Abstract
CACNA1C, encoding the Cav1.2 subunit of L-type Ca2+ channels, has emerged as one of the most prominent and highly replicable susceptibility genes for several neuropsychiatric disorders. Cav1.2 channels play a crucial role in calcium-mediated processes involved in brain development and neuronal function. Within the CACNA1C gene, disease-associated single-nucleotide polymorphisms have been associated with impaired social and cognitive processing and altered prefrontal cortical (PFC) structure and activity. These findings suggest that aberrant Cav1.2 signaling may contribute to neuropsychiatric-related disease symptoms via impaired PFC function. Here, we show that mice harboring loss of cacna1c in excitatory glutamatergic neurons of the forebrain (fbKO) that we have previously reported to exhibit anxiety-like behavior, displayed a social behavioral deficit and impaired learning and memory. Furthermore, focal knockdown of cacna1c in the adult PFC recapitulated the social deficit and elevated anxiety-like behavior, but not the deficits in learning and memory. Electrophysiological and molecular studies in the PFC of cacna1c fbKO mice revealed higher E/I ratio in layer 5 pyramidal neurons and lower general protein synthesis. This was concurrent with reduced activity of mTORC1 and its downstream mRNA translation initiation factors eIF4B and 4EBP1, as well as elevated phosphorylation of eIF2α, an inhibitor of mRNA translation. Remarkably, systemic treatment with ISRIB, a small molecule inhibitor that suppresses the effects of phosphorylated eIF2α on mRNA translation, was sufficient to reverse the social deficit and elevated anxiety-like behavior in adult cacna1c fbKO mice. ISRIB additionally normalized the lower protein synthesis and higher E/I ratio in the PFC. Thus this study identifies a novel Cav1.2 mechanism in neuropsychiatric-related endophenotypes and a potential future therapeutic target to explore.
INTRODUCTION
The CACNA1C gene, encoding the Cav1.2 subunit of L-type calcium channels (LTCCs), has emerged as a candidate susceptibility gene for multiple neuropsychiatric disorders1–3 including bipolar disorder (BD),4 schizophrenia (SCZ),5 major depressive disorder (MDD),5 and autism spectrum disorder (ASD).6 CACNA1C is additionally present in biological pathways that are shared between SCZ, BD, and MDD.7 The Cav1.2 LTCCs are a key source of Ca2+ entry into cells and activate calcium-mediated signaling pathways8 that are critical for normal neuronal development, dendritic growth, and neuronal plasticity.8–10 The critical role of Cav1.2 channels in normal brain function is further underscored by human imaging and clinical studies that have reported structural and functional brain alterations and behavioral abnormalities in human carriers of CACNA1C disease-associated single-nucleotide polymorphisms (SNPs).11
Neuropsychiatric disorders comprise a plethora of complex behavioral endophenotypes, many of which are shared across multiple disorders.11,12 While alterations in mood and emotion, including anxiety, form the core features of neuropsychiatric disorders, impairments in social and cognitive processing are prominent co-morbid symptoms that remain understudied and show limited improvement with current treatments.13 In humans, disease-associated SNPs in CACNA1C have been associated not only with heightened anxiety but also cognitive dysfunction and impaired facial emotional recognition,11 a feature required for normal social behavior, suggesting a potential role for Cav1.2 LTCCs in mediating these functions.
The prefrontal cortex (PFC) is the central anatomical structure mediating higher-order social and cognitive function by having a top-down influence on several sub-cortical regions.14,15 Because of its wide range of outputs, the PFC can have different modulatory effects on distinct cognitive domains. In CACNA1C SNP carriers, neuroimaging studies have identified alterations in PFC structure16 and changes in cognitive processing-dependent PFC activity,11 suggesting altered prefrontal cortical functioning. However despite the significant link between CACNA1C and neuropsychiatric disease, the underlying Cav1.2-mediated molecular mechanisms contributing to neuropsychiatric-related behaviors remain unknown.
One emerging hypothesis for the pathophysiological mechanisms mediating the cognitive impairments in diseases like SCZ and ASD is altered excitation to inhibition (E/I) balance, with higher E/I ratio reported in multiple mouse models with altered social and cognitive function.17–20 Furthermore, higher E/I in the PFC via optogenetic manipulation was recently demonstrated to directly impair both social behavior and fear responses.21 Separately, impairments in cognitive functioning have also been reported in neuropsychiatric disease-associated mouse models that have decreased expression of factors involved in mRNA translation.22–25
While the precise mechanism by which the identified SNPs in CACNA1C confer risk for neuropsychiatric disorders is unknown, lower CACNA1C transcript levels have been reported in the brains of CACNA1C SNP-carrying SCZ26 and BD patients,27 suggesting that decreased Cav1.2 signaling has the potential to impact brain function that can contibute to the disease symptoms observed in CACNA1C-associated disorders. Using a combination of genetic, behavioral, molecular and electrophysiological strategies, here we show that forebrain-specific cacna1c conditional knockout mice have lower levels of protein synthesis in the PFC, in parallel with lower levels of phospho-mTOR and higher levels of phospho-eIF2a, a mRNA translation initiation repressor. Pharmacologically suppressing the downstream effects of elevated phospho-eIF2α by the small molecule ISRIB, is sufficient to normalize the lower protein synthesis and higher E/I ratio in the PFC. Additionally, systemic treatment with ISRIB is able to rescue the deficit in sociability and elevated anxiety-like behavior. Together, our findings highlight a novel Cav1.2-mediated mechanism via increased eIF2α phosphorylation in the PFC that results in aberrant synaptic transmission and protein synthesis that may underlie neuropsychiatric-associated behavioral deficits.
MATERIALS AND METHODS
Detailed methods are provided in Supplementary Information.
Animals
In this study forebrain-specific cacna1c conditional knockout (fbKO) mice,28 their wild-type (WT) littermates, and homozygous cacna1c floxed (cacna1cfl/fl; Lee et al.28) mice, all on a C57BL/6J background were utilized. All procedures were conducted in accordance with the Weill Cornell Medicine Institutional Animal Care and Use Committee rules.
Stereotaxic surgery
For focal knockdown of cacna1c, AAV2/2-Cre-GFP was sterotaxically delivered directly into the PFC of adult cacna1cfl/fl mice.28 Placement of surgical injections was confirmed using GFP immunohistochemistry.28
Behavioral testing
All behavioral tests were performed in adult mice.
Three-chambered social approach
Social approach was performed using the three-chamber social interaction test.29 'Sociability' was measured as the amount of time the experimental animal spent with a stranger mouse compared to a novel object. Data is reported as time spent in the chambers as well as contact zones (a designated 1.5” area encircling the cup) containing the stranger mouse or novel object.
Fear conditioning
Context- and cue-associated fear conditioning30 was performed, with some modifications.
Water-based Y-maze
The Y-maze was used to measure working memory as well as short- (1 h) and long-term (24 h and 7 days) memory retention. Working memory was measured over a 6 min period as percent of spontaneous alternations (defined as consecutive entry in the three different arms over total arm entries).31 For the memory tests, mice were trained over five trials to locate a submerged platform in one arm of the Y-shaped maze and tested 1 h, 24 h and 7 days later for memory retention. Each test consisted of 5 trials (and reported as an average) with the latency and successes (calculated based on the errors made during each trial) to locate the submerged platform measured.
Morris water maze
Morris water maze (MWM) was performed as previously published32 with slight modifications.
Elevated plus maze
Anxiety was measured using elevated plus maze (EPM).28
Electrophysiological methods
Whole-cell recordings
Postnatal day (P) 30-P45 male mice were anesthetized with isoflurane, decapitated and brains dissected. Coronal slices (400 μm) were obtained and whole-cell recordings were performed.33
Molecular methods
All molecular experiments were performed on brain tissue from adult mice.
SUnSET
Protein synthesis was measured using a modified version of the SUnSET technique.22,34
Subcellular fractionation and immunoblotting
Total protein lysates or synaptoneurosomes35 from the PFC were used for Western blot analysis. For details on antibodies used see Supplementary Table 1.
BDNF ELISA
Mature BDNF levels were measured using the ELISA protocol.36
Drug treatment
A single dose of ISRIB (SML0843, Sigma, St. Louis, MO, USA) at 2.5 mg kg−1 (diluted in 6.25% DMSO and 6.25% PEG300) or vehicle was administered intraperitoneally 90 min prior to behavioral testing, protein analyses, or electrophysiological recordings.37
Statistics
GraphPad Prism (La Jolla, CA, USA) was used for all statistical analyses. All statistical results and tests used are included in the figure legends. Sample size was chosen based on prior experience followed by power calculation. If power was greater than 0.7, additional animals were not added to the experiment. For most behavioral analyses a two-way analysis of variance was performed followed by a Bonferroni post hoc test if a main effect (P<0.05) was observed. For independent sample sets, the D’Agostino and Pearson normality test was performed and for those that passed normality (P>0.05), a two-tailed Student’s t-test was performed with Welch’s correction if variances were significantly different. For electrophysiological recordings that passed the normality test, a one-way analysis of variance was performed followed by a Bonferroni post hoc test if a main effect (P<0.05) was observed. For sample sets that failed to pass normality (P<0.05), a Kruskal–Wallis H-test was performed with a Dunn’s uncorrected post hoc test if a main effect was observed.
RESULTS
Cacna1c forebrain knockout mice display decreased sociability and increased cue-associated fear responses
Cav1.2 LTCCs are located primarily at the postsynaptic membrane38 of excitatory neurons (Supplementary Figure 1) where they tightly regulate Ca2+-mediated gene expression and synaptic plasticity. Because of the high prevalence of these channels in excitatory neurons and their critical role in brain activity, we generated forebrain-specific cacna1c conditional knockout (fbKO) mice that have a loss of cacna1c in excitatory neurons of the forebrain. Cacna1c fbKO mice have elevated anxiety-like behavior,28 a symptom common across all CACNA1C-associated neuropsychiatric disorders, and deficient postnatal hippocampal neurogenesis,39 also associated with neuropsychiatric disorders40 making this an ideal mouse model to examine mechanisms downstream of Cav1.2 (cacna1c) that underlie neuropsychiatric-related behavioral and pathophysiological phenotypes.
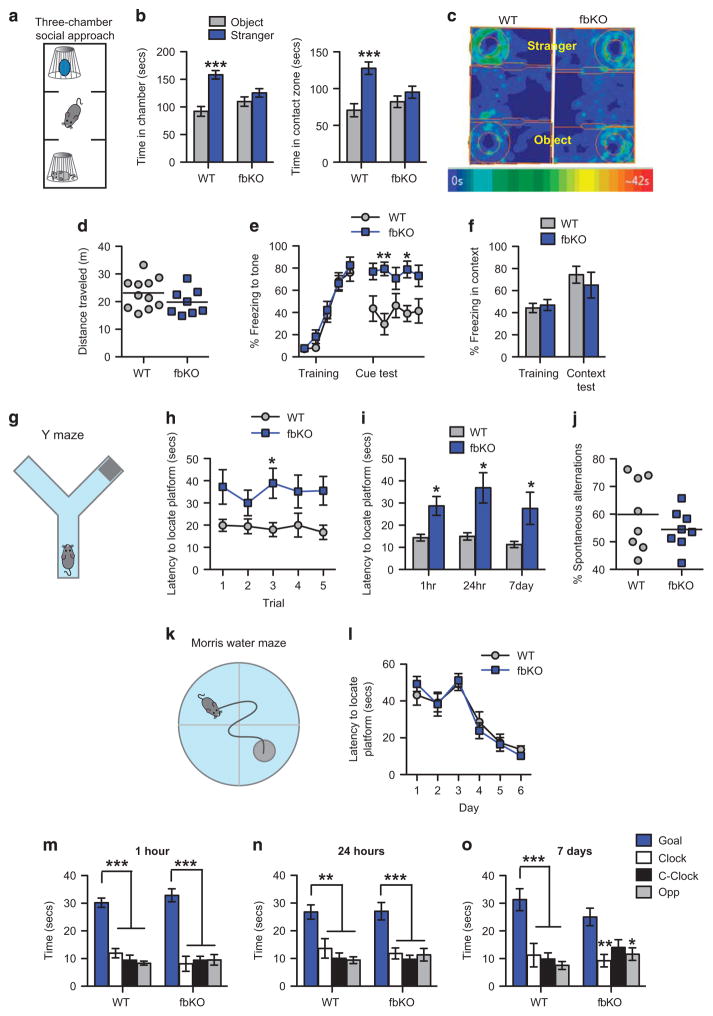
In most neuropsychiatric and neurodevelopmental disorders, including SCZ, BD, MDD and ASD, altered emotional regulation is often accompanied by impaired social behavior.12 Furthermore, human and animal studies have shown that anxiety and social interaction are intimately linked with overlapping neural mechanisms.41 Thus, we evaluated the role of cacna1c on social behavior utilizing the three-chamber social approach test. This behavior probes for voluntary initiation of social interaction with a novel conspecific mouse (Figure 1a) and recruits components of social anxiety.42,43 In the social approach test cacna1c fbKO mice displayed reduced sociability. While WT controls spent significantly more time in the chamber and contact zone containing the con-specific stranger mouse compared to the novel object, fbKO mice spent similar amounts of time with the stranger mouse and novel object (Figures 1b and c). This abnormal social behavior was not a result of decreased locomotor activity, as WT and fbKO mice traveled comparable distances during the social task (Figure 1d).
Figure 1.
Cacna1c fbKO mice display a deficit in sociability and impaired learning and memory. (a) Schematic of the three-chamber social approach apparatus. (b) In the three-chamber social approach test, WT but not fbKO adult mice spent significantly more time in the chamber (left; two-way ANOVA, genotype × chamber, F1,34 = 8.865, P = 0.0053) and contact zone (right; two-way ANOVA, genotype × zone, F1,34 = 6.6367, P =0.0165) containing the stranger mouse compared to the novel object (Bonferroni post hoc test, ***P<0.001 vs object; WT n = 11, fbKO n =8). (c) Representative heat maps showing time spent in the chambers (represented by rectangular compartments) and contact zones (represented by area between outer circle and inner circle) containing the stranger mouse and novel object (represented by inner circle). (d) WT and fbKO mice traveled similar distances during the social approach test (WT n =11, fbKO n =8). (e) In cue fear conditioning, WT and fbKO mice displayed similar percent freezing to the tone during training (two-way ANOVA, main effect of tone, F4, 60 = 55.06, P <0.0001) but during the twenty-four hour cue fear test, fbKO mice exhibited significantly higher freezing compared to WT mice (Two-way ANOVA, main effect of genotype, F1, 60 = 34.27, P <0.0001; Bonferroni post hoc test, *P <0.05, **P <0.01 vs WT). Each data point represents freezing during a single tone presentation (WT n =8, fbKO n =6). (f) During contextual fear conditioning, WT and fbKO mice displayed similar percent freezing to the context during training and the twenty-four hour test (WT n =6, fbKO n =6). (g) Schematic of the water-based Y-maze apparatus. (h) In the Y-maze during training, fbKO mice displayed increased latency in locating the submerged platform compared to WT mice (Two-way ANOVA, main effect of genotype, F1, 75 = 24.1, P <0.0001; Bonferroni post hoc test, *P <0.05 vs WT; WT n = 9, fbKO n = 8). (i) In the Y-maze memory tests, fbKO mice displayed significantly increased latency in locating the submerged platform at 1 h (Student’s t-test with Welch’s correction, t(8) = 3.188, P <0.05), 24 h (Student’s t-test with Welch’s correction, t(7) = 3.121, P <0.05) and 7 days (Mann–Whitney U-test, U = 13.00, P <0.05) post-training (*P<0.05, **P<0.01 vs WT; WT n =9, fbKO n =8). (j) WT and fbKO mice exhibit similar percent of spontaneous alternations during the working memory Y-maze task (WT n =8, fbKO n =8). (k) Schematic of the Morris water maze (MWM) apparatus. (l) In the MWM during training, WT and fbKO mice displayed similar latency in locating the submerged platform (Two-way ANOVA, main effect of day, F5, 72 = 26.41, P <0.0001; n = 7/genotype). (m–o) In the MWM probe tests, WT and fbKO mice spent significantly more time in the goal quadrant relative to the other quadrants at 1 h (m; Two-way ANOVA, main effect of quadrant, F3,48 = 70.41, P <0.0001), 24 h (n; Two-way ANOVA, main effect of quadrant, F3,48 = 23.01, P <0.0001) and 7 days (o; Two-way ANOVA, main effect of quadrant, F3,48 = 18.19, P <0.0001) post-training (Bonferroni post hoc test, *P<0.05, **P<0.01, ***P<0.001 vs Goal; n =7/genotype). Error bars represent mean ± s.e.m. ANOVA, analysis of variance; fbKO, forebrain knockout; WT, wild type.
Increased emotional reactivity to aversive stimuli is a prominent feature of many neuropsychiatric disorders and can be modeled in animals using the classical fear conditioning paradigm.44 Furthermore, trait anxiety, referring to an individuals predisposition to anxiety,41 has been associated with increased fear responses in both humans45 and rodents.46 Therefore, we next examined the role of cacna1c on fear-related behavior. We conducted cue- and context-associated fear conditioning, wherein mice acquire a conditioned fear response (freezing) by pairing an unconditioned stimulus (foot shock) with a conditioned stimulus (tone or context). During training, WT controls and fbKO mice displayed similar levels of freezing to both the cue (tone; Figure 1e) and context (Figure 1f), demonstrating no deficit in acquisition of fear or in sensitivity to foot shock. Interestingly, twenty-four hours later fbKO mice displayed higher freezing to the cue (Figure 1e) but not the context (Figure 1f). This heightened cue-associated fear response is consistent with our previous finding of elevated anxiety in these animals.28 Together, these findings demonstrated that loss of cacna1c in forebrain glutamatergic neurons reduces sociability and enhances cue-associated fear responses.
Cacna1c forebrain knockout mice exhibit selective deficits in learning and memory
Cognitive impairments are a prominent feature of neuropsychiatric disorders with deficits in PFC-specific cognitive functions being a hallmark of neuropsychiatric patients.13 In addition, in humans, cognitive function can be severely impacted by anxiety.47 Therefore, we next examined cognitive function in cacna1c fbKO mice utilizing the water-based Y-maze task (Figure 1g) that, in part, recruits the PFC and measures egocentric navigation (using internal cues) to locate a submerged platform. In this test, fbKO mice displayed a significant deficit in learning, with longer time (Figure 1h) and fewer successful attempts (Supplementary Figure 2a) in locating the submerged platform. Similarly, during the short- and long-term memory tests, fbKO mice exhibited a significant deficit in locating the platform (Figure 1i and Supplementary Figure 2b). These behavioral deficits were not a consequence of a generalized functional impairment in the PFC as fbKO mice showed no deficit in the Y-maze working memory-related task (Figure 1j, Supplementary Figures 2c–d), a PFC-dependent cognitive test that is often altered in SCZ patients.48
Next, to examine hippocampal-dependent learning and memory in fbKO mice, we employed the Morris water maze task (Figure 1k) that utilizes allocentric navigation (distal spatial cues). In this test, fbKO mice displayed no deficits in locating the hidden platform during learning (Figure 1l) or the memory probe tests (Figures 1m–o). Collectively, these results demonstrated that loss of cacna1c in forebrain glutamatergic neurons negatively impacts selective PFC-dependent but not hippocampal-dependent learning and memory.
Decreased sociability in mice with focal knockdown of cacna1c in the adult PFC
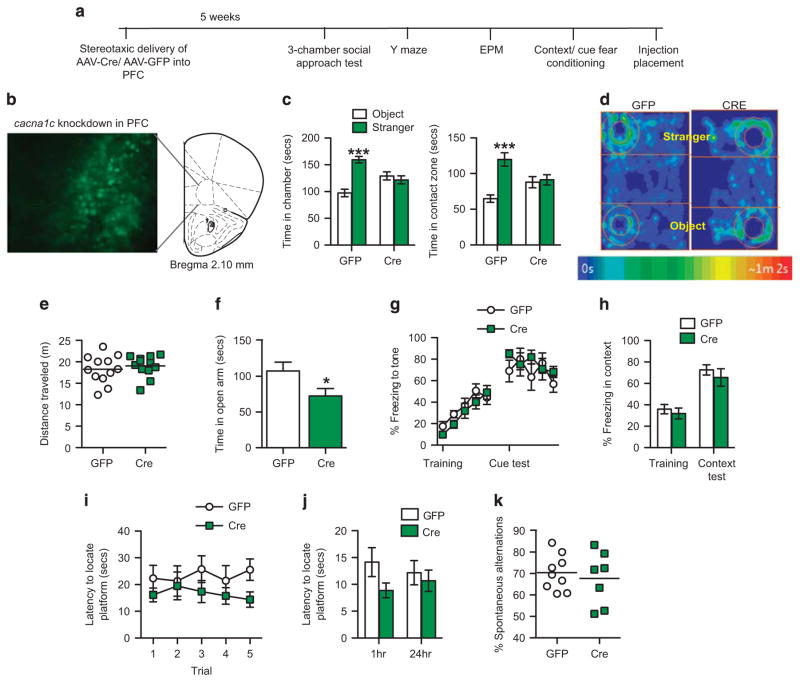
As neuroimaging studies have identified altered PFC structure and activity in CACNA1C SNP carriers11 and SCZ-associated SNPs result in decreased CACNA1C expression in the PFC,26 we wondered if focal ablation of cacna1c in the PFC of adult mice may be capable of inducing the behavioral deficits observed in cacna1c fbKO mice. Adenoassociated viral (AAV) vector-expressing Cre recombinase or control AAV-GFP was stereotaxically delivered into the PFC of adult cacna1cfl/fl mice (Figure 2b) and tested in the above-mentioned behaviors (Figure 2a). Interestingly, mice with a focal knockdown of cacna1c in the adult PFC (PFC-Cre), that have lower cacna1c mRNA levels (Supplementary Figure 3a), as well as Cav1.2 protein levels as previously revealed by immunohistochemistry,28 displayed a deficit in sociability in the three-chamber social approach test similar to that observed in fbKO mice (Figure 1b). While control (PFC-GFP) mice showed a preference for the chamber and contact zone containing the stranger mouse over the novel object, the PFC-Cre mice displayed no preference (Figures 2c and d). No difference in locomotor activity was observed as PFC-Cre and PFC-GFP mice traveled similar distances during the social task (Figure 2e). In addition, in agreement with our previous report,28 PFC-Cre mice displayed increased anxiety-like behavior spending significantly less time in the open arm of the elevated plus maze (EPM; Figure 2f). However, in contrast to the fbKO mice, knockdown of cacna1c in the adult PFC had no effect on cue fear conditioning (Figure 2g) or on learning and memory in the Y-maze (Figures 2i and j; Supplementary Figures 3b–c), suggesting a potential developmental role of Cav1.2 or involvement of additional brain regions for these tasks. Similar to fbKO mice, PFC-Cre mice displayed no deficits in working memory (Figure 2k; Supplementary Figures 3d–e) or contextual fear conditioning (Figure 2h). Thus, knockdown of cacna1c in the adult PFC is sufficient to drive the deficit in sociability and elevate anxiety-like behavior as observed in cacna1c fbKO mice, but does not impact learning and memory or fear associations.
Figure 2.
Focal knockdown of cacna1c in the adult PFC recapitulates the social deficit and elevated anxiety-like behavior. (a) Outline of experimental design. AAV-Cre or AAV-GFP was stereotaxically injected into the PFC of adult cacna1c floxed mice and 5 weeks later tested in a battery of behavioral tests. (b) Representative image of GFP-positive cells expressed by AAV-Cre-GFP in the PFC of cacna1c floxed mice. (c) In the three-chamber social approach test, GFP but not Cre mice spent significantly more time in the chamber (left; Two-way ANOVA, genotype × chamber, F1,44 = 24.28, P <0.0001) and contact zone (right; two-way ANOVA, genotype × zone, F1,44 = 11.71, P = 0.0014) containing the stranger mouse and novel object (Bonferroni post hoc test, ***P<0.001 vs WT; GFP n = 12, Cre n =12). (d) Representative heat maps showing time spent in the chambers and contact zones containing the stranger mouse and novel object during the social approach test. (e) GFP and Cre mice traveled similar distances during the social approach test (GFP n =12, Cre n =12). (f) Cre mice spent significantly less time in the open arm of the elevated plus maze compared to GFP mice (Student’s t-test, t(13) = 2.156, P = 0.05; *P = 0.05 vs GFP; GFP n = 8, Cre n = 7). (g) In cue fear conditioning, GFP and Cre mice displayed similar percent freezing to the tone during training (two-way ANOVA, main effect of tone, F4, 70 = 12.7, P <0.0001) and the test. Each data point represents freezing during a single tone presentation (GFP n = 8, Cre n = 8). (h) In contextual fear conditioning, GFP and Cre mice displayed similar percent freezing to the context during training and the test (GFP n =8, Cre n = 8). (i) In the Y-maze, during training, Cre mice displayed slightly lower latency (main effect of treatment, F1,75 = 5.374, P = 0.0232) in locating the submerged platform compared to GFP mice (GFP n =9, Cre n =8). (j) In the Y-maze memory tests, GFP and Cre mice displayed similar latencies in locating the submerged platform at 1 h and 24 h post-training (GFP n =9, Cre n =8). (k) GFP and Cre mice exhibit similar percent of spontaneous alternations during the working memory Y-maze task (GFP n =8, Cre n =8). Error bars represent mean ± s.e.m. ANOVA, analysis of variance; GFP, green fluorescent protein; PFC, prefrontal cortex.
Cacna1c forebrain knockout mice have decreased initiation of mRNA translation in the PFC
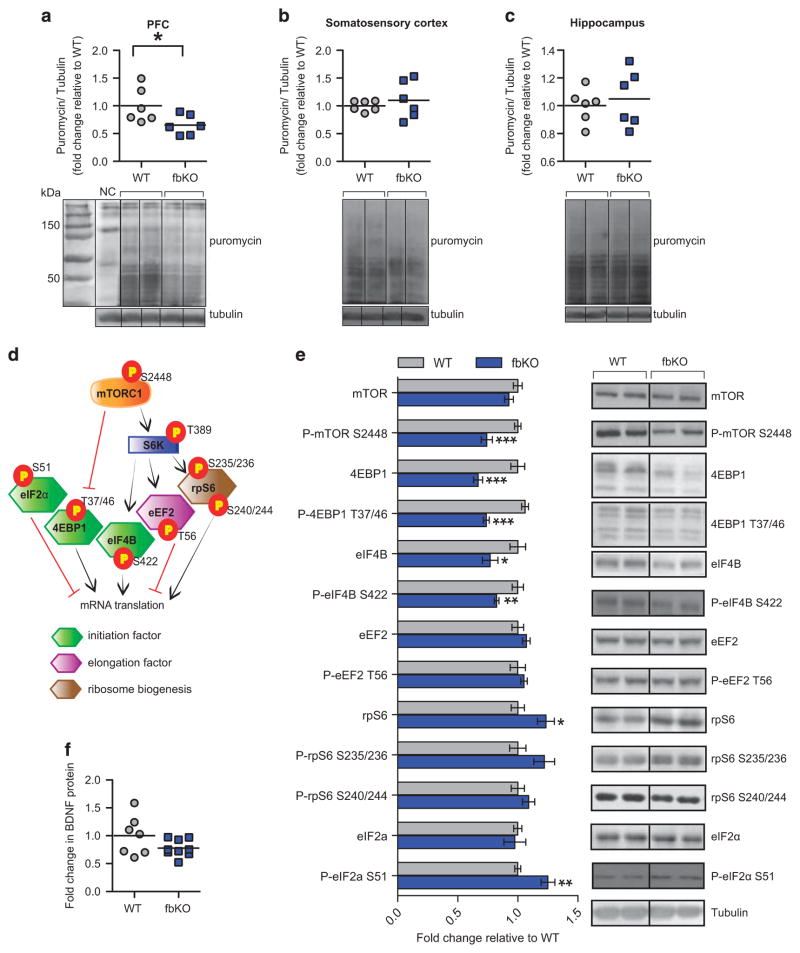
Alterations in protein synthesis, via dysregulated cap-dependent mRNA translation have been shown to underlie social deficits22,49 and anxiety.24 Therefore, we measured the rate of protein synthesis in the brain of cacna1c fbKO mice utilizing the SUnSET technique.34 Puromycin was injected into the lateral ventricle of WT and fbKO mice and levels of total puromycin-labeled proteins, indicative of active translation, were measured. We found significantly lower puromycin labeling in the PFC of fbKO mice (Figure 3a), with no differences in the somatosensory cortex (Figure 3b) or hippocampus (Figure 3c). Thus, loss of cacna1c in forebrain glutamatergic neurons results in lower de novo protein synthesis specifically in the PFC, consistent with the observed PFC- and not hippocampal-dependent behavioral deficits.
Figure 3.
Cacna1c fbKO mice exhibit lower protein synthesis and lower mRNA translation initiation factors in the PFC. (a–c) Immunoblot analysis (top) and representative blots (bottom) of puromycin-labeled protein. Compared to WT mice, fbKO mice displayed significantly lower levels of puromycin-labeled proteins in the PFC (a; Student’s t-test, t(10) = 2.330, P<0.05) but not the somatosensory cortex (b) or hippocampus (c; WT n =6, fbKO n = 6). (d) Schematic representation indicating the relevant phosphorylation sites (indicative of their activity) of mTORC1, its substrates, and eIF2α in their regulation of mRNA translation. (e) Immunoblot analysis (left) and representative blots of markers of mRNA translation in the PFC from total protein lysates. Compared to WT mice, fbKO mice displayed significantly lower levels of P-mTOR S2448 (Student’s t-test, t(12) = 4.410, P = 0.0009), and its downstream substrates 4EBP1 (Student’s t-test, t(12) = 4.739, P = 0.0005), P-4EBP1 T37/46 (Student’s t-test, t(11) = 8.627, P<0.0001), eIF4B (Student’s t-test, t(11) = 2.494, P<0.05), and P-eIF4B S422 (Student’s t-test, t(11) = 3.651, P =0.0038) as well as significantly higher levels of P-eIF2α S51 (Student’s t-test, t(12) = 3.972, P <0.01) in the PFC (n = 6–7/genotype). (f) WT and fbKO mice display similar levels of mature BDNF protein levels in the PFC (WT n =7, fbKO n =9). *P<0.05, **P<0.01, ***P<0.001 vs WT. Error bars represent mean ± s.e.m. fbKO, forebrain knockout; PFC, prefrontal cortex; WT, wild type.
Alteration in the mTORC1 pathway-dependent translation has been associated with behavioral and synaptic deficits in multiple mouse models of neuropsychiatric disorders.50 Furthermore, translation is tightly regulated at the level of initiation and elongation. Therefore, we quantified levels of several mRNA translation factors within the mTORC1 pathway (Figure 3d). In the PFC of cacna1c fbKO mice we found significantly lower levels of phosphorylated mTOR (P-mTOR) at serine (S) 2448, a marker of active mTORC1,50 together with significantly decreased expression of the eukaryotic translation initiation factors, 4EBP1 and eIF4B and their active phosphorylated forms. We observed no difference in levels of the mTORC1-regulated elongation factor eEF2 (Figure 3e), or its downstream target brain-derived neurotrophic factor (BDNF; Figure 3f), an important mediator of neuropsychiatric-related behavioral endophenotypes.51 In addition, even though higher levels of total rpS6 protein were present in the PFC of fbKO mice (Figure 3e), we observed no difference in the active, phosphorylated ribosomal protein rpS6, involved in ribosome biogenesis, a step necessary for mRNA translation.
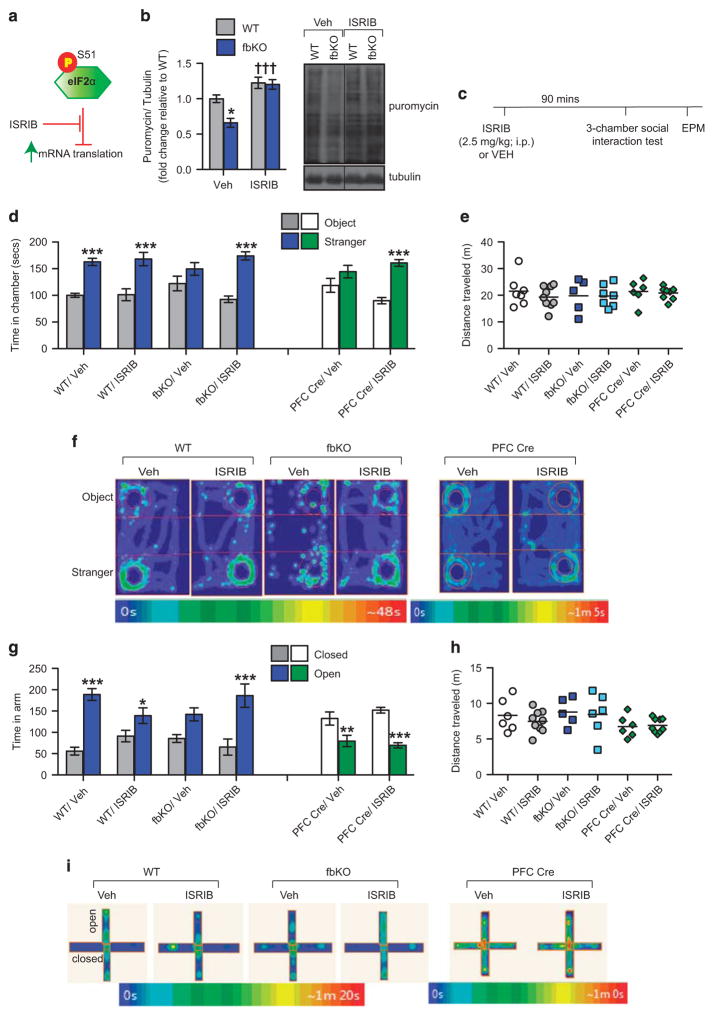
mRNA translation is also regulated by factors that are mTORC1-independent including eukaryotic initiation factor 2α (eIF2α; Figure 3d), which when phosphorylated at S51 inhibits initiation of mRNA translation.25 In the PFC of fbKO mice we found significantly higher levels of P-eIF2α S51 (Figure 3e), consistent with the decreased protein synthesis in this region (Figure 3a). Collectively these data indicate that in the PFC, loss of cacna1c in forebrain glutamatergic neurons results in lower activity of mTORC1-dependent factors and eIF2α, all of which are involved in initiation of cap-dependent mRNA translation. Systemic ISRIB normalizes the lower protein synthesis in the PFC Because of the lack of a direct pharmacological activator of mTORC1 to correct the lower protein synthesis in the PFC of cacna1c fbKO mice, we tested whether a recently discovered small molecule ISRIB (integrated stress response inhibitor)37 that potently blocks the inhibitory effects of higher P-eIF2α S51 on mRNA translation37 was sufficient to normalize the lower general protein synthesis in the PFC of fbKO mice and rescue the social deficit and anxiety-like behavior (Figure 4a). Adult WT and fbKO mice were injected with puromycin in the lateral ventricle concurrent with an acute injection of either ISRIB (2.5 mg kg −1, i.p.) or vehicle for a total of ninety minutes. While vehicle treated fbKO mice displayed lower puromycin labeling in the PFC, confirming lower protein synthesis (Figure 3a), ISRIB treatment significantly increased puromycin-labeled proteins to the level of WT controls (Figure 4b). These results demonstrated that acute treatment with ISRIB is sufficient to normalize the lower levels of general protein synthesis in the PFC of cacna1c fbKO mice.
Figure 4.
ISRIB normalizes the lower protein synthesis in the PFC and rescues the social deficit and elevated anxiety in cacna1c fbKO mice. (a) Schematic depiction of ISRIB’s inhibitory effect on the effect of P-eIF2α S51 on general mRNA translation. (b) Immunoblot analysis (left) and representative blots (right) of puromycin-labeled protein in the PFC. Compared to WT mice, vehicle treated fbKO mice displayed significantly lower levels of puromycin-labeled proteins that was normalized to WT levels with ISRIB treatment (Two-way ANOVA, main effect of genotype, F1,14 = 5.934, P = 0.0288, main effect of treatment, F1,14 = 26.78, P = 0.0001; Bonferroni post hoc test, *P <0.05 vs WT/Veh, †††P <0.001 vs fbKO/Veh; WT/Veh n =3, fbKO/Veh n =3, WT/ISRIB n =5, fbKO/ISRIB n = 6) (c) Outline of experimental design. Adult WT, fbKO and PFC-Cre mice were given a single systemic injection of either ISRIB (2.5 mg/kg, i.p) or vehicle and ninety minutes later tested in social approach followed immediately by EPM. (d) In the three-chamber social approach test, vehicle and ISRIB treated WT mice (WT/Veh and WT/ISRIB, respectively) spent significantly more time in the chamber containing the stranger mouse compared to the novel object (Two-way ANOVA, main effect of chamber, F1, 28 = 40.78, P <0.0001). In contrast, vehicle treated fbKO mice (fbKO/Veh) mice spent similar amounts of time in the chambers containing the stranger mouse and object while fbKO mice with ISRIB treatment (fbKO/ISRIB) displayed a normalization of this social deficit spending significantly more time with the stranger mouse compared to the object (Two-way ANOVA, treatment × chamber, F1, 20 = 8.12, P = 0.0099), looking similar to WT/Veh mice. Similarly, vehicle treated PFC Cre mice (PFC Cre/Veh) spent similar amounts of time in the chamber containing the stranger mouse and object whereas PFC Cre mice following ISRIB treatment (PFC Cre/ISRIB) exhibited a normalization of this social deficit by spending significantly more time with the stranger mouse compared to the novel object (Two-way ANOVA, treatment x chamber, F1, 26 = 6.535, P = 0.0168) looking similar to WT mice (Bonferroni post hoc test, ***P <0.0001 vs object; WT: Veh n = 7, ISRIB n = 9; fbKO: Veh n =5, ISRIB n =7; PFC-Cre: Veh n =6, ISRIB n =9). (e) All mice traveled similar distances during the social approach test (WT: Veh n =7, ISRIB n =9; fbKO: Veh n =5, ISRIB n =7; PFC Cre: Veh n =6, ISRIB n =9). (f) Representative heat maps showing time spent in the chambers containing the stranger mouse and novel object during the social approach test. (g) In the elevated plus maze, WT/Veh and WT/ISRIB mice spent significantly more time in the open arms relative to the closed arms of the maze, although there was a minor effect of ISRIB on anxiety- like behavior (Two-way ANOVA, treatment X arm, F1, 26 = 7.349, P = 0.0117). fbKO/Veh mice spent similar amounts of time in the open and closed arms of the maze while fbKO/ISRIB mice displayed normalization of this behavior spending significantly more time in the open arm relative to the closed arm of the maze (Two-way ANOVA, main effect of arm, F1, 18 = 19.64, P = 0.0003), looking similar to WT/Veh mice. In contrast, both PFC Cre/Veh and PFC Cre/ISRIB mice spent significantly less time in the open arm of the maze compared to the closed arm (Two-way ANOVA, main effect of arm, F1, 26 = 47.01, P <0.0001; Bonferroni post hoc test, *P <0.05, **P <0.01, ***P <0.001 vs closed arm; WT: Veh n =6, ISRIB n =9; fbKO: Veh n =5, ISRIB n =6; PFC Cre: Veh n =6, ISRIB n =9). (h) All mice traveled similar distances in the elevated plus maze (WT: Veh n =6, ISRIB n =9; fbKO: Veh n =5, ISRIB n =6; PFC Cre: Veh n =6, ISRIB n =9). (i) Representative heat maps showing time spent in the open and closed arms of the elevated plus maze. Error bars represent mean ±s.e.m. ANOVA, analysis of variance; EPM, elevated plus maze; fbKO, forebrain knockout; PFC, prefrontal cortex; WT, wild type.
Systemic ISRIB reverses the decreased sociability and increased anxiety in cacna1c forebrain knockout mice
To examine whether an acute treatment with ISRIB corrected the behavioral impairments, adult WT and fbKO mice were injected with either ISRIB (2.5 mg kg −1, i.p.) or vehicle and ninety minutes later tested in social approach followed immediately by elevated plus maze (Figure 4c). Remarkably, ISRIB was sufficient to rescue the social deficit in cacna1c fbKO mice. While vehicle treated fbKO mice displayed a lack of social preference, ISRIB treated mice spent significantly more time with the stranger mouse over the novel object, looking similar to social behavior in WT animals (Figures 4d and f). In WT mice, ISRIB treatment had no effect on social preference (Figures 4d and f). Moreover, ISRIB relieved the anxiogenic phenotype displayed by cacna1c fbKO mice in EPM. Compared to vehicle-treated fbKO mice that spent equal amount of time in the open and closed arms, ISRIB-treated fbKO mice spent significantly greater time in the open arms compared to the closed arms, looking similar to the behavior displayed by vehicle treated WT mice (Figures 4g and i). Interestingly, in WT mice, ISRIB treatment appeared to marginally increase anxiety (Figures 4g and i). ISRIB had no effect on locomotor activity in either the social interaction (Figure 4e) or anxiety (Figure 4h) behavioral tests. These results demonstrated that an acute systemic treatment with ISRIB in adulthood was sufficient to normalize the social deficit and elevated anxiety in cacna1c fbKO mice.
Systemic ISRIB reverses the decreased sociability but not the increased anxiety in adult PFC-specific cacna1c knockdown mice To test whether suppressing the effects of phosphorylated eIF2α with ISRIB can reverse the social deficit and anxiety-like behavior in adult PFC-specific cacna1c knockdown (PFC-Cre) mice, we also treated PFC-Cre mice with ISRIB and tested them in the social and anxiety behavioral tests (Figure 4c). While vehicle treated PFC-Cre mice displayed the sociability deficit, ISRIB treatment significantly increased the time spent in the chamber containing the stranger mouse over the novel object (Figures 4d and f). Surprisingly, in the EPM test, ISRIB was unable to normalize anxiety-like behavior. Both vehicle and ISRIB treated PFC-Cre mice spent more time in the closed arms of the maze (Figures 4g and i). ISRIB in PFC-Cre mice had no effect on locomotor activity during the social (Figure 4e) or anxiety behavioral tests (Figure 4h). These results suggest a convergent molecular mechanism underlying the social impairments in the developmental (fbKO) and adult (PFC-Cre) cacna1c-deficient mice but potentially divergent mechanisms mediating the anxiety-like behavior.
Cacna1c forebrain knockout mice exhibit higher excitatory-inhibitory balance in the PFC
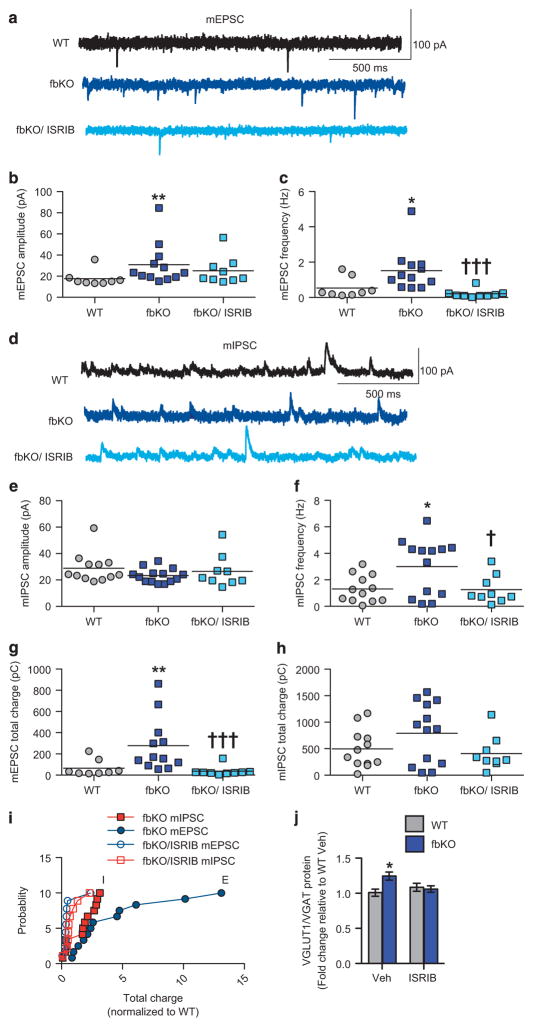
It has been hypothesized that excitatory/inhibitory (E/I) imbalance in the brain is one neuropathophysiological mechanism underlying the social deficits and anxiety-like behavior observed in patients with SCZ and ASD, as well as in rodent models.18,19 In particular, studies have identified altered excitatory neurotransmission52 and spine density53 in layer 5 pyramidal neurons of the PFC in mouse models of ASD that display social impairments. Additionally, mouse models that have impaired protein synthesis and exhibit these behavioral deficits, have altered E/I balance in the PFC.22,54,55 Therefore, to further explore the cellular mechanism underlying the behavioral deficits in cacna1c fbKO mice, we performed whole-cell patch clamp recordings in acute brain slices from layer 5 pyramidal neurons of the PFC. In the PFC of fbKO mice there was significantly reduced amplitude of nifedipine-sensitive L-type calcium currents (Supplementary Figures 4a–b), demonstrating effective loss of function of Cav1.2 channels paralleling lower Cav1.2 protein levels in synaptoneurosomes from the PFC of fbKO mice (Supplementary Figure 4c). Examination of miniature excitatory synaptic currents (mEPSCs) revealed significantly higher amplitude and frequency of mEPSCs in fbKO mice compared to WT controls that was normalized with ISRIB (Figures 5a–c). Interestingly, fbKO mice displayed no difference in the amplitude of miniature inhibitory synaptic currents (mIPSCs; Figures 5d–e) but significantly higher frequency that was normalized to WT levels with ISRIB (Figures 5d and f). Resting membrane potentials (RMPs) were comparable between WT (n = 7; −69.2 ± 1.0 mV) and fbKO (n = 9; − 69.1 ± 0.6 mV) mice. When measuring total synaptic charge transfer, reflecting the E/I ratio,23 we found significantly higher charge transfer for mEPSCs but not mIPSCs in fbKO mice that was normalized to WT levels with ISRIB (Figures 5g–i).
Figure 5.
Cacna1c fbKO mice exhibit higher excitatory/inhibitory ratio in layer 5 pyramidal neurons of the PFC that is normalized with ISRIB. (a) Representative traces of mEPSCs from layer 5 pyramidal neurons of the PFC. (b) fbKO mice displayed significantly higher amplitude of mEPSCs that remained unchanged with ISRIB (Kruskal–Wallis test, H =8.242, P =0.0162; Dunn’s uncorrected post hoc test, **P<0.01 vs WT; WT n =8, fbKO n =12, fbKO/ISRIB n =9 cells, from n =2–4 mice per group). (c) fbKO mice displayed significantly higher frequency of mEPSCs that was normalized to WT levels with ISRIB (Kruskal–Wallis test, H =16.74, P =0.0002; Dunn’s uncorrected post hoc test, *P<0.05 vs WT, †††P<0.0001 vs fbKO; WT n =8, fbKO n =12 cells, fbKO/ISRIB n =9 cells, from n =2–4 mice per group). (d) Representative traces of mIPSCs from layer 5 pyramidal neurons of the PFC. (e) fbKO mice displayed similar amplitude of mIPSCs compared to WT mice that was unchanged with ISRIB (WT n =12, fbKO n =13, fbKO/ISRIB n =9 cells, from n =2–4 mice per group). (f) fbKO mice displayed significantly higher frequency of mIPSCs that was normalized to WT levels with ISRIB (one-way ANOVA, F2, 33 = 4.909, P = 0.0141; Bonferroni post hoc test, *P <0.05 vs WT, †P<0.05 vs fbKO; WT n =12, fbKO n =13, fbKO/ISRIB n =9 cells, from n =4 mice per group). (g) fbKO mice displayed significantly higher total charge transfer for mEPSCs that was normalized with ISRIB (Kruskal–Wallis test, H =14.2, P =0.0008; Dunn’s uncorrected post hoc test, **P<0.01 vs WT, †††P<0.001 vs fbKO; WT n =8, fbKO n = 12, fbKO/ISRIB n =9 cells, from n =2–4 mice per group). (h) fbKO mice displayed similar total charge transfer for mIPSCs compared to WT mice that was unchanged with ISRIB (WT n =12, fbKO n =13, fbKO/ISRIB n =9 cells, from n = 2–4 mice per group). (i) Higher relative change in mEPSC than mIPSC total charge transfer for each fbKO neuron normalized to the mean WT value that was corrected by ISRIB. (j) Compared to WT/Veh mice, fbKO/Veh mice had significantly higher levels of VGLUT1/VGAT ratio in the synaptoneurosome from the PFC while fbKO/ISRIB mice showed normalization of this elevated ratio (two-way ANOVA, treatment × genotype, F1, 23 = 5.797, P = 0.0245; Bonferroni post hoc test, *P <0.05 vs WT; WT: Veh n = 6, ISRIB n = 7; fbKO: Veh n = 6, ISRIB n = 7). Error bars represent mean ±s.e.m. ANOVA, analysis of variance; E, excitatory; fbKO, forebrain knockout, I, inhibitory; mEPSCs, miniature excitatory post synaptic currents; mIPSCs, miniature inhibitory post synaptic currents; WT, wild type.
We additionally examined protein levels of excitatory and inhibitory presynaptic markers, vesicular glutamate (VGLUT1) and GABA (VGAT) transporters in synaptoneurosomes isolated from the PFC and evaluated their ratio, a molecular measure previously used as a correlate of altered E/I balance.56 In the PFC of fbKO mice, no difference in levels of VGLUT1 (Supplementary Figure 5b) but significantly lower VGAT (Supplementary Figure 5c) levels was observed. Furthermore, when comparing the VGLUT1/VGAT ratio, a molecular measure of E/I ratio, fbKO mice displayed a significantly higher ratio in the PFC (Figure 5j) consistent with the elevated E/I ratio. Remarkably, an acute treatment with ISRIB (Supplementary Figure 5a) was sufficient to normalize the higher VGLUT1/VGAT ratio in the PFC of fbKO (Figure 5j) that appeared to be a result of an increase in VGAT protein levels (Supplementary Figure 5c). These results demonstrate that loss of cacna1c in forebrain glutamatergic neurons results in higher E/I ratio in layer 5 pyramidal neurons that is normalized by ISRIB.
DISCUSSION
Here, we report that fbKO mice that have a loss of cacna1c in excitatory glutamatergic neurons of the forebrain and exhibit anxiety-like behavior,28 display reduced sociability, enhanced cue-associated fear responses and demonstrate selective impairments in learning and memory. The social deficit and increased anxiety-like behavior is recapitulated in mice with a focal knockdown of cacna1c in the adult PFC, identifying cacna1c in this region as a critical mediator of these behavioral deficits. Further exploration of the PFC of cacna1c fbKO mice identified higher E/I ratio, a physiological parameter that has been linked to neuropsychiatric-related phenotypes, as well as elevated VGLUT1/VGAT ratio, a molecular correlate of the higher E/I. Molecular studies in the PFC of cacna1c fbKO mice revealed decreased protein synthesis concurrent with downregulation of mTORC1-dependent mRNA translation initiation factors and upregulation of P-eIF2α S51, an inhibitor of mRNA translation. Finally, acute systemic treatment in cacna1c fbKO mice with the small molecule ISRIB that suppresses the effect of P-eIF2α S51 on mRNA translation was sufficient to normalize the lower protein synthesis and higher E/I balance in the PFC. Furthermore, ISRIB treatment was also able to correct the social deficit and elevated anxiety.
The precise functional contribution of CACNA1C SNPs to neuropsychiatric symptoms remains unknown. To date all disease-associated CACNA1C SNPs are intronic1 with both an increase57,58 and decrease26,27,58,59 in CACNA1C expression and function reported in human samples. Irrespective, given that Cav1.2 channels play a crucial role in various neuronal functions, we hypothesize that higher or lower Ca2+ influx in neurons can alter neuronal and brain function and impact behavior in patients. This hypothesis is supported by the Cav1.2 Timothy syndrome (TS) mouse model that harbors a mutation that results in higher Ca2+ influx, displays a similar social deficit and persistent increase in cue-associated fear memory60 as we report here in cacna1c fbKO mice. However, while the TS mouse displays increased freezing during the contextual fear memory test,60 the cacna1c fbKO mice do not, demonstrating some differences between the two mouse models. However, it is possible that the lack of a phenotype in cacna1c fbKO mice is due to a ceiling effect in freezing levels, a question that will be addressed in future studies using alternative fear conditioning protocols. Our behavioral findings in cacna1c fbKO mice are comparable to the low extraversion, a personality trait that is characterized by reduced social interactions61 and poor learning performance62 reported in human CACNA1C SNP carriers. Interestingly, while the cacna1c fbKO mice displayed elevated anxiety,28 the TS mouse did not,60 suggesting that human CACNA1C SNPs that have been associated with increased anxiety63,64 could possibly result from decreased cacna1c levels in the PFC. Interestingly, the lack of a deficit in the working memory task in the cacna1c fbKO mice that, in part, recruits the PFC, suggests that cacna1c deficiency may not affect working memory. This is supported by a previous study that identified intact working memory in patients with the SCZ-associated CACNA1C SNPs.65 However, as the cacna1c fbKO mice that we utilized lack cacna1c in excitatory, glutamatergic neurons, it is possible that this cell-type is not involved in working memory. This is supported by a recent finding of a role of the parvalbumin-positive interneurons of the PFC in working memory.66 Together, these preclinical animal model findings support the growing idea and evidence that brain region and cell-type specific loss of cacna1c may contribute to varying neuropsychiatric-related endophenotypes.59,67
The PFC has been identified as a key regulator of social cognition (reviewed in Bicks et al.17) and an important modulator of anxiety.68 Together with our previous findings,28 here we show that cacna1c in the PFC regulates both sociability and anxiety. As ISRIB treatment is sufficient to correct both, the social deficit and the elevated anxiety in cacna1c fbKO mice, our findings suggest overlapping anatomical and molecular mechanisms underlying these two endophenotypes. This is consistent with previous studies that have identified an overlap between anxiety and impaired social behavior.41 These include human studies that have reported increased social dysfunction with increased anxiety levels as well as animal studies that have identified increase in social interaction with anxiolytic treatments.41 Interestingly, ISRIB treatment in mice with knockdown of cacna1c in the adult PFC rescued only the social deficit and not the anxiety-like behavior highlighting the complexity of behavioral control at the circuit- and mechanistic level and suggests dissociability of anxiety and social behavior in certain conditions, such as that recapitulated by the adult PFC-specific cacna1c knockout model, that are possibly mediated by divergent neural mechanisms. It will be important in future studies to use such conditional and inducible knockout strategies to further examine cacna1c circuitry and mechanisms underlying complex behaviors such as anxiety and sociability. Future studies will also examine if ISRIB treatment directly in the PFC is sufficient to correct the social deficit and heightened anxiety.
The increase in E/I transmission and higher VGLUT1/VGAT ratio in the PFC of cacna1c fbKO mice, makes this a potential cellular perturbation mediating the behavioral impairments in these mice. This is supported by the observation that ISRIB in adult cacna1c fbKO mice normalizes the behavioral impairments, as well as the elevated E/I and VGLUT1/VGAT ratio. Our data in the cacna1c fbKO mouse model adds to the growing body of evidence that cortical E/I imbalance17–20 negatively impacts neuropsychiatric-related behavioral endophenotypes with higher prefrontal cortical E/I driving neuropsychiatric-related impairments in social behavior21,22,69 and anxiety.70
Converging lines of evidence suggest that exaggerated mRNA translation is one mechanism underlying higher E/I and neuropsychiatric-related behavioral impairments.19,25,50 Our finding that lower mRNA translation can also result in higher E/I and produce similar behavioral deficits suggests that divergent downstream mRNA translation mechanisms can eventually result in comparable physiological and behavioral outcomes. We find altered activity of two primary mRNA translation pathways, mTORC1 that regulates global protein synthesis,50 and eIF2α that regulates both global protein synthesis and also mRNA-specific translation.25 Although suppressing the effects of exaggerated phospho-eIF2α by ISRIB is sufficient to normalize the lower levels of general protein synthesis in the PFC in cacna1c fbKO mice, our data does not rule out the possibility of a role of eIF2α-specific mRNA translation of target genes (discussed below). Furthermore, ISRIB may initiate crosstalk between eIF2α and mTORC1 downstream pathways71 and regulate similar mRNA targets, a future line of investigation.
mTORC1 has been extensively studied in neurodevelopmental and neuropsychiatric disorders50 with most reporting an increase in mTORC1 activity (and thus increase in protein synthesis). In contrast, in cacna1c fbKO mice we find lower mTORC1 activity that parallels the lower protein synthesis. Interestingly lower mTORC1 has been reported in mice deficient in MeCP2,72 a downstream target of LTCCs73 that also display social deficits and elevated anxiety74 and have higher E/I.55 Furthermore lower mTORC1 has been reported in patients with idiopathic autism75 that exhibit core social behavioral impairments. Thus, lower mTORC1 activity can produce similar cellular and behavioral deficits as higher mTORC1 activity seen in several animal models of neuropsychiatric disease.
As compared to mTORC1, studies on the eIF2α pathway are limited, although a role in synaptic plasticity and memory has been reported. Phospho-mutant eIF2α (S51A) mice, with reduced levels of P-eIF2α S51, require a lower threshold to elicit the late-phase of hippocampal LTP (L-LTP) and exhibit enhanced memory,76 behavioral effects mimicked by ISRIB.37 Similarly, knockout of GCN2, an upstream kinase of eIF2α that has lower phospho-eIF2α, results in similar L-LTP and short-term spatial memory enhancement77 as seen in eIF2α S51A phospho-mutant mice, supporting our findings that higher P-eIF2α S51 can be detrimental to synaptic plasticity and behavior. Additionally, knockout of PERK, another upstream kinase of eIF2α results in elevated metabotropic glutamate receptor (mGluR)-LTD78 and enhanced fear memory.79 These effects on synaptic plasticity and memory enhancement have been suggested to be a consequence of selective increase in eIF2α-mediated translation of the transcription factor ATF4. ATF4 belongs to the CREB family of transcription factors80 that are highly regulated by LTCCs,81 and is a repressor of CREB-mediated transcription,82,83 suggesting the possibility that the deficits in cacna1c fbKO mice may be mediated by ATF4. However, a recent study has demonstrated the necessity of ATF4 for regulation of spine number, morphology and synaptic plasticity,84 suggesting that the effects of ATF4 may be finely tuned and also dependent on the disease model examined. Future studies will examine the contribution of the eIF2α/ATF4 pathway to cellular and behavioral phenotypes we have identified.
Another question to be addressed in future studies is the mechanism by which eIF2α contributes to the E/I imbalance, and how ISRIB corrects it. ATF4 has been shown to regulate EPSCs and maintain glutamatergic synapses.84 Another potential route of regulation of E/I imbalance is via P-eIF2α-selective translation of the oligophrenin-1 (OPHN1) mRNA, involved in regulation of cell surface levels of hippocampal glutamatergic AMPA receptors.85,86 Using VGLUT1 and VGAT as markers of excitatory and inhibitory synapses, we find lower levels of VGAT in the PFC of cacna1c fbKO mice that are restored to WT levels by ISRIB, suggesting that higher EPSPs could possibly result from decreased translation of VGAT or other markers of inhibitory transmission. A similar finding of elevated EPSP in parallel with increased P-eIF2α has been reported and suggested to be a result of impaired synthesis of inhibitory elements.87 Future studies will explore P-eIF2α regulation of both excitatory and inhibitory pre- and postsynaptic proteins that contribute to the E/I imbalance observed in the PFC of cacna1c fbKO mice.
The mechanism by which deficient Cav1.2 signaling impairs eIF2α and mTORC1 pathways remains unknown. One potential route is via activation of the PI3K/Akt pathway, implicated downstream of LTCCs,88 that activates mTORC1 via phosphoryla-tion and inhibition of the tuberous sclerosis complex TSC1/2,50 a molecule linked to ASD-related symptoms. The Akt/PI3K pathway also negatively modulates eIF2α phosphorylation via PKR-like ER kinase (PERK).89 Thus, we hypothesize that loss of cacna1c resulting in deficient Cav1.2 signaling may inhibit Akt that subsequently (1) suppresses phosphorylation of TSC1/2 and increases its inhibitory effect on mTORC1 phosphorylation, and separately (2) activates PERK and induces eIF2α phosphorylation.
Overall, the preclinical findings in this study provide evidence that the cacna1c forebrain knockout mouse model is a valuable animal model to study mechanisms downstream of Cav1.2 (cacna1c) that contribute to neuropsychiatric pathophysiology and also highlights how CACNA1C can exert such pleiotropic effects on psychopathology related to multiple neuropsychiatric disorders. This study identifies a novel in vivo role of cacna1c in glutamatergic neurons, demonstrates causality between altered expression of this gene and development of neuropsychiatric-related behaviors in mice, and identifies a unique molecular mechanism in cacna1c-deficient mice that merits further exploration for understanding cacna1c-mediated behavioral endophenotypes.
Supplementary Material
Acknowledgments
This work was supported by The Hartwell Foundation (AMR), the Weill Cornell Autism Research Program (AMR), the Weill Cornell Medicine Postdoctoral Fellowship (ZDK), the National Institutes of Health (5R01DA029122 to AMR; 5 R00 MH095825 05 and 1 R01 MH110553 01 to NVDMG), Leon Levy Foundation (NVDMG), and Citizens United for Research in Epilepsy (CURE; NVDMG). We thank Dr Jacqueline Crawley for technical assistance with establishing the social interaction behavioral apparatus and procedure in our laboratory. We thank Drs Eric Klann, Aditi Bhattacharya, Alexandra Cohen and Anni Lee for technical assistance with establishing the water-based Y-maze behavioral paradigm in our laboratory. We thank Dr Andrew Pieper for critical reading of the manuscript and Dr Héctor De Jesús-Cortés for his comments on the figures.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
ZDK and AMR contributed to the experimental design, data interpretation and wrote the paper. AC and NVDMG performed electrophysiological experiments and analyzed data. ZDK performed stereotaxic surgeries and analyzed data. ZDK and DKF conducted molecular and behavioral experiments. BR conducted molecular experiments. RCR and MB conducted behavioral experiments. MJG conducted electron microscopy experiments. All authors discussed and commented on the manuscript.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- 1.Bhat S, Dao DT, Terrillion CE, Arad M, Smith RJ, Soldatov NM, et al. CACNA1C (Cav1. 2) in the pathophysiology of psychiatric disease. Prog Neurobiol. 2012;99:1–14. doi: 10.1016/j.pneurobio.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Consortium C-DGotPG. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heyes S, Pratt WS, Rees E, Dahimene S, Ferron L, Owen MJ, et al. Genetic disruption of voltage-gated calcium channels in psychiatric and neurological disorders. Prog Neurobiol. 2015;134:36–54. doi: 10.1016/j.pneurobio.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira MA, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry. 2010;15:1016–1022. doi: 10.1038/mp.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Zhao L, You Y, Lu T, Jia M, Yu H, et al. Schizophrenia Related Variants in CACNA1C also Confer Risk of Autism. PLoS ONE. 2015;10:e0133247. doi: 10.1371/journal.pone.0133247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Network, Pathway Analysis Subgroup of Psychiatric Genomics C. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18:199–209. doi: 10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simms BA, Zamponi GW. Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron. 2014;82:24–45. doi: 10.1016/j.neuron.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Pasca SP, Portmann T, Voineagu I, Yazawa M, Shcheglovitov A, Pasca AM, et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med. 2011;17:1657–1662. doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabir ZD, Lee AS, Rajadhyaksha AM. L-type Ca channels in mood, cognition and addiction: Integrating human and rodent studies with a focus on behavioural endophenotypes. J Physiol. 2016;594:5823–5837. doi: 10.1113/JP270673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyoshi M, Morimura Y. Clinical manifestations of neuropsychiatric disorders. Neuropsychiatr Disord. 2010;(Part I):1–14. [Google Scholar]
- 13.Millan MJ, Agid Y, Brune M, Bullmore ET, Carter CS, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- 14.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 15.Riga D, Matos MR, Glas A, Smit AB, Spijker S, Van den Oever MC. Optogenetic dissection of medial prefrontal cortex circuitry. Front Syst Neurosci. 2014;8:230. doi: 10.3389/fnsys.2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, McIntosh AM, He Y, Gelernter J, Blumberg HP. The association of genetic variation in CACNA1C with structure and function of a frontotemporal system. Bipolar Disord. 2011;13:696–700. doi: 10.1111/j.1399-5618.2011.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal cortex and social cognition in mouse and man. Front Psychol. 2015;6:1805. doi: 10.3389/fpsyg.2015.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med. 2015;15:146–167. doi: 10.2174/1566524015666150303003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullins C, Fishell G, Tsien RW. Unifying views of autism spectrum disorders: a consideration of autoregulatory feedback loops. Neuron. 2016;89:1131–1156. doi: 10.1016/j.neuron.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson SB, Valakh V. Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron. 2015;87:684–698. doi: 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santini E, Huynh TN, MacAskill AF, Carter AG, Pierre P, Ruggero D, et al. Exaggerated translation causes synaptic and behavioural aberrations associated with autism. Nature. 2013;493:411–415. doi: 10.1038/nature11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gkogkas CG, Khoutorsky A, Ran I, Rampakakis E, Nevarko T, Weatherill DB, et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature. 2013;493:371–377. doi: 10.1038/nature11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huynh TN, Shah M, Koo SY, Faraud KS, Santini E, Klann E. eIF4E/Fmr1 double mutant mice display cognitive impairment in addition to ASD-like behaviors. Neurobiol Dis. 2015;83:67–74. doi: 10.1016/j.nbd.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buffington SA, Huang W, Costa-Mattioli M. Translational control in synaptic plasticity and cognitive dysfunction. Annu Rev Neurosci. 2014;37:17–38. doi: 10.1146/annurev-neuro-071013-014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roussos P, Mitchell AC, Voloudakis G, Fullard JF, Pothula VM, Tsang J, et al. A role for noncoding variation in schizophrenia. Cell Rep. 2014;9:1417–1429. doi: 10.1016/j.celrep.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gershon ES, Grennan K, Busnello J, Badner JA, Ovsiew F, Memon S, et al. A rare mutation of CACNA1C in a patient with bipolar disorder, and decreased gene expression associated with a bipolar-associated common SNP of CACNA1C in brain. Mol Psychiatry. 2014;19:890–894. doi: 10.1038/mp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee AS, Ra S, Rajadhyaksha AM, Britt JK, De Jesus-Cortes H, Gonzales KL, et al. Forebrain elimination of cacna1c mediates anxiety-like behavior in mice. Mol Psychiatry. 2012;17:1054–1055. doi: 10.1038/mp.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inan M, Zhao M, Manuszak M, Karakaya C, Rajadhyaksha AM, Pickel VM, et al. Energy deficit in parvalbumin neurons leads to circuit dysfunction, impaired sensory gating and social disability. Neurobiol Dis. 2016;93:35–46. doi: 10.1016/j.nbd.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Detrait E, brohez C, Hanon E, De Ryck M. Automation of continuous spontaneous alternation to increase the thoughput for in vivo screening of cognitive enhancers. Optimization of the ethovision system for the Y-maze test in mice. Proceedings of Measuring Behavior, 7th International Conference on Methods and Techniques in Behavioral Research; Eindhoven, The Netherlands. 2010; pp. 141–144. [Google Scholar]
- 32.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Che A, Girgenti MJ, LoTurco J. The dyslexia-associated gene DCDC2 is required for spike-timing precision in mouse neocortex. Biol Psychiatry. 2014;76:387–396. doi: 10.1016/j.biopsych.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods. 2009;6:275–277. doi: 10.1038/nmeth.1314. [DOI] [PubMed] [Google Scholar]
- 35.Tropea TF, Kabir ZD, Kaur G, Rajadhyaksha AM, Kosofsky BE. Enhanced dopamine D1 and BDNF signaling in the adult dorsal striatum but not nucleus accumbens of prenatal cocaine treated mice. Front Psychiatry. 2011;2:67. doi: 10.3389/fpsyt.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kabir ZD, Katzman AC, Kosofsky BE. Molecular mechanisms mediating a deficit in recall of fear extinction in adult mice exposed to cocaine in utero. PLoS ONE. 2013;8:e84165. doi: 10.1371/journal.pone.0084165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sidrauski C, Acosta-Alvear D, Khoutorsky A, Vedantham P, Hearn BR, Li H, et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. eLife. 2013;2:e00498. doi: 10.7554/eLife.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Biase V, Obermair GJ, Szabo Z, Altier C, Sanguesa J, Bourinet E, et al. Stable membrane expression of postsynaptic CaV1. 2 calcium channel clusters is independent of interactions with AKAP79/150 and PDZ proteins. J Neurosci. 2008;28:13845–13855. doi: 10.1523/JNEUROSCI.3213-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee AS, De Jesus-Cortes H, Kabir ZD, Knobbe W, Orr M, Burgdorf C, et al. The neuropsychiatric disease-associated gene cacna1c mediates survival of young hippocampal neurons. eNeuro. 2016;3 doi: 10.1523/ENEURO.0006-16.2016. ENEURO.0006-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kempermann G, Krebs J, Fabel K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry. 2008;21:290–295. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- 41.Allsop SA, Vander Weele CM, Wichmann R, Tye KM. Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front Behav Neurosci. 2014;8:241. doi: 10.3389/fnbeh.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stemmelin J, Cohen C, Terranova JP, Lopez-Grancha M, Pichat P, Bergis O, et al. Stimulation of the beta3-Adrenoceptor as a novel treatment strategy for anxiety and depressive disorders. Neuropsychopharmacology. 2008;33:574–587. doi: 10.1038/sj.npp.1301424. [DOI] [PubMed] [Google Scholar]
- 44.Hoffman KL. Modeling neuropsychiatric disorders in laboratory animals. 2016. [Google Scholar]
- 45.Indovina I, Robbins TW, Nunez-Elizalde AO, Dunn BD, Bishop SJ. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron. 2011;69:563–571. doi: 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sartori SB, Hauschild M, Bunck M, Gaburro S, Landgraf R, Singewald N. Enhanced fear expression in a psychopathological mouse model of trait anxiety: pharmacological interventions. PLoS ONE. 2011;6:e16849. doi: 10.1371/journal.pone.0016849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson OJ, Vytal K, Cornwell BR, Grillon C. The impact of anxiety upon cognition: perspectives from human threat of shock studies. Front Hum Neurosci. 2013;7:203. doi: 10.3389/fnhum.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lara AH, Wallis JD. The Role of Prefrontal Cortex in Working Memory: A Mini Review. Front Syst Neurosci. 2015;9:173. doi: 10.3389/fnsys.2015.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darnell JC, Klann E. The translation of translational control by FMRP: therapeutic targets for FXS. Nat Neurosci. 2013;16:1530–1536. doi: 10.1038/nn.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costa-Mattioli M, Monteggia LM. mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat Neurosci. 2013;16:1537–1543. doi: 10.1038/nn.3546. [DOI] [PubMed] [Google Scholar]
- 51.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duffney LJ, Zhong P, Wei J, Matas E, Cheng J, Qin L, et al. Autism-like deficits in shank3-deficient mice are rescued by targeting actin regulators. Cell Rep. 2015;11:1400–1413. doi: 10.1016/j.celrep.2015.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samaco RC, McGraw CM, Ward CS, Sun Y, Neul JL, Zoghbi HY. Female Mecp2(+/−) mice display robust behavioral deficits on two different genetic backgrounds providing a framework for pre-clinical studies. Hum Mol Genet. 2013;22:96–109. doi: 10.1093/hmg/dds406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W, Peterson M, Beyer B, Frankel WN, Zhang ZW. Loss of MeCP2 from forebrain excitatory neurons leads to cortical hyperexcitation and seizures. J Neurosci. 2014;34:2754–2763. doi: 10.1523/JNEUROSCI.4900-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sceniak MP, Lang M, Enomoto AC, Howell CJ, Hermes DJ, Katz DM. Mechanisms of functional hypoconnectivity in the medial prefrontal cortex of Mecp2 null mice. Cereb Cortex. 2016;26:1938–1956. doi: 10.1093/cercor/bhv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bigos KL, Mattay VS, Callicott JH, Straub RE, Vakkalanka R, Kolachana B, et al. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry. 2010;67:939–945. doi: 10.1001/archgenpsychiatry.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshimizu T, Pan JQ, Mungenast AE, Madison JM, Su S, Ketterman J, et al. Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol Psychiatry. 2015;20:162–169. doi: 10.1038/mp.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eckart N, Song Q, Yang R, Wang R, Zhu H, McCallion AS, et al. Functional Characterization of Schizophrenia-Associated Variation in CACNA1C. PLoS ONE. 2016;11:e0157086. doi: 10.1371/journal.pone.0157086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bader PL, Faizi M, Kim LH, Owen SF, Tadross MR, Alfa RW, et al. Mouse model of Timothy syndrome recapitulates triad of autistic traits. Proc Natl Acad Sci USA. 2011;108:15432–15437. doi: 10.1073/pnas.1112667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roussos P, Giakoumaki SG, Georgakopoulos A, Robakis NK, Bitsios P. The CACNA1C and ANK3 risk alleles impact on affective personality traits and startle reactivity but not on cognition or gating in healthy males. Bipolar Disord. 2011;13:250–259. doi: 10.1111/j.1399-5618.2011.00924.x. [DOI] [PubMed] [Google Scholar]
- 62.Dietsche B, Backes H, Laneri D, Weikert T, Witt SH, Rietschel M, et al. The impact of a CACNA1C gene polymorphism on learning and hippocampal formation in healthy individuals: a diffusion tensor imaging study. Neuroimage. 2014;89:256–261. doi: 10.1016/j.neuroimage.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 63.Erk S, Meyer-Lindenberg A, Linden DE, Lancaster T, Mohnke S, Grimm O, et al. Replication of brain function effects of a genome-wide supported psychiatric risk variant in the CACNA1C gene and new multi-locus effects. Neuroimage. 2014;94:147–154. doi: 10.1016/j.neuroimage.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 64.Erk S, Meyer-Lindenberg A, Schnell K, Opitz von Boberfeld C, Esslinger C, Kirsch P, et al. Brain function in carriers of a genome-wide supported bipolar disorder variant. Arch Gen Psychiatry. 2010;67:803–811. doi: 10.1001/archgenpsychiatry.2010.94. [DOI] [PubMed] [Google Scholar]
- 65.Hori H, Yamamoto N, Fujii T, Teraishi T, Sasayama D, Matsuo J, et al. Effects of the CACNA1C risk allele on neurocognition in patients with schizophrenia and healthy individuals. Sci Rep. 2012;2:634. doi: 10.1038/srep00634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murray AJ, Woloszynowska-Fraser MU, Ansel-Bollepalli L, Cole KL, Foggetti A, Crouch B, et al. Parvalbumin-positive interneurons of the prefrontal cortex support working memory and cognitive flexibility. Sci Rep. 2015;5:16778. doi: 10.1038/srep16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koester SE, Insel TR. Understanding how non-coding genomic polymorphisms affect gene expression. Mol Psychiatry. 2016;21:448–449. doi: 10.1038/mp.2015.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18:1394–1404. doi: 10.1038/nn.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang J, Xu W, Hsu YT, Yee AX, Chen L, Sudhof TC. Conditional neuroligin-2 knockout in adult medial prefrontal cortex links chronic changes in synaptic inhibition to cognitive impairments. Mol Psychiatry. 2015;20:850–859. doi: 10.1038/mp.2015.31. [DOI] [PubMed] [Google Scholar]
- 70.Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, et al. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zimmerman HR, Beckelman B, Yang W, Ma T. Program No 12602 2016 Neuroscience Meeting Planner. Society for Neuroscience, Online; San Diego, CA, USA: 2016. Interactions between the eIF2a and mTORC1 signaling pathways. [Google Scholar]
- 72.Ricciardi S, Boggio EM, Grosso S, Lonetti G, Forlani G, Stefanelli G, et al. Reduced AKT/mTOR signaling and protein synthesis dysregulation in a Rett syndrome animal model. Hum Mol Genet. 2011;20:1182–1196. doi: 10.1093/hmg/ddq563. [DOI] [PubMed] [Google Scholar]
- 73.Tao J, Hu K, Chang Q, Wu H, Sherman NE, Martinowich K, et al. Phosphorylation of MeCP2 at Serine 80 regulates its chromatin association and neurological function. Proc Natl Acad Sci USA. 2009;106:4882–4887. doi: 10.1073/pnas.0811648106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gemelli T, Berton O, Nelson ED, Perrotti LI, Jaenisch R, Monteggia LM. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biol Psychiatry. 2006;59:468–476. doi: 10.1016/j.biopsych.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 75.Nicolini C, Ahn Y, Michalski B, Rho JM, Fahnestock M. Decreased mTOR signaling pathway in human idiopathic autism and in rats exposed to valproic acid. Acta Neuropathol Commun. 2015;3:3. doi: 10.1186/s40478-015-0184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, et al. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Costa-Mattioli M, Gobert D, Harding H, Herdy B, Azzi M, Bruno M, et al. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature. 2005;436:1166–1173. doi: 10.1038/nature03897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trinh MA, Ma T, Kaphzan H, Bhattacharya A, Antion MD, Cavener DR, et al. The eIF2alpha kinase PERK limits the expression of hippocampal metabotropic glutamate receptor-dependent long-term depression. Learn Mem. 2014;21:298–304. doi: 10.1101/lm.032219.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trinh MA, Kaphzan H, Wek RC, Pierre P, Cavener DR, Klann E. Brain-specific disruption of the eIF2alpha kinase PERK decreases ATF4 expression and impairs behavioral flexibility. Cell Rep. 2012;1:676–688. doi: 10.1016/j.celrep.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sidrauski C, McGeachy AM, Ingolia NT, Walter P. The small molecule ISRIB reverses the effects of eIF2alpha phosphorylation on translation and stress granule assembly. eLife. 2015;4 doi: 10.7554/eLife.05033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 82.Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- 83.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 84.Pasini S, Corona C, Liu J, Greene LA, Shelanski ML. Specific downregulation of hippocampal ATF4 reveals a necessary role in synaptic plasticity and memory. Cell Rep. 2015;11:183–191. doi: 10.1016/j.celrep.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nadif Kasri N, Nakano-Kobayashi A, Van Aelst L. Rapid synthesis of the X-linked mental retardation protein OPHN1 mediates mGluR-dependent LTD through interaction with the endocytic machinery. Neuron. 2011;72:300–315. doi: 10.1016/j.neuron.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Di Prisco GV, Huang W, Buffington SA, Hsu CC, Bonnen PE, Placzek AN, et al. Translational control of mGluR-dependent long-term depression and object-place learning by eIF2alpha. Nat Neurosci. 2014;17:1073–1082. doi: 10.1038/nn.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rudell JB, Rechs AJ, Kelman TJ, Ross-Inta CM, Hao S, Gietzen DW. The anterior piriform cortex is sufficient for detecting depletion of an indispensable amino acid, showing independent cortical sensory function. J Neurosci. 2011;31:1583–1590. doi: 10.1523/JNEUROSCI.4934-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng F, Zhou X, Luo Y, Xiao H, Wayman G, Wang H. Regulation of brain-derived neurotrophic factor exon IV transcription through calcium responsive elements in cortical neurons. PLoS ONE. 2011;6:e28441. doi: 10.1371/journal.pone.0028441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mounir Z, Krishnamoorthy JL, Wang S, Papadopoulou B, Campbell S, Muller WJ, et al. Akt determines cell fate through inhibition of the PERK-eIF2alpha phosphorylation pathway. Sci Signal. 2011;4:ra62. doi: 10.1126/scisignal.2001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.