Abstract
Sleep apnea is a disorder, which increasingly affects people worldwide. Whether the associated hypoxic events during sleep are central or obstructive in origin, the end result is excessive daytime sleepiness and an increased risk for several comorbidities, such as cardiovascular and neurodegenerative disorders. Sleep apnea is diagnosed more frequently in men than women, suggesting a role of sex hormones in the pathology of the disease. Furthermore, there are sex differences in the development and progression of comorbid diseases associated with sleep apnea. Therefore, treatment of sleep apnea may be clinically relevant for prevention of subsequent sex-specific comorbid disorders. While the impact sleep apnea has on cardiovascular events has been the subject of many research studies, the role of sleep apnea in neurodegeneration is less established. Here we review known risk factors for sleep apnea and the implications of the observed sex differences in this disease. We also summarize the evidence and mechanisms for how sleep apnea may contribute to the onset of neurodegenerative disorders, such as Alzheimer’s disease and Parkinson’s disease.
Keywords: Androgens, estrogens, oxidative stress, inflammation, chronic intermittent hypoxia, hypertension
Sleep apnea is estimated to affect 26% of the population in the United States [1], although up to 80% of those with the disorder are undiagnosed [2]. The major outcome of this condition is repetitive reduction in inspired oxygen during sleep [3]. This may be due to either a loss of central control of breathing effort or a physical obstruction of the upper airway [3], leading to apneas and/or hypopneas. One measure of the severity of sleep apnea is the apnea/hypopnea index (AHI), which quantifies the number of times per hour an apnea or hypopnea occurs. Cutoff points for the diagnosis of mild, moderate, and severe sleep apnea are AHI ≥ 5, ≥ 15, and ≥ 30 respectively [4]. Treatment is considered necessary for cases of moderate and severe sleep apnea, but optional for mild sleep apnea [5]. The most common therapeutic options include use of an oral apparatus or continuous positive airway pressure (CPAP) to maintain an open airway during sleep [5, 6].
Sleep apnea comorbid disorders include neurodegenerative disorders, such as Alzheimer’s disease (AD) or Parkinson’s disease (PD). These types of diseases are progressive disorders, which lead to a loss of neurons in specific areas of the central nervous system. Significant loss of neurons results in a functional deficit associated with the affected region. The initial degradation is a slow insidious process, wherein the patient is often unaware of the disorder. Clinical symptoms of AD include loss of working and episodic memory, followed by impaired executive functions and eventual autonomic system deficits [7]. These do not become apparent until approximately 40% damage to neurons in hippocampal structures occurs [8]. PD symptoms of bradykinesia, tremor, rigidity, and postural instability [9] do not manifest until 70–80% of neurons in the substantia nigra are lost [10]. Because diagnosis generally occurs during advanced stages of the disease, therapeutic treatments are generally not efficacious [11–15]. Identification of modifiable risk factors which exacerbate neurodegeneration may prove to be crucial to improving therapeutic options. One of these factors may be sleep apnea.
The association between sleep apnea and neurodegeneration has only recently begun to be explored, but current evidence suggests people with sleep apnea are at higher risk for neurodegenerative diseases [3]. For example, Peng, et al., recorded deficient regional brain activity in men with severe obstructive sleep apnea, connecting this sleep disorder with pathologic neural consequences [16]. Sleep apnea has been associated with deficits in working memory [17] and overnight consolidation of motor skill acquisition [18]. Evidence exists to support the hypothesis sleep apnea can increase the risk of developing AD or PD [17, 19–22]. In a multi-ethnic study, people who carried a genetic predisposition for AD exhibited a significant decline in cognition as hypoxic events increased in severity [23]. Additionally, sleep apnea is linked with increased risk for sporadic PD [22]. Therefore, treatment of sleep apnea may improve cognitive deficits in people with AD and PD [24–27].
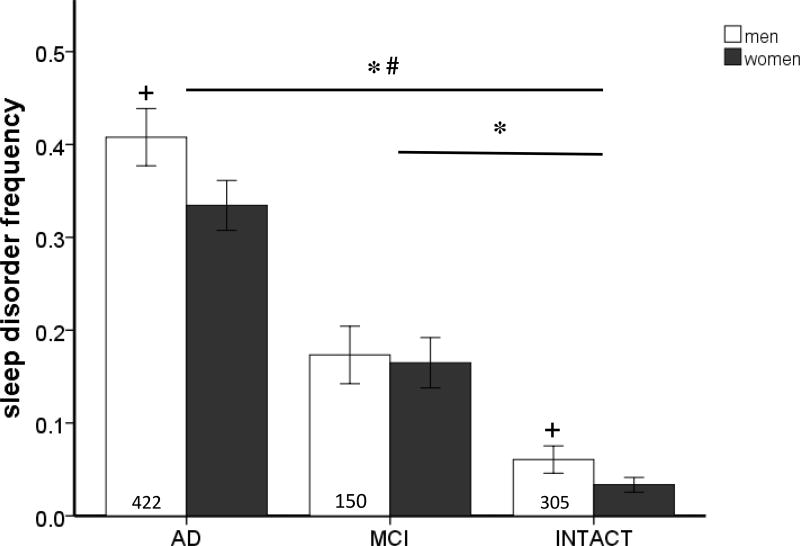
An increase in sleep disorders has been reported in association with both AD and PD [21–23, 28–36]. In our studies using the Texas Alzheimer’s Research Care and Consortium cohort (TARCC, a collaborative Alzheimer’s research effort funded by the State of Texas), we found participants with cognitive deficits are more likely to report a significant elevation in sleep disturbances than those who are cognitively intact or have mild cognitive impairment (figure 1, table 1). As dementia severity increases, so do sleep disturbances, which is evidenced by the higher number of sleep disturbances in AD patients versus mild cognitive impairment patients. Similar to our observations, other studies have documented an increased incidence of sleep apnea as the severity of PD progresses [21, 37, 38]. Although a few studies have not found the same association between sleep apnea and neurodegeneration [30, 34, 36], small sample sizes could be a contributing factor to this discrepancy. In general, literature indicates that sleep apnea increases the risk of neurodegeneration. However, it is unknown whether sleep apnea is a causative agent of AD or PD.
Figure 1.
The frequency of sleep disorders was obtained by TARCC participant or caregiver answers on the Neuropsychiatric Inventory Questionnaire [105] (Table 1). Participants with cognitive impairment reported more sleep disturbances than cognitively intact participants (Intact). Additionally, participants with Alzheimer’s disease (AD) reported more sleep disturbances than participants diagnosed with mild cognitive impairment (MCI) (F(2,1667) = 130.172, p < 0.05). Men reported more sleep disturbances than women (F(1,1667) = 3.805, p < 0.05). Analysis by ANOVA with Tukey post hoc testing. ; * versus INTACT; # versus MCI; + versus women (p = 0.07).
TABLE 1.
Characteristics of sample population used to determine frequency of sleep disturbances. Blood samples were provided by Caucasian men and women enrolled in the longitudinal research cohort of the Texas Alzheimer’s Research Care and Consortium (TARCC). Normal controls performed within normal limits on all cognitive testing. MCI was defined using Petersen’s criteria [103] and AD patients met consensus-based diagnosis for probable AD based on NINCDS-ADRDA criteria [104]. Institutional Review Board approval was obtained at each TARCC site and written informed consent was obtained from participants and/or caregivers.
| Men | Women | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variable | N | Mean | St. Dev. | N | Mean | St. Dev. |
| Age (years) | 877 | 72.79 | 8.88 | 1312 | 72.21 | 9.67 |
| min. age | max. age | min. age | max. age | |||
| 50 | 94 | 50 | 102 | |||
|
|
||||||
| N | % | N | % | |||
| hyperlipidemia | 557 | 63.51 | 725 | 55.26 | ||
| hypertension | 559 | 63.74 | 814 | 62.39 | ||
| obese | 192 | 21.89 | 355 | 24.92 | ||
| Alzheimer’s disease | 422 | 48.12 | 553 | 42.69 | ||
| mild cognitive impairment | 150 | 17.10 | 189 | 12.42 | ||
| cognitively intact | 305 | 34.78 | 570 | 44.65 | ||
| < high school diploma | 105 | 11.97 | 224 | 12.05 | ||
| high school diploma | 131 | 14.94 | 364 | 26.21 | ||
| ≤ 4 yrs. college | 403 | 45.95 | 525 | 43.00 | ||
| > college | 238 | 27.14 | 199 | 18.75 | ||
SEX DIFFERENCES IN SLEEP APNEA
Men are 2 – 3 times more likely to be diagnosed with sleep apnea than women, and the incidence in both sexes increases dramatically with age [1, 39–41]. This suggests sex hormones play a role in the development of sleep apnea. Estimates of the prevalence of sleep apnea indicate sex differences occur worldwide. In the United States, 24% of men and 9% of women are reported to experience an AHI ≥ 5 [41]. Similar results are observed in investigations conducted in Europe, Asia, and South America [1, 39, 42–44]. Several basic science studies indicate that biological sex differences are involved in the manifestation and progression of sleep apnea.
Sex has an impact on other risk factors, such as age and body weight, which can affect the onset and severity of sleep apnea. Men with sleep apnea are at higher risk for comorbid events than women [40], and have higher risk for sleep apnea during middle age than women [45]. In addition to more frequent incidence with age, men, unlike women, experience increasing AHI severity as they age [1, 39, 46]. Women diagnosed with sleep apnea are not only older at initial diagnosis, they are also diagnosed with less severe AHI than their male counterparts [45]. While the prevalence appears to increase as women enter menopause, the severity of AHI events does not [1, 41]. Interestingly, young women with polycystic ovary syndrome (PCOS), characterized by high testosterone levels, are at higher risk to develop obstructive sleep apnea [47]. This suggests that sex hormones (e.g. androgens and estrogens) may underlie these sex differences in sleep apnea onset, progression, and severity.
Sex-specific anatomical differences in adipose tissue deposition and airway size may account for some of the discrepancies in sleep apnea prevalence and severity. It has been postulated fat deposition around the neck contributes to airway constriction [3, 48, 49]. Sex hormones are crucial in determining the composition and deposition of adipose tissue, as well as the size of airway structures [50]. The result of this is a higher deposition of fat in upper body areas in men, such as around the neck and in the thoracic abdominal region, as opposed to women who are more likely to carry weight in their lower abdomen [40]. Interestingly, obesity and anatomical features contribute to the development and severity of sleep apnea in men, but not women [1, 39]. Men experience a positive correlation between AHI severity and all obesity measures, such as body mass index (BMI), waist-hip ratio, or neck circumference, regardless of age. The severity of AHI can be reduced by weight loss [45]. Neck circumference, narrow airway structure, and loss of muscle tone in a supine position can all be indicators of risk in men [45, 49].
Unlike men, associations between obesity and sleep apnea are not observed in women. Pre-menopausal women with sleep apnea tend to be more obese at lower AHI’s than men, and there is not an association between BMI and AHI severity [45]. Additionally, sleep apnea severity is not dependent on waist-hip ratios or neck circumference in women or affected by weight loss [39]. Although the prevalence of sleep apnea increases in post-menopausal women compared to pre-menopausal women, an association between obesity and sleep apnea is still not present in post-menopausal women, unlike what is observed in men [40, 45]. Pre-menopausal women do not experience the functional loss of airway musculature in a supine position observed in men [49], despite smaller anatomical structure. It is only post-menopausal women who experience a loss in respiratory function, which may be one contributor to the age-related increase in prevalence observed in women. Women with PCOS exhibit altered fat composition and deposition, which closely resembles patterns observed in men [51]. However, no studies have examined the impact of airway function on sleep apnea in PCOS women. The change in adipose tissue to resemble male characteristics may partially account for the elevated risk of sleep apnea in these women, and is further support for the idea sex hormones are contributing to observed sex differences.
In addition to distinctive anatomical differences, men and women with sleep apnea report different symptoms to primary care physicians [2, 40, 45, 52, 53]. Among patients referred for diagnostic sleep studies, men are more likely to report snoring and witnessed accounts of apneas and nocturnal gasping [45]. Women diagnosed with sleep apnea initially present with complaints of daytime sleepiness, fatigue or lack of energy, morning headaches, memory impairments, and enuresis more frequently than men do [45, 52, 53]. During sleep study assessments, men exhibit high AHI events during non-REM sleep phases [49]. Conversely, women are more likely to experience AHI events during both REM and non-REM sleep phases in conjunction with a lower supine AHI overall [49]. This results in more mild diagnoses of sleep apnea for women.
It is intriguing that each of the above risk factors can be modulated by sex hormones. Due to the patterns observed in middle aged men and women, sex hormones may differentially confer protection or exacerbate risk of sleep apnea and associated comorbidities [54, 55]. Sex hormones peak during young adulthood and then decline at different rates in men and women as they age. Testosterone and estrogen levels gradually decline as men age [54, 56, 57]. In contrast, estrogen drops abruptly in women as they enter menopause, while testosterone levels remain stable [58]. In men, the decline in testosterone levels has been associated with adiposity, less efficient sleep, and increased risk for cardiovascular and neurodegenerative events [54, 59, 60]. The role testosterone plays in the mechanisms of sleep apnea is the subject of current discussion and research studies within the aging and endocrinology fields [59, 61, 62]. Further investigation into the role of androgens and estrogens in the mechanisms of this disease are vital to determine how hormone therapy may differentially impact sleep apnea and its associated comorbidities in men and women.
SEX DIFFERENCES IN NEURODEGENERATION
Sex differences exist in the risk and symptoms of neurodegeneration, which parallel the pattern observed in sleep apnea. Aging, which is implicated in elevated incidence and severity of sleep apnea, is the primary risk factor for developing both AD and PD [9, 63]. While the risk for men to develop AD begins during middle age and increases linearly with aging, women appear to be protected from AD until reaching menopause [64]. At this point, the incidence rate in women climbs steeply until it reaches the same level as men. Interestingly, young women with PCOS are at higher risk to develop AD than young women without PCOS [65, 66], and appear to present a similar pattern of developing AD as men, supporting a role for androgens. Following transition from MCI to AD, men appear to have slower cognitive decline than women with AD, suggesting sex hormones impact disease progression [67]. Similarly, men are 1.3 to 2 times more likely to develop PD than women, are diagnosed at a younger age, and are more likely to experience motor deficits in their face, neck, and arms [9, 68, 69]. Women with PD suffer more often from tremor, postural deficits, and depression [9]. Due to the similarities between men and women with PCOS, it is likely hormones, as opposed to genetics, are major contributors to the patterns observed in sleep apnea and the onset of neurodegeneration.
COMMON MECHANISMS AND INTERACTIONS BETWEEN SLEEP APNEA AND NEURODEGENERTION
Using the Texas Alzheimer’s Research Care and Consortium (TARCC) cohort, we found men more frequently report sleep disturbances than their female counterparts (figure 1). This is similar to observations in other studies, in which men with PD report more daytime sleepiness than women with PD [9]. Post-mortem examination of androgens and estrogens in the brains of AD patients show men have a steeper decline in androgens, but not estrogens, than their healthy counterparts [70]. Men with PD also frequently experience a decline in bioavailable testosterone [71]. Women do not exhibit similar effects in either hormone. These observations support the hypothesis androgens may be fundamental to the mechanisms by which sleep apnea heightens the risk of neurodegeneration.
Hypertension, elevated inflammation, and oxidative stress are all common characteristics of sleep apnea and neurodegeneration, as well as risk factors for subsequent neurodegeneration [63, 72–76]. Clinical and basic science studies have reported sleep apnea contributes to the elevation of hypertension, inflammation, and oxidative stress [29, 77–82]. Indeed, hypertension, itself, is a primary risk factor for AD and PD and can exacerbate inflammation and oxidative stress through vascular dysfunction [63, 74, 76, 82–85]. In patients with PD, men are more likely than women to suffer from hypertension [69]. While the incidence of hypertension in AD does not appear to be different between men and women, women with mid-life hypertension are more likely to be diagnosed with dementia later in life than men with the same condition [86]. As women age, their risk of developing cognitive impairments increases if they have uncontrolled hypertension [87]. Therefore, the association of hypertension with sleep apnea may not only increase a person’s risk of subsequent neurodegeneration, it also appears to be a determining factor in the type of neurodegeneration experienced in a sex-dependent fashion.
Men with sleep apnea are more frequently diagnosed with hypertension than women with sleep apnea [88]. Typically, the hypertension is more severe in men than women, and their hypertension severity is positively associated with the severity of AHI [89]. Alternatively, women with sleep apnea experience milder hypertension, which is sustained independent of AHI severity and often resistant to pharmacological therapeutics [89]. In chronic intermittent hypoxia, an animal model of the hypoxic events experienced by patients with sleep apnea, sex differences in the manifestation of hypertension persist. Male rats are observed to experience an elevation in mean arterial pressure, similar to men with sleep apnea [77]. However, in a study comparing male and female responses to chronic intermittent hypoxia, gonadally intact female rats did not experience the sustained hypertension observed in male rats. In fact, only estrogen-deficient ovariectomized female rats exhibited an elevation in mean arterial pressure [90]. This indicates hormones play a crucial role in the impact of repetitive hypoxic events during sleep on vascular reactivity.
In addition to hypertension, the peripheral elevation in oxidative stress and inflammation documented in people with sleep apnea is integral to the mechanisms by which sleep apnea may preclude neurodegeneration. Children with sleep apnea have higher circulating inflammatory markers as well as elevated beta-amyloid expression, a marker often associated with cognitive impairment, than children without sleep apnea [91–93]. Treatment of sleep apnea in those children appears to improve these conditions. In adults with sleep apnea (in the absence of other comorbid conditions), an increase in inflammatory biomarkers was observed in both men and women [94]. Once again, sex differences were observed in the identity of which biomarkers were more highly expressed. In another study that investigated only men, Kaczmarek, et al. reported an increase in endothelial nitric oxide synthase, hypoxia-inducible factor 1 alpha, and vascular endothelial growth factor, indicative of elevated oxidative stress within endothelial cells with sleep apnea [82]. While anecdotal evidence exists to suggest a difference between men and women in sleep apnea induced vascular dysfunction and oxidative stress, that relationship remains to be definitively answered.
To further examine the impact of sleep apnea on physiology, investigators have used different animal models. A common animal model to examine the hypoxia associated with sleep apnea is chronic intermittent hypoxia, wherein room air is repetitively decreased to 8 – 10% of normal oxygen levels while the animal sleeps. Using this animal model, an increase in inflammation and oxidative stress markers in male rats exposed to chronic intermittent hypoxia has been observed [20, 29, 80, 95–98]. In our lab, increased oxidative stress and inflammatory dysregulation was observed in the periphery and the central nervous system, specifically within the entorhinal cortex and substantia nigra of male rats after only seven days exposure [29]. Damage to these areas is implicated in the onset of AD and PD, respectively [10, 11]. Chronic intermittent hypoxia models of longer duration and more severe hypoxic conditions induce inflammation and neuronal loss in the hippocampus of male rodents, which is indicative of advanced stage AD [97–101]. This suggests the hypoxic events experienced during sleep apnea may be responsible for triggering oxidative and inflammatory events within brain regions responsible for cognition and motor control. Studies investigating the mechanisms of sleep apnea in female rodents are scarce and represent a need for further research. Interestingly, treatment of sleep apnea is correlated with a reduction of inflammatory biomarkers associated with AD [79, 92]. In addition, treatment with anti-inflammatory agents appear to reduce the risk for neurodegeneration compared to the general population [9, 68, 92, 102]. It appears that addressing the underlying pathology of all stages of sleep apnea may be protective against the accumulation of neurodegenerative-inducing inflammation and oxidative stress.
CONCLUSION
Sleep apnea is a common disorder, which is underdiagnosed in most of the population. The observed sex differences in sleep apnea may be due to the actions of androgens and estrogens. The effects of sleep apnea go well beyond a lack of restfulness during the day to exacerbate the risk of cardiovascular and neurodegenerative diseases. It is possible sleep apnea contributes to neurodegeneration by inducing vascular dysfunction, resulting in elevation of oxidative stress and inflammation in a regional specific manner within the central nervous system. Preservation of vascular function may be influenced by sex-hormones. While the mechanisms of sleep apnea in cardiovascular disease have been highly investigated, its contributions to neurodegeneration are only recently beginning to be appreciated. Understanding how sleep apnea contributes to oxidative stress and inflammation within the central nervous system will provide valuable information in the search to treat neurodegeneration. Furthermore, investigation is needed into the contribution of sex and sex hormones to provide protection or exacerbation of risk for sleep apnea and its comorbidities.
Acknowledgments
This study was supported by the Texas Alzheimer’s Research and Care Consortium (TARCC) funded by the state of Texas through the Texas Council on Alzheimer’s Disease and Related Disorders. Research reported here was supported by the National Institutes of Health (NIH) R01 NS088514 to RLC and NIH training grant T32 AG 020494 to BDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep & breathing = Schlaf & Atmung. 2002;6(2):49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32(2):150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, Weinstein MD. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2009;5(3):263–76. [PMC free article] [PubMed] [Google Scholar]
- 6.Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, Boehlecke B, Brown TM, Coleman J, Jr, Friedman L, Kapen S, Kapur VK, Kramer M, Lee-Chiong T, Owens J, Pancer JP, Swick TJ, Wise MS. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29(3):375–80. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 7.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braak H, Braak E. Morphological criteria for the recognition of Alzheimer's disease and the distribution pattern of cortical changes related to this disorder. Neurobiol Aging. 1994;15(3):355–6. doi: 10.1016/0197-4580(94)90032-9. discussion 379-80. [DOI] [PubMed] [Google Scholar]
- 9.Lee A, Gilbert RM. Epidemiology of Parkinson Disease. Neurologic clinics. 2016;34(4):955–965. doi: 10.1016/j.ncl.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer's disease. Brain. 2015;138(Pt 10):2814–33. doi: 10.1093/brain/awv236. [DOI] [PubMed] [Google Scholar]
- 12.Bond M, Rogers G, Peters J, Anderson R, Hoyle M, Miners A, Moxham T, Davis S, Thokala P, Wailoo A, Jeffreys M, Hyde C. The effectiveness and cost-effectiveness of donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease (review of Technology Appraisal No. 111): a systematic review and economic model. Health Technol Assess. 2012;16(21):1–470. doi: 10.3310/hta16210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JJ. Parkinson's disease: health-related quality of life, economic cost, and implications of early treatment. Am J Manag Care. 2010;16(Suppl Implications):S87–93. [PubMed] [Google Scholar]
- 14.Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson's disease in the United States. Mov Disord. 2013;28(3):311–8. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- 15.Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, Jiang T, Zhu XC, Tan L. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;41(2):615–31. doi: 10.3233/JAD-132690. [DOI] [PubMed] [Google Scholar]
- 16.Peng DC, Dai XJ, Gong HH, Li HJ, Nie X, Zhang W. Altered intrinsic regional brain activity in male patients with severe obstructive sleep apnea: a resting-state functional magnetic resonance imaging study. Neuropsychiatric disease and treatment. 2014;10:1819–26. doi: 10.2147/NDT.S67805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagnon K, Baril AA, Gagnon JF, Fortin M, Decary A, Lafond C, Desautels A, Montplaisir J, Gosselin N. Cognitive impairment in obstructive sleep apnea. Pathologie-biologie. 2014;62(5):233–40. doi: 10.1016/j.patbio.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Landry S, Anderson C, Andrewartha P, Sasse A, Conduit R. The impact of obstructive sleep apnea on motor skill acquisition and consolidation. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2014;10(5):491–6. doi: 10.5664/jcsm.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J, Wohlleber ME, Ducca EL, Koushyk V, Glodzik L, Mosconi L, Ayappa I, Rapoport DM, de Leon MJ. I. Alzheimer's Disease Neuroimaging, Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964–71. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair D, Zhang SX, Ramesh V, Hakim F, Kaushal N, Wang Y, Gozal D. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med. 2011;184(11):1305–12. doi: 10.1164/rccm.201107-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou PS, Lai CL, Chou YH, Chang WP. Sleep apnea and the subsequent risk of Parkinson's disease: a 3-year nationwide population-based study. Neuropsychiatric disease and treatment. 2017;13:959–965. doi: 10.2147/NDT.S134311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeh NC, Tien KJ, Yang CM, Wang JJ, Weng SF. Increased Risk of Parkinson's Disease in Patients With Obstructive Sleep Apnea: A Population-Based, Propensity Score-Matched, Longitudinal Follow-Up Study. Medicine. 2016;95(2) doi: 10.1097/MD.0000000000002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson DA, Lane J, Wang R, Reid M, Djonlagic I, Fitzpatrick AL, Rapp SR, Charles LE, O'Hara R, Saxena R, Redline S. Greater Cognitive Deficits with Sleep-Disordered Breathing among Individuals with Genetic Susceptibility to Alzheimer's Disease: The Multi-Ethnic Study of Atherosclerosis. Annals of the American Thoracic Society. 2017 doi: 10.1513/AnnalsATS.201701-052OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, Liu L, Ayalon L, He F, Loredo JS. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc. 2008;56(11):2076–81. doi: 10.1111/j.1532-5415.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooke JR, Ayalon L, Palmer BW, Loredo JS, Corey-Bloom J, Natarajan L, Liu L, Ancoli-Israel S. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer's disease and obstructive sleep apnea: a preliminary study. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2009;5(4):305–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Harmell AL, Neikrug AB, Palmer BW, Avanzino JA, Liu L, Maglione JE, Natarajan L, Corey-Bloom J, Loredo JS, Ancoli-Israel S. Obstructive Sleep Apnea and Cognition in Parkinson's disease. Sleep Med. 2016;21:28–34. doi: 10.1016/j.sleep.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neikrug AB, Liu L, Avanzino JA, Maglione JE, Natarajan L, Bradley L, Maugeri A, Corey-Bloom J, Palmer BW, Loredo JS, Ancoli-Israel S. Continuous positive airway pressure improves sleep and daytime sleepiness in patients with Parkinson disease and sleep apnea. Sleep. 2014;37(1):177–85. doi: 10.5665/sleep.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng S, Stark CD, Stark RJ. Sleep apnoea and the neurologist. Practical neurology. 2017;17(1):21–27. doi: 10.1136/practneurol-2016-001524. [DOI] [PubMed] [Google Scholar]
- 29.Snyder B, Shell B, Cunningham JT, Cunningham RL. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiological Reports. 2017;5(9) doi: 10.14814/phy2.13258. e13258-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnulf I, Konofal E, Merino-Andreu M, Houeto JL, Mesnage V, Welter ML, Lacomblez L, Golmard JL, Derenne JP, Agid Y. Parkinson's disease and sleepiness: an integral part of PD. Neurology. 2002;58(7):1019–24. doi: 10.1212/wnl.58.7.1019. [DOI] [PubMed] [Google Scholar]
- 31.Diederich NJ, Vaillant M, Leischen M, Mancuso G, Golinval S, Nati R, Schlesser M. Sleep apnea syndrome in Parkinson's disease. A case-control study in 49 patients. Mov Disord. 2005;20(11):1413–8. doi: 10.1002/mds.20624. [DOI] [PubMed] [Google Scholar]
- 32.Ju YS, Videnovic A, Vaughn BV. Comorbid Sleep Disturbances in Neurologic Disorders. Continuum (Minneapolis, Minn.) 2017;23(4, Sleep Neurology):1117–1131. doi: 10.1212/CON.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 33.Buratti L, Viticchi G, Falsetti L, Cagnetti C, Luzzi S, Bartolini M, Provinciali L, Silvestrini M. Vascular impairment in Alzheimer's disease: the role of obstructive sleep apnea. J Alzheimers Dis. 2014;38(2):445–53. doi: 10.3233/JAD-131046. [DOI] [PubMed] [Google Scholar]
- 34.Dlugaj M, Weinreich G, Weimar C, Stang A, Dragano N, Wessendorf TE, Teschler H, Winkler A, Wege N, Moebus S, Mohlenkamp S, Erbel R, Jockel KH. Sleep-disordered breathing, sleep quality, and mild cognitive impairment in the general population. J Alzheimers Dis. 2014;41(2):479–97. doi: 10.3233/JAD-132132. [DOI] [PubMed] [Google Scholar]
- 35.Kim SJ, Lee JH, Lee DY, Jhoo JH, Woo JI. Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. Am J Geriatr Psychiatry. 2011;19(4):374–81. doi: 10.1097/JGP.0b013e3181e9b976. [DOI] [PubMed] [Google Scholar]
- 36.Lutsey PL, Norby FL, Gottesman RF, Mosley T, MacLehose RF, Punjabi NM, Shahar E, Jack CR, Jr, Alonso A. Sleep Apnea, Sleep Duration and Brain MRI Markers of Cerebral Vascular Disease and Alzheimer's Disease: The Atherosclerosis Risk in Communities Study (ARIC) PLoS One. 2016;11(7):e0158758. doi: 10.1371/journal.pone.0158758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R, Pezzela FR, Forbes A, Hogl B, Trenkwalder C. The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73(6):629–35. doi: 10.1136/jnnp.73.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schapira AH. Excessive daytime sleepiness in Parkinson's disease. Neurology. 2004;63(8 Suppl 3):S24–7. doi: 10.1212/wnl.63.8_suppl_3.s24. [DOI] [PubMed] [Google Scholar]
- 39.Punjabi NM. The Epidemiology of Adult Obstructive Sleep Apnea. Proceedings of the American Thoracic Society. 2008;5(2):136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye L, Pien GW, Weaver TE. Gender differences in the clinical manifestation of obstructive sleep apnea. Sleep Med. 2009;10(10):1075–84. doi: 10.1016/j.sleep.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. The New England journal of medicine. 1993;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 42.Saaresranta T, Hedner J, Bonsignore MR, Riha RL, McNicholas WT, Penzel T, Anttalainen U, Kvamme JA, Pretl M, Sliwinski P, Verbraecken J, Grote L. Clinical Phenotypes and Comorbidity in European Sleep Apnoea Patients. PLoS One. 2016;11(10):e0163439. doi: 10.1371/journal.pone.0163439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, Hamilton GS, Dharmage SC. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Medicine Reviews. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Shao C, Jiang JB, Wu HC, Wu SB, Yu BY, Tang YD. Clinical assessment and polysomnographic study of sleep apnea in a Chinese population of snorers. Journal of Zhejiang University. Science. B. 2015;16(3):215–23. doi: 10.1631/jzus.B1400236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basoglu OK, Tasbakan MS. Gender differences in clinical and polysomnographic features of obstructive sleep apnea: a clinical study of 2827 patients. Sleep & breathing = Schlaf & Atmung. 2017 doi: 10.1007/s11325-017-1482-9. [DOI] [PubMed] [Google Scholar]
- 46.Mehra R, Stone KL, Blackwell T, Ancoli Israel S, Dam TT, Stefanick ML, Redline S. Prevalence and correlates of sleep-disordered breathing in older men: osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2007;55(9):1356–64. doi: 10.1111/j.1532-5415.2007.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Helvaci N. Polycystic ovary syndrome and the risk of obstructive sleep apnea: a meta-analysis and review of the literature. 2017;6(7):437–45. doi: 10.1530/EC-17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohsenin V. Effects of gender on upper airway collapsibility and severity of obstructive sleep apnea. Sleep Medicine. 2003;4(6):523–529. doi: 10.1016/s1389-9457(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 50.Sathish V, Martin YN, Prakash Y. Sex Steroid Signaling: Implications for Lung Diseases. Pharmacology & therapeutics. 2015;150:94–108. doi: 10.1016/j.pharmthera.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Randeva HS, Tan BK, Weickert MO, Lois K, Nestler JE, Sattar N, Lehnert H. Cardiometabolic Aspects of the Polycystic Ovary Syndrome. Endocrine Reviews. 2012;33(5):812–41. doi: 10.1210/er.2012-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quintana-Gallego E, Carmona-Bernal C, Capote F, Sanchez-Armengol A, Botebol-Benhamou G, Polo-Padillo J, Castillo-Gomez J. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respiratory medicine. 2004;98(10):984–9. doi: 10.1016/j.rmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Lindberg E, Benediktsdottir B, Franklin KA, Holm M, Johannessen A, Jogi R, Gislason T, Real FG, Schlunssen V, Janson C. Women with symptoms of sleep-disordered breathing are less likely to be diagnosed and treated for sleep apnea than men. Sleep Med. 2017;35:17–22. doi: 10.1016/j.sleep.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 54.Araujo AB, Wittert GA. Endocrinology of the aging male, Best practice & research. Clinical endocrinology & metabolism. 2011;25(2):303–19. doi: 10.1016/j.beem.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacological reviews. 2010;62(2):155–98. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bassil N, Morley JE. Late-life onset hypogonadism: a review. Clinics in geriatric medicine. 2010;26(2):197–222. doi: 10.1016/j.cger.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Pinsky MR, Hellstrom WJ. Hypogonadism, ADAM, and hormone replacement. Therapeutic advances in urology. 2010;2(3):99–104. doi: 10.1177/1756287210369805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christensen A, Pike CJ. Menopause, obesity and inflammation: interactive risk factors for Alzheimer's disease. Frontiers in aging neuroscience. 2015;7:130. doi: 10.3389/fnagi.2015.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barrett-Connor E, Dam TT, Stone K, Harrison SL, Redline S, Orwoll E. The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. The Journal of clinical endocrinology and metabolism. 2008;93(7):2602–9. doi: 10.1210/jc.2007-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barron AM, Pike CJ. Sex hormones, aging, and Alzheimer's disease. Frontiers in bioscience (Elite edition) 2012;4:976–97. doi: 10.2741/e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grech A, Breck J, Heidelbaugh J. Adverse effects of testosterone replacement therapy: an update on the evidence and controversy. Therapeutic advances in drug safety. 2014;5(5):190–200. doi: 10.1177/2042098614548680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? The journal of sexual medicine. 2007;4(5):1241–6. doi: 10.1111/j.1743-6109.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- 63.Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(8) doi: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA - Journal of the American Medical Association. 2011;306(6):613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castellano CA, Baillargeon JP, Nugent S, Tremblay S, Fortier M, Imbeault H, Duval J, Cunnane SC. Regional Brain Glucose Hypometabolism in Young Women with Polycystic Ovary Syndrome: Possible Link to Mild Insulin Resistance. PLoS One. 2015;10(12):e0144116. doi: 10.1371/journal.pone.0144116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rasgon NL, Kenna HA. Insulin resistance in depressive disorders and Alzheimer's disease: revisiting the missing link hypothesis. Neurobiol Aging. 2005;26(Suppl 1):103–7. doi: 10.1016/j.neurobiolaging.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 67.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: risk factors and prevention. The Lancet. Neurology. 2016;15(12):1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 69.Szewczyk-Krolikowski K, Tomlinson P, Nithi K, Wade-Martins R, Talbot K, Ben-Shlomo Y, Hu MT. The influence of age and gender on motor and non-motor features of early Parkinson's disease: initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism & related disorders. 2014;20(1):99–105. doi: 10.1016/j.parkreldis.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 70.Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer's disease. Neurobiol Aging. 2011;32(4):604–13. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Melcangi RC, Giatti S, Garcia-Segura LM. Levels and actions of neuroactive steroids in the nervous system under physiological and pathological conditions: Sex-specific features. Neuroscience and biobehavioral reviews. 2015 doi: 10.1016/j.neubiorev.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 72.Bamberger ME, Landreth GE. Inflammation, apoptosis, and Alzheimer's disease. Neuroscientist. 2002;8(3):276–83. doi: 10.1177/1073858402008003013. [DOI] [PubMed] [Google Scholar]
- 73.Chao Y, Wong SC, Tan EK. Evidence of inflammatory system involvement in Parkinson's disease. Biomed Res Int. 2014;2014:308654. doi: 10.1155/2014/308654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57(2):132–40. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S26–36. doi: 10.1002/ana.10483. discussion S36-8. [DOI] [PubMed] [Google Scholar]
- 76.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015 doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 77.Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/DeltaFosB in central autonomic regions. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;301(1):R131–9. doi: 10.1152/ajpregu.00830.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Semenza GL, Prabhakar NR. Neural Regulation of Hypoxia-Inducible Factors and Redox State Drives the Pathogenesis of Hypertension in a Rodent Model of Sleep Apnea. J Appl Physiol (1985) 2015 doi: 10.1152/japplphysiol.00162.2015. jap 00162 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.May AM, Mehra R. Obstructive sleep apnea: role of intermittent hypoxia and inflammation. Seminars in respiratory and critical care medicine. 2014;35(5):531–44. doi: 10.1055/s-0034-1390023. [DOI] [PubMed] [Google Scholar]
- 80.Smith SM, Friedle SA, Watters JJ. Chronic intermittent hypoxia exerts CNS region-specific effects on rat microglial inflammatory and TLR4 gene expression. PLoS One. 2013;8(12):e81584. doi: 10.1371/journal.pone.0081584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 Pt 1):685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 82.Kaczmarek E, Bakker JP, Clarke DN, Csizmadia E, Kocher O, Veves A, Tecilazich F, O'Donnell CP, Ferran C, Malhotra A. Molecular biomarkers of vascular dysfunction in obstructive sleep apnea. PLoS One. 2013;8(7):e70559. doi: 10.1371/journal.pone.0070559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Del Rio R, Moya EA, Parga MJ, Madrid C, Iturriaga R. Carotid body inflammation and cardiorespiratory alterations in intermittent hypoxia. Eur Respir J. 2012;39(6):1492–500. doi: 10.1183/09031936.00141511. [DOI] [PubMed] [Google Scholar]
- 84.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 85.Zimmerman MC. Angiotensin II and angiotensin-1–7 redox signaling in the central nervous system. Current Opinion in Pharmacology. 2011;11(2):138–143. doi: 10.1016/j.coph.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Mungas DM, DeCarli C, Dean A, Whitmer RA. Female sex, early-onset hypertension, and risk of dementia. Neurology. 2017;89(18):1886–1893. doi: 10.1212/WNL.0000000000004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu PH, Masterman DA, Mulnard R, Cotman C, Miller B, Yaffe K, Reback E, Porter V, Swerdloff R, Cummings JL. Effects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly men. Archives of neurology. 2006;63(2):177–85. doi: 10.1001/archneur.63.2.nct50002. [DOI] [PubMed] [Google Scholar]
- 88.Hedner J, Bengtsson-Bostrom K, Peker Y, Grote L, Rastam L, Lindblad U. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J. 2006;27(3):564–70. doi: 10.1183/09031936.06.00042105. [DOI] [PubMed] [Google Scholar]
- 89.Yu Q, Yin G, Zhang P, Song Z, Chen Y, Zhang D, Hu W. Distinct associations between hypertension and obstructive sleep apnea in male and female patients. PLoS One. 2014;9(11):e113076. doi: 10.1371/journal.pone.0113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hinojosa-Laborde C, Mifflin SW. Sex differences in blood pressure response to intermittent hypoxia in rats. Hypertension. 2005;46(4):1016–21. doi: 10.1161/01.HYP.0000175477.33816.f3. [DOI] [PubMed] [Google Scholar]
- 91.Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;9(3):254–9. doi: 10.1016/j.sleep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kheirandish-Gozal L, Philby MF, Alonso-Alvarez ML, Teran-Santos J, Gozal D. Biomarkers of Alzheimer Disease in Children with Obstructive Sleep Apnea: Effect of Adenotonsillectomy. Sleep. 2016;39(6):1225–32. doi: 10.5665/sleep.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim J, Hakim F, Kheirandish-Gozal L, Gozal D. Inflammatory pathways in children with insufficient or disordered sleep. Respir Physiol Neurobiol. 2011;178(3):465–74. doi: 10.1016/j.resp.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bouloukaki I, Mermigkis C, Tzanakis N, Kallergis E, Moniaki V, Mauroudi E, Schiza SE. Evaluation of Inflammatory Markers in a Large Sample of Obstructive Sleep Apnea Patients without Comorbidities. Mediators of Inflammation. 2017;2017 doi: 10.1155/2017/4573756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Briancon-Marjollet A, Monneret D, Henri M, Joyeux-Faure M, Totoson P, Cachot S, Faure P, Godin-Ribuot D. Intermittent hypoxia in obese Zucker rats: cardiometabolic and inflammatory effects. Experimental physiology. 2016;101(11):1432–1442. doi: 10.1113/EP085783. [DOI] [PubMed] [Google Scholar]
- 96.Rosenzweig I, Williams SC, Morrell MJ. The impact of sleep and hypoxia on the brain: potential mechanisms for the effects of obstructive sleep apnea. Current opinion in pulmonary medicine. 2014;20(6):565–71. doi: 10.1097/MCP.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 97.Sapin E, Peyron C, Roche F, Gay N, Carcenac C, Savasta M, Levy P, Dematteis M. Chronic Intermittent Hypoxia Induces Chronic Low-Grade Neuroinflammation in the Dorsal Hippocampus of Mice. Sleep. 38(10):1537–46. doi: 10.5665/sleep.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126(2):313–23. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 99.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21(7):2442–50. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gozal D, Row BW, Kheirandish L, Liu R, Guo SZ, Qiang F, Brittian KR. Increased susceptibility to intermittent hypoxia in aging rats: Changes in proteasomal activity, neuronal apoptosis and spatial function. Journal of Neurochemistry. 2003;86(6):1545–1552. doi: 10.1046/j.1471-4159.2003.01973.x. [DOI] [PubMed] [Google Scholar]
- 101.Nair D, Dayyat EA, Zhang SX, Wang Y, Gozal D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of Sleep Apnea. PLoS ONE. 2011;6(5) doi: 10.1371/journal.pone.0019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl 1):S232–40. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.O'Bryant SE, Johnson L, Balldin V, Edwards M, Barber R, Williams B, Devous M, Cushings B, Knebl J, Hall J. Characterization of Mexican Americans with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2013;33(2):373–9. doi: 10.3233/JAD-2012-121420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 105.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. The Journal of neuropsychiatry and clinical neurosciences. 2000;12(2):233–9. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]