Abstract
Objective
To estimate the impact of NICE approval of tumour necrosis factor inhibitor (TNFi) therapies on the incidence of total hip replacement (THR) and total knee replacement (TKR) among rheumatoid arthritis (RA) patients in England and Wales.
Methods
Primary care data (Clinical Practice Research Datalink (CPRD)) for the study period (1995-2014) were used to identify incident adult RA patients. The age and sex-standardised 5-year incidence of THR and TKR was calculated separately for RA patients diagnosed in each six-months between 1995-2009. We took a natural experimental approach, using segmented linear regression to estimate changes in level and trend following the publication of NICE TA 36 in March 2002, incorporating a 1-year lag. Regression coefficients were used to calculate average change in rates, adjusted for prior level and trend.
Results
We identified 17,505 incident RA patients of whom 465 and 650 underwent THR and TKR surgery, respectively. The modeled average incidence of THR and TKR over the biologic-era was 6.57/1,000 person years (PYs) and 8.51/1,000 PYs, respectively, with projected (had pre-NICE TA 36 level and trend continued uninterrupted) figures of 5.63/1,000 PYs and 12.92 PYs, respectively. NICE guidance was associated with a significant average decrease in TKR incidence of -4.41/1,000 PYs (95% C.I. -6.88 to -1.94), equating to a relative 34% reduction. Overall, no effect was seen on THR rates.
Conclusions
Among incident RA patients in England and Wales, NICE guidance on TNFi therapies for RA management was temporally associated with reduced rates of TKR but not THR
Keywords: Pharmacoepidemiology, Rheumatoid Arthritis, Biologic Therapy, Arthroplasty of Hip, Arthroplasty of Knee
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease associated with pain, stiffness and swelling in affected joints (1). Joint damage is a central feature of RA (2), estimated to account for approximately 25% of disability in established disease (3). Data from the mid-late 20th century indicate that over half of incident RA patients required RA-related surgery over 30 years of follow-up (4). Despite some evidence suggesting that joint erosions can heal over time (3), such healing is generally rare (5) and reducing the risk of irreversible joint damage through early and ‘treat-to-target' disease management is emphasised in numerous guidelines.
The literature widely recognizes that the availability of biological disease modifying anti-rheumatic drugs (bDMARDs) over recent decades has revolutionised the management of RA (6–8). A wealth of randomised controlled trial (RCT) data has conclusively demonstrated the beneficial impact of bDMARDs on ACR response criteria and structural joint damage (8, 9). However, no such data exists concerning the impact of bDMARDs on the need for joint replacement - a costly consequence of joint failure that is not without its risks. During the “biologic era” there has been an emerging body of observational studies indicating the number and/or incidence of RA related joint surgery has generally been decreasing across numerous developed countries (10–20). Although the use of biologic therapies has been routinely offered as an explanatory factor, their role is not clear and estimation of their impact on the incidence of joint replacement remains lacking.
It has previously been suggested that when an RCT is likely to be unfeasible, a strong research alternative is to take advantage of naturally occurring events such as a national health policy change in order to carry out a quasi-experiment (21). In this context, our aim was to estimate the impact of the national institute for health and care excellence (NICE) approval of tumour necrosis factor inhibitor (TNFi) therapy (22) on the temporal trends of total hip (THR) and total knee replacement (TKR) among RA patients in England and Wales.
Methods
Study population and data sources
We used primary care health data from the Clinical Practice Research Datalink (CPRD) for the period Apr 1995 to Sept 2014. As of 2013, CPRD covered over 11.3 million patients from 674 UK practices and had a representative coverage of approximately 7% of the United Kingdom (23). Where available, we obtained linked secondary care Hospital Episode Statistics (HES) data for the same time period. Approximately 58% of UK CPRD practices participate in the CPRD linkage scheme (23) and previous research by CPRD has shown that linked practices/patients are representative of the CPRD population as a whole. HES data contains hospital admission records relating to each ‘finished consultant episode’ – the period of time an individual spends under the care of one NHS consultant. Mortality data were linked to the Office for National Statistics (ONS) database.
Incident RA patients within the study period were identified using a pre-defined READ code list (appendix table 1), as developed elsewhere (24), with the date of first recorded RA considered as diagnosis date. Data on BMI, smoking status and Charlson comorbidity score at date of RA diagnosis was also extracted from CPRD. Patients with either a prior or subsequent diagnosis of a different inflammatory arthritis (lupus, ankylosing spondylitis, psoriatic arthritis or crystal arthropathy) were excluded due to possible diagnosis or coding errors. Patients aged <18 years old were also excluded as were patients registered in a general practitioner (GP) practice outside England or Wales given that compliance to NICE guidance is only mandatory for these countries (appendix figure 1).
Intervention
Our defined intervention was the publication of the NICE technology appraisal (TA) 36 in March 2002. This provided guidance on the use of TNFi (etanercept and infliximab) for the treatment of RA, and stated that these therapies were recommended options for the treatment of adults with severe RA (Disease Activity Score (DAS) >5.1) who had already failed to respond to two conventional synthetic disease modifying anti-rheumatic drug (csDMARD) therapies.
Outcomes
We used CPRD Read codes as used previously (25, 26) to identify the occurrence of first THR and TKR after incident RA diagnosis. THR and TKR were considered separately so patients could potentially have both outcomes of interest. For validation purposes, we also identified first subsequent THR and TKR within the HES data using the classification of interventions and procedures coding system (OPCS4). Reporting of THR and TKR in HES has previously been shown to be comparable with the National Joint Registry (NJR) (27).
Statistical analysis
To assess the validity of the THR and TKR Read codes, we calculated sensitivity and specificity among the 62.6% of RA patients with both CPRD and HES data (appendix figure 1), considering HES as the reference standard. Agreement was defined as surgeries present in both data sources within a 60-day time period, with 30-day and 90-day time periods also explored. Given that good agreement was found (appendix table 2), we proceeded to use THR and TKR as reported in CPRD for the main analysis, and the whole study population (including those with no linked HES) were included.
An age and sex standardised time-series was derived by calculating 5-year incidence rates of THR and TKR among newly diagnosed RA patients within each 6 months between 1995-2009. We only concentrated on the first 5 years after diagnosis so that patients contributed the same follow-up irrespective of when they were diagnosed to allow valid rate comparisons throughout the study period. Patients were followed up from date of diagnosis until the first date of: outcome event, death, loss to follow-up or 5 years of follow-up.
A segmented linear regression was performed on the aggregated standardised time-series to estimate two parameters of interest associated with the publication of NICE TA 36: change in subsequent level of outcome and change in subsequent trend (28). Given that the NICE guidance recommended that only patients having a failed response to two (6 month) trials of csDMARD therapy should initiate a TNFi, a 1-year lag period following March 2002 was decided upon a-priori in which data were removed from the time-series. The regression model was specified as following: Yt=β0 + β1*timet + β2*interventiont + β3*post_intervention_timet + et. Here, Yt is the 5-year incidence among RA patients diagnosed at time point (i.e. 6-monthly period) t. β0 estimates the baseline level of the outcome just before the beginning of the time series. β1 estimates the pre-intervention trend, β2 the change in level between the time point immediately before vs. after the lag period and β3 the change in trend occurring immediately after the lag period. Analyses were based on 14 pre-intervention data points (Apr 1995 – Mar 2002) and 13 post-intervention data points (Apr 2003 – Sept 2009). Final model specification was derived using a backward-stepwise approach (p-entry 0.049; p-exit 0.20) to remove non-significant regression terms in order to maximise statistical power, although results from full models were also reported. Durbin-Watson statistics indicated no significant autocorrelation.
Regression coefficients were used to estimate an overall intervention effect by predicting what would have been observed post-intervention (i.e. counterfactual rates) had pre-intervention levels and/or trends continued uninterrupted, and comparing this with what was modelled using observed post-intervention data (28, 29). The midpoint of the post-intervention period was used to calculate the average difference over the post-intervention time points.
Sensitivity analyses
Due to likely delay in implementation of NICE recommendations, a 2-year lag period was used in a sensitivity analysis. A data-driven (with no pre-specified time point/intervention) approach was also conducted in order to identify where – if at all - any changes in trend occurred using a Joinpoint analysis (30). An uncorrelated errors model was specified, using the grid search method with a maximum of one Joinpoint and minimum of eight observations before and after (as recommended for an interrupted time series approach ). Model selection was carried out using permutation tests (Monte Carlo methods) with a significance level set to 0.05.
Results
Between 1995-2014 there were 23,830 incident RA patients identified, of whom 10,952 had HES linkage (appendix figure 1). Considering HES as the reference standard, THR in CPRD had 80.1% sensitivity and 98.4% specificity whilst TKR in CPRD had 83.5% sensitivity and 98.1% specificity (appendix table 2).
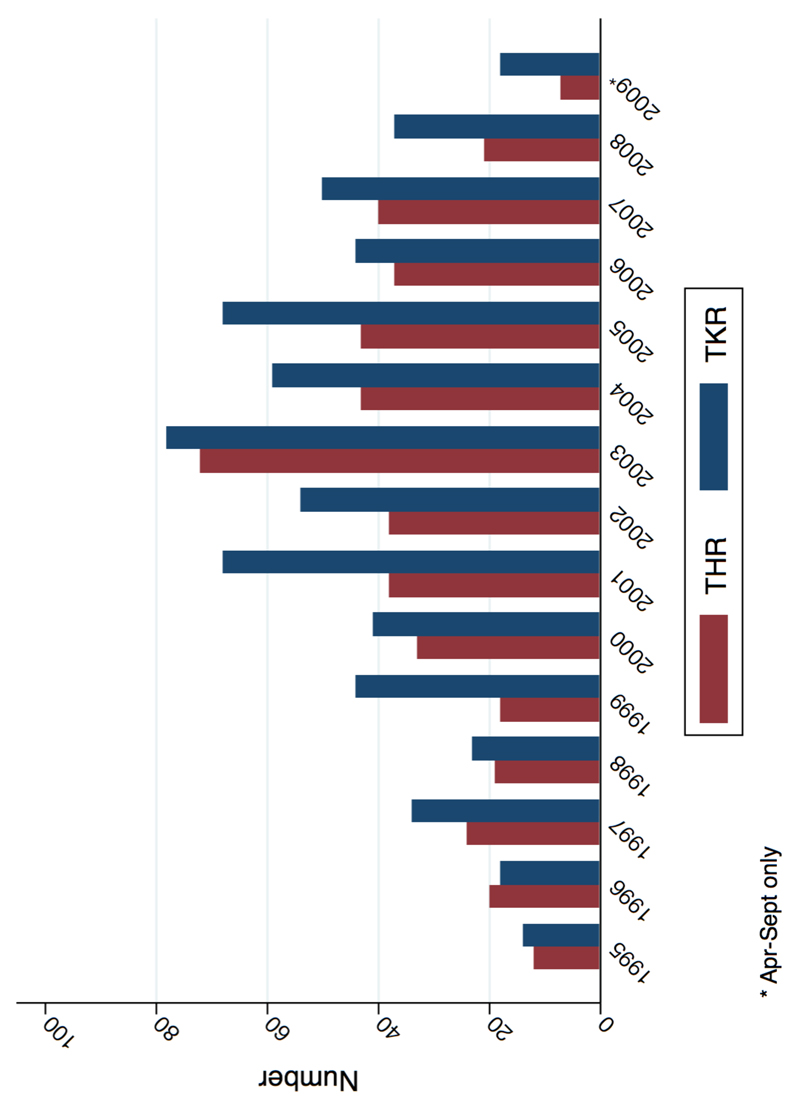
Included in the interrupted time series analysis were 17,505 incident RA patients diagnosed in CPRD between 1995-2009 (appendix figure 1). Patient characteristics are presented in appendix table 3. Mean age at RA diagnosis increased slightly from 58.7 in 1995 to 60.3 in 1999 (p=0.065), whilst the gender ratio remained fairly stable over the same time frame (70.4% to 66.3% female; p=0.12). Prevalence of obesity (BMI ≥30), as reported at time of RA diagnosis, increased from 11.5% in 1995 to 22.1% in 2009 (p=<0.001), whilst concurrent smoking status decreased from 27.9% to 20.9% (p=0.011). Overall there were 465 THRs and 650 TKRs occurring within 5-years of RA diagnosis (figure 1), yielding a crude incidence rate of 6.16/1,000 person years (PYs) (95% CI: 5.63 to 6.75) and 8.65/1,000 PYs (95% CI: 8.01 to 9.34), respectively. Median follow-up over the 5 years following RA diagnosis was 5.00 years (inter quartile range (IQR): 3.69-5.00) for THR and 5.00 years (IQR 3.60-5.00) for TKR, with median time-to-event being 1.96 (IQR: 0.95-3.24) and 2.32 (IQR: 1.02-3.58), respectively. Average mortality over the same time frame was 15.5% while loss-to-follow-up was 18.2%.
Figure 1.
Number of RA patients undergoing THR/TKR surgery within 5 years: stratified by year
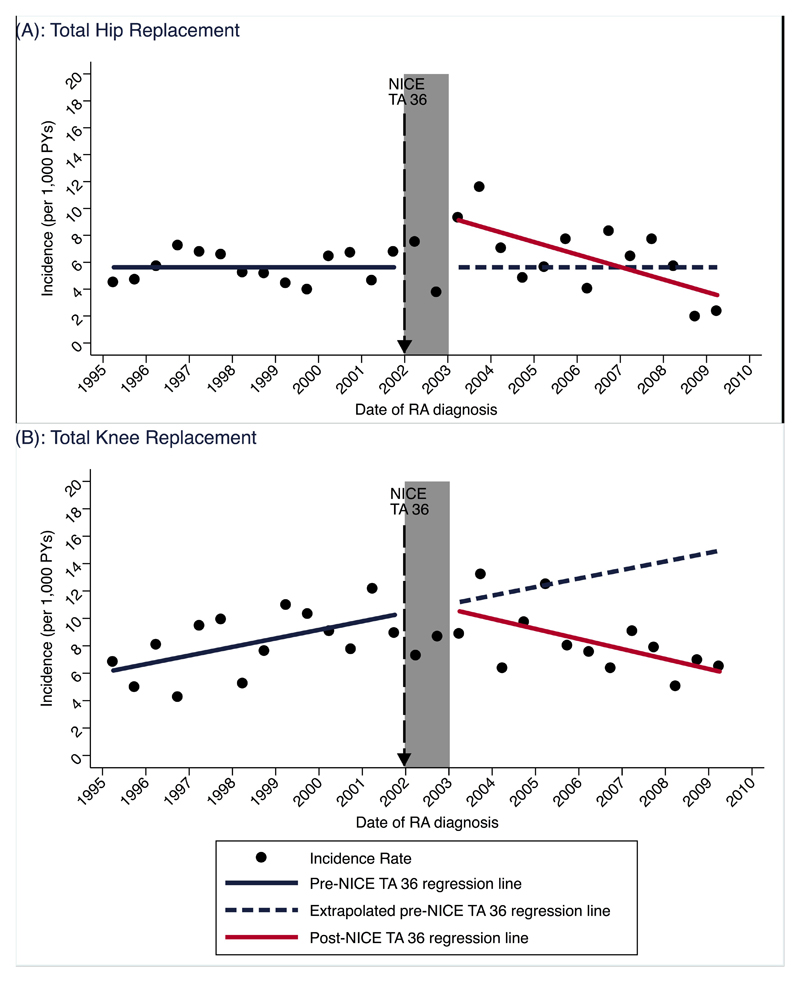
Age and sex standardised 5-year incidence of THR at the start of the study period was 5.63/1,000 PYs (95% CI: 4.74 to 6.54), which remained unchanged during the pre-TNFi period (Apr 1995 – Mar 2002) (Table 1 and Figure 2a). Immediately following the intervention lag period there was a level increase in the incidence rate by 3.97/1,000 PYs (95% CI: 1.79 to 6.14) but a subsequent downward trend of -0.47/1,000 PYs (95% CI: -0.71 to 0.22) per 6 months for the remainder of the post-NICE TA36 time period. Based on these coefficients, the estimated incidence of THR at the mid-point of the post-NICE TA36 time period was 6.57/1,000 person years (PYs). The incidence at the same time point estimated solely by extrapolating the pre-NICE TA36 level and trend was 5.63/1,000 PYs, therefore translating to no significant average change in rates (0.95/1,000 PYs (95% CI: -2.66 to 4.56)).
Table 1.
Temporal Trends in Joint Replacement Rates Among 17,505 Incident Rheumatoid Arthritis Patients Diagnosed From 1995 to 2009 (per 1,000 Person-Years)
| Parameter | Coefficient | Lower 95% C.I. | Upper 95% C.I. | p value |
|---|---|---|---|---|
| Total Hip Replacement | ||||
| Intercept | 5.63 | 4.73 | 6.52 | <0.001 |
| Trend 1 | - | - | - | - |
| Level change after NICE TA 36 | 3.97 | 1.79 | 6.14 | 0.001 |
| Trend change after NICE TA 36 1 | -0.49 | -0.71 | -0.22 | 0.001 |
| Total Knee Replacement | ||||
| Intercept | 5.89 | 3.83 | 7.94 | <0.001 |
| Trend 1 | 0.31 | 0.11 | 0.51 | 0.004 |
| Level change after NICE TA 36 | - | - | - | - |
| Trend change after NICE TA 36 1 | -0.68 | -1.08 | -0.28 | 0.002 |
- = p ≥ 0.2
per 6 months
Figure 2.
Standardised incidence of joint replacement within 5 years: (A) THR and (B) TKR
The incidence of TKR was 5.89/1,000 PYs (95% CI: 3.83 to 7.94) at the start of the study period, which increased by 0.31/1,000 PYs (95% CI: 3.83 to 7.94) per 6 months during the pre-intervention period (table 1, figure 2b). Immediately following the 1-year lag period there was a significant downward change in the prior upward trend by -0.68/1,000 PYs (95% CI: -1.08 to -0.28) per 6 months. Based on these coefficients the modeled incidence for the midpoint of the post-intervention period was 8.51/1,000 PYs, which was a significant 4.41/1,000 PYs (95% C.I. 6.88 to 1.98) lower compared to that estimated, had pre-intervention trends continued uninterrupted (table 2). This equated to an approximate relative 34% reduction.
Table 2.
Estimated Difference (per 1,000 Person Years) in Joint Replacement Rates Following Approval of TNFi in England and Wales
| Outcome | Without intervention 1 | With intervention 1 | Absolute Difference 2 | ||
|---|---|---|---|---|---|
| estimate | lower 95% C.I. | upper 95% C.I. | |||
| Total Hip Replacement | 5.63 | 6.57 | 0.95 | -2.66 | 4.56 |
| Total Knee Replacement | 12.92 | 8.51 | -4.41 | -6.88 | -1.94 |
Post-NICE TA 36 midpoint estimate (June 2006)
Calculated by comparing estimated values for midpoint of post-intervention period to counterfactual values for the same time point (i.e. post-intervention midpoint values estimated based solely on extrapolation of pre-intervention level and/or trend). Estimated from parsimonious models
In sensitivity analyses using a 2-year lag, THR rates remained flat during the study period while results for TKR remained unchanged from the main analysis (appendix figure 2). Conversely, Joinpoint analysis identified significant inflections in upward trends in the incidence of both THR and TKR, at the time points spanning Oct 2005 – Mar 2006 (P=0.034) and Apr 2001 – Sep 2001 (P=0.036), respectively. Results from the main analysis using full models were unchanged as when using parsimonious models (appendix table 4).
Discussion
Our results indicate that the introduction of TNFi therapies for the management of RA was associated with a significant reduction in TKR but not THR incidence among early RA patients within England and Wales. Specifically, whilst TKR incidence was increasing prior to TNFi approval, this upward trend was reversed following the start of the biologic era, yielding a relative 34% average reduction compared to counterfactual rates. The relationship between THR incidence and TNFi approval was less clear, with no significant average change in post-NICE TA 36 modelled rates compared to counterfactual values.
Treatments recommended by a NICE TA publication “as an option” should be available for use in the NHS within 3 months of the guidance being published (31). Although in reality this may not be the case for a particular patient due to delayed implementation at a local level, a population-level increase in the use of etanercept and infliximab (in line with the recommendations) would be expected (32, 33) given the authoritative and widespread reach of NICE.
The potential impact of TNFi therapy on need for joint replacement was not mentioned in the NICE TA 36 document, although the evidence base referred to indicates the plausibility of such a relationship. For example, results from a high quality RCT were summarised, in which 31% of patients on methotrexate alone experienced progression of structural damage at 54 weeks, whilst this was only 8% for patients on methotrexate plus infliximab (22). Indeed, the clinical effectiveness of both these therapies in terms of reduced erosive damage is well established (34–36) and joint replacement has been suggested as an important consideration in future economic modelling of the management of RA with bDMARDs (8, 37).
Our decrease in TKR rates is consistent with data from the UK NJR which indicate the percentage of knee replacements for which RA was an indication fell from 3% to 2% (2004 to 2010) (10, 38). Our data also complements and builds on prior studies from elsewhere in Europe and the US. Notably, in the Republic of Ireland the number of THR and TKR surgeries was found to increase dramatically from 1995 to 2010, whilst the number of those with a diagnosis of RA significantly decreased for TKR but remained stable for THR (18). A similar study from the US found significant reductions in the number of both TKR and THR patients who had RA as the primary diagnosis at surgery, whilst the numbers of TKR and THR for other reasons profoundly increased (16). Similar arthroplasty trends were reported in most (Norway (39), Finland (13)) but not all (Sweden (19)) Scandinavian countries.
We found the overall incidence of THR to be lower than TKR, which is supportive of milder RA involvement at the hip (40) and which may explain why TNFi approval was here associated with reduced rates of TKR but not THR. Previous estimates of hip joint synovitis in early RA range between approximately 20-40% (41, 42) which is considerably lower than the 60% estimated for the knee (43). Also worth mentioning is that while the knee joint featured in the 1987 ACR classification criteria and Disease Activity Score 28, the hip joint did not (44, 45).
However, our lack of access to joint replacement rates for the general population is a key limitation to our study. As such we recognise the need for caution in interpreting our findings. For example, it may be that the approximately stable incidence of THR we observed for RA patients may be a favourable outcome were THR incidence in the general population to have undergone a significant concurrent increase. Indeed, previous NJR data for the general population indicate an overall year-on-year increase in the raw number of both THR and TKR procedures carried out from 2003-2012 (46).
Furthermore, in evaluating the impact of NICE guidance on biologics, we cannot rule out other factors such as prescription rates of csDMARDs having markedly increased within this population (47), which may have contributed to a reduced need for joint replacement (48). Improvement in non-therapeutic aspects of RA management and increased awareness may likewise have played a role (49), as may have a gradually declining disease severity or changes in smoking prevalence or BMI, although we consider these reasons insufficient to explain the relatively sudden inflection observed in the TKR trend following NICE recommendations (50). Another intriguing possibility is that the mere availability of bDMARDS may have given rise to a greater impetus among clinicians to diagnose RA earlier in its natural history, and thereby contributed to a decline in 5-year TKR rates. Whilst unfortunately we do not have data on disease severity, we found the average comorbidity index to worsen over the study period and there was no evidence for a boom in incident RA diagnoses associated with the advent of biologics (appendix table 3). It is also reassuring that the annual proportion of RA patients migrating out of the CPRD remained below 5% for all the years under study.
Related to this point is that etanercept and infliximab prescriptions are not administered or captured in the primary care setting and were therefore not available for analysis. Whilst anecdotally the speed and intensity of therapy uptake was subject to regional variation, it’s been previously reported that by 2005 approximately 8,500 RA patients on TNFi had been recruited to the British Society for Rheumatology Biologics Register (BSRBR-RA) (51). For this reason we used a 2-year lag as a sensitivity analysis to allow for delayed implementation, which remained consistent with the overall finding of reduced incidence of TKR but not THR in the biologic era. The use of Joinpoint regression indicated a downward inflection was best-placed at the time point 6-12 months prior to NICE TA 36. This may suggest some role for the 2001 British Society for Rheumatology guidelines (upon which NICE TA 36 were based), although given the close temporal proximity this cannot be determined.
The trend change in THR rates as identified in the Joinpoint analysis was not consistent with the main analysis and was likely the product of particularly low values for the last two time points (figure 2a). This finding warrants further investigation regarding these methods, notably the sensitivity to the number of time points in pre- vs. post- intervention periods and the number of outcome events occurring per time point. We are currently exploring these issues with the use of simulated data. Further work will also include estimating the impact of TNFi therapies using healthcare data outside the UK with the inclusion of a non-RA control group.
Another caveat to our results is that they pertain to joint replacements within the first 5 years after RA diagnosis and so it is possible we have underestimated the impact of TNFi therapy by not considering longer-term outcomes. A fixed 5-year time window across the study period was used to prevent THR and TKR rates over time being influenced by underlying variation in the length of follow-up available (i.e. the bias of patients at the beginning of the study period systematically having longer enrolment in the database).
Our study also has several strengths. We studied a large sample of RA patients identified from a data source generalizable to the UK population in terms of age, sex and ethnicity (23). Rather than describing temporal trends of RA as an indicator for THR/TKR surgery, we consider our approach of using RA patients as the denominator to be preferable because this accounts for underlying changes in the incidence of RA over time. The interrupted time-series analysis is another strength as this quasi-experimental method controls for secular trends in the outcome prior to the intervention (21, 28) , and allows for comparison with counterfactual values. The importance of this approach is evident given that a conventional before-after comparison of time-to-TKR using a Cox regression model would have here masked the positive association and yielded no significant difference as there are two almost equal but opposite trends in existence (Figure 2b). The linkage to HES allowed us to carry out an internal validation of CPRD coding of THR and TKR, which we also supplemented by estimating the five and ten year cumulative incidence of a combined THR/TKR outcome (results not shown) that were very similar to reports from previous UK early RA inception cohorts, at approximately 7% and 12%, respectively .
In conclusion, we have demonstrated that approval of TNFi therapies in England and Wales was temporally associated with a significant and clinically meaningful decline in TKR incidence but no change in THR incidence among early (first 5 years post-diagnosis) RA patients.
Supplementary Material
Key Messages.
-
(1)
In England and Wales, TNFi approval for RA was associated with reduced TKR rates
-
(2)
In England and Wales, no change in THR rates was seen following TNFi approval
-
(3)
Further studies in non-UK settings are required to validate these findings
Acknowledgments
Study design: DPA, AJ, SH, CJE, NKA, CC. Study conduct: SH, DPA, RC,LD, AD, AJ. Statistical analysis: SH, DPA, AJ. Data interpretation: DPA, SH, LD, RC, AS, CJE, NKA, CC. Drafting manuscript: SH, DPA. Revising manuscript: All authors. Approving final manuscript: All authors.
The authors would like to thank Miss Susan Thwaite (National Rheumatoid Arthritis Society) for her role as patient and public representative and her role in the study steering committee.
DPA is funded by a National Institute for Health Research Clinician Scientist award (CS-2013-13-012). This article presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
RC reports grants from The Danish Rheumatism Association and from The Bjarne Jensen Foundation. LD reports personal fees from UCB, from MSD, from UCB, and from MSD. CE reports grants and personal fees from Pfizer, from Biogen and from Abbvie, and personal fees from UCB, from Roche, from Janssen, from Samsung bioepis, from Sandoz, from Celltrion, and from Mundipharma. NKA reports grants from BIOIBERICA and personal fees from BIOVENTUS, from REGENERON, and from SMITH & NEPHEW. CC has received consultancy fees and honoraria from Amgen, Danone, Eli Lilly, GSK, Medtronic, Merck, Nestle, Novartis, Pfizer, Roche, Servier, Shire, Takeda and UCB. AJ reports personal fees from Data Safety and Monitoring Board, from Servicer, from UK Renal Registry, from IDIAP Jordi GOI, Freshfields Bruckhaus Deringer and grants from Roche. DPA reports grants from Amgen, from UCB Biopharma, and from Les Laboratoires Servier. SH, AD and AS have nothing to disclose.
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8(11):656–64. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott DL, Pugner K, Kaarela K, Doyle DV, Woolf A, Holmes J, et al. The links between joint damage and disability in rheumatoid arthritis. Rheumatology (Oxford) 2000;39(2):122–32. doi: 10.1093/rheumatology/39.2.122. [DOI] [PubMed] [Google Scholar]
- 4.Massardo L, Gabriel SE, Crowson CS, O'Fallon WM, Matteson EL. A population based assessment of the use of orthopedic surgery in patients with rheumatoid arthritis. Journal of Rheumatology. 2002;29(1):52–6. [PubMed] [Google Scholar]
- 5.Dohn UM, Boonen A, Hetland ML, Hansen MS, Knudsen LS, Hansen A, et al. Erosive progression is minimal, but erosion healing rare, in patients with rheumatoid arthritis treated with adalimumab. A 1 year investigator-initiated follow-up study using high-resolution computed tomography as the primary outcome measure. Annals of the Rheumatic Diseases. 2009;68(10):1585–90. doi: 10.1136/ard.2008.097048. [DOI] [PubMed] [Google Scholar]
- 6.Scott DL. Biologics-based therapy for the treatment of rheumatoid arthritis. Clin Pharmacol Ther. 2012;91(1):30–43. doi: 10.1038/clpt.2011.278. [DOI] [PubMed] [Google Scholar]
- 7.Feldmann M, Williams RO, Paleolog E. What have we learnt from targeted anti-TNF therapy? Annals of the Rheumatic Diseases. 2010;69:97–9. doi: 10.1136/ard.2009.117143. [DOI] [PubMed] [Google Scholar]
- 8.Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther. 2011;33(6):679–707. doi: 10.1016/j.clinthera.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nam JL, Ramiro S, Gaujoux-Viala C, Takase K, Leon-Garcia M, Emery P, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2014;73(3):516–28. doi: 10.1136/annrheumdis-2013-204577. [DOI] [PubMed] [Google Scholar]
- 10.National Joint Registry for England and Wales: 1st Annual Report. 2004.
- 11.National Joint Registry for England and Wales: 5th Annual Report. 2008.
- 12.Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868–71. doi: 10.1136/ard.2009.112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331–7. doi: 10.3109/17453674.2013.810519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432–9. doi: 10.1002/art.38384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shourt CA, Crowson CS, Gabriel SE, Matteson EL. Orthopedic surgery among patients with rheumatoid arthritis 1980-2007: a population-based study focused on surgery rates, sex, and mortality. J Rheumatol. 2012;39(3):481–5. doi: 10.3899/jrheum.111056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.David G, Tandon N, Waters H, Gunnarsson C, Kavanaugh A. Rheumatoid Arthritis and Joint Replacement: Impact of Biologics. Americal Journal of Pharmaceutical Benefits. 2014;6(6):256–64. [Google Scholar]
- 17.Nystad TW, Fenstad AM, Furnes O, Havelin LI, Skredderstuen AK, Fevang BT. Reduction in orthopaedic surgery in patients with rheumatoid arthritis: a Norwegian register-based study. Scand J Rheumatol. 2015:1–7. doi: 10.3109/03009742.2015.1050451. [DOI] [PubMed] [Google Scholar]
- 18.Harty L, O'Toole G, FitzGerald O. Profound reduction in hospital admissions and musculoskeletal surgical procedures for rheumatoid arthritis with concurrent changes in clinical practice (1995-2010) Rheumatology (Oxford) 2015;54(4):666–71. doi: 10.1093/rheumatology/keu340. [DOI] [PubMed] [Google Scholar]
- 19.Hekmat K, Jacobsson L, Nilsson JA, Petersson IF, Robertsson O, Garellick G, et al. Decrease in the incidence of total hip arthroplasties in patients with rheumatoid arthritis--results from a well defined population in south Sweden. Arthritis Res Ther. 2011;13(2):R67. doi: 10.1186/ar3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh J, Y B, Watson S, Perez J, McGwin G, Ponce B. Trends in Joint Replacements Surgery in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(suppl 10) [Google Scholar]
- 21.Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. Bmj. 2015;350:h2750. doi: 10.1136/bmj.h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dillon A. Technological Appraisal Guidance - No. 36: Guidance on the use of etanercept and infliximab for the treatment of rheumatoid arthritis. National Institute for Clinical Excellence; 2002. Mar, [Google Scholar]
- 23.Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD) International journal of epidemiology. 2015 doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas SL, Edwards CJ, Smeeth L, Cooper C, Hall AJ. How accurate are diagnoses for rheumatoid arthritis and juvenile idiopathic arthritis in the general practice research database? Arthritis and rheumatism. 2008;59(9):1314–21. doi: 10.1002/art.24015. [DOI] [PubMed] [Google Scholar]
- 25.Culliford D, Maskell J, Judge A, Cooper C, Prieto-Alhambra D, Arden NK, et al. Future projections of total hip and knee arthroplasty in the UK: results from the UK Clinical Practice Research Datalink. Osteoarthritis Cartilage. 2015;23(4):594–600. doi: 10.1016/j.joca.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Prieto-Alhambra D, Javaid MK, Judge A, Maskell J, Cooper C, Arden NK, et al. Hormone replacement therapy and mid-term implant survival following knee or hip arthroplasty for osteoarthritis: a population-based cohort study. Ann Rheum Dis. 2015;74(3):557–63. doi: 10.1136/annrheumdis-2013-204043. [DOI] [PubMed] [Google Scholar]
- 27.National Joint Registry for England and Wales: 10th Annual Report. 2013.
- 28.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang F, Wagner AK, Soumerai SB, Ross-Degnan D. Methods for estimating confidence intervals in interrupted time series analyses of health interventions. J Clin Epidemiol. 2009;62(2):143–8. doi: 10.1016/j.jclinepi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statistics in medicine. 2000;19(3):335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 31.NICE. NICE technological appraisal guidance. NICE; 2017. [01/02/2017]. Available from: https://www.nice.org.uk/About/What-we-do/Our-Programmes/NICE-guidance/NICE-technology-appraisal-guidance. [Google Scholar]
- 32.Dayer MJ, Jones S, Prendergast B, Baddour LM, Lockhart PB, Thornhill MH. Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time-series analysis. Lancet. 2015;385(9974):1219–28. doi: 10.1016/S0140-6736(14)62007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawley S, Leal J, Delmestri A, Prieto-Alhambra D, Arden NK, Cooper C, et al. Anti-Osteoporosis Medication Prescriptions and Incidence of Subsequent Fracture Among Primary Hip Fracture Patients in England and Wales: An Interrupted Time-Series Analysis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016;31(11):2008–15. doi: 10.1002/jbmr.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis and rheumatism. 2004;50(4):1051–65. doi: 10.1002/art.20159. [DOI] [PubMed] [Google Scholar]
- 35.Lipsky PE, van der Heijde DM, Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. The New England journal of medicine. 2000;343(22):1594–602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 36.Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372(9636):375–82. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- 37.Barton P. Development of the Birmingham Rheumatoid Arthritis Model: past, present and future plans. Rheumatology (Oxford) 2011;50(Suppl 4):iv32–iv8. doi: 10.1093/rheumatology/ker244. [DOI] [PubMed] [Google Scholar]
- 38.National Joint Registry for England and Wales: 8th Annual Report. 2011.
- 39.Nymark T, Lauritsen JM, Ovesen O, Rock ND, Jeune B. Short time-frame from first to second hip fracture in the Funen County Hip Fracture Study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2006;17(9):1353–7. doi: 10.1007/s00198-006-0125-y. [DOI] [PubMed] [Google Scholar]
- 40.Lehtimaki MY, Kaarela K, Hamalainen MM. Incidence of hip involvement and need for total hip replacement in rheumatoid arthritis. An eight-year follow-up study. Scand J Rheumatol. 1986;15(4):387–91. doi: 10.3109/03009748609098209. [DOI] [PubMed] [Google Scholar]
- 41.Duthie RB, Harris CM. A radiographic and clinical survey of the hip joint in sero-positive rheumatoid arthritis. Acta Orthop Scand. 1969;40(3):346–64. doi: 10.3109/17453676908989513. [DOI] [PubMed] [Google Scholar]
- 42.Eberhardt K, Fex E, Johnsson K, Geborek P. Hip involvement in early rheumatoid arthritis. Ann Rheum Dis. 1995;54(1):45–8. doi: 10.1136/ard.54.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly IG. Surgical treatment of the rheumatoid hip. Ann Rheum Dis. 1990;49(Suppl 2):858–62. doi: 10.1136/ard.49.suppl_2.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and rheumatism. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 45.Fransen J, S G, van Riel Piet LCM. Rheumatoid Arthritis Measures. Arthritis & Rheumatism. 2003;49(5S):214–24. [Google Scholar]
- 46.National Joint Registry for England and Wales: 12th Annual Report. 2015.
- 47.Judge A, Wallace G, Prieto-Alhambra D, Arden NK, Edwards CJ. Can the publication of guidelines change the management of early rheumatoid arthritis? An interrupted time series analysis from the United Kingdom. Rheumatology (Oxford) 2015;54(12):2244–8. doi: 10.1093/rheumatology/kev268. [DOI] [PubMed] [Google Scholar]
- 48.Moura CS, Abrahamowicz M, Beauchamp ME, Lacaille D, Wang YS, Boire G, et al. Early medication use in new-onset rheumatoid arthritis may delay joint replacement: results of a large population-based study. Arthritis Research & Therapy. 2015;17 doi: 10.1186/s13075-015-0713-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luqmani R, Hennell S, Estrach C, Birrell F, Bosworth A, Davenport G, et al. British society for rheumatology and british health professionals in rheumatology guideline for the management of rheumatoid arthritis (the first two years) Rheumatology. 2006;45(9):1167–9. doi: 10.1093/rheumatology/kel215a. [DOI] [PubMed] [Google Scholar]
- 50.Penfold RB, Zhang F. Use of Interrupted Time Series Analysis in Evaluating Health Care Quality Improvements. Acad Pediatr. 2013;13(6):S38–S44. doi: 10.1016/j.acap.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Watson K, Symmons D, Griffiths I, Silman A. The British Society for Rheumatology biologics register. Ann Rheum Dis. 2005;64(Suppl 4):iv42–3. doi: 10.1136/ard.2005.042499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.