Abstract
Measurements of carbon isotope contents of plant organic matter provide important information in diverse fields such as plant breeding, ecophysiology, biogeochemistry and paleoclimatology. They are currently based on 13C/12C ratios of specific, whole metabolites, but we show here that intramolecular ratios provide higher resolution information. In the glucose units of tree-ring cellulose of 12 tree species, we detected large differences in 13C/12C ratios (>10‰) among carbon atoms, which provide isotopically distinct inputs to major global C pools, including wood and soil organic matter. Thus, considering position-specific differences can improve characterisation of soil-to-atmosphere carbon fluxes and soil metabolism. In a Pinus nigra tree-ring archive formed from 1961 to 1995, we found novel 13C signals, and show that intramolecular analysis enables more comprehensive and precise signal extraction from tree rings, and thus higher resolution reconstruction of plants’ responses to climate change. Moreover, we propose an ecophysiological mechanism for the introduction of a 13C signal, which links an environmental shift to the triggered metabolic shift and its intramolecular 13C signature. In conclusion, intramolecular 13C analyses can provide valuable new information about long-term metabolic dynamics for numerous applications.
Introduction
In-depth understanding of the earth system is required to preserve intact ecosystems and protect biodiversity, maintain food supplies and secure other resources in the context of ongoing environmental change. Measurements of stable carbon isotope ratios (13C/12C ratios, expressed as δ13C) have helped to develop such understanding by (inter alia) constraining global C cycle models1 and illuminating plant-environment interactions2. However, there are major uncertainties in earth system models due to incomplete characterisation of soil microbial, biogeochemical, plant physiological, and climatic processes. Notably, estimation of soil-to-atmosphere CO2 fluxes based on δ13C analysis is impeded by (inter alia) lack of knowledge about 13C fractionations by soil microbes3. Similarly, simulated C exchange fluxes between the atmosphere and biosphere are insufficiently constrained due to limited understanding of CO2 fertilization effects4, i.e., the increase in plant carbon sequestration associated with rising atmospheric [CO2].
Natural plant archives, including tree rings, enable 13C analyses over decadal to millennial time scales. This is important because covering such timeframes by direct monitoring or manipulative experiments is impossible, but it is essential for robustly constraining vegetation modules of earth system models and predicting changes in plant productivity under climate change. However, the information that can be extracted from archives is currently limited by lack of sufficient understanding of plant 13C fractionation. There are well-established differences in 13C abundances among intramolecular C positions in various metabolites, including glucose5–9, but extant studies in plant ecophysiology and the earth sciences report conventional 13C/12C measurements of whole molecules. These whole-molecule studies rely on the assumption that intramolecular variability is negligible. Here, we test this assumption and investigate the potential of intramolecular 13C measurements for extracting information from archives.
To analyse effects of intramolecular 13C variation, we distinguish two major 13C fractionation systems, diffusion-Rubisco (DR) fractionation and post-Rubisco (PR) fractionation. DR fractionation refers to the 13C fractionation by CO2 diffusion from ambient air into plant chloroplasts and Rubisco-mediated CO2 fixation2, previously called photosynthetic fractionation10. The rationale for the change in nomenclature is outlined below. Rubisco adds a single carbon from CO2 to ribulose-1,5-bisphosphate. Therefore, DR fractionation cannot cause intramolecular 13C variation, i.e. it is not position-specific. In contrast, PR fractionation denotes 13C fractionation by enzymes acting downstream of Rubisco. This type of fractionation is known to occur at individual C positions within metabolites11, i.e. it is position-specific. PR fractionation occurs at metabolic branch points9. Theoretically, events such as changes in metabolite allocation at an isotope-sensitive branch point will change the intramolecular 13C pattern. Thus, intramolecular 13C distributions should carry signals reflecting such shifts in metabolic branching.
To explore the potential of the intramolecular level, we measured intramolecular 13C distributions in the glucose units of tree-ring cellulose of an annually resolved Pinus nigra tree-ring series. The samples originate from a moisture limited site, and cover the period 1961–1995. We then conducted a comparative time-series analysis with conventional whole-molecule and intramolecular 13C/12C ratios. Furthermore, we measured the same distributions in samples of six angiosperm and five additional gymnosperm species from globally distributed sites.
We report six findings. First, 13C distributions show intramolecular differences of the order of 10‰. Second, while a signal due to DR fractionation is present at some C positions of Pinus nigra tree-ring glucose, it is attenuated or even absent at other positions. Third, the intramolecular approach enables better description and prediction of environmental variables. Fourth, Hierarchical Cluster Analysis revealed PR signals at several C positions. Fifth, environmental drivers control PR fractionation. Finally, we propose an ecophysiological mechanism for the origin of a PR signal linking an environmental shift with a defined metabolic shift, which leaves its isotopic signature in the tree-ring archive. We conclude that intramolecular 13C analysis greatly extends the information that can be extracted from tree-ring archives.
Intramolecular 13C fractionation: Concepts and nomenclature
As described above, we distinguish here between diffusion-Rubisco (DR) and post-Rubisco (PR) fractionation. Synonyms for DR and PR fractionation are photosynthetic fractionation, and post-photosynthetic or post-carboxylation fractionation, respectively10,12. Photosynthesis involves the action of several fractionating enzymes, e.g. Rubisco, transketolase, and aldolase13, but the term photosynthetic fractionation usually refers exclusively to fractionation by CO2 diffusion and Rubisco-catalysed carboxylation. Fractionations occurring downstream of Rubisco carboxylation have been called post-carboxylation fractionation, but other fractionating carboxylases occur in plants. Robust understanding of high-resolution intramolecular 13C fractionation requires unambiguous terminology. Therefore, we here introduce the terms DR and PR fractionation, which allow the classification of plant 13C fractionations into non-position-specific and position-specific processes.
13C discrimination, a measure of the 13C fractionation in plants, has been defined14 as:
| 1 |
where Ra and Rp are the 13C/12C ratios of a carbon source and plant sample, respectively. To screen for intramolecular 13C signals, suitable isotope parameters are required. In analogy to Δ, we define positional 13C discrimination as:
| 2 |
where Rpi is the 13C/12C ratio at carbon position i of a plant metabolite (see Fig. 1a for carbon assignments). With Ra and Rpi expressed in terms of the conventional δ scale as δ13Ca and δ13Cpi, respectively, Δi is given as:
| 3 |
A process known as triose phosphate cycling (TPC) involves scrambling of substantial proportions (20–25%) of carbon between symmetry-related carbon positions in tree-ring glucose and can potentially confound existing intramolecular 13C signals, particularly leaf-level signals. Below, we present a convenient method for removing the effect of TPC from observed intramolecular 13C distributions of hexoses and verify its suitability. TPC-free positional 13C discrimination, Δi′, is then given as:
| 4 |
and, in terms of δ, as:
| 5 |
where isotope parameters marked by a prime are free of TPC-related variation. Δi and Δi′ each have specific uses: Δi, denoting observed 13C abundances, is relevant when tree-ring glucose enters microbiological and biogeochemical processes; Δi′, denoting TPC-free 13C abundances, enables better understanding of 13C fractionation systems in plants.
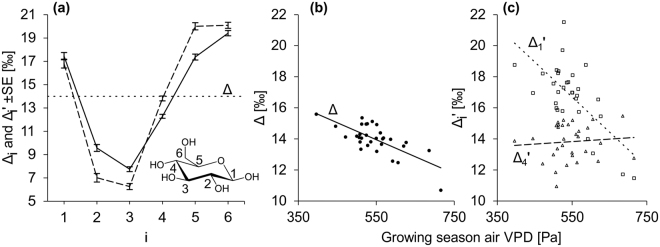
Figure 1.
Intramolecular 13C distributions and effects of growing season air vapour pressure deficit (VPD) on 13C discrimination. Data were acquired for tree-ring glucose of Pinus nigra laid down from 1961 to 1995 at a site in the Vienna basin. (a) Intramolecular 13C distributions (means over 31 years) expressed in terms of intramolecular 13C discrimination. Solid line, observed distribution (Δi); dashed line, TPC-free distribution (Δi′); dotted line, hypothetical distribution without positional 13C effects. Insert: Glucose unit of cellulose showing intramolecular locations of carbon positions, i. (b,c) Effects of VPD on whole-molecule 13C discrimination, Δ and on positional 13C discrimination at C-1 and C-4; Δ1′ and Δ4′, respectively. Linear regression demonstrates highly significant negative relationships between VPD and both Δ and Δ1′, and no detectable relationship between VPD and Δ4′ (ordinary least squares regressions, n = 31, Δ = −0.011VPD + 20.0, r = −0.72, p = 5.4*10−6; Δ1′ = −0.023VPD + 29.1, r = −0.68, p = 3*10−5; Δ4′ = 0.002VPD + 12.9, r = 0.09, p = 0.64).
Results
Tree-ring glucose exhibits a non-random intramolecular 13C distribution
First, we examined intramolecular 13C distributions by averaging all 31 annual distributions of the raw and TPC-free datasets (Δi and Δi′, respectively) of Pinus nigra. Both distributions show non-random patterns with intramolecular differences exceeding 10‰ (Fig. 1a; solid and dashed lines, respectively). Positional differences are more pronounced in Δi′ than in Δi. This is as expected, given that Δi′ is free of the influence of TPC, which causes partial averaging of positional 13C abundances (see below). We obtained similar intramolecular 13C distributions for six angiosperm and five additional gymnosperm species from different sites with global coverage (Fig. S1, Table S1). Our observations of distinct 13C patterns in tree-ring glucose are consistent with observations of glucose derived from other metabolites15–18. As mentioned above, DR fractionation cannot induce intramolecular 13C differences. Thus, observed patterns show that PR fractionations have clearly detectable effects.
The observable DR signal in tree-ring glucose is position-specific
Above, we show that tree-ring glucose exhibits a pronounced intramolecular 13C pattern, which can be attributed to PR fractionation effects. If this pattern varies over time, then intramolecular 13C abundances may carry unique information about long-term metabolic dynamics. Therefore, all subsequent analyses focus on properties related to temporal variability of the intramolecular 13C patterns (i.e. intramolecular 13C signals).
A tree ring formed in a particular year may have had significant input of stored glucose monomers from previous years. If so, 13C time series would exhibit autocorrelation signals. Therefore, we tested all 13C time series (Δ, Δi, Δi′) for autocorrelation, applying temporal lags of one to three years (see SI). We found no evidence of autocorrelation, showing that interannual carryover of signals is negligible. Thus, all subsequent analyses focused on conditions during the year of tree-ring formation.
DR fractionation may be affected by diverse environmental variables19. It is routinely evaluated by measurements of whole-molecule 13C discrimination, Δ20. An underlying assumption is that DR fractionation controls Δ. To search for the most influential environmental variable, we correlated Δ with air vapour pressure deficit, precipitation, soil moisture, air temperature, and global radiation during the growing season (VPD, PRE, SM, TMP, RAD, respectively; the method used to estimate the growing season is described in SI). VPD was found to be most strongly correlated with Δ (VPD, r = −0.72, p = 5*10−6; PRE, r = 0.44, p = 0.013; SM, r = 0.38, p = 0.038; TMP, r = −0.38, p = 0.033; RAD, r = −0.58, p = 7*10−4; n = 31). The strong negative VPD dependency is consistent with expectations for a moisture-limited site, published relationships and the well-established mechanisms underlying DR fractionation2,19. Thus, the variability of DR fractionation is reflected by the variability of VPD in the first approximation. This establishes VPD as a proxy of DR fractionation under given conditions.
If DR fractionation was the only temporally variable fractionation process in plants, its signal strength should be equal at all positional time series of 13C discrimination, Δi′ (see above). We tested this by analysing the linear relationships between Δi′ and VPD. We found that VPD signal strengths vary among Δi′ (Fig. S3). The largest deviations from uniformity were detected in Δ1′ and Δ4′ (Figs. 1b,c and S3). While the slope of the Δ1′~VPD regression is significantly steeper than the slope of the Δ~VPD regression (p = 0.02, see ANCOVA results in Table S4), the slope of the Δ4′~VPD regression is not significantly different from zero (p = 0.64). Thus, the VPD signal is stronger in Δ1′ than in Δ, and undetectable in Δ4′, which implies that the DR signal is transmitted into tree-ring glucose in a position-specific manner.
The intramolecular approach enables better description and prediction of environmental variables
Correlation coefficients for the Δ~VPD and Δ1′~VPD relationships are similar (r = −0.72 and −0.68, respectively). Thus, simple linear regression modelling provided no indications that Δi′ is superior to Δ as a proxy of environmental variables. Therefore, we tested the feasibility of capturing a higher-quality VPD signal using Δi′ in a more sophisticated modelling approach. Combining multiple linear regression modelling with automatic model selection, we generated a Δi′ model that describes VPD more precisely than the corresponding Δ model (VPD~Δ1′ + Δ3′ + Δ5′, adjR2 = 0.60, p = 4*10−6 vs. VPD~Δ, adjR2 = 0.50, p = 5.4*10−6). In contrast to R2, model evaluation by adjR2 takes the number of explanatory variables into account, enabling comparison of models with different numbers of explanatory variables. Next, we tested the predictive abilities of both models by 10-fold cross-validation. We found that the Δi′ model predicts VPD more precisely (Q2 = 0.52 vs. Q2 = 0.43, where Q2 denotes the cross-validated R2). These findings show that the intramolecular approach enables more precise description and prediction of VPD, and suggests that Δi′ might allow for improved climate reconstructions.
Tree-ring glucose contains several distinct intramolecular 13C signals
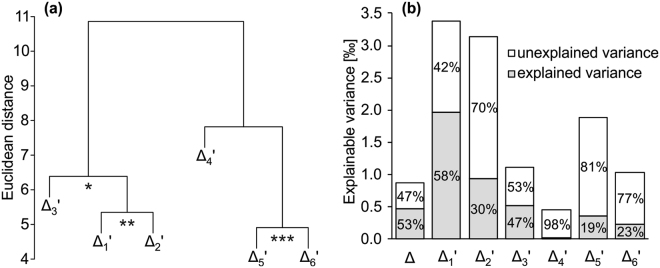
Due to the single carbon addition by Rubisco, DR fractionation equally affects all carbon entering photosynthesis (see above). However, the results presented above show that the DR signal is not equally distributed over all carbon positions of the downstream metabolite tree-ring glucose (Figs. 1b,c and S3), suggesting that PR fractionations influence Δi′, and have had varying effects in the 31-year tree-ring series. To confirm this implication, we screened for position-specific signals by hierarchical cluster analysis of Δi′. We found four clusters: Δ1′ to Δ2′, Δ3′, Δ4′, and Δ5′ to Δ6′ (Fig. 2a). Cluster formation and separation occur due to common and distinct variability, respectively. For instance, Δ1′ and Δ2′ as well as Δ5′ and Δ6′ share significantly correlated common signals (r = 0.54, p = 1.65*10−3, and r = 0.61, p = 2.36*10−4, respectively, n = 31). As Δ1′ and Δ6′ as well as Δ2′ and Δ5′ are uncorrelated (r = 0.08, p = 0.68, and r = 0.11, p = 0.71, respectively, n = 31), detected common signals are independent of each other. Thus, PR fractionations introduce 13C signals on top of the DR fractionation signal. Moreover, independence among clusters implies that intramolecular 13C patterns of tree-ring glucose vary on interannual timescales.
Figure 2.
Common variability among and components of variance in time-series of 13C discrimination. Data were acquired for tree-ring glucose of Pinus nigra laid down from 1961 to 1995 at a site in the Vienna basin. (a) Dendrogram showing clustering of time series of the TPC-free intramolecular 13C discrimination, Δi′. Asterisks denote the significance of correlation between Δi′ forming a cluster (*p ≤ 0.05; **p ≤ 10−2; ***p ≤ 10−3, n = 31). (b) De-convolution of the explainable component of variance in Δ, and Δi′ into an explained and an unexplained component of variance according to previous authors21. Explainable variance denotes the total variance minus estimated error variance. Explained variance denotes the component of variance accounted for by growing season air vapour pressure deficit. Unexplained varaince denotes the component of variance not accounted for by independent variables.
Ecophysiological information is better resolved on the intramolecular level
Observation of multiple intramolecular 13C signals implies that Δ is a composite of several signals with distinct physiological origins, and raises questions about the relative importance of DR and PR fractionation for Δ and Δi′. To address these questions, we first estimated the error variances in Δ and Δi′, which reflect random components of variance caused by finite measurement precision, which differs strongly between Δ and Δi′. Then, we calculated explainable components of variance, which may theoretically be linked to specific ecophysiological processes through modelling21. We then de-convoluted the explainable variance into a component explained by growing season air VPD and an unexplained component. With VPD as a proxy of DR fractionation (see above), this approach enables estimation of the relative importance of DR versus PR fractionation.
The explainable variance differs substantially among Δi′, from 0.45‰ for Δ4′ to 3.37‰ for Δ1′ (Fig. 2b). High values indicate substantial fractionation effects. From this perspective, Δ1′, Δ2′, Δ5′ have high potential, and Δ4′ has low potential for extracting ecophysiological information. In most Δi′, the unexplained component of variance exceeds the explained component. In Δ, both components of variance are similar. These findings suggest that PR fractionation has non-negligible effects on Δ and all Δi′. Moreover, they emphasise the generally high potential for extracting multiple ecophysiological signals from intramolecular-level 13C data, particularly novel signals reflecting dynamic regulation of enzyme reactions downstream of Rubisco.
Discussion
Intramolecular 13C distributions of tree-ring glucose are generally non-random (Fig. 1a and S1). This finding is consistent with previous observations of glucose derived from other species, tissues, and metabolites15–18. Detected intramolecular 13C differences exceed 10‰. Thus, they are an order of magnitude larger than intra-annual 13C variations of atmospheric CO222. Moreover, their magnitude is similar to 13C differences reported for distinct plant metabolites23, and to the whole 13C range reported for bulk plant materials, including C3 and C4 plants24.
Wood cellulose (composed of glucose units) is one of the largest global C pools25 and thus may strongly influence responses of the global C cycle to climatic changes. More specifically, wood cellulose is a major contributor to soil organic matter and, hence, subject to numerous biogeochemical transformations. These transformations are incompletely understood with respect to contributions of different microbial communities, turnover times of soil organic matter components, and responses to climatic changes26.
Isotopes are powerful tools for analysing soil C turnover and associated phenomena. However, their use requires information about both fractionation effects of microbial communities3 and the isotopic composition of soil substrates. For instance, soil cellulose decomposition occurs under both aerobic and anaerobic conditions via several metabolic pathways27. Because of the non-random 13C distribution of wood glucose (Fig. 1a, solid line and Fig. S1), different breakdown pathways will liberate CO2 with distinct δ13C fingerprints. The δ13C of liberated CO2 will equal the δ13C of substrate glucose, if glucose molecules are completely respired. If glucose is fermented (liberating C-3 and C-4), CO2 with approximately 2.5‰ more positive δ13C values will be released (Fig. S1). Although this reasoning neglects fractionation effects of decarboxylation reactions, it illustrates the association of distinct breakdown pathways with substantial 13C differences in respired CO2. Thus, it shows that considering positional 13C differences in soil organic matter will enable better characterisation of C turnover pathways and quantification of heterotrophic soil respiration. This, in turn, will help reduce uncertainties in regional- to global-scale models of terrestrial productivity, and earth system models28.
Our data provide the first proof of temporal variability in intramolecular 13C patterns; more specifically, interannual variation in the 13C patterns of glucose derived from Pinus nigra tree rings (Fig. 2a). As non-random intramolecular 13C patterns result from specific isotopic effects of enzymes acting downstream of Rubisco29, these observations establish a clear link between 13C abundances of plant organic matter and temporal variability in metabolic dynamics.
Our analyses show that intramolecular 13C abundances of tree-ring glucose contain information about the dynamics of both primary CO2 fixation and downstream metabolic processes. While DR fractionation explains much of the interannual variability of Δ, PR fractionations are clearly not negligible (Figs. 2a,b). This may explain why the sensitivity of whole-molecule δ13C values in tree rings to ecophysiological parameters is highly variable30, and why coefficients of determination (R2) obtained by attempts to model Δ rarely exceed 50%. This, in turn, suggests that multiple intramolecular signals are generally present in 13C datasets, and that intramolecular 13C analysis offers considerable scope to improve the resolution and robustness of 13C analyses.
While the mechanisms behind observed PR fractionation signals require further attention, intramolecular 13C ratios clearly offer more information than whole-molecule ratios (Figs. 2a,b). This will likely facilitate retrospective assessment of plant ecophysiological and environmental traits unrelated to the diffusion-Rubisco mechanism. To illustrate this point, we relate the magnitudes of observed Δi′~VPD dependencies to published magnitudes of enzyme isotope effects, and derive a hypothesis for the physiological origin of PR fractionations at glucose C-1 and C-2.
Δ1′ and Δ2′ exhibit higher degrees of explainable variance than any other Δi′, and are highly correlated with each other (Figs. 2a,b). In comparison, the correlation between Δ3′ and the average over Δ1′ and Δ2′ is less significant. Above, we established VPD as proxy of DR fractionation under given conditions, and we found significant VPD correlations with Δ1′ (r = −0.68, p = 3*10−5), Δ2′ (r = −0.49, p = 5.5*10−3) and Δ3′ (r = −0.51, p = 3.5*10−3). However, as shown in Figure S3, regression slopes between VPD and Δi′ decline in the order Δ1′ (b1′ = −0.0226 ± 0.0046SE ‰ Pa−1), Δ2′ (b2′ = −0.0156 ± 0.0052SE ‰ Pa−1) and Δ3′ (b3′ = −0.0116 ± 0.0037SE ‰ Pa−1). DR fractionation is not position-specific, and can therefore only introduce regression slopes of equal size. Significant VPD correlations suggest that the DR signal is present at Δ1′ to Δ3′. Above-average explainable variance, a strong common signal, and steeper VPD slopes indicate that Δ1′ and Δ2′ contain additional VPD-dependent PR signals. Thus, assuming that b3′ represents the common DR signal, the PR contributions to the Δ1′~VPD and Δ2′~VPD slopes are b1PR′ = b1′–b3′ and b2PR′ = b2′–b3′, respectively.
Phosphoglucose isomerase (PGI, EC 5.3.1.9) catalyses conversion of fructose-6-phosphate to glucose-6-phosphate (G6P), which is used in formation of starch or tree-ring cellulose. It is the only enzyme that simultaneously modifies C-1 and C-2 bonds of G6P and hence glucose units in tree-ring cellulose, and can therefore introduce isotope effects of substantial size at these positions (primary isotope effects). Hence, PGI is the most likely generator of the correlated PR signals in Δ1′ and Δ2′. Bacterial glucose isomerase (EC 5.3.1.5) has substantial equilibrium and kinetic isotope effects at glucose C-1 and C-2 (EIEC-1 = −13‰, EIEC-2 = 7‰, KIEC-1 = 5‰, KIEC-2 = 15‰), according to previous authors18, who hypothesis that plant PGI should have similar effects, as it operates by the same reaction mechanism. Hence, shifts of the PGI reaction from irreversible to equilibrium conditions will be accompanied by correlated 13C shifts at C-1 and C-2 of the reaction product, G6P. Magnitudes of these 13C shifts are proportional to the differences between corresponding kinetic and equilibrium isotope effects, and 13C shifts at C-1 and C-2 of G6P are linearly related as (KIEC-1-EIEC-1)/(KIEC-2-EIEC-2) = 2.25. That is, a given shift at G6P C-2 will be accompanied by a 2.25-fold larger shift at C-1. This ratio should equal the ratio of the PR contributions to the Δ1′~VPD and Δ2′~VPD regression slopes, b1PR′/b2PR′. We found that b1PR′/b2PR′ = 2.74 (+1.35SE, −0.60SE), which is consistent with a PGI-related mechanism introducing a 13C signal in Δ1′ and Δ2′.
From an ecophysiological perspective, the occurrence of PGI-driven fractionation is plausible for the following reasons. In isohydric plants like Pinus nigra, strong negative relationships between VPD and both stomatal conductance and intercellular [CO2] can be expected31. At high intercellular [CO2], plants photosynthesise at high rates, and stromal PGI is strongly displaced from equilibrium32–34. As intercellular [CO2] declines, plants photosynthesise at lower rates, and stromal PGI shifts towards equilibrium32. According to published isotope effects18, a shift towards equilibrium results in 13C enrichments at C-1 and C-2 of stromal G6P. From G6P, the signal is transmitted to transitory starch and the glucose units of tree-ring cellulose derived therefrom. Low intercellular [CO2], as induced by stomatal closure due to high VPD, is associated with 13C enrichment by the DR fractionation system. Consequently, DR and PR fractionation at C-1 and C-2 have synergistic effects, and lead to steeper Δ1′ ~ VPD and Δ2′ ~ VPD regression slopes. A regulated shift towards PGI equilibrium may putatively facilitate stabilisation of the Calvin-Benson cycle35, which is probably most important when intercellular [CO2] is low. Thus, a PGI-related mechanism can explain enhanced Δ1′ and Δ2′ fractionations and is ecophysiologically plausible.
Analysis of intramolecular variation in isotope ratios is intended to resolve multiple ecophysiological signals using several information channels. In that sense, it is conceptually related to the so-called “dual-isotope approach”; the independent, but simultaneous, examination of stomatal conductance and carbon assimilation through combined analysis of whole-molecule δ13C and δ18O of plant organic matter36. In its current form, however, application of such dual-isotope analysis depends on several assumptions, which impedes its widespread implementation37. One problem noted by the cited authors is that stomatal conductance and carbon assimilation are not the only processes that modulate isotope ratios. Our observation of PR fractionation, which the dual-isotope concept neglects, highlights this challenge.
The sensitivity of Δ to multiple ecophysiological variables (Figs. 2a,b) hinders attempts to model the 13C fractionation system of plants and to derive ecophysiological and environmental information from Δ measurements. Generally, deconvolution of several signals with only one observable variable is not feasible. In contrast, resolution of six partly independent intramolecular 13C variables (Fig. 2a) offers a conceptual shift from underdetermined towards fully or even overdetermined model systems. This development can potentially reduce numbers of confounding factors and (hence) model uncertainty. The most powerful approaches may combine intramolecular and multi-isotope techniques, which would offer the highest number of independent isotope information channels. In future, estimations of physiological and environmental parameters including source isotope compositions will most likely rely on such “multichannel” approaches.
Intrinsic water-use efficiency (iWUE) is defined as the ratio between the rates of carbon assimilation and transpiration. It is a major determinant of plant performance at water-limited sites2. DR fractionation is correlated with iWUE, and Δ is often used as proxy of iWUE19. Our results indicate that a purer DR signal can be obtained on the level of intramolecular 13C abundances. Thus, models based on Δi′ may provide better estimates of iWUE.
We found that a statistical model of VPD based on Δi′ has greater descriptive and predictive capacities than the corresponding Δ model. This finding is especially noteworthy given the lower achievable accuracy of Δi′ measurements compared to Δ measurements (SD ± 1‰ vs. SD ± 0.1‰, respectively). Currently, Δi′ measurements are time consuming and thus limited to small sample sets. We expect that Δi′ applications will improve markedly with anticipated analytical advancements and with the further elucidation of PR fractionation effects, which might allow more sophisticated mechanistic modelling.
Intramolecular 13C abundances are functions of environmental and related physiological variables, studied here at annual resolution. The approach is generally suitable for analysis of samples covering much longer timeframes38, far exceeding the scope of manipulation experiments or direct observation. However, upscaling to these timeframes requires an assessment of the temporal robustness of 13C signals. In nature, wood cellulose often persists for long periods, and is datable with high accuracy. Several tree-ring chronologies with annual resolution and calendric exactness encompass the entire Holocene39. Subfossil wood samples date back to the last interglacial period, ≈130,000 to 115,000 BP40,41. Thus, intramolecular 13C distributions in wood are promising archives of information about physiological and environmental conditions in past decades, centuries, and millennia. Position-specific isotope abundances may be particularly valuable for acquiring information (which is difficult to acquire by any other available technology) about the capacity of different plant species to acclimatise and adapt to long-term environmental changes. This, in turn, might aid attempts to identify suitable plants, cultivars and genotypes for changing environments.
We anticipate that intramolecular 13C measurements will complement whole-molecule stable isotope measurements and multi-isotope approaches in several applications. These include: prediction of 13C abundances of CO2 formed by different respiratory pathways; characterisation of the C metabolism of soil microbial communities; analyses of soil carbon turnover; elucidation of plants’ physiological responses to environmental changes and their long-term acclimatisation (in periods and conditions covered by calibrating data); and reconstructions of plant physiological and environmental traits based on mechanistic models (outside periods and conditions covered by calibrating data).
Methods
Additional information is provided under Supporting Information.
Site and samples
We used samples of annual rings of 19 Pinus nigra Arnold trees (two cores per tree) from the Bierhäuselberg site (Vienna region, Austria), which has shallow, very dry soil. Both the site and samples have been previously described in detail42. In addition, we used dated tree-ring samples, pooling 5–10 annual rings of 11 angiosperm and gymnosperm species from ecologically different sites with global coverage (Table S1).
Sample preparation
We carefully separated dated Pinus nigra tree rings (from 1961 to 1995) using a binocular microscope and a scalpel, and combined rings in annual pools. Thus, our data represent properties of the tree species at the site rather than individual trees. Pooled samples were ground (Retsch® MM400, Haan, Germany) and their glucose contents were converted into 1,2-O-isopropylidene-α-D-glucofuranose following a published protocol43. Samples of 11 additional angiosperm and gymnosperm species were processed in the same way, but in a final step their glucose contents were converted into 3,6-anhydro-1,2-O-isopropylidene-α-D-glucofuranose43. Checks by 1H NMR showed that sample purity was ≥99.9%.
13C EA-IRMS and 13C NMR spectroscopy
Conventional δ13CVPDB measurements of the glucose derivative were acquired for Pinus nigra samples. Quantitative 1D 13C NMR spectra were collected44 using a Bruker 400 MHz AVANCE III instrument equipped with a 5 mm BBFO SmartProbeTM (Bruker BioSpin GmbH, Rheinstetten, Germany). We recorded and processed 30 spectra per Pinus nigra sample and eight spectra per sample of the additional species using TopSpinTM 3.1 (Bruker BioSpin GmbH, Rheinstetten, Germany). We excluded Pinus nigra samples from 1977, 1978, 1981, and 1982 because they were too small.
Calculation of Δi and Δi′
Integration of 13C NMR spectra resulted in average signal integrals, Si, of specific carbon positions of the glucose derivatives, i = {C-1,…, C-6, C-q, C-Me1, C-Me2}. Each carbon is directly bound to one or two neighbouring carbons. Calculation of 13C molar equivalents, Si(c), considered corresponding signal satellites45. Removal of 13C variation related to TPC followed methods described below, eq. (8), and resulted in TPC-free 13C molar equivalents, Si(c)′. Calculation of positional 13C/12C ratios, expressed as δ13Cpi and δ13Cpi′ followed published procedures46. Calculation of positional discrimination, Δi, and the TPC-free positional discrimination, Δi′, followed eqs. (3) and (5), and incorporated reconstructed annual atmospheric δ13CO2 (=δ13Ca) for the northern hemisphere47. As the open canopy at our site presumably allows rapid mixing of biogenic and atmospheric CO2, errors in Δi and Δi′ due to the contribution of isotopically distinct biogenic CO2 should be minimal. Positional 13C deviations from the molecular average were calculated as Δδ13Ci = (Si(c)/(ΣSi(c)/n)−1)*103 with i = {C-1, …, C-6}.
Fractional redistribution of 13C signals between symmetry-related glucose carbon positions by heterotrophic triose phosphate cycling
When cellulose is synthesized, translocated sucrose is first broken down to hexoses, which are converted to UDP-glucose. During these reactions, 40 to 50% of the hexose phosphates generated are further broken down to triose phosphates, before use in cellulose synthesis. This is known as triose phosphate cycling (TPC). Triose phosphate isomerase equilibrates glyceraldehyde 3-phosphate (G3P) with dihydroxyacetone phosphate (DHAP), respectively derived from C4–6 and C1–3 portions of hexoses. Their equilibration causes carbon exchange between C1–3 and C4–6 portions of hexoses. Thus, in comparison to the hexose units of sucrose, approximately 20 to 25% of carbons in the UDP-glucose pool have been effectively redistributed between symmetry-related carbon positions, i.e. between C-1 and C-6, C-2 and C-5, C-3 and C-4. This implies that intramolecular 13C differences between these symmetry-related positions are partially levelled out by TPC. In the following text, we derive equations to back-calculate the intramolecular 13C distribution before TPC. Please note that the resulting TPC-free distribution does not represent the 13C distribution of any naturally occurring hexose. This is because both parts of sucrose, i.e. glucose and fructose, are used for cellulose synthesis, but differ with respect to their 13C distributions8.
Equation for removing the averaging effect of heterotrophic TPC
With y denoting the fraction of hexose phosphates cycling through triose phosphates, and with complete triose phosphate equilibration, the fraction of carbon redistributed between symmetry-related carbon positions is given by y/2. Then, the observed 13C abundance at a specific hexose carbon position, Ci, is given by:
| 6 |
and the observed 13C abundance of the symmetry-related carbon position, Cs, is given by:
| 7 |
Here, 13Ci′ and 13Cs′ denote TPC-free 13C abundances. Solving eqs (6) and (7) for 13Ci′, TPC-free 13C abundances are given by:
| 8 |
Validation of the procedure
Reported estimates of proportions of carbon redistributed by TPC include 20–25% in Quercus robur48, 25% and 19% in Quercus petraea and Picea abies, respectively49, and 19% in various riparian tree species50. Thus, the fraction of carbons redistributed by TPC seems to fall within a quite narrow range in all investigated species. Both phylogenetically and in terms of wood anatomy, Pinus nigra is closer to Picea abies than to Quercus species. Therefore, we chose y = 0.4 as a TPC factor for calculating TPC-free 13C abundances (13Ci′). Δi and Δi′ were then calculated as described above.
As TPC averages 13C abundances at symmetry-related hexose positions, it should lead to correlation between symmetry-related Δi values, and these correlations should be removed by the calculation of TPC-free Δi′ values. As expected for 13C abundances affected by TPC, Δi time series of symmetry-related glucose carbon positions correlate significantly (Table 1, values in boldface). In contrast, the TPC-free dataset, Δi′, does not exhibit such a correlation pattern, indicating that co-variation introduced by TPC was removed (Table 2). In mathematical terms, TPC causes weighted averaging of carbon abundances, eqs. (6) and (7). Like any averaging, it reduces variability. Accordingly, Δi′ exhibits more pronounced variation in its intramolecular distribution than Δi (Fig. 1a). Generally, averaging only has a net effect if differences are present, and the effect increases with the magnitude of the differences. This is reflected by the larger impact of removing TPC effects on Δ2 and Δ5 than on Δ1 and Δ6 (Fig. 1a).
Table 1.
Correlation coefficients and significance levels (*p ≤ 0.05; **p ≤ 10−2; ***p ≤ 10−3; ****p ≤ 10−4) obtained from the Δi cross-correlation analysis (n = 31).
| Δ1 | Δ2 | Δ3 | Δ4 | Δ5 | Δ6 | |
|---|---|---|---|---|---|---|
| Δ1 | 1 | |||||
| Δ2 | 0.60*** | 1 | ||||
| Δ3 | 0.31 | 0.52** | 1 | |||
| Δ4 | 0.00 | 0.31 | 0.38* | 1 | ||
| Δ5 | 0.37* | 0.42* | 0.24 | 0.39* | 1 | |
| Δ6 | 0.55** | 0.48** | 0.31 | 0.11 | 0.69**** | 1 |
Table 2.
Correlation coefficients and significance levels (*p ≤ 0.05; **p ≤ 10−2; ***p ≤ 10−3) obtained from the Δi′ cross-correlation analysis (n = 31).
| Δ1′ | Δ2′ | Δ3′ | Δ4′ | Δ5′ | Δ6′ | |
|---|---|---|---|---|---|---|
| Δ1′ | 1 | |||||
| Δ2′ | 0.54** | 1 | ||||
| Δ3′ | 0.31 | 0.48** | 1 | |||
| Δ4′ | −0.12 | 0.10 | −0.12 | 1 | ||
| Δ5′ | 0.11 | −0.07 | 0.03 | 0.32 | 1 | |
| Δ6′ | 0.08 | 0.19 | 0.21 | 0.06 | 0.61*** | 1 |
Environmental data
We acquired monthly means of precipitation, air temperature and global radiation from the Hohe Warte climate station (Central Institution for Meteorology and Geodynamics, Vienna, Austria, 16°22′ E, 48°15′ N, 203 m AMSL, WMO ID: 1103500). Deficits in air vapour pressure, VPD [Pa], were calculated following published procedures51. We acquired monthly means of soil moisture from a global grid dataset (CPC Soil Moisture V2, NOAA, OAR, ESRL, PSD, Boulder, Colorado, USA) for 16°15′ E, 48°15′ N. Both the climate station and the selected grid point are no more than a horizontal distance of 15 km from the sampling site with a negligible vertical offset. Thus, all data should represent site conditions well. In conifers, tracheids form over several months52. Thus, we calculated climate averages and sums of the growing season, which we estimated to extend from March to November (Fig. S2).
Statistical analyses
Statistical analyses were performed in R 1.0.143. We compared regression slopes by ANCOVA using two categories and type II sum of squares. For statistical description of VPD, we first fitted the maximal model, VPD~Δ1′ + Δ2′ + Δ3′ + Δ4′ + Δ5′ + Δ6′. We arrived at the minimal adequate model by stepwise model simplification based on Akaike’s information criterion using the step() function of the Stats package with default settings. To test the predictive abilities of the simple linear regression model, VPD~Δ, and the minimal adequate model from multiple linear regression modelling, VPD~Δ1′ + Δ3′ + Δ5′, we performed 10-fold cross-validation using cv.lm(m = 10) and CVlm(m = 10) functions of the DAAG package. We performed Hierarchical Cluster Analysis on z-scores of Δi′ using Euclidean distances and Ward’s fusion criterion for cluster formation53.
Data availability
The datasets generated and analysed during the current study are available from the corresponding authors on reasonable request.
Electronic supplementary material
Acknowledgements
This study was supported by the Swedish Research Council VR, the Kempe foundations, and the Knut and Alice Wallenberg Foundation (“NMR for Life” facility and grant 2015.0047). We thank Iain Robertson (Swansea University), Andrea Seim (University of Freiburg), Alan Talhelm (University of Idaho), John Marshall (SLU, Umeå), Steve Leavitt (University of Arizona), Liang Wei (University of Idaho), Richard Norby (Oak Ridge National Laboratory), and Kathy Allen (University of Melbourne) for contributing samples from angiosperm and gymnosperm trees.
Author Contributions
T.W. and J.S. conceived the study. T.W., I.E., M.G. and J.S. prepared samples and acquired data. T.W. and J.S. contributed new analytical tools. T.W., J.Y., D.F. and J.S. performed statistical analyses. T.W., J.Y., A.G. and J.S. interpreted statistical results. T.W. developed a method for removing isotope redistribution effects by triose phosphate cycling, and introduced an ecophysiological mechanism explaining fractionation effects at GLC C-1 and C-2. T.W., A.G., D.F. and J.S. wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-23422-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thomas Wieloch, Email: thomas.wieloch@umu.se.
Jürgen Schleucher, Email: jurgen.schleucher@umu.se.
References
- 1.Ciais P, Tans PP, Trolier M, White JW, Francey RJ. A large northern hemisphere terrestrial CO2 sink indicated by the 13C/12C ratio of atmospheric CO2. Science. 1995;269:1098–1102. doi: 10.1126/science.269.5227.1098. [DOI] [PubMed] [Google Scholar]
- 2.Farquhar GD, O’Leary MH, Berry JA. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Australian Journal of Plant Physiology. 1982;9:121–137. doi: 10.1071/PP9820121. [DOI] [Google Scholar]
- 3.Brüggemann N, et al. Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: a review. Biogeosciences. 2011;8:3457–3489. doi: 10.5194/bg-8-3457-2011. [DOI] [Google Scholar]
- 4.Ciais, P. et al. In Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. (eds C. Heinze, P. Tans & V. Vesala) 465–570 (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA; 2013).
- 5.Abelson PH, Hoering TC. Carbon isotope fractionation in formation of amino acids by photosynthetic organisms. Proceedings of the National Academy of Sciences of the United States of America. 1961;47:623–632. doi: 10.1073/pnas.47.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeNiro MJ, Epstein S. Mechanism of carbon isotope fractionation associated with lipid synthesis. Science. 1977;197:261–263. doi: 10.1126/science.327543. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt, H.L. & Gleixner, G. In Stable Isotopes - Integration of biological, ecological and biochemical processes. (ed H. Griffiths) 13–25 (BIOS Scientific Publishers Ltd, Oxford; 1998).
- 8.Gilbert A, Silvestre V, Robins RJ, Remaud GS, Tcherkez G. Biochemical and physiological determinants of intramolecular isotope patterns in sucrose from C3, C4 and CAM plants accessed by isotopic 13C NMR spectrometry: a viewpoint. Natural Product Reports. 2012;29:476–486. doi: 10.1039/c2np00089j. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt HL, Robins RJ, Werner RA. Multi-factorial in vivo stable isotope fractionation: causes, correlations, consequences and applications. Isotopes in Environmental and Health Studies. 2015;51:155–199. doi: 10.1080/10256016.2015.1014355. [DOI] [PubMed] [Google Scholar]
- 10.Badeck FW, Tcherkez G, Nogues S, Piel C, Ghashghaie J. Post-photosynthetic fractionation of stable carbon isotopes between plant organs-a widespread phenomenon. Rapid Communications in Mass Spectrometry. 2005;19:1381–1391. doi: 10.1002/rcm.1912. [DOI] [PubMed] [Google Scholar]
- 11.Tcherkez G, Mahé A, Hodges M. 12C/13C fractionations in plant primary metabolism. Trends in Plant Science. 2011;16:499–506. doi: 10.1016/j.tplants.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Gessler A, Tcherkez G, Peuke AD, Ghashghaie J, Farquhar GD. Experimental evidence for diel variations of the carbon isotope composition in leaf, stem and phloem sap organic matter in Ricinus communis. Plant, Cell and Environment. 2008;31:941–953. doi: 10.1111/j.1365-3040.2008.01806.x. [DOI] [PubMed] [Google Scholar]
- 13.Tcherkez G, Farquhar G, Badeck F, Ghashghaie J. Theoretical considerations about carbon isotope distribution in glucose of C3 plants. Functional Plant Biology. 2004;31:857–877. doi: 10.1071/FP04053. [DOI] [PubMed] [Google Scholar]
- 14.Farquhar GD, Richards RA. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Australian Journal of Plant Physiology. 1984;11:539–552. doi: 10.1071/PP9840539. [DOI] [Google Scholar]
- 15.Rossmann A, Butzenlechner M, Schmidt HL. Evidence for a nonstatistical carbon isotope distribution in natural glucose. Plant Physiology. 1991;96:609–614. doi: 10.1104/pp.96.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert A, Silvestre V, Robins RJ, Remaud GS. Accurate quantitative isotopic 13C NMR spectroscopy for the determination of the intramolecular distribution of 13C in glucose at natural abundance. Analytical Chemistry. 2009;81:8978–8985. doi: 10.1021/ac901441g. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert A, Silvestre V, Robins RJ, Tcherkez G, Remaud GS. A 13C NMR spectrometric method for the determination of intramolecular delta13C values in fructose from plant sucrose samples. New Phytologist. 2011;191:579–588. doi: 10.1111/j.1469-8137.2011.03690.x. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert A, Robins RJ, Remaud GS, Tcherkez G. Intramolecular 13C pattern in hexoses from autotrophic and heterotrophic C3 plant tissues. Proceedings of the National Academy of Sciences. 2012;109:18204–18209. doi: 10.1073/pnas.1211149109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarroll D, Loader NJ. Stable isotopes in tree rings. Quaternary Science Reviews. 2004;23:771–801. doi: 10.1016/j.quascirev.2003.06.017. [DOI] [Google Scholar]
- 20.Cernusak LA, et al. Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytologist. 2013;200:950–965. doi: 10.1111/nph.12423. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson MB, Dåbakk E, Korsman T, Renberg I. Quantifying relationships between near-infrared reflectance spectra of lake sediments and water chemistry. Environmental Science & Technology. 1996;30:2586–2590. doi: 10.1021/es950953a. [DOI] [Google Scholar]
- 22.Levin I, Graul R, Trivett NBA. Long-term observations of atmospheric CO2 and carbon isotopes at continental sites in Germany. Tellus B. 1995;47:23–34. doi: 10.3402/tellusb.v47i1-2.15996. [DOI] [Google Scholar]
- 23.Gleixner G, Scrimgeour C, Schmidt H-L, Viola R. Stable isotope distribution in the major metabolites of source and sink organs of Solanum tuberosum L.: a powerful tool in the study of metabolic partitioning in intact plants. Planta. 1998;207:241–245. doi: 10.1007/s004250050479. [DOI] [Google Scholar]
- 24.O’Leary MH. Carbon isotope fractionation in plants. Phytochemistry. 1981;20:553–567. doi: 10.1016/0031-9422(81)85134-5. [DOI] [Google Scholar]
- 25.Lorenz, K. & Lal, R. Carbon sequestration in forest ecosystems. (Springer Netherlands, 2010).
- 26.Bond-Lamberty B, et al. Soil respiration and bacterial structure and function after 17 years of a reciprocal soil transplant experiment. PLOS ONE. 2016;11:1–16. doi: 10.1371/journal.pone.0150599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Boer W, Folman LB, Summerbell RC, Boddy L. Living in a fungal world - Impact of fungi on soil bacterial niche development. FEMS Microbiology Reviews. 2005;29:795–811. doi: 10.1016/j.femsre.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Flato GM. Earth system models: an overview. Wiley Interdisciplinary Reviews: Climate Change. 2011;2:783–800. [Google Scholar]
- 29.Gleixner G, Schmidt H-L. Carbon isotope effects on the Fructose-1,6-bisphosphate aldolase reaction, Origin for non-statistical 13C distributions in carbohydrates. Journal of Biological Chemistry. 1997;272:5382–5387. doi: 10.1074/jbc.272.9.5382. [DOI] [PubMed] [Google Scholar]
- 30.Barbour MM, Song X. Do tree-ring stable isotope compositions faithfully record tree carbon/water dynamics? Tree Physiology. 2014;34:792–795. doi: 10.1093/treephys/tpu064. [DOI] [PubMed] [Google Scholar]
- 31.Roman DT, et al. The role of isohydric and anisohydric species in determining ecosystem-scale response to severe drought. Oecologia. 2015;179:641–654. doi: 10.1007/s00442-015-3380-9. [DOI] [PubMed] [Google Scholar]
- 32.Dietz K-J. A possible rate-limiting function of chloroplast hexosemonophosphate isomerase in starch synthesis of leaves. Biochimica et Biophysica Acta. 1985;839:240–248. doi: 10.1016/0304-4165(85)90004-2. [DOI] [Google Scholar]
- 33.Gerhardt R, Stitt M, Heldt HW. Subcellular metabolite levels in spinach leaves: Regulation of sucrose synthesis during diurnal alterations in photosynthetic partitioning. Plant Physiology. 1987;83:399–407. doi: 10.1104/pp.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schleucher J, Vanderveer P, Markley JL, Sharkey TD. Intramolecular deuterium distributions reveal disequilibrium of chloroplast phosphoglucose isomerase. Plant, Cell and Environment. 1999;22:525–533. doi: 10.1046/j.1365-3040.1999.00440.x. [DOI] [Google Scholar]
- 35.Sharkey TD, Weise SE. The glucose 6-phosphate shunt around the Calvin–Benson cycle. Journal of Experimental Botany. 2016;67:4067–4077. doi: 10.1093/jxb/erv484. [DOI] [PubMed] [Google Scholar]
- 36.Scheidegger Y, Saurer M, Bahn M, Siegwolf R. Linking stable oxygen and carbon isotopes with stomatal conductance and photosynthetic capacity: a conceptual model. Oecologia. 2000;125:350–357. doi: 10.1007/s004420000466. [DOI] [PubMed] [Google Scholar]
- 37.Roden J, Siegwolf R. Is the dual-isotope conceptual model fully operational? Tree Physiology. 2012;32:1179–1182. doi: 10.1093/treephys/tps099. [DOI] [PubMed] [Google Scholar]
- 38.Ehlers I, et al. Detecting long-term metabolic shifts using isotopomers: CO2-driven suppression of photorespiration in C3 plants over the 20th century. Proceedings of the National Academy of Sciences. 2015;112:15585–15590. doi: 10.1073/pnas.1504493112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedrich M, et al. The 12,460-year Hohenheim oak and pine tree-ring chronology from central Europe - A unique annual record for radiocarbon calibration and paleoenvironment reconstructions. Radiocarbon. 2004;46:1111–1122. doi: 10.1017/S003382220003304X. [DOI] [Google Scholar]
- 40.Roig FA, et al. Climate variability 50,000 years ago in mid-latitude Chile as reconstructed from tree rings. Nature. 2001;410:567–570. doi: 10.1038/35069040. [DOI] [PubMed] [Google Scholar]
- 41.van der Ham RWJM, et al. Plant remains from the Kreftenheye Formation (Eemian) at Raalte, The Netherlands. Vegetation History and Archaeobotany. 2008;17:127–144. doi: 10.1007/s00334-007-0115-9. [DOI] [Google Scholar]
- 42.Leal S, Eamus D, Grabner M, Wimmer R, Cherubini P. Tree rings of Pinus nigra from the Vienna basin region (Austria) show evidence of change in climatic sensitivity in the late 20th century. Canadian Journal of Forest Research. 2008;38:744–759. doi: 10.1139/X07-189. [DOI] [Google Scholar]
- 43.Betson TR, Augusti A, Schleucher J. Quantification of deuterium isotopomers of tree-ring cellulose using Nuclear Magnetic Resonance. Analytical Chemistry. 2006;78:8406–8411. doi: 10.1021/ac061050a. [DOI] [PubMed] [Google Scholar]
- 44.Chaintreau A, et al. Site-specific 13C content by quantitative isotopic 13C Nuclear Magnetic Resonance spectrometry: A pilot inter-laboratory study. Analytica Chimica Acta. 2013;788:108–113. doi: 10.1016/j.aca.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Zhang B-L, Trierweiler M, Jouitteau C, Martin GJ. Consistency of NMR and mass spectrometry determinations of natural-abundance site-specific carbon isotope ratios. The case of glycerol. Analytical Chemistry. 1999;71:2301–2306. doi: 10.1021/ac9812375. [DOI] [PubMed] [Google Scholar]
- 46.Silvestre V, et al. Isotopic 13C NMR spectrometry to assess counterfeiting of active pharmaceutical ingredients: Site-specific 13C content of aspirin and paracetamol. Journal of Pharmaceutical and Biomedical Analysis. 2009;50:336–341. doi: 10.1016/j.jpba.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 47.Leuenberger, M. In Terrestrial Ecology, Vol. 1. (eds T. E. Dawson & R. T. W. Siegwolf) 211–233 (Elsevier, 2007).
- 48.Hill SA, Waterhouse JS, Field EM, Switsur VR, Ap Rees T. Rapid recycling of triose phosphates in oak stem tissue. Plant, Cell and Environment. 1995;18:931–936. doi: 10.1111/j.1365-3040.1995.tb00603.x. [DOI] [Google Scholar]
- 49.Augusti A, Betson TR, Schleucher J. Hydrogen exchange during cellulose synthesis distinguishes climatic and biochemical isotope fractionations in tree rings. New Phytologist. 2006;172:490–499. doi: 10.1111/j.1469-8137.2006.01843.x. [DOI] [PubMed] [Google Scholar]
- 50.Roden JS, Ehleringer JR. Hydrogen and oxygen isotope ratios of tree-ring cellulose for riparian trees grown long-term under hydroponically controlled environments. Oecologia. 1999;121:467–477. doi: 10.1007/s004420050953. [DOI] [PubMed] [Google Scholar]
- 51.Abtew, W. & Melesse, A. M. In Evaporation and Evapotranspiration 53–62 (Springer Verlag, 2013).
- 52.Cuny HE, Rathgeber CBK, Frank D, Fonti P, Fournier M. Kinetics of tracheid development explain conifer tree-ring structure. New Phytologist. 2014;203:1231–1241. doi: 10.1111/nph.12871. [DOI] [PubMed] [Google Scholar]
- 53.Ward JH. Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association. 1963;58:236–244. doi: 10.1080/01621459.1963.10500845. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding authors on reasonable request.