Abstract
Mesenchymal stem cells (MSCs) are a major component of the tumor microenvironment (TME) and play a key role in promoting tumor progression. The tumor uses exosomes to co-opt MSCs and re-program their functional profile from normally trophic to pro-tumorigenic. These tumor-derived small vesicles called “TEX” carry and deliver a cargo rich in proteins and nucleic acids to MSCs. Upon interactions with surface receptors on MSCs and uptake of the exosome cargo by MSCs, molecular, transcriptional and translational changes occur that convert MSCs into producers of factors that are necessary for tumor growth and that also alter functions of non-tumor cells in the TME. The MSCs re-programmed by TEX become avid producers of their own exosomes that carry and deliver mRNA and miRNA species as well as molecular signals not only back to tumor cells, directly enhancing their growth, but also horizontally to fibroblasts, endothelial cells and immune cells in the TME, indirectly enhancing their pro-tumor functions. TEX-driven cross-talk of MSCs with immune cells blocks their anti-tumor activity and/or converts them into suppressor cells. MSCs re-programmed by TEX mediate pro-angiogenic activity and convert stromal cells into cancer-associated fibroblasts (CAFs). Although MSCs have a potential to exert anti-tumor activities, they largely provide service to the tumor using the multidirectional communication system established by exosomes in the TME. Future therapeutic options consider disruption of this complex vicious cycle by either molecular or gene-regulated silencing of pro-tumor effects mediated by MSCs in the TME.
Keywords: Mesenchymal stem cells (MSCs), tumor-derived exosomes (TEX), TEX-driven re-programming, MSC-derived exosomes, tumor microenvironment (TME), immune modulation in the TME
1. Introduction
1.1. Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are an important component of the tumor microenvironment (TME). MSCs are also referred to as “mesenchymal stromal cells.” This nomenclature, frequently used interchangeably, acknowledges the origin of MSCs from the stromal compartment and also implies that mesenchymal stromal cells have properties associated with stem cells. Indeed, MSCs are a population of adult multipotent cells capable of self-renewal and differentiation into osteoblasts, chondrocytes and adipocytes [1]. The most immature MSCs, i.e., true stem cells, can also differentiate into other embryonic lineages [2]. MSCs can be obtained from different tissues, e.g., bone marrow, adipose tissues, placenta or umbilical cord and can be propagated to give rise to cellular products that are used for therapy [3, 4]. It is because of their self-renewal capacity, multi-potency and immunomodulatory properties that MSCs have become an especially attractive tool for regenerative medicine [5, 6] and currently serve as a major cellular source for the replacement of damaged tissues or pathological lesions [7]. As MSCs do not express human leukocyte antigen (HLA) class II molecules, they have low immunogenicity [6, 8]. They are also able to suppress functions of various immune effector cells and promote activity of regulatory immune cells [8, 9]. These features of MSCs underpin their role as therapeutic agents in a variety of diseases, including the graft versus host disease (GVHD) or myocardial infarction [3, 10].
1.2. Mesenchymal stem cells in cancer
In cancer, MSCs play a crucial role in promoting tumor progression. On the one hand, they provide a framework for anchoring tumor cells in the form of tumor stroma and secrete factors that facilitate tumor growth [11]. On the other hand, MSCs present in the TME can transdifferentiate into M2-type macrophages, myeloid-derived suppressor cells (MDSC) or M2-type microphages under the influence of cytokines or chemokines [12–14].
As participants in re-programming of the TME, MSCs interact with the surrounding cells and communicate the messages for changes that are orchestrated by the tumor [11]. When the mechanisms responsible for MSCs interactions with other cells in the TME were searched for, it was discovered that MSCs secrete multiple bioactive factors with activities that can dramatically alter the key cellular functions of neighboring cells, such as survival, apoptosis, maturation and differentiation [15, 16]. This secretome-based paracrine activity of MSCs is now widely recognized as a cell-free strategy mediated by soluble factors present in MSCs conditioned media (CM) [10]. However, more recent studies showed that in addition to the soluble secretome, these CMs also contained particulate fractions with biological activities associated with MSCs. This finding suggested that a novel cellular mechanism involving a particulate nano-communication system operated by MSCs may be responsible for dissemination of messages from MSCs to various recipient cells [17, 18].
1.3. Exosomes as components of the tumor microenvironment
The objective of this review is to introduce extracellular vesicles (EVs) and, specifically, their smallest subset, exosomes, as the mediators of MSCs signaling that drives tissue re-programming in the TME. The focus of this review on exosomes, as opposed to all EVs, is deliberate. As discussed below, EVs represent a heterogeneous population of vesicles that may or may not share phenotypic and functional attributes. In the absence of firm criteria for the EVs’ nomenclature, the International Society of Extracellular Vesicles has encouraged investigators to establish minimal requirements for the characterization of EVs, with strict attention to integrity, size, molecular cargo and functionality of the vesicle population being evaluated [19]. Focusing this narrative on exosomes as opposed to EVs narrows the scope of inquiry to the currently most widely studied and better characterized subset of EVs.
2. Extracellular vesicles (EVs) and exosomes
EVs are produced by all cells and come in several different vesicular formats, each with a different cellular origin, distinct size as well as a varied molecular content [20]. Exosomes are the smallest subset of EVs (30–150nm in diameter) with a unique biogenesis. They originate from the endocytic compartment of the parent cell via a series of intraluminal invaginations taking place in the multivesicular bodies (MVBs). Consequently, their molecular content recapitulates, at least in part, the content of the parent cell [21]. Due to their endocytic origin exosomes are the only EVs that carry endosomal markers such as ALIX, TSG101 or syntenin-1 [21]. Microvesicles are larger than exosomes (500–1,000nm), are formed by “blebbing” or “pinching off” from the parent cell surface membrane and contain random assortments of cellular contents [22]. The largest EVs (1,000 to 5,000nm) are apoptotic bodies, which represent remnants of cells undergoing apoptosis and contain a vast array of cellular debris [22].
2.1. Characteristics of exosomes
Among various EVs, exosomes are of great current interest, because their molecular/genetic profiles approximate those of the parent cells and their ubiquitous presence in all body fluids [21, 23]. These traits qualify exosomes as potential candidates for circulating biomarkers expected to provide information about the molecular make-up and functions of the tissue-bound parent cells. If the parent cell is a tumor cell, for example, a proportion of exosomes present in plasma of cancer patients is likely to be tumor-derived and may be considered as a “liquid biopsy” of the tumor. Exosomes, due to their low immunogenicity, long half-life in the circulation and the ability to cross the blood-brain barrier, are also being tested as therapeutic nanodelivery systems for small interfering RNAs or other drugs [24].
Exosomes are enclosed by a protein-phospholipid bilayer membrane decorated by cell type-specific proteins, lipids and glycans. Exosome lumen is filled with various cellular proteins, nucleic acids, mRNA, miRNA and DNA, soluble factors, including cytokines and chemokines, enzymes and cofactors [25]. Components of the exosome cargo are biologically active, as isolated exosomes mediate cellular-crosstalk when co-incubated with recipient cells or upon delivery to experimental animals in vivo [26, 27]. Exosomes produced by different cell types carry distinct molecular and genetic components, and they may be “addressed” by the parent cell to reach a specific molecular address of the recipient cell. Upon contacting a local or distantly-located recipient cell, exosomes deliver signals that culminate in cellular re-programming [28, 29]. The mechanisms responsible for delivery and processing of the exosome cargo in recipient cells are not entirely understood, but may include the initial ligand-receptor type of binding on the cell surface followed by endocytosis or phagocytosis of exosomes [30]. Whether exosomes signal via cognate receptors on their surface or are internalized, delivering their content of nucleic acids to the recipient cells, the exosome-recipient cell interaction results in a loss or gain of functions in the recipient cell [31]. Recent attention has been focused on transfer of miRNAs by exosomes as a major mechanism of the recipient cell modifications [31].
To date, much of what is known about exosomes comes from studies of cell line supernatants, where all vesicles are products of the cultured cell. In contrast, exosomes present in body fluids are heterogeneous mixtures of vesicles derived from various cells. Currently, methods are being developed to isolate and characterize not only total exosome fractions from human body fluids but also to separate subsets of exosomes released by e.g., T cells or tumor cells, based on specific markers, such as e.g., CD3 or a tumor-associated antigen carried by these exosomes. Isolation from body fluids and subtyping of exosomes is an evolving science [32, 33]. Exosomal proteins, lipids and nucleic acids described in published studies have been listed in a data base, ExoCarta, which aims at the definition of specific molecular/genetic signatures of exosomes derived from different cell types [34]. It should be remembered, however, that almost all of the early studies were performed with exosomes derived from supernatants of cultured cell lines and the list of exosome components in the data base may not necessarily reflect the content of plasma-derived exosomes.
2.2. Tumor-derived exosomes (TEX)
Tumor cells are avid producers of exosomes, and tumor cell-derived exosomes, called “TEX” are ubiquitously present in the tumor milieu and in body fluids of all patients with cancer [27, 35]. The ratios of TEX/normal cell-derived exosomes in the plasma of cancer patients varies, but generally TEX represent a substantial proportion of total exosomes recovered from plasma, especially in patients with advanced malignancies [36]. In the TME, TEX are major participants in intercellular cross-talk. Serving as information transfer vehicles, TEX carry messages from the parent tumor cell to other normal or malignant cells in the TME, including MSCs [37]. As Figure 1 indicates, TEX can mediate autocrine, juxtacrine and paracrine signaling that the tumor cells establish and that is necessary for their survival [38]. Notably, TEX paracrine activities are not limited to the tumor site: TEX circulate and disseminate information to tissues and cells distant from the tumor.
Figure 1.
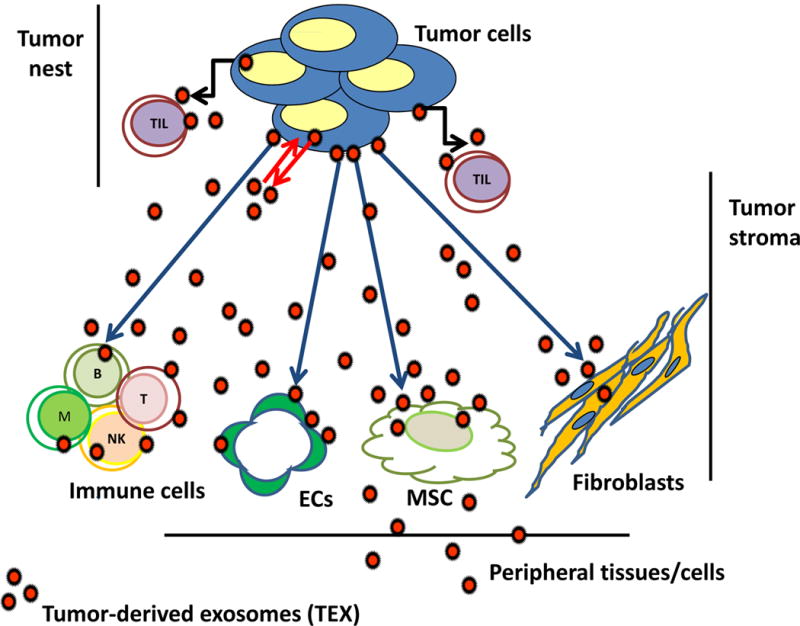
In the TME, tumor-derived exosomes (TEX) communicate with the tumor cells producing TEX via autocrine interactions (red arrows). Tumor-infiltrating T cells (TILs) are also targeted by TEX via juxtacrine signaling (black arrows). Other cells in the TME: MSCs, fibroblasts, ECs, or immune cells are reprogrammed by TEX via paracrine interactions (blue arrows). TEX travel freely in tissues and body fluids and carry information to distantly located tissues and cells.
2.3. MSC-derived exosomes
The tumor stroma is an essential component of the TME, with MSCs and their paracrine-based activity assuming a major role in the tumor-stroma cross-talk [11, 16]. In the TME, MSCs remodel the extracellular matrix (EMT), participate in the epithelial-mesenchymal transition, promote establishment of distant metastases, inhibit anti-tumor immune responses and support survival of leukemic cells in the bone marrow, creating a leukemia niche [11, 15, 16]. Literature is replete with evidence for the role of MSCs in progression and, to a lesser degree, also suppression of tumor growth and metastasis [reviewed in [11]. A broad variety of functions attributed to MSCs in cancer has been shown to be mediated by soluble factors these cells produce, including cytokines, chemokines and growth factors [reviewed in [16]. With the discovery of exosomes and realization that MSCs in the TME make and release an abundance of exosomes [39], the understanding of mechanisms underpinning interactions of MSCs with the tumor and vice versa has undergone a drastic change. MSCs are now viewed not only as recipients of signals emanating from the tumor but also as efficient producers of their own exosomes, which horizontally transfer information to neighboring cells and transform the cellular milieu into one supportive of the tumor survival (Figure 2). In this context, it is important to recognize that the relationship between the MSCs and tumor cells is bidirectional (Figure 2), and that the exosomes produced by MSCs that are re-programmed by TEX may exert profound effects on tumor growth [40]. This aspect of the MSCs biology is of intense interest, because of the potential for future therapeutic interventions it offers for cancer control via MSCs-derived exosomes [24].
Figure 2.
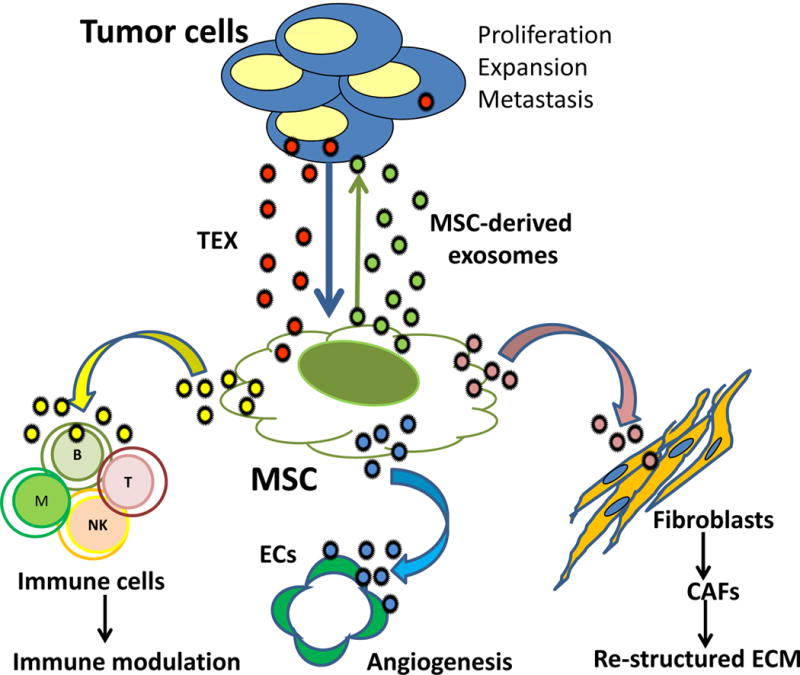
TEX-mediated re-programming in the TME. TEX re-program MSCs, converting them into highly efficient producers of MSC-derived exosomes. MSCs cross talk with tumor cells and other cells in the TME using exosomes. Tumor to MSC cross-talk is bidirectional. MSEs re-programmed by TEX horizontally deliver exosomes carrying individual messages to fibroblasts, ECs or immune cells. MSCs play a central role in conveying pro-tumorigenic signals to these cells. It is not yet clear whether exosomes produced by the re-programmed parent MSCs are distinct subsets of vesicles each carrying an individually “addressed” message to recipient cells or are equipped with a broad cargo of molecular/genetic components to convey distinct messages to different recipient cells. In this context, recipient cells and the TME determine the outcome of exosome-mediated information transfer. The MSC plays a central role in conveying pro-tumor messages to cells present in the TME.
3. MSCs re-programming by TEX
In the TME, the tumor-MSCs interactions begin at the inception of tumor growth, when MSCs are first attracted to the tumor and participate in the formation of the “pre-metastatic niche.” The recruitment of MSCs to tumor and their re-programming by TEX result in dramatic changes in the MSCs phenotype and functions. The coopted MSCs become pro-tumorigenic and convert into producers of factors promoting tumor progression. MSCs-derived exosomes mediating pro-tumorigenic effects maintain the cross-talk with the other cells in the TME, including tumor cells (Figure 2).
The ability of TEX to orchestrate re-programming of MSCs and effectively recruit their help in re-modeling of the TME has been the subject of intense recent studies. It is known that molecular signatures of TEX are distinct from those of exosomes derived from normal cells [27]. It has also been shown that TEX released by different types of tumor cells carry distinct molecular signatures [33]. TEX signatures contain molecular and genetic signals that are able to induce modifications in recipient MSCs [27, 41]. Valadi et al were the first to report that exosomes mediated transfer of mRNAs and miRNAs between various cells [42].
3.1. The molecular/genetic cargos of TEX
Table 1 indicates that the TEX surface is enriched in numerous biologically active proteins. The cargo of TEX isolated from plasma of patients with cancer is enriched in immunosuppressive receptor/ligands, including PD-1/PD-L1, Fas/FasL, TRAIL/TRAILR [27]. However, immunostimulatory molecules, such as CD80, OX40, OX40L, or CD70, are also present, as are the major histocompatibility complex (MHC) molecules and various tumor associated antigens (TAAs). The TEX content suggests that TEX are equipped to supply immune-inhibitory as well as immune-stimulatory proteins to recipient cells. TEX are also enriched in adhesion molecules, presumably to facilitate their surface interaction with recipient cells and subsequent entry into the cytosol to deliver nucleic acids carried in the TEX lumen [43]. TEX also carry oncogenic DNA (including KRAS, HRAS, BCR-ABL); oncogenic microRNAs (miR125b,130b,155) and mRNAs (e.g., BrR-ABL1) and oncogenic proteins (e.g., EGFRVIII). TEX were shown to elicit tumorigenic conversion of immortalized rodent fibroblasts, epithelial cells or stem cell lines [44]. TEX carry tetraspanins (CD9, CD63, CD81), which are often used as “exosome markers,” although they are not selective components of exosomes. While the TEX content varies depending on their cellular origin, TEX are well equipped to induce modifications in MSCs functions, as they carry most if not all of the factors previously reported to mediate MSCs differentiation [45]. For example, it is well known that VEGF and TGF-β1 are involved in angiogenesis as well as tumor metastasis [46, 47]. Both these factors are carried by TEX produced by various solid tumors or by leukemia cells [48], and these TEX have been shown to trigger MSCs differentiation into pro-angiogenic and pro-invasive myofibroblasts [49].
Table 1.
Molecular/genetic contents of tumor-derived exosomes (TEX)
| Molecular/genetic contenta | Selected examples |
|---|---|
| Exosome Surface | |
| Signal transduction receptor/ligands | G-proteins |
| Inhibitory | Fas/FasL, PD-1/PDL-1 |
| TRAIL/TRAIL-R, TGF-β/TGFR | |
| Stimulatory | CD80, CD86, OX40/OX40L |
| MHC molecules | Class I; class II |
| Adhesion molecules | EPCAM, ICAM, CD44 |
| Integrins | αvβ5, αvβ6, αvβ1, α6β4 |
| Tetraspanins | CD9, CD63, CD81, CD82 |
| Membrane fusion proteins | Rab25, Rab27b, Annexins |
| Tumor-associated antigens | MAAs, LAAsb |
| Chaperones | Hsp70, Hsp90 |
| APM components | TAP-1, TAP-2 |
| Transmembrane proteins | LAMPs, CXCR4 |
| Enzymes | CD39, CD73, CD26 |
| Lipids | Cholesterol, ceramides, sphingomyelins, phospholipids |
| Exosome Lumen | |
| ESCRT-associated components | ALIX, TSG-101, syntenin-1, dynamin |
| Nucleic acids | mRNAs, miRNAs, DNA |
| Cytosolic proteins | Histones, nucleoproteins, ribosomal proteins |
| Proteases | GTP-ases, PL-ases, MMPs |
| Growth factors | EGF, FGF |
| Suppressive factors | PGE2, adenosine |
The exosome components listed above were selected from a list of many other molecules as reported in the ExoCarta based on the frequency of literature reports describing their presence in TEX.
MAA= melanoma-associated antigens; LAA=leukemia associated antigens
3.2. TEX induce phenotypic and functional changes in MSCs
Emerging evidence for TEX serving as inducers of changes in MSCs that have been previously ascribed to tumor cells is largely derived from in vitro experiments, where isolated MSCs are co-incubated with TEX produced by cultured tumor cells [50]. Direct contact between cells in transwell-type experiments appears not to be required for reprogramming of MSCs. While soluble factors or chemokines and cytokines produced by the tumor are likely to be partly responsible for the induced modifications as suggested [16], newer evidence indicates the involvement of another mode of communication. First, in in vivo experiments with TEX in experimental animals have shown that TEX freely circulate and induce cell alterations in distantly-located cells and at distant tissue sites, suggesting delivery of messages protected from enzymatic degradation during transit [51]. Second, re-programming of MSCs by TEX appears to be a continuous and relentless process designed to introduce additional changes in MSCs to fulfill better the needs of tumor cells as they expand and metastasize. An epigenetic type of modifications mediated by genetic elements introduced by exosomes to recipient MSCs appears to be involved [52]
3.3. Mechanisms underpinning tumor to MSCs cross talk
The available data indicate that MSCs acquire the ability to perform a new and diverse set of functions upon exposure to TEX (Table 2). The cellular, molecular and genetic mechanisms responsible for re-programming of MSCs by TEX are under intense scrutiny. Cooperation of some sort between TEX and MSCs appears to be taking place, each partner contributing to the mechanism driving information transfer. The initial contact may be in the form of receptor-ligand signaling to which TEX contributes ligands decorating its membrane and MSCs contribute cognate receptors on their cell surface, leading to activation of one or many intracellular activation pathways in recipient cells [53]. This receptor-ligand type of interaction has been reported for exosomes carrying FasL, TRAIL, PD-L1, Wnt3a or TGF-β [38]. TEX carry numerous cell adhesion molecules (CAMs) and thus can readily fuse with adhesion receptors on MSCs allowing for the protein/gene transfer to the MSCs cytosol (Table 1). TEX carry integrins that determine tissue tropism of the vesicles. Thus, TEX-associated integrins α6β4 and α6β1 were reported to associate with lung metastasis, while exosomal integrin αvβ5 was linked to liver metastasis [54] Phagocytosis of TEX carrying opsonins or complement components by MSCs expressing Fc receptors (FcRs) and/or complement receptors (CRs) as well as endocytosis, to which TEX contribute opsonins or ligands and MSCs the clathrin-coated pits, provide other means of delivery of proteins and/or genes to MSCs [55]. In each instance, the protein/gene transfer can lead to a cellular response or to lysosomal degradation of the transferred materials [55]. It remains unclear whether the protein transfer alone is sufficient for re-programming of MSCs by TEX or whether the transfer of transcription factors and nucleic acids is obligatory. In this context, it is important to remember that some cells, e.g., T lymphocytes, do not readily internalize exosomes [29] and may depend on the protein-protein signaling for information transfer.
Table 2.
TEX re-program MSCs in the TME to mediate pro-tumorigenic functions
| Migration of MSC to the tumor site [68, 69] |
| Production of pro-inflammatory cytokines [71] |
| Conversion of primary tumor site to the “pro-metastatic niche” [86, 88] |
| Activation of intracellular molecular pathways [39, 53] |
| Promotion of tumor growth in vivo [105] |
| Induction of epithelial-to-mesenchymal transition (EMT) [75, 76] |
| Migration of tumor cells to metastatic sites in various tissues [69] |
| Recruitment and implantation of tumor cells in the bone marrow [79] |
| Alteration of the extracellular matrix [63] |
| Promotion of angiogenesis [104–106] |
| Immunomodulatory functions: |
| Suppression of immune effector cells [39, 97–101] |
| Expansion of immune regulatory cells [94, 97, 100] |
| Increased resistance to cancer therapies [116, 118] |
3.4. Nucleic acids transfer by TEX
Transfer of mRNA transcripts or of various miRNAs to MSCs by TEX is currently considered to be the major mechanism responsible for information transfer between the tumor and MSCs [reviewed in [56]. In particular, non-coding RNAs (ncRNAs), including miRNAs and long ncRNAs, have been featured as candidates responsible for the phenotypic/functional changes induced by TEX in recipient cells [57]. miRNAs act on RNA by silencing or post-transcriptionally regulating gene expression, while long ncRNAs participate in imprinting and gene dosage regulation, including histone modification and formation of ribonucleoprotein complexes [58]. More recent evidence indicates that exosomes may also transfer DNA, and thus can modify gene expression in recipient cells [59]. Huan et al reported that AML cell-derived exosomes labeled with the PKH26 dye were readily taken up by bone marrow stromal cells. These exosomes carried RNA transcripts relevant to leukemia pathogenesis such as FLT3, NPM1, CXCR4, MMP9 or IGF-1R [60]. Leukemia-derived exosomes also carried miR-150, which targets CXCR4, a cognate receptor for SDF-1α, decreasing its surface expression levels in recipient cells as well as migration toward SDF-1α. In addition, transcriptional activity of miR150 disturbed the CXCR4-CXCL12 axis, which is necessary for the retention of hematopoietic progenitor cells (HPCs) in the bone marrow and their normal differentiation. This study suggests that leukemia-derived exosomes promote leukemia growth by interfering with CXCR4-CXCL12 signaling [60]. Studies by Paggetti et al showed that CLL-derived exosomes taken up by MSCs ex vivo and in vivo transfer miRNAs and proteins, inducing an inflammatory phenotype and transforming MSCs into cancer-associated fibroblasts (CAFs) [61]. Among miRNAs known to be abundant in TEX produced by various tumor types and shown to exert priming effects on MSCs upon transfer are the miR17-92 cluster and miR21. The miR17-92 cluster includes seven different miRNAs that have pro-angiogenic activity and also target the E2F transcription factor family- a critical regulator of the cell cycle and apoptosis in recipient cells. The miR17-92 cluster can regulate angiogenesis by directly targeting anti-angiogenic factors such as thrombospondin and connective tissue growth factor [62]. This dual role of miR17-92 in angiogenesis may be regulated at the level of the TEX cellular origin or the nature of recipient cell. TEX may also be highly enriched in miR21, which is considered to be an oncogene, as it promotes cell proliferation, migration and invasiveness by targeting a number of tumor suppressor genes, including p53, PTEN, and antagonists of the RAS pathway [63]. It also enhances expression of VEGF and thus promotes angiogenesis [64]. A recent study investigated the cargo composition of human (h) MSC-derived exosomes by next-generation sequencing (NGS) and proteomic analysis [65]. These exosomes carried a large number of proteins known to support tumor growth (e.g., PDGFR-b, TIMP-1 and TIMP-2 and miRNAs with pro-tumorigenic functions (e.g., miR-21 and miR-34a) when delivered to MCF-7 breast cancer cells. Numerous other miRNA species are carried and transferred by exosomes from the tumor to MSCs and from re-programed MSCs to other cells in the TME (reviewed in [56]). Dissecting this complex network of gene-regulating elements is rapidly becoming the new frontier of cancer research [31].
4. MSC re-programmed by TEX cross-talk with tumor cells
MSCs service to tumors has been extensively studied (reviewed in [15]) and consists of different mechanisms, some involving direct transfer of growth factors to the tumor and others indirectly benefiting the tumor through re-structuring of its environment via modifications of non-tumor cells in the TME. Furthermore, the bulk of these direct as well as indirect interactions between cells appears to be contact-independent and is executed by exosomes. As new details of exosome-mediated information transfer emerge, the functional relevance of TEX to cancer progression is becoming increasingly clear: aside from utilizing the autocrine feed-back mechanism (Figure 1), TEX re-program the MSCs and engage them in pro-tumor activities. Such re-programmed MSCs become a rich source of exosomes that carry and deliver to the tumor factors necessary for its proliferation, differentiation, survival, invasion and metastasis [66]. Thus, exosome-mediated information transfer between the tumor and MSCs is a bidirectional process (Figure 2).
4.1. Functional profiles of MSC re-programmed by TEX
Table 2 lists various pro-tumor functions mediated by the MSCs that were reprogrammed by TEX. Remarkably, the modifications induced by TEX in MSCs mimic those reported previously to be induced directly by the tumor cells [40]. Lindoso et al reported, for example, that TEX produced by renal cancer stem cells promote migration of MSCs to the tumor and induce expression of the a pro-tumorigenic phenotype in these MSCs [67]. These changes in MSCs correlated with overexpression of genes involved in cell migration (CXCR4 and CXCR7), in the matrix remodeling (collagen type IV alpha 3 chain) and in angiogenesis or tumor growth (IL-8, OPN and myeloperoxidase) [67]. TEX in other tumor types were also reported to promote MSCs migration to the tumor site [68, 69]. Exosomes produced by primary or metastatic colorectal cancers (CRCs) were shown to reprogram colonic MSCs, inducing morphological and functional alterations which favored tumor growth and its metastasis [70]. TEX derived from prostate cancer, breast cancer or CLL cells were shown to induce MSCs differentiation into myelofibroblasts overexpressing alpha smooth muscle actin (αSMA) [71]. TEX produced by lung cancer cell line, A549, stimulated production and secretion of inflammatory cytokines in MSCs, including IL-6, IL-8 and MCP-1, via the NF-κB-TLR signaling pathway [72]. Further, this priming of MSCs was shown to involve activation of TLR 2/NF-κB signaling by interaction of HSP70 on the surface of TEX with the recipient cells. In several other studies examining TEX interactions with MSCs, enhanced secretion of IL-6 or IL-8 in recipient cells which, in turn, promoted cancer expansion [73, 74] and the epithelial to mesenchymal transition (EMT) [75, 76]. MSC-derived exosomes carrying growth factors and IL6 were shown to promote proliferation of multiple myeloma cells in vitro and in vivo [77]. Recent report provides evidence that AML blast-derived exosomes remodel MSCs in the bone marrow niche into leukemia-growth permissive cells and suppress normal hematopoiesis in vivo [78]. Either in vivo engrafted AML cells or AML-derived exosomes enhanced numbers and functions of MSCs. Further, AML exosomes induced broad downregulation of factors supporting normal hematopoiesis, such as CXCL12, KITL, IL-7 and IGF1, in MSCs. Disruption of exosome secretion in AML cells through targeting Rab27a, which is responsible for regulation of exosome release, significantly delayed leukemia development in mice [78]. In these experiments, TEX-MSCs cross-talk has first led to changes in the recipient cell phenotype that then enabled re-programmed MSCs to participate in cancer progression. The overall conclusion of these recent experiments is that exosomes released by the MSCs re-programmed by TEX are essential for is the promotion of tumor progression.
5. Cross-talk of the re-programmed MSCs with other cells in the TME
Under normal physiological conditions, MSCs are a rich source of exosomes [79], which modulate functions of various neighboring tissue cells. At various tissue sites, MSCs establish an intimate relationship with stromal cells, HPCs, immune cells or endothelial cells in the vascular compartment [80]. Such a relationship is beneficial for normal physiological functions of these cells. The critical roles of MSCs in normal hematopoiesis, tissue repair and vascularization as well as regulation of immune cell functions have been extensively evaluated [40]. Importantly, it is becoming evident that MSC-derived exosomes are responsible for many functions generally attributed to MSCs [81]. In the TME, this physiological exosome-driven communication network becomes deranged, and the normal reconstructive functions of MSCs become replaced by a new repertoire of pro-tumor activities mediated by the exosomes produced by the MSCs reprogrammed by TEX. These MSC-derived exosomes have the capacity to interact with multiple cell types in the TME and to ensure they adequately support tumor growth. They carry a complex cargo of molecules and genes comprising >850 unique gene products and >150 different miRNAs [82, 83] and thus have a potential to elicit diverse cellular responses in a broad variety of cells.
5.1. Re-programmed MSCs vs tumor stroma
The tumor-stroma interaction is critical for cancer progression, and it too is mediated via exosomes. A key function of MSC-derived exosomes in the TME is to ensure that stromal support is provided for the tumor. It has been shown that interaction of MSCs with cancer cells leads them to acquire the CAF-like phenotype characterized by expression of CXCL12, α-SMA and fibroblast surface protein (FSP) [84, 85]. In fact, conversion of fibroblasts and MSCs into CAFs is accomplished by TEX, and exosomes secreted by CAFs promote tumor growth [86]. TEX from primary CLL cells reprogrammed MSCs to the CAF phenotype characterized by increased NF-κB signaling and elevated secretion of cytokines and chemokines, which enhanced tumor cell survival in vitro and in vivo [61]. The well-known regenerative functions of MSCs are also mediated by exosomes as per recently reported evidence that CD63+ exosomes produced by BM-MSCs carry Wnt3a protein on their surface and are able to engage recipient cells expressing the cognate receptor (Frizzled) and activate the downstream Wnt pathway, leading to stimulation of fibroblast proliferation as well as up-regulation of endothelial cell activities [87]. It is likely that many of the above described effects can be attributed to miRNAs carried by exosomes, which are implicated in stroma-restructuring, angiogenesis and metastasis, including miR-200c, miR-146, miR-92, miR-301, miR-7g and miR-130b [88].
5.2. Re-programmed MSCs vs immune cells
An increasing number of studies report that MSC-derived exosomes mimic the well-known capability of MSCs to modulate activities of immune effector cells, including T and B lymphocytes, NK cells, monocytes/macrophages and dendritic cells (DC) [39, 89, 90]. The word “modulation” is frequently used to describe the effects of MSCs or exosomes they produce on immune cells. Presumably, this implies that MSCs and MSC-derived exosomes can inhibit as well as stimulate immune responses. The dual ability of TEX to mediate suppression and activation of immune responder cells is well known [91] and it has been shown that TEX carry and deliver both immunosuppressive and immunostimulatory signals to recipient cells [37]. It is less clear at present whether exosomes derived from non-transformed cells have the same ability for dual modulation of immune cells. In the context of the MSCs, which are known to be able to produce a vast array of immunostimulatory and immunoinhibitory cytokines [92], “modulation” seems to be an appropriate functional designation. Interestingly, recent studies show that MSC-derived exosomes mediate immune Immunosuppression rather than immune stimulation (Figure 3). Only infrequent reports mention immunopotentiating capabilities of MSCs [93] although none refer to MSC-derived exosomes. Further, not only re-programmed MSCs in the TME but also exosomes produced by MSCs isolated from the normal human or murine bone marrows (BM) are immunoinhibitory (Table 4). MSC-derived exosomes carry CD39 and CD73, ectonucleotidases catalyzing adenosine production [94] and, in parallel to MSCs, a plethora of other immunosuppressive factors, including indoleamine 2,3-dioxygenase (IDO), TGF-β, IL-6, PGE2, PD-1, galectin -1 and HLA-G5 [95–97].
Figure 3.
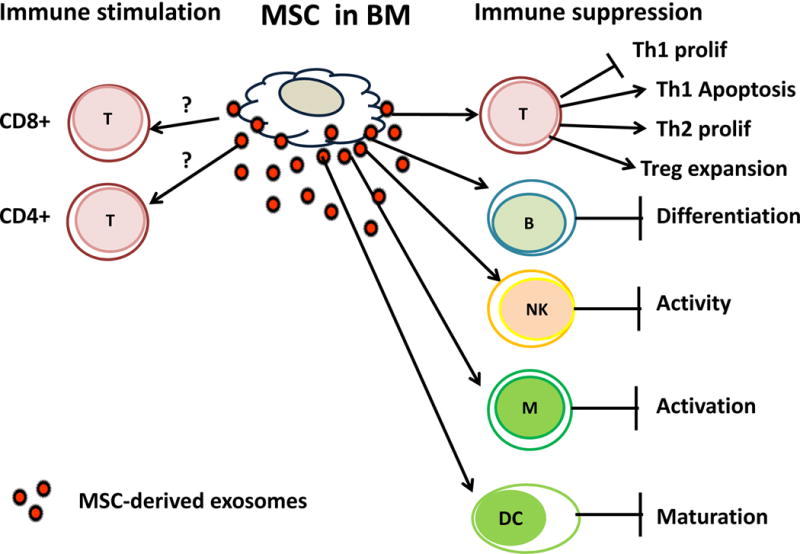
Immune modulation by exosomes produced by MSCs isolated from normal human or murine bone marrow. These exosomes co-incubated with normal human immune cells induce immune suppression. No convincing evidence is currently available to support immunostimulatory effects of MSC-derived exosomes. The potential mechanisms of immune suppression mediated by MSC-derived exosomes are shown in Table 4.
Table 4.
Immune modulation by MSC-derived exosomesa
| MSCs source | Immune function modulated | Effector mechanism | Reference |
|---|---|---|---|
| Normal human BM-MSC | ↓ Lymphocyte proliferation | TGF-β | 101, 102 |
| ↓ Survival | |||
| Normal human BM-MSC | ↓ T cell activation | TGF-β | 101 |
| ↑ Treg | |||
| ↑ Th2 TH1 TH17 | |||
| BM-derived MSC | ↓ CD3+ T cell proliferation | IDO | 98 |
| ↑ Apoptosis | IL-10 | ||
| ↑ Treg/Teff ratio | |||
| Normal human BM-MSC | ↓ B cell proliferation | IL-10 | 100 |
| ↓ T cell proliferation | TGF- β | ||
| Murine BM-MSC | ↓ T cell proliferation | PT-L1, TGF- β | 97 |
| ↑ Treg (CD4+CD25+FOXP3+) | |||
| Human ESC → MSC | Re-programmed THP-1 | ↑IL-10, TGF- β | 39 |
| ↑ Treg (CD4+CD25FOXP3+) | |||
| ↑ Survival of allogeneic skin graft in vivo | ↓IL-1β, IL-6, TNFα | ||
| Normal human BM-MSC | ↓ M0 activation | ↓TLR signaling | 99 |
MSC-derived exosomes used in co-incubation experiments with various immune cells were obtained from cultures of BM-MSC
The inhibitory effects of MSC-derived exosomes on functions of immune cells were demonstrated in several recent studies [81, 94, 98–100]. In co-cultures of PBMCs with exosomes isolated from MSCs, T-cell activation tended to be suppressed, and the fraction of regulatory T cells (Treg) was enlarged [101]. The authors of this study also reported that exposing CD4+ T cells to MSC-derived exosomes favored expansion of Th2 cells, while limiting proliferation of Th1 and Th17 cell subsets. The observed effects were associated with a shift in a cytokine profile of PBMC from pro-inflammatory IL-1β and TNF-α to anti-inflammatory TGF-β [101]. These results suggest that exosomes produced by normal MSCs can suppress T-cell activation and help maintain immune homeostasis. Perhaps, the immunoinhibitory effects of exosomes produced by normal MSC could be looked upon not as overt immune suppression but rather as down-regulation of T-cell activation, which is designed to prevent destructive inflammatory responses and thus protect tissue cells from damage by activated immune cells.
Several other studies show that MSC-derived exosomes lower the immune system activation through induction of anti-inflammatory cytokines and regulatory immune cells [39, 100, 101]. MSC-derived exosomes can activate monocytes by the TLR signaling pathway [39, 100, 101]. When monocytes are stimulated by MSC-derived exosomes they differentiate into macrophages which secrete IL-10, leading to Treg expansion [39] similar to effects induced by MSCs [102]. In the TME, these anti-inflammatory effects of MSCs are amplified, designating exosomes produced by MSCs as the major mechanism responsible for expansion of Treg and MDSCs [39]. Thus, MSC-derived exosomes contribute to maintaining of an immunosuppressive climate throughout the TME. However, in the normal tissue milieu, MSC-derived exosomes might protect tissue cells from an immune-mediated damage.
5.3. Re-programmed MSC vs endothelial cells
One of the tumor-promoting functions of MSCs is to support angiogenesis in the TME. In a seminal paper Skog et al demonstrated that glioblastoma-derived EVs carrying mRNA for Gluc were internalized by human brain microvascular endothelial cells (HBMVEC), which translated it to the Gluc protein and activity. Further, HBMVEC co-incubated with these EVs in the presence of angiogenic growth factors developed into tubular structures in matrigels [103]. This was the first demonstration that EVs in the TME transport RNAs and proteins promoting angiogenesis in the recipient cells. Since then the role of tumor-derived as well as MSC-derived exosomes in vessel growth has been confirmed in numerous studies [104–107]. Co-culture of tumor cells with MSCs or subcutaneous injections of MSC-tumor cell mixtures into nude mice promoted tumor growth associated with expansion in the tumor vasculature [105]. Further, MSC-derived exosomes upregulated VEGF expression in tumor cells by activating the extracellular signal-regulated kinase1/2 (ERK1/2) pathway. Inhibition of ERK1/2 activation or the pretreatment of exosomes with RNase abrogated these effects, confirming the crucial involvement of mRNAs delivered by exosomes in the promotion of angiogenesis [105]. MSC-derived exosomes also carried transcripts and proteins related to angiogenesis and proliferation, including VEGF, TGF-β, fibroblast growth factor (FGF) and cytokines, including IL-6, IL-8 and IL-1β [94, 95]. MSC-derived exosomes transfer miRNAs to endothelial cells (ECs). For example, Gong et al showed that MSC-derived exosomes carrying a pro-angiogenic miR-30b promoted tube formation with HUVEC, increased migration of EC and increased blood flow in tubes formed in matrigel plugs, following their implantation into mice [104]. The available data confirm the pro-angiogenic potency of MSC-derived exosomes in the TME and indicate that transfer by exosomes of mRNAs and relevant proteins to ECs is the mechanisms accounting for EC reprogramming.
6. Anti- tumor effects of MSC-derived exosomes
While exosomes produced by MSCs in the TME largely mediate pro-tumor effects, there is also evidence for anti-tumor activity of MSC-derived EVs. Bruno et al. reported that exosomes derived from normal human BM-MSCs inhibited growth and survival various human tumor cell lines [108]. In vivo inhibition of tumor growth by these exosomes was also reported in NOD/SCID mice [108, 109]. Studies from several other groups confirmed the tumor-inhibitory potential of MSC-derived exosomes on breast cancer or hepatocellular carcinoma cells [110–112]. However, the sources of EVs used in these experiments differed widely as did methods for EV isolation. It may be that exosomes and MVs mediate quite different or overlapping functions [113]. It may also be that exosomes from normal MSCs could be tumor inhibitory perhaps by up-regulating immune effector cell functions in vitro or in experimental animals, as suggested [109, 111], or by protecting tumor cell progenitors from destruction by e.g., radiation [114]. Interestingly, enrichment of the exosome cargo in certain pro-angiogenic miRNAs, eg., miR-100, and the ability of these exosomes to suppress VEGF production and thus angiogenesis in breast cancer cells through modulating the mTOR/HIF-1α signaling axis is directly related to anti-tumor effects they mediate [112]. Growth of glioma was reported to be inhibited by stromal cell-derived exosomes rich in miR-146b [115], and MSC-derived exosomes appear to be enriched in miR-23b which promotes breast cancer cell dormancy [116]. Thus, modulation by miRNAs carried in MSC-derived exosomes of vascular responses and/or tumor growth may be responsible for their anti-tumor activity.
Nevertheless, exosomes produced in the TME by TEX-conditioned MSCs carry immunosuppressive cargos, inhibit anti-tumor immune responses in vitro or in vivo and promote tumor growth [77]. At the same time, it should be remembered that exosomes carry rich and diverse cargos and that not all MSC-derived exosomes are equal [45]. It is also known that their interactions with immune cells could lead to immune response inhibition or to immune stimulation [38]. The dichotomy of exosome-mediated immune-modulatory functions has been previously discussed [91] and remains a subject of great interest. Similar dichotomy, albeit less frequently discussed, seems to exist in respect to other pro-tumor activities modulated by MSC-derived exosomes.
7. Conclusions
Exosomes produced by MSCs in the TME emerge as a new communication system operating between cells engaged in supporting tumor progression. MSCs, due to their abilities to produce a broad spectrum of cytokines, chemokines and growth factors, have been considered to be a key cell in the promotion of tumor growth (reviewed in [40, 56]). This is not surprising, as MSCs are well known for their salutary or rescue effects [117]. It has been shown that tumor cells are highly effective in altering the MSC functional phenotype from physiological to pathological activities, and that this alteration is associated with the expression of a new set of cytokines and chemokines in MSCs. However, the molecular/genetic underpinning of the transition has remained obscure. Rapidly emerging data focus on exosomes as a remarkably versatile and effective transport and delivery system of nucleic acids and proteins between cells engaged in the biological cross-talk in the TME. MSC-derived exosomes function largely via horizontal transfer of mRNAs, miRNAs and proteins, which upon delivery to targeted recipient cells alter the phenotypic and functional profiles of these cells. In the TME, these exosome-mediated alterations are largely pro-tumorigenic, although it appears that MSCs can also exert anti-tumor activities. These immunosuppressive inhibitory activities appear to be mediated by miRNAs which may be of interest as future cancer therapeutics.
Table 3.
Mechanisms involved in TEX-driven re-programming of MSCs in the tumor microenvironment
| Mechanism | Changes induced in re-programmed MSCs | References |
|---|---|---|
| Genetic | ||
| Uptake & transfer of miRNA species | ↑ Proliferation | 30, 55, 62 |
| ↑ Cellular activity via upregulation of common signaling pathways (TGF-β, AKT, β-catenin, Wnt, Notch) | 55, 56, 60, 72 | |
| ↑ Production of growth factors and cytokines necessary for TME re-modeling | 50, 55, 56, 60 | |
| ↑ Pro-angiogenic activity | 59, 62, 64 | |
| ↑ Immunoregulatory gene alterations | 49, 61 | |
|
| ||
| Proteomic | ||
| Surface receptor signaling | ⇅ Major molecular pathways | 37, 55, 63 |
| Uptake of TEX cargo | ⇅ Major molecular pathways | 29, 49, 67 |
| Transfer of: Enzymes | ↑ Immunosuppressive phenotype | 27, 41 |
| Cytokines/growth factors | ↑ Stromal remodeling | 39, 40, 48, 66 |
| ↑ Immune modulation | ||
| Survival proteins | ↑ Resistance to apoptosis | 71, 74 |
| Immunosuppressive molecules | ↓ Anti-tumor immune functions | 36, 38 |
| MHC molecules | Altered interactions with T lymphocytes | 25, 36, 37 |
|
| ||
| Metabolic | ||
| Changes in cellular metabolism | ↑ Hypoxia | 118, 119 |
| Switch from oxidative phosphorylation to glycolysis | ||
Acknowledgments
Funding:
This work was supported in part by NIH grants R0-1 CA168628 and R21 CA205644 to TLW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
The author indicates no potential conflicts of interest.
References
- 1.Lazennec G, Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit? Stem Cells. 2008;26(6):1387–94. doi: 10.1634/stemcells.2007-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Zhou H, Guo M, Bian C, Sun Z, Yang Z, Zeng Y, Ai H, Zhao RC. Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: clinical report. Biol Blood Marrow Transplant. 2010;16(3):403–12. doi: 10.1016/j.bbmt.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 4.El Omar R, Beroud J, Stoltz JF, Menu P, Velot E, Decot V. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng Part B Rev. 2014;20(5):523–44. doi: 10.1089/ten.TEB.2013.0664. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–49. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 6.Le Blanc K, Ringden O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(5):321–34. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 8.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 9.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 10.Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DP. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1(2):129–37. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Mol Cancer. 2017;16(1):31. doi: 10.1186/s12943-017-0597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–61. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen HW, Chen HY, Wang LT, Wang FH, Fang LW, Lai HY, Chen HH, Lu J, Hung MS, Cheng Y, Chen MY, Liu SJ, Chong P, Lee OK, Hsu SC. Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. J Immunol. 2013;190(10):5065–77. doi: 10.4049/jimmunol.1202775. [DOI] [PubMed] [Google Scholar]
- 14.Waterman RS, Henkle SL, Betancourt AM. Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS One. 2012;7(9):e45590. doi: 10.1371/journal.pone.0045590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norozi F, Ahmadzadeh A, Shahrabi S, Vosoughi T, Saki N. Mesenchymal stem cells as a double-edged sword in suppression or progression of solid tumor cells. Tumour Biol. 2016;37(9):11679–11689. doi: 10.1007/s13277-016-5187-7. [DOI] [PubMed] [Google Scholar]
- 16.Lazennec G, Lam PY. Recent discoveries concerning the tumor - mesenchymal stem cell interactions. Biochim Biophys Acta. 2016;1866(2):290–299. doi: 10.1016/j.bbcan.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70(20):3871–82. doi: 10.1007/s00018-013-1290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–22. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Thery C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25(6):364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol. 2016;36(3):301–12. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9:86. doi: 10.1186/1479-5876-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, Giebel B. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. Int J Mol Sci. 2017;18(7) doi: 10.3390/ijms18071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyiadzis M, Whiteside TL. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia. 2017 doi: 10.1038/leu.2017.91. [DOI] [PubMed] [Google Scholar]
- 26.Wen SW, Sceneay J, Lima LG, Wong CS, Becker M, Krumeich S, Lobb RJ, Castillo V, Wong KN, Ellis S, Parker BS, Moller A. The Biodistribution and Immune Suppressive Effects of Breast Cancer-Derived Exosomes. Cancer Res. 2016;76(23):6816–6827. doi: 10.1158/0008-5472.CAN-16-0868. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig S, Floros T, Theodoraki MN, Hong CS, Jackson EK, Lang S, Whiteside TL. Suppression of Lymphocyte Functions by Plasma Exosomes Correlates with Disease Activity in Patients with Head and Neck Cancer. Clin Cancer Res. 2017;23(16):4843–4854. doi: 10.1158/1078-0432.CCR-16-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep. 2016;6:20254. doi: 10.1038/srep20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller L, Simms P, Hong CS, Nishimura MI, Jackson EK, Watkins SC, Whiteside TL. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. OncoImmunology. 2017 doi: 10.1080/2162402X.2016.1261243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neviani P, Fabbri M. Exosomic microRNAs in the Tumor Microenvironment. Front Med (Lausanne) 2015;2:47. doi: 10.3389/fmed.2015.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7(3):789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong CS, Funk S, Muller L, Boyiadzis M, Whiteside TL. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles. 2016;5:29289. doi: 10.3402/jev.v5.29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson RJ, Kalra H, Mathivanan S. ExoCarta as a resource for exosomal research. J Extracell Vesicles. 2012;1 doi: 10.3402/jev.v1i0.18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 2011;96(9):1302–9. doi: 10.3324/haematol.2010.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whiteside TL. Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv Clin Chem. 2016;74:103–41. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyiadzis M, Whiteside TL. Information transfer by exosomes: A new frontier in hematologic malignancies. Blood Rev. 2015;29(5):281–90. doi: 10.1016/j.blre.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Whiteside TL. Exosomes carrying immunoinhibitory proteins and their role in cancer. Clin Exp Immunol. 2017 doi: 10.1111/cei.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233–44. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- 40.Dostert G, Mesure B, Menu P, Velot E. How Do Mesenchymal Stem Cells Influence or Are Influenced by Microenvironment through Extracellular Vesicles Communication? Front Cell Dev Biol. 2017;5:6. doi: 10.3389/fcell.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. 2016;126(4):1216–23. doi: 10.1172/JCI81136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 43.Kumar B, Garcia M, Murakami JL, Chen CC. Exosome-mediated microenvironment dysregulation in leukemia. Biochim Biophys Acta. 2016;1863(3):464–70. doi: 10.1016/j.bbamcr.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 44.Choi D, Lee TH, Spinelli C, Chennakrishnaiah S, D’Asti E, Rak J. Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. Semin Cell Dev Biol. 2017 doi: 10.1016/j.semcdb.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35(4):851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 46.Colak S, Ten Dijke P. Targeting TGF-beta Signaling in Cancer. Trends Cancer. 2017;3(1):56–71. doi: 10.1016/j.trecan.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Sia D, Alsinet C, Newell P, Villanueva A. VEGF signaling in cancer treatment. Curr Pharm Des. 2014;20(17):2834–42. doi: 10.2174/13816128113199990590. [DOI] [PubMed] [Google Scholar]
- 48.Hong CS, Muller L, Whiteside TL, Boyiadzis M. Plasma exosomes as markers of therapeutic response in patients with acute myeloid leukemia. Front Immunol. 2014;5:160. doi: 10.3389/fimmu.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chowdhury R, Webber JP, Gurney M, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget. 2015;6(2):715–31. doi: 10.18632/oncotarget.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109(31):E2110–6. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whiteside TL. The potential of tumor-derived exosomes for noninvasive cancer monitoring. Expert Rev Mol Diagn. 2015:1–18. doi: 10.1586/14737159.2015.1071666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma A. Bioinformatic analysis revealing association of exosomal mRNAs and proteins in epigenetic inheritance. J Theor Biol. 2014;357:143–9. doi: 10.1016/j.jtbi.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 53.Hong CS, Sharma P, Yerneni SS, Simms P, Jackson EK, Whiteside TL, Boyiadzis M. Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Scientific Reports. 2017 doi: 10.1038/s41598-017-14661-w. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lo Cicero A, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. 2015;35:69–77. doi: 10.1016/j.ceb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 56.Lopatina T, Gai C, Deregibus MC, Kholia S, Camussi G. Cross Talk between Cancer and Mesenchymal Stem Cells through Extracellular Vesicles Carrying Nucleic Acids. Front Oncol. 2016;6:125. doi: 10.3389/fonc.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 58.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 59.Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, Kalluri R. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289(7):3869–75. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huan J, Hornick NI, Shurtleff MJ, Skinner AM, Goloviznina NA, Roberts CT, Jr, Kurre P. RNA trafficking by acute myelogenous leukemia exosomes. Cancer Res. 2013;73(2):918–29. doi: 10.1158/0008-5472.CAN-12-2184. [DOI] [PubMed] [Google Scholar]
- 61.Paggetti J, Haderk F, Seiffert M, Janji B, Distler U, Ammerlaan W, Kim YJ, Adam J, Lichter P, Solary E, Berchem G, Moussay E. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015;126(9):1106–17. doi: 10.1182/blood-2014-12-618025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, Thomas-Tikhonenko A. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38(9):1060–5. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647–58. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y, Luo F, Wang B, Li H, Xu Y, Liu X, Shi L, Lu X, Xu W, Lu L, Qin Y, Xiang Q, Liu Q. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016;370(1):125–35. doi: 10.1016/j.canlet.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 65.Vallabhaneni KC, Penfornis P, Dhule S, Guillonneau F, Adams KV, Mo YY, Xu R, Liu Y, Watabe K, Vemuri MC, Pochampally R. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6(7):4953–67. doi: 10.18632/oncotarget.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Y, Bucan V, Baehre H, von der Ohe J, Otte A, Hass R. Acquisition of new tumor cell properties by MSC-derived exosomes. Int J Oncol. 2015;47(1):244–52. doi: 10.3892/ijo.2015.3001. [DOI] [PubMed] [Google Scholar]
- 67.Lindoso RS, Collino F, Camussi G. Extracellular vesicles derived from renal cancer stem cells induce a pro-tumorigenic phenotype in mesenchymal stromal cells. Oncotarget. 2015;6(10):7959–69. doi: 10.18632/oncotarget.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanchez CA, Andahur EI, Valenzuela R, Castellon EA, Fulla JA, Ramos CG, Trivino JC. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. 2016;7(4):3993–4008. doi: 10.18632/oncotarget.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anthony BA, Link DC. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014;35(1):32–7. doi: 10.1016/j.it.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lugini L, Valtieri M, Federici C, Cecchetti S, Meschini S, Condello M, Signore M, Fais S. Exosomes from human colorectal cancer induce a tumor-like behavior in colonic mesenchymal stromal cells. Oncotarget. 2016;7(31):50086–50098. doi: 10.18632/oncotarget.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012;40(1):130–8. doi: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- 72.Li X, Wang S, Zhu R, Li H, Han Q, Zhao RC. Lung tumor exosomes induce a pro-inflammatory phenotype in mesenchymal stem cells via NFkappaB-TLR signaling pathway. J Hematol Oncol. 2016;9:42. doi: 10.1186/s13045-016-0269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haga H, Yan IK, Takahashi K, Wood J, Zubair A, Patel T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J Extracell Vesicles. 2015;4:24900. doi: 10.3402/jev.v4.24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corrado C, Raimondo S, Saieva L, Flugy AM, De Leo G, Alessandro R. Exosome-mediated crosstalk between chronic myelogenous leukemia cells and human bone marrow stromal cells triggers an interleukin 8-dependent survival of leukemia cells. Cancer Lett. 2014;348(1–2):71–6. doi: 10.1016/j.canlet.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 75.Syn N, Wang L, Sethi G, Thiery JP, Goh BC. Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharmacol Sci. 2016;37(7):606–17. doi: 10.1016/j.tips.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Shi S, Zhang Q, Xia Y, You B, Shan Y, Bao L, Li L, You Y, Gu Z. Mesenchymal stem cell-derived exosomes facilitate nasopharyngeal carcinoma progression. Am J Cancer Res. 2016;6(2):459–72. [PMC free article] [PubMed] [Google Scholar]
- 77.Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, Azab F, Flores LM, Campigotto F, Weller E, Anderson KC, Scadden DT, Ghobrial IM. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123(4):1542–55. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar B, Garcia M, Weng L, Jung X, Murakami JL, Hu X, McDonald T, Lin A, Kumar AR, DiGiusto DL, Stein AS, Pullarkat VA, Hui SK, Carlesso N, Kuo YH, Bhatia R, Marcucci G, Chen CC. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia. 2017 doi: 10.1038/leu.2017.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Narayanan R, Huang CC, Ravindran S. Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells Int. 2016;2016:3808674. doi: 10.1155/2016/3808674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ. MSCs-Derived Exosomes: Cell-Secreted Nanovesicles with Regenerative Potential. Front Pharmacol. 2016;7:231. doi: 10.3389/fphar.2016.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Trapani M, Bassi G, Midolo M, Gatti A, Kamga PT, Cassaro A, Carusone R, Adamo A, Krampera M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci Rep. 2016;6:24120. doi: 10.1038/srep24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A, Lim SK. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int J Proteomics. 2012;2012:971907. doi: 10.1155/2012/971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38(1):215–24. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, Wymant J, Jones AT, Kynaston H, Mason MD, Tabi Z, Clayton A. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34(3):290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- 85.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70(23):9621–30. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 86.Luga V, Wrana JL. Tumor-stroma interaction: Revealing fibroblast-secreted exosomes as potent regulators of Wnt-planar cell polarity signaling in cancer metastasis. Cancer Res. 2013;73(23):6843–7. doi: 10.1158/0008-5472.CAN-13-1791. [DOI] [PubMed] [Google Scholar]
- 87.McBride JD, Rodriguez-Menocal L, Guzman W, Candanedo A, Garcia-Contreras M, Badiavas EV. Bone Marrow Mesenchymal Stem Cell-Derived CD63+ Exosomes Transport Wnt3a Exteriorly and Enhance Dermal Fibroblast Proliferation, Migration, and Angiogenesis In Vitro. Stem Cells Dev. 2017 doi: 10.1089/scd.2017.0087. [DOI] [PubMed] [Google Scholar]
- 88.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles Released from Human Renal Cancer Stem Cells Stimulate Angiogenesis and Formation of Lung Premetastatic Niche. Cancer Research. 2011;71(15):5346–5356. doi: 10.1158/0008-5472.Can-11-0241. [DOI] [PubMed] [Google Scholar]
- 89.Burrello J, Monticone S, Gai C, Gomez Y, Kholia S, Camussi G. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front Cell Dev Biol. 2016;4:83. doi: 10.3389/fcell.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marigo I, Dazzi F. The immunomodulatory properties of mesenchymal stem cells. Semin Immunopathol. 2011;33(6):593–602. doi: 10.1007/s00281-011-0267-7. [DOI] [PubMed] [Google Scholar]
- 91.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 93.Zhou Y, Day A, Haykal S, Keating A, Waddell TK. Mesenchymal stromal cells augment CD4+ and CD8+ T-cell proliferation through a CCL2 pathway. Cytotherapy. 2013;15(10):1195–207. doi: 10.1016/j.jcyt.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 94.Amarnath S, Foley JE, Farthing DE, Gress RE, Laurence A, Eckhaus MA, Metais JY, Rose JJ, Hakim FT, Felizardo TC, Cheng AV, Robey PG, Stroncek DE, Sabatino M, Battiwalla M, Ito S, Fowler DH, Barrett AJ. Bone marrow-derived mesenchymal stromal cells harness purinergenic signaling to tolerize human Th1 cells in vivo. Stem Cells. 2015;33(4):1200–12. doi: 10.1002/stem.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65(3):336–41. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 96.Kilpinen L, Impola U, Sankkila L, Ritamo I, Aatonen M, Kilpinen S, Tuimala J, Valmu L, Levijoki J, Finckenberg P, Siljander P, Kankuri E, Mervaala E, Laitinen S. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147(1–2):47–54. doi: 10.1016/j.imlet.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 98.Del Fattore A, Luciano R, Pascucci L, Goffredo BM, Giorda E, Scapaticci M, Fierabracci A, Muraca M. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015;24(12):2615–27. doi: 10.3727/096368915X687543. [DOI] [PubMed] [Google Scholar]
- 99.Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, Riches DW, Deiuliis G, Kaminski N, Boregowda SV, McKenna DH, Ortiz LA. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Conforti A, Scarsella M, Starc N, Giorda E, Biagini S, Proia A, Carsetti R, Locatelli F, Bernardo ME. Microvescicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem Cells Dev. 2014;23(21):2591–9. doi: 10.1089/scd.2014.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen W, Huang Y, Han J, Yu L, Li Y, Lu Z, Li H, Liu Z, Shi C, Duan F, Xiao Y. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64(4):831–40. doi: 10.1007/s12026-016-8798-6. [DOI] [PubMed] [Google Scholar]
- 102.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156(1):149–60. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gong M, Yu B, Wang J, Wang Y, Liu M, Paul C, Millard RW, Xiao DS, Ashraf M, Xu M. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget. 2017;8(28):45200–45212. doi: 10.18632/oncotarget.16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, Xu X, Wang M, Qian H, Xu W. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315(1):28–37. doi: 10.1016/j.canlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 106.Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, Montgomery EN, Mellema MS, Bardini RL, Contreras Z, Hoon M, Bauer G, Fink KD, Fury B, Hendrix KJ, Chedin F, El-Andaloussi S, Hwang B, Mulligan MS, Lehtio J, Nolta JA. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells. 2016;34(3):601–13. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell Physiol Biochem. 2015;37(6):2415–24. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- 108.Bruno S, Collino F, Deregibus MC, Grange C, Tetta C, Camussi G. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 2013;22(5):758–71. doi: 10.1089/scd.2012.0304. [DOI] [PubMed] [Google Scholar]
- 109.Bruno S, Collino F, Iavello A, Camussi G. Effects of mesenchymal stromal cell-derived extracellular vesicles on tumor growth. Front Immunol. 2014;5:382. doi: 10.3389/fimmu.2014.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, Kim YG, Jang JY, Kim CW. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013;8(12):e84256. doi: 10.1371/journal.pone.0084256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ko SF, Yip HK, Zhen YY, Lee CC, Lee CC, Huang CC, Ng SH, Lin JW. Adipose-Derived Mesenchymal Stem Cell Exosomes Suppress Hepatocellular Carcinoma Growth in a Rat Model: Apparent Diffusion Coefficient, Natural Killer T-Cell Responses, and Histopathological Features. Stem Cells Int. 2015;2015:853506. doi: 10.1155/2015/853506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N, Javan M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1alpha/VEGF signaling axis in breast cancer cells. Cell Oncol (Dordr) 2017 doi: 10.1007/s13402-017-0335-7. [DOI] [PubMed] [Google Scholar]
- 113.Whiteside TL, Boyiadzis M. Response commentary: exosomes vs microvesicles in hematological malignancies. Leukemia. 2017 doi: 10.1038/leu.2017.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wen S, Dooner M, Cheng Y, Papa E, Del Tatto M, Pereira M, Deng Y, Goldberg L, Aliotta J, Chatterjee D, Stewart C, Carpanetto A, Collino F, Bruno S, Camussi G, Quesenberry P. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia. 2016;30(11):2221–2231. doi: 10.1038/leu.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, Shu W, Jiang F, Chopp M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335(1):201–4. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K, Ochiya T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 117.Bernardo ME, Pagliara D, Locatelli F. Mesenchymal stromal cell therapy: a revolution in Regenerative Medicine? Bone Marrow Transplant. 2012;47(2):164–71. doi: 10.1038/bmt.2011.81. [DOI] [PubMed] [Google Scholar]
- 118.Ohyashiki JH, Umezu T, Ohyashiki K. Exosomes promote bone marrow angiogenesis in hematologic neoplasia: the role of hypoxia. Curr Opin Hematol. 2016;23(3):268–73. doi: 10.1097/MOH.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 119.Johnson SM, Dempsey C, Chadwick A, Harrison S, Liu J, Di Y, McGinn OJ, Fiorillo M, Sotgia F, Lisanti MP, Parihar M, Krishnan S, Saha V. Metabolic reprogramming of bone marrow stromal cells by leukemic extracellular vesicles in acute lymphoblastic leukemia. Blood. 2016;128(3):453–6. doi: 10.1182/blood-2015-12-688051. [DOI] [PubMed] [Google Scholar]


