Abstract
Ketamine, a drug with rapid antidepressant effects and well-described effects on slow wave sleep (SWS), is a useful intervention for investigating sleep–wake mechanisms involved in novel therapeutics. The drug rapidly (within minutes to hours) reduces depressive symptoms in individuals with major depressive disorder (MDD) or bipolar disorder (BD), including those with treatment-resistant depression. Ketamine treatment elevates extracellular glutamate in the prefrontal cortex. Glutamate, in turn, plays a critical role as a proximal element in a ketamine-initiated molecular cascade that increases synaptic strength and plasticity, which ultimately results in rapidly improved mood. In MDD, rapid antidepressant response to ketamine is related to decreased waking as well as increased total sleep, SWS, slow wave activity (SWA), and rapid eye movement (REM) sleep. Ketamine also increases brain-derived neurotrophic factor (BDNF) levels. In individuals with MDD, clinical response to ketamine is predicted by low baseline delta sleep ratio, a measure of deficient early night production of SWS. Notably, there are important differences between MDD and BD that may be related to the effects of diagnosis or of mood stabilizers. Consistent with its effects on clock-associated molecules, ketamine alters the timing and amplitude of circadian activity patterns in rapid responders versus non-responders with MDD, suggesting that it affects mood-dependent central neural circuits. Molecular interactions between sleep homeostasis and clock genes may mediate the rapid and durable elements of clinical response to ketamine and its active metabolite.
Keywords: Brain-derived neurotrophic factor (BDNF), Circadian, Major depressive disorder, Neuroplasticity, Slow wave sleep, Suicidality
1 Introduction
The glutamatergic N-methyl-D-aspartate (NMDA) receptor antagonist ketamine is a rapid-acting antidepressant with immediate clinical utility. It is also a valuable research intervention that provides insights into the development of novel treatments for mood disorders. Ketamine rapidly (within minutes to hours) reduces depressive symptoms in individuals with major depressive disorder (MDD) or bipolar disorder (BD), including in patients with treatment-resistant depression (Diazgranados et al. 2010a; Murrough et al. 2013; Zarate et al. 2012); treatment effects may last for up to 7 days in some individuals. Importantly, the drug may also reduce suicidal ideation in patients (DiazGranados et al. 2010b; Price et al. 2009; Zarate et al. 2012), suggesting an important treatment option for this population with urgent clinical needs.
Disrupted sleep patterns and circadian rhythms have long been associated with depressive disorders (Gillin et al. 1979; Wehr and Goodwin 1983). Relatedly, sleep deprivation (SD) was suggested to have rapid chronotherapeutic effects on both sleep homeostasis and mood (Borbély and Wirz-Justice 1982; Wirz-Justice and Van den Hoofdakker 1999). In addition, the mood stabilizer lithium was recently found to have beneficial molecular effects on the circadian clock; a full discussion of lithium’s effects on circadian function is beyond the scope of this chapter. We refer interested readers to several excellent reviews on this subject (Gould and Manji 2002; Lenox et al. 2002; McCarthy et al. 2012; Gould and Manji 2005). Interestingly, in addition to its antidepressant effects, ketamine has well-described effects on slow wave sleep (SWS) (Duncan et al. 2013b; Duncan and Zarate 2013), and is thus a useful intervention for investigating the sleep–wake mechanisms involved in novel therapeutics.
Recent research has identified both sleep homeostatic (sleep slow waves) and circadian components that both modulate and mediate the antidepressant and antisuicidal effects of ketamine. The relevant mechanism(s) of action is an area of active investigation, and includes release of glutamate, the major excitatory neurotransmitter in the brain. A sequence of molecular and cellular events follows this glutamate release. These include increased α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) neurotransmission, activation of the mammalian target of rapamycin (mTOR) signaling pathway, and increased brain-derived neurotrophic factor (BDNF) activity. Ketamine also activates clock-associated gene molecules (Bellet et al. 2011), including those within reward circuits of the nucleus accumbens (Zhao et al. 2014).
Taken together, the evidence gathered to date highlights a rich field of clinical and preclinical results that can be fruitfully explored to identify links between circadian/sleep wake systems and the development of novel rapid antidepressant treatments. To further understand the homeostatic process of sleep and how it relates to the development of novel therapeutics, recent investigations have focused on glutamatergic-associated molecular and SWS associations that parallel ketamine’s rapid antidepressant effects. This chapter will review current insights drawn from sleep and circadian system research related to the development of novel interventions.
2 Glutamatergic Function in Sleep and Circadian Systems
Distribution of metabotropic glutamate R2/R3 receptors is widespread, including in the prefrontal cortex (PFC), hippocampus, and the reticular and sensory thalamic nuclei, all of which have been implicated in neuropsychiatric syndromes (Wright et al. 2013). Glutamate is co-localized within orexin (also known as hypocretin) neurons of the dorsal lateral hypothalamus, which also have widespread distribution throughout the brain (Peyron et al. 1998). These neurons, whose deficiency is the cause of narcolepsy/cataplexy, become hyperactive after SD and metabolic challenge (Willie et al. 2001; Sakurai 2007).
As regards circadian regulation of sleep–wake patterns, synchronization between environmental light dark cycles and internal biological circadian rhythms is mediated in part by release of glutamate from the retinohypothalamic tract (RHT). The consequent light response of light sensitive clock genes (Roecklein et al. 2009) and their downstream molecular events – which ultimately affects sleep quality – are affected by this glutamate-initiated sequence of clock-associated effects.
Ketamine treatment elevates extracellular glutamate in the PFC (Bagley and Moghaddam 1997; Homayoun and Moghaddam 2007). In addition, glutamate plays a critical role as a proximal element in a ketamine-initiated molecular cascade that increases synaptic strength and plasticity, which ultimately results in rapidly improved mood. Glutamatergic effects are common to interventions with rapid antidepressant properties such as SD, electroconvulsive therapy (ECT) (Murck et al. 2009), and ketamine. For instance, SD (extended wakefulness) increases the availability of metabotropic glutamate receptors of subtype 5 (Hefti et al. 2013) and alters glutamatergic function (Benedetti et al. 2009). Increased cortical excitability, as measured by gamma activity, has been associated with ketamine treatment (Cornwell et al. 2012) as well as with increased prior wake time (Huber et al. 2013); both have glutamatergic underpinnings.
2.1 Ketamine-Induced Glutamatergic Signaling Initiates a Cascade of Molecular, Cellular, and BDNF-Associated Events
The rapid antidepressant properties of ketamine and one of its key metabolites, (2R,6R)-hydroxynorketamine (HNK) (Zanos et al. 2016), induce altered glutamatergic signaling and upregulation of AMPA receptors, consistent with previously described findings of increased synaptic strength and plasticity (Duman and Aghajanian 2012). Interestingly, in the study by Zanos and colleagues, (2R,6R)-HNK was essential to ketamine’s antidepressant effects. Furthermore, the (2R,6R)-HNK enantiomer was responsible for behavioral, EEG, electrophysiological, and cellular antidepressant-related actions in mice without inducing any ketamine-related side effects. The specific sequence from ketamine infusion to improved mood, cognition, and behavior includes: (1) increased glutamate release; (2) early activation and upregulation of AMPA receptors; and (3) activity-dependent release of BDNF (Li et al. 2010; Maeng and Zarate 2007). Ultimately, these events activate the mTOR signaling pathway, affecting downstream changes in dendritic spines and local synaptic protein synthesis, including BDNF (Duman and Aghajanian 2012). BDNF secretion, activation of the tropomyosin-receptor-kinase B (TrkB) receptor, and downstream trafficking lead to further dendritic structural complexity, spine and BDNF synthesis, synaptic plasticity, and strengthened local circuitry (Duman and Aghajanian 2012; Maeng and Zarate 2007).
Downstream to these events, polymorphisms such as BDNF Val66Met also contribute to the altered functional effects of BDNF trafficking (Chen et al. 2004; Egan et al. 2003). Enhanced synaptic plasticity and neuronal synchronization resulting from changes in critical local neuronal circuits – especially in areas involved in mood and behavior – converge to produce rapid antidepressant effects (Maeng and Zarate 2007; Zarate et al. 2006). As discussed later in Sects. 2.3, 3.1, and 4, below, changes in BDNF, SWS, and mood are present in patients who respond to ketamine.
2.2 Clues to Rapid Antidepressant Mechanisms of Ketamine and Sleep Deprivation Are Found Within Core Elements of Sleep and Circadian Systems
In mood disorders, both homeostatic and circadian processes are dysregulated, with significant impact on sleep–wake cycles, circadian rhythms of body temperature, hormones, behavior, and mood. Patients with MDD often, but not uniformly, exhibit disturbed sleep. For example, several studies have reported an absence of disturbed sleep patterns in individuals with MDD (Frey et al. 2012; Quitkin et al. 1985; Antonijevic 2006), suggesting that distinctions such as typical versus atypical subtypes, gender, or other co-morbidities may be involved. Disturbed sleep is generally characterized by decreased sleep and sleep continuity, low levels of SWS and slow wave activity (SWA, defined as EEG activity between 1 and 4 Hz in non-rapid eye movement (NREM) sleep), short rapid eye movement (REM) latency, and increased and early morning waking (Gillin et al. 1979; Duncan et al. 1979; Kupfer et al. 1985; Pillai et al. 2011; Benca et al. 1992). Many of these disturbances are consistent with abnormal function of the sleep homeostat (SWS deficiency) and central clock (temperature and hormone rhythms). Notably, many of these features have been incorporated within the two-process model of sleep–wake regulation (described next) in which a sleep homeostat (modeled by Process S) interacts with the central circadian clock (modeled by Process C) to influence human sleep behavior and mood. Numerous chronotherapeutic sleep interventions, such as partial SD and sleep phase advance have been evaluated within this model for their capacity to alter homeostatic mechanisms and to affect interactions between sleep homeostatic and circadian processes. For instance, the antidepressant properties of bright light – the major signal responsible for synchronizing internal circadian timing to the external day – are used during bright light therapy.
The two-process model of human sleep–wake regulation (Borbély 1982) has been used to measure the timing, duration, and structure of human sleep (Dijk and Czeisler 1995) with important implications for health and depression. In healthy individuals, extended prior wakefulness is associated with a homeostatic increase in the level of “S,” measured by increased SWS/SWA (a surrogate marker of “S”) during recovery sleep. The “S”-deficiency hypothesis of depression (Borbély 1987; Borbély and Wirz-Justice 1982) posits that both sleep disturbances and depressive symptoms are related to low levels of “S.” The rapid antidepressant effects of SD therapy in depression are hypothesized to increase “S” toward normal levels, as indicated by elevated SWS/SWA during early recovery (Borbély and Wirz-Justice 1982). Key elements of this hypothesis have further provided a basis for launching promising investigations regarding the molecular effects of SD and their role in rapid antidepressant effects, with predictions based on synaptic homeostasis (Tononi and Cirelli 2006).
The mechanism of action of rapid-acting antidepressants may be complex, involving temporal and quantitative differences in BDNF and synaptic plasticity (by SD and partial SD) on the one hand, and altered timing of the central clock (e.g., by bright light therapy) on the other. The timing of these events, along with the course of mood changes, provides important clues regarding their relative contribution to both the rapid expression of antidepressant response and the maintenance of that response (Kuhn et al. 2016). The fact that SD does not consistently elevate BDNF (Kuhn et al. 2016; Ibrahim et al. 2011) suggests that declines in BDNF after extended wakefulness might have a protective effect against excessive cortical excitability and plasticity (Kuhn et al. 2016). Both SD and bright light therapy [as well as selective serotonin reuptake inhibitors (SSRIs)] produce rapid changes (within 12 h) in both SWA levels and clock-gene expression (Cuesta et al. 2009; Uz 2005), respectively. However, the rapid antidepressant response latencies of these interventions vary, ranging from hours (in the case of ketamine, SD, and partial SD), to several days (bright light therapy and sleep phase advance), to weeks for SSRIs (Rush et al. 2006). The distinct molecular pathways activated by homeostatic versus circadian-linked interventions may contribute differently to the rapid onset of antidepressant effects, particularly compared to the persistence (durability) of any such effects.
Chronotherapies that control the duration of prior waking – and, therefore, sleep homeostatic mechanisms [e.g., dark therapy and “extended-night” interventions (Wehr et al. 1998; Wirz-Justice et al. 1999; Barbini et al. 2005)] – reduce symptoms of mania, hypomania, and rapid cycling in BD (Barbini et al. 2005; Wehr et al. 1998; Wirz-Justice et al. 1999). Thus, controlled sleep–wake schedules, regulation of sleep homeostasis, and underlying pathways of synaptic plasticity (including glutamatergic function) may inform our understanding of sleep homeostasis and SWS as modulating elements of mood and novel mechanisms of mood stabilizers. The role of interacting homeostatic and circadian systems in developing novel treatment interventions is discussed later.
2.3 Sleep Slow Waves and Evoked Potentials Are Markers of Synaptic Plasticity
Sleep EEG and evoked potentials are useful markers of altered synaptic plasticity in humans (Huber et al. 2004, 2006), and evidence from these studies is consistent with the synaptic homeostasis hypothesis (Tononi and Cirelli 2006). High-density EEG studies have shown that interventions such as rotation learning and high-frequency transcranial magnetic stimulation (TMS) associated with synaptic potentiation in local cortical circuits lead to local increases in SWA during subsequent sleep (Huber et al. 2004). Interventions such as arm immobilization, which is associated with synaptic depression, lead to a local reduction in SWA (Huber et al. 2006). Computer simulations indicate that sleep SWA directly reflects synaptic strength due to changes in neural synchronization and recruitment (Esser et al. 2007; Vyazovskiy et al. 2007).
In addition, several studies that directly examined the effects of BDNF on EEG SWS also noted a close relationship between SWA and BDNF (Faraguna et al. 2008; Huber et al. 2007). These studies found that SWA was increased by intrahemispheric infusion of BDNF, by behavioral interventions that increase central levels of BDNF, and by the plasticity-related genes Arc, Homer, and NGFI-A (Huber et al. 2007). Conversely, SWA was diminished by BDNF antagonism (Faraguna et al. 2008). The fact that acoustic suppression of SWA was associated with diminished perceptual learning (Aeschbach et al. 2008) suggests that decreased SWS levels could contribute to cognitive and memory deficits in some depressed patients. Interestingly, human carriers of the BDNF Met allele of the Val66Met polymorphism produce less SWS (Bachmann et al. 2012) and also display altered mood response to ketamine (Laje et al. 2012), thus establishing a link between genetic variants of BDNF, SWA, and mood.
3 Clinical Effects of Ketamine on Sleep and Mood
As noted above, ketamine increases SWS, memory, and plasticity, and elevates slow wave production (Campbell and Feinberg 1996; Feinberg and Campbell 1993) in addition to its antidepressant effects. Ketamine’s effects on SWS suggest a possible association between this agent’s rapid antidepressant effects and its plasticity-related effects. These effects on both mood and sleep have been specifically explored in MDD and BD.
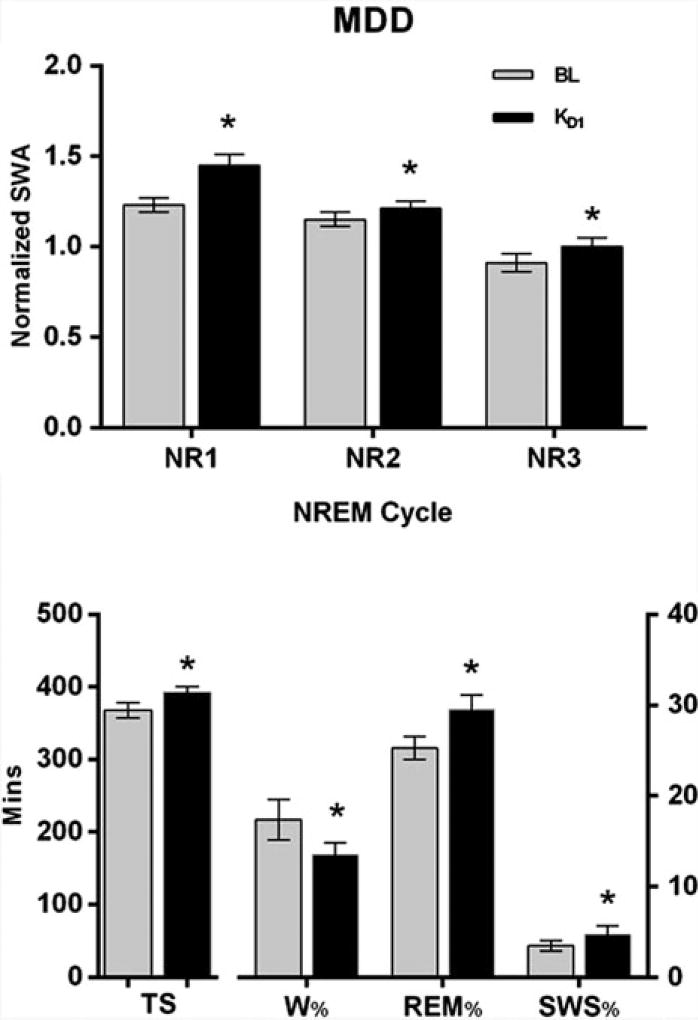
In MDD, rapid antidepressant response to ketamine was linked to decreased waking, as well as increased total sleep, SWS, SWA, and REM sleep (Fig. 1) (Duncan et al. 2013b). Furthermore, clinical response to ketamine was predicted in MDD by a low baseline delta sleep ratio (defined as the quotient of SWA in the first to the second NREM episode), a measure of deficient early night production of SWS (Duncan et al. 2013a), and the abnormal production of SWS during the sleep period. Normalizing the deficient early night production of SWS by increasing early production of SWS in the first non-REM period (Fig. 1, lower panel) appears critical to ketamine’s rapid antidepressant effects. Notably, although increased sleep slow waves were not present on Day 1 post-ketamine infusion, increased total sleep, REM sleep, and sleep efficiency (i.e., decreased time spent awake after sleep onset) were associated with antidepressant response (Duncan et al. 2013b) on Day 2 post-ketamine treatment, indicating their association with a more prolonged antidepressant response.
Fig. 1.
Slow wave activity (SWA, top) during non-REM episodes (cycles) 1–3 (NR1,2,3) and selected sleep measures (bottom) in patients with major depressive disorder (MDD; n = 30) compared during baseline (BL, grey bars) and the first night (D1) after ketamine infusion (KD1, black bars). All patients had severe, treatment-resistant MDD at the time of ketamine treatment. The lower panel shows that in patients with MDD, ketamine significantly improved sleep quality [increased Total Sleep (TS), slow wave sleep (SWS), and percent time spent in rapid eye movement sleep (REM %)], and decreased the percent of time spent awake (W%) on D1. Ketamine also increased early production of SWA during NR1, thus enhancing the nighttime decline in SWA across successive non-REM episodes. (* p < 0.05)
Increasing the early nighttime production of SWS (Fig. 1, upper panel) may be important to ketamine’s rapid antidepressant effects. Interestingly, sleep slow waves are markers of neuronal plasticity that are similarly increased using other (non-ketamine) novel therapies, further implicating sleep and circadian systems in mood response. For instance, the effects of repetitive TMS (rTMS) on plasticity (Cohen et al. 2010) involve both sleep–wake and/or circadian-dependent processes. Applying rTMS to the left dorsolateral prefrontal cortex (DLPFC) increased SWA at F3 (Saeki et al. 2013), indicating locally enhanced synaptic plasticity, similar to ketamine’s plasticity-related effects on SWS (Duncan et al. 2013b). In that rTMS study, SWA production was particularly enhanced during the first half of the sleep period (Saeki et al. 2013). This result echoes the early night effects of ketamine (see Fig. 1, top panel) in which ketamine elevated the early night peak in SWA, followed by a progressive decline in SWA levels during the remaining sleep period (Duncan et al. 2013b). This homeostatic nighttime decline, common to ketamine and to other novel therapeutics, is consistent with a pattern of synaptic downscaling that follows the gradual rise of daytime synaptic potentiation and elevated SWA at sleep onset.
Interestingly, an important discrepancy between MDD and BD was found when examining ketamine’s effects on SWS in BD patients who were receiving mood stabilizer therapy. Increased SWS production in response to ketamine was not observed in this sample of BD patients, in contrast to the results obtained for MDD patients. Although rapid antidepressant effects as well as improved sleep were observed (Duncan and Zarate 2013), ketamine infusions reduced SWA levels in BD during non-REM episodes 1 and 3. This result has two possible interpretations. First, the finding is consistent with sleep differences between MDD and BD patients. Genetic factors influence REM sleep expression (Kupfer and Ehlers 1989) and this, in turn, indirectly affects SWS expression; the result is thus consistent with underlying genetic differences between MDD and BD. These differences may stem from complex and unstable waking patterns during mania and hypomania (Linkowski et al. 1986) that potentially influence and confound measurement of SWS expression in BD. Second, the result is consistent with studies indicating that mood stabilizers per se influence SWS expression. The ongoing use of mood stabilizers (i.e., lithium) during ketamine infusion may thus have affected SWS and could confound any study of ketamine-induced slow wave production (Duncan and Zarate 2013).
More provocatively, this result could also suggest that the effect of mood stabilizers on SWS also contributes to their mood stabilizing properties. Controlling mood cycles by stabilizing day-to-day variations in SWS may be a property shared by dark-therapy/extended bed-rest interventions (i.e., chronotherapeutic interventions that impose fixed bed-rest schedules; these control the duration of prior wakefulness, thereby minimizing night-to-night fluctuations in SWS and ultimately stabilizing mood). This finding may echo a recent report that SWS levels correlate with mood (Eidelman et al. 2010) such that low SWS levels are associated with reduced future mania. Thus, a property of mood stabilizers might be their ability to minimize night-to-night fluctuations in SWS.
3.1 Ketamine’s Rapid Effects on Mood, Sleep Slow Waves, and BDNF Levels Are Consistent with Increased Synaptic Plasticity
Response to ketamine infusions also suggest links between neuroplasticity, SWS, and mood on the one hand, and the neurotrophin BDNF on the other. Increased BDNF levels have been associated with several rapid-acting antidepressant interventions (Duncan et al. 2013b; Giese et al. 2014; Haile et al. 2014; Gorgulu and Caliyurt 2009), underscoring its key role in the rapid response mechanism. Beyond the association with rapid mood response, the magnitude of BDNF and SWS levels were correlated in ketamine responders (Duncan et al. 2013b), a finding consistent with the preclinical effects of BDNF on SWS production (Huber et al. 2007). Furthermore, the magnitude of BDNF levels measured 4 h post-ketamine infusion predicted mood response 3 days post-infusion (Haile et al. 2014), suggesting that these acute levels might provide a marker of durable response.
3.2 Ketamine’s Rapid Antisuicidal Effects Reverse Late-Night Waking
An area of increasing interest is ketamine’s ability to rapidly (within minutes to hours) reduce suicidal thoughts in individuals with treatment-resistant depression (DiazGranados et al. 2010b; Murrough et al. 2015; Price et al. 2009, 2014; Zarate et al. 2012). No FDA-approved medications presently exist for acute suicidal thoughts, and rapid-acting treatments are critically needed for this psychiatric emergency. Ketamine’s impact on suicidal thoughts has been shown to occur independently of its antidepressant effects (Ballard et al. 2014) and has been associated with specific neuroimaging biomarkers, specifically glucose metabolism in the infralimbic cortex (BA 25) (Ballard et al. 2015). This suggests that investigations of ketamine use in suicidal individuals could identify potential biomarkers of antisuicidal response and elucidate the neurobiological underpinnings of acute suicide risk.
Interestingly, suicide risk has also been linked to sleep difficulties. Self-reported sleep difficulties are associated with subsequent death by suicide (Bernert et al. 2014; Gunnell et al. 2013), and the association between subjective sleep difficulties and suicidal thoughts and behaviors has been found to occur independently of depressive symptoms (Pigeon et al. 2012).
More objective measures of suicidal thoughts or past behaviors have been associated with polysomnography-defined increased REM sleep percentage, REM activity, REM duration, and decreased sleep efficiency (Agargun and Cartwright 2003; Sabo et al. 1991). Evidence of seasonality and suicidal behavior, including altered circadian psychomotor activity in suicidal individuals, suggests altered chronobiological activity in suicidal patients (Hiltunen et al. 2011; Verkes et al. 1996). Chronotherapeutics – including SD in conjunction with lithium, sleep phase advance, and bright light therapy (triple chronotherapy), as well as cognitive behavioral therapy with SD (Breitstein et al. 2014) – have been associated with rapid reductions in suicidal thinking (Benedetti et al. 2014; Sahlem et al. 2014), suggesting that sleep may be a particularly important modifiable risk factor for acutely suicidal patients.
Interestingly, ketamine’s antisuicidal effects may also involve the circadian sleep–wake system. First, the hours of 12:00 a.m. to 5:00 a.m. may be an especially high-risk time for suicide deaths (Perlis et al. 2016), suggesting a particular circadian interval of risk. Second, in initial investigations of this time period across EEG sleep, wakefulness over the course of the night, particularly in the 4:00–4:59 a.m. hour, was associated with suicidal thoughts the next day in a sample of depressed inpatients (Ballard et al. 2016); this effect was independent of age, gender, diagnosis (MDD vs. BD), or depressive symptom severity. Third, in evaluating wakefulness after ketamine administration, antisuicidal response (that is, complete next-day remission of suicidal thoughts) was associated with reduced wakefulness, even when adjusting for baseline sleep (Vande Voort et al. 2016). Taken together, these results point to the potential importance of sleep and circadian rhythms in ketamine’s antisuicidal effects and indicate the need for further evaluation of BDNF, clock-gene expression, and cortisol on this process.
4 Ketamine’s Interactive Effects on Sleep and Circadian Systems
While some ketamine studies indicate a relationship between BDNF levels and SWS amplitude, other studies describe a diurnal and/or circadian component to plasticity-evoked changes that implicate both sleep homeostatic/plasticity mechanisms and circadian-related processes. For example, effective rapid antidepressant interventions appear related to BDNF regulation as well as circadian and homeostatic sleep systems. The presence of a diurnal rhythm for BDNF in partial SD responders (Giese et al. 2014) suggests a similarity between circadian system variations in BDNF and the circadian effects of other rapid-acting treatment interventions. Similar to SD and sleep homeostatic challenges on clock-gene expression [for a review, see (Franken and Dijk 2009)], other novel therapeutics (such as rTMS) affect clock and circadian function. Plasticity associated with rTMS was found to correlate with cortisol awakening response, a circadian biomarker related to peripheral circadian CLOCK genes (Clow et al. 2014); in addition, rTMS treatment increased REM latency, another circadian marker, also consistent with linkage between rTMS antidepressant effects and circadian rhythms (Cohrs et al. 1998).
5 Ketamine Alters Circadian Amplitude and Timing in MDD
Interactions between molecular and neural elements of the circadian clock and the sleep homeostat regulate the expression and timing of the human sleep–wake pattern. Chronotherapies are hypothesized to alter mood, in part by altering the timing and expression of this central clock (Benedetti et al. 2007a). Bright light therapy (Wirz-Justice and Terman 2012), low-dose melatonin (Lewy et al. 2002), and the novel therapeutic drug agomelatine (Kasper and Hamon 2009; Kasper et al. 2010) share the common property of altering the timing and expression of the central clock. The fact that glutamate is a key transmitter that functions to resynchronize the timing of the central clock with the external light–dark cycle suggests that it may also alter central circadian timing during clinical intervention.
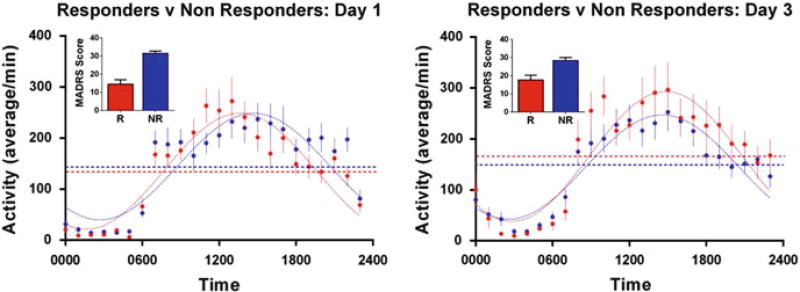
As noted above, glutamate levels are altered by ketamine infusion. As regards the circadian clock in particular, ketamine alters the timing and amplitude of circadian activity patterns in MDD rapid responders versus non-responders (Fig. 2). While consistent with preclinical studies of ketamine’s effects on circadian clock-gene-related molecules, the finding indicates that the effect is mood-dependent. Specifically, on day 1, the day after ketamine infusion (Fig. 2, left panel) rapid responders to ketamine showed phase-advanced (early) timing of the 24-h of activity pattern (as evidenced by higher AM and lower PM activity counts) relative to non-responders. Also, 2 days after the infusion (D3, right panel), responders showed an increased amplitude of the 24-h pattern of motor activity whereas non-responders did not, suggesting that the amplitude of the rhythm is more likely to be associated with durability of the mood response (given that the amplitude increases in day 3 responders) than with acute response – note, for instance, the similar amplitudes in day 1 responders versus non-responders. The mood-dependent properties may incorporate effects on clock-gene pathways, possibly within the expression of mood-associated circuits that contribute to durable clinical response. The early timing of the activity pattern on day 1 is also consistent with the beneficial effects of SD, which are associated with advanced timing of the circadian system (Benedetti et al. 2007b).
Fig. 2.
Day 1 and Day 3 patterns of wrist activity for ketamine-treated patients who responded (>50% decrease in Montgomery-Asberg Depression Rating Scale (MADRS) score) within 1 day of ketamine infusion compared with non-responders. (Left panel) Responders (R, red) compared with non-responders (NR, blue) 1 day after ketamine infusion (Day 1). (Right panel) Responders (R, red) who maintained the 50% decrease in MADRS score compared with patients who did not meet response criteria (NR, blue) on Day 3. The raw MADRS scores for each group are shown as inserts for each day. On Day 1, the phase of the 24-h pattern of activity in ketamine responders differed from non-responders (p = 0.0038). On Day 3 the amplitude (p = 0.0488) of the 24-h pattern significantly differed between responders and non-responders. Group sizes are: D1 (R = 21, NR = 30) and D3 (R = 13, NR = 35)
6 Do Homeostatic and Circadian Systems Interact to Affect Ketamine’s Antidepressant Properties?
SWS-associated neural plasticity (Duncan et al. 2013b) as well as clock genes and their associated molecules (Bunney and Bunney 2012, 2013) have both been linked to ketamine’s rapid antidepressant effects. Clear delineation of the temporal relationship between clock-gene activation versus sleep homeostatic mechanisms can inform our understanding of how these processes contribute to rapid antidepressant response. This requires close examination of day-to-day clinical mood changes on the one hand, and specific changes in clock genes and sleep homeostasis on the other.
In the case of sleep homeostasis, clinical studies have found that rapid antidepressant response and SWS-related changes in neural plasticity both occur within 12 h of ketamine infusion (Duncan et al. 2013b; Duncan and Zarate 2013). In contrast to conventional antidepressant treatments, rapid-acting interventions such as SD and ketamine infusion quickly increase SWS production, highlighting the role of increased “S” and SWS as markers of rapid mood response. Underlying the role of SWS-associated processes in the onset of rapid response are neurotrophin release and neuronal plasticity; these are produced both by the rapid-acting effects of SD and the rapid-acting antidepressant ketamine.
Clinical studies have not directly examined ketamine’s effects on clock-gene expression. In contrast, preclinical studies have shown rapid clock-gene-associated effects after ketamine infusion but, as discussed here, their role in mediating rapid antidepressant response is unclear. For example, like ketamine, SSRIs and bright light exposure produce rapid clock-gene related effects in preclinical studies (Cuesta et al. 2009; Uz 2005), as well as demonstrated effects in MDD (Li et al. 2013; Rush et al. 2006). However, unlike ketamine, SSRIs often require 5 weeks for their antidepressant effects to manifest (Posternak and Zimmerman 2005; Rush et al. 2006), which is inconsistent with a rapidly acting antidepressant clock-gene mechanism; nevertheless, a chronic mechanism involving clock gene effects might be relevant (Li et al. 2013). Similarly, bright light therapy has rapid effects on clock-gene expression but requires several days for an antidepressant response to manifest (Benedetti et al. 2007a; Duncan 2009). Thus, the extent to which clockgene effects are both necessary and sufficient for a rapid and/or durable antidepressant response is an important question that requires further examination of the specific circadian phase-dependent elements that both moderate and mediate rapid and durable response effects.
The fact that sleep homeostasis and clock genes interact at the molecular level (Franken 2013; Franken and Dijk 2009) allows for interacting effects that mediate clinical response. Subsequent to the initial events that trigger the cascade of molecular and plasticity-associated events – for which SWS is an important marker – the interaction between homeostatic and circadian clock processes (Archer et al. 2014) could be critical for sustaining antidepressant response.
7 Strategies for Prolonging Ketamine’s Mood Response
Although ketamine provides rapid relief from depressive symptoms – with a response rate of approximately 70% within 24 h (Machado-Vieira et al. 2010) – its effects are often short-lived, and most patients relapse within 7 days. Therefore, an important goal of ongoing research is to identify mechanisms of durable response to ketamine. This approach might include pairing activation of: (a) the acute rapid response mechanism with, (b) a response maintenance mechanism. The fact that repeated chronotherapies, or chronotherapies combined with conventional treatments, extend acute rapid antidepressant effects (e.g. of single SD or partial SD interventions), suggests that repeat chronotherapies might be effectively used for extending ketamine treatment, or that incorporating their mechanisms of action may extend beneficial effects, but this remains to be evaluated.
7.1 Sleep, Naps, and Post-ketamine Relapse
For an individual who has experienced a rapid antidepressant response to ketamine, the night of sleep that follows the infusion is not predictably followed by relapse. This contrasts with the fact that few SD responders (5–10%) remain euthymic after recovery sleep (Benedetti et al. 1999; Benedetti and Colombo 2011). Furthermore, the rapid clinical benefits of both SD and partial SD are: (a) reduced by naps and microsleeps during extended waking and prior to remission itself, and (b) reduced by naps and recovery sleep during remission (Benedetti and Colombo 2011; Hemmeter et al. 2010; Wiegand et al. 1987). The fact that relapses can be limited (or response extended) by repeated episodes of sleep phase advance (Benedetti et al. 2001; Berger et al. 1997) indicates that sleep per se is not sufficient for relapse, but that sleep stage, circadian phase, or other factors influence relapse (Wiegand et al. 1986, 1987, 1993) [see also (Hemmeter et al. 2007)]. To our knowledge, whether or not the extended antidepressant effects of sequentially applied chronotherapeutics (e.g., partial SD, sleep phase advance) are linked with extended cortical excitability and mood response remains unknown. However, the contribution of microsleeps, napping, and reduced sleep times on extending response and delaying relapse have not been carefully examined following ketamine.
Interestingly, the extension of chronotherapeutic benefits and their interaction with ECT and rTMS have also been investigated. One study found that rTMS (administered over the left DLPFC) prolonged the effects of SD (Eichhammer et al. 2002), but a second study found that active rTMS (left DLPFC) was not superior to sham rTMS in stabilizing the antidepressant effects of SD (Kreuzer et al. 2012; Eichhammer et al. 2002).
Drug interventions have also been used to extend the rapid antidepressant effects of chronotherapies. While chronotherapies, particularly bright light therapy, augment the effects of antidepressant medications (Lam and Kennedy 2015), the augmenting effects of an antidepressant and mood stabilizer (sertraline and lithium) with three chronotherapies (SD; bright light therapy; sleep phase advance) also showed rapid benefits in BD with persisting effects (Wu et al. 2009). In a study of 39 BD patients (13 of whom were treatment-resistant and 23 of whom were medicated with lithium and/or an SSRI), three interventions of SD plus bright light therapy reduced Hamilton Depression Rating Scale (HAM-D) scores by 50% in 26 of the 39 patients (Benedetti et al. 2007b). In a third study, the use of a mood stabilizer (lithium) combined with SD and bright light therapy alleviated suicidal ideation and mood symptoms (Benedetti et al. 2014). The benefits of combination therapies with ketamine have not been fully evaluated. Differences are evident in the observation that the antidepressant effects of ketamine in BD patients maintained on mood stabilizers (lithium and valproate) are not remarkably greater or more prolonged than those in drug-free MDD patients (Diazgranados et al. 2010a; Ionescu et al. 2015).
While naps blunt rapid antidepressant response to SD or partial SD (Dallaspezia and Benedetti 2011), pharmacological treatments that reduce microsleeps/naps (i.e., modafinil, caffeine, flumazenil) do not substantially enhance the rapid antidepressant response associated with SD (Beck et al. 2010; Hemmeter et al. 2007). However, one case report found that modafinil reduced napping and increased clinical benefit during SD (Even et al. 2005). On the other hand, enhanced vigilance associated with the benzodiazepine receptor antagonist flumazenil prolonged rapid antidepressant response to SD to the day after recovery sleep (Hemmeter et al. 2007), possibly by affecting the dynamics of process “S.”
7.2 Molecular Associations with Extended Response
Extended antidepressant response has also been investigated at the molecular level. One study found that the glycogen synthase kinase (GSK) promotor variant (rs334558*C) interacts with the long/short form of the serotonin transporter (5-HTTLPR) 5HT allele to extend post-SD antidepressant response after recovery sleep (Benedetti et al. 2012), suggesting a molecular target for serotonergic interventions that might prolong response. Extended mood benefits from SD by the drug flumazenil, which affects sleep homeostasis (Hemmeter et al. 2007), and by BDNF release subsequent to ketamine infusion (Haile et al. 2014), also suggest a path for investigating extended ketamine mood effects, sleep homeostasis, and plasticity (Laje et al. 2012; Bachmann et al. 2012). Whether the active ketamine metabolite 2R,6R-HNK (Zanos et al. 2016) could also extend clinical antidepressant response is an important question.
8 Summary and Future Directions
The past decade has been a period of steady progress in developing new strategies for rapidly treating mood disorders, and understanding the antidepressant mechanisms of the glutamatergic drug ketamine has been a critical part of this progress. Ketamine’s rapid effects on sleep and clock-gene related pathways have provided mechanistic antidepressant links with previously established, rapid-acting chronotherapeutic interventions. Overall, ketamine’s capacity to increase neurotrophic activity and synaptic strength, normalize sleep, and reinforce the circadian system are seen as critical elements for rapid antidepressant mood effects.
Moving forward, two important areas of clinical development are needed. First, the ability of repeated infusions to extend ketamine’s antidepressant effects is being evaluated. Extended effects are reminiscent of sequentially applied chronotherapeutic effects; elucidating whether repeated treatments are also associated with plasticity-associated sleep markers and molecular changes such as ketamine-associated cortical excitability will be critical for developing interventions with durable effects. Second, the potential antisuicidal effects of ketamine highlight the need for more research into the relationship between suicide risk, sleep, and circadian rhythms. One common pathway for ketamine’s rapid antidepressant effects may be normalization of the circadian sleep/wake system transcriptome that, in turn, may compensate for clock-gene variant effects or for insufficient sleep caused by numerous behavioral or environmental factors. The recent identification of ketamine’s active metabolite (2R,6R-HNK) (Zanos et al. 2016) will be an important element in identifying future novel treatments with fewer side effects and more durable clinical benefits.
Acknowledgments
The authors thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance.
References
- Aeschbach D, Cutler AJ, Ronda JM. A role for non-rapid-eye-movement sleep homeostasis in perceptual learning. J Neurosci. 2008;28(11):2766–2772. doi: 10.1523/JNEUROSCI.5548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agargun MY, Cartwright R. REM sleep, dream variables and suicidality in depressed patients. Psychiatry Res. 2003;119(1–2):33–39. doi: 10.1016/s0165-1781(03)00111-2. [DOI] [PubMed] [Google Scholar]
- Antonijevic IA. Depressive disorders – is it time to endorse different pathophysiologies? Psychoneuroendocrinology. 2006;31:1–15. doi: 10.1016/j.psyneuen.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Archer SN, Laing EE, Moller-Levet CS, van der Veen DR, Bucca G, Lazar AS, Santhi N, Slak A, Kabiljo R, von Schantz M, Smith CP, Dijk DJ. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc Natl Acad Sci U S A. 2014;111(6):E682–E691. doi: 10.1073/pnas.1316335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann V, Klein C, Bodenmann S, Schafer N, Berger W, Brugger P, Landolt HP. The BDNF Val66Met polymorphism modulates sleep intensity: EEG frequency- and state-specificity. Sleep. 2012;35(3):335–344. doi: 10.5665/sleep.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley J, Moghaddam B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. 1997;77(1):65–73. doi: 10.1016/s0306-4522(96)00435-6. [DOI] [PubMed] [Google Scholar]
- Ballard ED, Ionescu DF, Vande Voort JL, Niciu MJ, Richards EM, Luckenbaugh DA, Brutsche NE, Ameli R, Furey ML, Zarate CA., Jr Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161–166. doi: 10.1016/j.jpsychires.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Lally N, Nugent AC, Furey ML, Luckenbaugh DA, Zarate CA., Jr Neural correlates of suicidal ideation and its reduction in depression. Int J Neuropsychopharmacol. 2015;18(1) doi: 10.1093/ijnp/pyu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Vande Voort JL, Bernert RA, Luckenbaugh DA, Richards EM, Niciu MJ, Furey ML, Duncan WC, Zarate CA., Jr Nocturnal wakefulness is associated with next-day suicidal ideation in major depressive disorder and bipolar disorder. J Clin Psychiatry. 2016;77(7):825–831. doi: 10.4088/JCP.15m09943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbini B, Benedetti F, Colombo C, Dotoli D, Bernasconi A, Cigala-Fulgosi M, Florita M, Smeraldi E. Dark therapy for mania: a pilot study. Bipolar Disord. 2005;7(1):98–101. doi: 10.1111/j.1399-5618.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- Beck J, Hemmeter U, Brand S, Muheim F, Hatzinger M, Holsboer-Trachsler E. Modafinil reduces microsleep during partial sleep deprivation in depressed patients. J Psychiatr Res. 2010;44(13):853–864. doi: 10.1016/j.jpsychires.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Bellet MM, Vawter MP, Bunney BG, Bunney WE, Sassone-Corsi P. Ketamine influences CLOCK:BMAL1 function leading to altered circadian gene expression. PLoS One. 2011;6(8):e23982. doi: 10.1371/journal.pone.0023982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric-disorders – a metaanalysis. Arch Gen Psychiatry. 1992;49(8):651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Colombo C. Sleep deprivation in mood disorders. Neuropsychobiology. 2011;64(3):141–151. doi: 10.1159/000328947. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Colombo C, Barbini B, Campori E, Smeraldi E. Ongoing lithium treatment prevents relapse after total sleep deprivation. J Clin Psychopharmacol. 1999;19(3):240–245. doi: 10.1097/00004714-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Barbini B, Campori E, Fulgosi MC, Pontiggia A, Colombo C. Sleep phase advance and lithium to sustain the antidepressant effect of total sleep deprivation in bipolar depression: new findings supporting the internal coincidence model? J Psychiatr Res. 2001;35(6):323–329. doi: 10.1016/s0022-3956(01)00034-6. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Barbini B, Colombo C, Smeraldi E. Chronotherapeutics in a psychiatric ward. Sleep Med Rev. 2007a;11(6):509–522. doi: 10.1016/j.smrv.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Dallaspezia S, Fulgosi MC, Barbini B, Colombo C, Smeraldi E. Phase advance is an actimetric correlate of antidepressant response to sleep deprivation and light therapy in bipolar depression. Chronobiol Int. 2007b;24(5):921–937. doi: 10.1080/07420520701649455. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Calabrese G, Bernasconi A, Cadioli M, Colombo C, Dallaspezia S, Falini A, Radaelli D, Scotti G, Smeraldi E. Spectroscopic correlates of antidepressant response to sleep deprivation and light therapy: a 3.0 Tesla study of bipolar depression. Psychiatry Res. 2009;173(3):238–242. doi: 10.1016/j.pscychresns.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Dallaspezia S, Lorenzi C, Pirovano A, Radaelli D, Locatelli C, Poletti S, Colombo C, Smeraldi E. Gene-gene interaction of glycogen synthase kinase 3-beta and serotonin transporter on human antidepressant response to sleep deprivation. J Affect Disord. 2012;136(3):514–519. doi: 10.1016/j.jad.2011.10.039. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Riccaboni R, Locatelli C, Poletti S, Dallaspezia S, Colombo C. Rapid treatment response of suicidal symptoms to lithium, sleep deprivation, and light therapy (chronotherapeutics) in drug-resistant bipolar depression. J Clin Psychiatry. 2014;75(2):133–140. doi: 10.4088/JCP.13m08455. [DOI] [PubMed] [Google Scholar]
- Berger M, Vollmann J, Hohagen F, Konig A, Lohner H, Voderholzer U, Riemann D. Sleep deprivation combined with consecutive sleep phase advance as a fast-acting therapy in depression: an open pilot trial in medicated and unmedicated patients. Am J Psychiatry. 1997;154(6):870–872. doi: 10.1176/ajp.154.6.870. [DOI] [PubMed] [Google Scholar]
- Bernert RA, Turvey CL, Conwell Y, Joiner TE., Jr Association of poor subjective sleep quality with risk for death by suicide during a 10-year period: a longitudinal, population-based study of late life. JAMA Psychiat. 2014;71(10):1129–1137. doi: 10.1001/jamapsychiatry.2014.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbély A. Two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Borbély AA. The S-deficiency hypothesis of depression and the two-process model of sleep regulation. Pharmacopsychiatry. 1987;20(1):23–29. doi: 10.1055/s-2007-1017069. [DOI] [PubMed] [Google Scholar]
- Borbély AA, Wirz-Justice A. Sleep, sleep deprivation and depression. A hypothesis derived from a model of sleep regulation. Hum Neurobiol. 1982;1(3):205–210. [PubMed] [Google Scholar]
- Breitstein J, Penix B, Roth BJ, Baxter T, Mysliwiec V. Intensive sleep deprivation and cognitive behavioral therapy for pharmacotherapy refractory insomnia in a hospitalized patient. J Clin Sleep Med. 2014;10(6):689–690. doi: 10.5664/jcsm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney BG, Bunney WE. Rapid-acting antidepressant strategies: mechanisms of action. Int J Neuropsychopharmacol. 2012;15(5):695–713. doi: 10.1017/S1461145711000927. [DOI] [PubMed] [Google Scholar]
- Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry. 2013;73(12):1164–1171. doi: 10.1016/j.biopsych.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. NREM delta stimulation following MK-801 is a response of sleep systems. J Neurophysiol. 1996;76(6):3714–3720. doi: 10.1152/jn.1996.76.6.3714. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24(18):4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow A, Law R, Evans P, Vallence AM, Hodyl NA, Goldsworthy MR, Rothwell JR, Ridding MC. Day differences in the cortisol awakening response predict day differences in synaptic plasticity in the brain. Stress. 2014;17(3):219–223. doi: 10.3109/10253890.2014.905533. [DOI] [PubMed] [Google Scholar]
- Cohen DA, Freitas C, Tormos JM, Oberman L, Eldaief M, Pascual-Leone A. Enhancing plasticity through repeated rTMS sessions: the benefits of a night of sleep. Clin Neurophysiol. 2010;121(12):2159–2164. doi: 10.1016/j.clinph.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs S, Tergau F, Riech S, Kastner S, Paulus W, Ziemann U, Ruther E, Hajak G. High-frequency repetitive transcranial magnetic stimulation delays rapid eye movement sleep. Neuroreport. 1998;9(15):3439–3443. doi: 10.1097/00001756-199810260-00019. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CA., Jr Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry. 2012;72(7):555–561. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta M, Clesse D, Pevet P, Challet E. New light on the serotonergic paradox in the rat circadian system. J Neurochem. 2009;110(1):231–243. doi: 10.1111/j.1471-4159.2009.06128.x. [DOI] [PubMed] [Google Scholar]
- Dallaspezia S, Benedetti F. Chronobiological therapy for mood disorders. Expert Rev Neurother. 2011;11(7):961–970. doi: 10.1586/ern.11.61. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010a;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA., Jr Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010b;71(12):1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk D-J, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC. Non-pharmacological treatments and chronobiological aspects of bipolar disorder: implications for novel therapeutics. In: Zarate C, Manji H, editors. Bipolar depression: molecular neurobiology, clinical diagnosis and pharmacology. Birkhauser; Switzerland: 2009. pp. 95–116. [Google Scholar]
- Duncan WC, Jr, Zarate CA., Jr Ketamine, sleep, and depression: current status and new questions. Curr Psychiatry Rep. 2013;15(9):394. doi: 10.1007/s11920-013-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC, Jr, Pettigrew KD, Gillin JC. REM architecture changes in bipolar and unipolar depression. Am J Psychiatry. 1979;136(11):1424–1427. doi: 10.1176/ajp.136.11.1424. [DOI] [PubMed] [Google Scholar]
- Duncan WC, Jr, Selter J, Brutsche N, Sarasso S, Zarate CA., Jr Baseline delta sleep ratio predicts acute ketamine mood response in major depressive disorder. J Affect Disord. 2013a;145(1):115–119. doi: 10.1016/j.jad.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS, Yuan P, Brutsche N, Manji HK, Tononi G, Zarate CA. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2013b;16(2):301–311. doi: 10.1017/S1461145712000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Kharraz A, Wiegand R, Langguth B, Frick U, Aigner JM, Hajak G. Sleep deprivation in depression stabilizing antidepressant effects by repetitive transcranial magnetic stimulation. Life Sci. 2002;70(15):1741–1749. doi: 10.1016/s0024-3205(02)01479-0. [DOI] [PubMed] [Google Scholar]
- Eidelman P, Talbot LS, Gruber J, Hairston I, Harvey AG. Sleep architecture as correlate and predictor of symptoms and impairment in inter-episode bipolar disorder: taking on the challenge of medication effects. J Sleep Res. 2010;19(4):516–524. doi: 10.1111/j.1365-2869.2010.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser SK, Hill SL, Tononi G. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep. 2007;30(12):1617–1630. doi: 10.1093/sleep/30.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even C, Thuile J, Santos J, Bourgin P. Modafinil as an adjunctive treatment to sleep deprivation in depression. J Psychiatry Neurosci. 2005;30(6):432–433. [PMC free article] [PubMed] [Google Scholar]
- Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28(15):4088–4095. doi: 10.1523/JNEUROSCI.5510-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG. Ketamine administration during waking increases delta EEG intensity in rat sleep. Neuropsychopharmacology. 1993;9:41–48. doi: 10.1038/npp.1993.41. [DOI] [PubMed] [Google Scholar]
- Franken P. A role for clock genes in sleep homeostasis. Curr Opin Neurobiol. 2013;23(5):864–872. doi: 10.1016/j.conb.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Franken P, Dijk DJ. Circadian clock genes and sleep homeostasis. Eur J Neurosci. 2009;29(9):1820–1829. doi: 10.1111/j.1460-9568.2009.06723.x. [DOI] [PubMed] [Google Scholar]
- Frey S, Birchler-Pedross A, Hofstetter M, Brunner P, Gotz T, Munch M, Blatter K, Knoblauch V, Wirz-Justice A, Cajochen C. Young women with major depression live on higher homeostatic sleep pressure than healthy controls. Chronobiol Int. 2012;29:278–294. doi: 10.3109/07420528.2012.656163. [DOI] [PubMed] [Google Scholar]
- Giese M, Beck J, Brand S, Muheim F, Hemmeter U, Hatzinger M, Holsboer-Trachsler E, Eckert A. Fast BDNF serum level increase and diurnal BDNF oscillations are associated with therapeutic response after partial sleep deprivation. J Psychiatr Res. 2014;59:1–7. doi: 10.1016/j.jpsychires.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Duncan W, Pettigrew KD, Frankel BL, Snyder F. Successful separation of depressed, normal, and insomniac subjects by EEG sleep data. Arch Gen Psychiatry. 1979;36(1):85–90. doi: 10.1001/archpsyc.1979.01780010091010. [DOI] [PubMed] [Google Scholar]
- Gorgulu Y, Caliyurt O. Rapid antidepressant effects of sleep deprivation therapy correlates with serum BDNF changes in major depression. Brain Res Bull. 2009;80(3):158–162. doi: 10.1016/j.brainresbull.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Gould TD, Manji HK. The Wnt signaling pathway in bipolar disorder. Neuroscientist. 2002;8(5):497–511. doi: 10.1177/107385802237176. [DOI] [PubMed] [Google Scholar]
- Gould TD, Manji HK. Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology. 2005;30(7):1223–1237. doi: 10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- Gunnell D, Chang SS, Tsai MK, Tsao CK, Wen CP. Sleep and suicide: an analysis of a cohort of 394,000 Taiwanese adults. Soc Psychiatry Psychiatr Epidemiol. 2013;48(9):1457–1465. doi: 10.1007/s00127-013-0675-1. [DOI] [PubMed] [Google Scholar]
- Haile CN, Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Foulkes A, Iqbal S, Mahoney JJ, 3rd, De La Garza R, 2nd, Charney DS, Newton TF, Mathew SJ. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol. 2014;17(2):331–336. doi: 10.1017/S1461145713001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti K, Holst SC, Sovago J, Bachmann V, Buck A, Ametamey SM, Scheidegger M, Berthold T, Gomez-Mancilla B, Seifritz E, Landolt HP. Increased metabotropic glutamate receptor subtype 5 availability in human brain after one night without sleep. Biol Psychiatry. 2013;73(2):161–168. doi: 10.1016/j.biopsych.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Hemmeter U, Hatzinger M, Brand S, Holsboer-Trachsler E. Effect of flumazenil-augmentation on microsleep and mood in depressed patients during partial sleep deprivation. J Psychiatr Res. 2007;41(10):876–884. doi: 10.1016/j.jpsychires.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Hemmeter UM, Hemmeter-Spernal J, Krieg JC. Sleep deprivation in depression. Expert Rev Neurother. 2010;10(7):1101–1115. doi: 10.1586/ern.10.83. [DOI] [PubMed] [Google Scholar]
- Hiltunen L, Suominen K, Lonnqvist J, Partonen T. Relationship between daylength and suicide in Finland. J Circadian Rhythms. 2011;9:10. doi: 10.1186/1740-3391-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27(43):11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430(6995):78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- Huber R, Tononi G, Cirelli C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep. 2007;30(2):129–139. doi: 10.1093/sleep/30.2.129. [DOI] [PubMed] [Google Scholar]
- Huber R, Maki H, Rosanova M, Casarotto S, Canali P, Casali AG, Tononi G, Massimini M. Human cortical excitability increases with time awake. Cereb Cortex. 2013;23(2):332–338. doi: 10.1093/cercor/bhs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Duncan WC, Luckenbaugh DA, Yuan P, Machado-Vieira R, Zarate CAJ. Rapid antidepressant changes with sleep deprivation in major depressive disorder are associated with changes in vascular endothelial growth factor (VEGF): a pilot study. Brain Res Bull. 2011;86:129–133. doi: 10.1016/j.brainresbull.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Zarate CA., Jr A single infusion of ketamine improves depression scores in patients with anxious bipolar depression. Bipolar Disord. 2015;17:438–443. doi: 10.1111/bdi.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JC W, Kelsoe JR, Schachat C, Bunney BG, DeModena A, Golshan S, Gillin JC, Potkin SG, Bunney WE. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66(3):298–301. doi: 10.1016/j.biopsych.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Kasper S, Hamon M. Beyond the monoaminergic hypothesis: agomelatine, a new antidepressant with an innovative mechanism of action. World J Biol Psychiatry. 2009;10(2):117–126. doi: 10.1080/15622970902717024. [DOI] [PubMed] [Google Scholar]
- Kasper S, Hajak G, Wulff K, Hoogendijk WJ, Montejo AL, Smeraldi E, Rybakowski JK, Quera-Salva MA, Wirz-Justice AM, Picarel-Blanchot F, Bayle FJ. Efficacy of the novel antidepressant agomelatine on the circadian rest-activity cycle and depressive and anxiety symptoms in patients with major depressive disorder: a randomized, double-blind comparison with sertraline. J Clin Psychiatry. 2010;71(2):109–120. doi: 10.4088/JCP.09m05347blu. [DOI] [PubMed] [Google Scholar]
- Kreuzer PM, Langguth B, Schecklmann M, Eichhammer P, Hajak G, Landgrebe M. Can repetitive transcranial magnetic stimulation prolong the antidepressant effects of sleep deprivation? Brain Stimul. 2012;5(2):141–147. doi: 10.1016/j.brs.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Kuhn M, Wolf E, Maier JG, Mainberger F, Feige B, Schmid H, Burklin J, Maywald S, Mall V, Jung NH, Reis J, Spiegelhalder K, Kloppel S, Sterr A, Eckert A, Riemann D, Normann C, Nissen C. Sleep recalibrates homeostatic and associative synaptic plasticity in the human cortex. Nat Commun. 2016;7:12455. doi: 10.1038/ncomms12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer DJ, Ehlers CL. Two roads to rapid eye movement latency. Arch Gen Psychiatry. 1989;46(10):945–948. doi: 10.1001/archpsyc.1989.01810100087016. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Ulrich RF, Coble PA, Jarrett DB, Grochocinski VJ, Doman J, Matthews G, Borbély AA. Electroencephalographic sleep of younger depressives. Comparison with normals. Arch Gen Psychiatry. 1985;42(8):806–810. doi: 10.1001/archpsyc.1985.01790310068009. [DOI] [PubMed] [Google Scholar]
- Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, Kelmendi B, Simen A, McMahon FJ, Sanacora G, Zarate C., Jr Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry. 2012;72(11):e27–e28. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam RW, Kennedy SH. STAR*D and measurement-based care for depression: don’t toss out the baby. Can J Psychiatry. 2015;60(1):6–8. doi: 10.1177/070674371506000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenox RH, Gould TD, Manji HK. Endophenotypes in bipolar disorder. Am J Med Genet. 2002;114(4):391–406. doi: 10.1002/ajmg.10360. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Emens JS, Sack RL, Hasler BP, Bernert RA. Low, but not high, doses of melatonin entrained a free-running blind person with a long circadian period. Chronobiol Int. 2002;19(3):649–658. doi: 10.1081/cbi-120004546. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Liu LJ, LZ X, Gao L, Wang XF, Zhang JT, Lu L. Diurnal alterations in circadian genes and peptides in major depressive disorder before and after escitalopram treatment. Psychoneuroendocrinology. 2013;38(11):2789–2799. doi: 10.1016/j.psyneuen.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Linkowski P, Kerkhofs M, Rielaert C, Mendlewicz J. Sleep during mania in manic-depressive males. Eur Arch Psychiatry Neurol Sci. 1986;235(6):339–341. doi: 10.1007/BF00381002. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Baumann J, Wheeler-Castillo C, Latov D, Henter ID, Salvadore G, Zarate CA. The timing of antidepressant effects: a comparison of diverse pharmacological and somatic treatments. Pharmaceuticals (Basel) 2010;3:19–41. doi: 10.3390/ph3010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA., Jr The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep. 2007;9(6):467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Nievergelt CM, Kelsoe JR, Welsh DK. A survey of genomic studies supports association of circadian clock genes with bipolar disorder spectrum illnesses and lithium response. PLoS One. 2012;7(2):e32091. doi: 10.1371/journal.pone.0032091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murck H, Schubert MI, Schmid D, Schussler P, Steiger A, Auer DP. The glutamatergic system and its relation to the clinical effect of therapeutic-sleep deprivation in depression – an MR spectroscopy study. J Psychiatr Res. 2009;43(3):175–180. doi: 10.1016/j.jpsychires.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, Aan Het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Soleimani L, DeWilde KE, Collins KA, Lapidus KA, Iacoviello BM, Lener M, Kautz M, Kim J, Stern JB, Price RB, Perez AM, Brallier JW, Rodriguez GJ, Goodman WK, Iosifescu DV, Charney DS. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med. 2015;45(16):3571–3580. doi: 10.1017/S0033291715001506. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Grandner MA, Brown GK, Basner M, Chakravorty S, Morales KH, Gehrman PR, Chaudhary NS, Thase ME, Dinges DF. Nocturnal wakefulness as a previously unrecognized risk factor for suicide. J Clin Psychiatry. 2016;77(6):726–733. doi: 10.4088/JCP.15m10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73(9):e1160–e1167. doi: 10.4088/JCP.11r07586. [DOI] [PubMed] [Google Scholar]
- Pillai V, Kalmbach DA, Ciesla JA. A meta-analysis of electroencephalographic sleep in depression: evidence for genetic biomarkers. Biol Psychiatry. 2011;70(10):912–919. doi: 10.1016/j.biopsych.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Posternak MA, Zimmerman M. Is there a delay in the antidepressant effect? A meta-analysis. J Clin Psychiatry. 2005;66(2):148–158. doi: 10.4088/jcp.v66n0201. [DOI] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66(5):522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, Soleimani L, Charney DS, Foulkes AL, Mathew SJ. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. 2014;31(4):335–343. doi: 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitkin FM, Rabkin JG, Stewart JW, McGrath PJ, Harrison W, Davies M, Goetz R, Puig-Antich J. Sleep of atypical depressives. J Affect Disord. 1985;8:61–67. doi: 10.1016/0165-0327(85)90073-4. [DOI] [PubMed] [Google Scholar]
- Roecklein KA, Rohan KJ, Duncan WC, Rollag MD, Rosenthal NE, Lipsky RH, Provencio I. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J Affect Disord. 2009;114:279–285. doi: 10.1016/j.jad.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sabo E, Reynolds CF, 3rd, Kupfer DJ, Berman SR. Sleep, depression, and suicide. Psychiatry Res. 1991;36(3):265–277. doi: 10.1016/0165-1781(91)90025-k. [DOI] [PubMed] [Google Scholar]
- Saeki T, Nakamura M, Hirai N, Noda Y, Hayasaka S, Iwanari H, Hirayasu Y. Localized potentiation of sleep slow-wave activity induced by prefrontal repetitive transcranial magnetic stimulation in patients with a major depressive episode. Brain Stimul. 2013;6(3):390–396. doi: 10.1016/j.brs.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Sahlem GL, Kalivas B, Fox JB, Lamb K, Roper A, Williams EN, Williams NR, Korte JE, Zuschlag ZD, El Sabbagh S, Guille C, Barth KS, Uhde TW, George MS, Short EB. Adjunctive triple chronotherapy (combined total sleep deprivation, sleep phase advance, and bright light therapy) rapidly improves mood and suicidality in suicidal depressed inpatients: an open label pilot study. J Psychiatr Res. 2014;59:101–107. doi: 10.1016/j.jpsychires.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Uz T. Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neuroscience. 2005;134:1309–1316. doi: 10.1016/j.neuroscience.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Vande Voort JL, Ballard ED, Luckenbaugh DA, Bernert RA, Richards EM, Niciu MJ, Park L, Machado-Vieira R, Duncan WC, Zarate CA. Antisuicidal response following ketamine infusion is associated with decreased nighttime wakefulness in major depressive disorder and bipolar disorder. J Clin Psychiatry. 2016 doi: 10.4088/JCP.15m10440. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkes RJ, Kerkhof GA, Beld E, Hengeveld MW, van Kempen GM. Suicidality, circadian activity rhythms and platelet serotonergic measures in patients with recurrent suicidal behaviour. Acta Psychiatr Scand. 1996;93(1):27–34. doi: 10.1111/j.1600-0447.1996.tb10615.x. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Riedner BA, Cirelli C, Tononi G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep. 2007;30(12):1631–1642. doi: 10.1093/sleep/30.12.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr TA, Goodwin FK, editors. Psychobiology and psychopathology. Vol. 2. Boxwood Press; New York, NY: 1983. Circadian rhythms in psychiatry. [Google Scholar]
- Wehr TA, Turner EH, Shimada JM, Lowe CH, Barker C, Leibenluft E. Treatment of a rapidly cycling bipolar patient by using extended bed rest and darkness to stabilize the timing and duration of sleeo. Biol Psychiatry. 1998;43:822–828. doi: 10.1016/s0006-3223(97)00542-8. [DOI] [PubMed] [Google Scholar]
- Wiegand M, Berger M, Zulley J, von Zerssen D. The effect of trimipramine on sleep in patients with major depressive disorder. Pharmacopsychiatry. 1986;19:198–199. [Google Scholar]
- Wiegand M, Berger M, Zulley J, Lauer C, von Zerssen D. The influence of daytime naps on the therapeutic effect of sleep deprivation. Biol Psychiatry. 1987;22(3):389–392. doi: 10.1016/0006-3223(87)90157-0. [DOI] [PubMed] [Google Scholar]
- Wiegand M, Riemann D, Schreiber W, Lauer CJ, Berger M. Effect of morning and afternoon naps on mood after total sleep deprivation in patients with major depression. Biol Psychiatry. 1993;33:467–476. doi: 10.1016/0006-3223(93)90175-d. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Terman M. Chronotherapeutics (light and wake therapy) as a class of interventions for affective disorders. Handb Clin Neurol. 2012;106:697–713. doi: 10.1016/B978-0-444-52002-9.00042-5. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: what do we know, where do we go? Biol Psychiatry. 1999;46(4):445–453. doi: 10.1016/s0006-3223(99)00125-0. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Quinto C, Cajochen C, Werth E, Hock C. A rapid-cycling bipolar patient treated with long nights, bedrest and light. Biol Psychiatry. 1999;45:1075–1077. doi: 10.1016/s0006-3223(98)00289-3. [DOI] [PubMed] [Google Scholar]
- Wright RA, Johnson BG, Zhang C, Salhoff C, Kingston AE, Calligaro DO, Monn JA, Schoepp DD, Marek GJ. CNS distribution of metabotropic glutamate 2 and 3 receptors: transgenic mice and [(3)H]LY459477 autoradiography. Neuropharmacology. 2013;66:89–98. doi: 10.1016/j.neuropharm.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Wu JC, Kelsoe JR, Schachat C, Bunney BG, DeModena A, Golshan S, Gillin JC, Potkin SG, Bunney WE. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66(3):298–301. doi: 10.1016/j.biopsych.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA, Jr, Gould TD. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Eisinger BE, Driessen TM, Gammie SC. Addiction and reward-related genes show altered expression in the postpartum nucleus accumbens. Front Behav Neurosci. 2014;8:388. doi: 10.3389/fnbeh.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]