Abstract
Objective
To investigate the effects of focal (hemiablation) or total cryotherapy and minimum tumor temperature on patient-reported quality of life (QoL) in prostate cancer patients.
Methods
An IRB-approved database was reviewed for patients who underwent cryotherapy or active surveillance (AS). QoL questionnaire responses were collected and scores were analyzed for differences between focal versus total cryotherapy and very cold (<−76° C) versus moderate-cold (≥−76°C) minimum tumor temperatures.
Results
A total of 197 patients responded to a total of 547 questionnaires. Focal and total cryotherapy patients had initially lower sexual function scores relative to AS (year 1 mean difference focal: −31.7, p<0.001; total: −48.1, p<0.001). Focal cryotherapy was associated with a more rapid improvement in sexual function. Both focal and total cryotherapy sexual function scores were not statistically significantly different from the AS cohort by post-procedural year 4. Very cold and moderate-cold temperatures led to initially lower sexual function scores relative to AS (year 1 very cold: −38.1, p<0.001; moderate-cold: −30.7, p<0.001). Moderate-cold temperature scores improved more rapidly than those of very cold temperature. Neither very cold nor moderate-cold temperatures had a statistically significant difference in sexual function scores relative to AS by post-procedural year 4. Urinary function and bowel habits were not significantly different between focal and total cryotherapy, or very cold versus moderate-cold temperature groups.
Conclusion
Focal cryotherapy and moderate-cold (≥−76°C) temperature were associated with favorable sexual function relative to total cryotherapy and very cold temperature, respectively. No significant differences in urinary function or bowel habits were observed between groups.
Keywords: Quality of Life, cryotherapy, Active Surveillance, focal, cryosurgery
INTRODUCTION
Cryotherapy is an effective primary treatment modality for localized low and intermediate risk prostate cancer 1,2. Over the past decade, cryotherapy advancements have allowed for comparable rates of biochemical recurrence and higher quality of life (QoL) relative to traditional treatment modalities 3–5. However, sexual function after cryotherapy has been reported to initially decline following treatment 6,7. Thus, it is critical to identify modifiable parameters of the cryotherapy technique that do not negatively impact disease control, but may be associated with improved QoL outcomes.
Cryotherapy is the controlled freezing of the prostate gland 8. The rapid decrease in temperature results in protein denaturation, disruption of blood supply, and prostate cell apoptosis. The generally accepted therapeutic temperature of −40° C or colder should be obtained throughout the tumor and its periphery to ensure complete tissue destruction 9,10,11. Although cold temperatures may cause nerve damage, this is usually transient due to the resilience of the neural sheath 12. The extent of nerve damage exhibits temperature dependence 13–18. Therefore, because post-procedural QoL in terms of sexual function may be directly affected by the extent of cryo-injury in the neurovascular bundles, we chose to determine how minimum tumor temperature is associated with patient-reported quality of life following the cryotherapy procedure.
In patients with unilateral disease, cryotherapy may be used to hemiablate the disease-containing half of the prostate sparing the contralateral neurovascular bundle with the goal of maintenance of continence and potency 3,5,19. Robust evidence of oncological outcomes of focal therapy is lacking 3,20. Therefore, we analyzed the QoL in focal (hemiablation) cryotherapy patients, and how it compares to that in patients who underwent total cryotherapy or active surveillance (AS) 21. We investigated two modifiable parameters of cryotherapy: minimum tumor temperature and extent of cryoablation (focal and total gland ablation) to determine their association with post-procedural QoL (urinary, bowel, and sexual function) in prostate cancer patients.
MATERIAL & METHODS
Database maintenance
An IRB-approved institutional prostate cancer database was prospectively maintained with patient characteristics and questionnaire scores including the Expanded Prostate Cancer Index Composite (EPIC) and the International Index of Erectile Function Questionnaire (IIEF), valid and reliable questionnaires for the assessment of QoL in prostate cancer patients22,23.
Design and patients
Consented prostate cancer patients received EPIC and IIEF questionnaires at three-month intervals after the initiation of cryotherapy or enrollment in the AS protocol. Patients who received salvage cryotherapy were excluded. All other patients who underwent primary cryotherapy or AS from February 2011 to March 2017 and completed at least 1 questionnaire within the four years following treatment were included.
Cryotherapy technique
Patients were shown to be negative for metastasis via abdominal/pelvic imaging and bone scan. A single surgeon performed all cryotherapy procedures using the Galil Cryotherapy Surgical System (Galil Medical, Inc. Arden Hills, MN) 24. First, transrectal ultrasound (TRUS) was used to determine prostatic dimensions and locations of the urethra, peripheral zone, and distance to the prostatic capsule for optimal placement of 17-gauge (1.47 mm) cryoneedles or 2.4 mm cryoprobes, and thermocouples25. Candidates for focal cryotherapy were identified using a number of factors including PSA less than 10, unilateral disease, lack of extracapsular extension, and patient interest in maintaining sexual function. In focal cryotherapy, the cancer-containing half of the prostate was hemiablated, sparing the contralateral lobe and neurovascular bundle. In total cryotherapy, the prostate was ablated bilaterally. In both focal and total cryotherapy, prostatic tissue was cooled rapidly to a target of −40°C. The ice ball was directly visualized on TRUS during formation and was monitored throughout the procedure. Damage to the nerves was minimized using color Doppler TRUS, and urethral damage was avoided using a warming catheter. No rectal warming or saline block were employed. Two freeze-thaw cycles were performed. Temperatures were monitored with thermal sensors positioned at 5 mm, 15 mm, 25 mm, and 35 mm from the cryoneedle tip to ensure complete ablation of targeted tissue. The minimum temperature recorded out of all segments of all cryoneedles during a procedure was termed “minimum tumor temperature”, and used for subsequent analysis.
Patient cohort design and analysis
First, the entire cryotherapy group was separated into two cohorts: focal and total cryotherapy. Here, our focal cohort refers to men who underwent hemiablation of the prostate gland. Each group’s QoL scores were compared to the AS QoL scores and to each other at corresponding time points following the treatment. In the next portion of our study, the entire cryotherapy group was divided into two cohorts based on the minimum tumor temperature achieved during cryotherapy: moderate-cold and very cold. From database review, the median minimum tumor temperature in our cryotherapy cohort was determined to be −76° C. Because there are no reports to our knowledge to guide the choice of temperature (besides being −40° C or colder), the median minimum tumor temperature in our cryotherapy cohort was chosen as the cutoff between the two temperature groups. Thus the “very cold” group was defined as those who had minimum tumor temperatures below −76° C, and the “moderate-cold group” was defined as those who had minimum tumor temperatures of −76° C or above. Each of these groups was compared to each other and to AS patients. A t-test was used to compare the mean tumor temperature scores between the total and focal cryotherapy groups. The t-test was also used to compare preoperative mean PSA and age among cohorts. The chi-squared test was used to compare race, mean Gleason scores, and use of medical treatment for urinary or sexual function among cohorts. A p-value less than 0.05 was considered statistically significant.
Available patient symptom scores in each of the EPIC domains (urinary function, bowel habits, sexual function) and IIEF domains (erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall sexual satisfaction) were quantitated for cryotherapy and AS patients. Timing of the questionnaires was rounded to the nearest three months. EPIC and IIEF subdomain score means and standard deviations were obtained, and data were plotted as a function of time following procedure or AS enrollment. Statistical analyses were completed using Prism (GraphPad Software, Inc., La Jolla, CA), and results with p-values less than 0.05 were considered statistically significant. Mean scores of total curves were compared to one another using analysis of variance (ANOVA). A Bonferroni multiple comparison post-test was used to compare total, focal, very cold temperature, or moderate-cold temperature cryotherapy to AS patients. Then, a Bonferroni multiple comparison post-test was used to compare total cryotherapy scores to focal cryotherapy scores directly, and again used to compare very cold minimum tumor temperature patient scores to moderate-cold temperature patient scores directly. Mean scores were then similarly compared at 12 month intervals: 0–12 months (post-procedural year 1), 13–24 months (post-procedural year 2), 25–36 months (post-procedural year 3), 37–48 months (post-procedural year 4). P-values were obtained and indicated with asterisks within their respective time intervals on the graphs. Data were smoothed for graphical representation.
RESULTS
One hundred ninety-seven (129 cryotherapy [focal 89, total 38, very cold 52, moderate-cold 64; focal/total information and temperature information was not available for 2 and 13 patients, respectively] and 68 AS) patients were included in our study, with a median follow-up of 30 months post-procedure (Table 1). The mean minimum tumor temperature of the focal group was −74° C and the mean minimum tumor temperature of the total group was −70° C. These means were not statistically significantly different (p=0.21). Patient demographics and tumor characteristics are indicated in Table 1. The stratification of questionnaire sets by year and patient cohort is shown in Supplementary Table 1. Approximately 13% of our patients received hormonal therapy.
Table 1.
Characteristics of patients who underwent Cryotherapy or Active Surveillance as Primary Management for Prostate Cancer
| Cryotherapy (n=129) | AS (n=68) | Total patients (n=197) | p-value1 | |
|---|---|---|---|---|
| Survey packets (EPIC/IIEF) analyzed | 335 | 212 | 547 | |
| Median age | 69 | 66 | 68 | 0.007 |
| Focal | 68 | 0.00022 | ||
| Total | 74 | |||
| Very cold minimum tumor temperature | 70 | 0.663 | ||
| Moderate-cold minimum tumor temperature | 69 | |||
| Race or ethnic group - no. (%) | 0.0074 | |||
| White | 99/122 (81.1) | 63/66 (95.5) | 162/188 (86.0) | |
| Black | 15/122 (12.3) | 3/66 (4.5) | 18/188 (9.6) | |
| Hispanic | 5/122 (3.6) | 0/66 (0) | 5/188 (2.7) | |
| Asian | 1/122 (0.8) | 0/66 (0) | 1/188 (0.5) | |
| Other | 2/122 (1.6) | 0/66 (0) | 2/188 (1.0) | |
| Cryotherapy type – no. (%) | ||||
| Focal | 89/127 (70) | |||
| Total | 38/127 (30) | |||
| Very cold minimum tumor temperature | 52/116 (55) | |||
| Moderate-cold minimum tumor temperature | 64/116 (45) | |||
| Median PSA at treatment or initiation of AHS overall Gleason score | 6.1 | 5.0 | 5.7 | 0.08 |
| Median overall (%) | 7 | 6 | 6 | N/A5 |
| 0–6 | 48/128 (37.5) | 53/64 (82.8) | 101/192 (52.6) | |
| 7 (3+4, 4+3) | 55/128 (43.0) | 11/64 (17.2) | 66/192 (34.3) | |
| 8+ | 25/128 (19.5) | 0/64 (0) | 25/192 (13.0) | |
| Focal | 0.0542 | |||
| 0–6 | 37 | |||
| 7 (3+4, 4+3) | 39 | |||
| 8+ | 12 | |||
| Total | ||||
| 0–6 | 11 | |||
| 7 (3+4, 4+3) | 15 | |||
| 8+ | 12 | |||
| Very cold minimum tumor temperature | 0.133 | |||
| 0–6 | 23 | |||
| 7 (3+4, 4+3) | 23 | |||
| 8+ | 6 | |||
| Moderate-cold minimum tumor temperature | ||||
| 0–6 | 20 | |||
| 7 (3+4, 4+3) | 28 | |||
| 8+ | 16 | |||
| Prostate volume median (g) | 41 | 39 | 41 | 0.60 |
| Alpha-blocker / 5-alpha-reductase inhibitor use | ||||
| Focal | 48 | 0.562 | ||
| Total | 23 | |||
| Very cold minimum tumor temperature | 32 | 0.413 | ||
| Moderate-cold minimum tumor temperature | 33 | |||
| PDE5 inhibitor use | ||||
| Focal | 42 | 0.242 | ||
| Total | 14 | |||
| Very cold minimum tumor temperature | 25 | 0.553 | ||
| Moderate-cold minimum tumor temperature | 26 | |||
p-values were obtained for mean differences between cryotherapy and AS groups, unless otherwise noted
Comparison between focal and total cryotherapy group
Comparison between cryotherapy very cold minimum tumor temperature and moderate-cold minimum tumor temperature groups
Comparison of white versus black and other races/ethnicities combined
N/A, not applicable; p-value was not obtained due to the lack of AS patients with Gleason scores greater than or equal to 8.
Effect of focal versus total cryotherapy on patient QoL
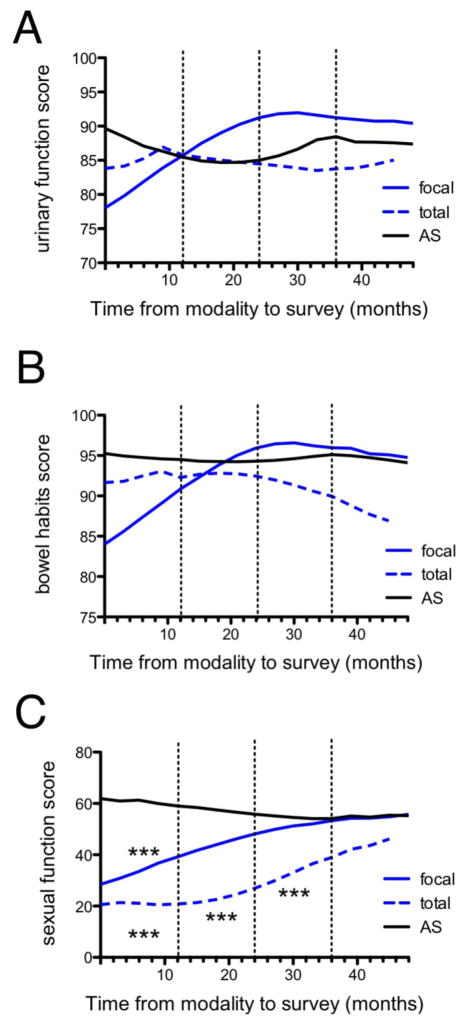
There were no statistically significant differences in urinary function (p=0.27) or bowel habits scores (p=0.12) among the total, focal cryotherapy and AS groups (Figures 1A and 1B). There was no statistically significant difference in alpha-blocker and 5-alpha-reductase inhibitor use (Table 1, p=0.56) or PDE5 inhibitor use (Table 1, p=0.24) between the total and focal cryotherapy groups. Both total and focal cryotherapy patients reported lower sexual function scores relative to AS soon after the procedure, then improved toward AS level scores, and were not significantly different from AS by post-procedural year 4 (Figure 1C). During the first post-procedural year, cryotherapy patients who underwent focal cryotherapy reported significantly lower sexual function scores relative to AS patients (mean difference: −31.7, p<0.001). Similarly, in the first post-procedural year, the total cryotherapy group reported lower scores than those of AS (−48.1, p<0.001). Over the next three years, the mean differences between focal cryotherapy sexual function scores and AS decreased with no significant difference by post-procedural year 2. On the contrary, total cryotherapy sexual function scores remained significantly lower compared to AS scores until post-procedural year 4 when the scores were no longer statistically significantly different. In post-procedural years 1–3, focal cryotherapy patients reported scores significantly better than those of total cryotherapy patients (year 1 mean difference: 16.4, p<0.05; year 2: 21.7, p<0.001; year 3: 24.8, p<0.01), but by year 4 there was no significant difference between the two groups (7.3, n.s.). Neither focal nor total cryoablation patient groups had a significant difference in sexual function score relative to AS patients by the post-procedural year 4. There was no statistically significant difference in mean minimum tumor temperatures between the total (70° C) and focal (74° C) groups (p=0.21).
Figure 1. Prostate cancer patient quality of life in the 4 years following total cryotherapy, focal cryotherapy, and AS as quantitated by EPIC questionnaire.
Urinary function (A), bowel habits (B), and sexual function (C) are shown as quantitated by the EPIC questionnaire over the four years following total cryotherapy (blue dashed line), focal cryotherapy (blue solid line), or AS (black line). Asterisks indicate statistically significant differences relative to AS. *p<0.05, **p<0.01, ***p<0.001.
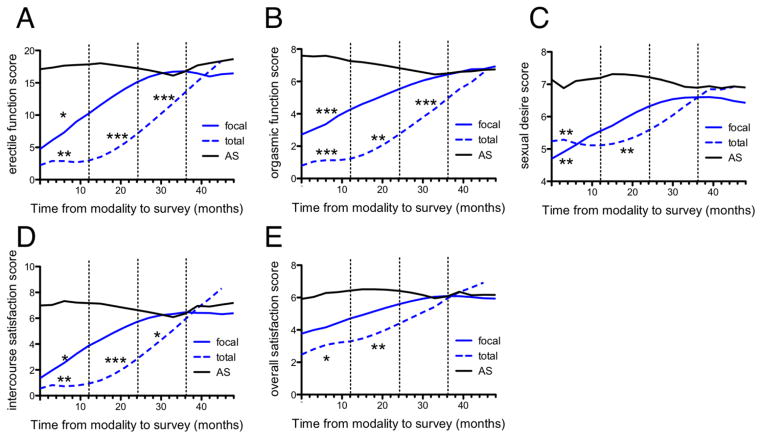
Focal cryotherapy patients were seen to have early lower scores in all IIEF sub-domains (post-procedural year 1 erectile function mean difference: −13.4, p<0.05; orgasmic function: 5.2, p<0.001; sexual desire: −2.8, p<0.01; intercourse satisfaction −6.1, p<0.05; overall satisfaction: −3.0, n.s.), but exhibited rapid improvement by post-procedural year 2, with no statistically significant difference compared to AS IIEF sub-domain scores. Similarly, the total cryotherapy patients had initial lower scores (post-procedural year 1 erectile function mean difference: −17.4, p<0.01; orgasmic function: 7.5, p<0.001; sexual desire: −2.8, p<0.01; intercourse satisfaction −7.3, p<0.01; overall satisfaction: −3.9, p<0.05) that improved over the following years. By post-procedural year 4, there was no difference between IIEF scores in any sub-domain of total cryotherapy patients and the AS cohort. Overall, both focal and total cryotherapy patients experienced initially lower sexual function scores, but rapidly improved in the following years. Focal patients had a more rapid improvement as compared to total cryotherapy patients.
Effect of minimum tumor temperature on cryotherapy patient QoL
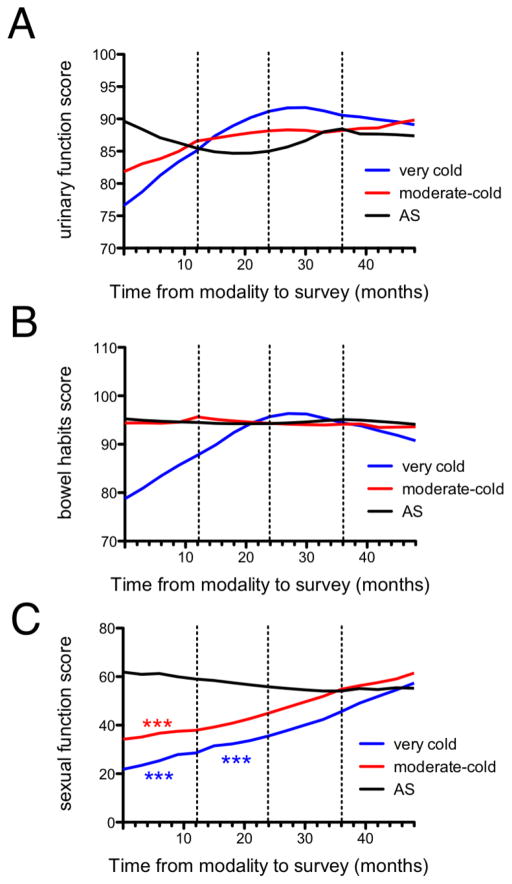
There was no statistically significant difference in urinary function (p=0.77) or bowel habits (p=0.15) scores among the very cold temperature, moderate-cold temperature, and AS groups either when analyzed for the entire time course or over yearly intervals (Figures 3A and 3B). There was no statistically significant difference in alpha-blocker and 5-alpha-reductase inhibitor use (Table 1, p=0.41) or PDE5 inhibitor use (Table 1, p=0.55) between very cold and moderate-cold cryotherapy groups. Sexual function scores were significantly different among the very cold temperature, moderate-cold temperature, and AS groups (p<0.0001) (Figure 3C). When analyzed at yearly intervals, the moderate-cold group had significantly lower scores than those of AS in the first post-procedural year (moderate-cold year 1: −30.7, p<0.001). The very cold temperature group scores were significantly lower than those of the AS group during the first 2 post-procedural years (year 1 mean difference: −38.1, p<0.001; year 2: −18.2, p<0.001). By post-procedural year 2, the moderate-cold temperature group scores were not significantly different from AS (−2.2, n.s.), and by post-procedural year 3 the very cold temperature group scores were not significantly different from AS (−13.5, n.s.). Overall, the moderate-cold temperature group exhibited a more rapid improvement in sexual function scores than the very cold temperature group. Given the lower proportion of focal cryotherapy in the moderate-cold group (59% focal) relative to the very cold group (78% focal), focal versus total cryoablation is unlikely to account for the difference in sexual function between the minimum tumor temperature groups.
Figure 3. Prostate cancer patient quality of life in the 4 years following very cold minimum tumor temperature cryotherapy, moderate-cold minimum tumor temperature cryotherapy, and AS as quantitated by EPIC questionnaire.
Urinary function (A), bowel habits (B), and sexual function (C) are shown as quantitated by the EPIC questionnaire over the four years following very cold minimum tumor temperature cryotherapy (blue line), moderate-cold minimum tumor temperature cryotherapy (red line), or AS (black line). Asterisks indicate statistically significant differences relative to AS. *p<0.05, **p<0.01, ***p<0.001.
In all IIEF subdomains, the very cold temperature group had a slower improvement in IIEF scores than the moderate-cold temperature group (Supplementary Figure 1A–E). Furthermore, in terms of intercourse satisfaction, the very cold temperature group scores remained significantly lower than those of the AS group at 4 years post-procedure (mean difference: −2.2; p<0.05), whereas the moderate-cold temperature did not (0.07, n.s.). In the very cold temperature group, there was minimal improvement over the four post-procedural years in the sexual desire (mean score ± SD year 1: 4.6 ± 0.8; year 4: 5.8 ± 1.0) and overall satisfaction subdomain scores (year 1: 3.8 ± 0.7; year 4: 5.1 ± 1.0). Overall, the very cold temperature group reported lower sexual function scores in the EPIC questionnaire and most of the IIEF subdomains relative to the moderate-cold group.
DISCUSSION
Cryotherapy has become an important modality in the primary treatment of low and intermediate risk prostate cancer1. Our study provides novel insight into the quality of life in prostate cancer patients following primary cryotherapy. Overall, our results indicate that, over the four years following the procedure, focal cryotherapy of the prostate gland and a moderate minimum tumor temperature are associated with superior patient-reported sexual function, relative to total cryotherapy and a very cold minimum tumor temperature, respectively. Advancements in imaging and cryotherapy technology continue to allow for minimal collateral damage in an attempt to preserve erectile function26. In our study, despite an early post-operative lower sexual function, we observed progressive recovery and no difference in EPIC sexual function scores between any of our cryotherapy groups and AS by the fourth post-procedural year.
Focal cryotherapy in prostate cancer treatment spares the contralateral neurovascular bundle8. Our results extend those of Shah et al., who systematically reviewed current literature and found erectile dysfunction to be present in 0–42% of men after having undergone focal cryotherapy27. In our focal cryotherapy cohort of patients, we observed no significant difference in erectile function relative to the AS cohort by the second post-procedural year. In contrast, our total cryotherapy cohort did not recover to scores similar to AS until post-procedural year 4. We attribute these differences to the sparing of the contralateral neurovascular bundle during focal cryotherapy.
A minimum cryotherapy temperature of −40°C is generally accepted as a therapeutic threshold 9,28,29. Both the very cold (below −76° C) and moderate-cold temperature groups (−76° C or above) improved in sexual function scores over the four post-procedural years. However, the very cold temperature group had generally worse scores and a slower improvement than the moderate-cold temperature group in terms of sexual function and its various subdomains. Therefore, the minimum tumor temperature should be chosen carefully, as it may have implications in the post-procedural QoL following cryotherapy. Additional studies are needed to further investigate and identify the optimal temperature range.
Nerve injury within the neurovascular bundles following cryotherapy is a possible complication 12,18. Studies have demonstrated that such injury is usually transient 30,31. The resilience of the neural sheath allows for axonal regeneration following cryo-injury. If the sheath is not irreparably damaged, both sensation and motor function return spontaneously over many months. Both moderate-cold minimum tumor temperature and focal cryotherapy may spare the architecture of the neural sheath and provide for an efficient scaffold for neural regeneration, resulting in a rapid improvement in sexual function scores.
Our analysis compared patient-reported QoL scores following cryotherapy treatment to scores of patients who underwent AS, at respective time points. Because our study is retrospective in nature, the baseline scores for cohorts were not available in sufficient number for inclusion in our analysis. However, our interpretations and conclusions in thus study were based only on comparison of QoL scores following cryotherapy to those scores following initiation of AS, not relative to baseline scores. Further, because our database review was retrospective in nature, it was subject to potential selection bias. For example, as indicated in Table 1, the mean age of the total cryotherapy population was greater than that of the focal cryotherapy population. In this case, the greater age as well as potentially greater comordities could contribute to a lower post-procedural QoL. To the contrary, the total cryotherapy cohort exhibited excellent urinary function, bowel habits, and sexual function scores, similar to the active surveillance cohort by post-procedure year 4. While hormonal therapy is known to be associated with adverse sexual function, only a small subset (~13%) of patients in our cryotherapy cohort underwent hormonal therapy. Because our study’s emphasis was specifically on cryotherapy outcomes, we chose to investigate patients irrespective of ongoing hormonal treatments. It is known that cryotherapy is an effective primary option for disease control in low and intermediate risk prostate cancer 1,2. The scope of the present study was to investigate QoL outcomes following cryotherapy, irrespective of disease control in our cohorts. Further studies are underway to investigate biochemical recurrence, PSA response, and disease control in our patient population. The variable annual response rates in our cohorts were an additional limitation. In particular, in the fourth year of our study, only 7 questionnaires were available from patients who underwent total cryotherapy (Supplementary Table 1). This may have partially accounted for the lack of significant difference in sexual function between the total cryotherapy cohort and the AS cohort seen in Figures 1C and 2. Although our institution’s cryotherapy database is among the largest single-center databases, patient sample size remains a limitation. Additional high-powered studies will be needed to further explore the effects of cryotherapy parameters on patient QoL.
Figure 2. Prostate cancer patient sexual function in the 4 years following total cryotherapy, focal cryotherapy, and AS as quantitated by IIEF questionnaire subdomains.
Erectile function (A), orgasmic function (B), sexual desire (C), intercourse satisfaction (D), and overall sexual satisfaction (E) are shown as quantitated by the IIEF questionnaire subdomains over the four years following total cryotherapy (blue dashed line), focal cryotherapy (blue solid line) or AS (black line). Asterisks indicate statistically significant differences relative to AS. *p<0.05, **p<0.01, ***p<0.001.
CONCLUSIONS
Post-procedure urinary function and bowel habit scores in the total cryotherapy, focal cryotherapy, moderate-cold cryotherapy (≥−76° C), and very cold (<−76° C) cryotherapy groups were not significantly different relative to the active surveillance group. The total cryotherapy group exhibited post-procedural sexual function scores initially lower than the active surveillance group. The total cryotherapy group sexual function scores then improved over the ensuing three years, and were similar to those of the active surveillance group by post-procedure year 4. The focal cryotherapy group exhibited a similar trend, but with a more rapid improvement, reporting similar sexual function scores to those of the active surveillance group by post-procedure year 2. A moderate-cold minimum tumor temperature (≥−76° C) was associated with a more rapid improvement in most sexual function scores relative to a very cold temperature (<−76° C).
Supplementary Material
Erectile function (A), orgasmic function (B), sexual desire (C), intercourse satisfaction (D), and overall sexual satisfaction (E) are shown as quantitated by the IIEF questionnaire subdomains over the four years following very cold minimum tumor temperature cryotherapy (blue line), moderate-cold minimum tumor temperature cryotherapy (red line), or AS (black line). Asterisks indicate statistically significant differences relative to AS. *p<0.05, **p<0.01, ***p<0.001.
Acknowledgments
Funding: G.T.W. was supported by Medical Scientist Training Program award T32GM008444 and National Research Service Award F30AI112252 from the NIH.
Footnotes
Conflict of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bahn D, de Castro Abreu AL, Gill IS, et al. Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. European urology. 2012;62(1):55–63. doi: 10.1016/j.eururo.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JK, Miller RJ, Ahmed S, Lotz MJ, Baust J. Ten-year biochemical disease control for patients with prostate cancer treated with cryosurgery as primary therapy. Urology. 2008;71(3):515–518. doi: 10.1016/j.urology.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 3.Perera M, Krishnananthan N, Lindner U, Lawrentschuk N. An update on focal therapy for prostate cancer. Nat Rev Urol. 2016;13(11):641–653. doi: 10.1038/nrurol.2016.177. [DOI] [PubMed] [Google Scholar]
- 4.Ritch CR, Katz AE. Prostate cryotherapy: current status. Curr Opin Urol. 2009;19(2):177–181. doi: 10.1097/mou.0b013e32831e16ce. [DOI] [PubMed] [Google Scholar]
- 5.Kongnyuy M, Lipsky MJ, Islam S, et al. Predictors of biochemical recurrence after primary focal cryosurgery (hemiablation) for localized prostate cancer: A multi-institutional analytic comparison of Phoenix and Stuttgart criteria. Urologic oncology. 2017 doi: 10.1016/j.urolonc.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Robinson JW, Donnelly BJ, Saliken JC, Weber BA, Ernst S, Rewcastle JC. Quality of life and sexuality of men with prostate cancer 3 years after cryosurgery. Urology. 2002;60(2 Suppl 1):12–18. doi: 10.1016/s0090-4295(02)01679-5. [DOI] [PubMed] [Google Scholar]
- 7.Anastasiadis AG, Sachdev R, Salomon L, et al. Comparison of health-related quality of life and prostate-associated symptoms after primary and salvage cryotherapy for prostate cancer. J Cancer Res Clin Oncol. 2003;129(12):676–682. doi: 10.1007/s00432-003-0472-4. [DOI] [PubMed] [Google Scholar]
- 8.Habibian DJ, Katz AE. Emerging minimally invasive procedures for focal treatment of organ-confined prostate cancer. Int J Hyperthermia. 2016;32(7):795–800. doi: 10.1080/02656736.2016.1195925. [DOI] [PubMed] [Google Scholar]
- 9.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37(3):171–186. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology. 2002;60(2 Suppl 1):40–49. doi: 10.1016/s0090-4295(02)01683-7. [DOI] [PubMed] [Google Scholar]
- 11.Bischof JC, Smith D, Pazhayannur PV, Manivel C, Hulbert J, Roberts KP. Cryosurgery of dunning AT-1 rat prostate tumor: thermal, biophysical, and viability response at the cellular and tissue level. Cryobiology. 1997;34(1):42–69. doi: 10.1006/cryo.1996.1978. [DOI] [PubMed] [Google Scholar]
- 12.Baust JG, Gage AA, Baust JM. Dermatological Cryosurgery and Cryotherapy. Springer; 2016. Principles of Cryoablation; pp. 9–16. [Google Scholar]
- 13.Beazley RM, Bagley DH, Ketcham AS. The effect of cryosurgery on peripheral nerves. Journal of Surgical Research. 1974;16(3):231–234. doi: 10.1016/0022-4804(74)90036-5. [DOI] [PubMed] [Google Scholar]
- 14.Breidenbach LM, Thomford N, Pace WG. Cryosurgery of tumors involving the facial nerve. Archives of Surgery. 1972;105(2):306–307. doi: 10.1001/archsurg.1972.04180080154025. [DOI] [PubMed] [Google Scholar]
- 15.Carter D, Lee P, Gill W, Johnston R. The effect of cryosurgery on peripheral nerve function. Journal of the Royal College of Surgeons of Edinburgh. 1972;17(1):25. [PubMed] [Google Scholar]
- 16.Mandeville AF, McCabe BF. Some observations on the cryobiology of blood vessels. The Laryngoscope. 1967;77(8):1328–1350. doi: 10.1288/00005537-196708000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Whittaker D. Degeneration and regeneration of nerves following cryosurgery. British journal of experimental pathology. 1974;55(6):595. [PMC free article] [PubMed] [Google Scholar]
- 18.Gage A, Baust J, Baust J. Experimental cryosurgery investigations in vivo. Cryobiology. 2009;59(3):229–243. doi: 10.1016/j.cryobiol.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nomura T, Mimata H. Focal therapy in the management of prostate cancer: an emerging approach for localized prostate cancer. Adv Urol. 2012;2012:391437. doi: 10.1155/2012/391437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. [Accessed 05/17/17, 2017];AUA Clinical Guidelines. 2017 https://www.auanet.org/guidelines/clinically-localized-prostate-cancer-new-(aua/astro/suo-guideline-2017)
- 21.Berg CJ, Habibian DJ, Katz AE, Kosinski KE, Corcoran AT, Fontes AS. Active Holistic Surveillance: The Nutritional Aspect of Delayed Intervention in Prostate Cancer. Journal of nutrition and metabolism. 2016;2016:2917065. doi: 10.1155/2016/2917065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 23.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 24.Onik GM, Cohen JK, Reyes GD, Rubinsky B, Chang Z, Baust J. Transrectal ultrasound-guided percutaneous radical cryosurgical ablation of the prostate. CANCER-PHILADELPHIA- 1993;72:1291–1291. doi: 10.1002/1097-0142(19930815)72:4<1291::aid-cncr2820720423>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 25.Ullal AV, Korets R, Katz AE, Wenske S. A report on major complications and biochemical recurrence after primary and salvage cryosurgery for prostate cancer in patients with prior resection for benign prostatic hyperplasia: a single-center experience. Urology. 2013;82(3):648–652. doi: 10.1016/j.urology.2013.04.052. [DOI] [PubMed] [Google Scholar]
- 26.Gao L, Yang L, Qian S, et al. Cryosurgery would be an effective option for clinically localized prostate cancer: a meta-analysis and systematic review. Scientific reports. 2016:6. doi: 10.1038/srep27490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah TT, Ahmed H, Kanthabalan A, et al. Focal cryotherapy of localized prostate cancer: a systematic review of the literature. Expert Rev Anticancer Ther. 2014;14(11):1337–1347. doi: 10.1586/14737140.2014.965687. [DOI] [PubMed] [Google Scholar]
- 28.Babaian RJ, Donnelly B, Bahn D, et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2008;180(5):1993–2004. doi: 10.1016/j.juro.2008.07.108. [DOI] [PubMed] [Google Scholar]
- 29.Hollister W, Mathew A, Baust J, Vary Buskirk R. Effects of freezing on cell viability and mechanisms of cell death in a human prostate cancer cell line. Molecular Urology. 1998;2(1):13–18. [Google Scholar]
- 30.Pradel W, Hlawitschka M, Eckelt U, Herzog R, Koch K. Cryosurgical treatment of genuine trigeminal neuralgia. British Journal of Oral and Maxillofacial Surgery. 2002;40(3):244–247. doi: 10.1054/bjom.2001.0765. [DOI] [PubMed] [Google Scholar]
- 31.Finelli PF. Ulnar neuropathy after liquid nitrogen cryotherapy. Archives of dermatology. 1975;111(10):1340–1342. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Erectile function (A), orgasmic function (B), sexual desire (C), intercourse satisfaction (D), and overall sexual satisfaction (E) are shown as quantitated by the IIEF questionnaire subdomains over the four years following very cold minimum tumor temperature cryotherapy (blue line), moderate-cold minimum tumor temperature cryotherapy (red line), or AS (black line). Asterisks indicate statistically significant differences relative to AS. *p<0.05, **p<0.01, ***p<0.001.