Abstract
Engaging both partners of a pregnant couple can enhance prevention of mother-to-child transmission of HIV and promote family health. We developed and piloted an intervention to promote couple collaboration in health during pregnancy and postpartum in southwestern Kenya. We utilized formative data and stakeholder input to inform development of a home-based couples intervention. Next, we randomized pregnant women to intervention (n = 64) or standard care (n = 63) arms, subsequently contacting their male partners for enrollment. In the intervention arm, lay health workers conducted couple home visits, including health education, couple relationship and communication skills, and offers of couple HIV testing and counseling (CHTC) services. Follow-up questionnaires were conducted 3 months postpartum (n = 114 women, 86 men). Baseline characteristics and health behaviors were examined by study arm using t-tests, chi-square tests, and regression analyses. Of the 127 women randomized, 96 of their partners participated in the study. Of 52 enrolled couples in the intervention arm, 94% completed at least one couple home visit. Over 93% of participants receiving couple home visits were satisfied and no adverse social consequences were reported. At follow-up, intervention couples had a 2.78 relative risk of having participated in CHTC during the study period compared with standard care couples (95% confidence interval: 1.63–4.75), and significant associations were observed in other key perinatal health behaviors. This pilot study revealed that a home-based couples intervention for pregnant women and male partners is acceptable, feasible, and has the potential to enhance CHTC and perinatal health behaviors, leading to improved health outcomes.
Keywords: : pregnancy, HIV infection, male engagement, couple interventions, Africa
Introduction
Despite demonstrated success of antiretroviral therapy (ART) for treating maternal HIV disease and prevention of mother-to-child transmission (PMTCT), HIV prevalence among mothers and infants in Kenya remains persistently high.1,2 While rates of antenatal HIV testing continue to increase, only half of women testing HIV positive receive the full course of ART and only a portion of these women complete the series of steps required for efficacious PMTCT, known as the “PMTCT cascade.”3–6 A recent systematic review found that loss to follow-up in PMTCT programs in sub-Saharan Africa was around 49%7 and several other studies have indicated limited retention despite improvements in implementation of these programs.8,9
Studies in sub-Saharan Africa suggest that fears of stigma and violence reduce pregnant women's acceptance of HIV testing during antenatal care (ANC) and limit participation in programs for PMTCT.10–13 Our research in Kenya found that fears and experiences of stigma, from a male partner in particular, decrease antenatal HIV testing, limit linkage to HIV care, and reduce the uptake of skilled childbirth services.14–16 Two systematic reviews suggest that stigma, violence, and discrimination continue to hinder PMTCT uptake across sub-Saharan Africa.17,18
Nondisclosure of HIV status between partners also limits PMTCT uptake in sub-Saharan Africa.19,20 Lack of disclosure to partners can have drastic health implications for HIV-positive pregnant women by limiting initiation of HIV care; increasing the risk of sexual transmission of HIV if their male partner is HIV negative; and increasing the likelihood of suboptimal adherence to PMTCT interventions.21–24 Disclosure of HIV status can increase access to social support, create closer relationships with others,20,25,26 and may increase the likelihood that a pregnant woman delivers in a facility and uses antiretrovirals for PMTCT.27
Women who initially test HIV negative in ANC and their male partners are another crucial group to include in PMTCT interventions, because they may feel “safe” after an initial HIV-negative test result at the clinic.28 Recent studies have found seroconversion rates close to 3% among previously HIV-negative pregnant women in sub-Saharan Africa.29,30 These groups are at high risk of becoming HIV infected during late pregnancy, receive no PMTCT services, have an increased risk of MTCT, and may constitute a significant proportion of cases of vertical transmission.29,31–33
Advocates and scholars across Africa have increasingly called for couple HIV testing and counseling (CHTC) during pregnancy to enhance PMTCT and family health.34,35 However, attendance by male partners at ANC visits varies widely across sub-Saharan Africa.36,37 While the majority of pregnant women in Kenya receive HIV testing, only a small percentage (4.5% in 2013) of their male partners had been tested for HIV within the last 12 months.5 One recent intervention study in Kenya found that home-based couple strategies may improve male uptake of HIV testing during pregnancy.38
We conducted the Jamii Bora (“better family” in Swahili) study, in which we developed and pilot tested a couple relationship-focused intervention to facilitate HIV testing and mutual disclosure within pregnant couples to increase use of PMTCT and family health services. The study was based on couple relationship theory. This article aims to present the intervention development process, the intervention as designed, baseline characteristics of the sample, and process and outcome data from this randomized pilot trial of the home-based couples intervention trial in rural southwestern Kenya.
Methods
Ethical considerations
This study was conducted in the Nyanza Region of Kenya during the period 2014–2017 and was approved by the Institutional Review Boards of the University of Alabama at Birmingham and the Kenya Medical Research Institute. Participants provided signed informed consent in the language of their choice (Luo, English, or Swahili). Participants who reported intimate partner violence (IPV) or major depression in questionnaires were provided with support and referrals. The study was supported by an independent safety monitoring committee, composed of members from Kenyan and international organizations.
The setting
The Nyanza Region has the highest HIV prevalence in Kenya, with ∼15% of adults 15–49 years of age testing HIV positive.39 Maternal mortality in Nyanza is 669 per 100,000 live births,40 four times the national target.41 The research took place in southern Nyanza, in Migori County, which borders Tanzania and Lake Victoria. This setting has had consistently high HIV prevalence among pregnant women (18%) and high rates of MTCT (7–10%),42 and our preliminary data at baseline indicated high rates of dropouts along the PMTCT cascade.43
Conceptual framework
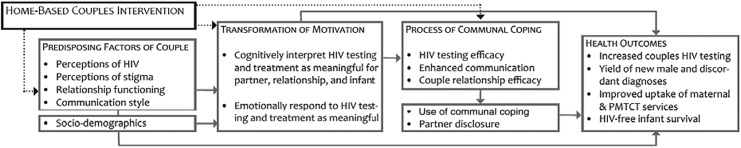
The conceptual framework for this study draws on Lewis et al.'s Interdependence Model of Health Behavior Change,44 which extends beyond an individually based understanding of health behavior change by positing that both partners can influence one another's health decisions and behaviors.45 By influencing the couple as a unit, interventions can help couples perceive potential health threats as impacting them jointly rather than individually, thus encouraging lasting change in health behaviors. We adapted this model (Fig. 1) for our home-based intervention, incorporating aspects of the Kenyan cultural setting elucidated in preliminary studies, such as the influence of extended family members and polygamous unions. Our qualitative work suggested that this model has relevance for understanding HIV-related decisions for pregnant couples in rural southwestern Kenya.46
FIG. 1.
Modified couple interdependence conceptual framework. Predisposing characteristics of couples include both intrinsic qualities (e.g., sociodemographic such as age, education, marital status) and variables that have the potential to be modified through intervention (e.g., perception of health threat and couple communication). Transformation of motivation helps couples move from a self-centered understanding of a health issue to a relationship-centered perspective.44 This process occurs when health issues are interpreted as having significance for the relationship or family, rather than simply for oneself.66 Communal coping is when couples make a joint assessment of a health threat and have a shared vision for managing that threat.67 It is influenced by outcome efficacy, or the couple's belief that a solution can be found to the health challenge, and couple relationship efficacy. Communal coping includes enhanced communication, joint decision-making, and working together to try new behaviors.
Formative work
Before the current study, we conducted qualitative research with HIV-positive pregnant women (n = 20), male partners (n = 20), and service providers (n = 16) to inform the development of an intervention in this setting.47 The results suggested that pregnant women preferred to be tested for HIV together as a couple, even if one or both partners had already been tested separately. Home visits were supported as a way to reduce costs and engage male partners who were reluctant to visit clinics. Participants discussed risks of home visits, including negative reactions of male partners to an unannounced visitor and community gossip. Importantly, they suggested that the program conduct home visits for all pregnant women and not just for HIV-infected women. Thus, before finalizing the intervention design, additional in-depth interviews were conducted with HIV-negative pregnant women (n = 20) and male partners (n = 20), using similar methods, with the addition of topics on couple relationship dynamics.46 Results suggested that a home-based couple intervention would also be acceptable for HIV-negative pregnant women and their partners. Given the importance attached to couple relationships and open communication about HIV, couple communication skills and counseling were highlighted as key components to be included in the intervention.
Development of the intervention
Through a series of meetings in three phases, we consulted with health workers and HIV program staff, community members, and other stakeholders (Table 1). These stakeholder discussions were used to refine the intervention procedures and content, develop the training program for home visitors, and create standard operating procedures for the study.
Table 1.
Stakeholder Process for Intervention Development
| Phase | Type of participants | No. of participants | Objectives | Products |
|---|---|---|---|---|
| Phase 1 | Representatives from local organizations involved in health promotion, health research, and provincial/local administration | 34 | Review the qualitative findings together and brainstorm on needed modifications to existing protocols for home visits and CHTC | Recommendations: the need for male partner consent and notification regarding upcoming home visits, avoiding jumping too quickly to the topic of HIV during the home visits, the need to involve key community gatekeepers, and how to craft community messaging to encourage study recruitment and retention. Stakeholders also recommended that the home visitors have good command of the cultural and social aspects of the community to communicate with and understand the couples, and also be competent in communication and HIV counseling and testing. |
| Phase 2 | Local community organizers and health workers, HIV clinicians, and a representative of the local Ministry of Health team | 5 | Get feedback on and refine the study manual that had been drafted for the pilot randomized controlled trial phase of the study | The team provided guidance on the study manual, including additional health topics to be included in the home visits, namely water and sanitation, birthing plans, immunization, HIV risk reduction and HIV retesting (including infant testing), and linkage to care. |
| Phase 3 | Local community members (chiefs, village elders, community health workers, and representatives of local community organizations) at the two initial study locations | 30 | Elucidate the best approaches for community entry and dissemination of information about the study | Recommendations were made regarding protecting confidentiality of study participants; fair distribution of study participation; socially acceptable features of a home visitor; how to deal with tricky situations such as polygamous marriages, miscarriages, and couple separations; appropriate length of time for a home visit; and an acceptable name for the study. |
CHTC, couple HIV testing and counseling; MOO, Manual of Operations.
The intervention
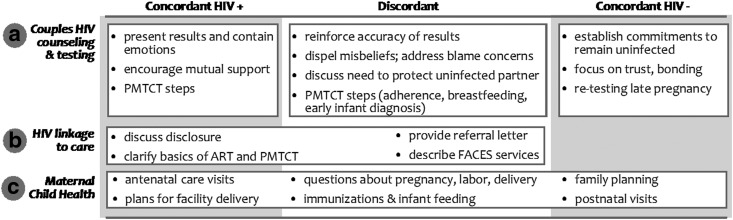
The resulting intervention consisted of home-based couple visits delivered by lay health workers, one male and one female, trained in couple counseling, including CHTC; maternal, child, and family health information; building couple relationship skills; and linkage to facility-based HIV prevention and treatment services. Two home visits were to be conducted during pregnancy—one soon after study enrollment and the next one around 1 month later—and one at ∼1 month postpartum. Intervention content was adapted for different serostatus couples (Fig. 2).
FIG. 2.
Intervention content by couple serostatus.
Health messages also differed by stage of pregnancy/postpartum, with the first visit focusing on topics such as ANC, nutrition, and malaria prevention; the second visit focusing on birth plans, danger signs, and PMTCT, and the third visit focusing on topics such as infant feeding, family planning, and men's health. Beyond offers of CHTC and health messages specific to the timing of the visit, each visit included a couple relationship/communication exercise, including exercises on the use of “I language,” listening skills (initiator and receiver), and negotiation skills.48 Couples had the opportunity to engage in CHTC at any of the three visits.
Pilot randomized controlled trial methods
Design
We conducted a pilot study of this intervention at five ANC clinics in Migori County. This pilot study used an individually randomized, controlled design to assess the acceptability and feasibility of the intervention and research methods, as well as to obtain preliminary data on short-term effects of the intervention on health behaviors. Outcome measures captured included CHTC uptake, use of Maternal and Child Health Services (ANC visits, health facility delivery, postnatal checkup for infant, postpartum checkup for woman), PMTCT behaviors for HIV-positive women (exclusive breastfeeding, ART use), and infant testing uptake for HIV-exposed infants.
Recruitment and inclusion
Inclusion criteria for pregnant women included the following: (a) 18 years or older, (b) had been offered HIV testing at ANC, (c) was currently living with a male partner, (d) was in a stable relationship with the male partner for at least 6 months, (e) had not yet participated in CHTC with this male partner, (f) had not yet disclosed her HIV status to her male partner, (g) did not have a known HIV-positive partner, and (h) gestational age <37 weeks. Male partners were the person identified by the pregnant woman as her primary partner and needed to be 18 years or older. We recruited HIV-negative women in roughly equal numbers to HIV-positive women each month, to ensure that these two groups were balanced over time. Each month, sites screened and recruited a target number of HIV-positive pregnant women first, followed by a similar number of HIV-negative pregnant women.
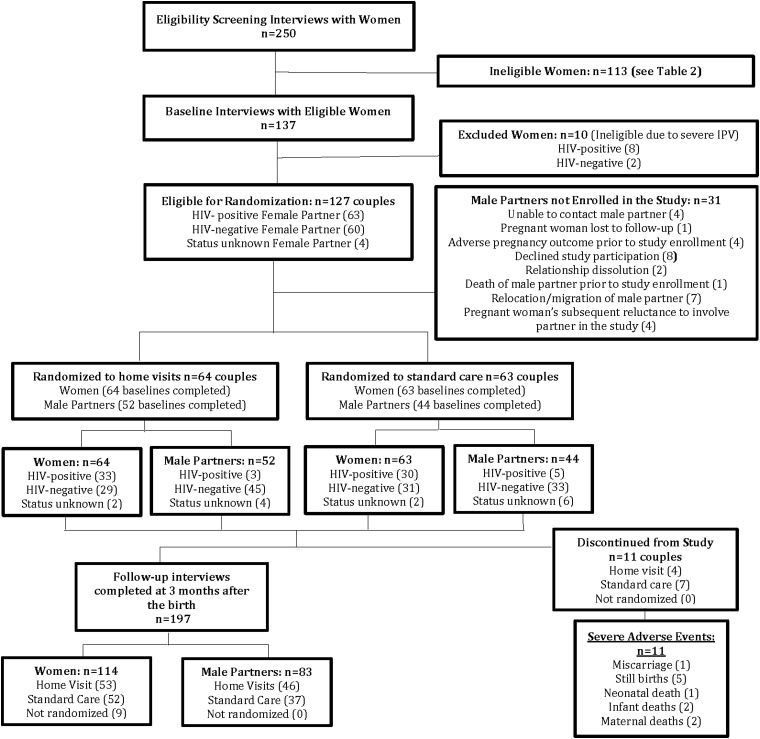
Pregnant women who met study inclusion criteria were asked if they would like to participate in a study about approaches for supporting pregnant couples on family health issues (including HIV). If interested, an initial informed consent process and a baseline questionnaire were administered, followed by a separate consent process for the randomization. Women reporting severe IPV in the past 6 months during the baseline questionnaire were ineligible for randomization. Eligible women were randomized to the intervention arm (home visits) or control arm (standard care). Male partners of randomized women were recruited into the study, asked to provide informed consent, and completed baseline questionnaires, after the pregnant women gave the study team permission to contact the male partners. Participant flow in the study is presented in Fig. 3.
FIG. 3.
Study flow chart.
Study arms
Once a woman was randomized, a lay health worker obtained detailed contact information and consulted with the woman about optimal times and ways to contact her male partner for potential inclusion in the study. Women in both arms of the study were given a letter for their male partners to invite them to participate in “a study we intend to carry out with pregnant women and their male partners, aimed at improving family life.” As described above, the intervention arm consisted of three couple home visits conducted by lay health workers. The control arm offered standard clinic-based ANC services, including the option for women and partners to return to the clinic for male partner HIV testing or CHTC. All ANC clients (women in both study arms) were given standard letters inviting their male partners to come to the clinic with their wives for ANC visits, as part of standard care at these sites. At ANC clinic visits, male partners who attended with their spouses were given priority and did not need to stand in line, unlike unaccompanied women. During standard ANC visits, male partners who attended were invited to participate in CHTC with their pregnant spouses and were given health education similar to what is given to unaccompanied women. A sample size of around 60 couples per study arm was targeted as appropriate for this initial pilot trial focusing on acceptability and feasibility of the intervention.
Data collection
Data to evaluate the intervention were captured from four sources: baseline questionnaires, couple visit forms, follow-up questionnaires, and medical records. Baseline questionnaires were conducted with pregnant women at the ANC clinic and male partners (in clinic or community location) after recruitment of the female partner. These questionnaires were programmed on tablet computers and administered by lay health workers. The questionnaires assessed sociodemographic characteristics of both women and male partners, couple relationship measures, and stigma. During the intervention period, lay health workers completed couple visit forms for each couple home or clinic visit. The form included topics covered during the visit, CHTC uptake and results, assessments of positive and negative life events,49 service linkages provided, and other process measures. This form, along with records of observations of visits by supervisors, was used to assess intervention fidelity. Follow-up questionnaires were conducted with women and male partners 3 months after the expected due date (EDD) of the baby on process and outcome measures, as well as all the measures collected at baseline. These questionnaires were administered to each participant individually during research visits by gender-matched independent interviewers. Medical records—data from medical records at the sites, were used to obtain non-self-report data on healthcare utilization for couples in the study; specifically, the medical records were used to confirm gestation at first ANC visit, current gestation at time of recruitment, the EDD, HIV serostatus of pregnant women at baseline, and couple HIV testing at the clinic.
Data analysis
In preliminary analyses, we described demographic characteristics of women and male partners using one-way frequency tables, as well as independent t-tests and chi-square tests to compare the baseline characteristics of women and men randomized to the intervention versus control groups. To examine potential generalizability of the research findings, we examined the screening data to identify the main reasons that potential participants were determined ineligible for the study, as well as examining reasons for male partner nonparticipation. To examine acceptability and feasibility of the intervention, we examined process and outcome indicators from the couple visit and follow-up questionnaire data, comparing indicators by study arm using chi-square tests and logistic regression analyses. Due to the prospective randomized controlled trial of the study design, odds ratios were converted to relative risks (RRs).
Results
Study participation
Of 250 pregnant women screened at the ANC clinics, 137 were determined eligible for the study and completed baseline questionnaires. Table 2 shows the distribution and overlap of the reasons that 109 women were determined ineligible. The main reasons for ineligibility were (1) not being in a stable relationship with a male partner for at least 6 months, often combined with not living with the male partner, and (2) having already tested for HIV together and/or disclosed HIV status, including those who had a male partner who had already disclosed his HIV-positive status to the woman. Ten women who reported severe IPV in the past 6 months in the baseline questionnaire were not randomized but were retained in the overall study and were contacted for follow-up questionnaires 3 months after the birth. None of the women, deemed eligible for the randomized portion of the study, refused participation.
Table 2.
Factors Influencing Study Ineligibility Status (N = 109)
| Frequency | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| (A) Gestation >36 weeks | (B) Age <18 | (C) Not in stable relationship for at least 6 months | (D) Male partner does not live in the same household at least one night per week | (E) Have already done CHTC with male partner | (F) Have already disclosed HIV status to male partner | (G) Know with certainty that male partner is HIV positive | Ineligibility code, signaling one or more factors influencing ineligibility | No. of potential participants in category | Percent of ineligible women |
| X | A | 1 | 0.9 | ||||||
| X | X | A, D | 1 | 0.9 | |||||
| X | X | X | X | A, E–G | 1 | 0.9 | |||
| X | X | A, F | 1 | 0.9 | |||||
| X | X | X | B–D | 4 | 3.7 | ||||
| X | X | X | X | B, E–G | 2 | 1.8 | |||
| X | X | B, F | 1 | 0.9 | |||||
| X | X | X | B, F, G | 1 | 0.9 | ||||
| X | X | C, D | 15 | 14 | |||||
| X | X | X | X | X | C–G | 1 | 0.9 | ||
| X | X | X | C, D, F | 1 | 0.9 | ||||
| X | X | X | C, E, F | 1 | 0.9 | ||||
| X | D | 16 | 14.7 | ||||||
| X | X | D, E | 2 | 1.8 | |||||
| X | X | X | D–F | 4 | 3.7 | ||||
| X | X | X | X | D–G | 1 | 0.9 | |||
| X | X | D, F | 5 | 4.6 | |||||
| X | X | X | D, F, G | 1 | 0.9 | ||||
| X | E | 5 | 4.6 | ||||||
| X | X | E, F | 17 | 15.6 | |||||
| X | X | X | E–G | 16 | 14.7 | ||||
| X | F | 5 | 4.6 | ||||||
| X | X | F, G | 6 | 5.5 | |||||
| X | G | 1 | 0.9 | ||||||
| Total | 109 | 100 | |||||||
Of the participants who completed the eligibility screen (250), 113 participants were ineligible for randomization into the study arms, of whom 4 had missing eligibility data. The above 109 participants with ineligibility data had between 1 and 5 reasons for exclusion. Categories/combinations with over 10% are presented in bold.
CHTC, couple HIV testing and counseling.
Randomization, male participation, and baseline characteristics
A total of 127 pregnant women were randomized into intervention or control arms. Ninety-six of their male partners (76%) were located and also enrolled in the study. Among women whose male partners could not be enrolled in the study (n = 31), reasons included the woman's subsequent lack of comfort with including her male partner in the study (4), relocation/migration of the male partner (7), male partner declining study participation (8), adverse pregnancy outcome (4), male partner unable to be contacted (4), relationship dissolution (2), death of male partner before enrollment (1), and the woman being lost to follow-up (1). Table 3 presents a comparison of characteristics of intervention versus control participants at baseline, separately for males and females. None of the differences by study arm were statistically significant.
Table 3.
Comparison of Intervention and Control Arm Participants at Baseline (N = 233)
| Women (n = 127) | Men (n = 96) | |||
|---|---|---|---|---|
| Variablesa | Intervention (n = 64) | Control (n = 63) | Intervention (n = 52) | Control (n = 44) |
| Median age | 23 (20–28) | 24 (20–30) | 32 (26–39) | 32 (27–41) |
| Median weeks of pregnancy at baseline | 26 (20–28) | 28 (20–32) | — | — |
| Median No. of pregnancies | 3 (2–4) | 3 (2–5) | — | — |
| Median No. of living children | 2 (1–3) | 2 (1–3) | 2 (1–4) | 2.5 (1–6) |
| Pregnancy intendedness | ||||
| Unintended pregnancyb | 38 (59.4) | 38 (60.3) | 18 (34.6) | 16 (36.4) |
| Major or severe depressionc | 1 (1.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Primary school education or less | 48 (75.0) | 43 (68.3) | 27 (51.9) | 24 (54.6) |
| Ownership of own mobile phone (not shared) | 38 (59.4) | 39 (61.9) | 47 (90.4) | 40 (90.9) |
| Works in farming or manual labor | 26 (40.6) | 16 (25.4) | 26 (50.0) | 18 (40.9) |
| Household food insecurity | ||||
| No hunger | 60 (93.8) | 58 (92.1) | 48 (92.3) | 40 (90.9) |
| Moderate hunger | 4 (6.3) | 5 (7.9) | 3 (5.8) | 4 (9.1) |
| Severe hunger | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Church attendance in the last 7 days | 42 (65.6) | 45 (71.4) | 31 (59.6) | 23 (52.3) |
| House with greater than one room | 50 (78.1) | 43 (68.3) | 38 (73.1) | 33 (75.0) |
| In a polygynous relationship | 12 (18.8) | 16 (25.4) | 9 (17.3) | 11 (25.0) |
| HIV positive at baselined | 33 (51.6) | 31 (49.2) | 3 (5.8) | 5 (11.4) |
| Serodiscordant couplesd (+/− or −/+) | 19 (36.5) | 12 (27.3) | ||
| Disclosed HIV test results to anyone besides health worker at baselinee | 4 (6.5) | 1 (1.6) | 8 (16.7) | 11 (28.2) |
Continuous variables are reported as medians and interquartile ranges in parentheses, and differences in these variables by study arm were tested with independent t-tests. Categorical variables are reported as ns, with percentages in parentheses, and differences in these variables were tested with chi-square tests. No differences between intervention and control were statistically significant.
Unintended pregnancy includes participants who wished to be pregnant at a later time or did not wish to be pregnant at any time.
Major or severe depression at the time of the questionnaire (referring to the last 2 weeks) was assessed using the Patient Health Questionnaire (PHQ)-9.65
Women's baseline HIV status was obtained from medical records, whereas men's baseline HIV status was self-reported. Serodiscordance is based on the individually reported HIV status for each partner in baseline questionnaires (home visit couples n = 52, standard care couples n = 44).
Excluding those responding refuse to answer, with resulting n's of 62 women in intervention, 61 women in control; 48 men in intervention and 39 men in control.
Follow-up questionnaires and study retention rates
All women and men enrolled in the study completed baseline questionnaires. Following randomization and baseline interviews, a total of 11 couples were subsequently discontinued from the study (4 from the intervention arm and 7 from the control arm) due to miscarriage (1), stillbirth (5), maternal death (2), or neonatal/infant death (3); leaving a sample of 60 women randomized to the intervention arm and 56 randomized to the control arm. If we consider only couples in which both partners were enrolled in the study, the resulting sample is 52 couples in the intervention arm and 44 couples in the control arm. The difference in the number of enrolled couples in the study arms (8 couples fewer in the control arm) appears to be both a function of the higher numbers of adverse events in the control arm and to less successful recruitment of males in the control arm. Those who were not discontinued from the study due to infant or maternal death were eligible to participate in the follow-up questionnaires 3 months after the birth. Of 126 eligible women, 114 could be located and participated in a follow-up questionnaire (90% retention rate). Of 96 eligible men, 83 could be located and participated in a follow-up questionnaire (86% retention rate). The study flow diagram is presented in Fig. 3.
Feasibility and acceptability of couple visits
Of the 52 enrolled couples in the intervention arm, 49 received at least 1 couple home visit. Eight couples completed only 1 home visit, 11 couples completed 2 visits, and 30 couples completed all 3 visits. Reported social consequences of home visits were overwhelmingly positive and included strengthened couple relationships, increased communication, and increased emotional support. No participants reported negative social consequences on the couple visit forms. Satisfaction with couple home visits was high, with 93.8% being very satisfied or satisfied.
Couple HIV testing and counseling
Thirty-three couples in the intervention arm participated in CHTC during a home visit (16 at first visit, 16 at second visit, and 1 at third visit). Three couples decided to test together at a health facility following a couple home visit. Four new HIV-positive diagnoses and seven serodiscordant couples were identified during the couple home visits, and all were successfully linked to HIV care. At follow-up, women in the intervention arm had almost three times higher RR for undergoing CHTC with their male partner during the study period, compared with women in the standard care arm (RR = 2.78; 95% confidence interval: 1.63–4.75) (Table 4).
Table 4.
Perinatal Health Behaviors by Study Arm
| Perinatal health behavior | Percent in intervention arm (n = 53) | Percent in control arm (n = 52) | Relative riska | 95% CI | p |
|---|---|---|---|---|---|
| Couple HIV testing and counselingb | 64 | 23 | 2.78 | 1.63–4.75 | <0.001 |
| Any male partner attendance of ANC visitsc | 52 | 43 | 1.21 | 0.76–1.91 | 0.425 |
| Giving birth at a health facilityd | 87 | 79 | 1.10 | 0.92–1.31 | 0.284 |
| Exclusive breastfeeding at 3 months postpartumd | 91 | 76 | 1.18 | 0.99–1.41 | 0.058 |
| Infant checkupd | 100 | 100 | — | — | — |
| Postpartum checkup for motherd | 72 | 50 | 1.43 | 1.04–1.98 | 0.027 |
| Postpartum family planning used | 79 | 77 | 1.03 | 0.84–1.26 | 0.774 |
Data from couple visit forms and follow-up questionnaires at 3 months postpartum.
Relative risks were not adjusted due to the randomized controlled design, with no significant differences detected between the intervention and control group.
Variable created using data from women's follow-up questionnaire, men's follow-up questionnaire, and couple visit forms.
Based on male follow-up questionnaire data (intervention n = 47, control n = 37).
Based on female follow-up questionnaire data.
ANC, antenatal care; CI, confidence interval.
Perinatal health behaviors
Positive trends and some significant associations were observed in several perinatal health behaviors: any male partner attendance at ANC visits (52% intervention vs. 43% control, p = 0.42); giving birth in a health facility (87% vs. 79%, p = 0.28); exclusive breastfeeding (90% vs. 76%, p = 0.06); and maternal postpartum checkup (72% vs. 50%, p = 0.03). Infant postnatal checkups were universal in both study arms (100%) and postpartum family planning use was similar in the two groups (79% vs. 77%, p = 0.77). Results of logistic regression analyses are presented in Table 4.
Discussion
This pilot study suggests that a home-based couple approach is acceptable and feasible in this rural southwestern Kenyan setting. The intervention model was developed using in-depth formative research and a robust community stakeholder process. The results indicate that couple home visits were appreciated by the majority of couples and facilitated delivery of health information and uptake of CHTC. Our approach focused on the especially vulnerable group of pregnant women, both HIV negative and HIV positive, who had not disclosed their HIV testing/status to their male partner at baseline. Despite this challenge, the study achieved good rates of engagement of male partners, high rates of delivery of the couple home visits, and excellent retention rates suggesting that a home-based couple intervention for pregnant women and male partners has the potential to enhance CHTC and other beneficial perinatal health behaviors leading to improved health outcomes.
These findings are promising given that male partners are clearly a key factor in retention of women and infants in the PMTCT cascade throughout sub-Saharan Africa. When male partners are uninvolved in HIV testing and ANC, women are less likely to accept ART,35,50 less likely to deliver in a health facility, and less likely to adhere to recommended care.51 Thus, many have advocated for engaging men in PMTCT.35,50–54 Yet, most early antenatal HIV testing strategies generally reached out to women only,37,54 making it immensely challenging for men to become involved.55 This is compounded by gender norms that limit men's ability to involve themselves in pregnancy and label ANC clinics as “female spaces.”55–57 Our research58 and that of others59,60 show that men themselves desire more involvement in PMTCT and antenatal services, but may not be reached by traditional clinic-based efforts. It is encouraging to see that CHTC is currently being emphasized in Kenya, with increasing numbers of couples being tested together in clinics.61 However, innovative methods are still needed to reach the majority of couples who do not come to the clinic together and to ensure that male partner involvement occurs in a safe and supportive way.34,57,60
The approach in this study was different than similar home-based couple interventions that have been tested in Kenya. The Home Based Partner Education (HOPE) study delivered a single couple home visit by a pair of counselors (one male and one female) to a larger sample of pregnant women and male partners in the same region of Kenya, and also obtained excellent results in terms of male partner testing (87%) and couple testing (77%) in the intervention arm,62 and was found to be cost-effective in reducing HIV-associated morbidity and mortality.63 The Jamii Bora approach differed in that it (a) focused on pregnant women who had not yet been able to disclose their HIV status; (b) included a series of three visits; (c) had an explicit focus on promoting couple communication and positive couple relationship dynamics; and (d) and was able to address a wide range of health topics around pregnancy and after the birth. Uptake of CHTC during the successive home visits for couples in the intervention arm revealed that some couples were not ready to accept CHTC at the initial home visit but accepted it at later visits, and ultimately that not all couples in the intervention arm decided to undergo CHTC at home. The current study, and subsequent larger efficacy studies, will assess whether this more intensive couple relationship-focused approach has wider benefits in terms of health behaviors and outcomes.
A considerable number of women in our study reported some form of recent IPV at baseline. No participants reported recent IPV at the time of home visits, but we cannot rule out the possibility that participants did not feel free to report violence when their partner was nearby. It appears to be critical to address IPV and related mental health issues as part of couple interventions, and to provide follow-up and support services.19,20 It is also notable that perinatal and maternal mortality was relatively high in this small sample (11 severe adverse events), representing ongoing challenges with high rates of perinatal, maternal, and infant mortality in this region.
Although potentially effective in enhancing family health, the Jamii Bora strategy is somewhat resource intensive. Three visits by a pair of counselors trained in couple counseling may not be feasible for every health center in low-resource settings. However, the home visitors were “lay” counselors—without advanced degrees—and we found that it was possible to train them to conduct couple counseling and address complex issues successfully. This increases the likelihood of this approach being feasible and sustainable. There is a need for cost-effectiveness analyses to assess the sustainability of this approach and determine if it should perhaps be targeted toward women/couples who do not respond to less-intensive efforts, such as an invitation letter for the couple to come to the health facility together, or distributing HIV self-testing kits to women for use together with their male partners.64 Thus, we plan to include relevant comparison groups and cost-effectiveness analyses in future studies of this promising home-based couples strategy.
Limitations
Despite the promise of this approach, there were some challenges. The study team was not able to engage all participants as couples, with some men being difficult to reach or refusing to participate, and some women (notably all of them HIV+) subsequently changing their minds about involving their male partner in the study. There were somewhat higher rates of discontinuation due to adverse events, as well as failure to recruit the male partner, in the control arm compared with the intervention arm (although not statistically significant), such that the control sample of couples was somewhat smaller than the intervention sample. Although the adverse events were not related to study participation, it is possible that study staff tried harder to recruit male partners if they knew that they were to be included in the intervention, or that men were less likely to participate in the study if they knew they would be in the control arm. The small sample size and short follow-up period of the pilot study suggest a need for larger studies that can assess long-term outcomes in terms of paternal, maternal, and child health. In addition, this study was specifically focused on supporting pregnant women who had not yet disclosed their HIV testing/status to their male partners, so the results cannot necessarily be extrapolated to all pregnant couples in this setting. Women who said they had disclosed their status to their male partner (even if their partner had not disclosed to them or they were unsure about their male partner's status) were not included in the study.
Acknowledgments
The authors thank the study participants and the dedicated researchers/lay counselors (Moses Okombo Ayany, Irene Awuor Jimbo, Jane Atieno June, Julius Odiwuor June, and Celestine Adhiambo Ngerhe) for their contributions to this study. The authors also acknowledge the important role of the KEMRI-UCSF Collaborative group, the Director of KEMRI, the Director of KEMRI's Centre for Microbiology, and the Kenya Ministry of Health for their support in conducting this research. The authors are also grateful for the support of Drs. Kenneth Ngure, Manuela Colombini, Beatrice Wamuti, Michael Kiragu, and Susan Meffert in monitoring the study. The research described in this article was supported by the US National Institute of Mental Health (NIMH), through grant R34MH102103. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health. This trial is registered at clinicaltrials.gov No. NCT02403583.
Authors' Contributions
J.M.T., E.A.B., A.M.H., Z.K., P.O., and L.A.D. conceptualized and designed the study. G.O. and E.W. acquired the data. A.J.R., P.L.M., and A.H. analyzed the data. J.M.T. wrote the article. L.A.D., E.A.B., A.M.H., Z.K., P.L.M., A.J.R., J.M.T., A.H., J.L.A., E.W., G.O., and P.O. reviewed and edited the article. All authors have read and approved the final version.
Author Disclosure Statement
The authors have no competing interests to declare.
References
- 1.Sirengo M, Muthoni L, Kellogg T, et al. . Mother-to-child transmission of HIV in Kenya: Results from a nationally representative study. J Acquir Immune Defic Syndr 2014;66(Suppl 1):S66–S74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bispo S, Chikhungu L, Rollins N, Siegfried N, Newell M. Postnatal HIV transmission in breastfed infants of HIV-infected women on ART: A systematic review and meta-analysis. J Int AIDS Soc 2017;20:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohler P, Okanda J, Kinuthia J, et al. . Community-based evaluation of PMTCT uptake in Nyanza Province, Kenya. PLoS One 2014;9:e110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stringer E, Ekouevi D, Coetzee D, et al. . Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in 4 African countries. JAMA 2010;304:293–302 [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS. Kenya AIDS Response Progress Report. 2014. Available at: www.unaids.org/sites/default/files/country/documents/KEN_narrative_report_2014.pdf (Last accessed April1, 2017)
- 6.WHO, UNAIDS, UNICEF. Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Towards Universal Access: Progress Report 2011. Geneva: WHO, 2011 [Google Scholar]
- 7.Sibanda E, Weller I, Hakim J, Cowan F. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: A systematic review and meta-analysis. AIDS 2013;27:2787–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnack A, Rempis E, Decker S, et al. . Prevention of mother-to-child transmission of HIV in Option B+ era: Uptake and adherence during pregnancy in western Uganda. AIDS Patient Care STDS 2016;30:110–119 [DOI] [PubMed] [Google Scholar]
- 9.Miller K, Muyindike W, Matthews LT, Kanyesigye M, Siedner MJ. Program implementation of Option B+ at a President's Emergency Plan for AIDS relief-supported HIV clinic improves clinical indicators but not retention in care in Mbarara, Uganda. AIDS Patient Care STDS 2017;31:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anigilaje E, Ageda B, Nweke N. Barriers to uptake of prevention of mother-to-child transmission of HIV services among mothers of vertically infected HIV-seropositive infants in Makurdi, Nigeria. Patient Prefer Adherence 2016;10:57–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kako P, Dubroskiy R. “You comfort yourself and believe in yourself”: Exploring lived experiences of stigma in HIV-positive Kenyan women. Issues Ment Health Nurs 2013;34:150–157 [DOI] [PubMed] [Google Scholar]
- 12.Cuca Y, Onono M, Bukusi E, Turan J. Factors associated with pregnant women's anticipations and experiences of HIV-related stigma in rural Kenya. AIDS Care 2012;24:1173–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill MM, Umutoni A, Hoffman HJ, et al. . Understanding antiretroviral treatment adherence among HIV-positive women at four postpartum time intervals: Qualitative results from the Kabeho study in Rwanda. AIDS Patient Care STDS 2017;31:153–166 [DOI] [PubMed] [Google Scholar]
- 14.Turan J, Hatcher A, Medema-Wijnveen J, et al. . The role of HIV-related stigma in utilization of skilled childbirth services in rural Kenya: A prospective mixed-methods study. PLoS Med 2012;9:e1001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turan J, Bukusi E, Onono M, Holzemer W, Miller S, Cohen C. HIV/AIDS stigma and refusal of HIV testing among pregnant women in rural Kenya: Results from the MAMAS study. AIDS Behav 2011;15:1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medema-Wijnveen J, Onono M, Bukusi E, Miller S, Cohen C, Turan J. How perceptions of HIV-related stigma affect decision-making regarding childbirth in rural Kenya. PLoS One 2012;7:e51492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okoli J, Lansdown G. Barriers to successful implementation of prevention-of-mother-to-child transmission (PMTCT) of HIV programmes in Malawi and Nigeria: A critical literature review study. Pan Afr Med J 2014;19:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gourlay A, Birdthistle I, Mburu G, Iorpenda K, Wringe A. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: A systematic review. J Int AIDS Soc 2013;16:18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colombini M, James C, Ndwiga C, Integra Team, Mayhew S. The risks of partner violence following HIV status disclosure, and health services responses: Narratives of women attending reproductive health services in Kenya. J Int AIDS Soc 2016;19:20766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeri I, El Ayadi A, Getahun M, et al. . “How can I tell?” Consequences of HIV status disclosure among couples in eastern African communities in the context of an ongoing HIV “test-and-treat” trial. AIDS Care 2016;28(Suppl 3):59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abaynew Y, Deribew A, Deribe K. Factors associated with late presentation to HIV/AIDS care in South Wollo Zone Ethiopia: A case-control study. AIDS Res Ther 2011;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gari T, Habte D, Markos E. HIV positive status disclosure among women attending art clinic at Hawassa University Referral Hospital, South Ethiopia. East Afr J Public Health 2010;7:87–91 [PubMed] [Google Scholar]
- 23.Bucagu M, Muganda J. Implementing primary health care-based PMTCT interventions: Operational perspectives from Muhima cohort analysis (Rwanda). Pan Afr Med J 2014;18:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jasseron C, Mandelbrot L, Dollfus C, et al. . Non-disclosure of a pregnant woman's HIV status to her partner is associated with non-optimal prevention of mother-to-child transmission. AIDS Behav 2013;17:488–497 [DOI] [PubMed] [Google Scholar]
- 25.Trinh T, Yatich N, Ngomoa R, et al. . Partner disclosure and early CD4 response among HIV-infected adults initiating antiretroviral treatment in Nairobi Kenya. PLoS One 2016;11:e0163594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salmen C, Hickey M, Fiorella K, et al. . “Wan Kanyakla” (We are together): Community transformations in Kenya following a social network intervention for HIV care. Soc Sci Med 2015;147:332–340 [DOI] [PubMed] [Google Scholar]
- 27.Spangler S, Onono M, Bukusi E, Cohen C, Turan J. HIV-positive status disclosure and use of essential PMTCT and maternal health services in rural Kenya. J Acquir Immune Defic Syndr 2014;67:S235–S242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rujumba J, Neema S, Byamugisha R, Tylleskar T, Tumwine J, Heggenhougen H. “Telling my husband I have HIV is too heavy to come out of my mouth”: Pregnant women's disclosure experiences and support needs following antenatal HIV testing in eastern Uganda. J Int AIDS Soc 2012;15:17429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyoyoko N, Umoh A. The prevalence and determinants of HIV seroconversion among booked ante-natal clients in the University of Uyo teaching hospital, Uyo Akwa Ibom State, Nigeria. Pan Afr Med J 2016;25:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinh T, Delaney K, Goga A, et al. . Impact of maternal HIV seroconversion during pregnancy on early mother to child transmission of HIV (MTCT) measured at 4–8 weeks postpartum in South Africa 2011–2012: A national population-based evaluation. PLoS One 2015;10:e0125525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawi JD, Mirambo M, Mogoma M, et al. . Sero-conversion rate of syphilis and HIV among pregnant women attending antenatal clinic in Tanzania: A need for re-screening at delivery. BMC Pregnancy Childbirth 2015;15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson L, Stinson K, Newell M, et al. . The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defic Syndr 2012;59:417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers A, Weke E, Kwena Z, et al. . Implementation of repeat HIV testing during pregnancy in Kenya: A qualitative study. BMC Pregnancy Childbirth 2016;16:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker S, Taulo F, Hindin M, Chipeta E, Loll D, Tsui A. Pilot study of home-based delivery of HIV testing and counseling and contraceptive services to couples in Malawi. BMC Public Health 2014;14:1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ezeanolue E, Obiefune M, Yang W, et al. . What do you need to get male partners of pregnant women tested for HIV in resource limited settings? The baby shower cluster randomized trial. AIDS Behav 2017;21:587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auvinen J, Kylma J, Suominen T. Male involvement and prevention of mother-to-child transmission of HIV in sub-Saharan Africa: An integrative review. Curr HIV Res 2013;11:169–177 [DOI] [PubMed] [Google Scholar]
- 37.Yende N, Rie AV, West N, Bassett J, Schwartz S. Acceptability and preferences among men and women for male involvement in antenatal care. J Pregnancy 2017;2017:4758017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osoti AO, John-Stewart G, Kiarie J, et al. . Home visits during pregnancy enhance male partner HIV counselling and testing in Kenya: A randomized clinical trial. AIDS 2014;28:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ministry of Health, National AIDS Control Council. Kenya HIV estimates report. Ministry of Health. Nairobi, Kenya, 2014 [Google Scholar]
- 40.Desai M, Phillips-Howard PA, Odhiambo FO, et al. . An analysis of pregnancy-related mortality in the KEMRI/CDC health and demographic surveillance system in western Kenya. PLoS One 2013;8:e68733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ministry of Public Health and Sanitation, Ministry of Medical Services. National Reproductive Health Strategy, 2009–2015. Nairobi: Republic of Kenya, 2009 [Google Scholar]
- 42.FACES. FACES—Family AIDS Care and Educational Service. Available at: www.faces-kenya.org (Last accessed January5, 2018)
- 43.Turan JM, Onono M, Steinfeld RL, et al. . Implementation and operational research: Effects of antenatal care and HIV treatment integration on elements of the PMTCT cascade: Results from the SHAIP cluster-randomized controlled trial in Kenya. J Acquir Immune Defic Syndr 2015;69:e172–e181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis MA, McBride CM, Pollak KI, Puleo E, Butterfield RM, Emmons KM. Understanding health behavior change among couples: An interdependence and communal coping approach. Soc Sci Med 2006;62:1369–1380 [DOI] [PubMed] [Google Scholar]
- 45.Kelley HH, Thibaut JW. Interpersonal Relations: A Theory of Interdependence. New York: Wiley, 1978 [Google Scholar]
- 46.Rogers AJ, Achiro L, Bukusi EA, et al. . Couple interdependence impacts HIV-related health behaviours among pregnant couples in southwestern Kenya: A qualitative analysis. J Int AIDS Soc 2016;19:21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walcott M, Hatcher A, Kwena Z, Turan JM. Acceptability and feasibility of approaches to facilitated HIV disclosure for pregnant women and partners in rural Kenya: A qualitative study. BMC Public Health 2013;13:1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood J. Communications Mosaics: An Introduction to the Field of Communication. Boston, MA: Cengage Learning, 2016 [Google Scholar]
- 49.Grinstead OA, Gregorich SE, Choi KH, Coates T. Positive and negative life events after counselling and testing: The voluntary HIV-1 counselling and testing efficacy study. AIDS 2001;15:1045–1052 [DOI] [PubMed] [Google Scholar]
- 50.Besada D, Rohde S, Goga A, et al. . Strategies to improve male involvement in PMTCT Option B+ in four African countries: A qualitative rapid appraisal. Global Health Action 2016;9:33507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yargawa J, Leonardi-Bee J. Male involvment and maternal health outcomes: Systematic review and meta-analysis. J Epidemiol Community Health 2015;69:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wesevich A, Mtande T, Saidi F, et al. . Role of male partner invovlement in ART retention and adherence in Malawi's Option B+ program. AIDS Care 2017;29:1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones D, Peltzer K, Weiss S, et al. . Implementing comprehensive prevention of mother-to-child transmission and HIV prevention for South African couples: Study protocol for a randomized controlled trial. Trials 2014;15:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brusamento S, Ghanotakis E, Tudor Car L, van-Velthoven M, Majeed A, Car J. Male involvement for increasing the effectiveness of prevention of mother-to-child HIV transmission (PMTCT) programmes. Cochrane Database Syst Rev 2012;10:CD009468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Theuring S, Jefferys L, Nchimbi P, Mbezi P, Sewangi J. Increasing partner attendance in antenatal care and HIV testing services: Comparable outcomes using written versus verbal invitations in an urban facility-based controlled intervention trial in Mbeya, Tanzania. PLoS One 2016;11:e0152734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jefferys L, Nchimbi P, Mbezi P, Sewangi J, Theuring S. Official invitation letters to promote male partner attendance and couple voluntary HIV counseling and testing in antenatal care: An implementation study in Mbeya Region, Tanzania. Reprod Health 2015;12:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morfaw F, Mbuagbaw L, Thabane L, et al. . Male involvement in prevention programs of mother to child transmission of HIV: A systematic review to identify barriers and facilitators. Syst Rev 2013;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walcott M, Hatcher A, Kwena Z, Turan J. Facilitating HIV status disclosure for pregnant women and partners in rural Kenya: A qualitative study. BMC Public Health 2013;13:1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koo K, Makin J, Forsyth B. Where are the men? Targeting male partners in preventing mother-to-child HIV transmission. AIDS Care 2013;25:43–48 [DOI] [PubMed] [Google Scholar]
- 60.Manjate Cuco R, Munguambe K, Bique Osman N, Degomme O, Temmerman M, Sidat M. Male partners' involvement in prevention of mother-to-child HIV transmission in sub-Saharan Africa: A systematic review. SAHARA J 2015;12:87–105 [DOI] [PubMed] [Google Scholar]
- 61.Ng'ang'a A, Waruiru W, Ngare C, et al. . The status of HIV testing and counseling in Kenya: Results from a nationally representative population-based survey. J Acquir Immune Defic Syndr 2014;66(Suppl 1):S27–S36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krakowiak D, Kinuthia J, Osoti AO, et al. . Home-based HIV testing among pregnant couples increases partner testing and identification of serodiscordant partnerships. J Acquir Immune Defic Syndr 2016;72(Suppl 2):S167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma M, Farquhar C, Ying R, et al. . Modeling the cost-effectiveness of home-based HIV testing and education (HOPE) for pregnant women and their male partners in Nyanza Province, Kenya. J Acquir Immune Defic Syndr 2016;72(Suppl 2):S174–S180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thirumurthy H, Masters SH, Mavedzenge SN, Maman S, Omanga E, Agot K. Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self-tests by HIV-negative female sex workers and women receiving antenatal and post-partum care in Kenya: A cohort study. Lancet HIV 2016;3:e266–e274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann 2002;32:509–515 [Google Scholar]
- 66.Rusbult CE, Van Lange PA. Interdependence, interaction, and relationships. Annu Rev Psychol 2003;54:351–375 [DOI] [PubMed] [Google Scholar]
- 67.Lyons RF, Mickelson KD, Sullivan MJL, Coyne JC. Coping as a communal process. J Soc Pers Relat 1998;15:579–605 [Google Scholar]