What you need to know.
Palliative radiotherapy offers effective symptom control for focal disease due to cancer
Increased analgesia, anti-emetics, and in some cases corticosteroids can help to reduce discomfort and side effects
Acute side effects of radiotherapy usually resolve within 4-6 weeks of completing treatment
Symptoms of cancer may deteriorate before improvement
For patients in the final weeks of life, the side effects and disruption of palliative radiotherapy may outweigh the benefits, and holistic palliative care may be more appropriate
Palliative radiotherapy offers a quick, inexpensive, and effective way of reducing many of the focal symptoms of advanced, incurable cancer, whether these arise from the primary tumour or from metastatic deposits. It can improve quality of life while being associated with limited treatment burden in terms of both hospital attendances and side effects.1 The average UK general practice oversees care for around 20 patients with terminal cancer each year with higher numbers seen in secondary care,2 3 while a Canadian survey of general practitioners found that 85% had provided care for patients with advanced cancer within the previous month.4 This article aims to update non-specialists on the benefits, practicalities, and side effects of palliative radiotherapy to ensure that patients are considered and referred for these treatments when appropriate.
Sources and selection criteria.
In developing this article, we used multiple sources. For each of the sites treated, we carried out a search of the Cochrane database to identify systematic reviews. Search terms used included “palliative AND radiotherapy AND bone metastases,” “spinal cord compression AND radiotherapy,” and “palliative radiotherapy AND lung cancer.” Where no Cochrane reviews were identified, we used Medline searches to identify other relevant systematic reviews and individual studies. We also searched our existing collections of relevant references and consulted appropriate experts where relevant studies could not be identified. In all cases we used the highest level of evidence available to inform this review, with more recent studies cited where possible. All searches were carried out between September 2017 and January 2018.
How is radiotherapy delivered?
Radiotherapy is delivered with linear accelerators (fig 1) in specialised cancer centres generally located in large urban areas (see box 1). High energy x rays are targeted to the disease site, causing DNA damage and cell death. Curative radiotherapy is routinely delivered over multiple, small daily doses (fractions) to reduce the risk of long term, permanent side effects in adjacent normal tissues.5 Palliative treatments require lower total doses, with the focus shifting to symptom control while minimising treatment burden. This change underpins the routine delivery of palliative radiotherapy using much shorter courses of larger fraction size (hypo-fractionation).
Fig 1.
Linear accelerator used to deliver radiotherapy
Box 1. Practicalities of palliative radiotherapy.
Anatomically targeted treatment during which the patient lies still on a relatively hard-topped treatment couch for about 15 minutes. The procedure itself is not associated with pain, but some may find the treatment position uncomfortable. Increased pain relief ahead of treatment can help. Occasionally this discomfort outweighs the benefits
Patients must be able to provide informed consent. In emergency situations (such as spinal cord compression) a decision may be made in the patient’s best interests if the patient lacks capacity and has no available representative
Patients must be able to follow verbal commands from radiographers outside the treatment room; a lack of capacity may make it difficult or even unsafe to deliver treatment. Sedation and anaesthesia are not routinely used for palliative radiotherapy
Palliative treatments are usually delivered as a single dose or a short course (usually over 1-3 weeks)
A close fitting mask maybe needed to ensure a consistent treatment position for treatments to the head, neck or upper chest (fig 2). This is generally well tolerated, even by more anxious patients
Re-treatment may be possible for recurrent symptoms, but side effects may be greater
Referrals and management of treatment related side effects can be discussed with the local radiotherapy department
Fig 2.
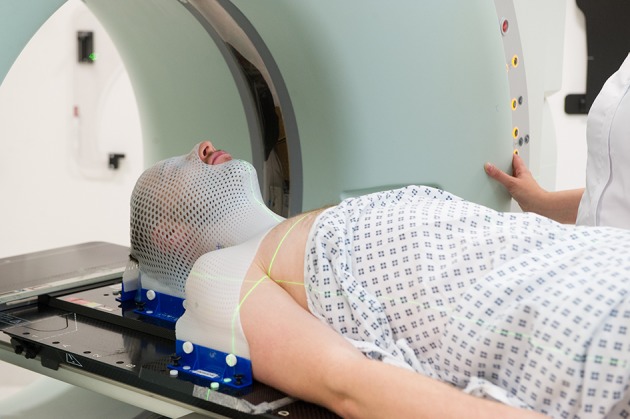
For radiotherapy to the head, neck, or upper chest, a close fitting mask maybe needed to ensure a consistent treatment position
Increasingly, advanced techniques are used to offer more precise treatment delivery, allowing increased dose to the tumour while maintaining limited dose to surrounding tissues (stereotactic radiotherapy) (see fig 3).
Fig 3.
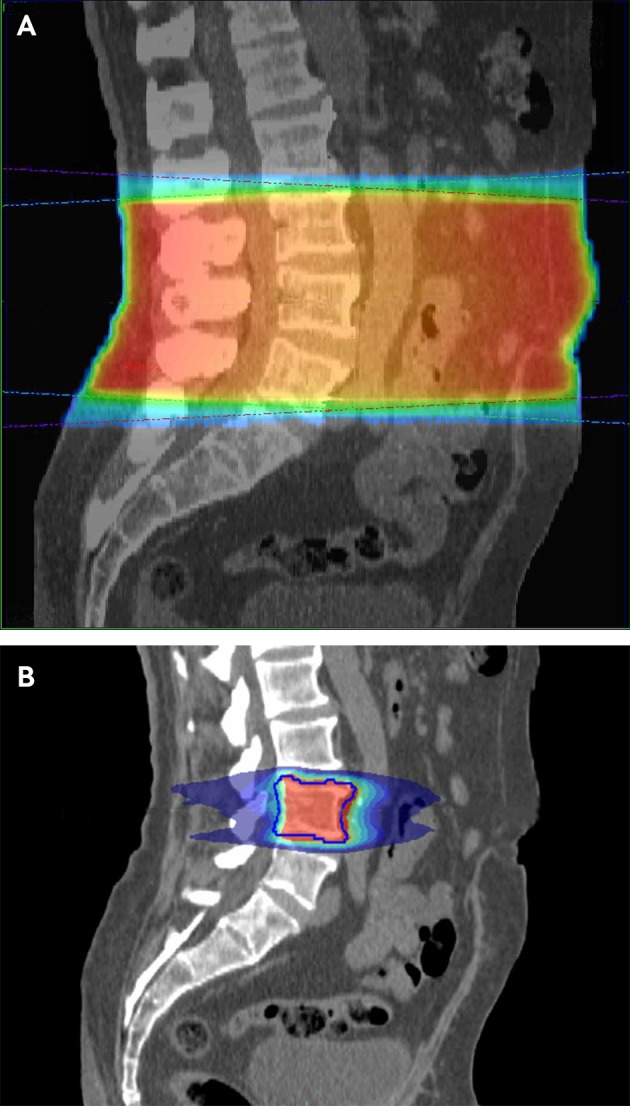
Computed tomograms showing the difference in radiotherapy dose distribution between simple, conventional palliative radiotherapy (A), and targeted stereotactic radiotherapy (B). The latter treatment plan allows a dose of roughly three times greater biological effectiveness to the target with significantly lower dose to surrounding tissue
What are the main barriers to referral for palliative radiotherapy?
Despite increasing numbers of radiotherapy treatment machines in the UK,6 national data highlight that radiotherapy use is lower than in Europe.7 8 9 10 Internationally, multiple population based studies have shown that the chances of receiving palliative radiotherapy are dictated not only by clinical need but also by factors such as age, deprivation, and distance from treatment centre.11 12 Questionnaire based studies suggest that a lack of understanding of the benefits of palliative radiotherapy among general practitioners and palliative care specialists may also be a barrier to referral.13 14 15
What are the indications for using palliative radiotherapy?
A wide range of focal symptoms from advanced cancer can be treated with palliative radiotherapy as described below (and in table 1). Patients can undergo radiotherapy alongside palliative systemic anticancer treatments.
Table 1.
Benefits of palliative radiotherapy for varying indications (evidence referenced is the highest level identified)
| Treatments assessed | Study and sample size | Endpoints | Results |
|---|---|---|---|
| Pain due to bone metastases | |||
| Single fraction radiotherapy v longer, more fractionated courses. | Chow et al 201216 (SR, 5617 patients, 25 trials) | Pain response, re-treatment rate, pathological fracture rate. Time point varied between trials |
60.7% response rate (OR for single v multiple fraction treatments 0.98 (95% CI 0.95 to 1.02)). 23.8% complete pain resolution. Re-treatment higher after single fraction (OR 2.6 (1.92 to 3.47)) No significant difference in pathological fracture rate (overall 3.2%, OR 1.10 (0.65 to 1.86)) |
| Sze et al 200417 (SR, 3487 painful sites, 11 trials) | Pain response, re-treatment rate, pathological fracture rate. Time point varied between trials |
59% response rate (OR for single v multiple fractions 1.03 (0.89 to 1.19)). 33% complete pain resolution. Re-treatment rate higher after single fraction (21.5% v 7.4%, OR 3.44 (2.67 to 4.43)). Fracture rate higher after single fraction (3% v 1.6%, OR 1.82 (1.06 to 3.11)) |
|
| Steenland et al 199918 (RCT, 1157 patients) | Pain response in remaining lifespan (1° endpoint), re-treatment rate, pathological fracture rate. Assessed weekly |
71% response rate; 35% complete resolution of pain; median time to benefit 3 weeks. Re-treatment rate higher after single fraction (25% v 7% (P<0.0001)). Fracture rate higher after single fraction (4% v 2% (P<0.05)) |
|
| Single 8 Gy fraction of radiotherapy v ibandronate infusion in metastatic prostate cancer | Hoskin et al 201519 (RCT, 470 patients) | Pain response at 4 weeks (1° endpoint), crossover, pathological fracture rate. Assessed 4 weekly |
53.1% response with radiotherapy v 49.5% with ibandronate (difference 3.7% (−12.4% to 5.0%), P=0.49). 24% crossover with ibandronate v 31% with radiotherapy. 3% fracture rate with ibandronate v 2% with radiotherapy (P=0.31) |
| Locally advanced lung cancer | |||
| Various palliative radiotherapy regimens | Stevens et al 201520 (SR, 3576 patients, 14 RCTs) | Control of thoracic symptoms, overall survival Time point varied between trials |
Pooled symptom response rates not reported due to study heterogeneity. Possibly better 1 year overall survival with higher dose regimens for patients with good performance status (33.3% (11.4% to 46.2%) v 25.6% (9.4% to 45.7%)), but unclear due to high study heterogeneity (n=1081, 8 trials). No survival improvement seen in poor performance status patients (risk ratio 0.96 (0.91 to 1.02) (n=911, 7 trials) |
| Various palliative radiotherapy regimens | Fairchild et al 200821 (SR, 3473 patients, 13 RCTs) | Control of thoracic symptoms, overall survival Time point varied between trials |
After high and low dose regimens, complete resolution of haemoptysis reported by 73.7% v 68.9% (P=0.19), improvement reported by 80.2% v 81.2%; 48.2% v 53.5% reported improved cough (P=0.04); 57.5% v 51.9% improved chest pain (P=0.43 with significant heterogeneity between studies). Individual RCTs reported improvement in shortness of breath in 35-40%22-24 One trial reported median time to response 5-7 weeks.24 1 year overall survival higher with high dose regimens (26.5% v 21.7%, P=0.002), at the expense of significantly increased oesophagitis |
| Locally advanced oesophageal and gastric cancer | |||
| External radiotherapy (40 Gy in 20 fractions, twice daily) | Kassam et al 200825 (phase I/II, 39 patients) | Dysphagia response at 56 days, survival, toxicity | Improved swallowing function reported by 69%, median time to benefit 4 weeks, duration of response 5.5 months |
| Oesophageal stenting with or without external radiotherapy | Javed et al 201026 (RCT, 84 patients) | Duration of dysphagia relief after stenting, overall survival | Duration of dysphagia relief increased with radiotherapy (7 v 3 months, P=0.002). Median overall survival increased with radiotherapy (180 v 120 days, P=0.009) |
| Oesophageal brachytherapy with or without external radiotherapy | Rosenblatt et al 201027 (RCT, 219 patients) | Dysphagia relief, overall survival | Improved duration of dysphagia relief with radiotherapy: at 200 days, 69.6% had not experienced a dysphagia event v 51.8% without radiotherapy (P=0.014 in multivariable modelling). No significant improvement in overall survival |
| Palliative radiotherapy for advanced gastric cancer | Tey et al 201728 (SR, 122 patients, 7 retrospective studies) | Reduction in gastric bleeding (response definitions varied) | Gastric bleeding reduced in 74% of patients (pooled analysis). Small numbers reported for pain and obstruction responses (n=18 and 33) |
| Malignant spinal cord compression | |||
| 20 Gy in 5 fractions v 30 Gy in 10 fractions radiotherapy | Rades et al 201629 (RCT, 203 patients (155 assessable)) | Motor function at 1 month, local control, overall survival | No significant differences in mobility (P=0.86), local control (P=0.51), or survival (P=0.68). 41.3% reported improved motor function after treatment, and 47.1% remained stable. Improvement in ambulation not reported. Median overall survival 3.2 months |
| 8 Gy single fraction v 16 Gy in 2 fractions radiotherapy | Maranzano et al 200930 (RCT, 327 patients (303 assessable)) | Symptom control (pain, motor, and sphincter function) at 1 month, toxicity, duration of response, overall survival | No significant difference in response rates or duration (P=0.40): median duration of response 5 months; median overall survival 4 months. 53% (95% CI 47% to 58%) achieved a pain response (25% (21% to 31%) complete resolution). 27% of non-ambulatory patients regained mobility after treatment (only 4% for those with paraplegia before treatment). 27% with sphincter disturbance regained control. Acute side effects were equivalent |
| 16 Gy in 2 fractions v split course (total dose 30 Gy in 8 fractions) | Maranzano et al 200531 (RCT, 300 patients (276 assessable)) | Symptom control (pain, motor, and sphincter function) at 1 month, toxicity, duration of response, overall survival | No significant difference in response rates or duration: median duration of response 3.5 months; median overall survival 4 months. 56.9% (51.1% to 62.7%) achieved a pain response (33.3% (27.7% to 38.9%) complete resolution). 35% of non-ambulatory patients regained mobility (not anyone with paraplegia). 14% with sphincter disturbance regained control. Acute side effects were equivalent |
| Radiotherapy (30 Gy in 10 fractions) with or without surgical decompression | Patchell et al 200532 (RCT, 101 patients (study stopped at interim analysis)) | Mobility (time point unclear), continence, corticosteroid use, pain control, overall survival | Post-treatment ambulation rates 84% with surgery v 57% with radiotherapy alone (odds ratio 6.2 (2.0 to 19.8), P=0.001). Continence was more likely after surgery, and doses of corticosteroids (P=0.009) and opiates (P=0.002) were lower. Median survival 126 days after surgery v 100 days after radiotherapy alone (multivariable analysis HR 0.60 (0.38 to 0.96), P=0.033) |
| Brain metastases | |||
| Whole brain radiotherapy (WBRT) (20 Gy in 5 fractions) v dexamethasone alone in non-small cell lung cancer | Mulvenna et al 201633 (RCT, 538 patients) | Overall survival, quality of life (measured in QALYs), use of corticosteroids | All patients received dexamethasone. No difference in overall survival with or without WBRT (median survival 9.2 weeks v 8.5 weeks, HR 1.06 (0.90 to 1.26)), or quality of life (mean QALY 46.4 v 41.7 days). |
| Effectiveness and adverse events after WBRT for adults with multiple brain metastases | Tsao et al 201234 (SR, 10 835 patients, 39 trials) | Overall survival, cerebral disease control, quality of life and symptom control | Unable to recommend one WBRT regimen over others due to lack of quality of life outcomes and no overall improvement in overall survival (n=3645, 8 trials). No improvement in survival (HR 1.08 (0.98 to 1.18)) or symptom control with addition of radio-sensitising drugs to WBRT. Toxicity increased. (n=2016, 6 trials). Addition of stereotactic radiotherapy to WBRT improved cerebral control (n=464, 3 trials). Improvement in overall survival only in individuals with a single metastasis and good performance status in one trial (n=333) (6.5 months v 4.9 months, P=0.03). Significantly reduced steroid doses after stereotactic radiotherapy shown in one trial (n=333) (52% v 33%, P=0.016). Addition of WBRT to stereotactic radiotherapy improved cerebral control (HR 2.61 (1.68 to 4.06), P<0.001) in pooled analysis (n=577, 3 trials) but not overall survival (HR 0.98 (0.71 to 1.35), P=0.88) (n=218, 2 trials) |
| Neurocognitive outcomes after stereotactic radiotherapy with or without WBRT. | Chang et al 200935 (RCT, 58 patients (trial stopped early after interim analysis)) | Neurocognitive outcomes at 4 months, cerebral disease control | Addition of WBRT resulted in lower CNS recurrence at 1 year (73% recurrence-free v 27%, P<0.001). This was at the cost of a higher probability of significantly reduced total recall at 4 months (mean posterior probability 52% v 24%). This difference persisted at 6 months. In practise, to avoid cognitive decline, regular MRI surveillance is often preferred over WBRT36 |
| Head and neck cancer | |||
| 30 Gy in 5 fractions radiotherapy delivered every 3 days | Porceddu et al 200737 (phase II, 37 patients) | Response rate, symptom control, quality of life, and toxicity. Overall and progression-free survival |
80% had an objective response at 2 weeks after treatment, 67% reported improved pain control, 33% felt their ability to eat solids was improved, 62% reported improved overall quality of life. 74% of patients experienced significant dysphagia during treatment, resolving by 4 weeks later. Median overall survival was 6.1 months (range 0.5–21) and progression-free survival 3.9 months (0.5–21). |
| 42 Gy in 12 fractions radiotherapy delivered twice daily in 4 fraction blocks repeated 4 weekly | Corry et al 200538 (phase II, 35 patients) | Response rate, symptom control, quality of life, and toxicity. Overall and progression-free survival |
53% objective response rate. Median overall survival 5.7 months (95% CI 3.4 to 9.3) and progression-free survival 3.1 months (2.2 to 6.1). 85% of patients experienced improved or stable dysphagia after treatment, 56% experienced improved pain control, 44% reported improved overall quality of life |
| Bladder cancer | |||
| 35 Gy in 10 fractions v 21 Gy in 3 fractions radiotherapy | Duchesne et al 200039 (RCT, 500 patients (272 assessable at 3 months)) | Symptomatic improvement at 3 months. Overall survival |
No significant difference for any endpoint (overall survival HR 0.99 (0.82 to 1.21), P=0.933). 51.4% reported symptom improvement (P=0.421 for comparison between arms). In patients experiencing these symptoms initially; haematuria improved in 88%; urinary frequency in 82%; nocturia in 64%, and dysuria in 72% of assessable patients at 3 months after treatment. Median overall survival 7.5 months |
| Rectal cancer | |||
| 30-39 Gy in 10-13 fractions | Cameron et al 201640 (prospective multicentre, 51 patients) | Symptomatic improvement at 3 months | Improvements in pain (77% (54% to 100%)), rectal dysfunction (90% (71% to 100%)), and bleeding (100%) |
| Gynaecological malignancies | |||
| Any external radiotherapy or brachytherapy regimen delivered palliatively to the cervix | van Lonkhuijzen et al 201141 (SR, 476 patients, 7 retrospective studies, 1 prospective study) | Symptomatic improvement | Wide heterogeneity in studies with variable time points and poor reporting limited this analysis. Bleeding improvement ranged from 45% to 100% of patients, pain reduction 31-100%, and discharge 15-100%. Toxicity not consistently reported |
| Locally advanced prostate cancer | |||
| Any palliative radiotherapy regimen delivered to the prostate | Cameron et al 201442 (SR, 315 patients, 9 retrospective studies) | Symptomatic improvement, quality of life, toxicity | Pooled response rates were 73% for haematuria, 80% pain, 63% bladder outlet obstruction, and 78% rectal symptoms. Toxicity was mild/moderate, though not systematically recorded. No reports of quality of life or patient reported outcomes |
RCT=Randomised controlled trial, SR=Systematic review, all phase II studies were non-randomised. OR=odds ratio. HR=hazard ratio. QALY=quality adjusted life year. CNS=central nervous system. MRI=magnetic resonance imaging.
Given that radiotherapy can only ever address focal disease, these treatments should supplement, not replace holistic palliative care. Assessment and support for all physical, psychological, and social needs, with strong communication between services, are necessary. Palliative radiotherapy rarely improves overall survival, which is reported to be a median of 5.2 months in one observational study.43 For patients with particularly limited prognosis, careful consideration of the appropriate level of intervention is essential; the potential benefits of treatment may be outweighed by expected side effects and treatment burden.
Pain due to bone metastases
Postmortem studies have detected bone metastases in up to 70% of patients with advanced cancer.44 Such metastases often cause localised pain and account for 35-40% of all palliative radiotherapy treatments.45 Pain may be constant or intermittent, can be neuropathic with a radiating dermatomal component and possible altered sensation, and often limits activities of daily living.46 Initial management combines analgesics and a holistic assessment of needs with interventions as required, such as home adaptations and walking aids.47 If, despite weak opioids, patients have persistent pain or side effects of medication, consider referral for radiotherapy.48 Metastases in long bones have a risk of pathological fracture. When this risk is assessed to be high, surgical stabilisation is often carried out before radiotherapy.49 50 51
Palliative radiotherapy provides pain relief in a median of 2-3 weeks for 60% of patients (table 1).16 17 Where pain recurs, retreatment can be considered after at least four weeks to allow response.52 Intravenous bisphosphonates offered equivalent pain relief to single fraction radiotherapy for metastatic prostate cancer in a single randomised controlled trial.19 This may be an alternative option for patients with prostate cancer naïve to bisphosphonates.
Symptoms due to locally advanced thoracic cancer
Lung cancer is the third commonest cancer in the UK and 28% of patients will present with locally advanced disease.53 54 Thoracic symptoms include dyspnoea (50%), chest pain (28%), cough (40%), haemoptysis (10%), and dysphagia (7%).22 Some of these local symptoms can be successfully palliated in about two thirds of patients, although the success rate varies with symptoms. More information is provided in table 1.20 21
Palliative radiotherapy to the mediastinum improved obstructive dysphagia from locally advanced oesophageal cancer in around two thirds of patients after a median of four weeks in a non-randomised phase I/II study.25 Given this delay in improvement and the risk of deterioration due to acute oesophagitis, patients with clinically significant dysphagia at baseline often undergo oesophageal stenting before radiotherapy.55 Radiotherapy improves durability of swallowing function after stenting.26 27 However, for patients with very limited prognosis, stenting alone can provide rapid relief of dysphagia, and this group is unlikely to benefit from the addition of palliative radiotherapy.
Symptomatic radiation pneumonitis (occurring in <5%) can occur from six weeks to six months after treatment that includes the lungs.20 21 Refer patients with cough and dyspnoea without another clear cause to the treating oncologist urgently for assessment and consideration of oral corticosteroids.
Pain and neurological compromise due to malignant spinal cord compression
Malignant spinal cord compression occurs when vertebral disease compresses the cord, either directly or as a result of vertebral collapse. More rarely, intraspinal or epidural metastases occur. Back pain is common, often occurring before neurological signs and symptoms, including sensory and motor disturbance and loss of sphincter control. Symptom progression varies, from neurological deterioration over hours to a gradual decline over weeks. Urgent magnetic resonance imaging (MRI) is required to confirm the diagnosis, and oral dexamethasone 16 mg once daily (with proton pump inhibitor) is routinely administered.56 57 Subsequent assessments target expected prognosis in order to guide management decisions.58 59
The median overall survival after a diagnosis of malignant spinal cord compression is 3-4 months.29 60 When predicted prognosis is more than six months, neurosurgical decompression may be considered before radiotherapy on the basis of a single randomised study showing improved neurological outcomes.32 Unfortunately, most patients have a prognosis of less than six months. For these patients, urgent palliative radiotherapy (within 24 hours of MRI confirmation) aims to reduce pain and retain or improve neurological function.56 The best neurological outcomes are seen in those retaining some movement before treatment or with gradual onset of neurological symptoms.61 For patients with established paraplegia, less than 10% regain mobility; in the absence of pain, and if the prognosis is very limited, holistic palliative care and appropriate social or nursing support may be more appropriate.62
Acute side effects reflect the vertebral level treated, while late radiation induced spinal cord myelopathy is rarely seen with palliative doses (<1%).63
Symptoms due to brain metastases
Brain metastases occur in 20-40% of individuals with systemic cancer.34 Presentation can be with seizures, focal neurology, or symptoms of raised intracranial pressure (nausea, vomiting, and headaches). Prognostic indices help to tailor treatment to the individual patient.64 65 For those with limited brain metastases and a life expectancy of more than six months, neurosurgery or stereotactic radiotherapy can be considered under discussion with the treating team, local neurosurgical or neuro-oncology teams, and patient.36 66
For those with more extensive cerebral disease who retain a good performance status, whole brain radiotherapy can be offered, although no high quality randomised data exists to support this over corticosteroids alone.67 Indeed, a recent trial demonstrated no survival or quality of life benefit from whole brain radiotherapy over steroids alone in patients with brain metastases from non-small cell lung cancer.33 This has resulted in a reduction in the use of whole brain radiotherapy in this situation, but extrapolation to other cancer diagnoses is unlikely to be justified.
Symptoms due to advanced head and neck cancer
Patients with locally advanced head and neck cancer often present with a range of difficult to control symptoms including pain, dysphagia or odynophagia, airway compromise, bleeding, and cosmetically distressing tumour bulk.68 These often frail patients have complex needs and require multidisciplinary support including specialist nursing and medical care, support from allied health professionals, palliative care, and community support with strong communication between services.
Prospective studies report improvement in pain control and quality of life in about 50-60% of patients after palliative radiotherapy, with improved ability to eat solids in 33%.37 38 Of note, in one UK series, 18% of patients required hospital admission during or immediately after treatment for nutrition, dehydration, and pain control.68
Symptoms due to advanced pelvic cancers
Locally advanced pelvic cancers can result in bleeding, discharge, bowel obstruction, urinary disturbance, and pelvic pain. Radiotherapy palliated bleeding in up to 90% of patients with advanced bladder, rectal, or gynaecological cancer and improved other symptoms for half to two thirds of patients.39 40 42 69 Acute side effects frequently occur, alongside temporary deterioration of existing symptoms. If abdominal discomfort or diarrhoea are severe or fail to resolve with simple measures, seek advice from the treating oncology team.
Bleeding, pain, and malodour due to skin cancers
Symptoms of bleeding, pain, and malodour due to advanced primary skin cancers responded to palliative radiotherapy in 61% of cases in a small observational study.70 Cutaneous disease—most commonly arising from breast cancer (metastases or primary), melanoma, and lung cancer71—can be treated similarly, although the evidence is extremely limited and there are no randomised comparisons with alternative approaches (such as surgical resection, electro-chemotherapy, photodynamic therapy, topical treatments).72 73
What are the most common side effects of palliative radiotherapy?
The side effects of radiotherapy are dictated by which tissues receive a substantial dose. For example, conventional radiotherapy to lumbar spine vertebral metastases will usually involve irradiation of the bowels, resulting in side effects related to both the bone metastasis and bowels (see fig 3). Additionally, treatment is associated with fatigue in at least two thirds of patients, and this can affect quality of life, limiting participation in preferred activities.74 75
Acute side effects of palliative radiotherapy usually resolve within 4-6 weeks of completing treatment. In routine practice, palliative prescribing of analgesia (including strong opiates) and antiemetics underpins the management of side effects. Randomised evidence is limited, and the recommendations for management of side effects (see table 2) are predominantly based on systematic reviews and guidelines.
Table 2.
Management of the acute side effects of palliative radiotherapy by organ or tissue
| Anatomical site | Side effects | Management | Supporting evidence |
|---|---|---|---|
| Bone | 35% of patient in the first week after treatment to bone metastases experience a pain flare. This resolves within a median of 3 days76 77 | Oral dexamethasone 8 mg once daily before treatment and for 4 days after, possibly with oral proton pump inhibitor | Rate of flare significantly reduced with dexamethasone (26% v 35%, P=0.05) (RCT, 298 patients)76 |
| Lung | Cough after treatment is not well documented but common in practice | Routinely managed with medication (such as weak opioids) | Limited evidence supporting any specific intervention (SR, 326 patients, 9 studies)78 |
| Mediastinum | Oesophagitis results in odynophagia or dysphagia in 14-22% of treated lung cancer patients (SR)20
21
79 and 28% of oesophageal cancer patients.25
Chest discomfort within the first few weeks after treatment |
Antacid mixed with local anaesthetic, simple analgesia, proton pump inhibitors, and soft bland diet. Dietetic referral and enteral feeding maybe required, particularly in patients with compromised swallow before treatment | Recommendation based on a recent literature review as no randomised evidence was identified to inform acute supportive management79 |
| Bowel or stomach | Nausea (such as seen during treatment to bone metastases in 61% or treatment for rectal cancer in 36%)40 80 | Antiemetics 30-60 minutes before, during, and after treatment (such as 5-HT3 receptor antagonists) | 5-HT3 antagonists reduced emesis compared with conventional antiemetics or placebo (SR of RCTs) and are recommended in international guidelines81 82 |
| Diarrhoea and abdominal discomfort during treatment for pelvic tumours in 20-40%,39 40 42 resolves within 6 weeks | Loperamide 2-4 mg and hyoscine butylbromide 20 mg as required. If diarrhoea severe (>6 bowel movements daily) or fails to improve within 12 hours, discuss with the treating oncology team | Recommendation based on regional guidelines and palliative prescribing as no randomised evidence identified83 84 | |
| Bladder | Dysuria, frequency, and nocturia. During the first few weeks after treatment in 33% and 20% of bladder and prostate cancer patients treated to the primary tumour39 42 | Simple analgesia, good fluid intake, and anticholinergic agents are used in routine care. Cranberry capsules can be considered |
Recommendation based on regional practise as no randomised evidence identified.85
Four small RCTs investigated role of cranberry supplements; two found reduced cystitis86 |
| Brain | Fatigue | Exercise, as possible, has been shown to reduce fatigue in cancer patients generally | Standardised mean difference in fatigue −0.27 (95% CI −0.37 to −0.17) with exercise (MA, 2648 patients, 38 trials).87
Small RCTs of psycho-stimulants show mixed results in cancer related fatigue (SR).88-90 No evidence in whole brain radiotherapy specifically |
| Headache (32%)33 | Simple analgesia with dexamethasone 4 mg once daily if persistent | Recommendation based on routine palliative care prescribing84 | |
| Nausea and vomiting (10-16%) | Antiemetics (such as cyclizine) and dexamethasone if persistent | Recommendation based on routine palliative care prescribing84 | |
| Otitis externa (5%) | Otitis externa is often asymptomatic, steroid drops can be used if troublesome | No randomised evidence identified91 | |
| Skin | Sunburn-like erythema over treated area, peaks late in treatment and for about 10 days afterwards. Severity is dictated by dose | Daily washing, unperfumed emollient creams or soaps, and non-adhesive dressings. For more severe reactions (with skin breakdown) the treating department should be contacted for advice | Recommendation based on regional guidelines92 93 as no strong conclusions reached in two literature reviews94-96 |
| Hair loss (most patients undergoing palliative radiotherapy to brain)33 97 98 | Wig referral before treatment can be arranged, although timing this can be difficult in the palliative setting | Information and alternative approaches to hair loss are available through a variety of websites99 100 | |
| Oral cavity and oropharynx | Oral or pharyngeal mucositis (63%) with pain and thickened secretions.37 Dysphagia (85%)37 and risk of aspiration pneumonia. Side effects peak at the end of treatment to two weeks beyond, then resolve over a month |
Oral hygiene, regular mouth washes (such as saline, sodium bicarbonate), topical analgesia or gels, nebulised saline and analgesia (including NSAIDs and opiates in appropriate formulations). Concerns for swallowing safety and nutritional status should be discussed with the treating team | No strong conclusions were reached for the management of existing mucositis in multiple SRs.101-103 Recommendations reflect national and international guidelines.101
104
105
Enteral feeding was required in 12% of patients in one observational UK series68 |
RCT=randomised controlled trial, SR=systematic review, MA=meta-analysis, NSAID=non-steroidal anti-inflammatory drug.
Long term side effects are uncommon in palliative radiotherapy, and management of these is led by the treating team with multidisciplinary involvement when required.106
What new treatments can we expect?
The radiotherapy dose delivered to a tumour is usually limited by likely side effects in surrounding tissues. Advanced techniques that offer treatments more closely matched to the tumour shape, delivered with computed tomography on the treatment couch immediately before radiotherapy, can more accurately target much higher radiotherapy doses to small focal disease sites. These more targeted stereotactic treatments are variously referred to as stereotactic body radiotherapy, stereotactic ablative body radiotherapy, and stereotactic radio-surgery. Figure 3 provides an example of the difference in radiotherapy dose distribution between the simple conventional palliative approach and these more complex treatments.
There is now the potential for these higher dose stereotactic treatments to be used to improve survival and quality of life in patients with metastatic disease. This is being investigated for “oligo-metastatic disease” (in which a patient has only a limited number of metastatic deposits, and the disease has not become widespread).115 For such patients, high dose stereotactic treatments can be used to ablate all macroscopic sites of disease, potentially resulting in superior overall survival. However, even the existence of the oligo-metastatic state remains controversial.110 A further possible role for these treatments is in more advanced disease, where a higher radiotherapy dose to a symptomatic metastasis might provide better and more durable symptom control while continuing to deliver treatment in a minimum number of fractions with limited toxicity to surrounding tissues.108 109 111 There are no randomised data to support either of these approaches currently. Their expected value remains controversial, and trials are now under way for a range of indications.107 108 109 112 113 116
An additional area of palliative radiotherapy in which significant advances are now being made is in the use of radionuclides. These treatments deliver radioactive isotopes to tumour tissue, either through anatomically targeted delivery (such as via the hepatic artery in metastatic colorectal cancer) or through the use of radiolabelled molecules or monoclonal antibodies which are preferentially taken up by the tumour or its microenvironment.117 Historically, their use has been limited to some relatively rare tumours, but novel agents are increasingly demonstrating benefits in a range of more common conditions such as metastatic prostate cancer.114 With trials ongoing internationally, these treatments are likely to be used more extensively over the next few years.
A patient’s perspective.
My late husband received palliative radiotherapy multiple times during his treatment for multiple myeloma. Early in the course of his disease, radiotherapy for back pain and spinal cord compression ensured that he was able to continue the gardening he had always enjoyed. Receiving treatment was never uncomfortable for him, but, as his general condition deteriorated towards the end of his life, he spent more time in hospital and the benefits of radiotherapy became less clear. He had a mask made for one of his treatments which covered his head and neck: he didn’t find this particularly uncomfortable and he was excited to show it to everyone. He even let his grandchildren play with it once treatment was completed.
Education into practice.
Think about the last time you saw a patient with advanced cancer. How much did you consider localised disease as a possible cause of their symptoms?
Would you feel confident referring them to discuss palliative radiotherapy to help treat their symptoms?
What else might you do differently as a result of reading this article?
How patients were involved in the creation of this article.
A patient representative (a relative of a previously treated patient) had the opportunity to review and comment on the draft manuscript. She did not feel any changes to the manuscript were needed but did share her experiences of her husband’s radiotherapy treatment.
Contributors: KS and AH conceived of the manuscript. KS wrote the initial draft manuscript and is guarantor. All authors contributed to manuscript revisions; defining the structure and content of the final draft. A patient representative and her family reviewed the draft manuscript and provided a carer’s perspective of the radiotherapy process. RP provided a generalist perspective. All authors approved the final draft.
Funding: While undertaking this work KS was funded by the Medical Research Council on a Clinical Research Training Fellowship (MR/N021339/1).
Competing interests: We have read and understood BMJ policy on declaration of interests and have no relevant interests to declare.
Provenance and peer review: Commissioned, based on an idea from the author; externally peer reviewed.
References
- 1. Lutz ST, Jones J, Chow E. Role of radiation therapy in palliative care of the patient with cancer. J Clin Oncol 2014;32:2913-9. 10.1200/JCO.2014.55.1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Research UK. Local cancer statistics. 2016. www.cancerresearchuk.org/cancer-info/cancerstats/local-cancer-statistics.
- 3.Health and social care information centre. General practice trends in the UK to 2015. 2016. http://content.digital.nhs.uk/media/21726/General-Practice-Trends-in-the-UK-to-2015/pdf/General_Practice_Trends_in_the_UK_to_2015.pdf.
- 4. Samant RS, Fitzgibbon E, Meng J, Graham ID. Barriers to palliative radiotherapy referral: a Canadian perspective. Acta Oncol 2007;46:659-63. 10.1080/02841860600979005 [DOI] [PubMed] [Google Scholar]
- 5.Joiner MC, van der Kogel A, eds. Basic clinical radiobiology 4th ed. CRC Press; 2009. www.crcpress.com/Basic-Clinical-Radiobiology-Fourth-Edition/Joiner-van-der-Kogel/p/book/9780340929667
- 6.Department of Health. Radiotherapy services in England 2012. 2012. www.gov.uk/government/uploads/system/uploads/attachment_data/file/213151/Radiotherapy-Services-in-England-2012.pdf
- 7. Hoskin PJ, Forbes H, Ball C, et al. Radiotherapy Clinical Information Group Variations in radiotherapy delivery in England—evidence from the national radiotherapy dataset. Clin Oncol (R Coll Radiol) 2013;25:531-7. 10.1016/j.clon.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 8. Round CE, Williams MV, Mee T, et al. Radiotherapy demand and activity in England 2006-2020. Clin Oncol (R Coll Radiol) 2013;25:522-30. 10.1016/j.clon.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 9. Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005;104:1129-37. 10.1002/cncr.21324 [DOI] [PubMed] [Google Scholar]
- 10.Independent Cancer Taskforce. Achieving world-class cancer outcomes—A strategy for England 2015-2020. 2015. www.cancerresearchuk.org/sites/default/files/achieving_world-class_cancer_outcomes_-_a_strategy_for_england_2015-2020.pdf
- 11. Huang J, Zhou S, Groome P, Tyldesley S, Zhang-Solomans J, Mackillop WJ. Factors affecting the use of palliative radiotherapy in Ontario. J Clin Oncol 2001;19:137-44. 10.1200/JCO.2001.19.1.137 [DOI] [PubMed] [Google Scholar]
- 12. Murphy JD, Nelson LM, Chang DT, Mell LK, Le QT. Patterns of care in palliative radiotherapy: a population-based study. J Oncol Pract 2013;9:e220-7. 10.1200/JOP.2012.000835 [DOI] [PubMed] [Google Scholar]
- 13. Halkett GK, Jiwa M, Meng X, Leong E. Referring advanced cancer patients for palliative treatment: a national structured vignette survey of Australian GPs. Fam Pract 2014;31:60-70. 10.1093/fampra/cmt068 [DOI] [PubMed] [Google Scholar]
- 14. Lutz S, Spence C, Chow E, Janjan N, Connor S. Survey on use of palliative radiotherapy in hospice care. J Clin Oncol 2004;22:3581-6. 10.1200/JCO.2004.11.151 [DOI] [PubMed] [Google Scholar]
- 15. Samant RS, Fitzgibbon E, Meng J, Graham ID. Family physicians’ perspectives regarding palliative radiotherapy. Radiother Oncol 2006;78:101-6. 10.1016/j.radonc.2005.11.008 [DOI] [PubMed] [Google Scholar]
- 16. Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24:112-24. 10.1016/j.clon.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 17. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Cochrane Database Syst Rev 2004;(2):CD004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol 1999;52:101-9. 10.1016/S0167-8140(99)00110-3 [DOI] [PubMed] [Google Scholar]
- 19. Hoskin P, Sundar S, Reczko K, et al. A multicenter randomized trial of ibandronate compared with single-dose radiotherapy for localized metastatic bone pain in prostate cancer. J Natl Cancer Inst 2015;107:djv197. 10.1093/jnci/djv197 [DOI] [PubMed] [Google Scholar]
- 20. Stevens R, Macbeth F, Toy E, Coles B, Lester JF. Palliative radiotherapy regimens for patients with thoracic symptoms from non-small cell lung cancer. Cochrane Database Syst Rev 2015;1:CD002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol 2008;26:4001-11. 10.1200/JCO.2007.15.3312 [DOI] [PubMed] [Google Scholar]
- 22. Sundstrøm S, Bremnes R, Aasebø U, et al. Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: a national phase III trial. J Clin Oncol 2004;22:801-10. 10.1200/JCO.2004.06.123 [DOI] [PubMed] [Google Scholar]
- 23. Macbeth FR, Bolger JJ, Hopwood P, et al. Medical Research Council Lung Cancer Working Party Randomized trial of palliative two-fraction versus more intensive 13-fraction radiotherapy for patients with inoperable non-small cell lung cancer and good performance status. Clin Oncol (R Coll Radiol) 1996;8:167-75. 10.1016/S0936-6555(96)80041-0 [DOI] [PubMed] [Google Scholar]
- 24. Kramer GWPM, Wanders SL, Noordijk EM, et al. Results of the Dutch national study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J Clin Oncol 2005;23:2962-70. 10.1200/JCO.2005.01.685 [DOI] [PubMed] [Google Scholar]
- 25. Kassam Z, Wong RKS, Ringash J, et al. A phase I/II study to evaluate the toxicity and efficacy of accelerated fractionation radiotherapy for the palliation of dysphagia from carcinoma of the oesophagus. Clin Oncol (R Coll Radiol) 2008;20:53-60. 10.1016/j.clon.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 26. Javed A, Pal S, Dash NR, et al. Palliative stenting with or without radiotherapy for inoperable esophageal carcinoma: a randomized trial. J Gastrointest Cancer 2012;43:63-9. 10.1007/s12029-010-9206-4 [DOI] [PubMed] [Google Scholar]
- 27. Rosenblatt E, Jones G, Sur RK, et al. Adding external beam to intra-luminal brachytherapy improves palliation in obstructive squamous cell oesophageal cancer: a prospective multi-centre randomized trial of the International Atomic Energy Agency. Radiother Oncol 2010;97:488-94. 10.1016/j.radonc.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 28. Tey J, Soon YY, Koh WY, et al. Palliative radiotherapy for gastric cancer: a systematic review and meta-analysis. Oncotarget 2017;8:25797-805. 10.18632/oncotarget.15554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rades D, Šegedin B, Conde-Moreno AJ, et al. Radiotherapy With 4 Gy × 5 Versus 3 Gy × 10 for metastatic epidural spinal cord compression: final results of the SCORE-2 trial (ARO 2009/01). J Clin Oncol 2016;34:597-602. 10.1200/JCO.2015.64.0862 [DOI] [PubMed] [Google Scholar]
- 30. Maranzano E, Trippa F, Casale M, et al. 8Gy single-dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicentre Italian trial. Radiother Oncol 2009;93:174-9. 10.1016/j.radonc.2009.05.012 [DOI] [PubMed] [Google Scholar]
- 31. Maranzano E, Bellavita R, Rossi R, et al. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. J Clin Oncol 2005;23:3358-65. 10.1200/JCO.2005.08.193 [DOI] [PubMed] [Google Scholar]
- 32. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. 10.1016/S0140-6736(05)66954-1 [DOI] [PubMed] [Google Scholar]
- 33. Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016;388:2004-14. 10.1016/S0140-6736(16)30825-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsao MN, Lloyd N, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev 2012;1:CD003869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. 10.1016/S1470-2045(09)70263-3 [DOI] [PubMed] [Google Scholar]
- 36. Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010;96:45-68. 10.1007/s11060-009-0073-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Porceddu SV, Rosser B, Burmeister BH, et al. Hypofractionated radiotherapy for the palliation of advanced head and neck cancer in patients unsuitable for curative treatment—“Hypo Trial”. Radiother Oncol 2007;85:456-62. 10.1016/j.radonc.2007.10.020 [DOI] [PubMed] [Google Scholar]
- 38. Corry J, Peters LJ, Costa ID, et al. The ‘QUAD SHOT’—a phase II study of palliative radiotherapy for incurable head and neck cancer. Radiother Oncol 2005;77:137-42. 10.1016/j.radonc.2005.10.008 [DOI] [PubMed] [Google Scholar]
- 39. Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys 2000;47:379-88. 10.1016/S0360-3016(00)00430-2 [DOI] [PubMed] [Google Scholar]
- 40. Cameron MG, Kersten C, Vistad I, et al. Palliative pelvic radiotherapy for symptomatic rectal cancer—a prospective multicenter study. Acta Oncol 2016;55:1400-7. 10.1080/0284186X.2016.1191666 [DOI] [PubMed] [Google Scholar]
- 41. van Lonkhuijzen L, Thomas G. Palliative radiotherapy for cervical carcinoma, a systematic review. Radiother Oncol 2011;98:287-91. 10.1016/j.radonc.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 42. Cameron MG, Kersten C, Guren MG, Fosså SD, Vistad I. Palliative pelvic radiotherapy of symptomatic incurable prostate cancer—a systematic review. Radiother Oncol 2014;110:55-60. 10.1016/j.radonc.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 43. Williams M, Woolf D, Dickson J, Hughes R, Maher J, Mount Vernon Cancer Centre Routine clinical data predict survival after palliative radiotherapy: an opportunity to improve end of life care. Clin Oncol (R Coll Radiol) 2013;25:668-73. 10.1016/j.clon.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 44. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s-9s. 10.1158/1078-0432.CCR-06-0931 [DOI] [PubMed] [Google Scholar]
- 45. Spencer K, Morris E, Dugdale E, et al. 30 day mortality in adult palliative radiotherapy—A retrospective population based study of 14,972 treatment episodes. Radiother Oncol 2015;115:264-71. 10.1016/j.radonc.2015.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roos DE, Turner SL, O’Brien PC, et al. Trans-Tasman Radiation Oncology Group, TROG 96.05 Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05). Radiother Oncol 2005;75:54-63. 10.1016/j.radonc.2004.09.017 [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization. WHO’s cancer pain ladder for adults. 2018. www.who.int/cancer/palliative/painladder/en/
- 48. Kane CM, Hoskin P, Bennett MI. Cancer induced bone pain. BMJ 2015;350:h315. 10.1136/bmj.h315 [DOI] [PubMed] [Google Scholar]
- 49. Koswig S, Budach V. [Remineralization and pain relief in bone metastases after after different radiotherapy fractions (10 times 3 Gy vs. 1 time 8 Gy). A prospective study] Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. 1999;175:500-8 10.1007/s000660050061. [DOI] [PubMed] [Google Scholar]
- 50. Townsend PW, Smalley SR, Cozad SC, Rosenthal HG, Hassanein RE. Role of postoperative radiation therapy after stabilization of fractures caused by metastatic disease. Int J Radiat Oncol Biol Phys 1995;31:43-9. 10.1016/0360-3016(94)E0310-G [DOI] [PubMed] [Google Scholar]
- 51. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res 1989;(249):256-64. [PubMed] [Google Scholar]
- 52. Chow E, van der Linden YM, Roos D, et al. Single versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol 2014;15:164-71. 10.1016/S1470-2045(13)70556-4 [DOI] [PubMed] [Google Scholar]
- 53. Walters S, Maringe C, Coleman MP, et al. ICBP Module 1 Working Group Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax 2013;68:551-64. 10.1136/thoraxjnl-2012-202297 [DOI] [PubMed] [Google Scholar]
- 54.Cancer Research UK. Lung cancer incidence statistics. 2015. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/incidence.
- 55. Dai Y, Li C, Xie Y, et al. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev 2014;(10):CD005048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Institute for Health and Care Excellence. Metastatic spinal cord compression in adults (quality standard QS56). www.nice.org.uk/guidance/qs56
- 57. George R, Jeba J, Ramkumar G, Chacko AG, Tharyan P. Interventions for the treatment of metastatic extradural spinal cord compression in adults. Cochrane Database Syst Rev 2015;(9):CD006716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186-91. 10.1097/01.brs.0000180401.06919.a5 [DOI] [PubMed] [Google Scholar]
- 59. Rades D, Douglas S, Veninga T, et al. Validation and simplification of a score predicting survival in patients irradiated for metastatic spinal cord compression. Cancer 2010;116:3670-3. 10.1002/cncr.25223 [DOI] [PubMed] [Google Scholar]
- 60. Maranzano E, Bellavita R, Rossi R, et al. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. J Clin Oncol 2005;23:3358-65. 10.1200/JCO.2005.08.193 [DOI] [PubMed] [Google Scholar]
- 61. Rades D, Douglas S, Huttenlocher S, et al. Validation of a score predicting post-treatment ambulatory status after radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys 2011;79:1503-6. 10.1016/j.ijrobp.2010.01.024 [DOI] [PubMed] [Google Scholar]
- 62.Royal College of Radiologists. Radiotherapy dose fractionation. 2nd ed. 2016. www.rcr.ac.uk/system/files/publication/field_publication_files/bfco163_dose_fractionation_2nd_ed_march2017.pdf
- 63. Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys 2010;76(Suppl):S42-9. 10.1016/j.ijrobp.2009.04.095 [DOI] [PubMed] [Google Scholar]
- 64. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. 10.1016/S0360-3016(96)00619-0 [DOI] [PubMed] [Google Scholar]
- 65. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008;70:510-4. 10.1016/j.ijrobp.2007.06.074 [DOI] [PubMed] [Google Scholar]
- 66. Kalkanis SN, Kondziolka D, Gaspar LE, et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010;96:33-43. 10.1007/s11060-009-0061-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Patil CG, Pricola K, Sarmiento JM, Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst Rev 2012;(9):CD006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kancherla KN, Oksuz DC, Prestwich RJD, et al. The role of split-course hypofractionated palliative radiotherapy in head and neck cancer. Clin Oncol (R Coll Radiol) 2011;23:141-8. 10.1016/j.clon.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 69. Yan J, Milosevic M, Fyles A, Manchul L, Kelly V, Levin W. A hypofractionated radiotherapy regimen (0-7-21) for advanced gynaecological cancer patients. Clin Oncol (R Coll Radiol) 2011;23:476-81. 10.1016/j.clon.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 70. Barnes EA, Breen D, Culleton S, et al. Palliative radiotherapy for non-melanoma skin cancer. Clin Oncol (R Coll Radiol) 2010;22:844-9. 10.1016/j.clon.2010.07.014 [DOI] [PubMed] [Google Scholar]
- 71. Wong CYB, Helm MA, Helm TN, Zeitouni N. Patterns of skin metastases: a review of 25 years’ experience at a single cancer center. Int J Dermatol 2014;53:56-60. 10.1111/j.1365-4632.2012.05635.x [DOI] [PubMed] [Google Scholar]
- 72. Spratt DE, Gordon Spratt EA, Wu S, et al. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: a meta-analysis. J Clin Oncol 2014;32:3144-55. 10.1200/JCO.2014.55.4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Adderley UJ, Holt IG. Topical agents and dressings for fungating wounds. Cochrane Database Syst Rev 2014;1:CD003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Radbruch L, Strasser F, Elsner F, et al. Research Steering Committee of the European Association for Palliative Care (EAPC) Fatigue in palliative care patients—an EAPC approach. Palliat Med 2008;22:13-32. 10.1177/0269216307085183 [DOI] [PubMed] [Google Scholar]
- 75. Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr 2004;2004:40-50. 10.1093/jncimonographs/lgh027 [DOI] [PubMed] [Google Scholar]
- 76. Chow E, Meyer RM, Ding K, et al. Dexamethasone in the prophylaxis of radiation-induced pain flare after palliative radiotherapy for bone metastases: a double-blind, randomised placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:1463-72. 10.1016/S1470-2045(15)00199-0 [DOI] [PubMed] [Google Scholar]
- 77. Loblaw DA, Wu JS, Kirkbride P, et al. Pain flare in patients with bone metastases after palliative radiotherapy--a nested randomized control trial. Support Care Cancer 2007;15:451-5. 10.1007/s00520-006-0166-y [DOI] [PubMed] [Google Scholar]
- 78. Molassiotis A, Bailey C, Caress A, Tan J-Y. Interventions for cough in cancer. Cochrane Database Syst Rev 2015;5:CD007881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Baker S, Fairchild A. Radiation-induced esophagitis in lung cancer. Lung Cancer (Auckl) 2016;7:119-27. 10.2147/LCTT.S96443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bone Pain Trial Working Party 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiother Oncol 1999;52:111-21. 10.1016/S0167-8140(99)00097-3 [DOI] [PubMed] [Google Scholar]
- 81. Salvo N, Doble B, Khan L, et al. Prophylaxis of radiation-induced nausea and vomiting using 5-hydroxytryptamine-3 serotonin receptor antagonists: a systematic review of randomized trials. Int J Radiat Oncol Biol Phys 2012;82:408-17. 10.1016/j.ijrobp.2010.08.060 [DOI] [PubMed] [Google Scholar]
- 82. Feyer PC, Maranzano E, Molassiotis A, Roila F, Clark-Snow RA, Jordan K, MASCC/ESMO Radiotherapy-induced nausea and vomiting (RINV): MASCC/ESMO guideline for antiemetics in radiotherapy: update 2009. Support Care Cancer 2011;19(Suppl 1):S5-14. 10.1007/s00520-010-0950-6 [DOI] [PubMed] [Google Scholar]
- 83.Northern Ireland Cancer Network. NICan acute oncolgy clinical guidelines. 2015. www.cancerni.net/sites/default/files/documents/NICaN%20Acute%20Oncology%20Clinical%20Guidelines_v1%20.9.pdf.
- 84.National Institute for Health and Care Excellence, British National Formulary. Medicines guidance: Prescribing in palliative care. https://bnf.nice.org.uk/guidance/prescribing-in-palliative-care.html
- 85.Leeds Teaching Hospitals NHS Trust. Radiotherapy to the bladder. 2017. http://flipbooks.leedsth.nhs.uk/LN000002.pdf
- 86. Hamilton K, Bennett NC, Purdie G, Herst PM. Standardized cranberry capsules for radiation cystitis in prostate cancer patients in New Zealand: a randomized double blinded, placebo controlled pilot study. Support Care Cancer 2015;23:95-102. 10.1007/s00520-014-2335-8 [DOI] [PubMed] [Google Scholar]
- 87. Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 2012;11:CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mücke M, Cuhls H, Peuckmann-Post V, Minton O, Stone P, Radbruch L, Mochamat Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev 2015;1:CD006788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol 2010;28:3673-9. 10.1200/JCO.2010.28.1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kerr CW, Drake J, Milch RA, et al. Effects of methylphenidate on fatigue and depression: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage 2012;43:68-77. 10.1016/j.jpainsymman.2011.03.026 [DOI] [PubMed] [Google Scholar]
- 91. Sourati A, Ameri A, Malekzadeh M. Ear toxicity. In: Acute side effects of radiation therapy. Springer, 2017: 47-51 10.1007/978-3-319-55950-6_5. [DOI] [Google Scholar]
- 92.Princess Royal Radiotherapy Review Team. Managing radiotherapy induced skin reactions. 2011. www.sor.org/system/files/news_story/201204/ltht-managingradiotherapyinducedskinreactions-oct2011.pdf
- 93.NHS Quality Improvement Scotland. Skincare of patients receiving radiotherapy. 2010. www.scan.scot.nhs.uk/Documents/SKINRADIOREV_BPS_MAR10%202.pdf.
- 94. Kumar S, Juresic E, Barton M, Shafiq J. Management of skin toxicity during radiation therapy: a review of the evidence. J Med Imaging Radiat Oncol 2010;54:264-79. 10.1111/j.1754-9485.2010.02170.x [DOI] [PubMed] [Google Scholar]
- 95. McQuestion M. Evidence-based skin care management in radiation therapy: clinical update. Semin Oncol Nurs 2011;27:e1-17. 10.1016/j.soncn.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 96. Kedge EM. A systematic review to investigate the effectiveness and acceptability of interventions for moist desquamation in radiotherapy patients. Radiography 2009;15:247-57 10.1016/j.radi.2008.08.002. [DOI] [Google Scholar]
- 97. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. 10.1200/JCO.2010.30.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. 10.1016/S0140-6736(04)16250-8 [DOI] [PubMed] [Google Scholar]
- 99.NHS Choices. Cancer and hair loss. 2017. https://www.nhs.uk/Livewell/cancer/Pages/Cancerandhairloss.aspx.
- 100.Macmillan Cancer Support. Changes to appearance and body image: Dealing with hair loss. www.macmillan.org.uk/information-and-support/coping/changes-to-appearance-and-body-image/dealing-with-hair-loss.
- 101. Lalla RV, Bowen J, Barasch A, et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014;120:1453-61. 10.1002/cncr.28592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Clarkson JE, Worthington HV, Furness S, McCabe M, Khalid T, Meyer S. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2010;1:CD001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Worthington HV, Clarkson JE, Eden OB. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2006;(2):CD000978. [DOI] [PubMed] [Google Scholar]
- 104.UKOMiC. Mouth care guidance and support in cancer and palliative care 2nd ed. 2015 www.ukomic.co.uk/pdf/UK_OM_Guidelines.pdf
- 105. Peterson DE, Bensadoun R-J, Roila F, ESMO Guidelines Working Group Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann Oncol 2011;22(Suppl 6):vi78-84. 10.1093/annonc/mdr391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Andreyev HJN, Davidson SE, Gillespie C, Allum WH, Swarbrick E, British Society of Gastroenterology. Association of Colo-Proctology of Great Britain and Ireland. Association of Upper Gastrointestinal Surgeons. Faculty of Clinical Oncology Section of the Royal College of Radiologists Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut 2012;61:179-92. 10.1136/gutjnl-2011-300563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Palma DA, Haasbeek CJA, Rodrigues GB, et al. Stereotactic ablative radiotherapy for comprehensive treatment of oligometastatic tumors (SABR-COMET): study protocol for a randomized phase II trial. BMC Cancer 2012;12:305. 10.1186/1471-2407-12-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. van der Velden JM, Verkooijen HM, Seravalli E, et al. Comparing conVEntional RadioTherapy with stereotactIC body radiotherapy in patients with spinAL metastases: study protocol for an randomized controlled trial following the cohort multiple randomized controlled trial design. BMC Cancer 2016;16:909. 10.1186/s12885-016-2947-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Braam P, Lambin P, Bussink J. Stereotactic versus conventional radiotherapy for pain reduction and quality of life in spinal metastases: study protocol for a randomized controlled trial. Trials 2016;17:61. 10.1186/s13063-016-1178-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Palma DA, Salama JK, Lo SS, et al. The oligometastatic state - separating truth from wishful thinking. Nat Rev Clin Oncol 2014;11:549-57. 10.1038/nrclinonc.2014.96 [DOI] [PubMed] [Google Scholar]
- 111.ClinicalTrials.gov. Study comparing stereotactic body radiotherapy vs conventional palliative radiotherapy (CRT) for spinal metastases. https://clinicaltrials.gov/ct2/show/NCT02512965
- 112.ClinicalTrials.gov. Conventional care versus radioablation (stereotactic body radiotherapy) for extracranial oligometastases. https://clinicaltrials.gov/ct2/show/NCT02759783
- 113.ClinicalTrials.gov. Stereotactic ablative radiotherapy for oligometastatic non-small cell lung cancer. https://clinicaltrials.gov/ct2/show/NCT02417662 [DOI] [PMC free article] [PubMed]
- 114. Parker C, Nilsson S, Heinrich D, et al. ALSYMPCA Investigators Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213-23. 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 115. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. 10.1200/JCO.1995.13.1.8 [DOI] [PubMed] [Google Scholar]
- 116.STAMPEDE Systemic Therapy in Advancing or Metastatic Prostate cancer: Evaluation of Drug Efficacy. www.stampedetrial.org/
- 117.NCRI. CTRad: identifying opportunities to promote progress in molecular radiotherapy research in the UK. 2016. www.ncri.org.uk/wp-content/uploads/2016/06/CTRad-promoting-research-in-MRT-UK-June-2016.pdf


