Abstract
Heterotopic ossification (HO) is a pathological phenomenon in which ectopic lamellar bone forms in soft tissues. HO involves many predisposing factors, including congenital and postnatal factors. Postnatal HO is usually induced by fracture, burn, neurological damage (brain injury and spinal cord injury) and joint replacement. Recent studies have found that patients who suffered from bone fracture combined with severe traumatic brain injury (S-TBI) are at a significantly increased risk for HO occurrence. Thus, considerable research focused on the influence of S-TBI on fracture healing and bone formation, as well as on the changes in various osteogenic factors with S-TBI occurrence. Brain damage promotes bone formation, but the exact mechanisms underlying bone formation and HO after S-TBI remain to be clarified. Hence, this article summarises the findings of previous studies on the relationship between S-TBI and HO and discusses the probable causes and mechanisms of HO caused by S-TBI.
The translational potential of this article: A better understanding of the probable causes of traumatic brain injury–induced HO can provide new perspectives and ideas in preventing HO and may support to design more targeted therapies to reduce HO or enhance the bone formation.
Keywords: Heterotopic ossification, Neurogenic heterotopic ossification, Severe traumatic brain injury
Introduction
Heterotopic ossification (HO) is a pathological phenomenon that causes bone formation in nonosseous tissues, including muscles and connective tissues [1]. This disease is usually induced by fracture, burn, neurological damage (brain injury and spinal cord injury) and joint replacement [2]. Patients with HO experience swelling of tissues, inflammation, pain, limited motion and joint adhesion. All these symptoms significantly affect the lives and emotions of patients [3], [4]. According to different inducements, HO can be divided into three types: myositis ossificans progressiva, traumatic HO and neurogenic HO [5]. By theory, HO may occur in any part of the body, but in most of the cases it occurs in the joints, such as in the hips, elbows, and shoulders. Depending on the inducements, the affected positions are different [4], [6].
Roberts [7] first described HO in patients with brain injury and a prolonged period of coma. Researchers thereby found an internal relationship between HO and the nervous system. Recent studies have found that patients who suffered from bone fracture combined with severe traumatic brain injury (S-TBI) are at a significantly increased risk for HO [8]. The incidence of neurogenic heterotopic ossification (NHO) in patients with traumatic brain injury (TBI) is about 20%, but the rate of NHO exceeds 50% when TBI and femur fracture are concomitant [5]. In some cases, HO may be caused by brain or spinal injuries, without other injuries existing [9]. Studies determined the effect of TBI on fracture healing and confirmed that patients who suffered from both fracture and S-TBI are expected to show a faster rate of fracture healing accompanied by hypertrophic callus formation or heterotopic ossifications [10], [11], [12]. Fracture healing and HO share common initiating events (inflammation, angiogenesis, chondrogenesis and osteogenesis) [13]. Several researchers have speculated that the consolidated callus formation is a form of local heterotopic bone formation [14], [15]. Spencer [16] conducted a histological analysis on the callus of a patient who suffered from fracture and S-TBI and found that the composition of callus is highly similar to that of the bone. Spencer also suggested that faster fracture healing and callus formation are indicative of HO [16].
Though TBI has been associated with faster fracture healing, enhanced callus formation and HO in both TBI patients and animal models, there are also evidences of enhanced bone loss and increased risk of fracture in brain injury patients [17], [18]. Animal studies in rodents with TBI are consistent with the clinical studies [19], [20], [21]. However, it should be noticed that, most of the bone loss after TBI occurs in the absence of fracture. The increased callus formation in TBI combined with bone fracture occurs in the early stages postinjury while the bone loss symptoms appear in a latter period [22], [23]. Faster bone fracture healing and HO are mostly linked to an S-TBI accompanied with breakdown of the blood–brain barrier, while the bone loss is usually investigated in a mild-TBI condition. Therefore, the condition and environment that enhanced bone loss or bone formation after TBI may have a great difference. Researchers have studied the association between TBI and HO for a long time, but the direct relationship between them remains unknown, and the pathological mechanism is yet to be elucidated. This article summarises the latest research findings and related perspectives about this issue, providing references for further understanding TBI-HO. Furthermore, the findings mentioned in this review may allow us to design more targeted therapies to reduce HO or enhance the bone formation.
Normally, Patients with brain injury are given corticosteroids, nonsteroidal antiinflammatory drugs, diphosphonates and radiotherapy as a preventative measure for HO [8]. Recently, several researchers proposed their hypotheses for the potential methods of HO prevention. Kan et al. [24] noticed that blocking of neuron-specific substance P (SP) signalling through the neurokinin 1 (NK1r) receptor can abrogate HO formation and suggested that inhibition of the SP receptor, NK1r, might therefore be a novel treatment for preventing the early events that lead to HO. Genêt et al. [25] found that ablation of phagocytic macrophages with clodronate-loaded liposomes reduced the size of NHO by 90%, supporting the conclusion that NHO is highly dependent on inflammation and phagocytic macrophages in soft tissues. All of these hypotheses or suggestions provide us with new perspectives and ideas for preventing HO.
Association between S-TBI and HO
Patients who suffered from fracture and TBI usually exhibit faster healing rate and increased callus formation [26]. Cadosch et al. [27] found that the time for bone union in patients with S-TBI was reduced by half when compared to patients with fracture alone. Callus formation in patients with S-TBI is 37–50% greater than that in patients with fracture alone. Consistently, studies in animal models combining TBI with bone fracture showed an increased volume of callus and a higher rate of gap bridging compared with the normal group or fracture group [22], [28], [29]. In addition, treating human osteoblasts with the serum of patients with S-TBI significantly promotes the proliferation of these cells. A number of studies also provided conclusive evidence for this view and found that serum after brain injury promotes the proliferation of osteoblasts and bone mesenchymal stem cells and the expression of intracellular alkaline phosphatase (ALP) [30], [31], [32].
In a subsequent study by Cadosch et al. [14], skeletal muscle cells were treated with the serum of patients with severe brain injury. The results showed that serum treatment significantly increases the level of intracellular ALP. A specific transcription factor of osteoblasts expresses and mineralises the formed nodule. These results indicate that the serum of S-TBI patients contains some humoural factors that promote the differentiation of osteogenitor cells in skeletal muscles into bone cells and mineralisation. In addition, blood–brain barrier permeability change, coma, mechanical ventilation, and inflammation caused by S-TBI are all important factors of HO induction. Table 1 summarises the relative factors in HO caused by S-TBI.
Table 1.
Relative factor in HO caused by S-TBI.
| Factors | Main function | |
|---|---|---|
| Osteogenic factors | BMP | Promotes differentiation of osteoblast and bone induction [33], [34] |
| Thrombin | Promotes the proliferation and inhibits the apoptosis of osteoblastic cells [35] | |
| TGF-β1 | Adjusts the proliferation and differentiation of bone cells [36], [37] | |
| VEGF | Induces angiogenesis and neurogenesis, and thereby promotes the healing of fractures and formation of new bone [38] | |
| Neuropeptide and hormones | CGRP | Improves blood supply of the damaged area and accelerate fracture healing [39], [40] |
| Substance P | Intensifies inflammatory response and promotes osteogenesis [41], [42] | |
| Leptin | Regulates the bone formation [43] | |
| Melatonin | Promotes the proliferation of normal bone cells and human osteoblast [44] | |
| Change of blood–brain barrier permeability | Osteogenic macromolecules within the central nervous system release from the damaged brain tissue [30], [45] | |
| Mechanical ventilation | Changes the electrolyte and acid–base balance in the body [46] | |
| Coma and immobilisation | Result in inadequate supply of local blood and oxygen | |
| Inflammation | Some inflammatory factors may promote bone formation [47], [48] | |
BMP = bone morphogenetic protein; CGRP = calcitonin gene-related protein; TGF-β1 = transforming growth factor β1; VEGF = vascular endothelial growth factor.
Effects of osteogenic factors released after TBI on fracture healing
Yang et al. [49] performed a comparative research on 74 patients who suffered from fracture alone or combined with S-TBI. The time for bridging callus formation and average thickness of callus were studied. The fractures were treated by intramedullary nailing. Compared with those of the control, the time for bridging callus formation in patients who suffered from fracture combined with S-TBI was significantly shorter, and the bone union was faster. The average thickness of callus was also significantly greater. This finding is due to the fact that S-TBI increases the levels of many osteogenic factors in the serum and therefore affects fracture healing. These inducible factors can promote ossification and play an important role in inducing the regeneration of normal or ectopic bone [50]. The increase in osteogenic factors in the body with S-TBI not only accelerates fracture healing and promotes callus formation but also has a close relationship to the incidence of HO.
Bone morphogenetic protein
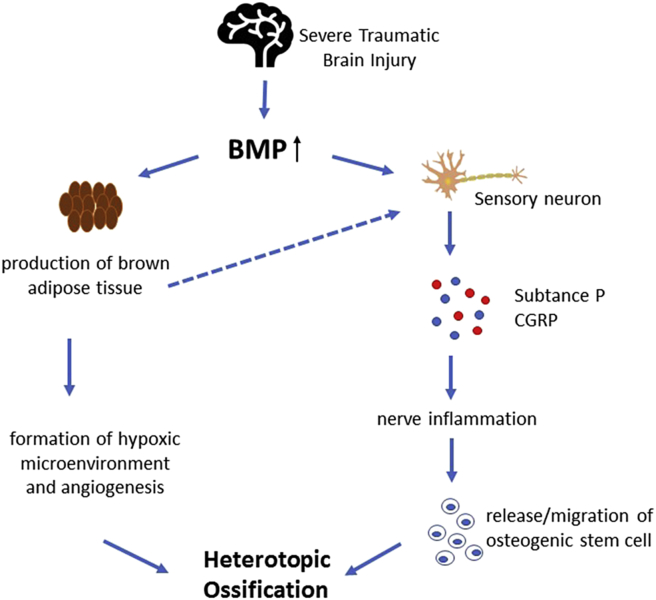
Bone morphogenetic protein (BMP) is an important member of the transforming growth factor β (TGF-β) superfamily. BMP2 and BMP4 are recognised as the most important factors for regulating bone formation. Both factors exert a strong effect on promoting osteoblast differentiation and bone induction [33], [34]. Wang et al. [51] revealed that implantation of 0.5–115 μg of partially purified recombinant human BMP-2A resulted in cartilage by Day 7 and bone formation by Day 14. Several studies have shown that BMP2 or BMP4 in combination with other factors, like vascular endothelial growth factor (VEGF) and TGF-β, can greatly promote endochondral bone formation [52], [53], [54]. The levels of BMP and its receptor, as well as the expression of its messenger RNA (mRNA), are all upregulated after brain injury [55], [56]. In addition, Scherbel et al. [8] used a well-established head injury model in rats and found that the muscles around the hips in animal models of S-TBI show an elevated level of BMP 2/4. However, the mRNA level of BMP 2/4 in the fracture group without TBI is upregulated at the fracture site 48 h postinjury but not at remote sites, including the hip muscles. This result explains why patients with TBI often acquire HO in the hip. Furthermore, BMP2 may act directly on sensory neurons, inducing the release of inflammatory cytokine SP and calcitonin gene-related protein (CGRP) [57]. BMP2 may also cause nerve inflammation, leading to nerve regeneration and migration or release of osteogenic stem cells or other stem cells [58]. Brown adipose tissue plays an important role in bone formation; it can promote the formation of hypoxic microenvironment and angiogenesis [59]. Abnormally elevated levels of BMP induce the production of brown adipose tissue, generating heat and stimulating sensory neurons to release SP and CGRP [58]. To sum up, the increased levels of BMP caused by fracture and S-TBI in concomitance produce multiple effects on osteogenesis and greatly increase the risk of HO (Fig. 1).
Figure 1.
An overview of the function of BMP on TBI–HO. After brain injury, the levels of BMP and its receptor, as well as the expression of its mRNA, are all upregulated. BMP subsequently acts on sensory neurons, induces the release of inflammatory cytokines, substance P and CGRP, and causes nerve inflammation, leading to nerve regeneration and migration or release of osteogenic stem cells or other stem cells. Abnormally elevated levels of BMP also induce the production of BAT, promoting the formation of hypoxic microenvironment and angiogenesis, generating heat and stimulating the sensory neurons to release SP and CGRP. BMP = bone morphogenetic protein; BAT = brown adipose tissue; CGRP = calcitonin gene-related protein; HO = heterotopic ossification; SP = substance P; TBI = traumatic brain injury.
Thrombin
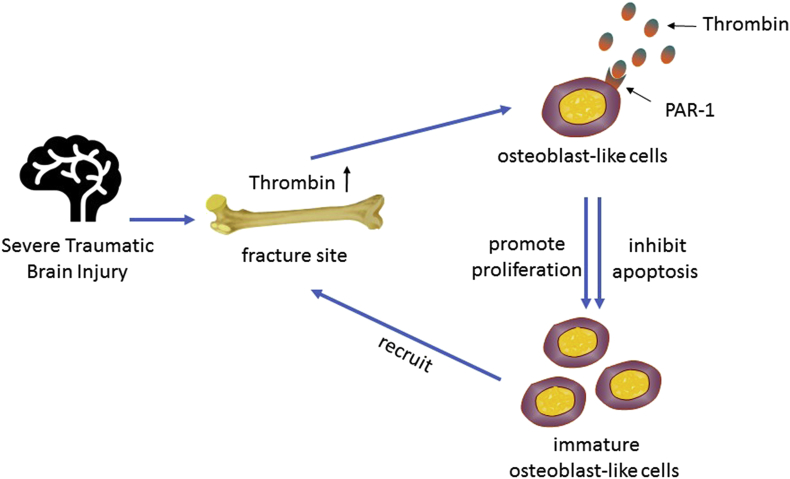
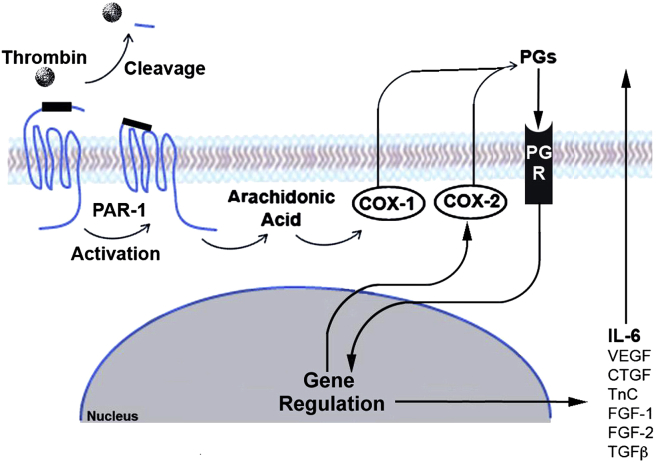
Thrombin is a serine protease that plays a key role in the coagulation cascade. TBI causes abnormal blood coagulation [60]. Thrombin in the brain is released immediately after the occurrence of haemorrhage, resulting in increased levels of thrombin at the injured area [61]. In 1978, Vandersande et al. [62] found that fracture alone is not the main reason for abnormal blood coagulation, sufficiently proving that a specific and close link exists between TBI and coagulation abnormalities. Many cellular responses to thrombin are mediated by protease-activated receptor-1 (PAR-1), which is expressed by osteoblasts in vivo. Therefore, in addition to its important role in thrombosis and haemostasis, thrombin also promotes many processes in osteoblastic cells [63]. For instance, thrombin can promote the proliferation of osteoblastic cells by activating PAR-1 and inhibit their apoptosis [35]. Cyclooxygenase is a key enzyme in the conversion of arachidonic acid to prostaglandins, playing an important role in the inflammatory response. Pagel et al. [64] proposed that fibroblast growth factor-1 and fibroblast growth factor-2, connective tissue growth factor, TGF-β1, tenascin C, VEGF and interleukin-6 (IL-6) induced by local prostaglandin are important factors in fracture healing. In addition, the activation of PAR-1 by thrombin after the injury could be a key adjustment for cyclooxygenase-2 expression. Therefore, thrombin may indirectly promote fracture healing by activating PAR-1. Wang et al. [65] indicated that thrombin protein can increase the level of early growth factors, regulatory factors and inflammatory angiogenic factor during inflammation and angiogenesis in bone repair. This fact proves that thrombin can promote fracture repair and new bone formation. Thrombin can also induce the osteogenic precursor cells that are less differentiated to migrate to the injury site [66]. However, thrombin can only promote proliferation for well-differentiated osteoblasts but is unable to induce them to migrate towards the injury sites. Abraham et al. [63] found that thrombin-induced PAR-1 activation reduces alkaline phosphatase activity. Meanwhile, the proliferation of osteoblasts is promoted. These findings indicate that the inhibition of ALP is inversely related to the proliferation of osteoblast-like cells, serving to maintain a population of immature osteoblast-like cells. These immature cells are recruited by thrombin and finally migrate to the site of injury, promoting bone healing. Some researchers believe that thrombin accelerates fracture healing and increases callus formation in patients who suffered from fractures and TBI [67]. Thus, an abnormal increase in thrombin after severe brain injury is likely to play an important role in increasing the incidence of HO (Figs. 2 and 3).
Figure 2.
Direct function of thrombin on fracture healing and HO. Thrombin in the brain is released immediately after haemorrhage, resulting in increased levels of thrombin at the injured area. Furthermore, thrombin promotes the proliferation and inhibits the apoptosis of osteoblastic cells by PAR-1 activation, serving to maintain a population of immature osteoblast-like cells. These immature cells are recruited by thrombin and finally migrate to the site of injury, promoting bone healing. HO = heterotopic ossification; PAR-1 = protease-activated receptor-1.
Figure 3.
Indirect function of thrombin on fracture healing and HO. The activation of PAR-1 on osteoblast-like cells by thrombin following the injury promotes the expression of COX-2, which is a key enzyme in the conversion of arachidonic acid to prostaglandins. Then, the local prostaglandin induces the release of FGF-1, FGF-2, CTGF, TGF-β1, TnC, VEGF, and IL-6 that play an important role in fracture healing. COX-2 = cyclooxygenase; CTGF = connective tissue growth factor; FGF = fibroblast growth factor; HO = heterotopic ossification; IL; PAR-1 = protease-activated receptor-1; TGF-β1 = transforming growth factor β1; TnC = tenascin C; VEGF = vascular endothelial growth factor. Note. From “Thrombin-stimulated growth factor and cytokine expression in osteoblasts is mediated by protease-activated receptor-1 and prostanoids,” by CN Pagel et al., 2009, Bone, 44, p.813–821. Copyright 2009, Elsevier. Reprinted with permission.
Transforming growth factor-β1
TGF-β1 is a polypeptide molecule that is rich in the bone matrix. This molecule can adjust the proliferation and differentiation of bone cells, promote new bone formation and inhibit bone resorption under certain conditions [36], [37]. TBI can increase the levels of TGF-β1 and the overexpression of its mRNA [68], [69], [70]. Ma et al. [71] found that in patients who suffered from fracture and TBI, the time for TGF-β1 expression to achieve the peak in the fracture site is synchronised with its time to reach the peak in the damaged brain and in the serum after the occurrence of TBI. Therefore, brain damage indirectly increases the content of TGF in vivo and further promotes bone growth and repair after the TGF has entered the systemic circulation through damaged blood–brain barrier. Their study also found that when TBI and fracture are concomitants, the expression of TGF-β1 in fractures is stronger than that in brain injury alone. This phenomenon indicates the importance of increased TGF-β1 after brain injury in bone healing and growth. TGF-β1 may serve to enrich the environment by which the NHO forms.
Vascular endothelial growth factor
Angiogenesis is an important step in bone formation because it increases the supply of oxygen and nutrients for bone formation and repair. VEGF plays a key role in angiogenesis. VEGF-dependent blood vessel invasion appears to be essential for coupling cartilage resorption with bone formation [72]. When TBI occurs, the following secondary pathological damage causes cerebral ischaemia and hypoxia, which upregulate the expression of hypoxia-inducible factor and finally increase the levels of VEGF in brain tissue [73]. Then, VEGF enters the fluid circulation through the blood–brain barrier, inducing angiogenesis and neurogenesis, which promote fracture healing and new bone formation. Osteoblasts, chondrocytes, and osteoclasts express VEGF receptors, and VEGF can act directly on these cells and play its role in promoting osteogenesis [38]. Wang et al. [74]found that mice overexpressing hypoxia-inducible factor-α in osteoblasts expressed high levels of VEGF and developed extremely dense and heavily vascularised long bones. Furthermore, VEGF influences other aspects of bone formation and development. In specific, it regulates bone remodelling by stimulating osteoblast differentiation [75]. Several studies showed that VEGF improved BMPs which induce bone formation and bone healing through modulation of angiogenesis [54], [76]. Mayr-Wohlfart et al. [77] proved that the proliferation of primary human osteoblasts could be stimulated by VEGF-A in a dose-dependent manner. Fiedler et al. [78]further confirmed that VEGF-A may facilitate bone formation in a direct fashion by inducing the migration of mesenchymal progenitors acting as a coupling factor for vasculogenesis and osteogenesis. Thus, the increased levels of VEGF after fracture combined with S-TBI may exert a great effect on the progress of bone healing and HO.
Effects of neuropeptide and hormones on fracture healing after brain injury
Bone tissue is rich in innervation. Many neuropeptides and hormones that exist in the nervous system exert great impact on the formation, development, and repair of bones. Brain damage increases the levels of some neurotrophic factors, which then enter the fluid circulation through the impaired blood–brain barrier and catalyse distal ectopic bone formation and fracture healing.
Calcitonin gene-related peptide
CGRP is a neuropeptide that is abundant in the sensory neurons which innervate the bone; it has been isolated from bone and shown to upregulate osteoblastic activity and downregulate osteoclastic activity [79], [80], [81]. Studies have shown that CGRP exerts a strong effect on vasodilation and can reach the fracture site through axonal transport, thereby improving the blood supply of the damaged area and accelerating fracture healing [40]. Zhang et al. [82] found that the synthesis and secretion of CGRP increase when fracture and cortical injury are concomitant compared with fracture alone. In several animal models, CGRP was shown to be required for HO development, and the neurogenic inflammation mediated by CGRP was suggested to initiate the formation of HO [83], [84]. Meanwhile, CGRP promotes the mitosis of stem cells and/or differentiation of bone cells to accelerate bone formation and inhibits bone resorption, thereby inducing HO [39].
Substance P
SP is a neuropeptide widely distributed in the nervous system [85], expressed by subsets of neurons in the central and peripheral nervous systems and also by nonneuronal cells including macrophages and T lymphocytes, cells involved at the earliest stages of preosseous fracture repair [23], [86], [87], [88]. When TBI occurs, the level of SP in the blood rises with the permeability of the blood–brain barrier increasing abnormally, thereby intensifying the inflammatory response. Inflammation is a predisposing factor of HO. In addition to immune modulation, SP stimulates the migration, proliferation, and mineralisation of mesenchymal stem cells, a cell type that is involved in HO formation, and promotes osteogenesis at the later stage of the differentiation and maturation of bone progenitor cells [41], [42]. Moreover, the SP receptor, NK1r, was demonstrated on chondrocytes, osteocytes, osteoblasts, osteoclasts and mast cells [89], [90], [91]. Kan et al. [24] have found that SP expression was upregulated in early preosseous sporadic HO and fibrodysplasia ossificans progressive (FOP) lesions and blocking SP secretion or function in the animal models prevented HO. They also tested SP expression in samples of heterotopic bone from patients with TBI and found SP upregulated in early lesions in acquired HO [24].
Leptin
Recent studies have found that levels of leptin in serum and cerebrospinal fluid (CSF) are significantly increased after TBI [92], [93]. Wang et al. [43] considered 64 male rats as experimental subjects to measure the serum levels of leptin in the sole brain injury group, sole fracture group, and fracture combined with TBI group and to elucidate its role in bone formation. Results showed that the serum levels of leptin were significantly higher in the fracture combined with TBI group than in the other groups in the first 4 and 8 weeks after surgery. The amount of callus also increased. Consistently, studies that looked at the impact of TBI on fracture healing in ob/ob mice found that leptin-deficient mice had significantly decreased fracture healing compared to wild-type mice, and application of local leptin can reverse the delay in healing efficiently [92], [94]. The signalling or long form of the leptin receptor has been found to be present in osteoblasts, chondrocytes and mesenchymal stem cells indicating that leptin may be of importance for skeletal bone growth and development [95], [96], [97]. Leptin acting on the hypothalamus can activate the sympathetic nerves and increase the β2 adrenergic receptor on the surface of osteoblasts, resulting in the inhibition of ossification [98]. When leptin acts peripherally, it not only promotes the mineralisation of bone and proliferation of osteoblasts but also inhibits the apoptosis of osteoblasts [99]. In normal circumstances, the two effects stay in a balanced state. However, direct and secondary brain damage could cause damage or dysfunction to the hypothalamus. This condition weakens the effect of ossification inhibition that is induced by leptin acting on the hypothalamus, thereby accelerating bone healing, increasing callus formation, and promoting HO [43].
Melatonin
In vertebrates, the roles of melatonin are numerous, and the biologic regulation of bone physiology is one of it [100], [101]. Melatonin can regulate calcium balance and bone metabolism, promote the proliferation and differentiation of osteoblast, suppress receptor activator for nuclear factor-κ B ligand (RANKL)-mediated osteoclast formation and stimulate the mineralisation of matrices [44], [102], [103]. In addition, melatonin is abundant in bone marrow, where precursors of bone cells are located, indicating that melatonin may act as an autacoid in bone cells [101]. Furthermore, melatonin may also impair osteoclast activity and bone resorption through its free radical scavenger and antioxidant properties [101]. When traumatic subarachnoid haemorrhage occurs due to brain damage, melatonin significantly increases in the CSF [104]. Considering the important role of melatonin in bone regulation, the increased content of melatonin caused by brain injury may also be an inducement for the fast healing of fractures and occurrence of HO in patients with bone fractures and S-TBI.
Interacting factors promoting bone formation
The cytokines, neuropeptides and hormones mentioned above not only promote bone formation on their own but also through a variety of interactions. For instance, TGF-β can enhance the osteogenic effect of BMP, promote the transcription and expression of thrombin receptor PAR-1 and enhance the promoting effects of thrombin on the proliferation of osteoblast-like cells [63]. BMP can increase the levels of platelet derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1), and VEGF [34]. In addition, the combination of VEGF and BMP4 can recruit mesenchymal stem cells to enhance cell survival and to induce cartilage formation in the early stages of endochondral bone formation [54]. The abnormal elevation of BMP can also induce the production of brown fats, and the heat production of brown fats can further stimulate sensory neurons to release SP and CGRP [58], [59]. Thrombin significantly upregulates the levels of VEGF and its receptors in osteoblasts [105]. These factors promote the expression of each other, resulting in cascade effects and jointly promoting bone growth, repair and formation.
Effect of TBI on blood–brain barrier permeability
The blood–brain barrier is a complex system consisting of brain microvascular endothelial cells, astrocyte cells, pericytes, perivascular macrophages and basement membrane. Patients with TBI often have damage and dysfunction of the blood–brain barrier [106]. The function of the blood–brain barrier significantly changes when TBI occurs. Then, the permeability of the blood–brain barrier increases. The osteogenic macromolecules within the central nervous system are released from the damaged brain tissue and act on the fracture through systemic circulation [30], [45]. The release of these factors causes further damage on the brain tissue and shows a close link to the incidence of HO.
Effect of coma and mechanical ventilation on HO
Patients with TBI more often go through a long-term coma and sustained mechanical ventilation compared with nonbrain damaged patients. Kampen et al. [107] found that TBI patients who suffered from HO usually have a more prolonged duration of coma and mechanical ventilation compared with those without HO. Although the exact link between these two factors and HO is not yet clear, prolonged mechanical ventilation might change the electrolyte and acid–base balance in the body, thereby increasing fluid pH, promoting calcium deposition and accelerating fracture healing and callus formation [46]. The occurrence of HO may also be due to the inadequate supply of local blood and oxygen that result from prolonged coma and immobilisation, which provide a good environment for HO.
Effect of inflammation on HO
Inflammation is an important factor causing HO. Certain inflammatory factors may promote bone formation while causing inflammation. For example, IL-6 can promote the differentiation of mesenchymal stem cells into bone cells, reduce the apoptosis of osteoblasts and promote angiogenesis during the repair and renewal of bones [108]. Furthermore, the activation of the immune system provides a good foundation for the occurrence of HO [83]. Brain injury is often associated with the activation of a number of immunological pathways. This phenomenon affects local and systemic immunisation and triggers the release of various inflammatory cytokines into the serum and CSF [47], [48]. Multiple inflammatory cytokines, such as increased levels of C-reactive protein, IL-6 and tumor necrosis factor-ɑ (TNF-ɑ), exist in the body of patients with TBI. As mentioned above, S-TBI can increase the levels of SP and CGRP, which recruit mast cells, thereby creating an inflammatory microenvironment that promotes HO [58].
Conclusion
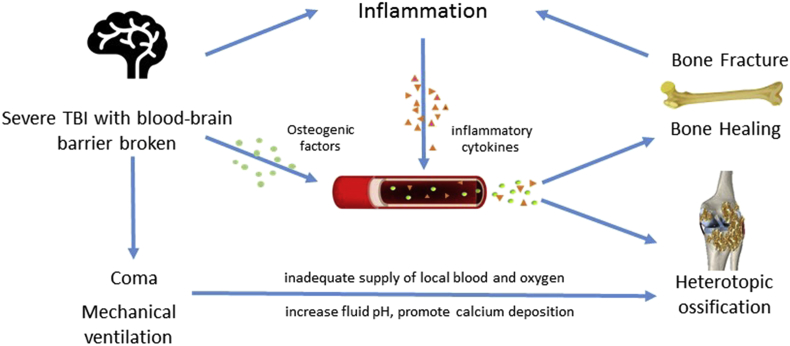
Many clinical and experimental studies have indicated that the mechanism of bone repair, growth and formation in patients who suffered from bone fracture and S-TBI is the result of the combined effects of multiple factors. First, bone fracture triggers the release of various cytokines that promote bone formation. Brain injury stimulates the body to release various cytokines when bone fractures are accompanied by S-TBI, generating an osteogenic effect. In addition, damage of the blood–brain barrier triggers the release of several osteogenic factors into the blood. Then, these factors act on the fracture sites locally through fluid circulation, promoting healing and increasing the risk of HO simultaneously. Second, the enhanced inflammatory response caused by S-TBI creates a good environment for the occurrence of HO, and the upregulation of some inflammatory factors in the body fluids accelerates bone formation. Moreover, patients who suffered from TBI are usually in a coma and need mechanical ventilation. These factors promote the incidence of HO. Overall, a close link exists between S-TBI and HO, and TBI influences many aspects of HO. Fig. 4 summarises the association between S-TBI and HO.
Figure 4.
Brain injury stimulates the body to release various cytokines when bone fractures are accompanied by S-TBI, generating an osteogenic effect. In addition, damage of the blood–brain barrier triggers the release of several osteogenic factors into the blood. Then, these factors act on the fracture sites locally through fluid circulation, promoting healing and increasing the risk of HO simultaneously. Second, the enhanced inflammatory response caused by S-TBI and bone fracture creates a good environment for the occurrence of HO, and the upregulation of some inflammatory factors in the body fluids accelerates bone formation. Moreover, patients who suffered from S-TBI are usually in a coma and need mechanical ventilation. Prolonged mechanical ventilation might change the electrolyte and acid–base balance in the body, thereby increasing fluid pH and promoting calcium deposition, and prolonged coma might result in inadequate supply of local blood and oxygen, which provide a good environment for HO. HO = heterotopic ossification; TBI = traumatic brain injury; S-TBI = severe traumatic brain injury.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Funding/support
This work was supported by the Science and Technology Innovation Fund of Shenzhen (no. JCYJ20160531174005444, JCYJ20150630114942279, GJHS20160331191611536, GJHS20150417103343353, GJHS20131212170736476, CXZZ20150529144128031), Guangdong Science and Technology Planning Project (no. 2013B050800005), Shenzhen Peacock Plan Foundation (KQCX2015033117354152) and 2015 SIAT innovative Youth Fund Project.
References
- 1.Davies O.G., Grover L.M., Eisenstein N., Lewis M.P., Liu Y. Identifying the cellular mechanisms leading to heterotopic ossification. Calcif Tissue Int. 2015;97:432–444. doi: 10.1007/s00223-015-0034-1. [DOI] [PubMed] [Google Scholar]
- 2.Adiguzel E., Uran A., Kesikburun S., Koroglu O., Demir Y., Yasar E. Knee pain relief with genicular nerve blockage in two brain injured patients with heterotopic ossification. Brain Inj. 2015;29:1736–1739. doi: 10.3109/02699052.2015.1075171. [DOI] [PubMed] [Google Scholar]
- 3.Ranganathan K., Loder S., Agarwal S., Wong V.W., Forsberg J., Davis T.A. Heterotopic ossification: basic-science principles and clinical correlates. J Bone Jt Surg Am. 2015;97:1101–1111. doi: 10.2106/JBJS.N.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Kuijk A.A., Geurts A.C., van Kuppevelt H.J. Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord. 2002;40:313–326. doi: 10.1038/sj.sc.3101309. [DOI] [PubMed] [Google Scholar]
- 5.Morgan W.E., Morgan C.P. Chiropractic care of a patient with neurogenic heterotopic ossification of the anterior longitudinal ligament after traumatic brain injury: a case report. J Chiropr Med. 2014;13:260–265. doi: 10.1016/j.jcm.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanden Bossche L., Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005;37:129–136. doi: 10.1080/16501970510027628. [DOI] [PubMed] [Google Scholar]
- 7.Roberts P. Heterotopic ossification complicating paralysis of intracranial origin. J Bone Jt Surg Br. 1968;50:70–77. [Google Scholar]
- 8.Scherbel U., Riess P., Khurana J., Born C., Delong W. Expression of bone morphogenic proteins in rats with and without brain injury and a tibia fracture. Univ Pennsylvania Orthop J. 2001;14:85–89. [Google Scholar]
- 9.Nauth A., Giles E., Potter B.K., Nesti L.J., O'Brien F.P., Bosse M.J. Heterotopic ossification in orthopaedic trauma. J Orthop Trauma. 2012;26:684–688. doi: 10.1097/BOT.0b013e3182724624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renfree K.J., Banovac K., Hornicek F.J., Lebwohl N.H., Villanueva P.A., Nedd K.J. Evaluation of serum osteoblast mitogenic activity in spinal cord and head injury patients with acute heterotopic ossification. Spine (Phila Pa 1976) 1994;19:740–746. doi: 10.1097/00007632-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Perkins R., Skirving A.P. Callus formation and the rate of healing of femoral fractures in patients with head injuries. J Bone Jt Surg Br. 1987;69:521–524. doi: 10.1302/0301-620X.69B4.3611150. [DOI] [PubMed] [Google Scholar]
- 12.Wildburger R., Zarkovic N., Egger G., Petek W., Zarkovic K., HP H. Basic fibroblast growth factor (BFGF) immunoreactivity as a possible link between head injury and impaired bone fracture healing. Bone Min. 1994;27:183–192. doi: 10.1016/s0169-6009(08)80192-4. [DOI] [PubMed] [Google Scholar]
- 13.Ausk B.J., Gross T.S., Bain S.D. Botulinum toxin-induced muscle paralysis inhibits heterotopic bone formation. Clin Orthop Relat Res. 2015;473:2825–2830. doi: 10.1007/s11999-015-4271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cadosch D., Toffoli A.M., Gautschi O.P., Frey S.P., Zellweger R., Skirving A.P. Serum after traumatic brain injury increases proliferation and supports expression of osteoblast markers in muscle cells. J Bone Jt Surg Am. 2010;92:645–653. doi: 10.2106/JBJS.I.00097. [DOI] [PubMed] [Google Scholar]
- 15.Morley J., Marsh S., Drakoulakis E., Hc Pape, Giannoudis P.V. Does traumatic brain injury result in accelerated fracture healing? Injury. 2005;36:363–368. doi: 10.1016/j.injury.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Spencer R.F. The effect of head injury on fracture healing. A quantitative assessment. J Bone Jt Surg Br. 1987;69:525–528. doi: 10.1302/0301-620X.69B4.3611151. [DOI] [PubMed] [Google Scholar]
- 17.Smith E., Comiskey C., Carroll A. Prevalence of and risk factors for osteoporosis in adults with acquired brain injury. Ir J Med Sci. 2016;185:473–481. doi: 10.1007/s11845-016-1399-5. [DOI] [PubMed] [Google Scholar]
- 18.Beaupre G.S., Lew H.L. Bone-density changes after stroke. Am J Phys Med Rehabil. 2006;85:464–472. doi: 10.1097/01.phm.0000214275.69286.7a. [DOI] [PubMed] [Google Scholar]
- 19.Brady R.D., Shultz S.R., Sun M., Romano T., van der Poel C., Wright D.K. Experimental traumatic brain injury induces bone loss in rats. J Neurotrauma. 2016;33:2154–2160. doi: 10.1089/neu.2014.3836. [DOI] [PubMed] [Google Scholar]
- 20.Yu H., Watt H., Mohan S. The negative impact of traumatic brain injury (TBI) on bone in a mouse model. Brain Inj. 2014;28:244–251. doi: 10.3109/02699052.2013.859735. [DOI] [PubMed] [Google Scholar]
- 21.Brady R.D., Grills B.L., Romano T., Wark J.D., O'Brien T.J., Shultz S.R. Sodium selenate treatment mitigates reduction of bone volume following traumatic brain injury in rats. 2016;16(4):369–376. [PMC free article] [PubMed] [Google Scholar]
- 22.Brady R.D., Grills B.L., Church J.E., Walsh N.C., McDonald A.C., Agoston D.V. Closed head experimental traumatic brain injury increases size and bone volume of callus in mice with concomitant tibial fracture. Sci Rep. 2016;6:34491. doi: 10.1038/srep34491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datar P., Srivastava S., Coutinho E., Govil G. Substance P structure, function, and therapeutics. Curr Top Med Chem. 2004;4:75–103. doi: 10.2174/1568026043451636. [DOI] [PubMed] [Google Scholar]
- 24.Kan L., Lounev V.Y., Pignolo R.J., Duan L., Liu Y., Stock S.R. Substance P signaling mediates BMP-dependent heterotopic ossification. J Cell Biochem. 2011;112:2759–2772. doi: 10.1002/jcb.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genet F., Kulina I., Vaquette C., Torossian F., Millard S., Pettit A.R. Neurological heterotopic ossification following spinal cord injury is triggered by macrophage-mediated inflammation in muscle. J Pathol. 2015;236:229–240. doi: 10.1002/path.4519. [DOI] [PubMed] [Google Scholar]
- 26.Wildburger R., Zarkovic N., Tonkovic G., Skoric T., Frech S., Hartleb M. Post-traumatic hormonal disturbances: prolactin as a link between head injury and enhanced osteogenesis. J Endocrinol Invest. 1998;21:78–86. doi: 10.1007/BF03350319. [DOI] [PubMed] [Google Scholar]
- 27.Cadosch D., Gautschi O.P., Thyer M., Song S., Skirving A.P., Filgueira L. Humoral factors enhance fracture-healing and callus formation in patients with traumatic brain injury. J Bone Jt Surg Am. 2009;91:282–288. doi: 10.2106/JBJS.G.01613. [DOI] [PubMed] [Google Scholar]
- 28.Locher R.J., Lünnemann T., Garbe A., Schaser K.D., Schmidt-Bleek K., Duda G. Traumatic brain injury and bone healing radiographic and biomechanical analyses of bone formation. J Musculoskelet Neuronal Interact. 2015;15:309–315. [PMC free article] [PubMed] [Google Scholar]
- 29.Tsitsilonis S., Seemann R., Misch M., Wichlas F., Haas N.P., Schmidt-Bleek K. The effect of traumatic brain injury on bone healing: an experimental study in a novel in vivo animal model. Injury. 2015;46:661–665. doi: 10.1016/j.injury.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 30.Bidner S.M., Rubins I.M., Desjardins J.V., Zukor D.J., Goltzman D. Evidence for a humoral mechanism for enhanced osteogenesis after head-injury. J Bone Jt Surg Am Vol. 1990;72a:1144–1149. [PubMed] [Google Scholar]
- 31.Klein B.Y., Shohami E., Reikhinshtein Y., Ben-Bassat H., Liebergall M. Serum-mediated osteogenic effects of head injury on cultured rat marrow stromal cells. Calcif Tissue Int. 1999;65:217–222. doi: 10.1007/s002239900686. [DOI] [PubMed] [Google Scholar]
- 32.Boes M., Kain M., Kakar S., Nicholls F., Cullinane D., Gerstenfeld L. Osteogenic effects of traumatic brain injury on experimental fracture-healing. J Bone Jt Surg Am. 2006;88:738–743. doi: 10.2106/JBJS.D.02648. [DOI] [PubMed] [Google Scholar]
- 33.Wozney J.M. The bone morphogenetic protein family and osteogenesis. Mol Reprod Dev. 1992;32:160–167. doi: 10.1002/mrd.1080320212. [DOI] [PubMed] [Google Scholar]
- 34.Scarfi S. Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World J Stem Cells. 2016;8:1–12. doi: 10.4252/wjsc.v8.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagel C.N., de Niese M.R., Abraham L.A., Chinni C., Song S.J., Pike R.N. Inhibition of osteoblast apoptosis by thrombin. Bone. 2003;33:733–743. doi: 10.1016/s8756-3282(03)00209-6. [DOI] [PubMed] [Google Scholar]
- 36.Pfeilschifter J., Seyedin S.M., Mundy G.R. Transforming growth factor beta inhibits bone resorption in fetal rat long bone cultures. J Clin Invest. 1988;82:680–685. doi: 10.1172/JCI113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noda M., Camilliere J.J. In vivo stimulation of bone formation by transforming growth factor-beta. Endocrinology. 1989;124:2991–2994. doi: 10.1210/endo-124-6-2991. [DOI] [PubMed] [Google Scholar]
- 38.Maes C., Goossens S., Bartunkova S., Drogat B., Coenegrachts L., Stockmans I. Increased skeletal VEGF enhances beta-catenin activity and results in excessively ossified bones. EMBO J. 2010;29:424–441. doi: 10.1038/emboj.2009.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shih C., Bernard G.W. Calcitonin gene related peptide enhances bone colony development in vitro. Clin Orthop Relat Res. 1997:335–344. [PubMed] [Google Scholar]
- 40.Lerner U.H., Persson E. Osteotropic effects by the neuropeptides calcitonin gene-related peptide, substance P and vasoactive intestinal peptide. J Musculoskelet Neuronal Interact. 2008;8:154–165. [PubMed] [Google Scholar]
- 41.Hong H.S., Lee J., Lee E., Kwon Y.S., Lee E., Ahn W. A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells. Nat Med. 2009;15:425–435. doi: 10.1038/nm.1909. [DOI] [PubMed] [Google Scholar]
- 42.Wang L., Zhao R., Shi X., Wei T., Halloran B.P., Clark D.J. Substance P stimulates bone marrow stromal cell osteogenic activity, osteoclast differentiation, and resorption activity in vitro. Bone. 2009;45:309–320. doi: 10.1016/j.bone.2009.04.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L., Yuan J.S., Zhang H.X., Ding H., Tang X.G., Wei Y.Z. Effect of leptin on bone metabolism in rat model of traumatic brain injury and femoral fracture. Chin J Traumatol. 2011;14:7–13. [PubMed] [Google Scholar]
- 44.Kesemenli C.C., Necmioglu S. The role of melatonin as a link between head injury and enhanced osteogenesis. Med Hypotheses. 2005;65:605–606. doi: 10.1016/j.mehy.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 45.Başkaya M.K., Muralikrishna Rao A., Doğan A., Donaldson D., Dempsey R.J. The biphasic opening of the blood–brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci Lett. 1997;226:33–36. doi: 10.1016/s0304-3940(97)00239-5. [DOI] [PubMed] [Google Scholar]
- 46.Sakellariou V.I., Grigoriou E., Mavrogenis A.F., Soucacos P.N., Papagelopoulos P.J. Heterotopic ossification following traumatic brain injury and spinal cord injury: insight into the etiology and pathophysiology. J Musculoskelet Neuronal Interact. 2012;12:230–240. [PubMed] [Google Scholar]
- 47.Morganti-Kossmann M.C., Hans V.H.J., Lenzlinger P.M., Dubs R., Ludwig E., Trentz O. TGF-beta is elevated in the CSF of patients with severe traumatic brain injuries and parallels blood–brain barrier function. J Neurotrauma. 1999;16:617–628. doi: 10.1089/neu.1999.16.617. [DOI] [PubMed] [Google Scholar]
- 48.Hayakata T., Shiozaki T., Tasaki O., Ikegawa H., Inoue Y., Toshiyuki F. Changes in Csf S100b and cytokine concentrations in early-phase severe traumatic brain injury. Shock. 2004;22:102–107. doi: 10.1097/01.shk.0000131193.80038.f1. [DOI] [PubMed] [Google Scholar]
- 49.Yang T.Y., Wang T.C., Tsai Y.H., Huang K.C. The effects of an injury to the brain on bone healing and callus formation in young adults with fractures of the femoral shaft. J Bone Jt Surg Br. 2012;94:227–230. doi: 10.1302/0301-620X.94B2.28193. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y.G., Lu S.B., Wang J.F. Osteoconduction and osteoinduction. Chin J Traumatol. 1996;12:332–333. [Google Scholar]
- 51.Wang E.A., Rosen V., D'Alessandro J.S., Bauduy M., Cordes P., Harada T. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990;87:2220–2224. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang Y.C., Kaigler D., Rice K.G., Krebsbach P.H., Mooney D.J. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res Off J Am Soc Bone Miner Res. 2005;20:848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y., He T., Liu J., Liu H., Zhou L., Hao W. Synergistic effects of overexpression of BMP2 and TGFbeta3 on osteogenic differentiation of bone marrow mesenchymal stem cells. Mol Med Rep. 2016;14:5514–5520. doi: 10.3892/mmr.2016.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng H., Wright V., Usas A., Gearhart B., Shen H.-C., Cummins J. Synergistic enhancement of bone formation and healing by stem cell–expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewen A., Soderstrom S., Hillered L., Ebendal T. Expression of serine/threonine kinase receptors in traumatic brain injury. Neuroreport. 1997;8:475–479. doi: 10.1097/00001756-199701200-00020. [DOI] [PubMed] [Google Scholar]
- 56.Guo Q., Zhang L. Effect of brain injury on expression of bone morphogenetic protein 2 in fracture healing process. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi Zhongguo Xiufu Chongjian Waike Zazhi Chin J Reparative Reconstr Surg. 2007;21:1040–1044. [PubMed] [Google Scholar]
- 57.Bucelli R.C., Gonsiorek E.A., Kim W.Y., Bruun D., Rabin R.A., Higgins D. Statins decrease expression of the proinflammatory neuropeptides calcitonin gene-related peptide and substance P in sensory neurons. J Pharmacol Exp Ther. 2008;324:1172–1180. doi: 10.1124/jpet.107.132795. [DOI] [PubMed] [Google Scholar]
- 58.Salisbury E., Rodenberg E., Sonnet C., Hipp J., Gannon F.H., Vadakkan T.J. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem. 2011;112:2748–2758. doi: 10.1002/jcb.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olmsted-Davis E., Gannon F.H., Ozen M., Ittmann M.M., Gugala Z., Hipp J.A. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol. 2007;170:620–632. doi: 10.2353/ajpath.2007.060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salehpour F., Bazzazi A.M., Porhomayon J., Nader N.D. Correlation between coagulopathy and outcome in severe head trauma in neurointensive care and trauma units. J Crit Care. 2011;26:352–356. doi: 10.1016/j.jcrc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 61.Xi G., Reiser G., Keep R.F. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? J Neurochem. 2003;84:3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- 62.Vandersande J.J., Boekhoutmussert R.J., Bouwhuishoogerwerf M.L., Veltkamp J.J. Head-injury and coagulation disorders. J Neurosurg. 1978;49:357–365. doi: 10.3171/jns.1978.49.3.0357. [DOI] [PubMed] [Google Scholar]
- 63.Abraham L.A., MacKie E.J. Modulation of osteoblast-like cell behavior by activation of protease-activated receptor-1. J Bone Miner Res Off J Am Soc Bone Miner Res. 1999;14:1320–1329. doi: 10.1359/jbmr.1999.14.8.1320. [DOI] [PubMed] [Google Scholar]
- 64.Pagel C.N., Song S.J., Loh L.H., Tudor E.M., Murray-Rust T.A., Pike R.N. Thrombin-stimulated growth factor and cytokine expression in osteoblasts is mediated by protease-activated receptor-1 and prostanoids. Bone. 2009;44:813–821. doi: 10.1016/j.bone.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 65.Wang H., Li X., Tomin E., Doty S.B., Lane J.M., Carney D.H. Thrombin peptide (TP508) promotes fracture repair by up-regulating inflammatory mediators, early growth factors, and increasing angiogenesis. J Orthop Res Off Publ Orthop Res Soc. 2005;23:671–679. doi: 10.1016/j.orthres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Karp J.M., Tanaka T.S., Zohar R., Sodek J., Shoichet M.S., Davies J.E. Thrombin mediated migration of osteogenic cells. Bone. 2005;37:337–348. doi: 10.1016/j.bone.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 67.Pu J.S., Yang T.F. Thrombin: a potential regulator between head injury and enhanced osteogenesis. Biosci Hypotheses. 2009;2:326–328. [Google Scholar]
- 68.Lindholm D., Castren E., Kiefer R., Zafra F., Thoenen H. Transforming growth factor-beta 1 in the rat brain: increase after injury and inhibition of astrocyte proliferation. J Cell Biol. 1992;117:395–400. doi: 10.1083/jcb.117.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krupinski J., Kumar P., Kumar S., Kaluza J. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke. 1996;27:852–857. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- 70.Logan A., Frautschy S.A., Gonzalez A.-M., Sporn M.B., Baird A. Enhanced expression of transforming growth factor β1 in the rat brain after a localized cerebral injury. Brain Res. 1992;587:216–225. doi: 10.1016/0006-8993(92)91000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma W., Niu Y.P. Serum content and expression of transforming growth factor beta 1 at fracture site of rats combined with traumatic brain injury in fracture healing. Chin J Clin Rehabil. 2006;10:88–91. [Google Scholar]
- 72.Gerber H.P., Vu T.H., Ryan A.M., Kowalski J., Werb Z., Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 73.Merrill M.J., Oldfield E.H. A reassessment of vascular endothelial growth factor in central nervous system pathology. J Neurosurg. 2005;103:853–868. doi: 10.3171/jns.2005.103.5.0853. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y., Wan C., Deng L., Liu X., Cao X., Gilbert S.R. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y.Q., Tan Y.Y., Wong R., Wenden A., Zhang L.K., Rabie A.B. The role of vascular endothelial growth factor in ossification. Int J Oral Sci. 2012;4:64–68. doi: 10.1038/ijos.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng H., Usas A., Olshanski A., Am Ho, Gearhart B., Gm Cooper. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res Off J Am Soc Bone Miner Res. 2005;20:2017–2027. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 77.Mayr-Wohlfart U., Waltenberger J., Hausser H., Kessler S., Gunther K.P., Dehio C. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30:472–477. doi: 10.1016/s8756-3282(01)00690-1. [DOI] [PubMed] [Google Scholar]
- 78.Fiedler J., Leucht F., Waltenberger J., Dehio C., Brenner R.E. VEGF-A and PlGF-1 stimulate chemotactic migration of human mesenchymal progenitor cells. Biochem Biophys Res Commun. 2005;334:561–568. doi: 10.1016/j.bbrc.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 79.Sample S.J., Hao Z., Wilson A.P., Muir P. Role of calcitonin gene-related peptide in bone repair after cyclic fatigue loading. PLoS One. 2011;6:1–10. doi: 10.1371/journal.pone.0020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sullivan M.P., Torres S.J., Mehta S., Ahn J. Heterotopic ossification after central nervous system trauma: a current review. Bone Jt Res. 2013;2:51–57. doi: 10.1302/2046-3758.23.2000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Naot D., Cornish J. The role of peptides and receptors of the calcitonin family in the regulation of bone metabolism. Bone. 2008;43:813–818. doi: 10.1016/j.bone.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 82.Zhang D., Zhang P., Wang Y., Han N., Tang C., Jiang B. The influence of brain injury or peripheral nerve injury on calcitonin gene-related peptide concentration variation and fractures healing process. Artif Cells Blood Substit Immobil Biotechnol. 2009;37:85–91. doi: 10.1080/10731190902743149. [DOI] [PubMed] [Google Scholar]
- 83.Convente M.R., Wang H., Pignolo R.J., Kaplan F.S., Shore E.M. The immunological contribution to heterotopic ossification disorders. Curr Osteoporos Rep. 2015;13:116–124. doi: 10.1007/s11914-015-0258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lazard Z.W., Olmsted-Davis E.A., Salisbury E.A., Gugala Z., Sonnet C., Davis E.L. Osteoblasts have a neural origin in heterotopic ossification. Clin Orthop Relat Res. 2015;473:2790–2806. doi: 10.1007/s11999-015-4323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lorente L., Martin M.M., Almeida T., Hernandez M., Ramos L., Argueso M. Serum substance P levels are associated with severity and mortality in patients with severe traumatic brain injury. Crit Care. 2015;19:192. doi: 10.1186/s13054-015-0911-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barbut D., Polak J.M., Wall P.D. Substance P in spinal cord dorsal horn decreases following peripheral nerve injury. Brain Res. 1981;205:289–298. doi: 10.1016/0006-8993(81)90340-1. [DOI] [PubMed] [Google Scholar]
- 87.Pinto F.M., Almeida T.A., Hernandez M., Devillier P., Advenier C., Candenas M.L. mRNA expression of tachykinins and tachykinin receptors in different human tissues. Eur J Pharmacol. 2004;494:233–239. doi: 10.1016/j.ejphar.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 88.Nelson Daniel A., Bost K.L. Non-neuronal mammalian tachykinin expression. Front Biosci. 2004;September;1:2166–2176. doi: 10.2741/1372. [DOI] [PubMed] [Google Scholar]
- 89.Millward-Sadler S.J., Mackenzie A., Wright M.O., Lee H.S., Elliot K., Gerrard L. Tachykinin expression in cartilage and function in human articular chondrocyte mechanotransduction. Arthritis Rheum. 2003;48:146–156. doi: 10.1002/art.10711. [DOI] [PubMed] [Google Scholar]
- 90.Goto T., Yamaza T., Kido M.A., Tanaka T. Light- and electron-microscopic study of the distribution of axons containing substance P and the localization of neurokinin-1 receptor in bone. Cell Tissue Res. 1998;293:87–93. doi: 10.1007/s004410051100. [DOI] [PubMed] [Google Scholar]
- 91.Okada T., Hirayama Y., Kishi S., Miyayasu K., Hiroi J., Fujii T. Functional neurokinin NK-1 receptor expression in rat peritoneal mast cells. Inflamm Res Off J Eur Histamine Res Soc [et al] 1999;48:274–279. doi: 10.1007/s000110050459. [DOI] [PubMed] [Google Scholar]
- 92.Khan S.N., DuRaine G., Ss Virk, Fung J., Dj Rowland, Ah Reddi. The temporal role of leptin within fracture healing and the effect of local application of recombinant leptin on fracture healing. J Orthop Trauma. 2013;27:656–662. doi: 10.1097/BOT.0b013e3182847968. [DOI] [PubMed] [Google Scholar]
- 93.Yan H., Zhang H.W., Fu P., Liu B.L., Jin W.Z., Duan S.B. Leptin's effect on accelerated fracture healing after traumatic brain injury. Neurol Res. 2013;35:537–544. doi: 10.1179/1743132813Y.0000000201. [DOI] [PubMed] [Google Scholar]
- 94.Graef F., Seemann R., Garbe A., Schmidt-Bleek K., Schaser K.D., Keller J. Impaired fracture healing with high non-union rates remains irreversible after traumatic brain injury in leptin-deficient mice. J Musculoskelet Neuronal Interact. 2017;17:78–85. [PMC free article] [PubMed] [Google Scholar]
- 95.Steppan C.M., Crawford D.T., Chidsey-Frink K.L., Ke H., Swick A.G. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92:73–78. doi: 10.1016/s0167-0115(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 96.Hess R., Pino A.M., Rios S., Fernandez M., Rodriguez J.P. High affinity leptin receptors are present in human mesenchymal stem cells (MSCs) derived from control and osteoporotic donors. J Cell Biochem. 2005;94:50–57. doi: 10.1002/jcb.20330. [DOI] [PubMed] [Google Scholar]
- 97.Reseland J.E., Syversen U., Bakke I., Qvigstad G., Lg Eide, Hjertner O. Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J Bone Min Res. 2001;16:1426–1433. doi: 10.1359/jbmr.2001.16.8.1426. [DOI] [PubMed] [Google Scholar]
- 98.Takeda S., Elefteriou F., Levasseur R., Liu X., Zhao L., Parker K.L. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 99.Wang L., Tang X., Zhang H., Yuan J., Ding H., Wei Y. Elevated leptin expression in rat model of traumatic spinal cord injury and femoral fracture. J Spinal Cord Med. 2011;34:501–509. doi: 10.1179/2045772311Y.0000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu J., Huang F., He H.W. Melatonin effects on hard tissues: bone and tooth. Int J Mol Sci. 2013;14:10063–10074. doi: 10.3390/ijms140510063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cardinali D.P., Ladizesky M.G., Boggio V., Cutrera R.A., Mautalen C. Melatonin effects on bone: experimental facts and clinical perspectives. J Pineal Res. 2003;34:81–87. doi: 10.1034/j.1600-079x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 102.Taylor A.C., Horvat-Gordon M., Moore A., Bartell P.A. The effects of melatonin on the physical properties of bones and egg shells in the laying hen. PLoS One. 2013;8:e55663. doi: 10.1371/journal.pone.0055663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakade O., Koyama H., Ariji H., Yajima A., Kaku T. Melatonin stimulates proliferation and type I collagen synthesis in human bone cells in vitro. J Pineal Res. 1999;27:106–110. doi: 10.1111/j.1600-079x.1999.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 104.Aiu M., Kiselev V.N., Loboda E.B. Cerebrospinal fluid melatonin in diseases of the nervous system. Zh Nevropatol Psikhiatr Im S S Korsakova. 1977;77:1814–1816. [PubMed] [Google Scholar]
- 105.Bluteau G., Pilet P., Bourges X., Bilban M., Spaethe R., Daculsi G. The modulation of gene expression in osteoblasts by thrombin coated on biphasic calcium phosphate ceramic. Biomaterials. 2006;27:2934–2943. doi: 10.1016/j.biomaterials.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 106.Shlosberg D., Benifla M., Kaufer D., Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van Kampen P.J., Martina J.D., Vos P.E., Hoedemaekers C.W., Hendricks H.T. Potential risk factors for developing heterotopic ossification in patients with severe traumatic brain injury. J Head Trauma Rehabil. 2011;26:384–391. doi: 10.1097/HTR.0b013e3181f78a59. [DOI] [PubMed] [Google Scholar]
- 108.Evans K.N., Forsberg J.A., Potter B.K., Hawksworth J.S., Brown T.S., Andersen R. Inflammatory cytokine and chemokine expression is associated with heterotopic ossification in high-energy penetrating war injuries. J Orthop Trauma. 2012;26:e204. doi: 10.1097/BOT.0b013e31825d60a5. [DOI] [PubMed] [Google Scholar]