Abstract
Background
This study evaluated the efficacy of an intervention combining the Valencia model of waking hypnosis with cognitive-behavioral therapy (VMWH-CBT) in managing cancer-related pain, fatigue, and sleep problems in individuals with active cancer or who were post-treatment survivors. We hypothesized that four sessions of VMWH-CBT would result in greater improvement in participants’ symptoms than four sessions of an education control intervention. Additionally, we examined the effects on several secondary outcome domains that are associated with increases in these symptoms (depression, pain interference, pain catastrophizing, and cancer treatment distress).
Methods
The study design was a randomized controlled crossover clinical trial comparing the VMWH-CBT intervention with education control. Participants (N = 44) received four sessions of both treatments, in a counterbalanced order (n = 22 per order condition).
Results
Participants were 89% female (N = 39) with mean age of 61 years (SD = 12.2). They reported significantly greater improvement after receiving the active treatment relative to the control condition in all the outcome measures. Treatment gains were maintained at 3-month follow-up.
Conclusions
This study supports the beneficial effects of the VMWH-CBT intervention relative to a control condition and that treatment gains remain stable. VMWH-CBT–trained clinicians should be accessible for managing symptoms both during and after cancer treatment, though the findings need to be replicated in larger samples of cancer survivors.
Keywords: cancer, fatigue, insomnia, oncology, pain, waking hypnosis
1| INTRODUCTION
Pain, fatigue, and sleep difficulties are the most common symptoms reported by individuals with cancer.1
Preliminary evidence supports the potential for non-pharmacological interventions in the management of cancer-related symptoms. Evidence for the efficacy of cognitive-behavioral therapy (CBT) is strong enough to recommend it as first-line treatment for cancer-related sleep problems.2 Moreover, evidence from functional neuroimaging studies supports the use of hypnosis for pain management.3 There is also evidence supporting the promise of hypnosis for managing cancer-related pain and other symptoms,4–6 and the combination of CBT with hypnosis has shown to be effective for fatigue management in patients undergoing radiotherapy for breast cancer.7,8
A form of hypnosis, the Valencia model of waking hypnosis (VMWH),9–12 may be particularly suited for helping patients better manage symptoms in their daily life. It consists of several standardized methods intended to be efficient, easy to learn, and easy to use in everyday life situations. It is based on waking hypnosis, the primary characteristic of which is that patients are able to use self-hypnosis with their eyes open, while engaged in other activities. This allows them to experience therapeutic suggestions whenever the need arises and to generalize the use of these skills across many situations. The model is versatile enough to be used with either relaxation or activation, depending on patients’ needs (ie, activation to cope with fatigue and relaxation to cope with sleep problems).
The purpose of this study was to evaluate the efficacy of the VMWH when combined with CBT13 in a sample of patients in active cancer treatment or post-treatment survivors and who also report bothersome pain, fatigue, or sleep problems. The primary hypothesis was that four sessions of VMWH-CBT would result in greater improvements in the symptoms than four sessions of an education control (EC) intervention. In addition, we examined in secondary analyses the effects of VMWH-CBT relative to EC on secondary outcome domains that are known to be associated with these symptoms, namely, depression, pain interference, pain catastrophizing, and cancer treatment–related distress. Finally, we evaluated the stability of any treatment gains in the primary and secondary outcomes at 3-month follow-up.
2| METHOD
2.1 | Design
The study was a randomized controlled crossover clinical trial comparing a treatment condition (VMWH-CBT) with an EC condition and single blinding, where it was necessary for patients and the intervention clinician to know the patient assignment order, but research staff collecting assessments did not know the intervention order of participants. All participants received four sessions of both treatments, in a counterbalanced order, leaving at least 1 week between interventions.
2.2 | Participants
The sample consisted of patients who had a cancer diagnosis and who were in either active treatment or post-treatment cancer survivors who presented with bothersome pain, fatigue, and/or sleep difficulties. Additional inclusion criteria were aged 18 years or older and able to read English and communicate in English or Spanish. Exclusion criteria included evidence for significant psychopathology that would interfere with study participation, including current suicidal ideation with intent or active psychosis or hallucinations (assessed with a psychological screening questionnaire over the phone), as well as severe cognitive impairment (determined by the clinician’s judgment during the first interaction with the participant on the phone).
2.3 | Interventions
The active treatment condition (VMWH-CBT) consisted of four sessions of treatment that combined training in self-hypnosis (VMWH) with CBT. We chose 4 sessions per condition to balance both (1) the need to have enough sessions for the treatment to be effective5,14,15 and (2) the need to have few enough sessions so that participants would not be overburdened. Participants first learned to identify and restructure any unhelpful thoughts regarding their symptoms using CBT methods. They also received training in a brief self-hypnosis method9 and learned how to use it to manage their symptoms, including current and (possible) future symptoms, using the VMWH exercises.12,16 Finally, during this intervention, participants received information about pain, fatigue, and sleep problems and learned behavioral strategies to cope with them during the study to facilitate the maintenance of treatment gains. The EC intervention consisted of four sessions of didactic lectures and discussions regarding their presenting symptoms, based on an education intervention used in a previous study,17 adapted to the symptoms that were the focus of the study. Participants in both conditions received a handbook with readings and exercises. They were assigned home activities and encouraged to read the materials as often as they found it helpful (EC) or to practice the skills taught (VMWH-CBT) approximately 3 times per day between sessions. Treatments were based on manuals developed by the study clinician (M.E.M.) with input from another study investigator (M.P.J.). Each session lasted approximately 1 hour. The VMWH-CBT treatment and EC manuals are available from the primary author (M.E.M.). All treatments were provided by the study clinician (M.E.M.).
2.4 | Measures
2.4.1 | Demographic and descriptive information
All participants provided demographic information and cancer history information (age, gender, marital status, and employment status; type of cancer and treatments).
2.4.2 | Primary outcome measures
Pain intensity was measured using 0–10 numerical rating scales of current pain and least, worst, and average pain during the past week. Such 0–10 scales have demonstrated their validity and reliability as measures of pain by their strong association with other measures of pain intensity, responsivity to pain treatment, and stability over time without intervening treatment.18
Fatigue was measured using the Patient-reported Outcomes Measurement Information System (PROMIS) 7-item fatigue short form, which has strong psychometric properties.19 Participants rated how often they experienced each item on a 5-point scale ranging from ‘never’ to ‘always’. As with all the PROMIS measures, the PROMIS Fatigue scores are reported on a T-score metric that is anchored to mean levels of each outcome in a healthy US general population.20
Sleep problems were assessed using the 9-item Medical Outcomes Survey Sleep Problem Index.21 The scale yields an Overall Sleep Problems Index, where higher scores indicate greater sleep impairment. There is support for the reliability and validity of the Medical Outcomes Survey Sleep measure.22
2.4.3 | Secondary outcome measures
Pain interference was measured using the 6-item PROMIS Pain Interference Short Form, which assesses the impact of pain on various areas of functioning.23 Scores are converted to T scores to be consistent with the PROMIS metric. This scale has demonstrated adequate psychometric characteristics.23
Depressive symptoms were assessed with the Patient Health Questionnaire Depression Scale (PHQ-8)24 that contains all items of PHQ-9 except the item on self-harm. PHQ-8 is considered valid as both a diagnostic and severity measure and has shown good psychometric properties in general and in patients with cancer.25
Pain catastrophizing was measured using the Pain Catastrophizing Scale (PCS).26 Participants indicate the degree to which they experienced each of 13 thoughts or feelings when experiencing pain. A total PCS score of 30 indicate a clinically relevant level of catastrophizing. The PCS has adequate to excellent internal consistency and satisfactory validity.
Cancer and treatment distress was measured by the Cancer Treatment Distress Scale,27 which has shown excellent psychometric properties. This scale consists of 22 items that assess how much distress or worry cancer or its treatment has caused in the past week.
2.5 | Procedures
Research assistants contacted individuals participating in previous studies who indicated an interest in being contacted about future studies and, if interested, screened them for eligibility. Also clinical oncology providers identified potentially eligible patients, suggested the study to them, and provided a brochure that included contact information for the study research assistants.
Eligible participants were randomly assigned to treatment order (ie, receiving the VMWH-CBT intervention first and then the EC intervention or vice versa). The randomization was blocked so that the allocation ratio was 1:1. The blocks had different sizes in different orders for each subgroup to prevent the study clinician (M.E.M.) from being able to predict the randomization order. In order to avoid unblinding the research staff who collected outcome data, the clinician prepared the materials for the condition assigned to each participant after they had consented for participation.
The primary and secondary outcome measures were administered by phone by research assistants who were blind to the study hypotheses and treatment condition. Outcome measures were administered at pretreatment, after the first set of four treatments was completed, after the second set of four treatments, and at 3-month follow-up. The measures of descriptive/demographic information were administered at pretreatment only. Participants did not receive any compensation for participation, and all study procedures were approved by the University of Washington Institutional Review Board. All participants provided signed informed consent.
2.6 | Data analyses
We first computed descriptive statistics for the demographic and cancer history variables. Next, we evaluated a possible treatment-order effect by performing a series of three repeated measure analyses of variance (ANOVAs), with both primary and secondary outcome measures as the dependent variables, and time (pretreatment, mid-treatment, and post-treatment) and treatment order (VMWH-CBT first vs EC first) as the independent variables. Because no significant order main effects or Time × Treatment-order interaction effects emerged from these analyses, we collapsed the analyses over the order variable for all subsequent analyses.
For descriptive purposes we computed the means and standard deviations of the scores for symptom severity at pretreatment and post-treatment for each intervention and for each primary outcome variable. We also computed the effect sizes (Cohen’s d) for pretreatment to post-treatment improvements in outcome measures for both interventions, as well as the percent of participants who showed meaningful improvements after each intervention for each outcome measure. For these responder analyses, we defined meaningful relief as an improvement in the outcome measures greater than half of the standard deviation of the baseline score.28 To test the primary study hypothesis, we performed a series of repeated measures ANOVAs (Bonferroni adjusted for multiple comparisons) to compare the change scores associated with each treatment condition for pain, fatigue, and/or sleep problems, including in analyses all participants who endorsed at least some level (greater than 0) of the outcome variable at pretreatment and collapsed across treatment order. We then repeated these analyses for the secondary outcome variables. Finally, to examine the stability of the changes from post-treatment to 3-month follow-up, we performed repeated measures ANOVAs (Bonferroni adjusted) to compare the means for each outcome measure across time from pretreatment to after both treatments and 3 months follow-up.
3| RESULTS
3.1 | Recruitment and demographic characteristics
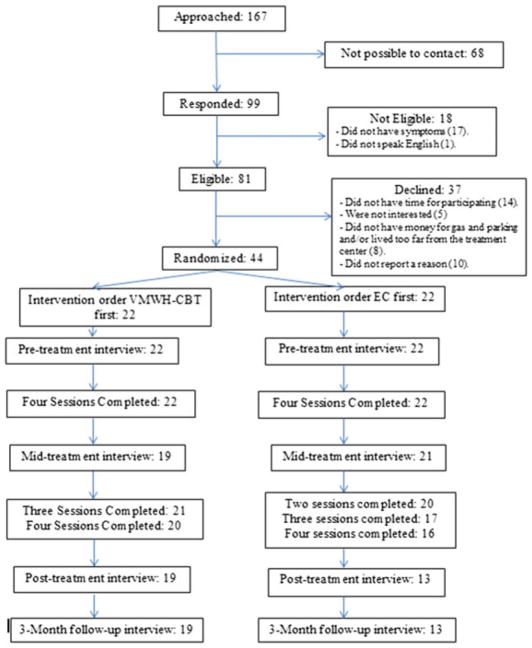
A total of 167 patients were identified as potential participants (Figure 1). We were able to contact and screen 99 of these. Forty-four (44.4% of those who were screened) were eligible and willing to participate, and were then randomized to receive the interventions. Eleven participants (25%) were recruited from individuals participating in previous studies who indicated an interest in being contacted about future studies. Twenty-four (55%) participants were referred from the Women’s Wellness Clinic at Seattle Cancer Care Alliance, and nine participants (20%) from cancer survivor groups in the Seattle metropolitan area. One bilingual participant received the sessions and assessments in her primary language (Spanish), although she followed all the reading materials in English. Twelve (27.3%) of the participants withdrew from the study: 4 were unable to be contacted for follow-up assessments; and 8 withdrew during treatment. Reasons for attrition included the following: medical issues prevented them from attending the sessions (n = 3), having too many personal problems to be able to follow the program (n = 1), death (n = 1), moving to another state (n = 1), having to travel often overseas (n = 1), and wanting to pursue more therapy with more sessions (n = 1). Participants’ demographic characteristics are presented in Table 1.
FIGURE 1.
Flow of participants through the study
TABLE 1.
Demographic characteristics of the sample
| Variable | N | % |
|---|---|---|
| Gender | ||
| Male | 5 | 11 |
| Female | 39 | 89 |
| Age | ||
| Mean (min–max)/SD | 60.95 (29–85) | 12.2 |
| Marital status | ||
| Single | 11 | 25 |
| Married | 29 | 66 |
| Separated/divorced | 4 | 9 |
| Employment status | ||
| Working | 18 | 41 |
| Not working | 21 | 48 |
| Retired | 5 | 11 |
| Type of cancer | ||
| GYN cancer | 29 | 66 |
| Prostate cancer | 2 | 5 |
| Leukemia | 2 | 5 |
| Soft tissue sarcoma | 1 | 2 |
| Lymphoma | 1 | 2 |
| Brain tumor | 1 | 2 |
| Unknown | 8 | 18 |
| Symptoms | ||
| Pain | 33 | 75 |
| Fatigue | 25 | 57 |
| Sleep problems | 40 | 91 |
| Pain, fatigue, and sleep problems | 17 | 39 |
Abbreviation: GYN, Gynecologic.
3.2 | Effects of the interventions on the outcome measures
Table 2 presents the means and standard deviations for the primary outcome measures (pain, fatigue, and sleep problems) assessed at pretreatment and post-treatment collapsed across treatment order and including all participants who endorsed at least some level (greater than 0) of the outcome variable at pretreatment. The same statistics are included for secondary outcome measures (depression, pain catastrophizing, cancer treatment distress, and pain interference). The effect sizes for pretreatment to post-treatment improvements in outcome measures ranged from 0.38 to 0.93 for the VMWH-CBT condition, being small (0.20–0.50) for pain interference, medium (0.50–0.80) for sleep problems, fatigue, and pain catastrophizing, and large (>0.80) for average pain intensity, depression, and cancer treatment distress. The effect sizes for the EC intervention ranged from −0.12 to 0.30, being small for all the outcome variables and below 0 for pain interference (which indicates a slight worsening in the outcome variable from pretreatment to post-treatment). As can be seen, across the outcome measures, the percentage of patients reporting a meaningful relief after the VMWH-CBT intervention ranged from 42% to 64%, whereas for the EC intervention it ranged from 19% to 45%.
TABLE 2.
Means, SDs, ANOVA result, and effect size for the outcome measures at pretreatment and post-treatment, collapsed across treatment order (including all participants who endorsed at least some level [greater than 0] of the outcome variable at pretreatment)
| Treatment Condition | N | Pretreatment Mean (SD) | Post-treatment Mean (SD) | P value* | Effect Size (η2P) for Time Effect | Effect Size (d) for Time Effect | % Who Obtained MR |
|---|---|---|---|---|---|---|---|
| Primary outcome measures | |||||||
| Average pain intensity (NRS-11) | |||||||
| VMWH-CBT | 25 | 2.76 (1.27) | 1.80 (1.22) | <.000 | 0.41 | 0.82 | 64 |
| Education control | 30 | 2.67 (1.28) | 2.33 (1.27) | .106 | 0.09 | 0.30 | 43 |
| Sleep problems (MOS-9) | |||||||
| VMWH-CBT | 32 | 44.57 (17.47) | 30.52 (14.29) | <.000 | 0.37 | 0.76 | 59 |
| Education control | 38 | 38.44 (16.55) | 35.01 (17.02) | .124 | 0.06 | 0.26 | 45 |
| Fatigue (PROMIS) | |||||||
| VMWH-CBT | 32 | 53.97 (7.20) | 48.36 (6.63) | <.000 | 0.37 | 0.75 | 56 |
| Education control | 38 | 52.86 (8.21) | 51.39 (8.26) | .095 | 0.07 | 0.28 | 34 |
| Secondary outcome measures | |||||||
| Depression (PHQ-8) | |||||||
| VMWH-CBT | 32 | 6.41 (4.07) | 3.63 (2.72) | <.000 | 0.44 | 0.87 | 56 |
| Education control | 38 | 5.43 (3.74) | 4.79 (4.01) | .182 | 0.05 | 0.22 | 34 |
| Pain interference (PROMIS) | |||||||
| VMWH-CBT | 32 | 52.98 (8.09) | 50.23 (6.21) | .038 | 0.13 | 0.38 | 44 |
| Education control | 37 | 51.91 (7.49) | 52.50 (6.97) | .454 | 0.02 | −0.12 | 19 |
| Pain catastrophizing (PCS) | |||||||
| VMWH-CBT | 24 | 14.13 (11.39) | 4.96 (6.49) | .004 | 0.30 | 0.65 | 50 |
| Education control | 30 | 11.73 (9.18) | 9.81 (9.75) | .266 | 0.04 | 0.21 | 33 |
| Cancer treatment distress (CTDX) | |||||||
| VMWH-CBT | 32 | 1.01 (.53) | 0.59 (0.44) | <.000 | 0.47 | 0.93 | 56 |
| Education control | 37 | 0.93 (.63) | 0.82 (0.55) | .107 | 0.07 | 0.28 | 32 |
Abbreviations: ANOVA, analysis of variance; NRS-11, 0–10 numerical rating scale; MOS-9, Medical Outcomes Survey Sleep Problem Index; PROMIS, Patient-reported Outcomes Measurement Information System; PHQ-8, Patient Health Questionnaire, Depression Scale; PCS, Pain Catastrophizing Scale; CTDX, Cancer Treatment Distress Scale.
% who obtained meaningful relief (MR) = change after treatment >½ SD.
P value for repeated measures ANOVA group by time interaction (P < .05, Bonferroni adjusted).
With respect to the planned between-group comparisons in pretreatment to post-treatment changes in the primary outcomes, we found significantly greater improvements (P < .001) following active treatment, relative to the control condition, for sleep problems, fatigue, and average pain intensity. For the secondary outcome variables, significant between-groups differences emerged for depression (P < .001), cancer distress (P < .001), pain interference (P < .05), and pain catastrophizing (P < .05).
3.3 | Maintenance of treatment gains on outcome measures
Table 3 presents the means and standard deviations for the outcome measures at three time points for those participants who endorsed the symptoms at a level greater than 0: pretreatment, after the participant had received both treatments, and at 3-month follow-up. There is a significant time effect (P ≤ .001, Bonferroni adjusted) for all outcome measures except for pain interference. The significant changes are reported from pretreatment to post-treatment, and there are no significant changes from post-treatment to 3-month follow-up.
TABLE 3.
Means, SDs, ANOVA result, and effect size for the outcome measures at pretreatment, after they had received both treatments, and at 3-month follow-up (including all participants who endorsed at least some level [greater than 0] of the outcome variable at pretreatment)
| Overcome Variable | N | Pretreatment Mean (SD) | After Both Treatments Mean (SD) | 3-Month Follow-up Mean (SD) | P value for Time Effect | Effect Size (η2PARC) for Time Effect |
|---|---|---|---|---|---|---|
| Primary outcome measures | ||||||
| Average pain | 22 | 2.86a (1.21) | 1.64b (1.26) | 1.86b (1.39) | .001 | 0.29 |
| Intensity (NRS-11) | 29 | 54.87a (6.54) | 48.52b (7.72) | 48.53b (9.10) | <.000 | 0.28 |
| Fatigue (PROMIS) | ||||||
| Sleep problems (MOS) | 29 | 46.65a (17.06) | 28.43b (14.13) | 27.99b (16.28) | <.000 | 0.41 |
| Secondary outcome measures | ||||||
| Depression (PHQ-8) | 29 | 6.38a (3.11) | 3.17b (2.67) | 3.41b (2.37) | <.000 | 0.33 |
| Pain interference (PROMIS) | 29 | 51.78a (8.24) | 49.60a (6.75) | 49.47a (7.51) | .168 | 0.06 |
| Pain Catastrophizing (PCS) | 20 | 13.05a (9.88) | 3.95b (3.78) | 3.70b (4.60) | <.000 | 0.39 |
| Cancer treatment distress (CTXD) | 29 | 0.94a (0.50) | 0.51b (0.43) | 0.57b (0.45) | <.000 | 0.37 |
Means with different subscripts are significantly different from one another (P < .05, Bonferroni adjusted).
P value for repeated measures ANOVA group by time interaction (P < .05, Bonferroni adjusted).
4| DISCUSSION
The findings support the primary hypothesis that the VMWH-CBT intervention results in clinically significant greater improvements in pain, fatigue, and sleep problems than an EC intervention. The same effects were found in the secondary outcomes assessing depression, pain catastrophizing, cancer treatment distress, and pain interference. The gains of the treatment in the primary and secondary outcomes remained stable up to the 3-month follow-up assessment for all outcome measures except for pain interference. The lower effects sizes for pain interference may be due to floor effects, as baseline scores for this measure were low for most of the participants. The effect sizes for all measures were larger following VMWH-CBT than following EC treatment. Moreover, the percentage of participants reporting a meaningful relief in their symptoms was higher after the VMWH-CBT intervention than after the EC for all measures. These results are consistent with previous research on hypnosis as an adjunct to CBT to improve fatigue in patients undergoing radiotherapy for breast cancer,7,8 and on hypnosis alone to reduce hot flashes,6 and to manage pain, fatigue, hot flashes, and sleep problems in women who are breast cancer survivors.5
This study has some important limitations. First, although the interventions were applied using a manual (ie, it was a highly standardized intervention), only one clinician, who was not blind to the hypotheses, provided both treatments to all the participants. Future studies should involve more clinicians when possible to control for the potential biasing effects of the therapist’s skills and expectancies. In addition, there were relatively few men in the sample. Thus, it is not clear if the findings would necessarily generalize to men with a history of cancer, although we know of no evidence suggesting that hypnotic or CBT approaches are more or less effective for women relative to men. Moreover, the aim of this study was to investigate the clinical benefits of the VMWH treatment when combined with CBT. As a result, we were not able to evaluate the relative contribution of each element to the overall benefits observed. Thus, further research is needed to identify the unique contributions of these treatment elements both alone and in combination and to evaluate their mechanisms. Finally, we did not measure expectancies for the treatments or evaluate the potential role of other mechanisms that could explain outcome (eg, brain activity, changes in self-efficacy). An important next step is to evaluate the role that such mechanism factors play in the benefits of this treatment.
Despite the study’s limitations, the findings make important new contributions to our understanding of the potential for non-pharmacological interventions to benefit individuals with cancer-related symptoms. To our knowledge, this is the first study testing the efficacy of the VMWH combined with CBT for managing the symptoms of pain, fatigue, and sleep problems in individuals with a history of cancer. The results support the beneficial effects of the intervention relative to an educational intervention that controls for the effects of time, therapist attention, and participation in a clinical trial, and indicate that the benefits are maintained for at least 3 months. Importantly, the intervention had no reported adverse effects. In fact, it can be viewed as empowering, as it teaches patients skills that they can use to better manage bothersome symptoms themselves. The VMWH in combination with CBT warrants further research as a promising intervention to help patients with cancer to better manage fatigue, pain, and sleep problems and to increase their quality of life.
Acknowledgments
This research was funded by a grant given to the first author by the Spanish Foundation of Science and Technology (Spanish Ministry of Science and Innovation) year 2011–2012.
References
- 1.Roscoe JA, Kaufman ME, Matteson-Rusby SE, et al. Cancer-related fatigue and sleep disorders. The Oncologist. 2007;12(Suppl 1):35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- 2.Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30:3687–3696. doi: 10.1200/JCO.2012.41.7238. [DOI] [PubMed] [Google Scholar]
- 3.Del Casale A, Ferracuti S, Rapinesi C, et al. Pain perception and hypnosis: findings from recent functional neuroimaging studies. Int J Clin Exp Hypn. 2015;63(2):144–170. doi: 10.1080/00207144.2015.1002371. [DOI] [PubMed] [Google Scholar]
- 4.Cramer H, Lauche R, Paul A, Langhorst J, Kummel S, Dobos GJ. Hypnosis in breast cancer care: a systematic review of randomized controlled trials. Integr Cancer Ther. 2014;14(1):5–15. doi: 10.1177/1534735414550035. [DOI] [PubMed] [Google Scholar]
- 5.Jensen MP, Gralow JR, Braden A, Gertz KJ, Fann JR, Syrjala KL. Hypnosis for symptom management in women with breast cancer: a pilot study. Int J Clin Exp Hypn. 2012;60(2):135. doi: 10.1080/00207144.2012.648057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elkins G, Marcus J, Stearns V, et al. Randomized trial of a hypnosis intervention for treatment of hot flashes among breast cancer survivors. J Clin Oncol. 2008;26:5022–5026. doi: 10.1200/JCO.2008.16.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montgomery GH, Kangas M, David D, Hallquist MN, Green S, Bovbjerg DHB. Fatigue during breast cancer radiotherapy: an initial randomized study of CBT plus hypnosis. Health Psychol. 2009;28(3):317–322. doi: 10.1037/a0013582. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery GH, David D, Kangas M, et al. Randomized controlled trial of a cognitive-behavioral therapy plus hypnosis intervention to control fatigue in patients undergoing radiotherapy for breast cancer. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.49.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capafons A. Rapid self-hypnosis: a suggestion method for self-control. Psicothema. 1998;10:571–581. [Google Scholar]
- 10.Capafons A. Hipnosis (Hypnosis) Madrid: Síntesis; 2001. [Google Scholar]
- 11.Capafons A. Clinical applications of “waking” hypnosis from a cognitive-behavioural perspective: from efficacy to efficiency. Contemporary Hypnosis. 2004;21:187–201. [Google Scholar]
- 12.Capafons A, Mendoza ME. “Waking” hypnosis in clinical practice. In: Rhue JW, Lynn SJ, Kirsch I, editors. Handbook of Clinical Hypnosis. 2. Washington, D.C: American Psychological Association; 2010. pp. 293–317. [Google Scholar]
- 13.Beck AT. Cognitive therapy: nature and relation to behavior therapy. Behav Ther. 1970;1:184–200. [Google Scholar]
- 14.Tan G, Rintala DH, Jensen MP, Fukui T, Smith D, Williams W. A randomized controlled trial of hypnosis compared with biofeedback for adults with chronic low back pain. Eur J Pain. 2015;19:271–280. doi: 10.1002/ejp.545. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MP, Ehde DM, Gertz KJ, et al. Effects of self-hypnosis training and cognitive restructuring on daily pain intensity and catastrophizing in individuals with multiple sclerosis and chronic pain. International Journal of Clinical and Experimental Hypnosis 2011. 2011;59:45–63. doi: 10.1080/00207144.2011.522892. [DOI] [PubMed] [Google Scholar]
- 16.Capafons A, Mendoza ME. The Valencia model of waking hypnosis and its clinical applications. In: Koester GD, Delisle PR, editors. Hypnosis: Theories, Research and Applications. New York: Nova Science Publishers Inc; 2009. pp. 237–270. [Google Scholar]
- 17.Ehde DM, Jensen MP. Feasibility of a cognitive restructuring intervention for treatment of chronic pain in persons with disabilities. Rehabil Psychol. 2004;49:254–258. [Google Scholar]
- 18.Jensen MP. Pain assessment in clinical trials. In: Wittink H, Carr D, editors. Pain management: Evidence, outcomes, and quality of life in pain treatment. Amsterdam: Elsevier; 2008. pp. 57–88. [Google Scholar]
- 19.Lai JS, Cella D, Choi S, et al. How item banks and their application can influence measurement practice in rehabilitation medicine: a PROMIS fatigue item bank example. Arch Phys Med Rehabil. 2011;92(10 Suppl):S20–S27. doi: 10.1016/j.apmr.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, Cella D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-reported Outcomes Measurement Information System (PROMIS) J Clin Epidemiol. 2010;63(11):1195–1204. doi: 10.1016/j.jclinepi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hays R, Stewart A. Sleep measures. In: Stewart A, Ware J, editors. Measuring Functioning and Well-being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1992. pp. 235–259. [Google Scholar]
- 22.Hays RD, Martin SA, Sesti AM, Spritzer KL. Psychometric properties of the medical outcomes study sleep measure. Sleep Med. 2005;6(1):41–44. doi: 10.1016/j.sleep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150(1):173–182. doi: 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 25.Thekkumpurath P, Walker J, Butcher I, et al. Screening for major depression in cancer outpatients: the diagnostic accuracy of the 9-item patient health questionnaire. Cancer. 2011;117(1):218–227. doi: 10.1002/cncr.25514. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 27.Syrjala KL, Yi JC, Langer SL. Psychometric properties of the cancer and treatment distress (CTXD) measure in hematopoietic cell transplantation patients. Psychooncology. 2016;25(5):529–535. doi: 10.1002/pon.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]