Abstract
Obesity enhances the risk of developing myelodysplastic syndromes. However, the effect of obesity on survival is unclear. Obese people present with monocytosis due to inflammatory signals emanating from obese adipose tissue. We hypothesized that obesity-induced myelopoiesis would promote the transition of myelodysplastic syndrome to acute myeloid leukemia and accelerate mortality in obesity. Obese Ob/Ob mice or their lean littermate controls received a bone marrow transplant from NUP98-HOXD13 transgenic mice, a model of myelodysplastic syndrome. The metabolic parameters of the mice were examined throughout the course of the study, as were blood leukocytes. Myeloid cells were analyzed in the bone, spleen, liver and adipose tissue by flow cytometry halfway through the disease progression and at the endpoint. Survival curves were also calculated. Contrary to our hypothesis, transplantation of NUP98-HOXD13 bone marrow into obese recipient mice significantly increased survival time compared with lean recipient controls. While monocyte skewing was exacerbated in obese mice receiving NUP98-HOXD13 bone marrow, transformation to acute myeloid leukemia was not enhanced. Increased survival of obese mice was associated with a preservation of fat mass as well as increased myeloid cell deposition within the adipose tissue, and a concomitant reduction in detrimental myeloid cell accumulation within other organs. The study herein revealed that obesity increases survival in animals with myelodysplastic syndrome. This may be due to the greater fat mass of Ob/Ob mice, which acts as a sink for myeloid cells, preventing their accumulation in other key organs, such as the liver.
Introduction
Obesity represents a major health risk and is independently associated with the development of a cluster of disorders commonly referred to as metabolic diseases, including type 2 diabetes mellitus (T2DM), cardiovascular disease, stroke, neurodegeneration, and liver diseases. Furthermore, causal associations between body mass index (BMI) and many cancers are becoming increasingly apparent.1–4
In the last decade, obesity has been associated not only with most forms of tumor-based cancer, but also with hematological malignancies.5 Obese patients have an increased risk of leukemia,6 and, in children, excess fat mass is linked both to enhanced incidence and lower overall survival for leukemia.7 In addition, obesity has been increasingly associated with an enhanced risk for the incidence of myelodysplastic syndromes (MDS).8,9
MDS encompass a group of bone marrow disorders characterized by defective hematopoiesis.10 The risk of progressing to leukemia is high in individuals with MDS, with approximately 30% proceeding to develop acute myeloid leukemia (AML), an aggressive hematopoietic malignancy with a low 5-year survival prognosis. While the environmental factors for developing MDS and its progression to AML remain poorly understood, recent epidemiological studies have revealed obesity to be associated with a small but significant increase in the risk of developing AML.11,12 While the exact biological mechanisms underpinning the increased leukemia risk in obesity are likely to be complex and multifactorial, the presence of chronic low-grade inflammation induced by obesity is thought to contribute to the increased cancer risk.13 Chronic low-grade inflammation is broadly characterized by alterations in circulating immuno-modulatory cytokines and leukocytes within the adipose tissue as well as the activation of stress pathways in metabolically important tissues.14 We and others have recently reported that obesity causes prominent monocytosis due to enhanced myelopoiesis and altered hematopoiesis driven by interleukin-1β (IL-1β).15,16 Interestingly, IL-1β, along with other myeloid promoting cytokines (i.e., IL-3, granulocyte-macrophage colony-stimulating factor [GM-CSF]) has also been shown to promote the progression of AML.17 Thus, we sought to investigate the influence of obesity on the transition of MDS to AML and survival. We hypothesized that obesity-induced inflammation would promote the progression of MDS to AML through heightened myelopoiesis and hematopoietic stress.
Methods
Detailed methods are available in Online Supplementary Methods
10-week-old male Ob/Ob mice, along with littermate lean controls, purchased from the Jackson Laboratory (USA), underwent a bone marrow transplant (BMT), receiving marrow from 6/8-week-old male wild-type (WT) C57bl/6 mice (Jackson Laboratory) or male NHD13 mice sourced from colonies maintained within the Alfred Medical Research Education Precinct (AMREP) Animal Centre. All animal experiments were approved by the AMREP Animal Ethics Committee and conducted in accordance with the National Health and Medical Research Council of Australia Guidelines for Animal Experimentation (Ethics E/1444/2014/B).
Results
Obese mice exhibit a hematopoietic phenotype after seven months of myelodysplastic syndrome
To determine the impact of obesity on the development of MDS, we performed bone marrow (BM) transplantation studies using NHD13 transgenic donor mice or WT littermate BM as a control into lean (Ob/+) and obese (Ob/Ob) recipient mice (Figure 1A). We considered the diet-induced obesity (DIO) model, but explicitly chose to conduct this experiment in Ob/Ob mice for the following reasons: i) Ob/Ob mice are guaranteed to maintain and gain weight following a BM transplant, which is in contrast to our prior experience whereby WT mice had limited weight gain post-BMT on a high fat diet, ii) the loss of leptin means these mice will feed consistently, and removes a variable of changes in feed patterns as the myelodysplasia progresses, iii) the diets are matched, ruling out effects of altered nutritional composition of standard chow and high fat diets, and iv) we have previously shown that Ob/Ob and DIO mice display enhanced myelopoiesis through the same mechanism (i.e., increased IL-1β production emanating from the adipose tissue).15 We acknowledge that leptin plays a role in hematopoiesis, but this is mainly restricted to the lymphoid system,18 which is suppressed in MDS and not likely to be a confounding factor in our experiment. Moreover, we have shown that supplementing Ob/Ob mice with leptin, while causing weight reduction, had no impact on myelopoiesis.15
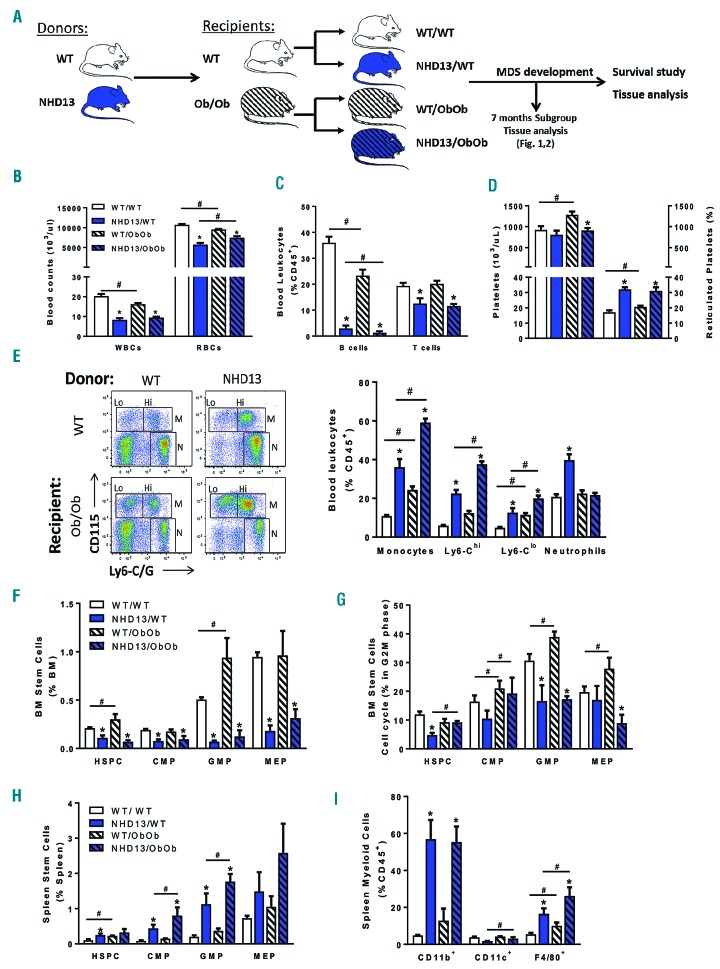
Figure 1.
Ob/Ob mice display a similar hematopoietic phenotype to wild-type mice when challenged with myelodysplastic syndrome despite pre-existing monocytosis. (A) Experimental overview: Ob/Ob mice and WT littermate controls transplanted with either WT or NHD13 bone marrow (BM) were followed until the development of MDS symptoms required euthanasia. Seven months after the bone marrow transplant, mice were bled for analysis (B–E) and a subset was culled for tissue analysis (F–I). (B) Blood counts obtained by CBC, (C) flow cytometry analysis of lymphocytes and (D) CBC platelet counts and flow cytometry analysis of reticulated platelets (% platelets). (E) myeloid cells analysis by flow cytometry on lysed blood. (F) Flow cytometry analysis of BM cells including long term stem cells and myeloid progenitors, and (G) cell cycle analysis (DAPI). (H–I) Flow cytometry analysis of spleen immune cells including (H) long term stem cells and myeloid progenitors and (I) myeloid populations. (A–E); n=12–16; (F–I); n=3. All data expressed as mean ± SEM. *P<0.05, for obesity effect; #P<0.05, for MDS effect as analyzed by 2-way ANOVA. WT: wild-type; WBC: white blood cell; RBC: red blood cell; HSPC: hematopoietic stem and progenitor cell; CMP: common myeloid progenitor; GMP: granulocyte-macrophage progenitor; MEP: megakaryocyte-erythroid progenitor.
The MDS model we chose to employ was the NHD13 transgenic mouse, which overexpresses a NUX98-HOXD13 fusion protein that has been associated with human MDS.19 These mice have an MDS phenotype that can develop into AML with a penetrance of ~30% within 14 months.19 Accordingly, we initially assessed the impact of obesity on hematopoiesis, and specifically myelopoiesis, seven months post-transplantation with BM from NHD13 transgenic donor mice, a time point at which all mice remained alive (i.e., the predicted halfway survival point of the model).
Total blood cell counts revealed the expected MDS features of decreased white blood cells (WBC) and red blood cells (RBC), effects that were observed in both lean and obese MDS mice (Figure 1B). As expected, the decrease in WBC counts was primarily due to lymphopenia (Figure 1C). Platelets were also significantly decreased in both lean and obese MDS mice. These changes occurred despite obese mice presenting with increased platelet numbers in the healthy state (Figure 1D). Both lean and obese MDS mice failed to compensate this thrombocytopenia with enhanced platelet production, as suggested by the increased percentage of newly formed reticulated platelets (Figure 1D). Next, we analyzed numbers of circulating myeloid cells to determine whether obesity influenced myelopoiesis. Consistent with our previous findings, obesity induced a significant increase in the proportion of monocytes when compared with lean controls, and this effect was strongly amplified in the presence of MDS (Figure 1E). Both Ly6-Chi and Ly6-Clo subsets contributed to this increase in the proportion of monocytes (Figure 1E). Interestingly, while the proportion of neutrophils was elevated in the lean NHD13/WT mice, this effect was absent in obese MDS mice (Figure 1E). Consistent with the known phenotype of MDS mice, defective hematopoiesis in BM progenitor cells was evident, with a decrease in the abundance and proliferation of all of the hematopoietic stem and progenitor cells occurring in both lean and obese MDS mice (Figure 1F,G). The obesity-induced increase in circulating monocytes could be explained by extramedullary myelopoiesis in the MDS mice, made apparent by increased hematopoietic stem and progenitor cells (HSPCs, also referred to as LSKs), common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs), and megakaryocyte-erythroid progenitors (MEPs) (Figure 1H). Consequently, a significant accumulation of CD11b+ myeloid cells and F4/80+ macrophages was also observed in the spleen (Figure 1I). Of note, the MDS pathology did not appear to significantly affect the metabolic function of lean or obese mice relative to their littermate controls (Online Supplementary Figure S1). Taken together, these data, at the half-way point in the pathogenesis of this model, demonstrated an enhanced monocyte response in obese MDS mice compared with lean MDS mice. Further, failing BM hematopoiesis appeared to promote a shift of this process to the spleen in the mice suffering from MDS.
Obese mice display prolonged survival when challenged with myelodysplastic syndrome despite increased myelopoiesis
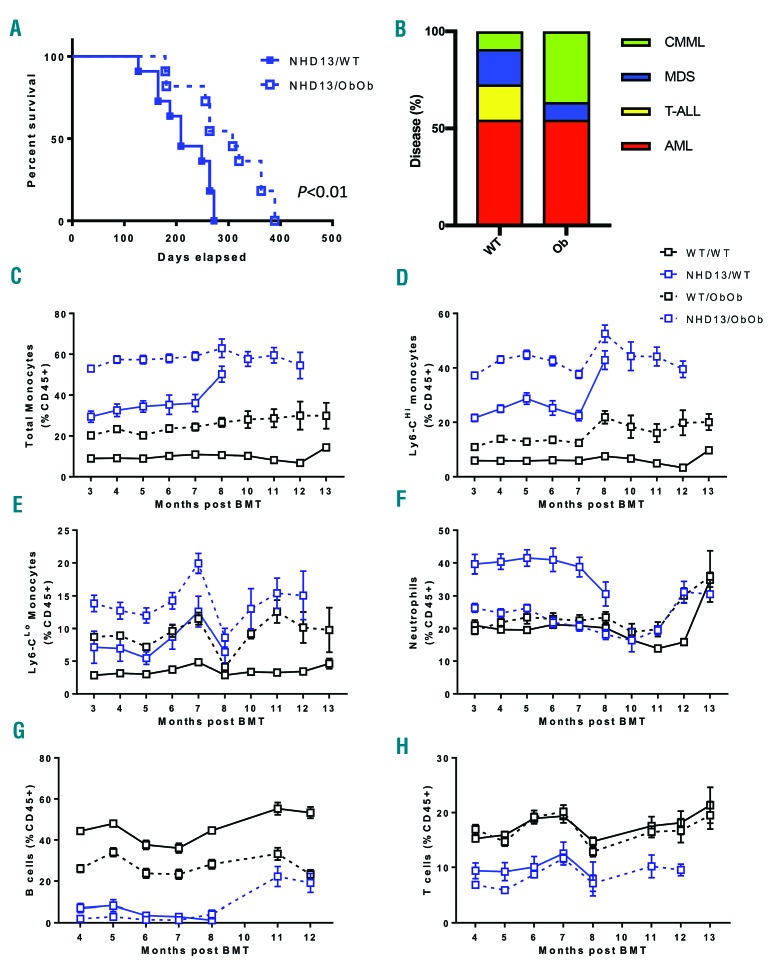
As the obese mice presented with increased myelopoiesis with increased splenic macrophages in the setting of MDS, we hypothesized that these mice would have reduced survival compared with the lean MDS mice. However, to our surprise, obese MDS mice had a significantly prolonged survival compared with their lean MDS counterparts (Figure 2A). Obesity appeared to influence the cause of death, by promoting the development of a chronic myelomonocytic leukemia (CMML) at the expense of T-cell acute lymphoblastic leukemia (T-ALL) and cytopenias (Figure 2B). Overall, the obese MDS mice lived an average of 100 days longer than the lean mice, a survival advantage that occurred despite an increase in the proportion of circulating monocytes, driven primarily by the inflammatory Ly6-Chi subset, this being consistently higher compared with those in the lean MDS counterparts (Figure 2C–E). Interestingly, while the proportion of neutrophils were higher in the lean NHD13 transplanted mice, neutrophils in obese MDS mice were comparable to mice without MDS (Figure 2F). It is unlikely that differences in circulating T cells or B cells could account for the improvement in longevity, as obese MDS mice were not protected from a sustained lymphopenia induced by the NHD13 BM compared with lean mice (Figure 2G,H). A similar situation was observed when looking at the total numbers of these circulating cells, with obese MDS mice having a trend to higher blood monocytes, driven by the Ly6-Chi subset (Online Supplementary Figure S2A–D). We also examined the abundance of ckit+ cells in the blood, which were unchanged, suggesting that refractory anemia with excess blasts was not occurring (Online Supplementary Figure S2E,F).
Figure 2.
Ob/Ob mice display prolonged survival when challenged with myelodysplastic syndrome despite preexisting monocytosis. Ob/Ob mice and WT littermate controls transplanted with either WT or NHD13 BM were followed for (A) Kaplan-Meier survival curve. (B) Proportion of disease contributing to death. Circulating myeloid cell populations, including (C) total monocytes, (D) Ly-6Chi monocytes, (E) Ly-6Clo monocytes and (F) neutrophils analyzed by flow cytometry on lysed blood. Circulating lymphoid populations, including (G) B cells and (H) T cells analyzed by flow cytometry on lysed blood. (A,B); n=16; (C–H); n=12–16. All data expressed as mean ± SEM. WT: wild-type; MDS: myelodysplastic syndrome; T-ALL: T-cell acute lymphoblastic leukemia; CMML: chronic myelomonocytic leukemia; AML: acute myeloid leukemia; BMT: bone marrow transplant.
Endpoint blood and spleen characteristics
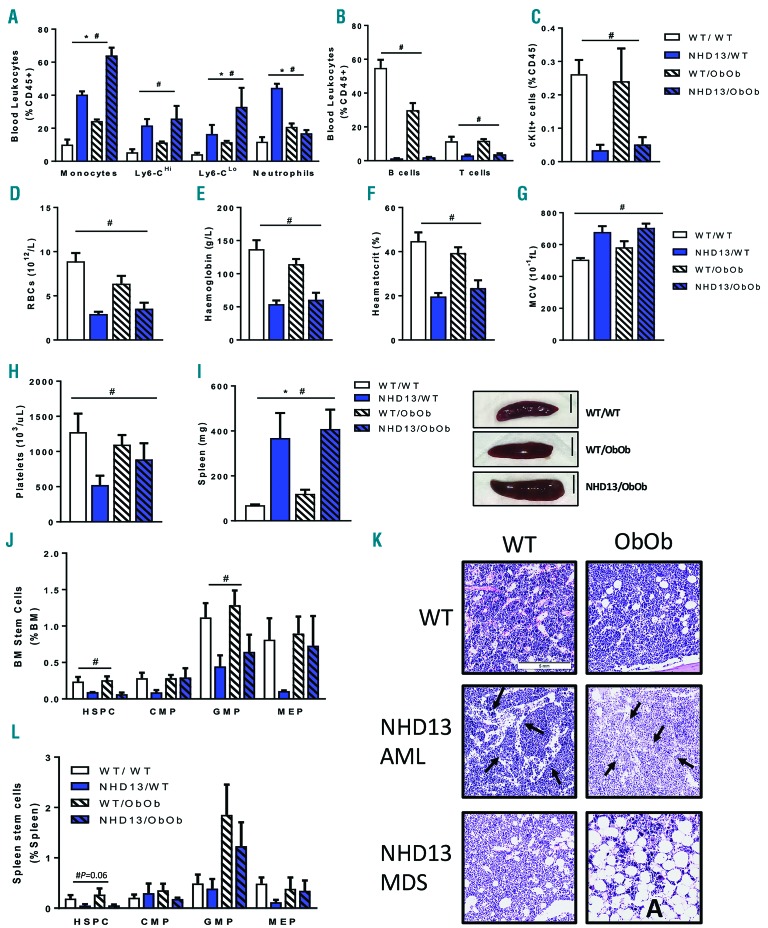
To explore why prolonged survival was observed in obese MDS mice relative to their lean counterparts, we first analyzed the key features of end-stage disease for MDS. Once the mice began to show the characteristic signs of the terminal stage of the disease, euthanasia was performed alongside healthy lean and obese controls for comparative analysis. Consistent with the data obtained throughout the course of the disease, monocytosis was observed in lean MDS mice, and this effect was exacerbated in the obese MDS mice (Figure 3A). The inefficient hematopoiesis which is characteristic of MDS occurred at similar levels in lean and obese MDS mice, both of which presented with severe lymphopenia, reduced circulating progenitor cells, and anemia (Figure 3B–F). Anemia was accompanied by macrocytosis, as demonstrated by the significantly increased mean corpuscular volume, indicative of RBC volume (Figure 3G). Platelet counts were also reduced with MDS, irrespective of obesity status (Figure 3H). Finally, prominent splenomegaly occurred in MDS mice, consistent with the monocytosis and aberrant extramedullary myelopoiesis observed at the seven-month time point (Figure 3I,J; Figure 1E,H). Consistent with MDS progression, and irrespective of body weight, we observed reduced stem cells in the BM with fewer HSPCs and GMPs (Figure 3K). Interestingly, obese mice transplanted with NHD13 BM showed a trend towards restored CMP and MEP populations, despite terminal stage of the disease (Figure 3L). Next, we compared the gross morphology of the BM between the groups. While processing the bones in the obese NHD13 mice we observed two distinct phenotypes, which we traced back to mice dying due to MDS or transformation to AML. Thus, in the NHD13 mice we took the opportunity to explore the phenotype amongst mice that had died of either MDS or AML (Figure 3L). As expected, the BM from lean control mice appeared normal. When we explored the marrow of lean NHD13 mice with AML, we noted characteristic crowding of the marrow and dilated vessels, with an abundant number of cells that appeared to be leaving the marrow, compared to the NHD13 mice with MDS which presented with more disperse marrow. Again, as expected, adipocytes were more abundant in the BM of obese control mice compared with lean control mice, along with more megakaryocytes, as we have previously described.20 However, the most notable change in the overall morphology was observed in the NHD13 obese mice. Those that died from AML had fewer adipocytes and more cellular marrow compared to the obese control mice, suggesting that the leukemic cells had used the lipid stored within these adipocytes for energy, similar to the interaction between HSPCs and adipocytes post-BMT.21 Interestingly, NHD13 obese mice that died from MDS had almost a complete lack of cells in the BM, which was loaded with adipocytes, suggesting that hematopoiesis was likely being supported by another organ, and at this end-stage of disease showed complete BM failure. In addition, stem cells and progenitor cells that were increased in the spleen at the seven-month time point appeared to have become exhausted and were rapidly maturing into myeloid cells (i.e., more GMPs; Figure 3L). Together, these data demonstrate a similar profile of hematological changes in obese and lean MDS mice at disease endpoint, with a clear difference being the adiposity observed in the marrow of the obese NHD13 mice that died due to MDS.
Figure 3.
Ob/Ob mice exhibit a similar disease phenotype at MDS/AML endpoint despite aggravated monocytosis. Ob/Ob mice and WT littermate controls transplanted with either WT or NHD13 bone marrow (BM) were followed until the development of MDS symptoms required euthanasia. Blood flow cytometry analysis of (A) myeloid cells, (B) lymphocytes and (C) progenitor cells. CBC analysis of (D) red blood cells, (E) hemoglobin, (F) hematocrit, (G) mean corpuscular volume and (H) platelets. (I) Spleen weights and representative images, scale 0.5cm. (J) Flow cytometry analysis of HSPCs and myeloid progenitors in the BM. (K) Representative images of BM from lean and Ob/Ob mice, arrows indicate dilated blood vessels, A indicates adipocytes. (L) Flow cytometry analysis of HSPCs in the spleen. (A–I); n=11–16; (J–L); n=4–8. All data expressed as mean ± SEM. *P<0.05, for obesity effect; #P<0.05, for MDS effect as analyzed by 2-way ANOVA. WT: wild-type; RBC: red blood cell; HSPC: hematopoietic stem and progenitor cell; CMP: common myeloid progenitor; GMP: granulocyte-macrophage progenitor; MEP: megakaryocyte-erythroid progenitor; MCV: mean corpuscular volume.
Obese mice present with remodeled adipose tissue in response to MDS
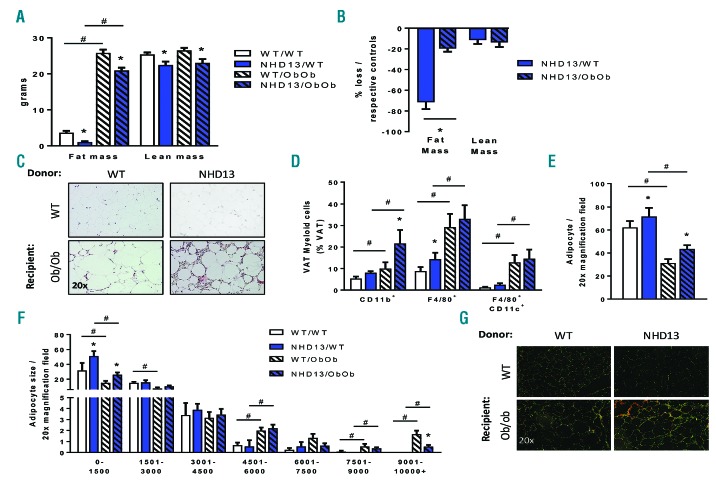
Given that the blood profile did not provide an explanation for the prolonged survival of the obese mice with MDS, we sought an alternate explanation. Interestingly, when analyzing tissues after sacrifice, we noted that lean MDS mice had lost almost all of their body fat (Figure 4A). In contrast, the obese mice, which started with substantially more fat mass, retained the majority of their adiposity when facing MDS. Indeed, when expressed as a percentage relative to mice without MDS, lean MDS mice had lost almost 75% of their fat mass at the time of sacrifice, while obese mice had only lost 20% of their fat mass overall, thus maintaining a stable percentage of body fat mass (Figure 4B, Online Supplementary Figure S3). Of note, examining endpoint lean mass revealed that obesity did not protect against muscle cachexia, as lean and obese MDS mice lost comparable amounts of lean mass (Figure 4A,B). However, at the 37-week time point, when all the lean NHD13 mice had died, the obese NHD13 still had lean mass equal to control WT mice, suggesting that their lean mass had not fallen to dire levels (Online Supplementary Figure S3B). Exploring the morphological phenotype of the epididymal fat pads, there was no major differences in gross morphology between the lean control and lean MDS mice. However, a massive infiltration of small nucleated cells surrounded most adipocytes in obese mice with MDS compared to control obese mice (Figure 4C). Using flow cytometry to identify this cell infiltrate, we observed significantly more macrophages, particularly CD11c+ pro-inflammatory macrophages, in the adipose tissue stromal vascular fraction of obese mice, but this was not impacted by MDS status (Figure 4D). However, the obese NHD13 transplanted mice had a significant and selective increase in CD11b+ cells, suggesting the accumulation of activated myeloid cells (Figure 4D). To reaffirm that the obese mice were unlikely to be prone to extramedular forms of leukemia (i.e., we saw no increase in circulating blood ckit+ cells; Online Supplementary Figure 2E,F), we performed an ex vivo migration assay whereby isolated ckit+ cells from WT or NHD13 mice were allowed to migrate to conditioned media from lean or obese mice. This revealed that independent of genotype, there was a suppressed migratory response to obese fat compared to lean, suggesting that ckit+ cells were not being encouraged to migrate to the obese adipose tissue and evolve into leukemic cells (Online Supplementary Figure 3D).
Figure 4.
Adipose tissue parameters in Ob/Ob mice subjected to MDS. Ob/Ob mice and WT littermate controls transplanted with either WT or NHD13 BM were followed until the development of MDS symptoms required euthanasia. After death, adipose tissues were dissected and analyzed. (A) Fat and lean mass measured by EchoMRI. (B) Percentage weight loss relative to respective control groups. (C) Representative H&E stained section of visceral adipose tissue (VAT). (D) Flow cytometry analysis of VAT stromal vascular fraction, including percentages of activated myeloid cells (CD11b+), macrophages (F4/80+) and pro-inflammatory macrophages (CD11c+). (E) Adipocyte number analysis and (F) adipocyte size analysis using Image Pro J. (G) PicroSirius red staining of VAT for collagen visualization in polarized light. (A–B); n=9–16; (C–G); n=4–8. All data expressed as mean ± SEM. *P<0.05, for obesity effect; #P<0.05, for MDS effect as analyzed by 2-way ANOVA. WT: wild-type.
The recruitment of immune cells into the adipose has been associated with fat tissue remodeling, thus we set out to analyze adipocyte characteristics.22 Quantification of adipocyte size revealed that the visceral adipose tissue (VAT) from obese MDS mice contained more smaller adipocytes and fewer large adipocytes, signifying extensive remodeling of the fat tissue in response to MDS, with a similar trend in the lean mice (Figure 4E,F). Consistent with increased immune cell recruitment and adipose tissue remodeling, we observed increased VAT fibrosis in the obese MDS animals when looking at collagen staining (Figure 4C–G). Given this data, we hypothesize that one mechanism by which obesity prolongs survival in mice with MDS is through a preservation of fat mass.
Impact of MDS on spleen and liver immune cell populations in WT and Ob/Ob mice
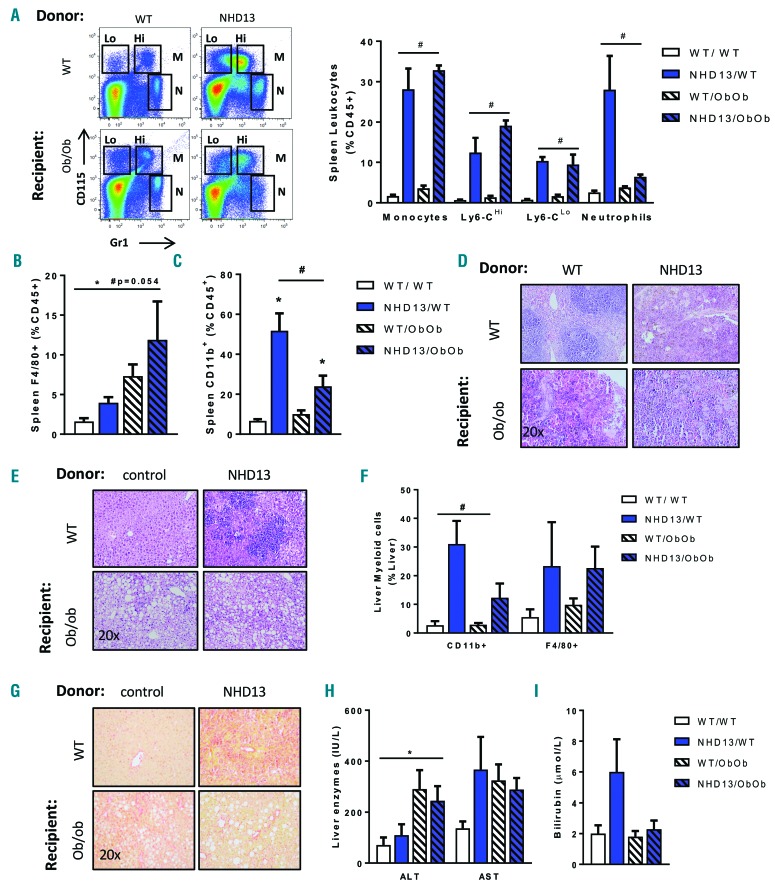
Given the increased recruitment of activated myeloid cells in the VAT of obese MDS mice and their prolonged survival, we hypothesized that the increased recruitment of myeloid cells to VAT may spare the recruitment of these cells to other organs. In the spleen, consistent with the splenomegaly and similar to the disease profile at seven months, we still observed a striking increase in Ly6-Chi and Ly6-Clo monocyte populations in both lean and obese MDS mice, consistent with MDS characteristics (Figure 3I and Figure 5A). Interestingly, the strong neutrophil accumulation we noted was restricted to the lean MDS mice, as obese MDS mice showed no increase in splenic neutrophils (Figure 5A). As observed in the VAT, and consistent with the obese phenotype, F4/80+ macrophages were increased in the spleens of obese mice, and there was a trend for MDS to potentiate this profile (Figure 5B). Strikingly, contrary to the VAT profile, there was a 7-fold increase in the abundance of splenic CD11b+ cells observed in lean MDS conditions compared to only a doubling in the obese animals (Figure 5C). This enhanced myeloid cell infiltration, particularly in the lean MDS mice, was supported by the gross morphology of the spleen (Figure 5D). Overall, it appeared that obese mice were partly protected from splenic myeloid cell accumulation.
Figure 5.
Spleen and liver parameters in response to MDS/AML in Ob/Ob mice. Spleen analysis by flow cytometry including (A) monocytes and neutrophils, (B) macrophages (F4/80+) and (C) activated myeloid cells (CD11b+). (D) Histological H&E staining of the spleen. Liver analysis by (E) histological H&E analysis, (F) flow cytometry analysis of hepatic immune cells, including activated myeloid cells (CD11b+) and macrophages (F4/80+) and (G) PicroSirius red staining of liver for collagen visualization. Plasma analysis of (H) liver enzymes alanine amino transferase (ALT) and aspartate amino transferase (AST) and (I) bilirubin. (A–G); n=4–8; (H–I); n=5–7. All data expressed as mean ± SEM. *P<0.05, for obesity effect; #P<0.05, for MDS effect as analyzed by 2-way ANOVA. WT: wild-type.
This prompted us to explore immune cell recruitment in the liver, where macrophages tend to home in on a context of obesity. Exploring the liver, initially at a macroscopic level, it appeared that MDS resulted in the preferential accumulation of immune cells in lean mice, and to a lesser extent in the obese MDS mice (Figure 5E). Quantitative flow cytometry data confirmed this observation, where MDS resulted in similar F4/80+ macrophages in lean and obese hepatic tissue, but significantly more hepatic CD11b+ myeloid cells in the lean animals (Figure 5F). These findings were correlated with the observed liver fibrosis, which appeared to be more prevalent in the livers of lean MDS mice (Figure 5G). Interestingly, the fatty liver phenotype observed in obese mice (Figure 5E) was associated with an increase in circulating liver enzymes aspartate transaminase (AST)/alanine transaminase (ALT) (Figure 5H). Although MDS did not influence ALT levels, lean MDS mice presented with increased AST levels when obese MDS mice showed no difference with their obese healthy counterparts (Figure 5H). Interestingly, in support of impaired liver function, lean MDS mice had higher levels of bilirubin, indicative of impaired uptake and degradation by the liver of these mice (Figure 5I), effects that were not apparent in the obese MDS mice. Finally, we assessed the basal levels of circulating creatine kinase as a proxy measure of muscle damage. This tended to be higher in the lean MDS mice, suggesting a possible impact of MDS on muscle integrity which did not appear to occur in their obese counterparts (Online Supplementary Figure S4).
Overall, these data support the idea that the obese VAT preferentially attracts specific myeloid cell populations in MDS mice which results in a concomitant decrease in the accumulation of these cells in other tissues, such as the spleen and liver.
Discussion
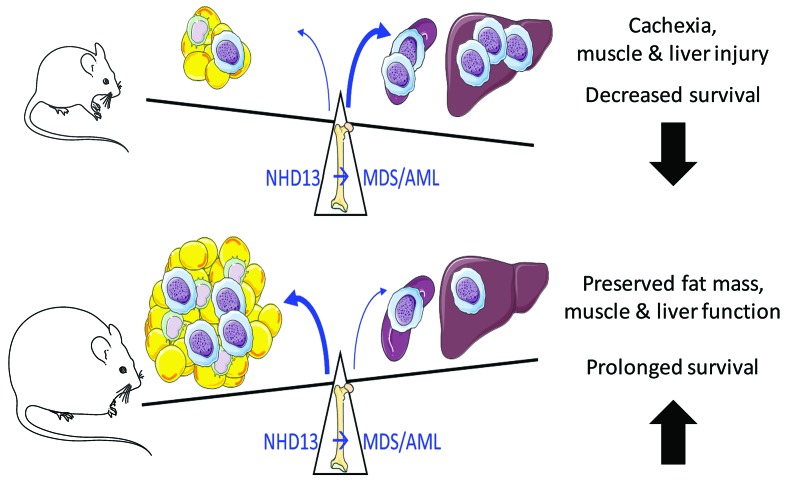
Obesity has become increasingly associated with cancer and is now recognized as a risk factor for many malignant pathologies.5,23 In addition to solid tumors, obesity has also been linked with different forms of leukemia.11 Obesity is associated with an increased prevalence of MDS, however the data is less clear regarding survival outcome in obese patients presenting with MDS. We have previously demonstrated that the obese adipose tissue interacts with the BM, and promotes monocytosis through the stimulation of the myeloid pathway via IL-1β. In this context, we hypothesized that this increased basal rate of myelopoiesis would contribute to MDS and the progression to AML, thereby decreasing survival. Surprisingly, while obesity-induced myelopoiesis was observed in the setting of MDS, this was associated with significantly improved survival. We hypothesize that the expanded adipose tissue in obese mice acts as a sink for the increased myeloid cells, sparing other vital organs from myeloid cell burden and subsequent dysfunction. Furthermore, we propose that the preservation of fat mass observed in obese MDS mice likely contributes to their survival advantage relative to their lean counterparts (Figure 6).
Figure 6.
Schematic overview of the proposed mechanism of enhanced survival in the obese mice transplanted with NHD13 bone marrow. MDS: myelodysplastic syndrome; AML: acute myeloid leukemia.
Our paradoxical findings highlight that obesity might not always be associated with enhanced mortality risk in people suffering from hematological disorders. One major difference between our pre-clinical data and the clinical course of MDS resides in the fact that patients with this disease may be treated with transfusions, chemotherapy, including epigenetic modifiers, and occasionally BMT. In general, studies supporting an obesity paradox in cancer tend to describe a ‘U-shape’ correlation between BMI and overall survival.24 This could indicate that increased energy stores in overweight and moderately obese patients could allow for longer survival, but severely obese patients with comorbidities would be at risk of decreased survival rate.
Consistent with this obesity paradox, there are accumulating reports demonstrating that adipose tissue can be critical for patient’s health and survival. In particular, subcutaneous adipose tissue has been associated with increased survival in several conditions, including the hematological disorder multiple myeloma.25–27 Adipose tissue is the main energy store in the human body, hence, conserving enough fat stores could lead to better survival outcomes by allowing slower rates of muscle proteolysis, another important source of energy. In addition, the adipose tissue has now been fully recognized as an endocrine organ impacting various bodily functions. Indeed, depletion of white adipose tissue has been associated with increased inflammatory signalling and disrupted circadian regulation.28 Low levels of adiponectin, one of the main hormones secreted by the VAT, has been associated with an increased risk of cancer and poor diagnosis.29 Thus, the maintenance of fat stores in the Ob/Ob mice transplanted with NHD13 may play a crucial role in their prolonged survival compared to the lean MDS that lose the majority of their VAT.
In the obese mice with MDS we found cells homed in to the VAT in significant numbers. In the absence of treatment, it appeared that the preferential homing of cells to the adipose tissue could partially protect other organs from infiltration, providing an explanation for the prolonged survival of obese mice when confronted with NHD13-induced MDS. Transformation from MDS to AML takes place in the stem and progenitor cells, not mature myeloid cells. Interestingly, transformation could have occurred in the obese VAT and at the same time prevented outgrowth of the leukemia, keeping these cells somewhat dormant. Prescience comes from the discovery that leukemic stem cells can reside within the VAT in a quiescent nature, conferring their protection from chemotherapy.30,31
One striking observation was the different cellular makeup of the BM between WT and obese mice that died of MDS. While the marrow of the WT mice that died of MDS displayed classical signs of dysplasia, with fewer hematopoietic cells, the marrow from the obese MDS mice was full of adipocytes, with few hematopoietic cells evident. Whether marrow adiposity alters the course of the disease requires further investigation. Of note, the bones of lean and obese mice that died from AML looked similar, thus there is a possibility that the leukemic cells, upon transition from MDS to AML, could have used this stored lipid in the early stages of proliferation as a source of energy, which in the lean mice would have come from peripheral organs (i.e., adipose tissue). The role of BM adipocytes is not well described, but was recently shown to play an important role in hematopoietic regeneration following ablation via chemotherapy or irradiation.21 Given the blood profile of the obese MDS mouse, where there was an increase in the Ly6-Chi monocytes, an early transition to CMML could be occurring; however, this hypothesis requires further investigation. Moreover, the mice that were deemed to have died from cytopenias could have potentially developed aplastic anemia, as the morphology of the bone marrow and numbers of circulating cells is similar to that described in this disease.
This study has a number of limitations which should be taken into account when interpreting our findings. Firstly, we used a genetic model of obesity, where leptin is deficient, thus causing hyperphagia. While this model is key in maintaining adiposity, it does not reflect a scenario where a change in diet drives obesity, in which, for example, changes in lipids may influence the transformation of the disease. Additionally, a role for leptin in the evolution of MDS cannot be excluded. Further, we opted to perform BMTs, as opposed to crossing the NHD13 mice with the Ob/Ob mice. Whether the hematopoietic stress associated with transplantation and engraftment altered the course of the disease is probably unlikely, but should be kept in mind. Finally, we only tested one model of MDS; whether this holds true in other models is yet to be determined.
In the study herein, we have demonstrated that obesity confers a survival advantage when mice are confronted with NHD13-induced MDS. It appears that the increased adiposity allows for dampening of the systemic leukemic insult, as leukemic cells preferentially home to the adipose tissue, protecting vital organs such as the liver and spleen. Additionally, maintaining adequate fat stores per se may also contribute to the improved survival observed in obese MDS mice. Our findings support the growing literature suggesting that despite increased incidences of MDS and AML in obese patients, their overall survival may not be different to lean patients and, in some instances, may even be prolonged. Thus, taken together with the recent findings of Carey et al.,17 it may also be important to understand the cytokine profile of the patients before treatment, to ensure more effective eradication of the leukemia and to promote the restoration of normal hematopoiesis (i.e., inhibiting IL-1β in obese patients).
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/4/597
Funding
This work was supported by NHMRC grants (APP1083138, APP1106154 and APP1142938) to AJM. MJK is a Russell Berrie Foundation Scholar in Diabetes Research from the Naomi Berrie Diabetes Centre. AJM is supported by a Career Development Fellowship from the NHMRC (APP1085752), a Future Leader Fellowship from the National Heart Foundation (100440) and a Centenary Award from CSL.
References
- 1.Khandekah MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11(12):886–895. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. [DOI] [PubMed] [Google Scholar]
- 3.Hildreth KL, Van Pelt RE, Schwartz RS. Obesity, insulin resistance, and Alzheimer’s disease. Obesity (Silver Spring). 2012;20(8):1549–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masuoka HC, Chalasani N. Nonalcoholic fatty liver disease: an emerging threat to obese and diabetic individuals. Ann N Y Acad Sci. 2013;1281:106–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer. 2008;122(6):1418–1421. [DOI] [PubMed] [Google Scholar]
- 7.Orgel E, Genkinger JM, Aggarwal D, Sung L, Nieder M, Ladas EJ. Association of body mass index and survival in pediatric leukemia: a meta-analysis. Am J Clin Nutr. 2016;103(3):808–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma X, Lim U, Park Y, et al. Obesity, lifestyle factors, and risk of myelodysplastic syndromes in a large US cohort. Am J Epidemiol. 2009;169(12):1492–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy F, Kroll ME, Pirie K, Reeves G, Green J, Beral V. Body size in relation to incidence of subtypes of haematological malignancy in the prospective Million Women Study. Br J Cancer. 2013;108(11):2390–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361(19):1872–1885. [DOI] [PubMed] [Google Scholar]
- 11.Poynter JN, Richardson M, Blair CK, et al. Obesity over the life course and risk of acute myeloid leukemia and myelodysplastic syndromes. Cancer Epidemiol. 2016;40:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo JJ, Reagan JL, Ingham RR, et al. Obesity but not overweight increases the incidence and mortality of leukemia in adults: a meta-analysis of prospective cohort studies. Leuk Res. 2012;36(7):868–875. [DOI] [PubMed] [Google Scholar]
- 13.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11(12):886–895. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. [DOI] [PubMed] [Google Scholar]
- 15.Nagareddy PR, Kraakman M, Masters SL, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19(5):821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer K, DelProposto J, Morris DL, et al. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab. 2014;3(6):664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey A, Edwards DKt, Eide CA, et al. Identification of Interleukin-1 by functional screening as a key mediator of cellular expansion and disease progression in acute Myeloid leukemia. Cell Rep. 2017;18(13):3204–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol. 1996;6(9):1170–1180. [DOI] [PubMed] [Google Scholar]
- 19.Lin YW, Slape C, Zhang Z, Aplan PD. NUP98–HOXD13 transgenic mice develop a highly penetrant, severe myelodysplastic syndrome that progresses to acute leukemia. Blood. 2005;106(1):287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraakman MJ, Lee MK, Al-Sharea A, et al. Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J Clin Invest. 2017;127(6):2133–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou BO, Yu H, Yue R, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19(8):891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest. 2017;127(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donohoe CL, Lysaght J, O’Sullivan J, Reynolds JV. Emerging concepts linking obesity with the hallmarks of cancer. Trends Endocrinol Metab. 2017;28(1):46–62. [DOI] [PubMed] [Google Scholar]
- 24.Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18(9):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindauer E, Dupuis L, Muller HP, Neumann H, Ludolph AC, Kassubek J. Adipose tissue distribution predicts survival in amyotrophic lateral sclerosis. PLoS One. 2013;8(6):e67783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeoka Y, Sakatoku K, Miura A, et al. Prognostic effect of low subcutaneous adipose tissue on survival outcome in patients with Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2016;16(8):434–441. [DOI] [PubMed] [Google Scholar]
- 27.Antoun S, Bayar A, Ileana E, et al. High subcutaneous adipose tissue predicts the prognosis in metastatic castration-resistant prostate cancer patients in post chemotherapy setting. Eur J Cancer. 2015;51(17):2570–2577. [DOI] [PubMed] [Google Scholar]
- 28.Tsoli M, Schweiger M, Vanniasinghe AS, et al. Depletion of white adipose tissue in cancer cachexia syndrome is associated with inflammatory signaling and disrupted circadian regulation. PLoS One. 2014;9(3): e92966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katira A, Tan PH. Evolving role of adiponectin in cancer-controversies and update. Cancer Biol Med. 2016;13(1):101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye H, Adane B, Khan N, et al. Adipose tissue functions as a reservoir for leukemia stem cells and confers chemo-resistance. Blood. 2015;126(23):845–845. [Google Scholar]
- 31.Ye H, Adane B, Khan N, et al. Leukemic stem cells evade chemotherapy by metabolic adaptation to an adipose tissue niche. Cell Stem Cell. 2016;19(1):23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.