Abstract
Background
Adolescent anxiety and depression are highly prevalent psychiatric disorders that are associated with altered molecular and neurocircuit profiles. Recently, increased mitochondrial DNA copy number (mtDNA-cn) has been found to be associated with several psychopathologies in adults, especially anxiety and depression. The associations between mtDNA-cn and anxiety and depression have not, however, been investigated in adolescents. Moreover, to date there have been no studies examining associations between mtDNA-cn and brain network alterations in mood disorders in any age group.
Methods
The first aim of this study was to compare salivary mtDNA-cn between 49 depressed and/or anxious adolescents and 35 well-matched healthy controls. The second aim of this study was to identify neural correlates of mtDNA-cn derived from diffusion tensor imaging (DTI) and tractography, in the full sample of adolescents.
Results
There were no diagnosis-specific alterations in mtDNA-cn. However, there was a positive correlation between mtDNA-cn and levels of anxiety, but not depression, in the full sample of adolescents. A subnetwork of connections largely corresponding to the left fronto-occipital fasciculus had significantly lower fractional anisotropy (FA) values in adolescents with higher than median mtDNA-cn.
Limitations
Undifferentiated analysis of free and intracellular mtDNA and use of DTI-based tractography represent this study’s limitations.
Conclusions
The results of this study help elucidate the relationships between clinical symptoms, molecular changes, and neurocircuitry alterations in adolescents with and without anxiety and depression, and they suggest that increased mtDNA-cn is associated both with increased anxiety symptoms and with decreased fronto-occipital structural connectivity in this population.
Keywords: mitochondrial DNA, adolescent depression, anxiety, MRI, DTI, brain connectivity
Introduction
Adolescent anxiety and depression are both highly prevalent with numerous long-term negative health consequences (Polanczyk et al., 2015). To advance understanding of these disorders and improve prevention and treatment approaches, comprehensive research at multiple levels of analysis is required, as suggested in the Research Domain Criteria (RDoC) framework (Insel et al., 2010). There is, however, a persistent gap in research that would span more than two levels of analysis. Specifically, the relationship between clinical symptoms (self-reports), molecular changes (intracellular markers), and neurocircuitry alterations in adolescents with anxiety and depression remains largely unclear.
One promising intracellular marker that has been studied in adults with depression and anxiety is the mitochondrial DNA copy number (mtDNA-cn) in blood cells or in saliva. An increase of mtDNA-cn has been found in some studies to be associated with stress and several psychopathologies in adults, especially anxiety and depression (Cai et al., 2015; Tyrka et al., 2016; Wang et al., 2017; Edwards et al., 2016) but this marker has not yet been studied in adolescents, near the time of onset of these disorders.
Depending on the cell and tissue type, each human cell contains between several hundred and over a thousand mitochondria, each carrying 2-10 copies of mtDNA (Robin and Wong, 1988). The human mtDNA is a double-stranded, closed circular molecule encoding 37 genes essential for normal mitochondrial functioning. It has been found that the relative content of mtDNA increases with age (Lee et al., 1998) and that the mtDNA content is positively correlated with the level of oxidative stress (Lee et al., 2000), although non-trivial relationships between blood levels of circulating cell-free mtDNA and an antioxidant enzyme have also been observed (Lindqvist et al., 2018). Mitochondrial biogenesis serves the energy demands of the cell, and one possible explanation of the observed correlations within cells is that the increase in mtDNA content may be a mechanism to prepare cells to respond to endogenous or exogenous oxidative stress through cell-cycle arrest (Lee et al., 2000). An increase in mtDNA-cn may therefore indicate a feedback mechanism and may serve as an index of compensatory mitochondrial biogenesis in the case of increased oxidative stress levels.
Mitochondria are also increasingly recognized as a signaling platform involved in fundamental events in the formation and plasticity of neuronal circuits (Cheng et al., 2010). Specifically, there is evidence that changes in mitochondrial bioenergetics can have major effects on the brain circuitry (Picard & McEwen, 2014). Disturbances in mitochondrial functions and signaling have been suggested to play roles in impaired neuroplasticity and neuronal degeneration in Alzheimer’s disease, Parkinson’s disease, stroke, and psychiatric disorders (Cheng et al., 2010). Whereas studies of the association between mtDNA-cn and psychiatric disorders remain sparse, studies linking mtDNA-cn and brain structure and function are completely lacking. A large body of literature demonstrated abnormal brain circuitry in various psychiatric disorders (reviewed by Cao et al., 2015; Rubinov & Bullmore, 2013; Griffa et al., 2013; Menon, 2011), including fronto-striatal white matter hypoconnectivity in both adult (Korgaonkar et al., 2014) and adolescent depression (LeWinn et al., 2014, Tymofiyeva et al., 2017). It remains unclear whether mtDNA-cn is linked to structural brain connectivity. Understanding alterations of mitochondrial biogenesis and neural correlates of these alterations in psychiatric disorders may help establish more effective therapeutic strategies for these disorders and thus lead to better outcomes for affected individuals.
To fill this significant knowledge gap and help address these unanswered questions, the first aim of this study was to compare salivary mtDNA-cn between clinically depressed and/or anxious adolescents and well-matched healthy controls. Salivary mtDNA was chosen for two reasons: 1) saliva is much easier to collect in adolescents than blood and 2) the largest and most impactful study in the field by Cai et al. used saliva to assess mtDNA content in humans, while their animal work produced comparable results for blood and saliva (Cai et al., 2015). Based on previous studies in adults linking higher mtDNA-cn with Major Depressive Disorder (MDD) (Cai et al., 2015; Tyrka et al., 2016; Wang et al., 2017; Edwards et al., 2016) and anxiety disorders (Tyrka et al., 2016), we hypothesized a higher mtDNA-cn in adolescents with MDD, with or without comorbid anxiety, compared to well-matched healthy controls. The second aim was to identify structural neurocircuitry correlates of mtDNA-cn in adolescents using diffusion tensor imaging (DTI). Based on the previously reported negative effects of disturbances in mitochondrial bioenergetics on the brain circuitry (Picard & McEwen, 2014; Cheng et al., 2010), we hypothesized that high mtDNA-cn would be associated with white matter hypoconnectivity.
Materials and Methods
Participants and Clinical Information
The Institutional Review Boards at the University of California San Diego (UCSD), University of California San Francisco (UCSF), Rady Children’s Hospital in San Diego, and the County of San Diego approved this study. All participants in the study provided written informed assent and their parent(s) or legal guardian(s) provided written informed consent in accordance with the Declaration of Helsinki.
The study protocol, recruitment procedures, clinical and diagnostic assessments, and inclusion/exclusion criteria have been previously described (LeWinn et al. 2014, Tymofiyeva et al., 2017) and are included here in brief. A subset of 84 postpubertal adolescents from Tymofiyeva et al. (2017), who had both usable DTI and mtDNA-cn data were included in this study. This dataset consisted of 49 adolescents with MDD according to the DSM-IV (mean age at time of scan 16.1±1.3yrs. [13.1–17.9], 27 females) and 35 well-matched healthy controls (HC) (mean age at time of scan 16.1±1.4yrs. [13.2–17.9], 22 females). Five of the depressed subjects were using psychotropic medication (three subjects - Selective Serotonin Reuptake Inhibitors (SSRIs), one subject - quetiapine and amitriptyline, and one - dexmethylphenidate) at the time of the scanning and 44 were unmedicated. The MDD and HC groups were well matched on age, gender, Tanner pubertal stage, Hollingshead socioeconomic status, and intelligence. Details of the groups’ characteristics can be found in Table 1.
Table 1.
Demographic and clinical characteristics of the study participants
| Number of participants in final analysis (n) | MDDa 49 |
HCa 35 |
Statisticb,c | p value | Effect Size (95% CI) | Signif. |
|---|---|---|---|---|---|---|
|
|
||||||
| Gender (M / F) | 22 / 27 | 13 / 22 | χ2(1.00) = 0.24 | 0.63 | ||
| Age at time of scan (years) | 16.1 ± 1.3 (13.1-17.9) | 16.1 ± 1.4 (13.2-17.9) | t(70.76) = 0.11 | 0.91 | g=0.02 (−0.41; 0.46) | |
| Hollingshead Socioeconomic Score | 40 ± 39 (11-70)† | 29 ± 23 (0-66)† | W = 1014 | 0.15 | PS=0.38 (0.26; 0.51) | |
| Tanner Score | 4 ± 0.5 (3-5)† | 4 ± 0.5 (3-5)† | W = 976 | 0.26 | PS=0.3 (0.2; 0.43) | |
| Wechsler Abbreviated Scale of Intelligence | 101.4 ± 12.4 (77-129) | 104.8 ± 9.1 (84-125) | t(81.95) = −1.45 | 0.15 | g=−0.32 (−0.76; 0.12) | |
| Children’s Depression Rating Scale (Standardized) | 71.9 ± 8.6 (55-85) | 33.9 ± 5.8 (30-55) | t(81.77) = 24.28 | <0.001 | g=5.32 (4.40; 6.25) | *** |
| Reynolds Adolescent Depression Scale (Standardized) | 65.4 ± 8.5 (35-78) | 41.9 ± 7.7 (30-56) [1] | t(75.33) = 13.10 | <0.001 | g=2.87 (2.25; 3.49) | *** |
| Multidimensional Anxiety Scale for Children (Standardized) | 59.9 ± 9.8 (32-78) [2] | 41.8 ± 9.2 (26-61) [2] | t(71.54) = 8.48 | <0.001 | g=1.86 (1.34; 2.38) | *** |
Mean ± SD (min - max) or median ± interquartile range (min - max) if indicated by†. The optional number in [] indicated the number of missing data points.
Statistic: W, Wilcox rank sum test; χ2, χ2 test for equality of proportions; t, Student’s t test.
Statistics for clinical scales refer only to participants included in the final analysis.
Abbreviations: MDD, Major depressive disorder; HC, healthy control; M, male; F, female. CI, Confidence Interval; SD, standard deviation; g, Hedge’s g;
p<0.001.
Clinical and Self-Report Assessments
Depression severity was assessed for each participant using both a clinician-administered scale, the Children’s Depression Rating Scale-Revised (CDRS-R) (Poznanski & Mokros, 1996), and a self-report scale, the Reynolds Adolescent Depression Scale (RADS-2) (Reynolds, 2002). As part of the original study, the CDRS-R scores were used to further differentiate the study groups: controls with CDRS-R T-scores higher than 54 and MDD participants with T-scores lower than 55 were excluded. Because the CDRS-R was used, in part, to determine group assignment, the RADS-2 scores were used in the present study for group and correlation analyses. The RADS-2 standardized scores and empirically derived clinical cutoff scores provide an indication of the clinical severity of the individual’s depressive symptoms. This 30-item self-report measures the four basic dimensions of depression: Dysphoric Mood, Anhedonia/Negative Affect, Negative Self-Evaluation, and Somatic Complaints, whereas the depression total score represents the overall severity of depressive symptomatology. Anxiety symptoms were assessed with the Multidimensional Anxiety Scale for Children (MASC) (March et al., 1997). The MASC consists of four main scales, three of which have subscales: Physical Symptoms (Tense/Restless, Somatic/Autonomic), Social Anxiety (Humiliation/Rejection, Performance Fears), Harm Avoidance (Perfectionism, Anxious Coping), and Separation/Panic (March, 1998). The MASC also yields a Total Scale score and an Anxiety Disorder Index score to identify youth who may meet criteria for an anxiety disorder.
mtDNA Data Acquisition, Processing and Analysis
The mtDNA data were extracted from saliva collected in an Oragene DNA kit (DNA Genotek, Kanata, Ontario, Canada). The personnel who performed the assay received deidentified samples and were blind to all other measurements. Detection of a 69bp fragment of the ND1 gene in mtDNA (nucleotides 3485-3553) and an 87bp fragment of RNase P (TaqMan® Copy Number Reference Assay, human, RNase P, cat# 4403328, Life Technologies) by a TaqMan multiplex assay was used to determine the relative copy number of mtDNA per diploid nuclear genome. This assay was adapted from previous published methods (He et al., 2002). The primer and probe sequences for ND1 were:
| ND1-forward | 5′-CCCTAAAACCCGCCACATCT-3′ |
| ND1-reverse | 5′-GAGCGATGGTGAGAGCTAAGGT-3′ |
| ND1-FAM probe | 5′ FAM-CCATCACCCTCTACATCACCGCCC-TAMRA-3′ |
The reaction contained 12.5ng of genomic DNA, 100nM of ND1 probe, 300nM of ND1-forward primer and ND1-reverse primer each, 1X RNase P copy number Reference Assay, 1X LightCycler® 480 Probe Master (Roche, cat# 04902343001) in a 10ul reaction. All samples were run in triplicate wells in 384-well plates in a Roche LightCyler 480. PCR conditions were 95°C 10min for 1 cycle; 45 cycles of 95°C 10sec, 60°C 30sec, 72°C 1sec with data acquisition at 72°C. Crossing point (Cp) for each well was derived by the LightCycler 480 program using the second derivation method. Relative copy number per diploid genome was calculated by the following formula: Relative mtDNA copy number=POWER{2, (CpND1-CPRNaseP)}*2. The interassay coefficient of variation (CV) was 3.4%.
MRI Data Acquisition and Network Construction
To address the second goal of the study, structural brain connectivity was assessed using MRI connectomics approach that allows for non-invasively mapping and analyzing brain networks. The acquisition, preprocessing, network construction and statistical analyses (Network-Based Statistic, NBS) steps were similar to our previous study (Tymofiyeva et al., 2017) and are briefly described below.
The data were collected using a 3T MRI system (MR750, GE Healthcare, Milwaukee, Wisconsin, USA) at the UCSD Center for Functional Magnetic Resonance Imaging (CFMRI). High-resolution anatomical T1-weighted images were acquired using a fast spoiled gradient recalled (SPGR) pulse sequence (TR/TE=8.1/3.17ms, flip angle=12°, slice thickness=1mm, FOV=250×250mm, 256×256matrix, 0.98×0.98×1mm voxels). The diffusion-weighted images were acquired using a dual spin echo, single-shot echo-planar imaging (EPI) sequence, 30 directions, b-value=1500s/mm2, TR/TE=7200/86.5ms, FOV=180×180mm, 96×96matrix, 1.875×1.875×2.5mm voxels, two averages.
The T1-weighted images were bias-field-corrected, skull-stripped, and transformed to MNI152 space using an affine transform in FSL (Smith et al., 2004). A quality assurance step was performed on DTI data as previously described (LeWinn et al., 2014). DTI reconstruction and deterministic whole-brain streamline fiber tractography were performed using the Diffusion Toolkit (Wang et al., 2007) with Fiber Assignment by Continuous Tracking (FACT) and a threshold angle of 35°.
Cerebral segmentation into 90 regions of interest (ROIs) was performed in the DTI space using the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) and intermediate registration to T1-weighted images in MNI space. The ROIs were dilated by one voxel and used as network nodes. Connections between AAL ROIs were calculated using the average fractional anisotropy (FA) along the streamlines as weights. The FA-weighted connections were stored as a 90×90 connectivity matrix, in which each row/column corresponded to a distinct node (brain ROI).
Statistical Analyses
The statistical analyses were performed using IBM SPSS Statistics software (version 25). Outlier analysis was performed using Tukey’s method (Tukey, 1977) and extreme cases of mtDNA-cn with values >3 times the interquartile range (IQR) were removed. Two models were tested. In the first model, mtDNA-cn was modeled as a function of the diagnostic group (MDD vs HC). Since the Shapiro-Wilks test of normality was significant for both groups, MDD and HC, we used a non-parametric approach, specifically, the independent-samples Mann-Whitney U test. Accounting for age was not considered necessary after linear regression analysis showed no significant relationship between age and mtDNA-cn in either of the groups, MDD or HC.
Because there was no statistical indication that the distribution of mtDNA differed between the two groups, and because we were interested in assessing depressive and anxiety symptoms in a continuous, not only dichotomous, manner, we pooled the two groups together and performed the remaining analyses in the full sample. Bivariate correlations were performed between mtDNA-cn and depressive and anxiety symptoms in the full sample.
In the second model tested in this study, structural brain connectivity was modeled as a function of mtDNA-cn (high-mtDNA-cn vs low mtDNA-cn groups, using median split in the full sample). To assess edge-wise differences in the connectivity matrices between the low- and high-mtDNA-cn groups, we utilized the NBS approach implemented in Matlab (Zalesky et al., 2010). Alpha threshold was set at the default value of 0.05. NBS also requires a choice of the primary threshold by the user, which can lead to differences in the resulting topologies. However, the control of family-wise error rate (FWER) is guaranteed irrespective of the threshold choice (Zalesky et al., 2010). We performed a t-test with 5 000 permutations and the strictest primary threshold chosen experimentally.
Results
The MDD and HC adolescent groups showed the expected significant differences in levels of depression and anxiety with the MDD group having greater depression and anxiety on all scales (CDRS-R, RADS-2, MASC) (all p<0.001; see Table 1). The MDD and HC groups did not significantly differ on age, gender, pubertal stage, IQ, and socioeconomic status.
mtDNA-cn Analysis
Based on the outlier analysis using Tukey’s method, one adolescent with MDD was removed from the analysis. The remaining 83 adolescents’ datasets were included in the analysis. There was no significant difference in mtDNA-cn between MDD and HC groups (independent-samples Mann-Whitney U test resulted in p=0.412, Fig. 1).
Figure 1.
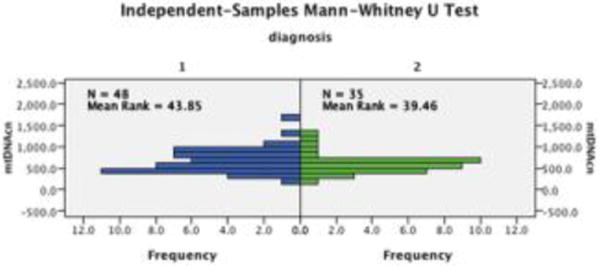
Results of the independent-samples Mann-Whitney U test: there was no significant difference in mtDNA-cn between MDD (1) and HC (2) groups (p=0.412).
In the pooled sample of the adolescents, both parametric and non-parametric correlations between mtDNA-cn and depressive symptoms, RADS-2 T-scores, were not significant: Pearson correlation r=0.080, p=0.474, N=82; Spearman’s rho=0.046, p=0.682, N=82 (one HC subject did not have RADS-2 scores available). However, the correlation between mtDNA-cn and anxiety symptoms measured using MASC T-scores was significant: Pearson correlation r=0.254, p=0.024, N=79; Spearman’s rho=0.226, p=0.046, N=79 (two HC and two MDD subjects did not have MASC scores available). At the same time, MASC was highly correlated with RADS-2: Pearson correlation r=0.770, p<0.001, N=79. We report Pearson correlation coefficient in addition to Spearman correlation coefficients, since Pearson correlation coefficient is most commonly used and demonstrates robustness to departures from normality in larger datasets (Bishara & Hittner, 2012).
Since we found a statistically significant correlation result for mtDNA levels and anxiety, we further explored different subscales of the MASC scale (Table 2). The analyses revealed that the correlation was largely driven by Somatic/Autonomic and Humiliation/Rejection subscales, both showing Spearman’s rho>0.2 (Table 2). In this case, we only report the Spearman’s rho, because we are not testing specific hypotheses but rather look for the largest contributing factor. Correction for multiple comparisons is not needed, since no hypothesis testing is performed in this case.
Table 2.
Non-parametric correlations (Spearman’s rho) of MASC subscales T-scores with mtDNA-cn in 78 adolescents.
| Correlation Coefficient | p (2-tailed) | |
|---|---|---|
| MASC Total | .226 | .046 |
| Physical Symptoms | .209 | .064 |
| Tense/Restless | .188 | .096 |
| Somatic/Autonomic | .238 | .034 |
| Harm Avoidance | .046 | .690 |
| Perfectionism | -.054 | .636 |
| Anxious Coping | .077 | .503 |
| Social Anxiety | .227 | .045 |
| Humiliation/Rejection | .239 | .034 |
| Performance Fears | .122 | .283 |
| Separation/Panic | .127 | .264 |
| Anxiety Disorder Index | .242 | .032 |
Removing medicated subjects, however, resulted in a non-significant correlation between mtDNA-cn and anxiety (Pearson r=0.181, p=0.122, N=74; Spearman’s rho=0.145, p=0.217, N=74) (Fig. S1). The medicated and non-medicated subjects differed significantly both with respect to anxiety levels (t=−2.069, p=0.042, independent samples t-test assuming equal variances) and mtDNA-cn (t=−2.067, p=0.042, independent samples t-test assuming equal variances). An additional mediation analysis indicated that the effect of medication on mtDNA-cn may be mediated by anxiety (the direct effect of medication status on mtDNA-cn became non-significant, beta std.=0.173, p=0.127, when adding MASC to the linear regression, whereas the MASC effect remained close to significant, beta std.=0.215, p=0.06). This suggests that the loss of significance when removing the medicated subjects from the analysis is likely due to the decreased N.
Since there was no statistically significant group difference in mtDNA-cn, we pooled MDD and HC adolescents together and split them into high-mtDNA and low mtDNA groups (using median split), in order to examine, whether their brains differed with respect to structural connectivity.
The results of the NBS analysis at the threshold of 3.4, p<0.05, and 1 000 permutations are presented in Figure 2. The results were confirmed with the false discovery rate (FDR) analysis. In particular, significantly lower FA was observed in adolescents with high (above-median) mtDNA-cn in the following connections: between the orbital part of the left superior frontal gyrus (L. Frontal_sup_orb) and the left Heschl’s gyrus (t=3.49), between the orbital part of the left middle frontal gyrus (L. Frontal_mid_orb) and the left Heschl’s gyrus (t=3.43), between the left olfactory cortex (L. Olfactory) and the left Heschl’s gyrus (t=3.41), between the right olfactory cortex (R. Olfactory) and the left Heschl’s gyrus (t=3.41), and between the left cuneus cortex (L. Cuneus) and the left Heschl’s gyrus (t=3.51).
Figure 2.
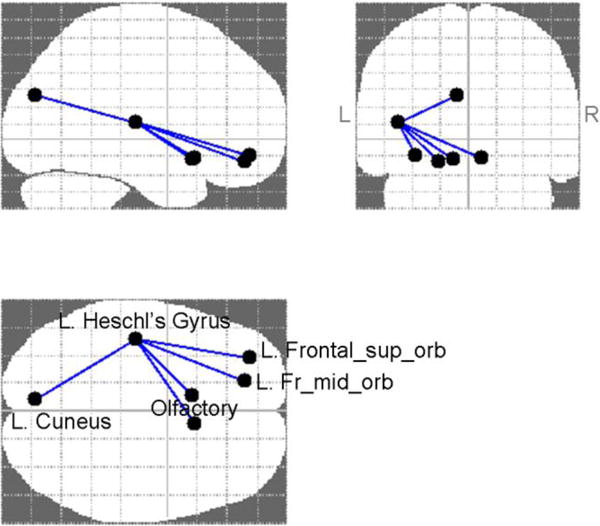
NBS results at the primary threshold of 3.4, p<0.05, 1 000 permutations, depicting hypoconnected edges in the high-mtDNA group; confirmed with false discovery rate (FDR) analysis.
Figure 3 shows an example participant’s tractography streamlines going through the L. Heschl’s gyrus, which demonstrate correspondence to the white matter track known as the fronto-occipital fasciculus (FOF).
Figure 3.
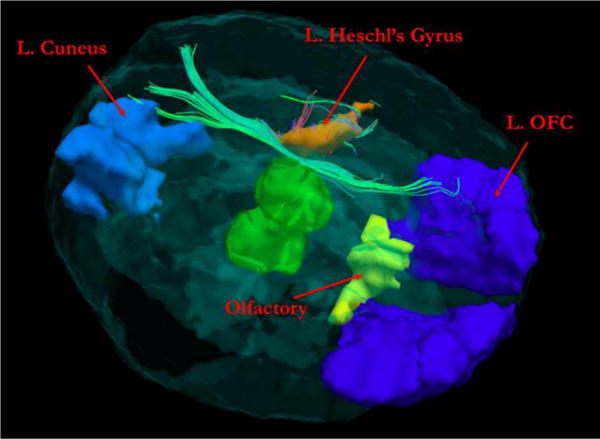
Example of study participant’s tractography streamlines going through the L. Heschl’s Gyrus, largely corresponding to the fronto-occipital fasciculus. The thalamus is displayed in the center of the brain for a reference. L. OFC: left orbitofrontal cortex.
After removing the medicated subjects, the NBS analysis produce results similar to those listed above for the full dataset: hypoconnected links between L. Frontal_sup_orb and the left Heschl’s gyrus (t=3.51), between the L. Frontal_mid_orb and the left Heschl’s gyrus (t=3.42), between the L. Olfactory and the left Heschl’s gyrus (t=3.42), and between the L. Cuneus and the left Heschl’s gyrus (t=3.50).
Discussion
Our first hypothesis regarding high levels of mtDNA copy number being associated with adolescent depression was based on certain previous studies in adult MDD (Cai et al., 2015; Tyrka et al., 2016; Wang et al., 2017; Edwards et al., 2016) and it was not confirmed. Interestingly, in a recent longitudinal study by Verhoeven et al., the authors found no evidence for an association between depressive symptoms and mtDNA-cn in a community-based sample of depressed adults (either between-person or within-person) (Verhoeven et al., 2017). Moreover, a decrease of mtDNA-cn was observed in adult MDD in another study (Chang et al., 2015). It is important to consider the developmental aspect of this molecular marker. Depressive illness is often a chronic and recurrent disorder, and mtDNA copy number has been shown to positively correlate with the duration of the longest episode (Edwards et al., 2016). One can therefore speculate that the inconsistent findings of increased mtDNA-cn in adult, but not adolescent depression, may be explained by the effect of accumulated oxidative stress during the comparatively longer duration of depressive illness in adults. This might also be a reason why He et al. did not find any association between leukocyte mtDNA-cn in blood and MDD in young adults (He et al., 2014). The severity of symptoms might also play a role and recent studies found significantly higher mtDNA-cn in suicide attempters (Lindqvist et al., 2016) and completers compared to controls (Otsuka et al., 2017).
Notably, we observed a significant positive correlation between mtDNA-cn and levels of anxiety, but not depression, in a mixed group of adolescents, which was—in exploratory analyses—particularly driven by Somatic/Autonomic and Humiliation/Rejection subscales. This finding can also potentially explain the discrepancy with the published literature on mtDNA in adult depression, as we describe here. A recent study by Steenkamp et al. showed a positive correlation between peripheral oxidative stress and anxiety, but not depression symptoms, in physically healthy, medication-free individuals with MDD (Steenkamp et al., 2017). Specifically, they found that higher plasma levels of F2-isoprostanes and glutathione disulfide (GSSG) were associated with higher anxiety scores. The continuous relationships between severity of anxiety symptoms and oxidative stress marker levels showed small to medium effect sizes. If anxiety but not depression is associated with oxidative stress, which in turn may lead to increased mtDNA-cn, this could explain our findings in adolescents. At the same time, symptoms of generalized and social anxiety are known to be highly comorbid with depression symptomatology (Hettema, 2008) and, therefore, previous mtDNA-cn findings may have been mis-attributed to depression rather than anxiety. This highlights the importance of mapping biomarkers onto dimensional symptoms in addition to categorical DSM diagnoses (Steenkamp et al., 2017).
It should be noted that the correlation between mtDNA-cn and anxiety in the adolescent subjects in our study was non-significant when the five medicated subjects were removed from the sample. The medicated and non-medicated subjects differed significantly both with respect to anxiety levels and mtDNA-cn. Our additional analysis indicated that the effect of medication on mtDNA-cn may be mediated by anxiety, suggesting that the loss of significance is likely due to the decreased sample size. From a psychopharmacological perspective, it needs to be mentioned that the major categories of drugs to treat affective disorders are known to impact mitochondrial function, and new-generation anti-depressive medications have preferential action on mitochondrial metabolism (Adzic et al., 2016). Future studies are needed to exclude the possibility that the effect of increased mtDNA-cn in anxious and depressed subjects is driven by antidepressant medication.
Our second aim was to identify network-level neural correlates of mtDNA-cn across all subjects. We used white matter microstructural properties, particularly fractional anisotropy (FA), as a proxy for structural connectivity between brain regions. FA has been widely used in studies of neuroplasticity, reflecting alterations in white matter fiber organization (including axon branching, sprouting, packing density, axon diameter, fiber crossing and the number of axons), as well as changes in myelin thickness and morphology (Zatorre et al., 2012). Since FA is impacted by multiple factors listed above (and some of them, such as crossing fibers, can misleadingly decrease FA without connectivity decrease), this metric cannot be used as a quantitative measure of brain connectivity (Jones et al., 2013). Nevertheless, impressive and useful qualitative results are continually obtained using FA, for example, in a recent neuroplasticity study with learning (Schlaffke et al., 2017).
In our study, we observed a significant difference in FA-weighted brain networks between adolescents with high and low mtDNA-cn. Specifically, those with mtDNA-cn above the median displayed weaker fronto-occipital connections in the left hemisphere compared to those with mtDNA-cn below the median. The finding persisted after excluding medicated subjects. It augments the finding in a sample partially overlapping with the sample of this study, showing that adolescent MDD was associated with the right fronto-striatal white matter hypoconnectivity (Tymofiyeva et al., 2017).
From previous studies of mitochondrial genetics it is known that dysfunctional mtDNA can influence plasticity of neuronal circuits (Picard & McEwen, 2014). In primates, even subtle changes in mitochondrial bioenergetics have shown major effects on the brain, which at least in part seems to be caused by their influence on synaptic transmission. It is not yet known whether these findings may be extended to humans (Picard & McEwen, 2014). In our study, fronto-occipital connections in the left hemisphere, with L. Heschl’s gyrus as an intermediate node, showed difference between the high- and low-mtDNA-cn groups. The fronto-occipital fasciculus (FOF), also known as the inferior fronto-occipital fasciculus (IFO) is a large white matter tract connecting the frontal, temporal and occipital lobes. It constitutes one of the major efferent and afferent neuronal projections to the frontal lobes and connects the prefrontal cortex with auditory and visual association cortex in the temporal lobe (Kier et al., 2004). Heschl’s gyrus contains the primary auditory cortex and is involved in hearing, which is commonly impaired in patients with mtDNA defects (Chinnery et al., 2000). It is possible that, since fronto-temporo-occipital connections develop more slowly than other regions (Lebel et al., 2008), they are particularly vulnerable to early life stress and therefore they are the ones showing the difference between the high- and low-mtDNA-cn groups in our study. The vulnerability of IFO to different forms of stress is supported by previous studies. For example, in a study by Huang et al., adolescents exposed to childhood maltreatment had significantly lower FA values in the left IFO (Huang et al., 2012). Bergamino et al. recently applied a free-water correction algorithm to female MDD patients’ DTI data, which revealed low-FA clusters in the left IFO of the patients compared to controls, with significant correlations of DTI measures with reported stress levels (Bergamino et al., 2015). Finally, patients with obsessive compulsive disorder (OCD), which is associated with considerable anxiety, displayed structural hypoconnectivity of the inferior fronto-occipital fasciculus bilaterally, that correlated with symptom severity and neuropsychological performance (Garibotto et al., 2010).
Based on the results of this study and previous literature, we would like to suggest a tentative model (Fig. 4) that describes relationships between different units of analysis as suggested by the Research Domain Criteria (RDoC) (Insel et al., 2010). The units of analysis considered here include clinical symptoms (self-reports), molecular changes, and neurocircuitry alterations. According to this model, the environmental impact (such as childhood trauma) can play a causal role in depressive and anxiety symptoms (Hovens et al., 2010; Li et al., 2016), which themselves represent a form of chronic stress. Chronic stress promotes oxidative stress throughout the body (Aschbacher et al., 2013). Specifically, anxiety but not depression is reportedly associated with increased levels of oxidative stress (Steenkamp et al., 2017), which in turn leads to increased mtDNA-cn as a compensatory feedback mechanism (Lee et al., 2000). In this model, the disturbance of the mtDNA-cn negatively affects brain plasticity (Picard & McEwen, 2014) and leads to white matter hypoconnectivity in distinct brain regions, which may represent neurocircuitry that is vulnerable to stress during brain development (namely, the fronto-temporo-occipital circuit that develops more slowly than other regions [Lebel et al., 2008]). Other interpretations of the results are possible, which presuppose different chains of events. For example, inherited interpersonal polymorphic differences in mtDNA (haplogroups) and other common mtDNA mutations may be modifying physiological responses to psychosocial stressors and in this way mediating the association between stress exposure and later mental illness (Picard et al., 2015). In this view, stress-reactive axes are under mitochondrial regulation, whereby mitochondrial dysfunction alters the perceived physiological severity of various stressors (Picard et al., 2015). To represent this alternative model, Figure 4 would need to be modified, in which the “mtDNA-cn” block would be placed between the “Environmental factors” and “Anxiety and depression symptoms” blocks. Being preserved across tissues, mitochondrial bioenergetics could be playing the role of systemic neuroendocrine modulator, explaining why some individuals show hypothalamic-pituitary-adrenal (HPA) hyperresponsiveness to psychological stress (Picard et al., 2015). This “filtered” stress would then lead to neural remodeling and altered fronto-temporo-occipital connectivity.
Figure 4.
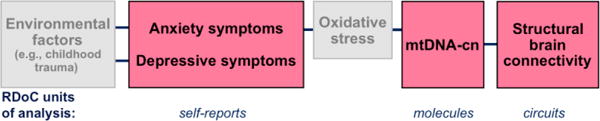
The hypothesized model of adolescent depression and anxiety with multiple units of analysis. The hypothesis of oxidative stress mediating the link between anxiety symptoms and mtDNA-cn has not been tested in this study (the light gray box).
Several methodological limitations should be taken into account when interpreting our findings. First, in this study saliva was collected in the stabilizing solution in the Oragene kit, which lysed the cells, so the relative copy number of mtDNA per diploid nuclear genome was a mixture of the intracellular mtDNA and free mtDNA in saliva. Future research should include separate analyses of free and intracellular mtDNA, as important differences have been observed between the two quantities (Lindqvist et al., 2018). Second, a DTI-based tractography method was employed in this work to reconstruct structural brain networks. Although DTI is most widely used, it has a limited capacity for resolving the fiber crossing issue and may result in misleading information about fiber tracts orientation (Farquharson et al., 2013). High-order reconstruction methods may be better at resolving complex fiber crossings (Tuch et al., 2002), however, even these more sophisticated tractography methods do not consistently show superior sensitivity and specificity (Thomas et al., 2014).
In summary, we did not observe differences in the mtDNA copy number between adolescents with clinical depression and well-matched healthy controls, although such differences were previously detected in some studies in adults. However, we observed a significant positive correlation between mtDNA-cn and levels of anxiety in a mixed group of adolescents. Notably, our study also suggests that there is a difference in white matter brain networks between adolescents with high versus low mtDNA-cn, specifically in the fronto-occipital connections of the left hemisphere. Since this study is the first to report these findings, our results will need to be confirmed in future studies. The results of our study highlight the importance of mapping biomarkers onto dimensional symptoms in addition to categorical DSM diagnoses and investigating the relationships between different units of analysis: molecular changes, neurocircuitry alterations, and clinical symptoms. We hope that our findings will help guide the design of future studies and contribute to improved prevention and treatment of adolescent anxiety and depression.
Supplementary Material
Highlights.
Mitochondrial DNA copy number (mtDNA-cn) did not differ in depressed adolescents.
mtDNA-cn positively correlated with anxiety in a mixed cohort of adolescents.
A hypoconnected subnetwork was found in adolescents with high mtDNA-cn.
The subnetwork largely corresponded to the left fronto-occipital fasciculus.
Acknowledgments
We would like to thank all of the study participants and their parents who made this work possible.
Funding sources
This study was supported by NCCIH R21AT009173 to OT, TTY and EHB; by NICHD R01HD072074 to DX and OT; by UCSF Research Evaluation and Allocation Committee (REAC) and J. Jacobson Fund to OT, EHB, TTY and DX; by the American Foundation for Suicide Prevention PDF-1-064-13 to TCH; by the Swedish Research Council 350-2012-303 to EHB; by NIMH R01MH085734 to TTY; by the Brain and Behavior Research Foundation (formerly NARSAD) to TTY; by the Swedish Research Council (registration number 2015-00387), Marie Sklodowska Curie Actions, Cofund (Project INCA 600398), the Swedish Society of Medicine, the Söderström-Königska Foundation, the Sjöbring Foundation, OM Persson Foundation and the province of Scania (Sweden) state grants (ALF) to DL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health of other funding agencies. The funding agencies did not play any role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Roles
Conceived and designed the study: OT EHB TCH CGC DL OMW KZL TTY. Performed data acquisition: TTY. Analyzed the data: OT TCH CGC JL KZL JPY SPB. Contributed materials/analysis tools: KZL MDS DX. Wrote the paper: OT EHB TCH CGC DL OMW JL KZL MDS LKMH JPY SPB DX TTY. All authors have approved the final article.
Contributors
Olga Tymofiyeva (OT)
Eva Henje Blom (EHB)
Tiffany C. Ho (TCH)
Colm G. Connolly (CGC)
Daniel Lindqvist (DL)
Owen M. Wolkowitz (OMW)
Jue Lin (JL)
Kaja Z. LeWinn (KZL)
Matthew D. Sacchet (MDS)
Laura KM Han (LKMH)
Justin P. Yuan (JPY)
Sarina P. Bhandari (SPB)
Duan Xu (DX)
Tony T. Yang (TTY)
Conflict of interest:
JL is a consultant to Telomere Diagnostics, formerly Telome Health, and owns stock in the company. The company had no role in this research or in writing this article. The remaining authors declare no conflict of interest.
References
- Adzic M, Brkic Z, Bulajic S, Mitic M, Radojcic MB. Antidepressant Action on Mitochondrial Dysfunction in Psychiatric Disorders. Drug Dev Res. 2016;77:400–406. doi: 10.1002/ddr.21332. [DOI] [PubMed] [Google Scholar]
- Aschbacher K, O’Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, Epel E. Good Stress, Bad Stress and Oxidative Stress: Insights from Anticipatory Cortisol Reactivity. Psychoneuroendocrinology. 2013;38:1698–1708. doi: 10.1016/j.psyneuen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamino M, Pasternak O, Farmer M, Shenton ME, Paul Hamilton J. Applying a free-water correction to diffusion imaging data uncovers stress-related neural pathology in depression. Neuroimage Clin. 2015;10:336–342. doi: 10.1016/j.nicl.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersani FS, Morley C, Lindqvist D, Epel ES, Picard M, Yehuda R, Flory J, Bierer LM, Makotkine I, Abu-Amara D, et al. Mitochondrial DNA copy number is reduced in male combat veterans with PTSD. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:10–17. doi: 10.1016/j.pnpbp.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Bishara AJ, Hittner JB. Testing the significance of a correlation with non-normal data: Comparison of Pearson, Spearman, transformation, and resampling approaches. Psychological Methods. 2012;17:399–417. doi: 10.1037/a0028087. [DOI] [PubMed] [Google Scholar]
- Cai N, Chang S, Li Y, Li Q, Hu J, Liang J, Song L, Kretzschmar W, Gan X, Nicod J, et al. Molecular Signatures of Major Depression. Current Biology. 2015;25:1146–1156. doi: 10.1016/j.cub.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Wang Z, He Y. Connectomics in psychiatric research: advances and applications. Neuropsychiatr Dis Treat. 2015;11:2801–2810. doi: 10.2147/NDT.S63470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Jou SH, Lin TT, Lai TJ, Liu CS. Mitochondria DNA Change and Oxidative Damage in Clinically Stable Patients with Major Depressive Disorder. PLOS ONE. 2015;10:e0125855. doi: 10.1371/journal.pone.0125855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Hou Y, Mattson MP. Mitochondria and neuroplasticity. ASN Neuro. 2010;2(5):e00045. doi: 10.1042/AN20100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery PF, Elliott C, Green GR, Rees A, Coulthard A, Turnbull DM, Griffiths TD. The spectrum of hearing loss due to mitochondrial DNA defects. Brain. 2000;123(Pt 1):82–92. doi: 10.1093/brain/123.1.82. [DOI] [PubMed] [Google Scholar]
- Di Monte DA. Mitochondrial DNA and Parkinson’s disease. Neurology. 1991;41:38–42. doi: 10.1212/wnl.41.5_suppl_2.38. [DOI] [PubMed] [Google Scholar]
- Edwards AC, Aggen SH, Cai N, Bigdeli TB, Peterson RE, Docherty AR, Webb BT, Bacanu SA, Flint J, Kendler KS. Chronicity of Depression and Molecular Markers in a Large Sample of Han Chinese Women. Depress Anxiety. 2016;33:1048–1054. doi: 10.1002/da.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquharson S, Tournier JD, Calamante F, Fabinyi G, Schneider-Kolsky M, Jackson GD, et al. White matter fiber tractography: why we need to move beyond DTI. J Neurosurg. 2013;118:1367–1377. doi: 10.3171/2013.2.JNS121294. [DOI] [PubMed] [Google Scholar]
- Garibotto V, Scifo P, Gorini A, Alonso CR, Brambati S, Bellodi L, Perani D. Disorganization of anatomical connectivity in obsessive compulsive disorder: A multi-parameter diffusion tensor imaging study in a subpopulation of patients. Neurobiology of Disease. 2010;37:468–476. doi: 10.1016/j.nbd.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Griffa A, Baumann PS, Thiran JP, Hagmann P. Structural connectomics in brain diseases. Neuroimage. 2013;80:515–526. doi: 10.1016/j.neuroimage.2013.04.056. [DOI] [PubMed] [Google Scholar]
- He L, Chinnery PF, Durham SE, Blakely EL, Wardell TM, Borthwick GM, Taylor RW, Turnbull DM. Detection and quantification of mitochondrial DNA deletions in individual cells by real-time PCR. Nucleic Acids Res. 2002;30:e68. doi: 10.1093/nar/gnf067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Tang J, Li Z, Li H, Liao Y, Tang Y, Tan L, Chen J, Xia K, Chen X. Leukocyte Mitochondrial DNA Copy Number in Blood Is Not Associated with Major Depressive Disorder in Young Adults. PLOS ONE. 2014;9:e96869. doi: 10.1371/journal.pone.0096869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM. What is the genetic relationship between anxiety and depression? Am J Med Genet C Semin Med Genet. 2008;148C:140–146. doi: 10.1002/ajmg.c.30171. [DOI] [PubMed] [Google Scholar]
- Hovens JGFM, Wiersma JE, Giltay EJ, Van Oppen P, Spinhoven P, Penninx BWJH, Zitman FG. Childhood life events and childhood trauma in adult patients with depressive, anxiety and comorbid disorders vs. controls. Acta Psychiatrica Scandinavica. 2010;122:66–74. doi: 10.1111/j.1600-0447.2009.01491.x. [DOI] [PubMed] [Google Scholar]
- Huang H, Gundapuneedi T, Rao U. White Matter Disruptions in Adolescents Exposed to Childhood Maltreatment and Vulnerability to Psychopathology. Neuropsychopharmacology. 2012;37:2693–2701. doi: 10.1038/npp.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. Am J Neuroradiol. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar MS, Fornito A, Williams LM, Grieve SM. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol Psychiatry. 2014;76:567–574. doi: 10.1016/j.biopsych.2014.02.018. [DOI] [PubMed] [Google Scholar]
- Kvickström P, Eriksson B, van Westen D, Lätt J, Elfgren C, Nilsson C. Selective frontal neurodegeneration of the inferior fronto-occipital fasciculus in progressive supranuclear palsy (PSP) demonstrated by diffusion tensor tractography. BMC Neurology. 2011;11:13. doi: 10.1186/1471-2377-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lee HC, Lu CY, Fahn HJ, Wei YH. Aging- and smoking-associated alteration in the relative content of mitochondrial DNA in human lung. FEBS Letters. 1998;441:292–296. doi: 10.1016/s0014-5793(98)01564-6. [DOI] [PubMed] [Google Scholar]
- Lee HC, Yin PH, Lu CY, Chi CW, Wei YH. Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem J. 2000;348:425–432. [PMC free article] [PubMed] [Google Scholar]
- LeWinn KZ, Connolly CG, Wu J, Drahos M, Hoeft F, Ho TC, Simmons AN, Yang TT. White matter correlates of adolescent depression: structural evidence for frontolimbic disconnectivity. J Am Acad Child Adolesc Psychiatry. 2014;53:899–909. doi: 10.1016/j.jaac.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, D’Arcy C, Meng X. Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: systematic review, meta-analysis, and proportional attributable fractions. Psychol Med. 2016;46:717–730. doi: 10.1017/S0033291715002743. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Fernström J, Grudet C, Ljunggren L, Träskman-Bendz L, Ohlsson L, Westrin Å. Increased plasma levels of circulating cell-free mitochondrial DNA in suicide attempters: associations with HPA-axis hyperactivity. Transl Psychiatry. 2016;6:e971. doi: 10.1038/tp.2016.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Picard M, Ohlsson L, Bersani FS, Fernström J, Westrin Å, Hough CM, Lin J, Reus VI, Epel ES, Mellon SH. Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in Major Depressive Disorder. Neuropsychopharmacology. doi: 10.1038/s41386-017-0001-9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS. Manual for the Multidimensional Anxiety Scale for Children (MASC) Toronto: Multi-Health Systems, Inc; 1998. [Google Scholar]
- March JS, Parker JDA, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Otsuka I, Izumi T, Boku S, Kimura A, Zhang Y, Mouri K, Okazaki S, Shiroiwa K, Takahashi M, Ueno Y, et al. Aberrant telomere length and mitochondrial DNA copy number in suicide completers. Scientific Reports. 2017;7:3176. doi: 10.1038/s41598-017-03599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, McEwen BS. Mitochondria impact brain function and cognition. Proc Natl Acad Sci USA. 2014;111:7–8. doi: 10.1073/pnas.1321881111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, McManus MJ, Gray JD, Nasca C, Moffat C, Kopinski PK, Seifert EL, McEwen BS, Wallace DC. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc Natl Acad Sci USA. 2015;112:E6614–E6623. doi: 10.1073/pnas.1515733112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual Research Review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry. 2015;56:345–365. doi: 10.1111/jcpp.12381. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB. Children’s Depression Rating Scale—Revised (CDRS-R) Western Psychological Services; Los Angeles: 1996. [Google Scholar]
- Reynolds WM. RADS-2, Reynolds Adolescent Depression Scale: Professional Manual. Psychological Assessment Resources; Lutz, FL: 2002. [Google Scholar]
- Robin ED, Wong R. Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. Journal of cellular physiology. 1988;136(3):507–513. doi: 10.1002/jcp.1041360316. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Bullmore E. Fledgling pathoconnectomics of psychiatric disorders. Trends in Cognitive Sciences. 2013;17:641–647. doi: 10.1016/j.tics.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Schlaffke L, Leemans A, Schweizer LM, Ocklenburg S, Schmidt-Wilcke T. Learning Morse Code Alters Microstructural Properties in the Inferior Longitudinal Fasciculus: A DTI Study. Front Hum Neurosci. 2017;11:383. doi: 10.3389/fnhum.2017.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Steenkamp LR, Hough CM, Reus VI, Jain FA, Epel ES, James SJ, Morford AE, Mellon SH, Wolkowitz OM, Lindqvist D. Severity of anxiety - but not depression - is associated with oxidative stress in Major Depressive Disorder. J Affect Disord. 2017;219:193–200. doi: 10.1016/j.jad.2017.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Ye FQ, Irfanoglu MO, Modi P, Saleem KS, Leopold DA, Pierpaoli C. Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci USA. 2014;111:16574–16579. doi: 10.1073/pnas.1405672111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48:577–582. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory Data Analysis. Addison-Wesley; 1977. [Google Scholar]
- Tymofiyeva O, Connolly CG, Ho TC, Sacchet MD, Henje Blom E, LeWinn KZ, Xu D, Yang TT. DTI-based connectome analysis of adolescents with major depressive disorder reveals hypoconnectivity of the right caudate. J Affect Disord. 2017;207:18–25. doi: 10.1016/j.jad.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Price LH, Kao HT, Porton B, Philip NS, Welch ES, Carpenter LL. Alterations of Mitochondrial DNA Copy Number and Telomere Length With Early Adversity and Psychopathology. Biol Psychiatry. 2016;79:78–86. doi: 10.1016/j.biopsych.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, Révész D, Picard M, Epel EE, Wolkowitz OM, Matthews KA, Penninx BWJH, Puterman E. Depression, telomeres and mitochondrial DNA: between- and within-person associations from a 10-year longitudinal study. Mol Psychiatry. 2017;00:1–8. doi: 10.1038/mp.2017.48. [DOI] [PubMed] [Google Scholar]
- Wang R, Benner T, Sorensen AG, Wedeen VJ. Diffusion Toolkit: a software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med. 2007;3720 [Google Scholar]
- Wang X, Sundquist K, Rastkhani H, Palmér K, Memon AA, Sundquist J. Association of mitochondrial DNA in peripheral blood with depression, anxiety and stress- and adjustment disorders in primary health care patients. Eur Neuropsychopharmacol. 2017 doi: 10.1016/j.euroneuro.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


