Chronic lymphocytic leukemia (CLL), the most frequent leukemia in adults, is characterized by an accumulation of monoclonal B cells with a mature phenotype in the blood and lymphoid organs. Despite considerable advances in the treatment of patients, CLL remains an incurable disorder,1,2 and therefore novel therapies are needed. New compounds harboring a trifluorinated thiazoline scaffold have recently been described for their pro-apoptotic properties.3 Indeed, a diaryl trifluorothiazoline, named fluorizoline, demonstrated a high apoptosis induction in different cancer cells in vitro by binding to prohibitin (PHB) 1 and 2.3–5 Very recently, fluorizoline was reported to efficiently induce apoptosis in CLL cells derived from patients, showing synergistic effects with other drugs used in CLL treatment such as the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib.6 However, none of these studies reported any assessment of fluorizoline efficacy in vivo. Here, we confirmed that fluorizoline strongly induces apoptosis in primary CLL cells through the induction of NOXA. However, we also demonstrated that fluorizoline, in contrast to ibrutinib, fails to control CLL development in a relevant mouse model of aggressive disease.
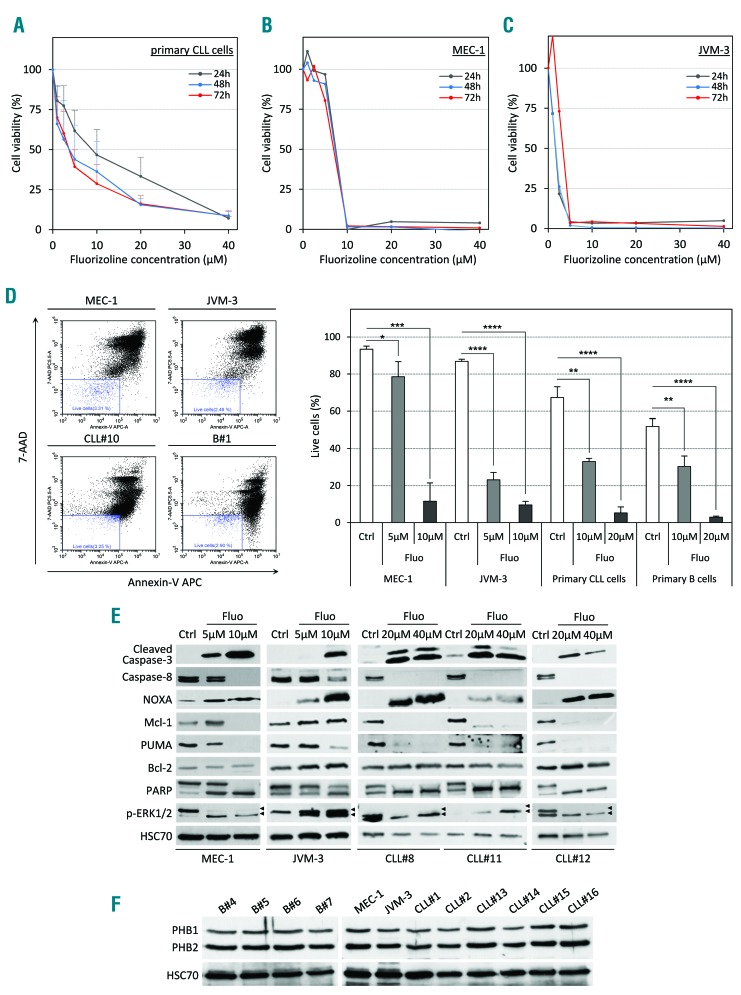
First, we isolated peripheral blood mononuclear cells (PBMC) from CLL patients (>95% CD5+CD19+ cell purity) and tested the cytotoxicity of fluorizoline ex vivo (Online Supplementary Methods). We observed that CLL cells were sensitive to treatment, as mirrored by the low inhibitory concentrations (IC50) after 24 hours (h), 48 h, and 72 h of culture (9 μM, 4 μM, and 4 μM IC50, respectively) (Figure 1A). Both the MEC-1 and JVM-3 CLL cell lines were sensitive to the drug as well (7.5 μM and 1.5 μM IC50, respectively) (Figure 1B and C). The strong decrease in cell viability was confirmed for MEC-1 and JVM-3 cell lines, and primary CLL cells by the quantification of annexin V/7-AAD-positive cells (Figure 1D). Fluorizoline also induced apoptosis in healthy B cells. To understand the mechanisms leading to apoptosis induction, we examined the expression and cleavage of proteins playing major roles in the cell death cascade. In cell lines and patients’ cells, both caspase-3 and -8 were cleaved after fluorizoline treatment (24 h) along with PARP cleavage in a dose-dependent manner (Figure 1E). We also observed an increased expression of the pro-apoptotic Bcl-2 homology domain 3 (BH3)-only protein NOXA and depletion of the anti-apoptotic molecule Mcl-1. However, fluorizoline-induced cell death appeared to be Bcl-2-independent as its level remained unchanged upon treatment. Finally, we observed a dose-dependent decrease in Phospho-ERK1 and an increase in ERK2 phosphorylation. The binding of fluorizoline to prohibitins is required to induce apoptosis.4 Therefore, we examined the expression of PHB1 and PHB2 in purified normal B and CLL cells. Indeed, both prohibitins are expressed in high levels in both populations with no detectable difference between them (Figure 1F and Online Supplementary Figure S1).
Figure 1.
Fluorizoline (fluo) efficiently induces apoptosis in chronic lymphocytic leukemia (CLL) cells in vitro. (A–C) Cytotoxic dose response of fluo on primary human CLL cells (A), CLL cell lines MEC-1 (B) and JVM-3 (C) was analyzed by CCK8 assay. Peripheral blood mononuclear cells (PBMC) from 7 CLL patients as well as the CLL cell lines MEC-1 and JVM-3 were treated with increasing doses of fluo ranging from 1 μM to 40 μM for 24 hours (h) (gray line), 48 h (blue line) or 72 h (red line). (D) Representative scatter plots illustrating annexin-V/7-AAD staining of cells after 24 h of treatment (left panel). MEC-1 (upper left plot), JVM-3 (upper right plot), primary CLL cells (lower left plot, patients CLL#8 to CLL#10), and normal B cells (lower right plot, donors B#1 to B#3) were treated with 5–20 μM fluo or with equivalent volume of the vehicle dimethylsulfoxide (DMSO) for 24 h and stained with annexin V-APC and 7-AAD prior to analysis by flow cytometry. Viable cells (annexin V-APC negative/7-AAD negative, blue gate) were determined and are expressed as percentage of total cell population. Data represent average percentage of live cells (right panel) from independent experiments (n=3). *P<0.05, **P < 0.01; ***P < 0.001; ****P<0.0001. (E) MEC-1, JVM-3 and primary CLL cells (patients CLL#8, CLL#11 and CLL#12) were treated with 5 μM to 40 μM fluorizoline or with equivalent volume of the vehicle DMSO (Ctrl) for 24 h, lysed and proteins were analyzed by western blot with the indicated antibodies. HSC70 protein served as a loading control. (F) The expression of PHB1 and PHB2 was analyzed by western blot in total cell lysates from normal B cells (donors B#4 to B#7), MEC-1 and JVM-3 cells, and primary CLL B cells (patients CLL#1, CLL#2 and CLL#13 to CLL#16). HSC70 protein served as a loading control.
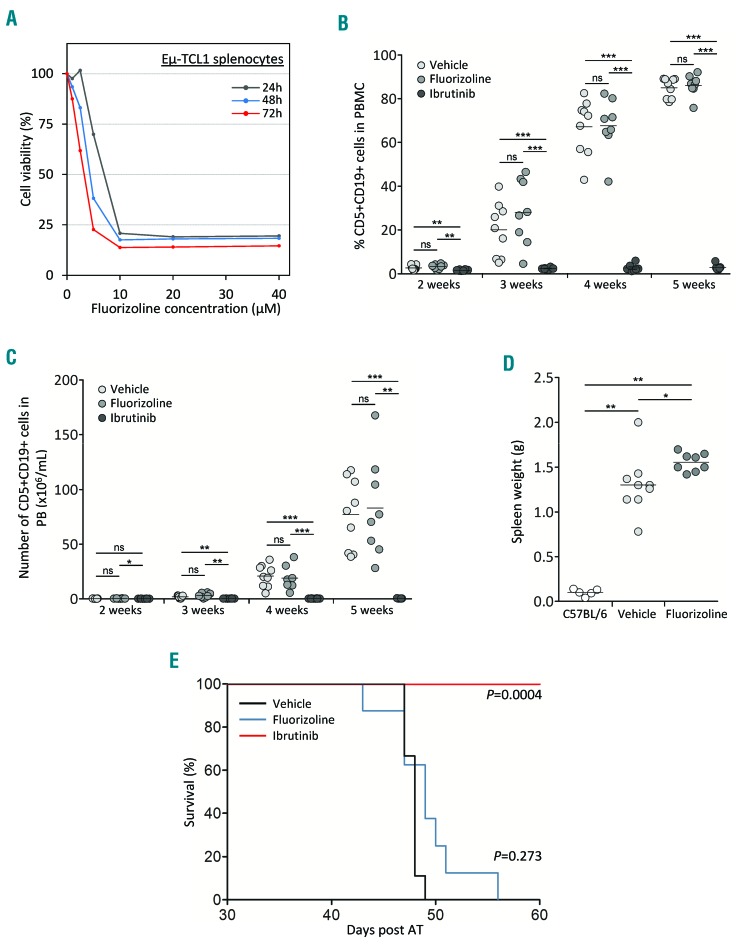
Next, we sought to confirm the therapeutic benefit of fluorizoline in the Eμ-TCL1 mouse model of CLL. For this purpose, we first prepared splenocytes from diseased Eμ-TCL1 mice and observed a similar toxicity of the compound towards murine CLL cells in vitro. Indeed, a concentration of 10 μM killed 80–90% of cells depending on the incubation period (Figure 2A). Then, we performed adoptive transfer (AT) of splenocytes from diseased Eμ-TCL1 to C57BL/6 recipients (Online Supplementary Methods). After five days, animals were randomized to treatment with either fluorizoline, ibrutinib, or control vehicles. Fluorizoline was administered by intraperitoneal injections three times a week at 15 mg/kg, a dose ten times higher than the dose of another PHB-binding compound successfully used for the treatment of murine CLL.7 The animals were treated for five weeks and monitored weekly for both the blood cell count and the percentage of leukemic CD5+CD19+ cells. Interestingly, while the animals receiving ibrutinib maintained an almost undetectable tumor load in the circulation, the animals treated with vehicle or fluorizoline became very rapidly (3 weeks) leukemic, as reflected by the increase in the percentage and the number of CD5+CD19+ CLL cells in the blood (Figure 2B and C). We also quantified by flow cytometry the proportion of immune cells in the blood of mice treated with fluorizoline or vehicle. After five weeks of treatment, no significant difference in B- and T-cell number was observed (Online Supplementary Figure S2). While monocytes increased and granulocytes decreased in a manner concomitant with the development of CLL cells, no difference was measured in presence of fluorizoline (Online Supplementary Figure S2). Moreover, fluorizoline did not control disease development in the spleen as indicated by enlarged spleens, which were even larger than in vehicle-treated animals (Figure 2D), as well as a high proportion of CLL cell infiltration (>96%). In addition, a survival analysis based on the Kaplan-Meier estimate did not indicate any statistical difference between the groups treated with vehicle or fluorizoline (P=0.273), whereas all animals treated with ibrutinib survived until the end of the experiment (P=0.0004) (Figure 2E).
Figure 2.
Fluorizoline (fluo) fails to control chronic lymphocytic leukemia (CLL) development in vivo. (A) Cytotoxic dose response of fluo on primary murine CLL cells (Eμ-TCL1 splenocytes). Splenocytes from a diseased Eμ-TCL1 mouse were treated with increasing doses of fluo ranging from 1 μM to 40 μM for 24 hours (h) (gray line), 48 h (blue line) or 72 h (red line). (B and C) Tumor load in the peripheral blood (PB) of individual mice. CLL development of AT-TCL1 mice treated either with 15 mg/kg intraperitoneal (i.p.) fluo (n=8) or ibrutinib in drinking water (Ibrutinib) (n=8) or equivalent doses of vehicles (vehicle) (n=10) was monitored weekly and is depicted by both the percentage (B) and the number (C) of CD5+ CD19+ CLL cells. (D) Spleen weight (g) of mice treated either with 15 mg/kg i.p. fluo (n=8) or ibrutinib in drinking water (n=8) or equivalent doses of vehicles (vehicle) (n=10). (E) Survival analysis of mice upon fluo and ibrutinib treatment. AT-TCL1 mice were treated either with 15 mg/kg i.p. fluo (red line) or ibrutinib in drinking water (blue line) or equivalent doses of vehicles (black line). Overall survival was analyzed using the Kaplan-Meier method and is shown as the percentage of viable mice in the observed period of time. (B–D) *P<0.05; **P<0.01; ***P<0.001. ns: not significant; PBMC: peripheral blood mononuclear cells.
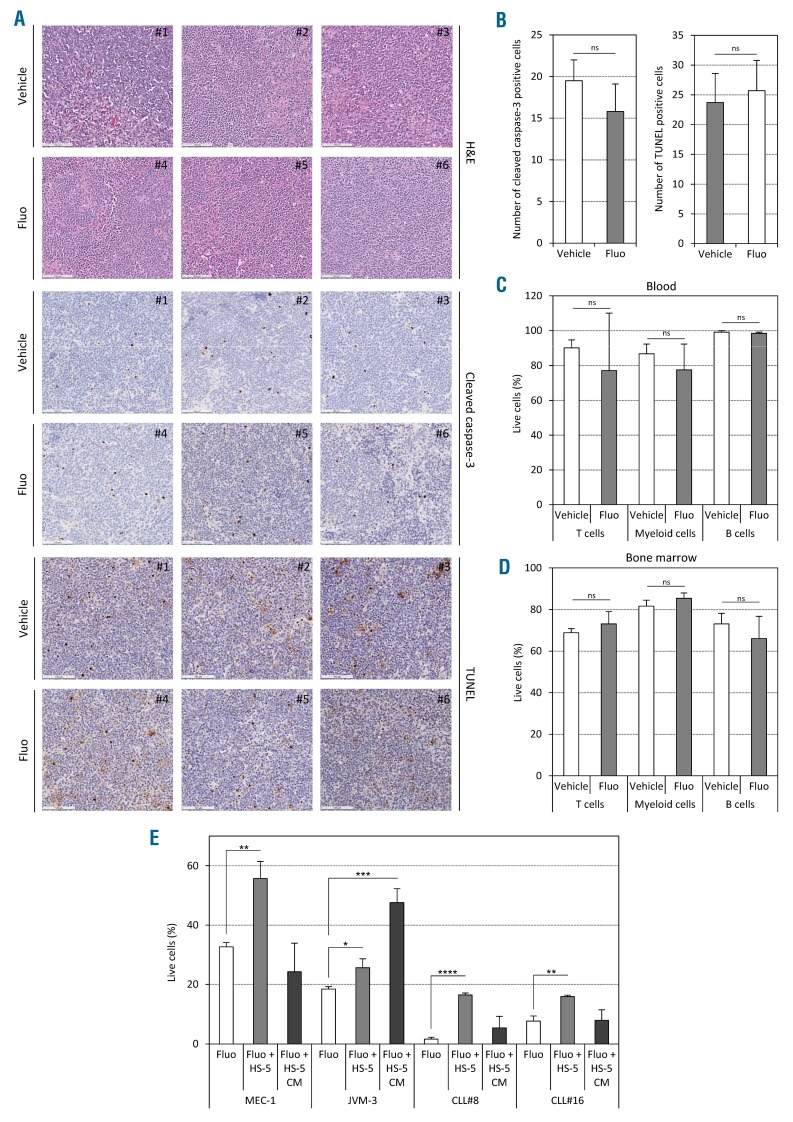
Furthermore, we investigated the induction of cell death in several organs after in vivo treatment. The absence of apoptosis was shown in situ by immunohistochemistry staining against cleaved caspase-3 and TUNEL assay in spleen sections (Figure 3A and B). Similarly, no difference in the percentage of live cells was observed for B cells, T cells, and myeloid cells in the blood and the bone marrow (Figure 3C and D). We also analyzed if the microenvironment could have any protective capabilities by co-culturing CLL cells with stromal cells. Indeed, we observed a significant protection from cell death by co-culturing CLL cells with HS-5 cells. The fact that conditioned medium had a protective effect only for JVM-3 cells, but not for CLL cells, suggests that direct cell contact is required for protection of human primary CLL cells (Figure 3E).
Figure 3.
Fluorizoline (fluo) does not induce apoptosis in vivo. (A–D) AT-TCL1 mice were treated with either 15 mg/kg fluo or equivalent volume of dimethylsulfoxide (DMSO) as vehicle, three times a week for two weeks (5 mice per group). (A and B) Immunohistochemical staining performed on indicated spleen sections using hematoxylin & eosin (H&E), anti-cleaved caspase-3 antibody, and TUNEL assay, respectively (scale bars, 100 μm). The panels show a representative picture for each animal (A) and the respective quantifications (10 fields per mouse in 3 mice per group) (B). (C and D) Cell viability was assessed by annexin-V/7-AAD staining in T cells, myeloid cells and B cells from blood (C) and bone marrow (D) of treated AT-TCL1 mice. (E) Cell viability was assessed in vitro in chronic lymphocytic leukemia (CLL) cells co-cultured on HS-5 stromal cells or with HS-5 conditioned medium only in presence of 5 μM (for cell lines) or 20 μM (for patients’ cells) fluo or equivalent volume of DMSO as vehicle. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. ns: not significant.
Moreover, fluorizoline is likely poorly bioavailable. Indeed, its structure suggests a lipophilic character with, as a main consequence, a rapid transfer from the blood to the fatty compartment and, therefore, a decreased bioavailability. Adsorption of fluorizoline to lipids and proteins in vivo could also reduce the free bioavailable fraction. In addition, the metabolization of fluorizoline (e.g. hydrolysis, oxidation and/or conjugation) into non-active by-products, could also explain the difference observed between cell cultures and animal experiments.
In conclusion, despite promising apoptosis-inducing effects in vitro, fluorizoline was unsuccessful in inducing apoptosis and limiting CLL progression in vivo. The absence of toxicity towards CLL cells and normal cells present in the peripheral circulation strongly points toward the low bioavailability of the drug or its rapid systemic clearance in vivo. Efforts should be made to modify the compound to improve its overall efficacy.
Supplementary Material
Acknowledgments
We thank Prof. Carlo Croce and Prof. John Byrd (OSU, OH) for the kind gift of Eμ-TCL1 mouse and Dr. Martina Seiffert (DKFZ Heidelberg, Germany) for providing the animals. We also thank Dr Brice Appenzeller (LIH, Human Biomonitoring Research Unit) for helpful discussions.
Footnotes
Funding: this project was supported by Télévie (7.4563.15, 7.4508.16, and 7.4502.17) and the Luxembourg Institute of Health (HEMATEXO project). Finally we thank Dr. Joshua Brown-Clay (LIH, LECR-Cytoskeleton and Cancer Progression) for editing the manuscript.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Ten Hacken E, Burger JA. Microenvironment interactions and B-cell receptor signaling in Chronic Lymphocytic Leukemia: Implications for disease pathogenesis and treatment. Biochim Biophys Acta. 2016;1863(3):401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kipps TJ, Stevenson FK, Wu CJ, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3:16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez-Perarnau A, Preciado S, Palmeri CM, et al. A trifluorinated thiazoline scaffold leading to pro-apoptotic agents targeting prohibitins. Angew Chem Int Ed Engl. 2014;53(38):10150–10154. [DOI] [PubMed] [Google Scholar]
- 4.Moncunill-Massaguer C, Saura-Esteller J, Perez-Perarnau A, et al. A novel prohibitin-binding compound induces the mitochondrial apoptotic pathway through NOXA and BIM upregulation. Oncotarget. 2015;6(39):41750–41765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pomares H, Palmeri CM, Iglesias-Serret D, et al. Targeting prohibitins induces apoptosis in acute myeloid leukemia cells. Oncotarget. 2016;7(40):64987–65000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosialls AM, Pomares H, Iglesias-Serret D, et al. The prohibitin-binding compound fluorizoline induces apoptosis in chronic lymphocytic leukemia cells through the upregulation of NOXA and synergizes with ibrutinib, AICAR or venetoclax. Haematologica. 2017;102(9):1587–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas DM, Edwards RB, Lozanski G, et al. The novel plant-derived agent silvestrol has B-cell selective activity in chronic lymphocytic leukemia and acute lymphoblastic leukemia in vitro and in vivo. Blood. 2009;113(19):4656–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.