Abstract
Oxidative stress (OS) has been linked to the etiology and development of leukemia as reactive oxygen species (ROS) and free radicals have been implicated in leukemogenesis. OS has beneficial and deleterious effects in the pathogenesis and progression of leukemia. High-dose chemotherapy, which is frequently used in leukemia treatment, is often accompanied by ROS-induced cytotoxicity. Thus, the utilization of chemotherapy in combination with antioxidants may attenuate leukemia progression, particularly for cases of refractory or relapsed neoplasms. The present review focuses on exploring the roles of OS in leukemogenesis and characterizing the associations between ROS and chemotherapy. Certain examples of treatment regimens wherein antioxidants are combined with chemotherapy are presented, in order to highlight the importance of antioxidant application in leukemia treatment, as well as the conflicting opinions regarding this method of therapy. Understanding the underlying mechanisms of OS generation will facilitate the elucidation of novel approaches to leukemia treatment.
Keywords: oxidative stress, reactive oxygen species, leukemogenesis, chemotherapy
1. Introduction
Oxidative stress (OS) refers to the cellular environment conditions that result from an imbalance between the generation of reactive oxygen species (ROS) and the response of the antioxidant defense systems (1). ROS are short-lived highly reactive molecules and serve a critical role in the progression of OS. ROS were identified as free radicals for the first time in 1954 by Gerschman (2). They are metabolites produced during normal cellular processes, which serve important roles in activities such as promoting health and longevity (3) and antimicrobial phagocytosis by cells of the innate immune system (4,5). The over-generation of ROS without an adequate response from the innate antioxidant system to maintain the homeostasis eventually leads to OS. ROS serve a dual role in tumorigenicity, particularly in hematologic malignancies. ROS can induce the activation of cell death processes, including apoptosis, which provides a mechanism for cancer treatment (6); however, it can also facilitate carcinogenesis by protecting the cell from apoptosis and promoting cell survival, inducing proliferation (7), migration (8), metastasis (9) and drug-resistance (10,11). It has been reported that OS is involved in the development of a number of hematologic malignancies, including acute myeloid leukemia (AML), chronic myeloid leukemia (CML), myelodysplastic syndrome (MDS) and acute lymphoblastic leukemia (ALL) (12–16). Numerous methods including the use of chemotherapeutic agents and radiation are reported to generate ROS or other free radicals in patients undergoing cancer therapy.
The present review focused on exploring the role of OS in leukemogenesis and determining the association between ROS and chemotherapy, as well as highlighting the importance of antioxidant application in leukemia treatment. Improving current understanding of the underlying mechanisms of OS generation in leukemogenesis will facilitate significant progress in developing novel therapeutic measures for various types of leukemia.
2. OS and the generation of ROS
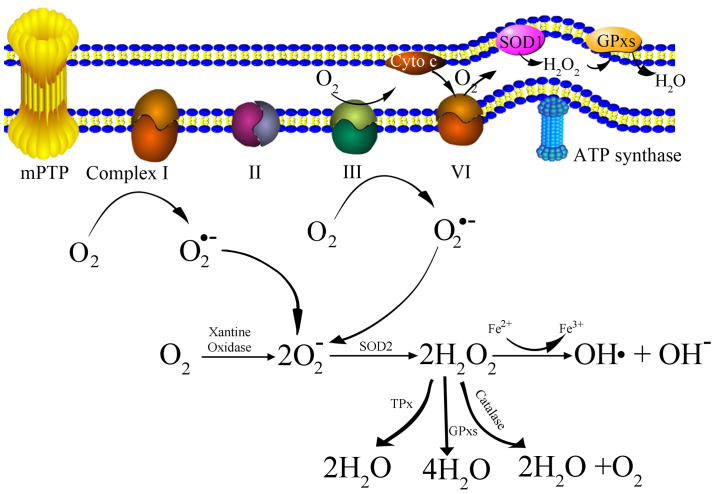
OS is a biochemical condition that occurs when intracellular antioxidants are unable to neutralize pro-oxidants, such as ROS. Mitochondria are the primary sites for oxidative phosphorylation, which produces massive highly reactive and unstable oxygen, thus oxidizing a large number of molecules to form ROS (17). ROS are generated intracellularly within various compartments and through multiple mechanisms (Table I). Mitochondria-derived ROS consist of singlet oxygen (O2), superoxide anions (O2•-), hydrogen peroxide (H2O2), nitric oxide (NO•), hydroxyl radicals (OH•) and hydroxyl ions (OH-). The generation of mitochondria-derived ROS is presented as a schematic in Fig. 1. Initially, oxygen is catalyzed to transform into a superoxide anion by xanthine oxidase (XO) (17,18), or by mitochondrial respiratory chain complexes I (NADH dehydrogenase) and III (bc1 complex) either in the matrix or in the intermembrane space (19). Subsequently, the superoxide anion is converted to H2O2 by superoxide dismutase (SOD). H2O2 can be detoxified to H2O and O2 with glutathione peroxidase, catalase (CAT) or thioredoxin peroxidase (TPx) (20). It can also be transformed into an OH• and an OH-via the Fenton reaction (21).
Table I.
Major intracellular sources of ROS.
| Reactive oxygen species | Intracellular sources | Compartment |
|---|---|---|
| O2 | Fenton reaction | Mitochondria |
| Lipid peroxidation chain reactions | Cytosol | |
| Haber-Weiss reaction | Peroxisomes | |
| Superoxide dismutase (SOD)-mediated reaction | Nucleus | |
| Catalase-mediated reaction | Plasma membrane | |
| Glutathione peroxidase-mediated reaction | Endoplasmic reticulum | |
| Xanthine oxidase (XO)-mediated reaction | Lysosome | |
| All membranes | ||
| OH• | Proton-catalyzed decomposition of peroxynitrite | Mitochondria |
| Fenton reaction | Cytosol | |
| Haber-Weiss reaction | Endoplasmic reticulum | |
| Decomposition of ozone (O3) | Lysosome | |
| Beckman-Radi-Freeman pathway | ||
| H2O2 | Superoxide dismutase (SOD)-mediated reaction | Mitochondria |
| NADPH oxidase-mediated reaction | Cytosol | |
| Cytochrome P450-mediated reaction | Peroxisomes | |
| Xanthine oxidase (XO)-mediated reaction | Plasma membrane | |
| Monoamine oxidases (MAO)-mediated reaction | Endosomes | |
| Peroxisomal fatty acid oxidation | Endoplasmic reticulum | |
| Flavin adenine dinucleotide (FAD)-mediated reaction | Lysosome | |
| Antibody-catalyzed water (H2O) oxidation | Nucleus | |
| Electron-transfer flavoprotein pathway | ||
| O2•− | Fenton reaction | Mitochondria |
| NADH/NADPH oxidase (NOX)-mediated reaction | Cytosol | |
| Xanthine oxidase (XO)-mediated reaction | Plasma membrane | |
| Lipoxygenase pathway | Peroxisomes | |
| Cyclooxygenase pathway | Nucleus | |
| Cytochrome P450 monooxygenase reaction | Endoplasmic reticulum | |
| Mitochondrial oxidative phosphorylation | ||
| Electron-transfer flavoprotein reaction | ||
| Hemoglobin auto-oxidation (within erythrocyte) | ||
| Nitric oxide synthases (NOS)-mediated reaction | ||
| HOCL, HOBr, HOI, and HOSCN | Eosinophil peroxidase (EPX)-mediated reaction (within eosinophil granulocytes) | Cytosol |
| Myeloperoxidase (MPO)-dependent oxidation (within neutrophil granulocytes) | Endoplasmic reticulum | |
| Lysosome | ||
| Vacuole | ||
| Plasma membrane | ||
| Mitochondria | ||
| Nucleus | ||
| OH− | Fenton reaction | Mitochondria |
| Haber-Weiss reaction | Cytosol | |
| Hydroperoxide (ROOH) decomposition | Endoplasmic reticulum | |
| Lysosome | ||
| O2•2− | Peroxide is unstable molecule. Hydrogen peroxide is more stable molecule | Mitochondria |
| formed as described above. | Cytosol | |
| Peroxisomes | ||
| Plasma membrane | ||
| Endosomes | ||
| Endoplasmic reticulum | ||
| Lysosome | ||
| Nucleus | ||
| O3 | Ozone (O3) is unstable molecule generated during antibody catalyzed | Cytosol |
| oxidation of H2O to H2O2 | Mitochondria | |
| NO• | Nitric oxide synthases (NOS)-mediated nitrite (NO2-) reduction | Cytosol |
| Xanthine oxidase (XO) reducing nitrates and nitrites | Peroxisomes | |
| Endoplasmic reticulum | ||
| Plasma membrane | ||
| Nucleus | ||
| ONOO− | Fenton reaction | Mitochondria |
| Rapid reaction of singlet oxygen (O2) and nitric oxide radical (NO•) | Cytosol | |
| The reaction of hydrogen peroxide (H2O2) with nitrite (NO2-) | Lysosome | |
| Endoplasmic reticulum | ||
| Nucleus | ||
| Peroxisomes | ||
| ROO•/RCOO•(Peroxyl radical) | Lipid peroxidation chain reactions | Cytosol |
| Synthesis of eicosanoids | Plasma membrane | |
| Hydroperoxide (ROOH) decomposition induced by heat or radiation | Peroxisomes | |
| ROOH reaction with transition metal ions and other oxidants capable | Endoplasmic reticulum | |
| of abstracting hydrogen | Mitochondria | |
| Nucleus | ||
| Lysosome | ||
| All membranes | ||
| HO2 | Fenton reaction | Mitochondria |
| Cytosol | ||
| Endoplasmic reticulum | ||
| Lysosome | ||
| ROOH/RCOOH | Lipoxygenase-mediated reaction | Cytosol |
| Oxidation of biomolecules, including lipids, proteins and DNA | Plasma membrane | |
| Cyclooxygenase reaction | Nucleus | |
| Cytochrome P450 monooxygenase reaction | Endoplasmic reticulum | |
| Heme-peroxidase turnover | Mitochondria | |
| Peroxisomes | ||
| Lysosome | ||
| R•, RO•, R-S• | Hydroperoxide (ROOH) decomposition induced by heat or radiation | Cytosol |
| ROOH reaction with transition metal ions and other oxidants capable | Plasma membrane | |
| of abstracting hydrogen | Mitochondria | |
| Lipid peroxidation chain reactions | Lysosome | |
| Peroxisomes | ||
| Endoplasmic reticulum | ||
| Nucleus | ||
| All membranes | ||
| CO3•− | The reaction between peroxynitrite and CO2 | Mitochondria |
| SOD-mediated reaction | Cytosol | |
| XO-mediated reaction | Peroxisomes | |
| Metal-ion catalyzed decomposition of HCO4− | Endoplasmic reticulum | |
| Peroxisomes | ||
| Lysosome | ||
| Vacuole |
Major intracellular sources of ROS. O2, singlet oxygen; OH•, hydroxyl radical; H2O2, hydrogen peroxide; O2•-, superoxide anion; HOCL, HOBr, HOI, HOSCN, hypochlorous acid and associated species; OH-, hydroxyl ion; O2•2−, peroxide; O3, ozone; NO•, nitric oxide radical; ONOO-, peroxynitrite; ROO•/RCOO•, peroxyl radical; HOO•, hydroperoxy radical; ROOH/RCOOH, organic hydroperoxide; R•; RO• R-S•, Organic radicals; CO3•-, carbonate radical; SOD, superoxide dismutase; XO, xanthine oxidase; HCO4-, peroxymonocarbonate.
Figure 1.
Schematic representation of the generation of mtROS. Complex I, NADH dehydrogenase; II, succinate dehydrogenase; III, bc 1 complex; IV, cytochrome c oxidase; V, ATP synthase; Cyto c, cytochrome c; mPTP, mitochondrial permeability transition pore; SOD, superoxide dismutase; GPxs, glutathione peroxidase; TPx, thioredoxin peroxidase; mtROS, mitochondrial derived reactive oxygen species.
3. Basic ways OS causes cell injury
OS causes cell injury predominantly via the following three basic pathways: Lipid peroxidation of membranes; oxidative modification of proteins; and DNA damage (17). Lipid peroxidation affects cell membranes and other lipid-containing structure via a process known as the ‘chain reaction of lipid peroxidation’. The critical intermediate products of this reaction are hydroperoxides (LOOHs), which can disturb the membrane structure and endanger cells (22,23). It has been reported that the direct secondary products of lipid peroxidation are aldehydes, malondialdehyde (MDA) and 4-hydroxynonenal/4-hydroxy-2-nonenal (HNE) (24). These products are considered to be the markers of OS, and their unique property of a no-charge structure allows them to easily permeate through membranes and into the cytosol, thus causing far-reaching and damaging effects inside and outside the cells, rendering them superior to ROS (25,26). There is evidence that HNE and MDA can cause protein or nucleic acid damage by modifying the amino acid residues to form stable adducts or covalent adducts with nucleic acids and membrane lipids (27,28).
Oxidative modification of proteins is another pathway by which OS causes cell damage, and thus serves a critical role in aging and cancer (29). MDA and HNE can react with and covalently modify numerous proteins, including amyloid-β peptide, collapsing response mediator protein-2 (CRMP2) and heat shock protein 70 (HSP70) (17,27,28). HNE- and MDA-protein adducts, including alpha-enolase (ENO1), phosphoglycerate kinase 1 (PGK1), triosephosphate isomerase (TPI) and pyruvate kinase (PK), are reported to be involved in cellular senescence and cancer (30–33). Besides MDA and HNE, ROS-mediated protein oxidation also can be measured via the concentration of carbonyl groups, advanced oxidation protein products (AOPPS), advanced glycation end products (AGE) and S-nitrosylated proteins, which are considered to be novel markers for OS due to their long half-life and their ease of detection (34).
With respect to oxidative DNA damage, ROS and products of lipid peroxidation can have an effect on genomic and mitochondrial DNA, leading to various types of DNA damage (35,36). The replication of damaged DNA prior to repair results in DNA mutations and genomic instability, subsequently leading to a variety of disorders and tumorigenesis. The molecule 8-oxoGuanine (8-OHG) and its nucleoside form 8-OHdG are considered to be indicators of oxidative DNA damage in vivo and in vitro (37,38). The presence of 8-OHG in the DNA caused a G-T and a C-A transversion, as 8-OHG allows the incorporation of cytosine and adenine nucleotides opposite the lesion during DNA replication (39,40). Numerous studies have reported that 8-OHG/8-OHdG is involved in carcinogenesis and altered level of them demonstrated an association with pathogenesis of aging associated disease and cancer (41–43). For example, Ames and colleagues have found the age-dependent accumulation of 8-OHdG in DNA from various aged rat organs (44) and increased levels of 8-OHdG and OH8Gua were shown in senescent human diploid fibroblast (45). Mitochondrial dysfunction and the lack of protective mechanisms mean that mitochondrial DNA can be more easily and extensively exposed to ROS than nuclear DNA, which can result in irreversible DNA damage. In general, ROS and other OS-products attack cells through a variety of intricate pathways. The lipid peroxidation of membranes, the oxidative modification of proteins and DNA damage are the major known mechanisms for oxidative cell damage. Improved understanding the molecular mechanisms associated with OS will assist in the development of novel and reliable treatments, as well as preventive measures, for various types of cancer, particularly for leukemia.
4. Dual role of OS in leukemogenesis
Leukemia develops when hematopoietic stem cells (HSC) lose the capacity to differentiate normally into mature blood cells at various stages during maturation and differentiation (46). Hypoxia has emerged as a key regulator of stem cell biology and maintains HSC quiescence with a condition of metabolic dormancy based on anaerobic glycolysis, which causes low production of ROS and high antioxidant defense (47,48). While hematopoietic cell differentiation is accompanied by changes in oxidative metabolism, including a decrease in anaerobic glycolysis and an increase in oxidative phosphorylation, thus producing high levels of ROS (49–51). Furthermore, evidences have indicated that leukemia stem cells (LSC) are more dependent on oxidative respiration and are more sensitive to OS, compared with normal HSCs (16). Although OS has been linked to the etiology and development of leukemia, numerous chemotherapeutic drugs exert their biological effects via the induction of OS in affected cells. Thus OS serves a dual role in leukemogenesis. ROS have a pathogenic role in various leukemia models, including CML, MDS and AML (14,52). First, BCR-ABL induces ROS production, which then contributes to malignant transformation, cell growth, resistance to apoptosis and increased DNA damage (53–55). Second, FLT3-ITD mutants induce increased production of ROS, which are responsible for increased DNA double-strand breaks and repair errors (56). Third, activated mutant Ras (N-Ras or H-Ras) induces the production of superoxide and H2O2 in human CD34+ cells through the stimulation of NOX-1 (NADPH oxidase 1) activity; this effect promotes the growth factor-independent proliferation of these cells (57).
Conversely, ROS and lipid peroxidation by-products are reported to be involved in mitochondria-derived apoptosis and the induction of cell death (6). It has been reported that ROS or lipid peroxidation by-products primarily react to cardiolipin molecules in the inner mitochondrial membrane (IMM), which disturbs the cytochrome c-cardiolipin interaction and promotes the release of cytochrome c into the cytoplasm, finally resulting in caspase activation and causing cell death (58,59). It has also been demonstrated that HNE reacts with the surrounding molecules near the site of its formation, thereby stimulating chain-reactions of mitochondria-derived apoptosis (60). A recent study explored the molecular mechanisms responsible for the leukemogenesis effect of MLL-AF9 and revealed an essential role of MEIS1 (61). MEIS1 expression in these leukemia types limits the extent of OS and responses for leukemia cell survival, while MEIS1 knockdown in MLL-AF9 leukemic cells induces ROS production and the inhibition of leukemic cell growth. Furthermore, a prior study published by our group demonstrated that increased intracellular ROS levels are important for the induction of cell death and the downregulation of BCR-ABL (62).
Furthermore, ROS participate in numerous cell growth pathways by interfering with the regulation of certain genes and signal transduction pathways, including tumor protein p53 mutation, activator protein-1 (AP-1) activation, vascular endothelial growth factor (VEGF) or rat sarcoma/mitogen activated protein kinase (Ras/MAPK), nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) signal pathway and the phosphatidylinositide 3-kinase/protein kinase B (PI3K/AKT) pathway (63). Ras/MAPK cascades consisting of mitogen-activated protein kinase (ERK1/2), c-Jun N-terminal kinase (JNK), p38 and 14-3-3β binds to big mitogen-activated protein kinase 1 (BMK1/ERK5) pathways (64) are involved in cytokines and growth factors signaling transmission. The latter, including tumor necrosis factor (TNF)-α, interferon gamma (IFN-γ), epidermal growth factor (EGF) and platelet-derived growth factor (PDGF), bind to their receptors under extracellular or intracellular stimuli and subsequently activate a series of MAP kinases (MAPKKK, MAPKK, MAPK). The activated MAPKs phosphorylate various substrate proteins, resulting in the regulation of various cellular activities (65–67). Each of aforementioned processes may be a target of ROS regulation. For example, it has been demonstrated that ROS activates the receptors of EGF and PDGF without corresponding ligands, thus stimulating Ras and activating the ERK pathway (68,69). Furthermore, in certain cells, treatment with H2O2 leads to the phosphorylation and activation of phospholipase C-γ (PLC-γ), and results in the generation of inositol trisphosphate (IP3) and diacylglycerol (DAG). The increase of the IP3 and DAG induces the release of calcium from intracellular stores, and activates numerous forms of Protein Kinase C (PKC), leading to the activation of Ras and Raf and the initiation of ERK signaling (70,71). Akt is a serine/threonine kinase, recruited to the cell membrane by PI3K and activated via phosphorylation. The end result of PI3K/Akt pathway activation is the stimulation of growth pathways and the inhibition of apoptosis, or vice versa. ROS not only activate PI3K directly to amplify its downstream signaling, but also concurrently inactivate its negative regulator PTEN (72). For example, it is reported that ROS can induce the phosphorylation of PTEN via casein kinase II, thus urging it to enter the proteolytic degradation pathway (73). ROS influence the NK-κB pathway mainly through inhibiting IκBα phosphorylation and degradation, thus activating the NK-κB pathway. In addition, IKK is the primary target for ROS through S-glutathionylation of the IKKβ on cysteine 179, resulting in the inhibition of IKKβ activity (74,75).
5. Association between OS and chemotherapy during leukemia treatment
The current therapy for leukemia primarily consists of high-dose cytotoxic chemotherapy with or without allogeneic stem cell transplantation. However, chemotherapeutic treatments are often accompanied by elevated ROS levels, and cause drug-intolerance or resistance correspondingly (75). The underlying mechanisms may be closely associated with the aforementioned ROS-mediated signaling pathway. Chemotherapy impairs the mitotic and metabolic process of cancer cells, involving various signal transmission abnormalities or sub-cellular organ damage, thus causing excess ROS production. Angsutararux et al (75) studied doxorubicin (DOX)-induced cardiotoxicity, and proposed that DOX is particularly harmful to the heart due to its exceptional effects on mitochondria, which are the home of ROS. Petrola et al (76) performed a clinical trial to evaluate OS through detecting the levels of MDA and nitrite in patients with CML undergoing treatment with 1st and 2nd generation TKIs. The results indicated that TKIs caused significantly high concentration of ROS in patients CML who were undergoing these treatments, and that oxidative damage markers could indicate resistance to TKIs. Furthermore, it has been demonstrated that anthracyclines, including DOX, a type of important component of current cancer treatment, generate high levels of ROS and cause severe chemotherapy-associated cardiotoxicity (77–79). Therefore, combinations of antioxidants and chemotherapeutic agents perhaps have promising synergistic effects (80). The role of OS in DOX-induced cardiotoxicity can be attenuated in a transgenic mouse model containing high levels of cardiac metallothionein, a potent antioxidant (81). Nakayama et al (82) conducted a systematic review of published clinical trials to examine the effects of dietary antioxidants taken concurrently with chemotherapy or radiation therapy. The results indicated that glutathione (GSH), vitamin E and N-acetysteine (NAC) were the most frequently used antioxidant supplements in combination with chemotherapy or radiation therapy for kinds of cancer treatments, including leukemia. GSH combined with cisplatin (CDDP)-based chemotherapy accord for 88% of all the experiments (23/26), and adding GSH to CDDP-based chemotherapy could improve the antitumor response against solid tumors and hematological malignancies; some also revealed a neuroprotective effect. Another study reported a trend of longer clinical PFS and OS in patients with CML when they were treated with vitamin A in combination with standard chemotherapy, although this trend was not statistically significant (83).
However, there are conflicting opinions regarding the administration of antioxidants during cancer therapy. Certain researchers suppose that it may reduce the effectiveness of chemotherapies, which are based on increasing oxidative stress. For example, Hewish et al (84) revealed that cytarabine was toxic to MLH1 and MLH2 deficient tumor cells, but this cytotoxicity was reduced by antioxidants. In general, the combination of antioxidants and chemotherapy is a promising strategy for cancer treatment, a number of other studies have argued their antagonistic effects. Further studies on the use of this specific combined therapy are required, and further synergistic effects must be investigated and elucidated.
6. Conclusions
Leukemia is a type of hematological neoplasm characterized by the abnormal proliferation and circulation of immature clonal hematopoietic cells in the blood or bone marrow (85). OS has been implicated in leukemogenesis and serves an important role in cell proliferation and cell signaling regulation. Abnormalities in the oxidative-antioxidative balance have been observed in numerous cases of leukemia neoplasm, including ALL, B-CLL and MM.
Indeed, leukemia cells produce higher concentrations of ROS than non-leukemic cells83. OS has beneficial and deleterious effects on leukemogenesis. On the one hand, it promotes leukemia progression through activating oncogenes, including Ras and VEGF, and the NF-κB signal transduction pathway. Conversely, mitochondria-derived apoptosis can olso be induced by OS and causes cell death. It is difficult to separate the oncogenic properties from the tumor suppressive activity. Therefore, an improved understanding of the association between OS and leukemogenesis will provide more insight for leukemia treatments. Chemotherapy is a commonly used strategy for leukemia treatment. However, the current cytotoxic drugs available for use in standard leukemia therapy are often accompanied by elevated ROS production, and cause drug-intolerance or resistance. Thus, targeting ROS levels during chemotherapy could constitute a novel approach for various types of leukemia, particularly for those of refractory and relapsed hematological neoplasms. Indeed, studies have demonstrated that antioxidant treatments combined with chemotherapy are effective in leukemia therapy, but their concurrent negative effects have also been recorded. Therefore, further studies are required to explore the synergistic effects, long-term effects and consequences of using these combination therapies. Potentially, targeted OS therapy in combination with chemotherapy or other strategies may become a clinically useful therapeutic approach for various types of hematological diseases in the near future.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81670178, 81370645), the Hangzhou Science and Technology Bureau (grant no. 20140633B06) and the Special Scientific Construction Research Funds of National Chinese Medicine Clinical Research Center, SATCM (grant no. JDZX2015113).
Competing interests
The authors declare that they have no competing interests.
References
- 1.Imbesi S, Musolino C, Allegra A, Saija A, Morabito F, Calapai G, Gangemi S. Oxidative stress in oncohematologic diseases: An update. Expert Rev Hematol. 2013;6:317–325. doi: 10.1586/ehm.13.21. [DOI] [PubMed] [Google Scholar]
- 2.Gerschman R, Gilbert D, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and X-irradiation: A mechanism in common. 1954. Nutrition. 2001;17:162. [PubMed] [Google Scholar]
- 3.Asthana J, Yadav AK, Pant A, Pandey S, Gupta MM, Pandey R. Specioside ameliorates oxidative stress and promotes longevity in Caenorhabditis elegans. Comp Biochem Physiol C Toxicol Pharmacol. 2015;169:25–34. doi: 10.1016/j.cbpc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Weyemi U, Caillou B, Talbot M, Ameziane-El-Hassani R, Lacroix L, Lagent-Chevallier O, Al Ghuzlan A, Roos D, Bidart JM, Virion A, et al. Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr Relat Cancer. 2010;17:27–37. doi: 10.1677/ERC-09-0175. [DOI] [PubMed] [Google Scholar]
- 6.Khoshtabiat L, Mahdavi M, Dehghan G, Rashidi MR. Oxidative stress-induced apoptosis in chronic myelogenous leukemia K562 cells by an active compound from the dithio-carbamate family. Asian Pac J Cancer Prev. 2016;17:4267–4273. [PubMed] [Google Scholar]
- 7.Cheng D, Zhao L, Xu Y, Ou R, Li G, Yang H, Li W. K-Ras promotes the non-small lung cancer cells survival by cooperating with sirtuin 1 and p27 under ROS stimulation. Tumour Biol. 2015;36:7221–7232. doi: 10.1007/s13277-015-3429-8. [DOI] [PubMed] [Google Scholar]
- 8.Weyemi U, Lagente-Chevallier O, Boufraqech M, Prenois F, Courtin F, Caillou B, Talbot M, Dardalhon M, Al Ghuzlan A, Bidart JM, et al. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene. 2012;31:1117–1129. doi: 10.1038/onc.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu D, Shen Z, Liu J, Chen J, Liu Y, Hu C, Li Z, Li Y. The ROS-mediated activation of STAT-3/VEGF signaling is involved in the 27-hydroxycholesterol-induced angiogenesis in human breast cancer cells. Toxicol Lett. 2016;264:79–86. doi: 10.1016/j.toxlet.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Lina M, Hongfei M, Yunxin X, Dai W, Xilin Z. The mechanism of ROS regulation of antibiotic resistance and antimicrobial lethality. Yi Chuan. 2016;38:902–909. doi: 10.16288/j.yczz.16-157. [DOI] [PubMed] [Google Scholar]
- 11.Das DS, Ray A, Das A, Song Y, Tian Z, Oronsky B, Richardson P, Scicinski J, Chauhan D, Anderson KC. A novel hypoxia-selective epigenetic agent RRx-001 triggers apoptosis and overcomes drug resistance in multiple myeloma cells. Leukemia. 2016;30:2187–2197. doi: 10.1038/leu.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Udensi UK, Tchounwou PB. Dual effect of oxidative stress on leukemia cancer induction and treatment. J Exp Clin Cancer Res. 2014;33:106. doi: 10.1186/s13046-014-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battisti V, Maders LD, Bagatini MD, Santos KF, Spanevello RM, Maldonado PA, Brulé AO, Araújo Mdo C, Schetinger MR, Morsch VM. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clin Biochem. 2008;41:511–518. doi: 10.1016/j.clinbiochem.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Chung YJ, Robert C, Gough SM, Rassool FV, Aplan PD. Oxidative stress leads to increased mutation frequency in a murine model of myelodysplastic syndrome. Leuk Res. 2014;38:95–102. doi: 10.1016/j.leukres.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pawlowska E, Blasiak J. DNA repair-a double-edged sword in the genomic stability of cancer cells-the case of chronic myeloid leukemia. Int J Mol Sci. 2015;16:27535–27549. doi: 10.3390/ijms161126049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Testa U, Labbaye C, Castelli G, Pelosi E. Oxidative stress and hypoxia in normal and leukemic stem cells. Exp Hematol. 2016;44:540–560. doi: 10.1016/j.exphem.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Kudryavtseva AV, Krasnov GS, Dmitriev AA, Alekseev BY, Kardymon OL, Sadritdinova AF, Fedorova MS, Pokrovsky AV, Melnikova NV, Kaprin AD, et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. 2016;7:44879–44905. doi: 10.18632/oncotarget.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanganahalli BG, Joshi PG, Joshi NB. Xanthine oxidase, nitric oxide synthase and phospholipase A(2) produce reactive oxygen species via mitochondria. Brain Res. 2005;1037:200–203. doi: 10.1016/j.brainres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Aprioku JS. Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J Reprod Infertil. 2013;14:158–172. [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao K, Zeng Q, Bai J, Li J, Xia L, Chen S, Zhou B. Enhanced organic pollutants degradation and electricity production simultaneously via strengthening the radicals reaction in a novel Fenton-photocatalytic fuel cell system. Water Res. 2017;108:293–300. doi: 10.1016/j.watres.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin H, Xu L, Porter NA. Free radical lipid peroxidation: Mechanisms and analysis. Chem Rev. 2011;111:5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 24.Breitzig M, Bhimineni C, Lockey R, Kolliputi N. 4-Hydroxy-2-nonenal: A critical target in oxidative stress? Am J Physiol Cell Physiol. 2016;311:C537–C543. doi: 10.1152/ajpcell.00101.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Negre-Salvayre A, Auge N, Ayala V, Basaga H, Boada J, Brenke R, Chapple S, Cohen G, Feher J, Grune T, et al. Pathological aspects of lipid peroxidation. Free Radic Res. 2010;44:1125–1171. doi: 10.3109/10715762.2010.498478. [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Wang J, Fan X, Ansari GA, Khan MF. Protein adducts of malondialdehyde and 4-hydroxynonenal contribute to trichloroethene-mediated autoimmunity via activating Th17 cells: Dose- and time-response studies in female MRL+/+ mice. Toxicology. 2012;292:113–122. doi: 10.1016/j.tox.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domingues RM, Domingues P, Melo T, Pérez-Sala D, Reis A, Spickett CM. Lipoxidation adducts with peptides and proteins: Deleterious modifications or signaling mechanisms? J Proteomics. 2013;92:110–131. doi: 10.1016/j.jprot.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab Rev. 1998;30:225–243. doi: 10.3109/03602539808996310. [DOI] [PubMed] [Google Scholar]
- 30.Sharma NK, Sethy NK, Bhargava K. Comparative proteome analysis reveals differential regulation of glycolytic and antioxidant enzymes in cortex and hippocampus exposed to short-term hypobaric hypoxia. J Proteomics. 2013;79:277–298. doi: 10.1016/j.jprot.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Chen XL, Zhou L, Yang J, Shen FK, Zhao SP, Wang YL. Hepatocellular carcinoma-associated protein markers investigated by MALDI-TOF MS. Mol Med Rep. 2010;3:589–596. doi: 10.3892/mmr_00000302. [DOI] [PubMed] [Google Scholar]
- 32.Hu H, Zhu W, Qin J, Chen M, Gong L, Li L, Liu X, Tao Y, Yin H, Zhou H, et al. Acetylation of PGK1 promotes liver cancer cell proliferation and tumorigenesis. Hepatology. 2017;65:515–528. doi: 10.1002/hep.28887. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad SS, Glatzle J, Bajaeifer K, Bühler S, Lehmann T, Königsrainer I, Vollmer JP, Sipos B, Ahmad SS, Northoff H, et al. Phosphoglycerate kinase 1 as a promoter of metastasis in colon cancer. Int J Oncol. 2013;43:586–590. doi: 10.3892/ijo.2013.1971. [DOI] [PubMed] [Google Scholar]
- 34.Singh RK, Tripathi AK, Tripathi P, Singh S, Singh R, Ahmad R. Studies on biomarkers for oxidative stress in patients with chronic myeloid leukemia. Hematol Oncol Stem Cell Ther. 2009;2:285–288. doi: 10.1016/S1658-3876(09)50039-8. [DOI] [PubMed] [Google Scholar]
- 35.Rahal ON, Fatfat M, Hankache C, Osman B, Khalife H, Machaca K, Muhtasib HG. Chk1 and DNA-PK mediate TPEN-induced DNA damage in a ROS dependent manner in human colon cancer cells. Cancer Biol Ther. 2016;17:1139–1148. doi: 10.1080/15384047.2016.1235658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen CY, Yen CY, Wang HR, Yang HP, Tang JY, Huang HW, Hsu SH, Chang HW. Tenuifolide B from cinnamomum tenuifolium stem selectively inhibits proliferation of oral cancer cells via apoptosis, ROS generation, mitochondrial depolarization, and DNA damage. Toxins (Basel) 2016;8:pii: E319. doi: 10.3390/toxins8110319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kikuchi A, Takeda A, Onodera H, Kimpara T, Hisanaga K, Sato N, Nunomura A, Castellani RJ, Perry G, Smith MA, Itoyama Y. Systemic increase of oxidative nucleic acid damage in Parkinson's disease and multiple system atrophy. Neurobiol Dis. 2002;9:244–248. doi: 10.1006/nbdi.2002.0466. [DOI] [PubMed] [Google Scholar]
- 38.Weidner AM, Bradley MA, Beckett TL, Niedowicz DM, Dowling AL, Matveev SV, LeVine H, III, Lovell MA, Murphy MP. RNA oxidation adducts 8-OHG and 8-OHA change with Aβ42 levels in late-stage Alzheimer's disease. PLoS One. 2011;6:e24930. doi: 10.1371/journal.pone.0024930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991;349:431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- 40.Sunaga N, Kohno T, Shinmura K, Saitoh T, Matsuda T, Saito R, Yokota J. OGG1 protein suppresses G:C->T:A mutation in a shuttle vector containing 8-hydroxyguanine in human cells. Carcinogenesis. 2001;22:1355–1362. doi: 10.1093/carcin/22.9.1355. [DOI] [PubMed] [Google Scholar]
- 41.Lovell MA, Soman S, Bradley MA. Oxidatively modified nucleic acids in preclinical Alzheimer's disease (PCAD) brain. Mech Ageing Dev. 2011;132:443–448. doi: 10.1016/j.mad.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 43.Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, Fraifeld VE. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev. 2013;12:661–684. doi: 10.1016/j.arr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Fraga CG, Shigenaga MK, Park JW, Degan P, Ames BN. Oxidative damage to DNA during aging: 8-hydroxy-2′-deoxyguanosine in rat organ DNA and urine; Proc Natl Acad Sci USA; 1990; pp. 4533–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN. Oxidative DNA damage and senescence of human diploid fibroblast cells; Proc Natl Acad Sci USA; 1995; pp. 4337–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vyas P, Jacobsen SE. Clever leukemic stem cells branch out. Cell stem cell. 2011;8:242–244. doi: 10.1016/j.stem.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 47.Nombela-Arrieta C, Silberstein LE. The science behind the hypoxic niche of hematopoietic stem and progenitors. Hematology Am Soc Hematol Educ Program. 2014;2014:542–547. doi: 10.1182/asheducation-2014.1.542. [DOI] [PubMed] [Google Scholar]
- 48.Bigarella CL, Liang R, Ghaffari S. Stem cells and the impact of ROS signaling. Development. 2014;141:4206–4218. doi: 10.1242/dev.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rönn RE, Guibentif C, Saxena S, Woods NB. Reactive oxygen species impair the function of CD90+ hematopoietic progenitors generated from human pluripotent stem cells. Stem cells. 2017;35:197–206. doi: 10.1002/stem.2503. [DOI] [PubMed] [Google Scholar]
- 50.Cao Y, Fang Y, Cai J, Li X, Xu F, Yuan N, Zhang S, Wang J. ROS functions as an upstream trigger for autophagy to drive hematopoietic stem cell differentiation. Hematology. 2016;21:613–618. doi: 10.1080/10245332.2016.1165446. [DOI] [PubMed] [Google Scholar]
- 51.Kaur A, Jankowska K, Pilgrim C, Fraser ST, New EJ. Studies of hematopoietic cell differentiation with a ratiometric and reversible sensor of mitochondrial reactive oxygen species. Antioxid Redox Signal. 2016;24:667–679. doi: 10.1089/ars.2015.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hole PS, Darley RL, Tonks A. Do reactive oxygen species play a role in myeloid leukemias? Blood. 2011;117:5816–5826. doi: 10.1182/blood-2011-01-326025. [DOI] [PubMed] [Google Scholar]
- 53.Sattler M, Verma S, Shrikhande G, Byrne CH, Pride YB, Winkler T, Greenfield EA, Salgia R, Griffin JD. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J Biol Chem. 2000;275:24273–24278. doi: 10.1074/jbc.M002094200. [DOI] [PubMed] [Google Scholar]
- 54.Kim JH, Chu SC, Gramlich JL, Pride YB, Babendreier E, Chauhan D, Salgia R, Podar K, Griffin JD, Sattler M. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood. 2005;105:1717–1723. doi: 10.1182/blood-2004-03-0849. [DOI] [PubMed] [Google Scholar]
- 55.Koptyra M, Falinski R, Nowicki MO, Stoklosa T, Majsterek I, Nieborowska-Skorska M, Blasiak J, Skorski T. BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood. 2006;108:319–327. doi: 10.1182/blood-2005-07-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sallmyr A, Fan J, Datta K, Kim KT, Grosu D, Shapiro P, Small D, Rassool F. Internal tandem duplication of FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and misrepair: Implications for poor prognosis in AML. Blood. 2008;111:3173–3182. doi: 10.1182/blood-2007-05-092510. [DOI] [PubMed] [Google Scholar]
- 57.Hole PS, Pearn L, Tonks AJ, James PE, Burnett AK, Darley RL, Tonks A. Ras-induced reactive oxygen species promote growth factor-independent proliferation in human CD34+ hematopoietic progenitor cells. Blood. 2010;115:1238–1246. doi: 10.1182/blood-2009-06-222869. [DOI] [PubMed] [Google Scholar]
- 58.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 59.Liu Z, Lin H, Ye S, Liu QY, Meng Z, Zhang CM, Xia Y, Margoliash E, Rao Z, Liu XJ. Remarkably high activities of testicular cytochrome c in destroying reactive oxygen species and in triggering apoptosis; Proc Natl Acad Sci USA; 2006; pp. 8965–8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong H, Yin H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015;4:193–199. doi: 10.1016/j.redox.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roychoudhury J, Clark JP, Gracia-Maldonado G, Unnisa Z, Wunderlich M, Link KA, Dasgupta N, Aronow B, Huang G, Mulloy JC, Kumar AR. MEIS1 regulates an HLF-oxidative stress axis in MLL-fusion gene leukemia. Blood. 2015;125:2544–2552. doi: 10.1182/blood-2014-09-599258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou W, Zhu W, Ma L, Xiao F, Qian W. Proteasome inhibitor MG-132 enhances histone deacetylase inhibitor SAHA-induced cell death of chronic myeloid leukemia cells by an ROS-mediated mechanism and downregulation of the Bcr-Abl fusion protein. Oncol Lett. 2015;10:2899–2904. doi: 10.3892/ol.2015.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- 65.Pimienta G, Pascual J. Canonical and alternative MAPK signaling. Cell cycle. 2007;6:2628–2632. doi: 10.4161/cc.6.21.4930. [DOI] [PubMed] [Google Scholar]
- 66.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 67.Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. MAP kinases. Chem Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 68.León-Buitimea A, Rodríguez-Fragoso L, Lauer FT, Bowles H, Thompson TA, Burchiel SW. Ethanol-induced oxidative stress is associated with EGF receptor phosphorylation in MCF-10A cells overexpressing CYP2E1. Toxicol Lett. 2012;209:161–165. doi: 10.1016/j.toxlet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lei H, Kazlauskas A. Growth factors outside of the platelet-derived growth factor (PDGF) family employ reactive oxygen species/Src family kinases to activate PDGF receptor alpha and thereby promote proliferation and survival of cells. J Biol Chem. 2009;284:6329–6336. doi: 10.1074/jbc.M808426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Franklin RA, Atherfold PA, McCubrey JA. Calcium-induced ERK activation in human T lymphocytes occurs via p56(Lck) and CaM-kinase. Mol Immunol. 2000;37:675–683. doi: 10.1016/S0161-5890(00)00087-0. [DOI] [PubMed] [Google Scholar]
- 71.Dann SG, Golas J, Miranda M, Shi C, Wu J, Jin G, Rosfjord E, Upeslacis E, Klippel A. p120 catenin is a key effector of a Ras-PKCε oncogenic signaling axis. Oncogene. 2014;33:1385–1394. doi: 10.1038/onc.2013.91. [DOI] [PubMed] [Google Scholar]
- 72.Leslie NR, Downes CP. PTEN: The down side of PI 3-kinase signalling. Cell Signal. 2002;14:285–295. doi: 10.1016/S0898-6568(01)00234-0. [DOI] [PubMed] [Google Scholar]
- 73.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 74.Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, et al. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta; Proc Natl Acad Sci USA; 2006; pp. 13086–13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Angsutararux P, Luanpitpong S, Issaragrisil S. Chemotherapy-induced cardiotoxicity: Overview of the roles of oxidative stress. Oxid Med Cell Longev. 2015;2015:795602. doi: 10.1155/2015/795602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petrola MJ, de Castro AJ, Pitombeira MH, Barbosa MC, Quixadá AT, Duarte FB, Gonçalves RP. Serum concentrations of nitrite and malondialdehyde as markers of oxidative stress in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Rev Bras Hematol Hemoter. 2012;34:352–355. doi: 10.5581/1516-8484.20120091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheuk DK, Sieswerda E, van Dalen EC, Postma A, Kremer LC. Medical interventions for treating anthracycline-induced symptomatic and asymptomatic cardiotoxicity during and after treatment for childhood cancer. Cochrane Database Syst Rev: CD008011. 2016 doi: 10.1002/14651858.CD008011.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bartlett JJ, Trivedi PC, Yeung P, Kienesberger PC, Pulinilkunnil T. Doxorubicin impairs cardiomyocyte viability by suppressing transcription factor EB expression and disrupting autophagy. Biochem J. 2016;473:3769–3789. doi: 10.1042/BCJ20160385. [DOI] [PubMed] [Google Scholar]
- 79.Piegari E, Russo R, Cappetta D, Esposito G, Urbanek K, Dell'Aversana C, Altucci L, Berrino L, Rossi F, De Angelis A. MicroRNA-34a regulates doxorubicin-induced cardiotoxicity in rat. Oncotarget. 2016;7:62312–62326. doi: 10.18632/oncotarget.11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sabnis HS, Bradley HL, Tripathi S, Yu WM, Tse W, Qu CK, Bunting KD. Synergistic cell death in FLT3-ITD positive acute myeloid leukemia by combined treatment with metformin and 6-benzylthioinosine. Leuk Res. 2016;50:132–140. doi: 10.1016/j.leukres.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun X, Zhou Z, Kang YJ. Attenuation of doxorubicin chronic toxicity in metallothionein-overexpressing transgenic mouse heart. Cancer Res. 2001;61:3382–3387. [PubMed] [Google Scholar]
- 82.Nakayama A, Alladin KP, Igbokwe O, White JD. Systematic review: Generating evidence-based guidelines on the concurrent use of dietary antioxidants and chemotherapy or radiotherapy. Cancer Invest. 2011;29:655–667. doi: 10.3109/07357907.2011.626479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meyskens FL, Jr, Kopecky KJ, Appelbaum FR, Balcerzak SP, Samlowski W, Hynes H. Effects of vitamin A on survival in patients with chronic myelogenous leukemia: A SWOG randomized trial. Leuk Res. 1995;19:605–612. doi: 10.1016/0145-2126(95)00032-J. [DOI] [PubMed] [Google Scholar]
- 84.Hewish M, Martin SA, Elliott R, Cunningham D, Lord CJ, Ashworth A. Cytosine-based nucleoside analogs are selectively lethal to DNA mismatch repair-deficient tumour cells by enhancing levels of intracellular oxidative stress. Br J Cancer. 2013;108:983–992. doi: 10.1038/bjc.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kennedy JA, Barabé F. Investigating human leukemogenesis: From cell lines to in vivo models of human leukemia. Leukemia. 2008;22:2029–2040. doi: 10.1038/leu.2008.206. [DOI] [PubMed] [Google Scholar]