 368 (31/yr) | 123 |
| Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Di Sante M, Morelli A, Cirino G, Wallace JL. | Inhibition of H2S generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. | Gastroenterology. 129:1210–24, 2005. | 382 (32/yr) | 124 |
| 2006 | ||||
| Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim HR, Jeon SB, Jeon WK, Chae HJ, Chung HT. | H2S inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. | Free Radic Biol Med. 41:106–19, 2006. | 411 (35/yr) | 125 |
| Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. | H2S is an endogenous modulator of leukocyte-mediated inflammation. | FASEB J. 20:2118–20, 2006. | 645 (58/yr) | 126 |
| 2007 | ||||
| Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. | H2S attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. | Proc Natl Acad Sci USA. 104:15560–5, 2007. | 777 (78/yr) | 127 |
| Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. | The novel proangiogenic effect of H2S is dependent on Akt phosphorylation. | Cardiovasc Res. 76:29–40, 2007. | 302 (30/yr) | 128 |
| Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. | H2S mediates the vasoactivity of garlic. | Proc Natl Acad Sci USA. 104:17977–82, 2007. | 508 (51/yr) | 129 |
| 2008 | ||||
| Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. | Characterization of a novel, water-soluble H2S -releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. | Circulation. 117:2351–60, 2008. | 404 (45/yr) | 130 |
| Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. | H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. | Science. 322:587–90, 2008. | 1402 (155/yr) | 113 |
| Furne J, Saeed A, Levitt MD. | Whole tissue H2S concentrations are orders of magnitude lower than presently accepted values. | Am J Physiol Regul Integr Comp Physiol. 295:R1479–85, 2008. | 417 (46/yr) | 131 |
| 2009 | ||||
| Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H. | A source of H2S and a mechanism of its release in the brain. | Antioxid Redox Signal. 11:205–14, 2009. | 322 (40/yr) | 132 |
| Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D, Tang X, Ren Y, Tang C, Du J. | Role of H2S in the development of atherosclerotic lesions in apolipoprotein E knockout mice. | Arterioscler Thromb Vasc Biol. 29:173–9, 2009. | 240 (30/yr) | 133 |
| Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. | H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. | J Biol Chem. 284: 11601–12, 2009. | 244 (31/yr) | 134 |
| Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. | 3-Mercaptopyruvate sulfurtransferase produces H2S and bound sulfane sulfur in the brain. | Antioxid Redox Signal. 11:703–14, 2009. | 550 (69/yr) | 135 |
| Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. | H2S mediates cardioprotection through Nrf2 signaling. | Circ Res. 105:365–74, 2009. | 424 (53/yr) | 136 |
| Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. | Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. | J Biol Chem. 284:22457–66, 2009. | 290 (36/yr) | 137 |
| Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. | H2S signals through protein S-sulfhydration. | Sci Signal. 2:ra72, 2009. | 600 (75/yr) | 138 |
| Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. | Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces H2S. | J Biochem. 146:623–6, 2009. | 345 (43/yr) | 139 |
| Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabo C. | H2S is an endogenous stimulator of angiogenesis. | Proc Natl Acad Sci USA. 106:21972–7, 2009. | 432 (54/yr) | 34 |
| 2010 | ||||
| Kimura Y, Goto Y, Kimura H. | H2S increases glutathione production and suppresses oxidative stress in mitochondria. | Antioxid Redox Signal. 12:1–13, 2010. | 364 (52/yr) | 140 |
| Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. | H2S mediates O2 sensing in the carotid body. | Proc Natl Acad Sci USA. 107:10719–24, 2010. | 234 (33/yr) | 141 |
| 2011 | ||||
| Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. | H2S as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. | Circ Res. 109:1259–68, 2011. | 310 (52/yr) | 114 |
| Shatalin K, Shatalina E, Mironov A, Nudler E. | H2S: a universal defense against antibiotics in bacteria. | Science. 334:986–90, 2011. | 240 (40/yr) | 49 |
| Krishnan N, Fu C, Pappin DJ, Tonks NK. | H2S-induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. | Sci Signal. 4:ra86, 2011. | 211 (35/yr) | 142 |
| 2012 | ||||
| Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. | H2S-linked sulfhydration of NF-κB mediates its antiapoptotic actions. | Mol Cell. 45:13–24, 2012. | 296 (60/yr) | 143 |
| Fu M, Zhang W, Wu L, Yang G, Li H, Wang R. | H2S metabolism in mitochondria and its regulatory role in energy production. | Proc Natl Acad Sci USA. 109:2943–8, 2012. | 180 (36/yr) | 144 |
| Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Módis K, Panopoulos P, Asimakopoulou A, Gerö D, Sharina I, Martin E, Szabo C. | H2S and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. | Proc Natl Acad Sci USA. 109:9161–6, 2012. | 278 (42/yr) | 145 |
| Filipovic MR, Miljkovic JLj, Nauser T, Royzen M, Klos K, Shubina T, Koppenol WH, Lippard SJ, Ivanovi-Burmazovi I. | Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. | J Am Chem Soc. 134:12016–27, 2012. | 171 (34/yr) | 146 |
| 2013 | ||||
| Módis K, Coletta C, Erdélyi K, Papapetropoulos A, Szabo C. | Intramitochondrial H2S production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics | FASEB J. 27: 601–611, 2013. | 118 (30/yr) | 147 |
| Kimura Y, Mikami Y, Osumi K, Tsugane M, Oka J, Kimura H. | Polysulfides are possible H2S-derived signaling molecules in rat brain. | FASEB J. 27:2451–7, 2013. | 146 (37/yr) | 148 |
| Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A. | Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). | Br J Pharmacol. 169:922–32, 2013. | 135 (34/yr) | 149 |
| Szabo C, Coletta C, Chao C, Módis K, Szczesny B, Papapetropoulos A, Hellmich MR. | Tumor-derived H2S, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. | Proc Natl Acad Sci USA. 110:12474–9, 2013. | 165 (41/yr) | 150 |
| Shibuya N, Koike S, Tanaka M, Ishigami-Yuasa M, Kimura Y, Ogasawara Y, Fukui K, Nagahara N, Kimura H. | A novel pathway for the production of H2S from D-cysteine in mammalian cells. | Nat Commun. 4:1366, 2013. | 170 (42/yr) | 151 |
| 2014 | ||||
| King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. | H2S cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. | Proc Natl Acad Sci USA. 111:3182–7, 2014. | 131 (43/yr) | 152 |
| Eberhardt M, Dux M, Namer B, Miljkovic J, Cordasic N, Will C, Kichko TI, de la Roche J, Fischer M, Suárez SA, Bikiel D, Dorsch K, Leffler A, Babes A, Lampert A, Lennerz JK, Jacobi J, Martí MA, Doctorovich F, Högestätt ED, Zygmunt PM, Ivanovic-Burmazovic I, Messlinger K, Reeh P, Filipovic MR. | H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. | Nat Commun. 5:4381, 2014. | 116 (38/yr) | 153 |
| 2015 | ||||
| Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, Longchamp A, Treviño-Villarreal JH, Mejia P, Ozaki CK, Wang R, Gladyshev VN, Madeo F, Mair WB, Mitchell JR. | Endogenous hydrogen sulfide production is essential for dietary restriction benefits. | Cell. 160:132–44. 2015. | 94 (47/yr) | 85 |
| Cortese-Krott MM, Kuhnle GG, Dyson A, Fernandez BO, Grman M, DuMond JF, Barrow MP, McLeod G, Nakagawa H, Ondrias K, Nagy P, King SB, Saavedra JE, Keefer LK, Singer M, Kelm M, Butler AR, Feelisch M. | Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. | Proc Natl Acad Sci USA. 112: E4651–60, 2015 | 76 (38/yr) | 155 |
Table 4. Decisive moments and future directions in hydrogen sulfide research.
initial motivation to enter the field, and areas of future interest.
| Investigator | Current affiliation | What and was the decisive moment when you decided to start working in the field of H2S biology? | What do you consider the most important tasks to be addressed in the future of H2S biology? |
|---|---|---|---|
| Giuseppe Cirino | University of Naples | In 2000/2001, we were working at the development of NSAIDS releasing NO when we came across this second mediator we thought to start a side project. The data turned out to be so interesting that the side project became the main project in a few years. | The interplay among H2S, the NO pathway and eicosanoids in health and disease. To define if the H2S pathway is a feasible target to develop therapeutics in major disease areas (cardiovascular, inflammation and cancer). |
| Martin Feelisch | University of Southampton, UK | n 1986, during my thesis work on the molecular mode of action of nitrovasodilators I “messed around” with sulfide (inorganic salts and H2S gas) and many other small thiols to study soluble guanylate cyclase activation. Although I noticed that it (redox)modulated the responses of several NO-donors I didn’t make much of it at the time as the focus of my work was on NO. I re-entered the field for real in 2012 by which time the NO/H2S cross-talk story had surfaced across all disciplines. | How the different forms of sulfur work at a systems level, a topic that will require the analysis of metabolite fluxes under dynamic conditions. |
| Hideo Kimura | National Center of Neurology and Psychiatry Kodaira, Tokyo, Japan | In 1993, in the library of Salk Institute I found a metabolic map showing mammalian enzymes which can produce H2S. Subsequently, I came across papers by Goodwin et al., Warenycia et al., and Savage and Gould, reporting the endogenous sulfide in mammalian brains. | Regulation is not well understood (including polysulfides). |
| David Lefer | Louisiana State University, New Orleans, LA, USA | In 2005, while working at Albert Einstein College of Medicine. We received a call from a group at Ikaria to discuss the potential use of H2S-based therapies during heart attack. We also felt that since H2S was an endogenously produced gas that it might behave similar to nitric oxide (NO) and this was of great interest to us. | To further define the precise role of each of the 3 enzymes that produce H2S and map them to organs and disease states. We should also define how H2S, NO, and CO interact under physiological and pathological states. We also need to develop far better H2S releasing and donating drugs with long half lives and oral activity. |
| Philip Moore | National University of Singapore, SIngapore | In 2000, I was working on NO and NOS inhibitors and looking for something ‘fresh’ to study. I suggested to a student doing her final year undergraduate project that we take a look at the effect of H2S on the guinea pig ileum in the organ bath – ‘classical’ pharmacology! The results were obvious and clear cut, very interesting and in parts difficult to interpret - which turns out to be a very good description of my experience with H2S over the following two decades. | Many things still need to be properly investigated. There are unresolved question about the sensitivity and specificity of assays as well as not only how but where to measure H2S in vivo? Add to this the multitude of possible targets for this gas within the cell and the picture is extraordinarily complicated. Building on the knowledge obtained to date and then translating it to develop useful therapeutics will be the challenge for the next decade. |
| Peter Nagy | National Institute of Oncology, Budapest, Hungary | In 2004, I often could smell the distinct odor of H2S during my mechanistic studies on the redox reactions of cysteine derivatives and other biological sulfur species. This made me excited about exploring whether the rich chemical properties of the smallest thiol molecule (sulfide) could contribute to regulation of living systems. When I looked into the scientific literature I noticed that a few investigators already started to research H2S biology. I conducted my first experiments in 2007. | To further elucidate the underlying chemical mechanisms of sulfide’s biological actions with special focus on the roles of reactive sulfur species regulated pathways in cancer biology. To elucidate the specific biological actions of these molecules in order to be able to most precisely target them for therapy and to avoid side effects. |
| Evgeny Nudler | New York University Langone Medical Center, New York, NY, USA | Around 2009 we’ve published several papers on the protective role of NO in bacteria. I was thinking that, by analogy with mammals, H2S should be an important signaling molecule in bacteria as well. When we looked at hundreds of sequenced bacterial genomes we realized that virtually all of them have paralogs of mammalian 3MST or CBS/CSE. We started to examine whether H2S endows bacteria with some evolutionary advantages. | With respect to bacteria it would be important to establish H2S-mediated signaling pathways. To my knowledge, protein S-sulfhydration has not yet been investigated in bacteria. |
| Andreas Papapetropoulos | University of Athens, Greece | In 2006 I invited Drs. C. Szabo and G. Cirino to a Greek Pharmacological Society Meeting. At dinner, we discussed current projects and H2S emerged as a new molecule that was potentially important in the cardiovascular system. It shared many characteristics with NO, a molecule I had been working with for 15 years. Later this year, in collaboration with Ikaria we started working on H2S and angiogenesis. | Accurately measuring H2S levels in cells, tissues and biological fluids, understanding how production of H2S is regulated short term so that it can exert its signaling functions, how deregulation of H2S levels contributes to the development of disease, discovering better pharmacological tools to modulate its levels. |
| Csaba Szabo | University of Texas, Galveston, TX, USA | 2002. Through my interest in vascular biology, I became aware of the studies of Dr. Kimura and Dr. Wang in the early 2000’s. In 2006, I started became Chief Scientific Officer of the company Ikaria and led research on various aspects of H2S biology and started developing an injectable H2S formulation IK-1001 and progressed it into human trials. | To translate the many basic observations in the field of H2S into novel therapeutic agents. In general: to discover and develop H2S biosynthesis inhibitors and H2S donors and to match them with the appropriate diseases (development indications). In specific: to introduce CBS inhibitors into clinical trials for cancer. |
| John Wallace | University of Calgary and Antibe Therapeutics Inc., Canada | Early 2003. Myself and Giuseppe Cirino had a discussion about the possibility that H2S may be a more ‘benign’ molecule than NO with respect to being protective in the GI tract. We began to explore the development of H2S-releasing NSAIDs. | Development of simpler but reliable methods for measuring H2S production (and localization) in vivo. |
| Matt Whiteman | University of Exeter Medical School, UK | 2000. I’d followed some of the early work as a side-interest but it wasn’t until Phil Moore gave a talk on H2S/NO cross-talk at the National University of Singapore in 2003 where I was working at the time that I became ‘properly’ interested. Since then H2S has been shown to be critical for mitochondrial function and I started working on developing novel slow- release mitochondria-targeted H2S delivery molecules for therapeutic and agricultural use. The side-interest has now become an obsession. | There is a very long list. Primarily for me (i) following through successful studies in animal models to establishing the clinical efficacy of mitochondria- targeted H2S donors and (ii) determining how much of the published effects of H2S and / or its intermediates are real and not just test-tube phenomena. |
Table 3.
Conferences on hydrogen sulfide biology.
| Meeting | Date | Location | Main organizer(s) |
|---|---|---|---|
| 1st International Conference of Hydrogen Sulfide in Biology and Medicine | June 26–28, 2009 | Shanghai, China | Rui Wang & Yi Zhun Zhu |
| 1st European Conference on the Biology on Hydrogen Sulfide | June 15–18, 2012 | Smolenice, Slovakia | Karol Ondrias |
| 2nd International Conference on Hydrogen Sulfide Biology and Medicine | September 20–22, 2012 | Atlanta, GA, USA | David Lefer |
| 2nd European Conference on the Biology of Hydrogen Sulfide | September 8–11, 2013 | Exeter, UK | Matt Whiteman |
| 3rd International Conference on Hydrogen Sulfide in Biology and Medicine | June 4–6, 2014 | Tokyo, Japan | Hideo Kimura |
| 3rd European Conference on the Biology of Hydrogen Sulfide | May 3–6, 2015 | Athens, Greece | Andreas Papapetropoulos |
| 4th International Conference on the Biology of Hydrogen Sulfide | June 3–5, 2016 | Naples, Italy | Giuseppe Cirino |
| 5th World Congress on Hydrogen Sulfide in Biology and Medicine* | June 1–3, 2018 | Toronto, Canada | John Wallace |
Planned. Please note that with this meeting, the European and International conferences will be merged and are going to be held every 2 years.
From the highly cited reports highlighted in Table 2, as well as thousands of additional studies published over the last decade, it became increasingly clear that H2S, at low concentrations (or low, steady rates of generation, in case of H2S donor compounds), has an entirely different pharmacological profile than what was previously characterized in the toxicological literature (where, typically, high concentrations were used to demonstrate adverse/noxious effects). Hence, the often-mentioned concept of the “bell-shaped pharmacological profile of H2S” was born. For example, at low concentrations, H2S was found to be cytoprotective [118,119,124,127,152] (as opposed to the cytotoxicity observed at higher concentrations). In addition, H2S turned out to have antioxidant/free radical and oxidant-scavenging effects [119,120,140], in addition to the previously known pro-oxidant and cytotoxic effects. H2S, at low concentrations, was also recently recognized to exert protective effects against DNA damage [156,157] - as opposed to its previously established DNA-damaging actions seen at high concentrations. Various anti-inflammatory effects of H2S have also been demonstrated [122], in stark contrast to the previous concept that proclaimed that H2S promotes inflammation. Finally, H2S, when exogenously administered at low concentrations, or when endogenously produced at biological rates, was found to exert stimulatory effects on cellular respiration [144,147,150,158,159] - in stark contrast to the well-established inhibitory effect on Complex IV, which only became apparent at higher concentrations.
With the use of pharmacological inhibitors, as well as mice lacking various H2S-producing enzymes, and by using transient and stable silencing of H2S-producing enzymes, the functional role of endogenously produced H2S is being delineated in more detail (as opposed to testing the effect of low concentrations of exogenously applied H2S on biological systems). There were also significant advances made in the field of H2S-mediated signaling, including the concept of sulfhydration (post-translational modification of protein cysteine residues, with functional consequences), which is prominently induced by polysulfides [114,138,142,143]. Indeed, polysulfides are emerging as a distinct class of sulfur-containing biological signaling molecules, with properties that are different from those of H2S [148]. A separate pathway of H2S production, from D-cysteine, has also been discovered by Kimura and his group in 2013, with relevance for the kidney and possibly other organs, as well [151]. Another area within the growing field of H2S biology relates to the interactions of H2S with various oxygen-and nitrogen-derived species, which includes functional interactions (for example at the level of the Akt signaling and the cGMP signaling) [145,152] as well as the recognition of the biological roles of various hybrid S/N species [146,155].18
The above mechanistic revelations (as well as many additional lines of studies that are too numerous to be mentioned here in detail) have also led to new concepts focusing on pharmacological supplementation (donation) of H2S, for therapeutic benefit, for pathophysiological states where H2S levels are diminished (either due to decreased production or increased consumption), including cardiovascular disease states (e.g. ischemia-reperfusion, vascular disease) [118,127,133] and to induce cytoprotection against the toxic side effects of various drugs [124]. These effects, at least in part, may also be related to the newly recognized proangiogenic effects [34,128] of H2S. It may be worth mentioning that the roots of the cardiovascular protective effect of H2S can be traced back to a parallel line of studies that began in the 50’s, focusing on balneotherapy (beneficial effects of H2S-containing spring baths) [162–164].19 The first long-acting H2S donor, synthesized and characterized by Philip Moore’s group in Singapore, was designated as GYY4137; this compound was found to exert beneficial blood pressure and vascular effects in the initial report [130], and was subsequently used in many dozens of follow-up studies and showed therapeutic benefit in many systems. The synthesis of GYY4137 was followed by the synthesis and characterization of multiple classes of H2S donors, some of them with targeting to various organelles (e.g. mitochondria) [156,165]. Another interesting group of H2S donors is the polysulfides, which include garlic-derived natural compounds, with beneficial vascular effects [129]. Several groups have also began synthesizing H2S donor compounds where a known drug (e.g. a non-steroidal anti-inflammatory) was coupled with a H2S donor group; the most advanced member of this type of compounds have progressed into Phase II clinical trials [166].
In contrast to cardiovascular diseases, where H2S levels are decreased, it was also recognized that in many other disease conditions - e.g. various forms of systemic inflammation [122], as well as several forms of cancer, including colon cancer [150] - circulating H2S levels are increased due to the upregulation of H2S-producing enzymes; in these conditions, pharmacological inhibition of H2S biosynthesis shows therapeutic potential.
The current article cannot cover all aspects of the explosion in the field that occurred over the last decade. The state-of-the-art of H2S biology can be overviewed in general reviews and monographs [116,167–174] as well as in specialized review articles focusing on the roles of H2S in the regulation of nervous system [175–179], cardiovascular system [180–186], gastrointestinal system [187–190], renal system [191–193], metabolic and mitochondrial aspects [66, 194–196], cellular signaling [197–202], and the biochemistry of the various H2S-producing enzymes [203–205]. Separate articles focus on the details of the chemical properties and reactivity of H2S and its functional interactions with other reactive species including oxygen-derived oxidants and free radicals and NO [206–214]. Other review articles focus on pharmacological donors and inhibitors of H2S biosynthesis [215–218], and on the therapeutic aspects and translational potential of H2S biology [218–225].
Although the field of H2S biology has dramatically expanded over the last decade, many topics and issues remain that will demand continuing attention. Some of these “hot” issues are listed in Table 4 by investigators currently active in the field. It is hoped that the current article not only gives a somewhat accurate overview of the field, but also stimulates future work in this area.
Acknowledgments
The work of the author in the field of H2S is supported by grants from the National Institutes of Health (R01GM107846 and R01GMCA175803) and the Cancer Prevention Research Institute of Texas (DP150074). The helpful comments of Drs. Martin Feelisch, Hideo Kimura, Andreas Papapetropoulos and Matt Whiteman and are appreciated. The English translation of the Russian-language articles by Dr. Nadiya Druzhyna is also appreciated.
Abbreviations
- ATP
adenosine triphosphate
- CBS
cystathionine-β-synthase
- CSE
cystathionine gamma lyase
- H2S
hydrogen sulfide
- 3-MST
3-mercaptopyruvate sulfurtransferase
- NO
nitric oxide
- ROS
reactive oxygen species
Footnotes
Hippocrates’ counsel (“When you come to a patient’s house, you should ask him what sort of pain he has, what caused them, how many days he has been ill, whether the bowels are working and what sort of food he eats”), was extended by Ramazzini as follows: “I may venture to add one more question: What occupation does he follow?”
“I am inclined to think that some volatile acid is given off by this camerine of filth when workers disturb it… such effluvia ought, one would think, to impair the lungs. Nevertheless, it is only against the eyes that these foul exhalations wage ruthless war, and they attack them so cruelly with their piercing stings that they rob them of life…”
Subsequent work revealed that the copper-H2S reaction has multiple biological implications. For instance, in biological contexts, the binding of H2S to copper plays a key role in the H2S-mediated inhibition of mitochondrial Complex IV [4] (see, for more detail, below), and H2S-copper reactions are responsible for the sensitive detection of H2S by the olfactory nerves [5].
As a follower of the erroneous “phlogiston theory”, which was dominant in his time, he considered it a combination of sulfur, phlogiston and heat. (Through separate lines of work, Scheele is also credited as the co-discoverer of oxygen or “fire air” as well as the elements chlorine and manganese.)
The eggy smell of eggs, then, at least in part, is due to H2S: H2S production by eggs has been subsequently confirmed by several groups (e.g. 33). However, whether H2S in eggs has any biological role remains unclear. It is worth noting that in 2009, we have demonstrated (34) that H2S stimulates angiogenesis in chicken chorioallantoic membranes (developing chick embryo), and that inhibitors of H2S production suppress angiogenesis in this experimental system.
In mammalian systems, thiosulfate has been long considered an inactive degradation product of H2S, until multiple lines of evidence (52,53) showed that thiosulfate can, in fact, act as a substrate for mammalian H2S biosynthesis.
From the evolutionary standpoint, the correct way of stating would be to say that the bacterial enzymes have mammalian homologs.
It should be noted, however, that this electrochemical sensor is not absolutely specific for H2S and also produces a signal with other small volatile sulfhydryl compounds such as methanethiol.
We have subsequently repurposed this device to measure, in animals and in human volunteers, exhaled H2S levels (basal levels as a result of physiological processes, and increased levels after H2S donor administration) [60,61].
Originally the word was spelled with one “s”; later on, the current spelling (transsulfuration) became widely accepted. Also, mammalian transulfuration was later re-named as “reverse transulfuration”, while the bacterial transulfuration system became known as “forward transulfuration”.
It is remarkable that in a 50-page comprehensive biography prepared by Klaus Hofmann for the National Academy of Sciences published in 1987 - although the concept of transulfuration is discussed in detail - there is no mention of Du Vigneaud’s contribution to the biological production of H2S. Nor is the pioneering work of Du Vigneaud mentioned in any of the last decades’ review articles focusing on pathways of endogenous H2S production.
Many of these early reports can only be found in the early German and French scientific literature.
Subsequent work, by Kimura’s group demonstrated that polysulfides, on their own (i.e. without conversion to H2S) act as biological mediators involved, among others, in the post-translational modification of proteins via sulfhydration [100].
Not surprisingly, a lot of the toxicological work on H2S comes from Alberta, Canada, where H2S exposure in the petrochemical industry has been a serious problem. Warenycia and co-workers were based at the University of Edmonton, and this particular, pioneering piece of work was supported by Alberta Community and Occupational Health through the Heritage Trust Research Program.
The absolute concentrations of biologically produced H2S have been and remain controversial; it is now widely accepted that in these early reports, the absolute values of H2S production have been overestimated due to methodological issues.
“Although the exact function of this sulfide is unknown, there are enzymatic processes that lead to the formation of H2S and to its rapid metabolism in vivo. Therefore, it remains to be seen whether endogenous levels are of physiologic significance with respect to mechanisms underlying the control of neuronal excitability. Furthermore, it will be of interest to determine whether elevation of intraneuronal or CNS sulfide levels correlations with known pathophysiological states…” [63].
One of these people was Professor Snyder himself. Although it took more than 10 years after Abe and Kimura’s paper until Snyder’s first paper on H2S appeared in the literature, his group has contributed significantly to the field, among others by identifying the process of sulfhydration (posttranslational modification of proteins) (see below for further details).
(The roots of the chemical interactions between H2S and nitrogen-containing reactive species can be traced back to the German chemistry literature [160,161]; however, the recognition that such species have biological relevance is a recent one.
Much of this information can be found in the Russian-language literature. For instance, in the study by Kubli, rabbits were feed with cholesterol during 25–30 days (to induce hypercholesterinemia) and for 120–140 days (to induce atherosclerosis). H2S baths with H2S concentrations of 150–180 mg/kg were applied in the course of 12–15 procedures, every other day, at 37 °C, for 15 min. Control group received regular water bath. Activity of cytochrome oxidase was measured in heart, liver, kidney, adrenal glands and hypothalamus by colorimetric assay. The study found that experimental atherosclerosis lead to decreased activity of cytochrome oxidase in all studied organs even at early stage of pathology (25–30 days); H2S bath increased activity of cytochrome oxidase, particular in heart and hypothalamus. In another study by N.R. Chepikova, atherosclerosis was induced in by cholesterol feeding and via decreasing thyroid function by 6-methylthiouracyl for 170 days. H2S bath therapy was administered similar to the rabbit study outlined above. H2S baths improved hemodynamic state in atherosclerosis dogs; restored arterial blood pressure and improved vascular function.
Author Disclosure Statement
C.S. is a founder, officer and shareholder of CBS Therapeutics Inc., an UTMB spin-off company focusing on therapeutic approaches around H2S biosynthesis inhibition in cancer cells.
Author contributions
CS – conceived the topic, researched the literature, interviewed - via email - several investigators working in the field of H2S, and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramazzini B. Mutinae (Modena) 1700. De Morbis Artificum Diatriba. Antonii Capponi. [Google Scholar]
- 2.Felton JS. The heritage of Bernardino Ramazzini. Occup Med. 1997;47:167–179. doi: 10.1093/occmed/47.3.167. [DOI] [PubMed] [Google Scholar]
- 3.Ramazzini B. The Latin text of 1713, rev. with translation and notes by W. C. Wright. University of Chicago Press; Chicago, IL: 1940. De Morbis Anificum Diatriba; pp. 151–157. [Google Scholar]
- 4.Nicholls P, Kim JK. Oxidation of sulphide by cytochrome aa3. Biochim Biophys Acta. 1981;637:312–20. doi: 10.1016/0005-2728(81)90170-5. [DOI] [PubMed] [Google Scholar]
- 5.Duan X, Block E, Li Z, Connelly T, Zhang J, Huang Z, et al. Crucial role of copper in detection of metal-coordinating odorants. Proc Natl Acad Sci USA. 2012;109:3492–7. doi: 10.1073/pnas.1111297109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupuyturen M. Rapport sur une espéce de mephitisme des fosses d’aisance, produite par le gas azote. Journal de Medicine. 1806;IX:187–213. [Google Scholar]
- 7.Mitchell CW, Davenport SJ. Hydrogen sulphide literature. Public Health Rep. 1924;39:1–13. [Google Scholar]
- 8.Chaussier F. Précis d’experiences faites sur les animaux avec le gaz hydrogéne sulfuré. J Gen de Med, Chir et Pharm Paris. 1908;15:19–39. [Google Scholar]
- 9.Beauchamp RO, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. A critical review of the literature on hydrogen sulfide toxicity. CRC Crit Rev Toxicol. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- 10.Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Ann Rev Pharmacol Toxicol. 1992;32:109–34. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- 11.Milby TH, Baselt RC. Hydrogen sulfide poisoning: clarification of some controversial issues. Am J Ind Med. 1999;35:192–5. doi: 10.1002/(sici)1097-0274(199902)35:2<192::aid-ajim11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 12.Knight LD, Presnell SE. Death by sewer gas: case report of a double fatality and review of the literature. Am J Forensic Med Pathol. 2005;26:181–5. [PubMed] [Google Scholar]
- 13.Support of Summary Information on the Integrated Risk Information System (IRIS) U.S. Environmental Protection Agency; Washington, DC: Jun, 2003. Toxicological Review of Hydrogen Sulfide. (CAS No. 7783-06-4) [Google Scholar]
- 14.Guidotti TL. Hydrogen sulfide intoxication. In: Lotti M, Bleecker ML, editors. Handbook of Clinical Neurology. Vol. 131. Elsevier; 2015. [DOI] [PubMed] [Google Scholar]
- 15.Toxicological Profile for Hydrogen Sulfide and Carbonyl Sulfide. US Department of Health and Human Services Public Health Service - Agency for Toxic Substances and Disease Registry; Nov, 2016. [PubMed] [Google Scholar]
- 16.Attene-Ramos MS, Wagner ED, Plewa MJ, Gaskins HR. Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res. 2006;4:9–14. doi: 10.1158/1541-7786.MCR-05-0126. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson RA, Roth SH, Jian Zheng AZ. Inhibition of respiratory and bioenergetic mechanisms by hydrogen sulfide in mammalian brain. J Toxicol Environment Health. 1998;54:491–507. doi: 10.1080/009841098158773. [DOI] [PubMed] [Google Scholar]
- 18.Khan AA, Schuler MM, Prior MG, Yong S, Coppock RW, Florence LZ, et al. Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicol Appl Pharmacol. 1990;103:482–90. doi: 10.1016/0041-008x(90)90321-k. [DOI] [PubMed] [Google Scholar]
- 19.Dorman DC, Moulin FJM, McManus BE, Mahle KC, James RA, Struve MF. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol Sci. 2002;65:18–25. doi: 10.1093/toxsci/65.1.18. [DOI] [PubMed] [Google Scholar]
- 20.Dorman DC, Struve MF, Gross EA, Brenneman KA. Respiratory tract toxicity of inhaled hydrogen sulfide in Fischer-344 rats, Sprague-Dawley rats, and B6C3F1 mice following subchronic (90-day) exposure. Toxicol Appl Pharmacol. 2004;198:29–39. doi: 10.1016/j.taap.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Roberts ES, Thomas RS, Dorman DC. Gene expression changes following acute hydrogen sulfide (H2S)-induced nasal respiratory epithelial injury. Toxicol Pathol. 2008;36:560–567. doi: 10.1177/0192623308317422. [DOI] [PubMed] [Google Scholar]
- 22.Ammann HM. A new look at physiologic respiratory response to hydrogen sulfide poisoning. J Hazardous Mater. 1986;13:369–374. [Google Scholar]
- 23.Kaufmann S, Rosenthal J. Über die Wirkungen der Schwefelwasserstoffs auf den Tierischen Organismus. Arch f Anat Physiol u Wissensch Med Leipz. 1895:659–675. [Google Scholar]
- 24.Krahl ME, Keltch AK, Neubeck CE, Clowes GH. Studies on cell metabolism and cell division: V. Cytochrome oxidase activity in the eggs of Arbacia Punctulata. Gen Physiol. 1941;24:597–617. doi: 10.1085/jgp.24.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chance B, Schoener B. High and low energy states of cytochromes. I. In mitochondria. J Biol Chem. 1965;241:4567–4573. [PubMed] [Google Scholar]
- 26.Nicholls P. The effect of sulphide on cytochrome aa3. Isoteric and allosteric shifts of the reduced a-peak. Biochm Biophys Acta. 1975;396:24–35. doi: 10.1016/0005-2728(75)90186-3. [DOI] [PubMed] [Google Scholar]
- 27.Nicholls P, Marshall DC, Cooper CE, Wilson MT. Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem Soc Trans. 2013;41:1312–6. doi: 10.1042/BST20130070. [DOI] [PubMed] [Google Scholar]
- 28.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 29.Volpato GP, Searles R, Yu B, Scherrer-Crosbie M, Bloch KD, Ichinose F, et al. Inhaled hydrogen sulfide: a rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology. 2008;108:659–668. doi: 10.1097/ALN.0b013e318167af0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seitz DH, Fröba JS, Niesler U, Palmer A, Veltkamp HA, Braumüller ST, et al. Inhaled hydrogen sulfide induces suspended animation, but does not alter the inflammatory response after blunt chest trauma. Shock. 2012;37:197–204. doi: 10.1097/SHK.0b013e31823f19a0. [DOI] [PubMed] [Google Scholar]
- 31.Li RQ, McKinstry AR, Moore JT, Caltagarone BM, Eckenhoff MF, Eckenhoff RG, et al. Is hydrogen sulfide-induced suspended animation general anesthesia? J Pharmacol Exp Ther. 2012;341:735–742. doi: 10.1124/jpet.111.187237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gayon MU. Note presentee par M. Pasteur. Sur les alterations des oeufs, a l’occasion d’une Note de MM. A. Bechamp et G. Eustache. Ann Ecole Normale Super Sér2. 1877;IV:205. [Google Scholar]
- 33.Germs AC. Hydrogen sulphide production in eggs and egg products as a result of heating. J Sci Food Agric. 1973;24:7–16. doi: 10.1002/jsfa.2740240103. [DOI] [PubMed] [Google Scholar]
- 34.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA. 2009;106:21972–7. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beijerinck WM. Über Spirillum Desulfuricans als Ursache von Sulfatreduction. Zentralbl Bakteriol Parasitenk Infektionskr Hyg Abt Orig. 1895;21:104–114. [Google Scholar]
- 36.Schardinger R. Beitrag zur hygieneschen Beurteilung des Trinkwassers. Zentralbl Bakteriol Parasitenk Infektionskr Hyg Abt Orig. 1984;16:833–859. [Google Scholar]
- 37.Durham HE. A simple method for demonstrating the production of gas by bacteria. Brit Med J. 1898;i:1387. doi: 10.1136/bmj.1.1952.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orlowski MA. Hydrogene sulfure comme produit des certaines bacteries. J med milit Russe, cited in: Jber Fortschr path Microorg. 1897;11:528. [Google Scholar]
- 39.Myers JT. The production of hydrogen sulphide by bacteria. J Bacteriol. 1920;5:231–52. doi: 10.1128/jb.5.3.231-252.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodler M, Vadon V, Pekár K. Ability to form H2S in various bacteria. Zentralbl Bakteriol Orig. 1968;206:117–22. [PubMed] [Google Scholar]
- 41.Clarke PH. Hydrogen sulphide production by bacteria. J Gen Microbiol. 1953;8:397–407. doi: 10.1099/00221287-8-3-397. [DOI] [PubMed] [Google Scholar]
- 42.Massidda A. Production of H2S In Brucella. Nuovi Ann Ig Microbiol. 1964;15:424–31. [PubMed] [Google Scholar]
- 43.Stutzenberger FJ, BennettT EO. Sensitivity of mixed populations of Staphylococcus aureus and Eschericia coli to mercurials. Appl Microbiol. 1965;13:570–4. doi: 10.1128/am.13.4.570-574.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadota H, Ishida Y. Production of volatile sulfur compounds by microorganisms. Ann Rev Microbiol. 1972;26:127–38. doi: 10.1146/annurev.mi.26.100172.001015. [DOI] [PubMed] [Google Scholar]
- 45.Basic A, Blomqvist S, Carlén A, Dahlén G. Estimation of bacterial hydrogen sulfide production in vitro. J Oral Microbiol. 2015;7:28166. doi: 10.3402/jom.v7.28166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubois T, Dancer-Thibonnier M, Monot M, Hamiot A, Bouillaut L, Soutourina O, et al. Control of Clostridium difficile physiopathology in response to cysteine availability. Infect Immun. 2016;84:2389–405. doi: 10.1128/IAI.00121-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Artman M. The production of hydrogen sulphide from thiosulphate by Escherichia coli. J Gen Microbiol. 1956;14:315–22. doi: 10.1099/00221287-14-2-315. [DOI] [PubMed] [Google Scholar]
- 48.Seiflein TA, Lawrence JG. Two transsulfurylation pathways in Klebsiella pneumoniae. J Bacteriol. 2006;188:5762–5774. doi: 10.1128/JB.00347-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shatalin K, Shatalina E, Mironov A, Nudler E. H2S: a universal defense against antibiotics in bacteria. Science. 2011;334:986–90. doi: 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- 50.Mironov A, Seregina T, Nagornykh M, Luhachack LG, Korolkova N, Lopes LE, et al. Mechanism of H2S-mediated protection against oxidative stress in Escherichia coli. Proc Natl Acad Sci USA. 2017;114:6022–6027. doi: 10.1073/pnas.1703576114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matoba Y, Yoshida T, Izuhara-Kihara H, Noda M, Sugiyama M. Crystallographic and mutational analyses of cystathionine β-synthase in the H2S-synthetic gene cluster in Lactobacillus plantarum. Protein Sci. 2017;26:763–783. doi: 10.1002/pro.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olson KR, Deleon ER, Gao Y, Hurley K, Sadauskas V, Batz C, et al. Thiosulfate: a readily accessible source of hydrogen sulfide in oxygen sensing. Am J Physiol Regul Integr Comp Physiol. 2013;305:R592–603. doi: 10.1152/ajpregu.00421.2012. [DOI] [PubMed] [Google Scholar]
- 53.Snijder PM, Frenay AR, de Boer RA, Pasch A, Hillebrands JL, Leuvenink HG, et al. Exogenous administration of thiosulfate, a donor of hydrogen sulfide, attenuates angiotensin II-induced hypertensive heart disease in rats. Br J Pharmacol. 2015;172:1494–504. doi: 10.1111/bph.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rizzo AA. The possible role of hydrogen sulfide in human periodontal disease. I. Hydrogen sulfide production in periodontal pockets. Periodontics. 1967;5:233–6. [PubMed] [Google Scholar]
- 55.Horowitz A, Folke LE. Hydrogen sulfide and periodontal disease. Periodontal Abstr. 1972;20:59–62. [PubMed] [Google Scholar]
- 56.Tonzetich J, McBride BC. Characterization of volatile sulphur production by pathogenic and non-pathogenic strains of oral Bacteroides. Arch Oral Biol. 1981;26:963–9. doi: 10.1016/0003-9969(81)90104-7. [DOI] [PubMed] [Google Scholar]
- 57.Fedorowicz Z, Aljufairi H, Nasser M, Outhouse TL, Pedrazzi V. Mouthrinses for the treatment of halitosis. Cochrane Database Syst Rev. 2008;8:CD006701. doi: 10.1002/14651858.CD006701.pub2. [DOI] [PubMed] [Google Scholar]
- 58.Willis CL, Gibson GR, Holt J, Allison C. Negative correlation between oral malodour and numbers and activities of sulphate-reducing bacteria in the human mouth. Arch Oral Biol. 1999;44:665–70. doi: 10.1016/s0003-9969(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi T, Hanabusa M, Hosoya N, Chiba T, Yoshida T, Morito A. Enamel surface changes caused by hydrogen sulfide. J Conserv Dent. 2015;18:427–430. doi: 10.4103/0972-0707.168794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Insko MA, Deckwerth TL, Hill P, Toombs CF, Szabo C. Detection of exhaled hydrogen sulphide gas in rats exposed to intravenous sodium sulphide. Br J Pharmacol. 2009;157:944–51. doi: 10.1111/j.1476-5381.2009.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toombs CF, Insko MA, Wintner EA, Deckwerth TL, Usansky H, Jamil K, et al. Detection of exhaled hydrogen sulphide gas in healthy human volunteers during intravenous administration of sodium sulphide. Br J Clin Pharmacol. 2010;69:626–36. doi: 10.1111/j.1365-2125.2010.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suarez F, Furne J, Springfield J, Levitt M. Insights into human colonic physiology obtained from the study of flatus composition. Am J Physiol. 1997;272:G1028–33. doi: 10.1152/ajpgi.1997.272.5.G1028. [DOI] [PubMed] [Google Scholar]
- 63.Levine J, Ellis CJ, Furne JK, Springfield J, Levitt MD. Fecal hydrogen sulfide pro- duction in ulcerative colitis. Am J Gastroenterol. 1998;93:83–87. doi: 10.1111/j.1572-0241.1998.083_c.x. [DOI] [PubMed] [Google Scholar]
- 64.Carbonero F, Benefiel AC, Gaskins HR. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat Rev Gastroenterol Hepatol. 2012;9:504–518. doi: 10.1038/nrgastro.2012.85. [DOI] [PubMed] [Google Scholar]
- 65.Yazici C, Wolf PG, Kim H, Cross TL, Vermillion K, Carroll T, et al. Race-dependent association of sulfidogenic bacteria with colorectal cancer. Gut. 2017 doi: 10.1136/gutjnl-2016-313321. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blachier F, Beaumont M, Andriamihaja M, Davila AM, Lan A, Grauso M, et al. Changes in the luminal environment of the colonic epithelial cells and physiopathological consequences. Am J Pathol. 2017;187:476–486. doi: 10.1016/j.ajpath.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 67.Szabo C, Ransy C, Módis K, Andriamihaja M, Murghes B, Coletta C, et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br J Pharmacol. 2014;171:2099–122. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tomasova L, Konopelski P, Ufnal M. Gut bacteria and hydrogen sulfide: the new old players in circulatory system homeostasis. Molecules. 2016;21:E1558. doi: 10.3390/molecules21111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Toliver-Kinsky T, Shatalin K, Nudler E, Szabo C. Systemic infection and combined burn and infection: Contribution of host-derived and bacteria-derived hydrogen sulfide production to bacterial clearance. Nitric Oxide. 2015;47:S46–S47. [Google Scholar]
- 70.De Cormis L. Release of hydrogen sulfide into an atmosphere containing sulfur dioxide. C R Acad Sci. 1968;266:683–685. [Google Scholar]
- 71.Yamasaki H, Cohen MF. Biological consilience of hydrogen sulfide and nitric oxide in plants: Gases of primordial earth linking plant, microbial and animal physiologies. Nitric Oxide. 2016;55–56:91–100. doi: 10.1016/j.niox.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Fenchel TM, Riedel RJ. The sulfide system: a new biotic community underneath the oxidized layer of marine sand bottoms. Mar Biol. 1970;7:255–268. [Google Scholar]
- 73.Jannasch HW, Wirsen CO. Morphological survey of microbial mats near deep-sea thermal vents. Appl Environ Microbiol. 1981;41:528–38. doi: 10.1128/aem.41.2.528-538.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jannasch HW, Mottl MJ. Geomicrobiology of deep-sea hydrothermal vents. Science. 1985;229:717–25. doi: 10.1126/science.229.4715.717. [DOI] [PubMed] [Google Scholar]
- 75.Gaill F. Aspects of life development at deep sea hydrothermal vents. FASEB J. 1993;7:558–65. doi: 10.1096/fasebj.7.6.8472894. [DOI] [PubMed] [Google Scholar]
- 76.Sylvan JB, Toner BM, Edwards KJ. Life and death of deep-sea vents: bacterial diversity and ecosystem succession on inactive hydrothermal sulfides. MBio. 2012;3:e00279–11. doi: 10.1128/mBio.00279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goffredi SK, Childress JJ, Desaulniers NT, Lallier FJ. Sulfide acquisition by the vent worm Riftia pachyptila appears to be via uptake of HS−, rather than H2S. J Exp Biol. 1997;200:2609–16. doi: 10.1242/jeb.200.20.2609. [DOI] [PubMed] [Google Scholar]
- 78.Kundell FA. A suggested pioneer organism for the Wächtershäuser origin of life hypothesis. Orig Life Evol Biosph. 2011;41:175–98. doi: 10.1007/s11084-010-9217-y. [DOI] [PubMed] [Google Scholar]
- 79.Mulkidjanian AY, Bychkov AY, Dibrova DV, Galperin MY, Koonin EV. Open questions on the origin of life at anoxic geothermal fields. Orig Life Evol Biosph. 2012;42:507–16. doi: 10.1007/s11084-012-9315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2007;104:20618–22. doi: 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vozdek R, Hnízda A, Krijt J, Kostrouchová M, Kožich V. Novel structural arrangement of nematode cystathionine β-synthases: characterization of Caenorhabditis elegans CBS-1. Biochem J. 2012;443:535–47. doi: 10.1042/BJ20111478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma DK, Vozdek R, Bhatla N, Horvitz HR. CYSL-1 interacts with the O2-sensing hydroxylase EGL-9 to promote H2S-modulated hypoxia-induced behavioral plasticity in C. elegans. Neuron. 2012;73:925–40. doi: 10.1016/j.neuron.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Módis K, Wolanska K, Vozdek R. Hydrogen sulfide in cell signaling, signal transduction, cellular bioenergetics and physiology in C. elegans. Gen Physiol Biophys. 2013;32:1–22. doi: 10.4149/gpb_2013001. [DOI] [PubMed] [Google Scholar]
- 84.Qabazard B, Li L, Gruber J, Peh MT, Ng LF, Kumar SD, et al. Hydrogen sulfide is an endogenous regulator of aging in Caenorhabditis elegans. Antioxid Redox Signal. 2014;20:2621–30. doi: 10.1089/ars.2013.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160:132–44. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei Y, Kenyon C. Roles for ROS and hydrogen sulfide in the longevity response to germline loss in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2016;113:E2832–41. doi: 10.1073/pnas.1524727113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hofmann Klaus. Vincent du Vigneaud (1901–1978): A Biographical Memoir. National Academy of Sciences; [PubMed] [Google Scholar]
- 88.du Vigneaud V, Loring HS, Craft H. The oxidation of the sulfur of homocystine, methionine, and S-methylcysteine in the animal body. J Biol Chem. 1934;105:481. [Google Scholar]
- 89.du Vigneaud V, Brown GB, Chandler JP. The synthesis of ll-S-(β-amino- βcarboxyethyl) homocysteine and the replacement by it of cystine in the diet. J Biol Chem. 1942;143:59. [Google Scholar]
- 90.Binkley F, du Vigneaud V. The formation of cysteine from homocysteine and serine by liver tissue of rats. J Biol Chem. 1942;144:507. [Google Scholar]
- 91.Hanson H, Eisfeld G. Intermediary sulfur metabolism. III. Formation of hydrogen sulfide from cystine and cysteine by the liver. Z Gesamte Inn Med. 1952;7:801–10. [PubMed] [Google Scholar]
- 92.Chatagner F, Sauret-Ignazi G. Role of transamination and pyridoxal phosphate in the enzymatic formation of hydrogen sulfide from cysteine by the rat liver under anaerobiosis. Bull Soc Chim Biol (Paris) 1956;38:415–28. [PubMed] [Google Scholar]
- 93.Hylin JW, Wood JL. Enzymatic formation of polysulfides from mercaptopyruvate. J Biol Chem. 1959;234:2141–4. [PubMed] [Google Scholar]
- 94.Braunstein AE, Goryachenkova EV, Lac ND. Reactions catalysed by serine sulfhydrase from chicken liver. Biochim Biophys Acta. 1971;171:366–368. doi: 10.1016/0005-2744(69)90173-9. [DOI] [PubMed] [Google Scholar]
- 95.Costa MT, Wolf AM, Giarnieri D. Cleavage of cystine by cystathionase. Enzymologia. 1972;43:271–9. [PubMed] [Google Scholar]
- 96.Yao K. Effects of several unusual sulfur-containing amino acids on rat liver cystathionine-gamma-lyase. Physiol Chem Phys. 1975;7:401–8. [PubMed] [Google Scholar]
- 97.Akopyan TN, Braunstein AE, Goryachenkova EV. Beta-cyanoalanine synthase: purification and characterization. Proc Natl Acad Sci USA. 1975;72:1617–21. doi: 10.1073/pnas.72.4.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Allsop J, Watts RW. Methionine adenosyltransferase, cystathionine beta-synthase and cystathionine gamma-lyase activity of rat liver subcellular particles, human blood cells and mixed white cells from rat bone marrow. Clin Sci Mol Med Suppl. 1975;48:509–513. doi: 10.1042/cs0480509. [DOI] [PubMed] [Google Scholar]
- 99.Ubuka T, Yuasa S, Ishimoto Y, Shimomura M. Desulfuration of l-cysteine through transamination and transsulfuration in rat liver. Physiol Chem Phys. 1977;9:241–6. [PubMed] [Google Scholar]
- 100.Kimura H. Hydrogen sulfide and polysulfide signaling. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2017.7076. in press. [DOI] [PubMed] [Google Scholar]
- 101.Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuo SM, Lea TC, Stipanuk MH. Developmental pattern, tissue distribution, and subcellular distribution of cysteine: alpha-ketoglutarate aminotransferase and 3-mercaptopyruvate sulfurtransferase activities in the rat. Biol Neonate. 1983;43:23–32. doi: 10.1159/000241634. [DOI] [PubMed] [Google Scholar]
- 103.Warenycia MW, Goodwin LR, Benishin CG, Reiffenstein RJ, Francom DM, Taylor JD, et al. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem Pharmacol. 1989;38:973–981. doi: 10.1016/0006-2952(89)90288-8. [DOI] [PubMed] [Google Scholar]
- 104.Goodwin LR, Francom D, Dieken FP, Taylor JD, Warenycia NW, Reiffenstein RJ, et al. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J Anal Toxicol. 1989;13:105–109. doi: 10.1093/jat/13.2.105. [DOI] [PubMed] [Google Scholar]
- 105.Savage JC, Gould DH. Determination of sulfides in brain tissue and rumen fluid by ion-interaction reversed-phase high-performance liquid chromatography. J Chromatogr. 1990;526:546–545. doi: 10.1016/s0378-4347(00)82537-2. [DOI] [PubMed] [Google Scholar]
- 106.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–71. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Snyder S. The rotten smell of memory: it’s a gas. Science News. 1996;149:116. [Google Scholar]
- 108.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–31. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 109.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–16. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002;283:H474–80. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 111.Tang G, Wu L, Liang W, Wang R. Direct stimulation of KATP channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol Pharmacol. 2005;68:1757–64. doi: 10.1124/mol.105.017467. [DOI] [PubMed] [Google Scholar]
- 112.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–90. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109:1259–68. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–8. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 115.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 116.Wang R. Signaling pathways for the vascular effects of hydrogen sulfide. Curr Opin Nephrol Hypertens. 2011;20:107–12. doi: 10.1097/MNH.0b013e3283430651. [DOI] [PubMed] [Google Scholar]
- 117.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 118.Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, et al. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–63. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 119.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–7. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 120.Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, et al. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem. 2004;90:765–8. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 121.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287:H2316–23. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 122.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, et al. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–8. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 123.Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, et al. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539–48. doi: 10.1002/hep.20817. [DOI] [PubMed] [Google Scholar]
- 124.Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–24. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 125.Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim HR, et al. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med. 2006;41:106–19. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 126.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–20. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 127.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560–5. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 129.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, et al. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci USA. 2007;104:17977–82. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–60. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 131.Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1479–85. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- 132.Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal. 2009;11:205–14. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 133.Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D, et al. Role of H2S in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:173–9. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 134.Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem. 2009;284:11601–12. doi: 10.1074/jbc.M808026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, et al. 3- Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–14. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 136.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, et al. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–74. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem. 2009;284:22457–66. doi: 10.1074/jbc.M109.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, et al. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–6. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 140.Kimura Y, Goto Y, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 141.Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, et al. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA. 2010;107:10719–24. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Krishnan N, Fu C, Pappin DJ, Tonks NK. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal. 2011;4:ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, et al. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fu M, Zhang W, Wu L, Yang G, Li H, Wang R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci USA. 2012;109:2943–8. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Módis K, Panopoulos P, et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA. 2012;109:9161–6. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Filipovic MR, Miljkovic JL, Nauser T, Royzen M, Klos K, Shubina T, et al. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S- nitrosothiols. J Am Chem Soc. 2012;134:12016–27. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Módis K, Coletta C, Erdélyi K, Papapetropoulos A, Szabo C. Intramitochondrial H2S production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013;27:601–611. doi: 10.1096/fj.12-216507. [DOI] [PubMed] [Google Scholar]
- 148.Kimura Y, Mikami Y, Osumi K, Tsugane M, Oka J, Kimura H. Polysulfides are possible H2S-derived signaling molecules in rat brain. FASEB J. 2013;27:2451–7. doi: 10.1096/fj.12-226415. [DOI] [PubMed] [Google Scholar]
- 149.Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, et al. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE) Br J Pharmacol. 2013;169:922–32. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Szabo C, Coletta C, Chao C, Módis K, Szczesny B, Papapetropoulos A, et al. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci USA. 2013;110:12474–9. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shibuya N, Koike S, Tanaka M, Ishigami-Yuasa M, Kimura Y, Ogasawara Y, et al. A novel pathway for the production of H2S from D-cysteine in mammalian cells. Nat Commun. 2013;4:1366. doi: 10.1038/ncomms2371. [DOI] [PubMed] [Google Scholar]
- 152.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, et al. H2S cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci USA. 2014;111:3182–7. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Eberhardt M, Dux M, Namer B, Miljkovic J, Cordasic N, Will C, et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat Commun. 2014;5:4381. doi: 10.1038/ncomms5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, et al. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem Biophys Res Commun. 2006;343:303–10. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- 155.Cortese-Krott MM, Kuhnle GG, Dyson A, Fernandez BO, Grman M, DuMond JF, et al. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc Natl Acad Sci USA. 2015;112:E4651–60. doi: 10.1073/pnas.1509277112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Szczesny B, Módis K, Yanagi K, Coletta C, Le Trionnaire S, Perry A, et al. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide. 2014;41:120–30. doi: 10.1016/j.niox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Szczesny B, Marcatti M, Zatarain JR, Druzhyna N, Wiktorowicz JE, Nagy P, et al. Inhibition of hydrogen sulfide biosynthesis sensitizes lung adenocarcinoma to chemotherapeutic drugs by inhibiting mitochondrial DNA repair and suppressing cellular bioenergetics. Sci Rep. 2016;6:36125. doi: 10.1038/srep36125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yong R, Searcy DG. Sulfide oxidation coupled to ATP synthesis in chicken liver mitochondria. Comp Biochem Physiol B Biochem Mol Biol. 2011;129:129–37. doi: 10.1016/s1096-4959(01)00309-8. [DOI] [PubMed] [Google Scholar]
- 159.Goubern M, Andriamihaja M, Nübel T, Blachier F, Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007;21:1699–706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- 160.Cox E, Jeffrey G, Stadler H. Structure of dinitrososulphite ion. Nature. 1948;162:770. [Google Scholar]
- 161.Degener E, Seel F. Zur Kenntnis der Salze der Nitrosohydroxylaminsulfonsäure. I. Chemischer Konstitutionsbeweis der Nitrosohydroxylaminsulfonate. Z Anorg Allg Chem. 1956;285:129–133. [Google Scholar]
- 162.Chepikova NR. The effect of hydrogen sulfide baths on the circulation and the processes of functional adaptation in dogs with experimental atherosclerosis. Vopr Kurortol Fizioter Lech Fiz Kult. 1966;31:497–504. [PubMed] [Google Scholar]
- 163.Lavrov VP. The effect of Matsesta hydrogen sulfide baths on the status of the myocardium and coronary arteries in experimental atherosclerosis. Vopr Kurortol Fizioter Lech Fiz Kult. 1968;33:313–6. [PubMed] [Google Scholar]
- 164.Kubli SKh. Effect of hydrogen sulfide baths on the activity of tissue cytochrome oxidase in rabbits with experimental arteriosclerosis. Vopr Kurortol Fizioter Lech Fiz Kult. 1970;35:315–7. [PubMed] [Google Scholar]
- 165.Le Trionnaire S, Perry A, Szczesny B, Szabo C, Winyard PG, Whatmore JL, et al. The synthesis and functional evaluation of a mitochondria-targeted hydrogen sulfide donor, (10-oxo-10-(4-(3-thioxo-3H-1,2-dithiol-5-yl)phenoxy)decyl) triphenylphosphonium bromide (AP39) J Med Chem Comm. 2014;5:728–736. [Google Scholar]
- 166.Wallace JL, Vaughan D, Dicay M, MacNaughton WK, de Nucci G. Hydrogen sulfide-releasing therapeutics: translation to the clinic. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2017.7068. in press. [DOI] [PubMed] [Google Scholar]
- 167.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2017;6:917–35. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 168.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Ann Rev Pharmacol Toxicol. 2011;51:169–87. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 169.Whiteman M, Le Trionnaire S, Chopra M, Fox B, Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin Sci (Lond) 2011;121:459–88. doi: 10.1042/CS20110267. [DOI] [PubMed] [Google Scholar]
- 170.Olson KR. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid Redox Signal. 2012;17:32–44. doi: 10.1089/ars.2011.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Olson KR, DeLeon ER, Liu F. Controversies and conundrums in hydrogen sulfide biology. Nitric Oxide. 2014;41:11–26. doi: 10.1016/j.niox.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 172.Papapetropoulos A, Whiteman M, Cirino G. Pharmacological tools for hydrogen sulphide research: a brief, introductory guide for beginners. Br J Pharmacol. 2015;172:1633–7. doi: 10.1111/bph.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Whiteman M, Moore PK, editors. Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide (Handbook of Experimental Pharmacology) Springer; 2016. [Google Scholar]
- 174.Olson KR, Straub KD. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology (Bethesda) 2016;31:60–72. doi: 10.1152/physiol.00024.2015. [DOI] [PubMed] [Google Scholar]
- 175.Tan BH, Wong PT, Bian JS. Hydrogen sulfide: a novel signaling molecule in the central nervous system. Neurochem Int. 2010;56:3–10. doi: 10.1016/j.neuint.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 176.Rong W, Kimura H, Grundy D. The neurophysiology of hydrogen sulfide. Inflamm Allergy Drug Targets. 2011;10:109–17. doi: 10.2174/187152811794776295. [DOI] [PubMed] [Google Scholar]
- 177.Zhang X, Bian JS. Hydrogen sulfide: a neuromodulator and neuroprotectant in the central nervous system. ACS Chem Neurosci. 2014;5:876–83. doi: 10.1021/cn500185g. [DOI] [PubMed] [Google Scholar]
- 178.Kida K, Ichinose F. Hydrogen sulfide and neuroinflammation. Handb Exp Pharmacol. 2015;230:181–9. doi: 10.1007/978-3-319-18144-8_9. [DOI] [PubMed] [Google Scholar]
- 179.Paul BD, Snyder SH. Modes of physiologic H2S signaling in the brain and peripheral tissues. Antioxid Redox Signal. 2015;22:411–23. doi: 10.1089/ars.2014.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res. 2014;114:730–7. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang R, Szabo C, Ichinose F, Ahmed A, Whiteman M, Papapetropoulos A. The role of H2S bioavailability in endothelial dysfunction. Trends Pharmacol Sci. 2015;36:568–78. doi: 10.1016/j.tips.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yang G, Wang R. H2S and blood vessels: an overview. Handb Exp Pharmacol. 2015;230:85–110. doi: 10.1007/978-3-319-18144-8_4. [DOI] [PubMed] [Google Scholar]
- 183.van Goor H, van den Born JC, Hillebrands JL, Joles JA. Hydrogen sulfide in hypertension. Curr Opin Nephrol Hypertens. 2016;25:107–13. doi: 10.1097/MNH.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 184.Katsouda A, Bibli SI, Pyriochou A, Szabo C, Papapetropoulos A. Regulation and role of endogenously produced hydrogen sulfide in angiogenesis. Pharmacol Res. 2016;113:175–185. doi: 10.1016/j.phrs.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kanagy NL, Szabo C, Papapetropoulos A. Vascular biology of hydrogen sulfide. Am J Physiol Cell Physiol. 2017;312:C537–C549. doi: 10.1152/ajpcell.00329.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Meng G, Zhao S, Xie L, Han Y, Ji Y. Protein S-sulfhydration by hydrogen sulfide in cardiovascular system. Br J Pharmacol. 2017 doi: 10.1111/bph.13825. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–71. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 188.Magierowski M, Magierowska K, Kwiecien S, Brzozowski T. Gaseous mediators nitric oxide and hydrogen sulfide in the mechanism of gastrointestinal integrity, protection and ulcer healing. Molecules. 2015;20:9099–123. doi: 10.3390/molecules20059099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Singh SB, Lin HC. Hydrogen sulfide in physiology and diseases of the digestive tract. Microorganisms. 2015;3:866–89. doi: 10.3390/microorganisms3040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Blachier F, Beaumont M, Andriamihaja M, Davila AM, Lan A, Grauso M, et al. Changes in the luminal environment of the colonic epithelial cells and physiopathological consequences. Am J Pathol. 2017;187:476–486. doi: 10.1016/j.ajpath.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 191.Koning AM, Frenay AR, Leuvenink HG, van Goor H. Hydrogen sulfide in renal physiology, disease and transplantation--the smell of renal protection. Nitric Oxide. 2015;46:37–49. doi: 10.1016/j.niox.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 192.Feliers D, Lee HJ, Kasinath BS. Hydrogen sulfide in renal physiology and disease. Antioxid Redox Signal. 2016;25:720–731. doi: 10.1089/ars.2015.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cao X, Bian JS. The role of hydrogen sulfide in renal system. Front Pharmacol. 2016;7:385. doi: 10.3389/fphar.2016.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Olson KR. Mitochondrial adaptations to utilize hydrogen sulfide for energy and signaling. J Comp Physiol B. 2012;182:881–97. doi: 10.1007/s00360-012-0654-y. [DOI] [PubMed] [Google Scholar]
- 195.Módis K, Bos EM, Calzia E, van Goor H, Coletta C, Papapetropoulos A, et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part II. Pathophysiological and therapeutic aspects. Br J Pharmacol. 2014;171:2123–46. doi: 10.1111/bph.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Suliman HB, Piantadosi CA. Mitochondrial biogenesis: regulation by endogenous gases during inflammation and organ stress. Curr Pharm Des. 2014;20:5653–62. doi: 10.2174/1381612820666140306095717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kabil O, Motl N, Banerjee R. H2S and its role in redox signaling. Biochim Biophys Acta. 2014;1844:1355–66. doi: 10.1016/j.bbapap.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kimura H. Signaling molecules: hydrogen sulfide and polysulfide. Antioxid Redox Signal. 2015;22:362–76. doi: 10.1089/ars.2014.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kimura H. Physiological roles of hydrogen sulfide and polysulfides. Handb Exp Pharmacol. 2015;230:61–81. doi: 10.1007/978-3-319-18144-8_3. [DOI] [PubMed] [Google Scholar]
- 200.Paul BD, Snyder SH. H2S: a novel gasotransmitter that signals by sulfhydration. Trends Biochem Sci. 2015;40:687–700. doi: 10.1016/j.tibs.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ju Y, Fu M, Stokes E, Wu L, Yang G. H2S-mediated protein S-sulfhydration: a prediction for its formation and regulation. Molecules. 2017;22:E1334. doi: 10.3390/molecules22081334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sen N. Functional and molecular insights of hydrogen sulfide signaling and protein sulfhydration. J Mol Biol. 2017;429:543–561. doi: 10.1016/j.jmb.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kabil O, Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal. 2014;20:770–82. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rose P, Moore PK, Zhu YZ. H2S biosynthesis and catabolism: new insights from molecular studies. Cell Mol Life Sci. 2017;74:1391–1412. doi: 10.1007/s00018-016-2406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Banerjee R. Catalytic promiscuity and heme-dependent redox regulation of H2S synthesis. Curr Opin Chem Biol. 2017;37:115–121. doi: 10.1016/j.cbpa.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li Q, Lancaster JR., Jr Chemical foundations of hydrogen sulfide biology. Nitric Oxide. 2013;35:21–34. doi: 10.1016/j.niox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ono K, Akaike T, Sawa T, Kumagai Y, Wink DA, Tantillo DJ, et al. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: implications of their possible biological activity and utility. Free Radic Biol Med. 2014;77:82–94. doi: 10.1016/j.freeradbiomed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nagy P. Mechanistic chemical perspective of hydrogen sulfide signaling. Methods Enzymol. 2015;554:3–29. doi: 10.1016/bs.mie.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 209.Cuevasanta E, Möller MN, Alvarez B. Biological chemistry of hydrogen sulfide and persulfides. Arch Biochem Biophys. 2017;617:9–25. doi: 10.1016/j.abb.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 210.Szabo C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: mechanisms and implications. Am J Physiol Cell Physiol. 2017;312:C3–C15. doi: 10.1152/ajpcell.00282.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Olas B. Hydrogen sulfide as a “double-faced” compound: one with pro- and antioxidant effect. Adv Clin Chem. 2017;78:187–196. doi: 10.1016/bs.acc.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 212.Cirino G, Vellecco V, Bucci M. Nitric oxide and hydrogen sulfide: the gasotransmitter paradigm of the vascular system. Br J Pharmacol. 2017 doi: 10.1111/bph.13815. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cortese-Krott MM, Butler AR, Woollins JD, Feelisch M. Inorganic sulfur-nitrogen compounds: from gunpowder chemistry to the forefront of biological signaling. Dalton Trans. 2016;45:5908–19. doi: 10.1039/c5dt05034k. [DOI] [PubMed] [Google Scholar]
- 214.Kevil C, Cortese-Krott NN, Nagy P, Papapetropoulos A, Feelisch M, Szabo C. Cooperative interactions between NO and H2S: Chemistry, biology, physiology, pathophysiology. In: Ignarro L, Freeman B, editors. Nitric Oxide. Elsevier; 2017. pp. 57–84. [Google Scholar]
- 215.Cortese-Krott MM, Koning A, Kuhnle GGC, Nagy P, Bianco CL, Pasch A, Wink DA, Fukuto JM, Jackson AA, van Goor H, Olson KR, Feelisch M. The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid Redox Signal. 2017;27:684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kashfi K, Olson KR. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem Pharmacol. 2013;85:689–703. doi: 10.1016/j.bcp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kashfi K. Anti-cancer activity of new designer hydrogen sulfide-donating hybrids. Antioxid Redox Signal. 2014;20:831–46. doi: 10.1089/ars.2013.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Whiteman M, Perry A, Zhou Z, Bucci M, Papapetropoulos A, Cirino G, Wood ME. Phosphinodithioate and phosphoramidodithioate hydrogen sulfide donors. Handb Exp Pharmacol. 2015;230:337–63. doi: 10.1007/978-3-319-18144-8_17. [DOI] [PubMed] [Google Scholar]
- 219.Szabo C, Papapetropoulos A. International Union of Basic and Clinical Pharmacology. Pharmacological modulation of hydrogen sulfide (H2S) levels: H2S donors and inhibitors of H2S biosynthesis. Pharmacol Rev. 2017 doi: 10.1124/pr.117.014050. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chan MV, Wallace JL. Hydrogen sulfide-based therapeutics and gastrointestinal diseases: translating physiology to treatments. Am J Physiol Gastrointest Liver Physiol. 2013;305:G467–73. doi: 10.1152/ajpgi.00169.2013. [DOI] [PubMed] [Google Scholar]
- 221.Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov. 2015;14:329–45. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 222.Salloum FN. Hydrogen sulfide and cardioprotection--Mechanistic insights and clinical translatability. Pharmacol Ther. 2015;152:11–7. doi: 10.1016/j.pharmthera.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 223.Szabo C. Gasotransmitters in cancer: from pathophysiology to experimental therapy. Nat Rev Drug Discov. 2016;15:185–203. doi: 10.1038/nrd.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ianaro A, Cirino G, Wallace JL. Hydrogen sulfide-releasing anti-inflammatory drugs for chemoprevention and treatment of cancer. Pharmacol Res. 2016;111:652–658. doi: 10.1016/j.phrs.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 225.Zhang JY, Ding YP, Wang Z, Kong Y, Gao R, Chen G. Hydrogen sulfide therapy in brain diseases: from bench to bedside. Med Gas Res. 2017;7:113–119. doi: 10.4103/2045-9912.208517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zheng Y, Yu B, De La Cruz LK, Roy Choudhury M, Anifowose A, Wang B. Toward hydrogen sulfide based therapeutics: critical drug delivery and developability issues. Med Res Rev. 2017 doi: 10.1002/med.21433. in press. [DOI] [PubMed] [Google Scholar]