Significance
Lignin valorization is critical for economic viability of future biorefineries but is hindered due to the challenges of engineered bio-chassis such as the slow kinetics of substrate uptake, aromatics toxicity, and cost. Here, an autoregulatory system involving a vanillin autoinducible promoter is demonstrated with an aromatics transporter in Escherichia coli that is induced by lignin-derived aromatics and simultaneously converted to value-added platform chemical with diverse applications. In addition to alleviating toxicity, the engineered E. coli strain eliminates the need for an external inducer such as isopropyl β-d-1-thiogalactopyranoside during fermentation, thereby significantly reducing the process cost. This study demonstrates an autoregulatory system for aromatics bioconversion and serves as a platform for future strain development for lignin valorization.
Keywords: lignin valorization, vanillin-inducible promoter, aromatics transporter, autoregulatory, catechol
Abstract
Efficient lignin valorization could add more than 10-fold the value gained from burning it for energy and is critical for economic viability of future biorefineries. However, lignin-derived aromatics from biomass pretreatment are known to be potent fermentation inhibitors in microbial production of fuels and other value-added chemicals. In addition, isopropyl-β-d-1-thiogalactopyranoside and other inducers are routinely added into fermentation broth to induce the expression of pathway enzymes, which further adds to the overall process cost. An autoregulatory system that can diminish the aromatics’ toxicity as well as be substrate-inducible can be the key for successful integration of lignin valorization into future lignocellulosic biorefineries. Toward that goal, in this study an autoregulatory system is demonstrated that alleviates the toxicity issue and eliminates the cost of an external inducer. Specifically, this system is composed of a catechol biosynthesis pathway coexpressed with an active aromatic transporter CouP under induction by a vanillin self-inducible promoter, ADH7, to effectively convert the lignin-derived aromatics into value-added chemicals using Escherichia coli as a host. The constructed autoregulatory system can efficiently transport vanillin across the cell membrane and convert it to catechol. Compared with the system without CouP expression, the expression of catechol biosynthesis pathway with transporter CouP significantly improved the catechol yields about 30% and 40% under promoter pTrc and ADH7, respectively. This study demonstrated an aromatic-induced autoregulatory system that enabled conversion of lignin-derived aromatics into catechol without the addition of any costly, external inducers, providing a promising and economically viable route for lignin valorization.
Lignin is a vast but underutilized renewable resource which is an amorphous, highly branched heteropolymer composed of phenylpropanoid units, accounting for 18–40% of plant cell walls on a weight basis (1–3). Lignin is the only source of renewable aromatics on Earth. Effective lignin valorization would yield more than 10-fold added value than just burning it for energy production (4). However, lignin is highly resistant to microbial as well as chemical attack because it contains phenylpropanoid units cross-linked via C–C and ether C–O bonds (5). Recent advances in synthetic biology (SynBio) are starting to enable engineering of new biosynthetic routes for lignin valorization to produce value-added chemicals using versatile and efficient microbial factories (4, 6–8). In the natural environment, the biodegradation of lignin occurs through a mixed population of microorganisms such as some white- and/or brown-rot fungi and proteo- and actinobacteria that synergistically break down lignin (9, 10). However, slow growth, difficulty with cultivation, and the low activity of ligninolytic enzymes secreted by wild-type ligninolytic strains pose a challenge for their utilization without strain engineering. The extremely slow kinetics of microbial lignin depolymerization and their poor environmental adaptability makes most naturally existing ligninolytic microbes unsuitable to be used in biorefineries for lignin valorization (8, 11). In fact, the ligninolysis of wood chips by white-rot fungi takes several weeks to months to achieve quantifiable significant results (12) and it is difficult to realize industrial production of ligninolytic enzymes. Therefore, robust engineered microbes or microbial consortia that can effectively depolymerize lignin are highly desired. Escherichia coli as a cell factory is well-established due to its unparalleled fast growth and readily available genetic tools for gene manipulation. However, when engineering E. coli as the chassis for lignin valorization, a few issues must be overcome first: (i) the absence of an effective transport system in E. coli to uptake aromatic compounds, (ii) the aromatics to be valorized are themselves potent growth and fermentation inhibitors and, (iii) the overall process cost needs to be reduced to improve the economic viability and to be cost-competitive with petroleum routes of chemical synthesis. To solve these challenging bottlenecks and improve the conversion efficiency of lignin valorization in the industrially robust E. coli, elegant biotechnological solutions are highly desired.
One of most commonly used expression systems in E. coli is the inducible expression system that relies on the T7 promoter, where the induction of protein expression is triggered by the addition of isopropyl-β-d-1-thiogalactopyranoside (IPTG) as the most effective inducer. However, the optical density of the culture needs to be monitored to ensure that IPTG is added at the optimal cell density to induce expression. The addition of IPTG to medium also imparts potential toxicity to the cells (13). Moreover, the high cost of IPTG that must to be added to the medium during fermentation would limit large-scale industrial utilization for low-cost fuel and chemical production in future biorefineries. Reducing the cost is especially important for developing a cost-effective lignin valorization platform. Moreover, lignin-derived aromatic compounds are commonly present in the hydrolysate of pretreated lignocellulosic biomass. Several studies have reported that the lignin depolymerization products such as vanillin have an inhibitory effect on enzyme activity and cell growth (14–17). Therefore, an autoregulatory system utilizing an aromatics-inducible promoter can diminish the toxicity issue to some extent and circumvent the addition of inducers and therefore overcome the above-mentioned limitations of cost and toxicity. Incorporating such capability into microbial cell factories enables the development of a “smart” and low-cost lignin valorization platform. In such an autoregulatory system the lignin-derived aromatics can be used both as the substrate and the inducer. The engineered cells would then be responsive to the lignin substrate and automatically overexpress the corresponding enzymes to convert the substrates to value-added compounds when the substrates are present and sensed by the microbes. In a recent study, the ADH7 promoter of Saccharomyces cerevisiae was found to be vanillin-inducible and able to induce protein synthesis even under severe vanillin stress (18). We therefore envision that this ADH7 promoter could potentially be suitable for the construction of an autoregulatory system for vanillin bioconversion, creating a smart SynBio chassis that enables both lignin valorization and a check on toxicity by keeping the concentrations of the substrate low.
For overcoming the second challenge of slow kinetics for aromatics uptake, one potential approach could be to increase the uptake of aromatic substrate by incorporating a transporter for aromatic compounds, thereby improving substrate availability inside the cell. Previous successful examples of microbial systems that apply nonnative transporters include the enhanced accumulation of arsenic in engineered E. coli and S. cerevisiae as “arsenic biosorbents” by overexpressing transporter proteins specifically targeting arsenite (19, 20). Recently, a transporter (CouP) that has high binding affinity to a range of phenylpropanoid ligands with Kd values in the nanomolar range has been identified from the purple photosynthetic bacterium Rhodopseudomonas palustris and characterized by Salmon et al. (6). The potential ability of CouP in the active transport of aromatic substrates makes it an ideal candidate to be incorporated into the engineered E. coli system for more efficient lignin valorization.
In this study, a synthetic pathway based on vanillin degradation (LigV and LigM) from the aromatic-catabolizing bacterium Sphingomonas paucimobilis SKY-6 (21) and a protocatechuate decarboxylase (aroY) (22) has been reconstructed in an engineered E. coli strain to covert a lignin-derived aromatic vanillin to catechol (Fig. 1). Catechol has been identified as a powerful and versatile building block for the synthesis of a wide range of polymeric materials with interesting structures, properties, and functions as well as high-value chemicals (23). In this synthetic pathway, vanillin is transformed by the vanillin dehydrogenase (LigV) to vanillate followed by the conversion of vanillate-O-demethylase (LigM) into protocatechuate, which is further converted into catechol by protocatechuate decarboxylase (aroY) (Fig. 1). We demonstrate here a smart, autoinducible, and efficient system for vanillin bioconversion by utilizing the vanillin-inducible promoter ADH7. We have realized the self-regulation of E. coli for the production of vanillic acid and catechol from lignin-derived aromatics using vanillin as an example. We further integrated an engineering strategy of expression of the aromatics transporter CouP in this engineered E. coli host. The resulting cell factory exhibits significantly improved yield because of the increased substrate availability by transporting the aromatics substrate inside the cells. The synthetic pathway was spontaneously turned on for vanillin bioconversion when the aromatic substrate was sensed by the engineered E. coli, thereby (i) eliminating the need for expensive external inducers and (ii) keeping a check on the level of fermentation inhibitor by turning the pathway on and bioconverting it to a less-toxic intermediate. This efficient and cost-effective cell factory system could prove to be valuable for lignin conversion and valorization and benefit future lignocellulosic biorefineries and a bio-based economy.
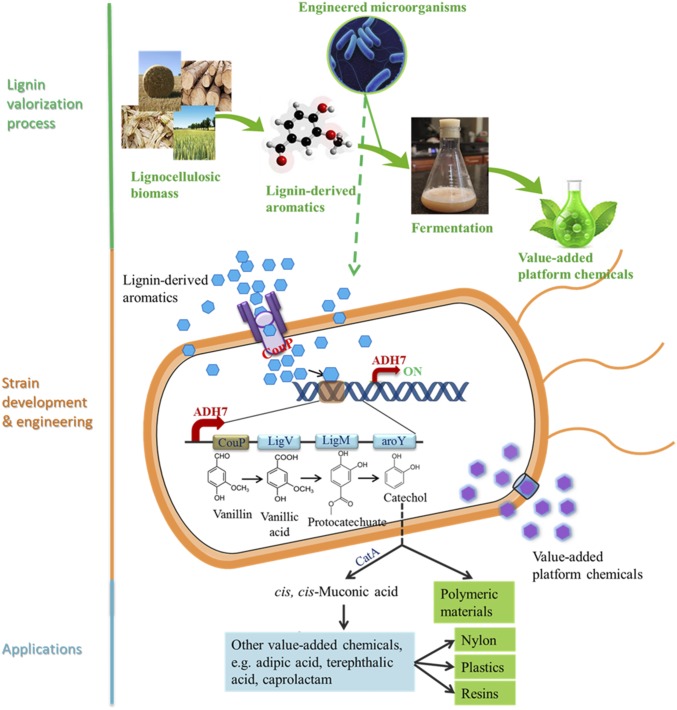
Fig. 1.
Schematic presentation of an autoregulatory microbial system for valorizing lignin-derived aromatics into value-added chemicals. A catechol biosynthesis pathway coexpressed with an aromatic transporter CouP under the induction of vanillin-inducible promoter ADH7. The transporter (blue) represents other unknown aromatics transporters.
Results
The Growth Inhibition of Vanillin, Vanillic Acid, and Catechol on E. coli Strain DH1.
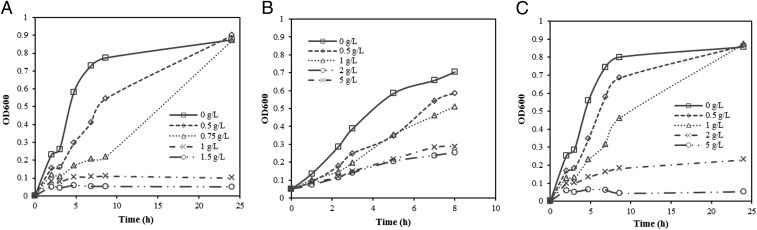
Microbial fermentation is inhibited by the presence of a small amount of aromatics derived from lignin from lignocellulosic biomass pretreatment, such as vanillin and phenols (14, 15). To investigate the inhibitory effects of aromatics on the growth of the E. coli strain DH1 in this study and select the optimum concentration of aromatics as the substrate, a range of concentrations of vanillin, vanillic acid, and catechol were added into the fermentation broths. As shown in Fig. 2, vanillic acid has the highest growth inhibition on the strain DH1, and growth was totally inhibited at the concentration of 1.5 g/L vanillic acid in the fermentation broth (Fig. 2A). While catechol has the least growth inhibition on strain DH1, the cells still exhibited about half of the growth rate at a concentration of 5 g/L, compared with when no catechol was added in the fermentation broth, as shown in the Fig. 2B. Vanillin displayed medium growth inhibition on strain DH1, and growth was completely inhibited at the concentration of 5 g/L vanillin in the fermentation broth, as shown in Fig. 2C. The strain still exhibited about 80% growth rate at 0.5 g/L of vanillin, which, therefore, was selected as the substrate concentration in the following study.
Fig. 2.
Growth inhibition of E. coli due to the presence of lignin-derived aromatics. E. coli strain DH1 is selected as an expression host in this study, which is a derivative of strain MM294 with mutated alleles recA1 and gyrA96 to increase the expression plasmid stability. E. coli DH1 growth curves in the presence of different concentrations of (A) vanillic acid, (B) catechol, and (C) vanillin.
Self-Induced Production of Vanillic Acid and Catechol Under a Vanillin-Inducible Promoter.
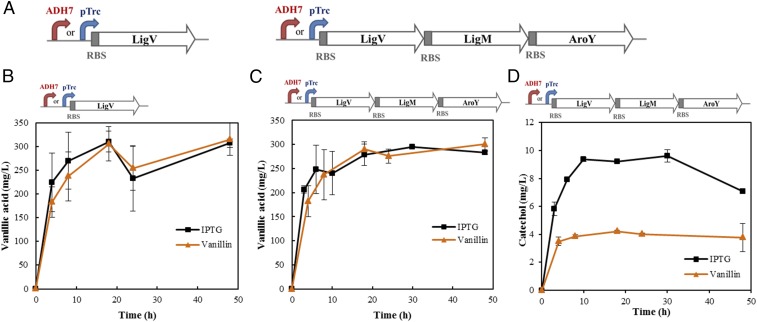
In our previous study we demonstrated the bioconversion of vanillin into catechol and cis, cis-muconic acid with an engineered E. coli strain expressing a synthetic pathway under the IPTG-inducible promoter (8). The relatively high cost of IPTG is prohibitive and makes it unfeasible as an inducer, especially in microbial fermentation at industrial scales. Therefore, in this study we conceptualized a vanillin autoregulatory system to improve the process economics of vanillin bioconversion through expression of the synthetic pathway under a vanillin-inducible promoter, ADH7 (18). A single vanillin dehydrogenase (LigV) (24) and the catechol biosynthesis pathway were both expressed under the ADH7 promoter and pTrc promoter (Fig. 3A), respectively, to investigate the promoter’s induction ability and to compare the induction strength. The details of the ligV, ligM, and aroY genes used in this study are provided in Table 1. As shown in Fig. 3B, both strains expressing LigV under the pTrc and ADH7 promoter yielded a significant amount of vanillic acid in the fermentation broth, indicating that the ADH7 promoter enables effective protein synthesis under the 0.5 g/L of vanillin induction and the bioconversion of vanillin into vanillic acid. Further expression of 3-O-methylgallate-O-demethylase (LigM) and protocatechuate decarboxylase (AroY) under ADH7 promoter resulted in the production of catechol from vanillin (Fig. 3D), but at a relatively lower level. These results successfully validate our concept that an autoregulatory engineered E. coli chassis expressing the catechol biosynthesis pathway under a vanillin-inducible promoter can effectively utilize vanillin not only as an inducer but as a substrate for the bioconversion of vanillin to catechol without the need for IPTG induction.
Fig. 3.
Self-inducible production of vanillic acid and catechol under a vanillin-inducible promoter. (A) Schematic of constructs for the expression of LigV/catechol biosynthesis pathway under promoter ADH7 (vanillin) or pTrc (IPTG). (B) Vanillic acid production from the strains expressing the enzyme LigV under promoter ADH7 (vanillin) or pTrc (IPTG). (C) Vanillic acid and (D) catechol production from the strains expressing the catechol biosynthesis pathway (LigV, LigM, and aroY) under promoter ADH7 (vanillin) or pTrc (IPTG).
Table 1.
Genes used in this study
| Gene | GenBank accession no. | Amino acids | Function | Source |
| ligV | KX774254 | 480 | Vanillin dehydrogenase | Sphingobium sp. SYK-6 |
| ligM | KX774255 | 471 | 3-O-methylgallate-O-demethylase | Sphingobium sp. SYK-6 |
| aroY | KX774258 | 502 | Protocatechuate decarboxylase | Klebsiella pneumonia subsp. |
| CouP | 4JB2_A | 370 | Coumarate transporter | R. palustris |
The strain expressing vanillin dehydrogenase LigV under ADH7 promoter produced lesser amount of vanillic acid than that produced from the strain expressing LigV under pTrc promoter, suggesting that pTrc promoter may have stronger strength than that of vanillin-inducible promoter ADH7. This premise was confirmed through the expression of the catechol biosynthesis pathway under ADH7 and pTrc promoter, respectively. The strain expressing the catechol biosynthesis pathway under pTrc promoter produced twice the amount of catechol of that produced under ADH7 promoter, which verified the weaker strength of ADH7 than pTrc promoter in terms of driving the expression of multiple pathway genes, as shown in Fig. 3D.
In addition, the lower production titer of vanillic acid in the early stage of induction (Fig. 3 B and C) may indicate that either ADH7 promoter needs longer response time to the inducer vanillin or that vanillin has slower up-take rate than that of the glucose analog IPTG, which may necessitate the expression of effective aromatics transporters in the E. coli strain DH1 for enhanced bioconversion of vanillin.
Yield Improvement of Vanillic Acid and Catechol with the Expression of Aromatics Transporter CouP.
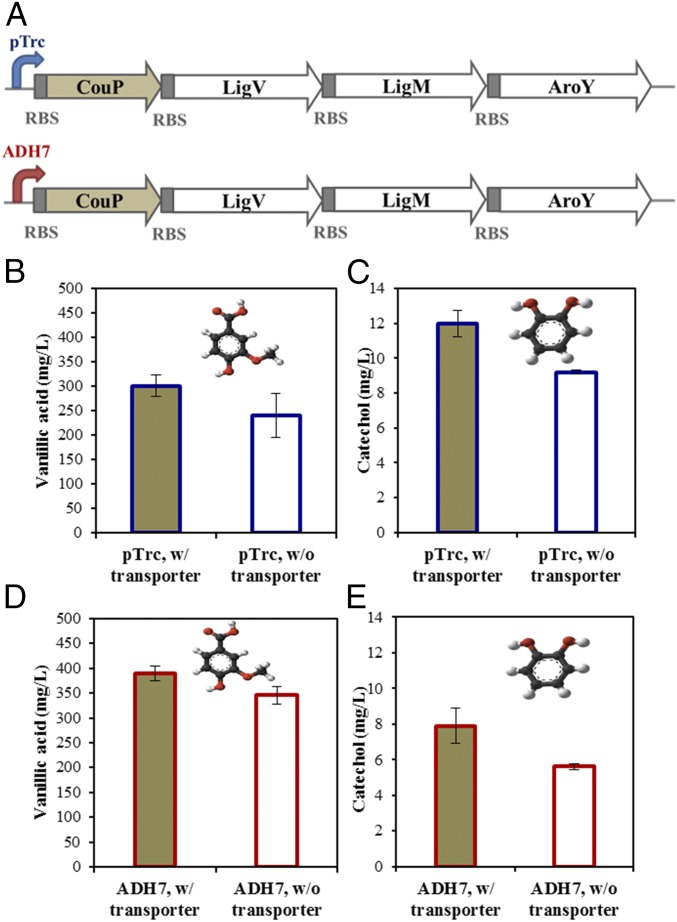
Microbial valorization of lignin-derived aromatics requires the efficient transport of these lignin-derived aromatic substrates across the microbial host-cell membrane. The aromatic compounds (particularly the lipophilic aromatics) can enter the cells by passive diffusion at high concentrations in the media (millimolar range), but the active aromatics transporter increases the efficiency and rate of the aromatic substrate acquisition and conversion (6, 25). In recent studies, several aromatic transporters from non-E. coli bacteria have been identified and demonstrated the ability to transport lignin-derived aromatics and other aromatic hydrocarbons (6, 26). A periplasmic binding protein, CouP, of an ABC system from R. palustris has been implicated as an active transporter for these aromatics, such as coumarate, ferulate, caffeate, and cinnamate (6). In this study, to further increase the yield of catechol, this aromatic transporter CouP was coexpressed with catechol biosynthesis pathway in the E. coli strain DH1 (Fig. 4A) and its effect on the bioconversion of vanillin was investigated. As shown in Fig. 4B and Fig. S2, the strain coexpressing the transporter CouP with the catechol biosynthesis pathway genes produced about 10–35% more vanillic acid at early fermentation stage (20-h fermentation after induction) than the strain expressing only catechol biosynthesis pathway genes. As the fermentation progressed the titers of vanillic acid produced from both constructs reached similar levels, as shown in Fig. S2. In terms of catechol, the final product of the pathway, the strain coexpressing the transporter CouP and catechol biosynthesis pathway produced about 30% more catechol than the strain expressing only catechol biosynthesis pathway within 10 h of induction by IPTG (Fig. 4C and Fig. S2), indicating the effective transport of the vanillin by CouP transporter and higher transport rate than vanillin passive diffusion. Similar to the formation of vanillic acid, the accumulation of catechol at the end of fermentation trended to levels similar to the fermentation of both strains expressing catechol biosynthesis pathway only or with transporter CouP incorporated, as shown in Fig. S2. Most likely there are other transporters known to facilitate the uptake of hydrophobic compounds, such as FadL protein and the aromatic amino acid (tryptophan) permease (26, 27), which may uptake vanillin as well but at a slower rate, or vanillin eventually saturated in the cytoplasm of the cells by passive diffusion after a longer period of time, although passive diffusion may have slower transport rate. The further screening of alternative aromatics transporters with higher transport rate may further improve the productivity of the target products.
Fig. 4.
Production of vanillic acid and catechol with the expression of aromatic transporter CouP under pTrc promoter (IPTG) or ADH7 promoter (vanillin). (A) Schematic of the constructs for the expression of catechol biosynthesis pathway with the aromatic transporter CouP under promoter pTrc and promoter ADH7, respectively. (B) Vanillic acid and (C) catechol production from the strains expressing the catechol biosyntheis pathway with (CVMY) and without (VMY) the aromatic transporter CouP under promoter pTrc. (D) Vanillic acid and (E) catechol production from the strains expressing the catechol biosyntheis pathway with (ACVMY) and without (AVMY) the aromatic transporter CouP under promoter ADH7.
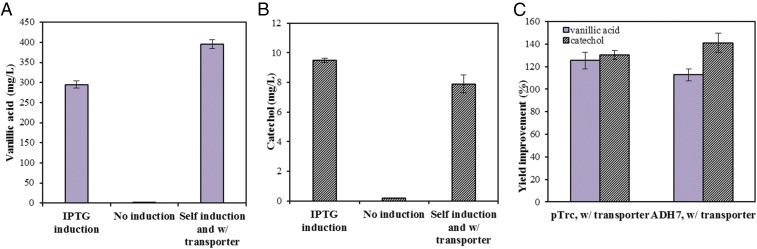
Self-Inducible Production of Vanillic Acid and Catechol with the Integration of Both Transporter and Promoter in the Engineered Chassis.
The vanillin-inducible promoter ADH7 resulted in a lower yield of vanillic acid than that of pTrc promoter in the early stage of fermentation, signifying the need for engineering for effective aromatic transporters in the E. coli strain DH1 for the bioconversion of vanillin. Therefore, we coexpressed the aromatic transporter CouP with the catechol biosynthesis pathway under the ADH7 promoter in the strain DH1 and compared the yields of vanillic acid and catechol with the engineered strain expressing the catechol biosynthesis pathway without the transporter CouP under ADH7 promoter. All of the strains constructed in this study are listed in Table 2. As shown in Figs. 4 and 5, the strain coexpressing pathway genes with transporter CouP produced about 40% more catechol than the strain that did not coexpress the transporter CouP. The strain expressing the transporter CouP yielded 10% more vanillic acid than that of the strain without the coexpression of the transporter CouP. Catechol is relatively sensitive to oxygen and can be oxidized slowly (28, 29). After long hours of incubation, the oxidation rate could be faster than the production rate, which may result in the decrease of catechol titer. This may explain why we observed a decrease of catechol production after 30 h of fermentation in some of the experiment (Fig. S2B).
Table 2.
Details of strains utilized
| Strains | Plasmids contained | Promoters and pathway genes |
| LigV | pBbE1a-LigV | pTrc, LigV |
| AligV | pBbE1a-adh7-LigV | ADH7, LigV |
| VMY | pBbE1a-LigVM-aroY | pTrc, LigV, LigM, AroY |
| AVMY | pBbE1a-adh7-LigVM-aroY | ADH7, LigV, LigM, AroY |
| CVMY | pBbE1a-CouP-LigVM-aroY | pTrc, CouP, LigV, LigM, AroY |
| ACVMY | pBbE1a-adh7-CouP-LigVM-aroY | ADH7, CouP, LigV, LigM, AroY |
Fig. 5.
Comparison of (A) vanillinc acid and (B) catechol produced from strains with no IPTG induction and with IPTG induction under pTrc promoter and self-induction under ADH7 promoter with transporter. (C) Percentage of yield improvement of the strain expressing the catechol biosynthesis pathway with transporter compared with the one without transporter under pTrc or ADH7 promoter.
Discussion
The drivers behind the urgent need to develop economically viable, sustainable, biorefining technologies for renewable fuels and chemicals production include energy security, economic development, and environmental concerns. Lignin, the second-most-abundant plant polymer on Earth after cellulose, is underutilized by its direct combustion for energy (4). Moreover, lignin is the only renewable feedstock that is composed of aromatic building blocks (4). Because of its vast supply and aromatic-rich content, lignin has great potential to be valorized to produce value-added chemicals. Being phenolic in nature, the depolymerization of lignin results in a multitude of aromatic compounds such as vanillin, syringaldehyde, ferulic acid, guaiacol, phenol, syringol, allyl guaiacol, and so on (5, 30–32). Developing microbial factories for the efficient bioconversion of these lignin-derived aromatics is of crucial importance for economically viable lignin upgrading strategies and enabling a bio-based economy and sustainability.
Catechol is one of the valuable platform chemicals that can potentially be produced from lignin-derived aromatics. Catechol and its further upgraded products such as muconic acid have been produced in engineered yeast from glucose through the shikimate pathway (22, 33). In a recent study, a bacterial coculture incorporating two engineered E. coli strains has been developed for the production of muconic acid from glucose and xylose mixture (34). Wang et al. (35) also reported the production of catechol from the aromatic compound benzoate by a benzoate-utilizing Pseudomonas strain. Until recently, only limited work has been done using lignin-derived aromatics as the substrates for the production of muconic acid and adipic acid. Vardon et al. (7) engineered Pseudomonas putida KT2440, a native muconic acid producer, to convert lignin-derived monomers such as coniferyl acohol, ferulate, vanillin, and p-coumarate to catechol and muconic acid through both the catechol and protocatechuate branches of the β-ketoadipate pathway. In their study, P. putida KT2440 was engineered to replace the PcaHG gene encoding a protocatechuate 3,4 dioxygenase with AroY, which diverted the metabolism of protocatechuate to the synthesis of catechol. The CatBC gene encoding dioxygenases was deleted to eliminate further metabolism of muconic acid and a phenol monooxygenase gene was inserted to enable the conversion of phenol to catechol.
In this study, through the expression of a catechol biosynthesis pathway under a vanillin-inducible promoter, ADH7, we have successfully demonstrated an autoregulatory microbial cell factory for the valorization of lignin-derived aromatics using vanillin as an example into valuable chemicals such as vanillic acid and catechol that further serve as platform chemicals for the production of a variety of high-value chemicals and polymer precursors. Furthermore, the coexpression of an associated aromatics transporter CouP with catechol biosynthesis pathway under pTrc or ADH7 promoter improved the catechol yield by 30% and 40%, respectively (Fig. 5C). This self-inducible system eliminates the need for costly IPTG inducer since the substrate vanillin serves as the inducer as well. Another important potential application of our autoregulatory system is that in addition to the ability to convert vanillin to value-added products, it can also serve to detoxify the aromatic compounds when they are copresent with other microbially fermentable substrates such as biomass-derived sugars. For example, although thermochemical depolymerization is an effective route to valorize lignin (1, 5, 30), these lignin-derived aromatics, such as vanillin (36, 37), are reported as potent fermentation inhibitors in the microbial production of fuels and other value-added chemicals (14–17). Bioconversion of these heterogeneous organic molecules to simplified product streams with lesser or no toxicity can potentially benefit the subsequent bioconversion of other substrates of interest. In our system, the product of our autoregulatory system is catechol, which has fewer inhibitory effects on the growth of E. coli than vanillin (Fig. 2) and is an intermediate compound that can be further converted to other value-added compounds, such as muconic acid and adipic acid (7, 8). Moreover, the resulting microbial cell factory is a promising platform whose function is inducible by lignin-derived aromatics, and it offers a promising path forward for the production of fuels and chemicals from lignocellulosic biomass. For example, by integrating this autoregulatory system with the biosynthesis pathway from sugars, one-pot approaches could be developed to coprocess and convert pretreated lignocellulosic liquor composed of sugars and lignin-derived aromatics into value-added chemicals. This envisioned one-pot approach minimizes separation challenges before fermentation with the additional benefit of reducing the toxicity of the vanillin to the sugar-conversion strain.
This study is a demonstration of developing an autoregulatory system for the bioconversion of lignin-derived aromatics. Lignin valorization is being explored in many research laboratories across the globe to improve the economics of biofuel production from cellulosic sugars. To provide a sense of yields, however, compared with the work on catechol or muconic acid production from sugar as a substrate, the catechol titer produced from lignin-derived vanillin in this system is still low. We observed substantial accumulation of the intermediate vanillic acid during the conversion by our engineered E. coli strain (Figs. 4 and 5), which is in agreement with the study in P. putida (7) and is likely due to the transcriptional or translational regulation of the enzymes in the pathways. To further increase titer, the currently introduced pathway in E. coli needs to be optimized to minimize vanillic acid accumulation and maximize the catechol production. In addition, the strength of the vanillin-inducible promoter ADH7 could be enhanced by further strain engineering. A detailed screening of engineered promoter with varying strength could lead to a tunable system for promoter strength and maximization of protein expression according to variables present in the fermentation liquor. Further metabolic engineering such as removing any bottleneck of the pathway as well as fermentation conditions optimization will likely lead to enhancement in the productivity of this engineered strain with an autoregulatory switch. In conclusion, we believe that the autoregulatory system we engineered in this study will aid in developing a low-cost and reliable platform for the valorization of lignin-derived aromatics and provide fertile ground for future studies.
Materials and Methods
Details of the materials and methods used in this study, including plasmid construction, strains, cultivation conditions, and HPLC analysis, are provided in SI Materials and Methods. The constructed strains are listed in Table 2, and Table S1 lists the oligonucleotides used for constructing the plasmids.
Supplementary Material
Acknowledgments
This project was supported by Laboratory Directed Research and Development Program 16-0758 of Sandia National Laboratories (Principal Investigator S.S.). Sandia National Laboratories is a multimission laboratory managed and operated by National Technology and Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International, Inc., for the US Department of Energy’s National Nuclear Security Administration under Contract DE-NA0003525. S.S. acknowledges the Joint BioEnergy Institute for strains BY4742 and DH1 and partial funding support by the Office of Science, Office of Biological and Environmental Research of the US Department of Energy under Contract DE-AC02-05CH11231.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720129115/-/DCSupplemental.
References
- 1.Ragauskas AJ, et al. Lignin valorization: Improving lignin processing in the biorefinery. Science. 2014;344:1246843. doi: 10.1126/science.1246843. [DOI] [PubMed] [Google Scholar]
- 2.Welker CM, et al. Engineering plant biomass lignin content and composition for biofuels and bioproducts. Energies. 2015;8:7654–7676. [Google Scholar]
- 3.Eudes A, Liang Y, Mitra P, Loqué D. Lignin bioengineering. Curr Opin Biotechnol. 2014;26:189–198. doi: 10.1016/j.copbio.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Tuck CO, Pérez E, Horváth IT, Sheldon RA, Poliakoff M. Valorization of biomass: Deriving more value from waste. Science. 2012;337:695–699. doi: 10.1126/science.1218930. [DOI] [PubMed] [Google Scholar]
- 5.Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM. The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev. 2010;110:3552–3599. doi: 10.1021/cr900354u. [DOI] [PubMed] [Google Scholar]
- 6.Salmon RC, Cliff MJ, Rafferty JB, Kelly DJ. The CouPSTU and TarPQM transporters in Rhodopseudomonas palustris: Redundant, promiscuous uptake systems for lignin-derived aromatic substrates. PLoS One. 2013;8:e59844. doi: 10.1371/journal.pone.0059844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vardon DR, et al. Adipic acid production from lignin. Energy Environ Sci. 2015;8:617–628. [Google Scholar]
- 8.Wu W, et al. Lignin valorization: Two hybrid biochemical routes for the conversion of polymeric lignin into value-added chemicals. Sci Rep. 2017;7:8420. doi: 10.1038/s41598-017-07895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown ME, Chang MCY. Exploring bacterial lignin degradation. Curr Opin Chem Biol. 2014;19:1–7. doi: 10.1016/j.cbpa.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Leonowicz A, et al. Biodegradation of lignin by white rot fungi. Fungal Genet Biol. 1999;27:175–185. doi: 10.1006/fgbi.1999.1150. [DOI] [PubMed] [Google Scholar]
- 11.Abd-Elsalam HE, El-Hanafy AA. Lignin biodegradation with ligninolytic bacterial strain and comparison of Bacillus subtilis and Bacillus sp. isolated from Egyptian soil. Environ Sci. 2009;5:39–44. [Google Scholar]
- 12.Husaini A, Fisol F. Lignocellulolytic enzymes produced by tropical white rot fungi during biopulping of Acacia mangium wood chips. J Biochem Technol. 2011;3:245–250. [Google Scholar]
- 13.Dvorak P, et al. Exacerbation of substrate toxicity by IPTG in Escherichia coli BL21(DE3) carrying a synthetic metabolic pathway. Microb Cell Fact. 2015;14:201. doi: 10.1186/s12934-015-0393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakai S, et al. Effect of lignocellulose-derived inhibitors on growth of and ethanol production by growth-arrested Corynebacterium glutamicum R. Appl Environ Microbiol. 2007;73:2349–2353, and erratum (2007) 73:6328. doi: 10.1128/AEM.02880-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai Z, Gao Z, He B, Wu B. Effect of lignocellulose-derived inhibitors on the growth and D-lactic acid production of Sporolactobacillus inulinus YBS1-5. Bioprocess Biosyst Eng. 2015;38:1993–2001. doi: 10.1007/s00449-015-1440-5. [DOI] [PubMed] [Google Scholar]
- 16.Um B-H, Karim M, Henk L. Effect of sulfuric and phosphoric acid pretreatments on enzymatic hydrolysis of corn stover. Appl Biochem Biotechnol. 2003;105:115–125. doi: 10.1385/abab:105:1-3:115. [DOI] [PubMed] [Google Scholar]
- 17.Alterthum F, Ingram LO. Efficient ethanol production from glucose, lactose, and xylose by recombinant Escherichia coli. Appl Environ Microbiol. 1989;55:1943–1948. doi: 10.1128/aem.55.8.1943-1948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen TTM, Iwaki A, Izawa S. The ADH7 promoter of Saccharomyces cerevisiae is vanillin-inducible and enables mRNA translation under severe vanillin stress. Front Microbiol. 2015;6:1390. doi: 10.3389/fmicb.2015.01390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen MWY, Shah D, Chen W, Da Silva N. Enhanced arsenate uptake in Saccharomyces cerevisiae overexpressing the Pho84 phosphate transporter. Biotechnol Prog. 2012;28:654–661. doi: 10.1002/btpr.1531. [DOI] [PubMed] [Google Scholar]
- 20.Singh S, Mulchandani A, Chen W. Highly selective and rapid arsenic removal by metabolically engineered Escherichia coli cells expressing Fucus vesiculosus metallothionein. Appl Environ Microbiol. 2008;74:2924–2927. doi: 10.1128/AEM.02871-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masai E, Katayama Y, Fukuda M. Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci Biotechnol Biochem. 2007;71:1–15. doi: 10.1271/bbb.60437. [DOI] [PubMed] [Google Scholar]
- 22.Curran KA, Leavitt JM, Karim AS, Alper HS. Metabolic engineering of muconic acid production in Saccharomyces cerevisiae. Metab Eng. 2013;15:55–66. doi: 10.1016/j.ymben.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Mishra S, Sachan A, Sachan SG. Production of natural value-added compounds: An insight into the eugenol biotransformation pathway. J Ind Microbiol Biotechnol. 2013;40:545–550. doi: 10.1007/s10295-013-1255-9. [DOI] [PubMed] [Google Scholar]
- 24.Masai E, et al. Characterization of ligV essential for catabolism of vanillin by Sphingomonas paucimobilis SYK-6. Biosci Biotechnol Biochem. 2007;71:2487–2492. doi: 10.1271/bbb.70267. [DOI] [PubMed] [Google Scholar]
- 25.Sonoki T, et al. Enhancement of protocatechuate decarboxylase activity for the effective production of muconate from lignin-related aromatic compounds. J Biotechnol. 2014;192:71–77. doi: 10.1016/j.jbiotec.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Hearn EM, Patel DR, van den Berg B. Outer-membrane transport of aromatic hydrocarbons as a first step in biodegradation. Proc Natl Acad Sci USA. 2008;105:8601–8606. doi: 10.1073/pnas.0801264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Díaz E, Ferrández A, Prieto MA, García JL. Biodegradation of aromatic compounds by Escherichia coli. Microbiol Mol Biol Rev. 2001;65:523–569. doi: 10.1128/MMBR.65.4.523-569.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas S, Wornat MJ. The effects of oxygen on the yields of polycyclic aromatic hydrocarbons formed during the pyrolysis and fuel-rich oxidation of catechol. Fuel. 2008;87:768–781. [Google Scholar]
- 29.Baila J, Kiss T, Jameson RF. Copper(II)-catalyzed oxidation of catechol by molecular oxygen in aqueous solution. Inorg Chem. 1992;31:58–62. [Google Scholar]
- 30.Pandey MP, Kim CS. Lignin depolymerization and conversion: A review of thermochemical methods. Chem Eng Technol. 2011;34:29–41. [Google Scholar]
- 31.Varanasi P, et al. Survey of renewable chemicals produced from lignocellulosic biomass during ionic liquid pretreatment. Biotechnol Biofuels. 2013;6:14. doi: 10.1186/1754-6834-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J, et al. Rapid room temperature solubilization and depolymerization of polymeric lignin at high loadings. Green Chem. 2016;18:6012–6020. [Google Scholar]
- 33.Averesch NJH, Krömer JO. Tailoring strain construction strategies for muconic acid production in S. cerevisiae and E. coli. Metab Eng Commun. 2014;1:19–28. doi: 10.1016/j.meteno.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Pereira B, Li Z, Stephanopoulos G. Engineering Escherichia coli coculture systems for the production of biochemical products. Proc Natl Acad Sci USA. 2015;112:8266–8271. doi: 10.1073/pnas.1506781112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang CL, Takenaka S, Murakami S, Aoki K. Isolation of a benzoate-utilizing Pseudomonas strain from soil and production of catechol from benzoate by transpositional mutants. Microbiol Res. 2001;156:151–158. doi: 10.1078/0944-5013-00096. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt D, Regenbrecht C, Hartmer M, Stecker F, Waldvogel SR. Highly selective generation of vanillin by anodic degradation of lignin: A combined approach of electrochemistry and product isolation by adsorption. Beilstein J Org Chem. 2015;11:473–480. doi: 10.3762/bjoc.11.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sainsbury PD, et al. Breaking down lignin to high-value chemicals: The conversion of lignocellulose to vanillin in a gene deletion mutant of Rhodococcus jostii RHA1. ACS Chem Biol. 2013;8:2151–2156. doi: 10.1021/cb400505a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.