Abstract
The large majority of cases of the autosomal dominant human disease fibrodysplasia ossificans progressiva (FOP) are caused by gain-of-function Arg206His mutations in the BMP type I receptor ACVR1 (ALK2). The Arg206His mutation is located in the GS domain of the type I receptor. This region is normally phosphorylated by the BMP type II receptor, which activates the type I receptor to phosphorylate its substrate, the signal transducer Smad1/5/8. A small subset of patients with FOP carry variant mutations in ACVR1 altering Gly328 to Trp, Glu or Arg. Since these mutations lie outside the GS domain, the mechanism through which ACVR1 Gly328 mutations cause disease remains unclear. We used a zebrafish embryonic development assay to test the signaling of human ACVR1 Gly328 mutant receptors comparing them to the Arg206His mutant. In this assay increased or decreased BMP pathway activation alters dorsal-ventral axial patterning, providing a sensitive assay for altered BMP signaling levels. We expressed the human ACVR1 Gly328 mutant receptors in zebrafish embryos to investigate their signaling activities. We found that all ACVR1 Gly328 human mutations ventralized wild-type embryos and could partially rescue Bmp7-deficient embryos, indicating that these mutant receptors can activate BMP signaling in a BMP ligand-independent manner. The degree of ventralization or rescue was similar among all three Gly328 mutants. Smad1/5 phosphorylation, a readout of BMP receptor signaling, was mildly increased by ACVR1 Gly328 mutations. Gene expression analyses demonstrate expanded ventral and reciprocal loss of dorsal cell fate markers. This study demonstrates that Gly328 mutants increase receptor activation and BMP ligand-independent signaling through Smad phosphorylation.
Keywords: FOP, BMP signaling, ACVR1, Smad1/5, zebrafish, dorsal-ventral embryonic patterning
1. INTRODUCTION
Fibrodysplasia ossificans progressiva (FOP, MIM# 135100) is a debilitating human autosomal dominant disease with episodic heterotopic ossification (HO) of soft tissues and characteristic malformation of the great toes. These classic features are sufficient to make a clinical diagnosis of FOP in most cases (Kaplan et al., 2009). FOP is caused by mutations in the Bone Morphogenetic Protein (BMP) type I receptor ACVR1 (also called ALK2) (Shore et al., 2006).
BMPs belong to the highly conserved transforming growth factor beta (TGFβ) superfamily of ligands, and play important roles during embryogenesis and skeletal formation. BMPs signal on the cell surface through tetramers of two type II receptors and two type I receptors. The type II receptor phosphorylates the type I receptor in the glycine-serine-rich (GS) domain, thus activating its serine-threonine kinase, which can then phosphorylate Smad1/5/8. Phosphorylated Smad1/5/8 (P-Smad) in complex with Smad4 then accumulates in the nucleus where it regulates gene expression depending on its developmental and tissue specific context (reviewed in Sieber et al, 2009; Bragdon et al., 2011; Dutko and Mullins, 2011; Wang et al., 2014).
All patients with FOP have mutations in ACVR1 with >95% carrying the recurring mutation Arg206His in the GS domain (Kaplan et al., 2009; Zhang et al., 2013). Studies in cell culture assays and zebrafish embryos show that this mutation renders the ACVR1 receptor constitutively active and confers increased BMP signaling activity in the absence of BMP ligand (Shen et al, 2009; van Dinther et al., 2009; Fukuda et al., 2010; Song et al., 2010; Chaikuad et al., 2012). In silico modeling indicates that the introduction of histidine at position 206 alters the structure of the activation domain of the mutant ACVR1 protein (Groppe et al., 2007).
A small subset of patients (<5%) has an atypical form of FOP. Kaplan et al., 2009, classified these patients into two categories: patients with the classic defining FOP features and additional uncommon manifestations are designated as “FOP-plus”; whereas patients with manifestations distinct from the range of classic FOP features e.g. a milder or more severe phenotype, including age of onset of HO after 10 years of age, mild or no toe deformities, or more severe digit deficits are considered “variant FOP”. Multiple reports of atypical FOP with varying disease severity have been published in the last several years (Kaplan et al., 2009; Carvalho et al., 2010; Hüning and Gillessen-Kaesbach, 2014; Kaplan et al., 2015).
Three different mutations in codon Gly328 of the kinase domain of ACVR1 have been confirmed in at least ten patients with atypical FOP (Kaplan et al., 2009; Carvalho et al., 2010; reviewed in Hüning and Gillessen-Kaesbach, 2014). Interestingly, the three mutations in codon 328 are associated with a milder (Gly328Arg) or a variant (Gly328Trp, Gly328Glu) disease phenotype compared to classic FOP with the Arg206His mutation. Five patients (including three patients from a single family) with a heterozygous ACVR1 Gly328Arg mutation had a later age of onset of HO (>10 years) or no HO and normal or only minimal morphological changes of the great toes. In contrast, two patients with a Gly328Trp mutation and three patients with a Gly328Glu mutation had the classic feature of FOP with childhood onset of HO. In addition, these five patients had severe deficits of digits, mild cognitive impairment and ectodermal signs with aplastic toenails and sparse scalp hair (Kaplan et al., 2009; Carvalho et al., 2010). In silico modeling of ACVR1 Gly328 mutations did not indicate significantly altered conformation of the protein kinase domain, however, this residue is positioned near the GS domain in the three-dimensional receptor structure and so these substitutions could alter receptor function (Kaplan et al., 2009; Petrie et al., 2009; Chaikaud et al., 2012).
The early zebrafish embryo has proven to be an excellent assay system for examining BMP pathway component function, including the human classic FOP Arg206His mutation in ACVR1 (Shen et al., 2009; Little and Mullins, 2009). Zebrafish embryonic development depends on a tightly regulated BMP activity gradient that specifies distinct tissues along the dorsal-ventral axis as a morphogen (Tucker et al., 2008; Hashiguchi and Mullins, 2013, Zinski et al, 2017). Mutations causing a loss or increase of BMP signaling have been described for many genes in the BMP signaling pathway (Schulte-Merker et al., 1997; Nguyen et al., 1998; Connors et al., 1999; Hild et al., 1999; Miller-Bertoglio et al., 1999; Dick et al., 2000; Schmid et al., 2000; Mintzer et al., 2001; Yabe et al., 2003). One of these components is Acvr1l (previously called Alk8), the zebrafish paralog to the human ACVR1 receptor. ACVR1 (ALK2) and Acvr1l share 87% protein homology in the GS and protein-kinase domains and expression of wild-type human ACVR1 can rescue the zygotic acvr1l zebrafish mutant lost-a-fin (laf) (Shen et al., 2009). Our knowledge of zebrafish BMP signaling during early development, together with the relative ease of genetic manipulation of zebrafish embryos make it a valuable tool to study the activities of the human ACVR1 mutant receptors.
The effect of ACVR1 Gly328 mutations on BMP signaling has not yet been studied in vivo. By misexpressing the ACVR1 Gly328 mutant (Glu328, Trp328, and Arg328) receptors during early zebrafish development, we demonstrate that ACVR1 Gly328 mutant receptors, like the classic ACVR1 Arg206His receptor, increase BMP signaling activity and can phosphorylate Smad1/5 in a BMP7 ligand-independent manner. This is corroborated by gene expression studies confirming expansion of markers of ventral cell fate and loss of dorsal cell fate. Thus, although the Gly328 mutations are not in the GS domain, they also can confer BMP ligand-independent gain-of-function signaling, suggesting that they may similarly alter ACVR1 receptor function in FOP disease development.
2. MATERIAL AND METHODS
2.1 Plasmid constructs
ACVR1 Trp328 and ACVR1 Glu328 in pcDNA3.1D/V5-His-TOPO were generously provided by M. Xu. The Arg328 plasmid was generated by site directed mutagenesis using a FLAG-tagged ACVR1 WT plasmid (Shen et al., 2009). All fragments were cloned into pCS2+ vector and confirmed by Sanger sequencing. The His206 plasmid is previously described (Shen et al., 2009).
2.2 mRNA microinjections and analysis
Zebrafish bmp7sb1aub mutant embryos were injected at the one-cell stage with bmp7a mRNA to completely rescue the phenotype; rescued homozygous mutant embryos were then raised to adulthood, as described previously (Schmid et al., 2000). Crosses between bmp7sb1aub/sb1aub adults provided exclusively bmp7sb1aub/sb1aub mutant embryos.
Rescue of zygotic acvr1ltm100b mutant embryos was also tested by injecting embryos from crosses of acvr1ltm100b/+ adults with mRNA of the human FOP ACVR1 mutants, as previously described (Mintzer et al, 2001; Shen et al., 2009).
Plasmids of pCS2+ carrying human ACVR1 Trp328 and ACVR1 Glu328 were linearized with KpnI and pCS2+ plasmids of ACVR1 Arg328 and ACVR1 His206 were linearized with NotI. mRNA was obtained using the mMESSAGE mMACHINE SP6 kit (Ambion). ACVR1 mRNA with variant mutation (Trp328 [100 pg], Glu328 [150 pg], Arg328 [100 pg]) or His206 [25 pg] was injected into the yolk of wild type (WT) or bmp7sb1aub/sb1aub embryos at the one-cell stage, as described previously (Westerfield, 2000). Phenotypes were assessed and classified at 24 hours post fertilization (hpf) for severity of ventralization or dorsalization (Mullins et al., 1996; Nguyen et al., 1998; Shen et al., 2009; Little and Mullins, 2009). Embryos were anaesthetized in 0.2% tricaine and embedded in 2% methylcellulose prior to imaging. Specimens were imaged and photographed with an MZ12.5 stereomicroscope equipped with a ColorSNAP-cf digital camera.
2.3 P-Smad1/5 immunostaining and confocal microscopy
Early and mid-gastrula embryos were fixed in 4% PFA in PBS overnight. Fixed embryos were dechorionated and deyolked followed by several washes in PBS buffer with 0.1% Triton. After blocking with NCS-PBS buffer with 0.1% Triton, embryos were suspended in a 1:100 dilution of phospho-Smad1/5/8 (P-Smad1/5) primary rabbit antibody (Cell Signaling 9511L) followed by a 1:500 dilution of Alexaflour 647 anti-rabbit secondary antibody (Invitrogen) to label P-Smad1/5 protein and 1:10,000 dilution of Sytox Orange (Invitrogen) to label nuclei. Embryos were washed, cleared in benzylbenzoate:benzylalcohol (2:1), and mounted on glass coverslips using silicon wafers.
Embryos were visualized using a Zeiss 710 confocal line-scanning microscope with a Zeiss LD LCI Plan-Apochromat 25x/0.8 Imm Corr DIC Objective. To quantify P-Smad1/5 immunofluorescence levels, a single representative z-slice per embryo was divided into 18 subunits. P-Smad1/5 intensity was measured in all nuclei and averaged across one subunit. The result was plotted in arbitrary units (AU) according to position in the embryo as either individual data or a compounded average across all embryos of the same injection condition. Image processing was done using MATLAB.
2.4 Whole-mount in situ hybridization
Whole-mount in situ hybridization was carried out as described (Schumacher et al., 2011) using chordin (Miller-Bertoglio et al., 1997), gsc (Schulte-Merker et al., 1994), gata2 (Detrich et al., 1995), and eve1 (Joly et al., 1993) on embryos collected at the 60% epiboly stage. Stained specimens were mounted in methylcellulose prior to imaging with an MZ12.5 stereomicroscope equipped with a ColorSNAP-cf digital camera.
3 RESULTS
3.1 ACVR1 Gly328 mutant receptors ventralize wild-type (WT) embryos
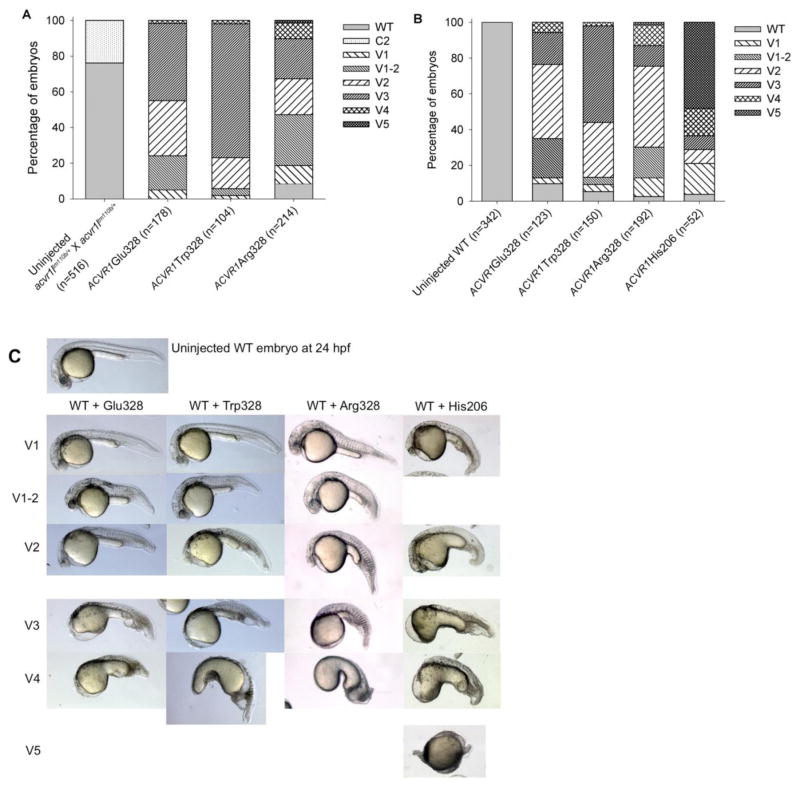
Zygotic null mutant embryos of the human ACVR1 paralog in zebrafish, acvr1ltm110b, exhibit a loss of ventral tail tissue, a class 2 (C2) dorsalization, due to decreased BMP signaling (Mintzer at al., 2001). To investigate whether the G328 variant mutations encode functional ACVR1 receptors, human ACVR1 Glu328, Trp328, and Arg328 mRNAs were injected into embryos of acvr1ltm110b/+ incrosses. As expected for a recessive trait, about one quarter of the uninjected control group fell into the C2 dorsalization category. In contrast, when the three variant Gly328 mutant human mRNAs were injected into acvr1l mutant embryos, no dorsalized embryos were observed and instead ventral tail tissues were fully restored (Fig. 1A).
Figure 1. Effect of Gly328 mutant receptor expression in acvr1ltm110b and WT embryos.
A. mRNA misexpression in embryos from incrosses of acvr1ltm110b/+ fish. One quarter are acvr1ltm110b/tm110b mutant embryos. Phenotypes were scored at 24 hpf.
B. mRNA misexpression in WT embryos. Phenotypes were scored at 24 hpf.
C. Ventralization phenotypes are indistinguishable between WT embryos injected with ACVR1 Gly328 mutant mRNAs and His206 mRNA.
hpf – hours post fertilization; C2 – lack of ventral tail fin and vein; V1 – mild ventralization with diminished eye size and broadening of ventral tail fin; V1-2 – loss of notochord, but eye present; V2 – lack of eye, reduced head tissue, loss of notochord; V3 – lack of head tissue; V4 – greatly enlarged yolk extension; V5 – radially ventralized; WT – wild type.
Next, mRNA for each variant was injected into WT embryos using the classic human ACVR1 His206 mutant mRNA as a positive control. All variant mutations caused mild to moderate ventralization at 24 hours post fertilization (hpf), similar to WT embryos injected with ACVR1 His206 (Fig. 1B,C) but unlike human WT ACVR1, which does not ventralize WT embryos (Shen et al., 2009). Only a small fraction of embryos fell into the V4 or V5 category, whereas the majority of embryos were mildly (V1, V1-2) to moderately (V2, V3) ventralized. Increasing the amount of injected mRNA of ACVR1 Glu328 into WT embryos by 3-fold increased the proportion of embryos with the most severe degrees of ventralization (V4 and V5) (Suppl. Fig. S1). These results indicate that ACVR1 Glu328, Trp328, and Arg328 mRNAs encode functional receptors and that these mutations, like ACVR1 His206, increase BMP signaling in the early zebrafish embryo.
3.2 ACVR1 codon 328 mutant mRNAs rescue BMP7-deficient embryos
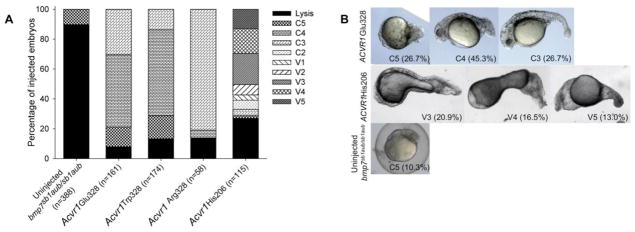
Since the obligate ligand in dorsal-ventral patterning of the zebrafish embryo is a BMP2b-BMP7a heterodimer (Little and Mullins, 2009), loss-of-function mutants of either bmp2b or bmp7a causes a loss of BMP signaling upstream of the Acvr1l receptor (Nguyen et al., 1998; Schmid et al., 2000). The zygotic recessive mutation bmp7asb1aub encodes a null allele of Bmp7a (Schmid et al., 2000). bmp7sb1aub/sb1aub embryos are severely dorsalized (C5 phenotype) and typically die by the 15-somite stage (Schmid et al., 2000). Previous work has shown that injection of ACVR1 WT mRNA into Bmp7a-deficient embryos fails to rescue the phenotype, whereas His206 mRNA injection causes ventralization, indicating BMP ligand-independent signaling by the mutant human receptor (Shen et al., 2009).
To determine whether Gly328 mutant ACVR1 receptors also confer ligand-independent signaling, mRNA of each was injected into bmp7asb1aub/sb1aub embryos. All three mutant receptors partially rescued the majority of bmp7asb1aub/sb1aub embryos to a C4 or C3 dorsalized phenotype (Fig. 2A), indicating ligand-independent signaling activity. Complete rescue or ventralization was never observed for the Gly328 receptors (Fig. 2B, Suppl. Fig. S2). Tripling the amount of Glu328 mRNA injected did not improve the proportion of embryos rescued or the observed phenotype (Suppl. Fig. S1) in contrast to the effect observed in WT embryos. These results contrast those of the ACVR1 His206 group of embryos: overexpression yielded severe ventralization in a large fraction of both WT and bmp7sb1aub/sb1aub embryos (Fig. 1 and 2, Shen et al., 2009).
Figure 2. Effect of human Gly328 mutant receptor expression in bmp7sb1aub/sb1aub embryos.
A. mRNA misexpression in bmp7sb1aub/sb1aub mutant embryos. Phenotypes were scored at 24 hpf.
B. Phenotypes observed in bmp7sb1aub/sb1aub embryos injected with ACVR1 Glu328 mRNA and ACVR1 His206 mRNA. In parantheses, percent embryos with the phenotype. Classifications as in Figure 1 legend; C3 – lack of ventral tail fin and vein, truncated tail, and at least 14 somites present; C4 – preserved head tissue, less than 14 somites evident; C5 – disordered tissue development without discernible anatomical structures; lysis – dead embryos (C5 embryos typically lyse prior to 24 hpf).
3.3 ACVR1 codon 328 mutant mRNA increased Smad1/5 phosphorylation in WT and Bmp7-deficient embryos
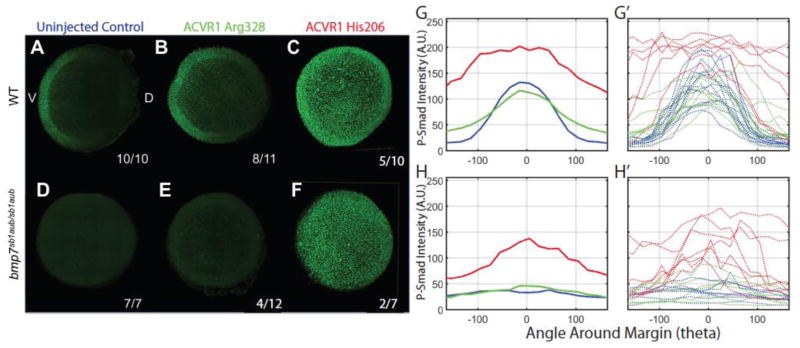
Western blot analysis of proteins from WT embryos injected with His206 mRNA demonstrated increased Smad1/5 phosphorylation (P-Smad1/5) at a mid-gastrula stage (Shen et al., 2009). Here, we immunostained embryos to visualize P-Smad1/5 expression and distribution in WT and homozygous bmp7sb1aub/sb1aub embryos at an early gastrula stage after injection of either His206 or Arg328 mutant mRNAs. In uninjected WT controls, P-Smad1/5 formed a gradient with peak intensity ventrally and no staining dorsally in the shield area (the dorsal organizer in zebrafish embryos) (Fig 3A), as described previously (Tucker et al., 2008; Zinski et al., 2017). In a majority of Arg328 mRNA injected WT embryos (8 of 11) (Fig 3B), P-Smad1/5 staining was expanded toward the shield area. In these embryos the P-Smad1/5 gradient was retained with highest levels ventrally. In contrast, all of the WT embryos injected with His206 mRNA displayed increased P-Smad1/5 activation with the majority of embryos (7 of 10) showing a loss of the P-Smad1/5 gradient (Fig 3C). Consistently, ACVR1 Arg328 mRNA caused a less overall increase in P-Smad1/5 levels than His206 mRNA.
Figure 3. Whole-mount immunostaining of P-Smad1/5.
WT (A, B, C) and bmp7sb1aub/sb1aub (D, E, F) embryos at mid-gastrulation. Uninjected control embryos in A, D (blue); human ACVR1 Arg328 mRNA injected in B, E (green); ACVR1 His206 mRNA injected in C, F (red). Animal pole views, dorsal (when evident) to the right. P-Smad1/5 quantification in arbitrary units (A.U.) of ACVR1 Arg328 mRNA injected embryos (green graphs) and ACVR1 His206 mRNA injected embryos (red graphs) compared to wild type (G, G′) and bmp7sb1aub/sb1aub (H, H′). The aggregate information is depicted in G and H; measurements for individual embryos are shown in G′ and H′.
We next examined the ability of the FOP mutant mRNAs to induce P-Smad1/5 in bmp7a null mutant embryos. Uninjected homozygous bmp7sb1aub/sb1aub embryos did not express P-Smad1/5 beyond background levels (7 of 7 embryos; Fig 3D). In Arg328 injected embryos, 8 of 12 were indistinguishable from uninjected controls on visual inspection (not shown), and 4 of 12 had slightly increased P-Smad1/5 (Fig 3E). His206 mRNA resulted in high P-Smad1/5 immunostaining in all 7 injected bmp7sb1aub/sb1aub embryos, two of which lacked a P-Smad1/5 gradient (Fig 3F), consistent with the severely ventralized phenotype observed (Fig. 2).
Quantification of P-Smad1/5 fluorescence for both Arg328 and His206 confirmed variably elevated P-Smad1/5 staining (Fig. 3G′, H′), consistent with the phenotypic range of ventralization observed in the injected embryos at 24 hpf (Fig. 1, 2). P-Smad1/5 levels were not significantly elevated in the Arg328 group over uninjected controls when averaged over all injected embryos, although significant changes were observed in some individual embryos (Fig 3G′, H′). This contrasted a marked increase in P-Smad1/5 intensity in the His206 group (Fig. 3G, H). This is expected as the degree of ventralization was lower in the ACVR1 Gly328 injected group compared to the strongly ventralizing mutation His206 (Fig. 1, 2).
3.4 ACVR1 Gly328 mutants cause altered expression of markers of ventral and dorsal cell types
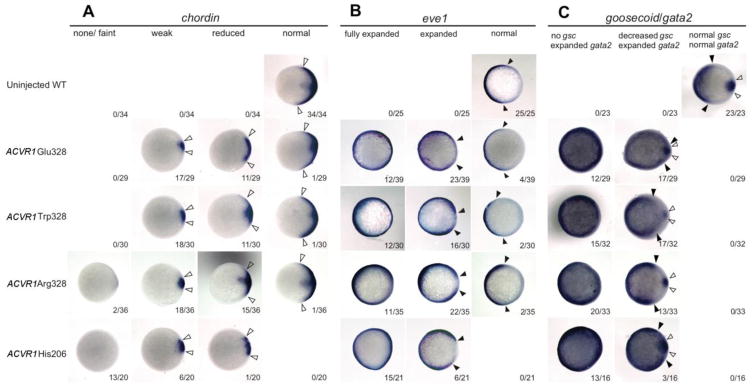
In the zebrafish embryo, the BMP signaling pathway is a tightly regulated network that patterns tissues along the dorsal-ventral embryonic axis (reviewed in Langdon and Mullins, 2011; Zinski et al., 2017). Thus, changes in gastrula BMP signaling shift or ablate the expression patterns of ventral and dorsal gastrula markers (Mullins et al., 1996; Hammerschmidt et al., 1996; Miller-Bertoglio et al., 1999; Mintzer et al., 2001). Accordingly, overexpression of ACVR1 His206 in WT embryos caused an expansion of ventral markers and a reduction in dorsal markers compared to controls (Shen et al., 2009).
To examine how misexpression of Gly328 mutant receptors affects dorsoventral patterning, we investigated the expression of dorsal markers (chordin, goosecoid [gsc]) and ventral markers (eve1, gata2) in WT embryos injected with Gly328 mRNA or His206 mRNA. We found that expression of dorsal and ventral markers was altered in the Gly328 group, but was more strongly affected by His206, consistent with the P-Smad1/5 immunostaining analysis. Expression of chordin and gsc was reduced in almost all embryos, with the proportion of embryos showing complete loss of chordin highest with His206 (Fig. 4A and C). We found that eve1 and gata2 were expanded in almost all injected embryos, but were most expanded in the His206 group (Fig. 4B and C). In summary, marker expression was altered in almost all injected embryos although less severely in the Gly328 groups than the His206 group. This is consistent with the observed phenotypic distribution at 24 hpf and confirms the hyper-activating effect of ACVR1 Gly328 mutations on the BMP signaling pathway.
Figure 4. Gene expression analysis.
Whole-mount in situ hybridization of chordin (A), eve1 (B), and goosecoid and gata2 (C) in WT embryos injected with human ACVR1 mutant mRNAs, compared to uninjected controls. Animal pole views with dorsal to the right. Closed arrows delineate the borders of the ventral markers, open arrows delineate the extent of the dorsal markers.
4. DISCUSSION
This study is the first in vivo examination of the effect of disease causing mutations in the human ACVR1 serine-threonine kinase domain, a domain highly conserved across species from zebrafish to humans. The replacement of a small non-polar amino acid (glycine) with a large non-polar (tryptophan) or a negatively charged amino acid (glutamic acid) in codon 328 has been associated with a more severe human FOP phenotype than the classic ACVR1 Arg206His mutation, whereas the exchange with a positively charged amino acid (arginine) is found in four patients with a milder presentation of FOP (Kaplan et al., 2009; Carvalho et al., 2010).
Given the striking differences in FOP patient clinical phenotypes with Gly328Trp/Glu and Gly328Arg mutations, the similarity in the phenotypes after injection of Gly328 mutant mRNAs in zebrafish embryos is noteworthy. The effect of each ACVR1 Gly328 mutant receptor and the classic Arg206His mutation in WT embryos was remarkably similar (Fig. 1), whereas the Gly328 mutants were less potent than Arg206His in bmp7sb1aub/sb1aub mutant embryos (Fig. 2, 3). The ACVR1 Gly328 mutant receptors only partially rescued the dorsalized phenotype of Bmp7 ligand-deficient mutant embryos, whereas Arg206His fully rescued and ventralized them. Detection of ventral and dorsal cell fate markers by in situ hybridization (Fig. 4) confirmed that the ventralizing effects of overexpressed ACVR1 Gly328 mutant mRNAs are due to changes in patterning during gastrulation, similar to the effect we observed by the GS-domain mutation Arg206His (Shen et al., 2009).
The classic FOP mutation ACVR1 Arg206His has been suggested to cause a conformational change of the receptor, permitting leaky activation of the receptor and therefore increased BMP signaling in the absence of ligand (Groppe et al., 2007; Shen et al., 2009; Groppe et al., 2011). Although the 206 and 328 residues reside in different domains of the ACVR1 receptor, structural analyses indicate that protein folding brings these domains into proximity of each other with potential functional interactions (Groppe et al., 2007; Chaikuad et al., 2012).
Impaired binding of FKBP12 has been suggested as a possible mechanism of increased ACVR1 activity in FOP patients (Kaplan et al., 2008; Shen et al., 2009) and decreased binding of FKBP12 to Arg206His ACVR1 has been demonstrated (Groppe et al., 2011). A recent study (Chaikuad et al., 2012) examined FKBP12 interaction with Arg328 and Glu328 through treatment with FK506, a competitor for FKBP12 binding. Codon 328 mutants, as well as the Arg206His mutant retained inhibition by FKBP12 suggesting that impaired FKBP12 binding does not play a significant role in the disease mechanism. However, we cannot exclude that a significant effect is caused by a subtle reduction in FKBP12 binding to the ACVR1 mutants.
ACVR1 Gly328 mutations also have been suggested to lead to a more permissive state of activation (Kaplan et al. 2009). Introducing a bulky side chain (Trp) or a charged amino acid (Glu, Arg) into a surface area involved in protein-protein interaction could significantly affect function without changing the tertiary structure of the protein, explaining how different amino acids cause the same effects in our zebrafish assay. This possibility is suggested by the crystal structure of the ACVR1 cytoplasmic domain in complex with FKBP12 or dorsomorphin. The Gly328 mutations introduce side chains that may break bonds necessary for auto-inhibition and promote an active kinase conformation of the ACVR1 receptor (Chaikuad et al., 2012).
Recent studies show that the Arg206His mutant ACVR1 receptor can be activated by binding Activin A (Hatsell et al., 2015; Hino et al., 2015; reviewed in Wolken at al., 2017). Whereas Activin A has an inhibitory effect on the ACVR1 WT receptor, these studies suggest that the binding of Activin A to the Arg206His mutant receptor can cause heterotopic ossification, although the presence of Activin A alone without an inflammatory stimulus may be insufficient to cause the episodic flare-ups seen in FOP. A recent study overexpressing variant mutant receptors including Glu328 and Trp328, but not Arg328, in cultured immortalized mouse embryonic fibroblasts (iMEFs) showed activation of the variant receptors leading to increased levels of pSmad1/5/8 when stimulated with BMP4 or Activin A (Haupt et al., 2017). It will be interesting to determine if the activity of the variant ACVR1 mutations is enhanced by Activin A ligand in vivo as well.
Our study shows that the Gly328 mutant mRNAs can partially rescue the dorsalized phenotype of bmp7-deficient embryos, showing that the mutant receptor is active in the absence of Bmp7 ligand. We similarly found that the Arg206His ACVR1 human receptor can rescue mutant embryos deficient for either or both Bmp7 or Bmp2 (Fig 2, Shen et al., 2009), with possibly increased potency compared to the Gly328 mutants. The notion of ligand-independent signaling is supported by an in vitro study with iMEFs that were transfected with ligand-deficient ACVR1 mutant plasmids. All mutant receptors retained the ability to induce Smad1/5/8 phosphorylation, although the residual activity was lowest for the Glu328 and Trp328 constructs (Haupt et al., 2017). Ectopic expression of Activin has been shown to be a potent inducer of mesoderm in vertebrates (Smith et al., 1990). mRNA sequencing data in zebrafish embryos indicate that Activin A is first expressed at 30 hpf (http://www.ebi.ac.uk/gxa/experiments/E-ERAD-475) and therefore would not be present at the time of our assays. However, experiments in medaka embryos suggest that maternal Activin protein is present at earlier stages (Wittbrodt & Rosa, 1994). Further studies will be required to evaluate the role of Activin A in activating these FOP mutant receptors in our zebrafish assays.
The phenotypic similarities between zebrafish embryos overexpressing Glu/Trp328 and Arg328 were unexpected given their different clinical phenotypes in human patients, however, the patient number is still small making it difficult to make generalized conclusions yet on their phenotypic effects. In our study, the effect on early zebrafish development and BMP signaling is comparable among the three ACVR1 Gly328 mutations. It is possible that these mutations have a more divergent effect at other developmental stages or in different cellular environments. In addition, our study used an overexpression system that increases the number of receptors on the cell surface, which could mask differences in effects of the various mutants. The equilibrium among different receptors could also influence phenotypic expression. These questions could be further explored in zebrafish transgenic or knock-in models expressing a single copy of a mutant ACVR1 receptor under the control of the endogenous promoter. Alternatively, developing a zebrafish embryo mRNA injection assay to quantitatively compare protein levels of each variant with phenotypic readouts might also allow one to distinguish more specifically between their activities. Given that a heterozygous knock-in mouse with the His206 mutation (Acvr1R206H/+) is perinatal lethal (Chakkalakal et al., 2012), whereas human newborns with the same mutation are healthy, a stable transgenic zebrafish model would also allow for more studies into the developmental context of FOP-causing mutations.
5. CONCLUSION
This work is among the first to explore the effects of the serine-threonine kinase domain ACVR1 Gly328 mutations in vivo. We demonstrate that ACVR1 Glu328, Trp328, and Arg328 mutations, like the classic Arg206His mutation, increase BMP signaling activity and can phosphorylate Smad1/5 in a BMP ligand-independent manner. Additional studies are needed to further elucidate the pathological mechanism of these mutated ACVR1 receptors and to explore the causes of the differing patient phenotypes of variant FOP mutations.
Supplementary Material
Highlights.
Variant kinase domain mutations of the BMP type I receptor ACVR1 of human fibrodysplasia ossificans progressiva (FOP) patients were characterized in zebrafish
Variant receptors, like the classic GS-domain FOP receptor, caused overactivity of BMP signaling
BMP ligand independent signaling was found for the variant ACVR1 mutant receptors
Acknowledgments
We are grateful to Meiqi Xu for the gift of Trp328 and Glu328 plasmids, Shawn Little for the ACVR1 WT and ACVR1 His206 (cFOP) plasmids, and James Dutko for use of bmp7sb1aub/sb1aub fish. We would like to thank the Busch-Nentwich lab for providing RNA-seq data. Funding for this research was provided by a T32 Postdoctoral Research Training Grant (NIH/NIGMS “Medical Genetics Research Training” T32GM008638) to B.E.M. and by NIH grant R01-GM56326 and a Cali Developmental Grant to M.C.M. This study was also supported by the International Fibrodysplasia Ossificans Progressiva Association, the Center for Research in FOP and Related Disorders, and the Cali/Weldon Professorship to E.M.S.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
B.E.M.: preparation of manuscript, study design, data collection and analysis. M.H. and J.Z.: data collection and analysis. E.M.S. and M.C.M.: preparation of manuscript, study design, data analysis. All authors reviewed the manuscript prior to submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagarova J, Vonner AJ, Armstrong KA, Börgermann J, Lai CSC, Deng DY, Beppu H, Alfano I, Filippakopoulos P, Morrell NW, et al. Constitutively active ALK2 receptor mutants require type II receptor cooperation. Mol Cell Biol. 2013;33:2413–2424. doi: 10.1128/MCB.01595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: a critical review. Cell Signaling. 2011;23(4):609–620. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Carvalho DR, Navarro MMM, Martins BJAF, Coelho KEFA, Mello WD, Takata RI, Speck-Martins CE. Mutational screening of ACVR1 gene in Brazilian fibrodysplasia ossificans progressiva patients. Clinical genetics. 2010;77(2):171–176. doi: 10.1111/j.1399-0004.2009.01256.x. [DOI] [PubMed] [Google Scholar]
- Chaikuad A, Alfano I, Kerr G, Sanvitale CE, Boergermann JH, Triffitt JT, von Delft F, Knapp S, Knaus P, Bullock AN. Structure of the Bone Morphogenetic Protein Receptor ALK2 and Implications for Fibrodysplasia Ossificans Progressiva. J Biol Chem. 2012;287:36990–36998. doi: 10.1074/jbc.M112.365932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal SA, Zhang D, Culbert AL, Convente MR, Caron RJ, Wright AC, Maidment ADA, Kaplan FS, Shore EM. An Acvr1 R206H knock-in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res. 2012;27:1746–1756. doi: 10.1002/jbmr.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors SA, Trout J, Ekker M, Mullins MC. The role of tolloid/minifin in dorsoventral pattern formation of the zebrafish embryo. Development. 1999;126:3119–30. doi: 10.1242/dev.126.14.3119. [DOI] [PubMed] [Google Scholar]
- Detrich H3, Kieran MW, Chan FY, Barone LM, Yee K, Rundstadler JA, Pratt S, Ransom D, Zon LI. Intraembryonic hematopoietic cell migration during vertebrate development. Proc Natl Acad Sci. 1995;92:10713–10717. doi: 10.1073/pnas.92.23.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A, Hild M, Bauer H, Imai Y, Maifeld H, Schier AF, Talbot WS, Bouwmeester T, Hammerschmidt M. Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development. 2000;127:343–354. doi: 10.1242/dev.127.2.343. [DOI] [PubMed] [Google Scholar]
- Dinther MV, Visser N, de Gorter DJ, Doorn J, Goumans MJ, de Boer J, ten Dijke P. ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteo- blast differentiation and bone formation. J Bone Miner Res. 2010;25:1208–1215. doi: 10.1359/jbmr.091110. [DOI] [PubMed] [Google Scholar]
- Dutko JA, Mullins MC. SnapShot: BMP signaling in development. Cell. 2011;145:636–6. doi: 10.1016/j.cell.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J, Noguchi Y, Iwakiri K, Kondo T, Kurose J, et al. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morpho- genetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem. 2009;284:7149–56. doi: 10.1074/jbc.M801681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe JC, Shore EM, Kaplan FS. Functional modeling of the ACVR1 (R206H) mutation in FOP. Clin Orthop Relat Res. 2007;462:87–92. doi: 10.1097/BLO.0b013e318126c049. [DOI] [PubMed] [Google Scholar]
- Groppe JC, Wu J, Shore EM, Kaplan FS. In vitro analyses of the dysregulated R206H ALK2 kinase-FKBP12 interaction associated with heterotopic ossification in FOP. Cells Tissues Organs. 2011;194:291–295. doi: 10.1159/000324230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, Van Eeden FJ, Granato M, Brand M, Furutani-Seiki M, Haffter P, Heisenberg CP, et al. Dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development. 1996;123:95–102. doi: 10.1242/dev.123.1.95. [DOI] [PubMed] [Google Scholar]
- Hashiguchi M, Mullins MC. Anteroposterior and dorsoventral patterning are coordinated by an identical patterning clock. Development. 2013:1970–1980. doi: 10.1242/dev.088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsell SJ, Idone V, Wolken DMA, Huang L, Kim HJ, Wang L, Wen X, Nannuru KC, Jimenez J, Xie L, Das N, et al. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Science translational medicine. 2015;7(303):303ra137–303ra137. doi: 10.1126/scitranslmed.aac4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt J, Xu M, Shore EM. Variable signaling activity by FOP ACVR1 mutations. Bone. 2017 doi: 10.1016/j.bone.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hild M, Dick A, Rauch GJ, Meier A, Bouwmeester T, Haffter P, Hammerschmidt M. The smad5 mutation somitabun blocks Bmp2b signaling during early dorsoventral patterning of the zebrafish embryo. Development. 1999;126:2149–2159. doi: 10.1242/dev.126.10.2149. [DOI] [PubMed] [Google Scholar]
- Hino K, Ikeya M, Horigome K, Matsumoto Y, Ebise H, Nishio M, Sekiguchi K, Shibata M, Nagata S, Matsuda S, Toguchida J. Neofunction of ACVR1 in fibrodysplasia ossificans progressiva. Proc Natl Acad Sci. 2015;112(50):15438–15443. doi: 10.1073/pnas.1510540112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüning I, Gillessen-Kaesbach G. Fibrodysplasia ossificans progressiva: clinical course, genetic mutations and genotype-phenotype correlation. Molecular syndromology. 2014;5(5):201–211. doi: 10.1159/000365770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly JS, Joly C, Schulte-Merker S, Boulekbache H, Condamine H. The ventral and posterior expression of the zebrafish homeobox gene eve1 is perturbed in dorsalized and mutant embryos. Development. 1993;119:1261–1275. doi: 10.1242/dev.119.4.1261. [DOI] [PubMed] [Google Scholar]
- Kaplan FS, Shen Q, Lounev V, Seemann P, Groppe J, Katagiri T, Pignolo RJ, Shore EM. Skeletal metamorphosis in fibrodysplasia ossificans progressiva (FOP) J Bone Miner Metab. 2008;26:521–530. doi: 10.1007/s00774-008-0879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, et al. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009;30:379–390. doi: 10.1002/humu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Kobori JA, Orellana C, Calvo I, Rosello M, Martinez F, Lopez B, Xu M, Pignolo RJ, Shore EM, Groppe JC. Multi-system involvement in a severe variant of fibrodysplasia ossificans progressiva (ACVR1 c. 772G> A; R258G): A report of two patients. American Journal of Medical Genetics Part A. 2015;167(10):2265–2271. doi: 10.1002/ajmg.a.37205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Lee KH, Zon L, Hammerschmidt M, Schulte-Merker S. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development. 1997;124:4457–66. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- Langdon Y, Mullins MC. Maternal and zygotic control of zebrafish dorsoventral axial patterning. Annu Rev Genet. 2011;45:357–77. doi: 10.1146/annurev-genet-110410-132517. [DOI] [PubMed] [Google Scholar]
- Le VQ, Wharton KA. Hyperactive BMP signaling induced by ALK2R206H requires type II receptor function in a Drosophila model for classic fibrodysplasia ossificans progressiva. Dev Dyn. 2012;241:200–214. doi: 10.1002/dvdy.22779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nature Cell Biol. 2009;11:637–643. doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Bertoglio VE, Fisher S, Sanchez A, Mullins MC, Halpern ME. Differential regulation of chordin expression domains in mutant zebrafish. Dev Biol. 1997;192:537–550. doi: 10.1006/dbio.1997.8788. [DOI] [PubMed] [Google Scholar]
- Miller-Bertoglio V, Carmany-Rampey A, Fürthauer M, Gonzalez EM, Thisse C, Thisse B, Halpern ME, Solnica-Krezel L. Maternal and Zygotic Activity of the Zebrafish ogon Locus Antagonizes BMP Signaling. Dev Biol. 1999;214:72–86. doi: 10.1006/dbio.1999.9384. [DOI] [PubMed] [Google Scholar]
- Mintzer KA, Lee MA, Runke G, Trout J, Whitman M, Mullins MC. lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally and zygotically in dorsoventral pattern formation. Development. 2001;128:859–69. doi: 10.1242/dev.128.6.859. [DOI] [PubMed] [Google Scholar]
- Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Heisenberg CP, Jiang YJ, et al. Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development. 1996;123:81–93. doi: 10.1242/dev.123.1.81. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, Mullins MC. Ventral and Lateral Regions of the Zebrafish Gastrula, Including the Neural Crest Progenitors, Are Established by a bmp2b/swirl Pathway of Genes. Dev Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- Petrie KA, Lee WH, Bullock AN, Pointon JJ, Smith R, Russell RGG, Brown MA, Wordsworth BP, Triffitt JT. Novel mutations in ACVR1 result in atypical features in two fibrodysplasia ossificans progressiva patients. PloS one. 2009;4:e5005. doi: 10.1371/journal.pone.0005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B, Furthauer M, Connors SA, Trout J, Thisse B, Thisse C, Mullins MC. Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development. 2000;127(5):957–967. doi: 10.1242/dev.127.5.957. [DOI] [PubMed] [Google Scholar]
- Schumacher JA, Hashiguchi M, Nguyen VH, Mullins MC. An Intermediate Level of BMP Signaling Directly Specifies Cranial Neural Crest Progenitor Cells in Zebrafish. PloS one. 2011;6:e27403. doi: 10.1371/journal.pone.0027403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Merker S, Hammerschmidt M, Beuchle D, Cho KW, De Robertis EM, Nusslein-Volhard C. Expression of zebrafish goosecoid and no tail gene products in wild-type and mutant no tail embryos. Development. 1994;120:843–852. doi: 10.1242/dev.120.4.843. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–63. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T, Mundlos S, Seeman P, Kaplan FS, Mullins MC, et al. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest. 2009;119:3462. doi: 10.1172/JCI37412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho T-J, Choi IH, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–7. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009;20:343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Smith JC, Price BMJ, Van Nimmen K, Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990;345(6277):729–731. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- Song GA, Kim HJ, Woo KM, Baek JH, Kim GS, Choi JY, Ryoo HM. Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem. 2010;285:22542–53. doi: 10.1074/jbc.M109.094557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel SE, Grunwald DJ, Myers PZ. Lithium perturbation and goosecoid expression identify a dorsal specification pathway in the pregastrula zebrafish. Development. 1993;117:1261–1274. doi: 10.1242/dev.117.4.1261. [DOI] [PubMed] [Google Scholar]
- Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14:108–119. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, Idowu O. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes & diseases. 2014;1(1):87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. A guide for the laboratory use of zebrafish (Danio rerio) 4. Univ. of Oregon Press; Eugene: 2000. The zebrafish book. [Google Scholar]
- Wittbrodt J, Rosa FM. Disruption of mesoderm and axis formation in fish by ectopic expression of activin variants: the role of maternal activin. Genes & Development. 1994;8(12):1448–1462. doi: 10.1101/gad.8.12.1448. [DOI] [PubMed] [Google Scholar]
- Wolken DMA, Idone V, Hatsell SJ, Yu PB, Economides AN. The obligatory role of Activin A in the formation of heterotopic bone in Fibrodysplasia Ossificans Progressiva. Bone. 2017 doi: 10.1016/j.bone.2017.06.011. S8756-3282(17), 30210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe T, Shimizu T, Muraoka O, Bae YK, Hirata T, Nojima H, Kawakami A, Hirano T, Hibi M. Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development. 2003;130(12):2705–2716. doi: 10.1242/dev.00506. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang K, Song L, Pang J, Ma H, Shore EM, Kaplan FS, Wang P. The phenotype and genotype of fibrodysplasia ossificans progressiva in China: a report of 72 cases. Bone. 2013;2:386–91. doi: 10.1016/j.bone.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinski J, Bu Y, Wang X, Dou W, Umulis D, Mullins MC. Systems biology derived source-sink mechanism of BMP gradient formation. eLife. 2017;6:e22199. doi: 10.7554/eLife.22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinski J, Tajer B, Mullins MC. TGF-β Family Signaling in Early Vertebrate Development. Cold Spring Harb Perspect Biol. 2017 doi: 10.1101/cshperspect.a033274. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.