Abstract
Transcellular retrograde signaling from the postsynaptic target cell to the presynaptic neuron plays critical roles in the formation, maturation, and plasticity of synaptic connections. We here review recent progress in our understanding of the retrograde signaling at developing central synapses. Three forms of potential retrograde signals—membrane-permeant factors, membrane-bound factors, and secreted factors—have been implicated at both developing and mature synapses. Although many of these signals may be active constitutively, retrograde factors produced in association with activity-dependent synaptic plasticity, e.g., long-term potentiation and long-term depression, are of particular interest, because they may induce modification of neuronal excitability and synaptic transmission, functions directly related to the processing and storage of information in the nervous system.
Neural information coded by the action potential is transmitted through a chemical synapse in the anterograde direction by release of neurotransmitters, neuropeptides, and other protein factors from the presynaptic terminal. These molecules produce immediate changes in the membrane potential as well as long-term structural and metabolic changes in the postsynaptic cell. Over the past several decades, it has become increasingly clear that information exchange at the synapse is bidirectional: the postsynaptic cell also provides a variety of retrograde signals to the presynaptic neuron. This reciprocal interaction is crucial for the differentiation and maintenance of the presynaptic cell as well as the formation and maturation of the synapse. The general notion of retrograde signaling involves postsynaptic production of a signal, either constitutively or triggered by synaptic activity, that acts on the presynaptic neuron through the following mechanisms. First, the retrograde signal can be carried by a membrane-permeant molecule that diffuses across the plasma membranes from the postsynaptic cell directly into the presynaptic nerve terminal. Second, membrane-impermeant but soluble factors can be packaged and secreted via exocytotic vesicles by the postsynaptic cell and exert the retrograde action by binding and activation of receptors on the presynaptic membrane. Third, direct signaling through the synaptic cleft may be accomplished through mediation of physically coupled pre- and postsynaptic membrane-bound proteins, including transmembrane proteins as well as those secreted and immobilized in the extracellular matrix within the synaptic cleft. This review will summarize recent progress in the study of retrograde regulation at central synapses, with a focus on the role of retrograde interaction in the formation and activity-dependent plasticity of synapses. Some aspects of this subject have been more extensively reviewed elsewhere (1). An excellent review of signaling at developing neuromuscular junctions (NMJs) has also appeared recently (2).
Retrograde Signaling During Synaptogenesis
Our present understanding of the process of synaptogenesis is based largely on studies of developing NMJs (2, 3). For central nervous system neurons, the mechanisms governing synapse formation and the signaling molecules involved in this process are still poorly understood. As the elementary unit for information processing and storage in the brain, the central synapse must be formed and regulated under appropriate control. Synapses are formed only between specific pre- and postsynaptic partners and stabilized at specific locations on the dendrite. Synapse formation is accompanied by a coordinated development of pre- and postsynaptic molecular and structural specializations, which requires exchanges of information between pre- and postsynaptic cells at all stages of synapse development, via anterograde as well as retrograde signaling.
(i) Signaling Before Synaptic Contact.
After long-range axon pathfinding, growth cones approach their target cell, and the process of synapse formation begins. It is likely that navigation of the growth cone is stalled and axon differentiation begins in response to factors secreted from the target cell. A potential candidate for such a stalling or “synaptogenic” signal is WNT-7a, which is secreted by granule cells in the cerebellum and found to induce axon and growth cone remodeling in mossy fibers via presynaptic Frizzled receptors (4). The latter activates an intracellular signaling cascade that represses the activity of glycogen synthase 3β kinase, an enzyme known to regulate the microtubule cytoskeleton, and produces stable loop-like microtubules in the stalled growth cone. The loop-like conformation of microtubules is similar to that adopted during synapse formation (5, 6). At the same time, synapsin I, known to be involved in synapse formation, maturation, and synaptic vesicle transport (7, 8), was found to be clustered at the remodeled areas of the mossy fiber. Other potential “synaptogenic” factors include molecules that serve for the guidance of the axon. For example, a target-secreted neurotrophin, brain-derived neurotrophic factor (BDNF), is not only a chemotropic factor for axon growth in culture (9) and in vivo (10) but also a factor that promotes presynaptic transmitter secretion (11, 12).
(ii) Signaling During Initial Cell–Cell Contact and Recognition.
Before synaptogenesis and formation of postsynaptic spines, motile filopodia extending from the developing dendrites may increase the probability of encounters between the dendrite and approaching axon (13). The interaction between specific pre- and postsynaptic membrane components will lead to selective adhesion and physical stabilization of the contact. In addition, downstream signaling may be triggered in both pre- and postsynaptic cytoplasm, resulting in structural differentiation and changes in local subcellular activities.
It is generally assumed that the selective formation of synapses among the large variety of neuronal types (14, 15) depends on specific recognition molecules on the neuronal surface. Several groups of candidates have now been proposed. One is the cadherin family of adhesion molecules (16, 17), which are localized at synapses and have sufficient molecular diversity. Cadherins display homophilic binding preferences and exhibit synapse specificity. For example, different cadherins have been found to segregate into different synapses on the same neuron, and specific cadherin expression patterns are correlated with neuronal connection patterns in the brain (18). Interfering with cadherin function in vitro leads to the reduction of long-term potentiation (LTP) (19), suggesting a role of cadherin in synapse remodeling that may underlie LTP. In olfactory systems, targeting of the olfactory neuron axons to the specific glomerulus in the olfactory bulb may be accomplished through the cell-specific odorant receptors expressed by the olfactory neurons (20). On the basis of potential molecular diversity (21), the family of neurexins and their ligands neuroligins may serve as pre- and postsynaptic markers for central neurons. Many variants of neurexin exhibit differential expression patterns in the brain, leading to the suggestion that a given neuronal subpopulation expresses a unique set of neurexins that contribute to the specificity of neuronal contacts (22). Similarly, Drosophila Dscam, a family of putative axon guidance receptors with extraordinary molecular diversity, may also contribute to the specificity of neuronal connectivity (23). Finally, it remains a distinct possibility that the set of transmitter receptors and ion channels, which often uniquely identify a specific neuronal type, may serve in a combinatorial manner as cell-type-specific markers for cell–cell recognition via extracellular domains of these membrane proteins (24).
At NMJs, muscle contact can induce an immediate increase of Ca2+-dependent neurotransmitter release from the growth cone (25, 26). In neuron–neuron contact, the interaction between pre- and postsynaptic membrane components may also trigger exocytosis in both pre- and postsynaptic cells, resulting in secretion of transmitters and other factors, as well as insertion of synaptic membrane components. It was found in vitro that the preassembled complex of synaptic vesicle proteins, calcium channels, endocytotic proteins, and large dense-core synaptic vesicles is transported as a unit within the axon at a speed close to that predicted for kinesin-mediated microtubule-based transport (27). Physical contact between an axon and a dendrite stops the movement of the packets and induces the assembly of the presynaptic active zone, suggesting that adhesion between the pre- and postsynaptic cells may trigger the insertion of presynaptic membrane components and associated fusion machinery into the plasma membrane (28). Adhesion and membrane insertion thus may be linked events, as shown by the finding in epithelial cells that the site of cell–cell adhesion also becomes a site of exocytosis (29).
(iii) Signals for Synaptic Differentiation and Maturation.
After the initial stabilization of synaptic contact via binding of specific pre- and postsynaptic membrane components, further signaling events are required for subsequent differentiation of synaptic specializations involving recruitment and clustering of synaptic vesicles, neurotransmitter receptors, and ion channels. As discussed above, physical interaction between pre- and postsynaptic membrane proteins may directly trigger intracellular signaling that leads to synaptic differentiation. Recently, membrane protein-mediated signaling pathways have been shown to take part in the formation of central synapses. Scheiffele et al. (30) demonstrated that contact-mediated intercellular signaling via neuroligin–neurexin interaction is sufficient to drive presynaptic terminal differentiation in vitro. In this study, contact with cocultured nonneuronal cells expressing neuroligin-1 was found to trigger clustering of synaptic vesicles in the pontine axons, and those contact sites exhibit functional and morphological properties of neuron–neuron synapses. This neuroligin activity requires the extracellular domain of the protein and can be inhibited by addition of soluble β-neurexin (neuroligin-1 receptor). Although the presynaptic localization of neurexins remains to be demonstrated, neuroligin-1 has been clearly shown to localize to the postsynaptic compartment at excitatory synapses (31). Neuroligin–neurexin interaction at the cell–cell contact may nucleate the assembly of transmitter release machinery through a presynaptic protein scaffold (32–35).
Protein–protein interactions across the synaptic junction could simultaneously induce retrograde as well as anterograde signaling. In cultured cortical neurons, Dalva et al. (36) found that ephrinB1 binding to the EphB receptor induces an interaction of EphB with the NMDA subtype of glutamate receptors through extracellular domains of these proteins, leading to synaptic recruitment of NMDA receptors. Treatment of ephrinB1 for several days increases the number of NMDA receptor-containing postsynaptic specializations as well as that of presynaptic release sites. The kinase activity of EphB is involved in the formation of NMDA receptor-containing postsynaptic specializations, although it is not required for the initial interaction of EphB and NMDA receptors. The localization of ephrinB and EphB was not examined in the latter study, although it has been shown that during early development, EphB is present on axons and ephrinB is localized on target cells (37), and that EphB is localized on the postsynaptic cell in the adult hippocampus (38). It is possible that EphB/NMDA receptor complex forms at postsynaptic sites, and a signal is transmitted to the nascent presynaptic site through ephrinB, because the ephrinB/EphB complex is capable of reciprocal signaling (39). In addition to membrane proteins, secreted factor Narp (neuronal-activity-regulated pentraxin) is known to induce AMPA receptor clustering in spinal cord neurons (40). Similar to the action of agrin at NMJs (2), Narp may serve as a nerve terminal-derived anterograde factor for triggering clustering of postsynaptic transmitter receptors.
Real-time imaging of the trafficking of synaptic vesicle proteins suggests that individual synaptic connections may form relatively quickly (27, 41). In hippocampal cultures, stimulation-driven recycling of synaptic vesicle has been observed as soon as 30 min after the initial axodendritic contact, whereas the recruitment of glutamate receptors, presumably to the postsynaptic membrane, appears to be delayed by another 40 min (41). This finding suggests that the timing of each differentiation event is controlled in a coordinated manner. It is unclear whether a postsynaptic retrograde signal is continuously required during the assembly of presynaptic terminal and whether the efficacy of assembly is regulated by the retrograde signal(s). The delayed postsynaptic differentiation is consistent with the idea that presynaptic differentiation and release of anterograde signals are required before postsynaptic differentiation can proceed.
Maturation of synapses involves acquisition of the full complement of pre- and postsynaptic components required for stable synaptic transmission with characteristic physiological and biochemical phenotype. Much of this process must depend on gene regulation and new protein synthesis. For example, developing sympathetic neurons switch their neurotransmitter phenotype from noradrenergic to cholinergic and peptidergic after innervation of the sweat gland and periosteum (42, 43), a process induced by target-derived retrograde factors. Although the switching of transmitter type requires the expression of a set of new enzymes for transmitter metabolism, possibly affecting the entire presynaptic neuron, retrograde determination of other more subtle presynaptic phenotypes can be restricted only to a subset of presynaptic nerve terminals. In the cricket central nervous system, synaptic terminals of a single neuron can exhibit synaptic facilitation on one target neuron but synaptic depression on another (44). Similarly, in rat neocortical slices, evoked postsynaptic currents from a single pyramidal neuron can undergo short-term facilitation or depression, depending on whether the type of the target interneuron is bitufted or multipolar (45). Thus a target-dependent retrograde signal can produce a local synapse-specific differentiation of the presynaptic nerve terminals. It remains largely unclear how the neuron produces a persistent synapse-specific modification that requires a specific set of proteins. One interesting possibility is local protein synthesis at the pre- and postsynaptic sites triggered by localized retrograde and anterograde signals (46).
One family of protein factors that may play a significant role in retrograde signaling is neurotrophin. Nerve growth factor (NGF), the first member in the neurotrophin family, is known to be a target-derived factor that promotes the survival and differentiation of presynaptic sympathetic and sensory neurons (47). Secreted NGF is internalized by the nerve terminal and transported retrogradely to the cell body to exert its trophic effects (48, 49). Other members of neurotrophins, including BDNF, neurotrophin 3 (NT-3), and neurotrophins 4/5 (NT-4/5), have all been shown to promote survival of specific populations of neurons (50). Neurotrophins also promote synaptic maturation, as shown by accelerated maturation of quantal secretion of transmitters (51, 52) and enhanced expression of neuropeptides (53) and transmitter receptors (54). Moreover, synapse density is increased in the superior cervical ganglia of transgenic mice overexpressing BDNF and decreased in BDNF knockout mice (55). Mice lacking the neurotrophin receptors TrkB and TrkC showed decreased density of synaptic vesicles, as well as reduced axonal arborization and synaptic density (56). BDNF knockout mice also showed presynaptic structural defects (57). All these presynaptic effects can be regarded as long-term global trophic effects of neurotrophins, which may be released by the postsynaptic target neuron in a constitutive manner. As discussed below, there is good evidence that neurotrophin may also be involved in the acute synapse-specific modulation of the presynaptic neuron induced by repetitive synaptic activity.
Retrograde Signaling in Activity-Dependent Synaptic Plasticity
(i) Developmental Refinement.
The function of the nervous system relies on precise synaptic circuits. Those circuits, initially formed by the guidance of molecular cues, establish an adult pattern through synaptic rearrangement that involves weakening and eliminating inappropriate inputs and strengthening and elaborating connections at appropriate locations. It has been known that in many parts of the nervous system, neuronal activities play an important role in this developmental process (2, 58, 59). A cellular mechanism for activity-dependent refinement of synaptic connections is based on the Hebb's postulate, which states that correlated activities of pre- and postsynaptic cells are directly responsible for the synaptic modification (60). Indeed, repetitive correlated pre- and postsynaptic activities have been shown to induce persistent functional modifications of synapses, i.e., LTP and long-term depression (LTD), in many developing nervous systems (61–67). Whether these functional modifications are causally related to the activity-dependent structural refinement of synaptic connections remains to be established (68, 69).
An attractive hypothesis for activity-dependent refinement is based on activity-dependent postsynaptic secretion and retrograde action of neurotrophins (70). Correlated activity determines the level of their release by the postsynaptic cell and may also regulate their actions on the presynaptic neuron. Neurotrophins are known to exert acute effects on synaptic function—in promoting transmitter secretion (11, 71, 72) or altering postsynaptic responses (73, 74)—as well as on the dendritic and axonal morphology (75–77). Neural activities up-regulate the expression of neurotrophins (50, 70, 78–80), and synaptic activity can trigger the secretion of neurotrophins (74). Most interestingly, the acute action of neurotrophin is likely to be spatially restricted, because it binds tightly to the cell surface or extracellular matrix after secretion (81). Consistent with this idea, synaptic potentiation induced by postsynaptically secreted NT-4 at developing NMJs in culture is localized only to the activated synapse, with other synapses made by the same presynaptic neuron unaffected (82). Moreover, the synaptic potentiation by BDNF is greatly enhanced if the presynaptic cell is active during the time of BDNF presentation (83). The spatial and temporal specificity of neurotrophin action makes neurotrophin an attractive candidate molecule for activity-dependent synaptic modulation. In the mammalian visual cortex, neurotrophins have been shown to be essential for the development of ocular dominance columns (78, 84). Local infusion of BDNF or NT-4/5 (85) or removal of endogenous BDNF or NT-4/5 by local infusion of TrkB-IgG (86) delayed or prevented the eye-specific segregation of thalamocortical afferents. These results strongly support a role of neurotrophins in activity-dependent development of neural circuits.
(ii) Short-Term Plasticity of GABAergic Inputs.
In the hippocampus and cerebellum, a brief period of membrane depolarization of pyramidal or granule cells induces a transient decrease of inhibitory transmission onto the depolarized cells. Such depolarization-induced suppression of inhibition (DSI) requires voltage-dependent Ca2+ influx into the postsynaptic cell (87). However, the expression of DSI is presynaptic, because DSI does not affect the sensitivity of the postsynaptic membrane to inotophoresed GABA or quantal size of miniature GABAergic events (88, 89). Thus DSI is mediated by retrograde signals initiated by Ca2+ influx into the postsynaptic cell. Recent studies suggest that cannabinoids can be such a signal (90–92). In hippocampal slices, antagonist of cannabinoid receptor-1 (CB1), which is localized on GABAergic axon terminals, blocks DSI. A synthetic CB1 agonist or natural CB1 ligand can acutely depress basal GABAergic transmission. In addition, postsynaptic Ca2+ uncaging alone mimics DSI, and this effect can be blocked by the CB1 antagonist. Thus endogenous cannabinoids released by the depolarized pyramidal neurons can mediate a transient down-regulation of GABAergic transmission.
(iii) Retrograde Signals Associated with Induction of LTP/LTD.
Repetitive synaptic activity can induce persistent increase or decrease of synaptic efficacy, known as LTP or LTD, respectively. In many parts of the nervous system, induction of LTP/LTD depends on the activation of the postsynaptic cell. For example, in the CA1 region of the hippocampus, NMDA receptor-mediated Ca2+ influx is critical for the induction of LTP/LTD. The NMDA receptor can be opened only when there is glutamate binding and sufficient depolarization of the membrane potential to remove the Mg2+ block. Although there is general agreement for the postsynaptic locus for the induction of LTP/LTD, whether the cellular change underlying synaptic modification occurs in the pre- or postsynaptic cell has been a long-lasting debate. Recent studies have shown that AMPA receptors are inserted into or removed from the postsynaptic membrane after induction of LTP or LTD, respectively (93–95), and that blocking exocytosis or endocytosis in the postsynaptic cell prevents generation of LTP or LTD (96–98), suggesting that receptor redistribution can account for synaptic modification. However, a presynaptic expression mechanism cannot be fully excluded. By studying single excitatory synapses between hippocampal neurons, it was found that glutamate receptors (including AMPA and NMDA receptors) are not saturated by glutamate released from a single vesicle (99, 100). The above result suggests that synaptic strength may be significantly influenced by the cleft neurotransmitter concentration, which can be regulated by a presynaptic mechanism, e.g., through regulating the size of fusion pore of the synaptic vesicle (101, 102). The LTP phenomenon is always associated with an increased frequency of miniature synaptic currents mediated by AMPA receptors. This increase can be explained by an increased presynaptic release probability or a pure postsynaptic mechanism through which initial “silent” synapses (composed only of NMDA receptors) are switched to functional synapses after insertion of AMPA receptors (103). Indeed, there is much evidence suggesting that silent synapses acquire AMPA-type responses after induction of LTP (104–106). Interestingly, Liu and colleagues (102) found that in cultured hippocampal neurons, silent synapses actually contained functional AMPA receptors, revealed by focal glutamate application. Interference of presynaptic vesicle fusion can revert functional to silent transmission. The result can be explained by different binding kinetics of NMDA and AMPA receptors. NMDA receptors are less sensitive to glutamate when it is present for only a very short time than when it is present for a longer time. Thus, a prolonged pulse of glutamate leads to high occupancy of NMDA receptors but low occupancy of AMPA receptors, whereas a much briefer but high concentration pulse leads to more activation of AMPA receptors (107). Liu and colleagues' result implies that maturation of silent synapses involves changes of presynaptic secretion through SNARE-mediated fusion. If the expression of LTP indeed involves a presynaptic locus, then a retrograde signal from the postsynaptic cell is needed to induce those presynaptic changes. On the other hand, another potential mechanism underlying LTP is the formation of new synapses (97). Insertion of new receptors at the postsynaptic membrane can be interpreted as reflecting early events of a process that leads to the splitting or budding of spines (108, 109). Eventually, the presynaptic terminal will undergo a concomitant split, so that a new synapse will be formed. This structural change again needs retrograde communication from the postsynaptic cell. Here, we summarize three forms of potential retrograde signaling associated with induction of LTP: signaling by membrane permeable factors, by membrane-bound adhesion proteins, and by secreted protein factors.
Nitric oxide.
Membrane-permeant factors, including arachidonic acid, platelet-activating factor, nitric monoxide (NO), or carbon monoxide (CO), have been suggested as the potential retrograde messenger associated with synaptic modification (1, 110). For example, NO is released in a Ca2+-dependent manner on activation of NMDA receptors (111, 112). Exogenously applied NO enhances transmitter release in an activity-dependent (pairing with weak tetanus), NMDA receptor-independent manner (113), whereas extracellular application of a membrane-impermeant NO scavenger inhibits the induction of LTP (114, 115). In mutant mice lacking both neuronal and endothelial isoforms of NO synthase, LTP is significantly reduced (116). Consistent with the role of retrograde messenger, postsynaptic injection of the NO synthase inhibitor blocks induction of LTP (115). Photolytic release of NO from postsynaptically injected NO donor, paired with weak tetanus, causes rapid potentiation that is blocked by an extracellular NO scavenger. In contrast, potentiation induced by NO released from presynaptically injected donor is not blocked by the scavenger (117). These results support the notion that NO is produced postsynaptically, travels through the extracellular space, and acts directly in the presynaptic neuron to produce LTP.
Adhesion molecules.
Membrane-bound factors at the synapse have been implicated to be involved in LTP (118). In mice lacking neural cell adhesion molecule (NCAM), LTP is abolished in both the CA1 and CA3 areas (119, 120). Application of functional blocking antibodies to NCAM inhibits induction of CA1 LTP (121). Similarly, blocking of cadherin function with antibodies or a peptide significantly reduced LTP, without affecting basal transmission or short-term plasticity (19). Interestingly, disruption of cadherin binding is effective in reducing LTP only when it occurs during induction, suggesting that cadherin plays a signaling role in synaptic plasticity (122). Transsynaptic signaling through cadherin–cadherin complex could result in direct structural rearrangement of the pre-synaptic active zone and postsynaptic density or trigger other signaling cascades involved in the induction of LTP. However, there is no evidence yet to support a direct link between the cadherin adhesion system and the presynaptic release machinery or postsynaptic signal transduction apparatus.
Neurotrophins.
Among the secreted molecules, neurotrophin is a potential retrograde signal. Release of BDNF can be triggered by membrane depolarization in a Ca2+-dependent manner (123). Exogenous application BDNF can acutely modify synaptic efficacy (11, 71, 72, 124). In some other studies, although basic transmission is not affected, BDNF promotes synaptic function by permitting tetanus-induced LTP (12). Most neurotrophin effects are observed on the presynaptic site through an increase of transmitter release, e.g., increase in the frequency but not the amplitude of miniature excitatory postsynaptic currents, reduction in paired-pulse facilitation, and the coefficient of variation (71, 72, 125). The enhancement of synaptic transmission by BDNF at the presynaptic level is likely to be caused by increased mitogen-activated protein kinase-dependent phosphorylation of synaptic vesicle protein synapsin, which results in acutely facilitated evoked glutamate release (126). Genetic deletion of BDNF in mice disrupts normal induction of LTP in the CA1 region of the hippocampus. This defect is rescued via reintroducing BDNF by transfecting hippocampal slices with BDNF-expressing adenovirus or by supplying exogenous BDNF (127, 128). BDNF has been shown to act through the TrkB receptor presynaptically but not postsynaptically to modulate LTP (129, 130), consistent with the role of BDNF as a retrograde signal. On the basis of the morphological effects of neurotrophins on axon and dendrites, it is proposed that neurotrophins can mediate late-phase LTP as synaptic morphogens (70). In this model, endogenous neurotrophins released under low-level activity have a permissive role, in that the trophic effect endows synapses with the ability to undergo LTP. Intensive synaptic activity associated with LTP that results in transient high-level calcium elevation leads to release of higher-level neurotrophins that may play an instructive role by inducing morphological changes that lead to the formation of new synaptic connections.
(iv) Global Presynaptic Modification Associated with LTP/LTD.
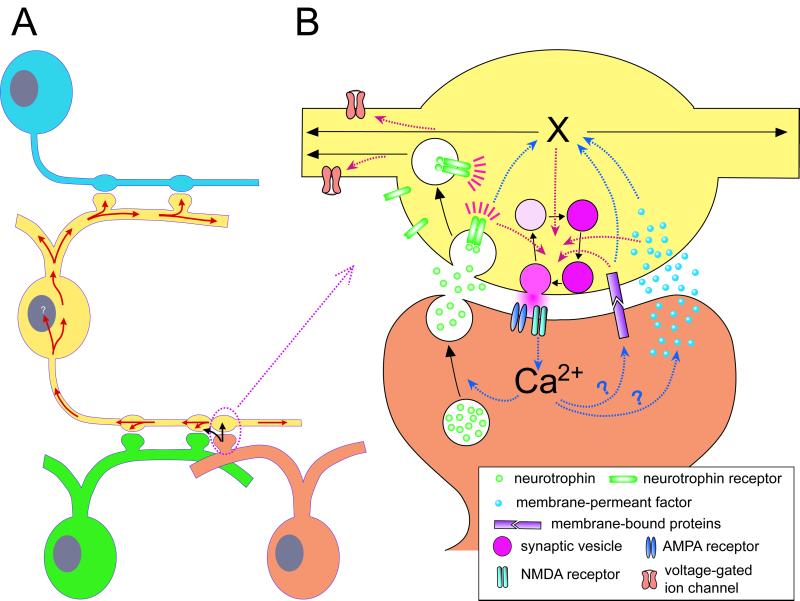
If a retrograde signal associated with synaptic modification by activity is readily diffusible in the extracellular space, one would expect this diffusible signal may also affect other nearby synapses. Indeed, some experimental results are consistent with this idea. DSI induced on one pyramidal cell spreads to other nondepolarized cells within 20 μm of the depolarized cell (90, 131). In hippocampal slices, LTP induced at synaptic inputs on a single CA1 pyramidal neuron spreads to synapses formed by the same set of afferent fibers on the neighboring neurons (132, 133) or to adjacent synapses made by different inputs onto the same postsynaptic cell (134). There is also evidence suggesting that the retrograde effect can propagate intracellularly in the presynaptic neuron. In Xenopus nerve-muscle cultures, LTD induced at one neuromuscular synapse can spread to synapses made by the same neuron onto another myocyte, apparently by signaling within the neuronal cytoplasm, because rapid clearance of extracellular fluid does not prevent the spread of depression (135). In networks of cultured hippocampal neurons, LTD and LTP induced at glutamatergic synapses were found to spread retrogradely to the input synapses on the dendrites of the presynaptic neuron (backpropagation), as well as laterally to synapses made by divergent outputs of the presynaptic neuron (presynaptic lateral propagation) (136, 137). Although presynaptic lateral propagation may be accounted for by the possibility of local spread of a diffusible retrograde signal between postsynaptic spines of different neurons sharing the same presynaptic bouton (“multisynapse” bouton; see ref. 138), backpropagation of potentiation/depression from nerve terminal to dendrites requires long-range signaling. Moreover, spread of LTP is restricted to the presynaptic neuron and there is no further up- or downstream propagation, suggesting that an intracellular signal confined within the presynaptic cytoplasm is responsible for the observed backpropagation. Interestingly, immediately after induction of LTP by correlated spiking, the intrinsic excitability of the presynaptic neuron is persistently enhanced, as revealed by the decreased threshold for spiking and reduced variability of interspike intervals (139). Modification of presynaptic excitability is at least in part caused by increased activation and decreased inactivation of voltage-gated sodium channels. Presynaptic inhibition of protein kinase C abolished changes in excitability without affecting LTP. In the above studies, the induction of LTP/LTD depends on postsynaptic NMDA receptors, thus the change in intrinsic excitability of the presynaptic cell or in synaptic transmission of its input synapses directly implicates the involvement of a transsynaptic signal (see Fig. 1).
Figure 1.
Presynaptic spread of retrograde signals associated with the induction of LTP. (A) Schematic diagram showing the direction of spread of potentiation signals in the presynaptic cytoplasm after induction of LTP at hippocampal synapses in cell cultures (see ref. 137). The site of induction of LTP is marked by the dotted circle. Retrograde signal(s) associated with the induction of LTP (black arrow) may trigger another presynaptic cytosolic signal that propagates throughout the presynaptic cytoplasm (red arrow) and may affect other synapses in close vicinity (black arrow). (B) Three potential mechanisms of retrograde signaling associated with LTP at hippocampal CA1 synapses. Correlated pre- and postsynaptic activity results in postsynaptic Ca2+ influx through NMDA receptors and a cascade of events that lead to three potential forms of retrograde signaling: Secreted factors (e.g., neurotrophins) are released via exocytosis and diffuse across the synaptic cleft to activate presynaptic receptors. Membrane-permeant factors (e.g., NO) directly diffuse from the postsynaptic cytoplasm to the presynaptic cell. Changes in the postsynaptic membrane proteins convey signals in the postsynaptic cytoplasm to the presynaptic cell via their physical linkages to presynaptic membrane receptors. All three forms of retrograde action may modulate presynaptic release machinery, vesicle recycling and refilling, and trigger cytosolic signals (X) for long-range presynaptic propagation of the potentiation signal. Retrograde transport of endocytic vesicles containing internalized neurotrophin–receptor complexes can also propagate the retrograde signal to other parts of the presynaptic neuron.
The mechanism for long-range cytoplasmic propagation in the presynaptic cell is unknown. It is possible that a transsynaptic retrograde signal, generated after the induction of synaptic plasticity, triggers another cytosolic signal that propagates throughout the presynaptic neuron, or the retrograde signal itself serves as the propagating signal (see Fig. 1). The identity of the cytosolic propagating signal remains to be elucidated. Given that the change in presynaptic excitability or propagation of potentiation/depression occurs with a fast onset (within a few minutes), this signal must travel with a speed of at least a few micrometers per second. Regenerative waves of second messenger (e.g., Ca2+, InsP3, cAMP) can be good candidates, because it is known that these waves can be generated at local sites and propagate over long distance across the entire cell, with a speed in the range of 8–100 μm/sec (140). A Ca2+ wave can be generated by Ca2+-induced Ca2+ release or coupled with InsP3-induced Ca2+ release. A Ca2+–cAMP-coupled wave is also possible, because there is a Ca2+-sensitive form of adenylate cyclase in the nervous system, and Ca2+ release can be modulated by protein phosphorylation via cAMP-dependent protein kinase. cAMP is known to be involved in different forms of synaptic plasticity and diffuses rapidly in the cytoplasm (141). Zheng et al. (142) have shown that local application of a membrane-permeable cAMP analogue at the growth cone of one neurite of a multipolar neuron resulted in growth inhibition of other neurites, suggesting a long-range intracellular signaling after local elevation of cAMP/protein kinase A activity. Rapid axonal transport can also be driven by motor proteins associated with microtubules. In hippocampal cultures, restricted application of glutamate to the cell body of presynaptic neuron resulted in LTP of its output synapses, which could be blocked by pretreatment with colchicine that disrupts axonal transport (143). In sympathetic ganglia, axotomy of postganglionic fibers resulted in the withdrawal of synaptic contacts of ganglionic cells. The effect can be mimicked by colchicine treatment and prevented by exogenous application of nerve growth factor (NGF) (144, 145). Recently, retrograde axonal transport of ligand–receptor complexes has been demonstrated for many trophic factors, including NGF, BDNF, NT-3, and NT-4 (49, 146). Transported neurotrophin–receptor complexes can exert its biochemical effect and propagate the signal en route to other parts of the neuron. Whatever the propagating signals, they may result in the change of presynaptic excitability by regulating voltage-gated ion channels on the axon or change of synaptic transmission by regulating the release machinery of the presynaptic terminals and postsynaptic responsiveness of synapses located on the dendrite of the presynaptic cell.
Concluding Remarks
It is well known that the development and maintenance of various neuronal functions depend on the trophic support of the target tissue, through uptake of trophic factors by the nerve terminal and retrograde axonal transport of the factors to the neuronal cell body. It has become clear only recently that retrograde signaling can occur in a manner that depends on the pattern of synaptic activity and over a much faster time scale than previously realized. Activity-dependent retrograde factors, as described above in association with the induction of LTP/LTD, are likely to play important modulatory roles in neuronal excitability and synaptic transmission, functions directly related to processing and storage of information in the neural network. They may exert their actions either locally at the presynaptic nerve terminal or globally throughout the entire presynaptic neuron. Major tasks remain in the identification of these retrograde signals and in understanding the mechanism of action of these signals and the implications for functions of the neural network.
Acknowledgments
We thank Li I. Zhang and Kang Shen for helpful discussions and comments. Work done in the authors' lab was supported by a grant from the National Institutes of Health.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- LTP
long-term potentiation
- LTD
long-term depression
- DSI
depolarization-induced suppression of inhibition
- NMJ
neuromuscular junction
Footnotes
This paper was presented at the Inaugural Arthur M. Sackler Colloquium of the National Academy of Sciences, “Neural Signaling,” held February 15–17, 2001, at the National Academy of Sciences in Washington, DC.
References
- 1.Fitzsimonds R M, Poo M M. Physiol Rev. 1998;78:143–170. doi: 10.1152/physrev.1998.78.1.143. [DOI] [PubMed] [Google Scholar]
- 2.Sanes J R, Lichtman J W. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 3.Burden S J. Genes Dev. 1998;12:133–148. doi: 10.1101/gad.12.2.133. [DOI] [PubMed] [Google Scholar]
- 4.Hall A C, Lucas F R, Salinas P C. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 5.Dent E W, Callaway J L, Szebenyi G, Baas P W, Kalil K. J Neurosci. 1999;19:8894–8908. doi: 10.1523/JNEUROSCI.19-20-08894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roos J, Hummel T, Ng N, Klambt C, Davis G W. Neuron. 2000;26:371–382. doi: 10.1016/s0896-6273(00)81170-8. [DOI] [PubMed] [Google Scholar]
- 7.Chin L S, Li L, Ferreira A, Kosik K S, Greengard P. Proc Natl Acad Sci USA. 1995;92:9230–9234. doi: 10.1073/pnas.92.20.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosahl T W, Spillane D, Missler M, Herz J, Selig D K, Wolff J-R, Hammer R E, Malenka R C, Südhof T C, Wolff J R, et al. Nature (London) 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- 9.Song H J, Ming G L, Poo M M. Nature (London) 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 10.Tucker K L, Meyer M, Barde Y A. Nat Neurosci. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- 11.Lohof A M, Ip N Y, Poo M M. Nature (London) 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 12.Figurov A, Pozzo-Miller L D, Olafsson P, Wang T, Lu B. Nature (London) 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 13.Ziv N E, Smith S J. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 14.Parra P, Gulyas A I, Miles R. Neuron. 1998;20:983–993. doi: 10.1016/s0896-6273(00)80479-1. [DOI] [PubMed] [Google Scholar]
- 15.MacNeil M A, Masland R H. Neuron. 1998;20:971–982. doi: 10.1016/s0896-6273(00)80478-x. [DOI] [PubMed] [Google Scholar]
- 16.Serafini T. Cell. 1999;98:133–136. doi: 10.1016/s0092-8674(00)81008-9. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro L, Colman D R. Neuron. 1999;23:427–430. doi: 10.1016/s0896-6273(00)80796-5. [DOI] [PubMed] [Google Scholar]
- 18.Redies C. Prog Neurobiol. 2000;61:611–648. doi: 10.1016/s0301-0082(99)00070-2. [DOI] [PubMed] [Google Scholar]
- 19.Tang L X, Hung C P, Schuman E M. Neuron. 1998;20:1165–1175. doi: 10.1016/s0896-6273(00)80497-3. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Nemes A, Mendelsohn M, Axel R. Cell. 1998;93:47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]
- 21.Missler M, Fernandez-Chacon R, Sudhof T C. J Neurochem. 1998;71:1339–1347. doi: 10.1046/j.1471-4159.1998.71041339.x. [DOI] [PubMed] [Google Scholar]
- 22.Ullrich B, Ushkaryov Y A, Sudhof T C. Neuron. 1995;14:497–507. doi: 10.1016/0896-6273(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 23.Schmucker D, Clemens J C, Shu H, Worby C A, Xiao J, Muda M, Dixon J E, Zipursky S L. Cell. 2000;101:671–684. doi: 10.1016/s0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- 24.Poo M M. Annu Rev Neurosci. 1985;8:369–406. doi: 10.1146/annurev.ne.08.030185.002101. [DOI] [PubMed] [Google Scholar]
- 25.Xie Z P, Poo M M. Proc Natl Acad Sci USA. 1986;83:7069–7073. doi: 10.1073/pnas.83.18.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai Z, Peng H B. Neuron. 1993;10:827–837. doi: 10.1016/0896-6273(93)90199-2. [DOI] [PubMed] [Google Scholar]
- 27.Ahmari S E, Buchanan J, Smith S J. Nat Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- 28.Roos J, Kelly R B. Nat Neurosci. 2000;3:415–417. doi: 10.1038/74773. [DOI] [PubMed] [Google Scholar]
- 29.Grindstaff K K, Yeaman C, Anandasabapathy N, Hsu S C, Rodriguez-Boulan E, Scheller R H, Nelson W J. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- 30.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 31.Song J Y, Ichtchenko K, Sudhof T C, Brose N. Proc Natl Acad Sci USA. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl T W, Sudhof T C. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- 33.Maximov A, Sudhof T C, Bezprozvanny I. J Biol Chem. 1999;274:24453–24456. doi: 10.1074/jbc.274.35.24453. [DOI] [PubMed] [Google Scholar]
- 34.Davis G W. Neuron. 2000;26:551–554. doi: 10.1016/s0896-6273(00)81190-3. [DOI] [PubMed] [Google Scholar]
- 35.Rao A, Levi S. Neuron. 2000;27:3–5. doi: 10.1016/s0896-6273(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 36.Dalva M B, Takasu M A, Lin M Z, Shamah S M, Hu L, Gale N W, Greenberg M E. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 37.Flanagan J G, Vanderhaeghen P. Annu Rev Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 38.Buchert M, Schneider S, Meskenaite V, Adams M T, Canaani E, Baechi T, Moelling K, Hovens C M. J Cell Biol. 1999;144:361–371. doi: 10.1083/jcb.144.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruckner K, Klein R. Curr Opin Neurobiol. 1998;8:375–382. doi: 10.1016/s0959-4388(98)80064-0. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien R J, Xu D, Petralia R S, Steward O, Huganir R L, Worley P. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 41.Friedman H V, Bresler T, Garner C C, Ziv N E. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 42.Habecker B A, Malec N M, Landis S C. J Neurosci. 1996;16:229–237. doi: 10.1523/JNEUROSCI.16-01-00229.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asmus S E, Parsons S, Landis S C. J Neurosci. 2000;20:1495–1504. doi: 10.1523/JNEUROSCI.20-04-01495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis G W, Murphey R K. J Neurosci. 1993;13:3827–3838. doi: 10.1523/JNEUROSCI.13-09-03827.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Nat Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- 46.Martin K C, Casadio A, Zhu H X, Rose J C, Chen M, Bailey C H, Kandel E R. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- 47.Levi-Montalcini R. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 48.Hendry I A, Stockel K, Thoenen H, Iversen L L. Brain Res. 1974;68:103–121. doi: 10.1016/0006-8993(74)90536-8. [DOI] [PubMed] [Google Scholar]
- 49.DiStefano P S, Friedman B, Radziejewski C, Alexander C, Boland P, Schick C M, Lindsay R M, Wiegand S J. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- 50.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 51.Wang T, Xie K, Lu B. J Neurosci. 1995;15:4796–4805. doi: 10.1523/JNEUROSCI.15-07-04796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liou J C, Fu W M. J Neurosci. 1997;17:2459–2468. doi: 10.1523/JNEUROSCI.17-07-02459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nawa H, Pelleymounter M A, Carnahan J. J Neurosci. 1994;14:3751–3765. doi: 10.1523/JNEUROSCI.14-06-03751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narisawa-Saito M, Carnahan J, Araki K, Yamaguchi T, Nawa H. Neuroscience. 1999;88:1009–1014. doi: 10.1016/s0306-4522(98)00496-5. [DOI] [PubMed] [Google Scholar]
- 55.Causing C G, Gloster A, Aloyz R, Bamji S X, Chang E, Fawcett J, Kuchel G, Miller F D. Neuron. 1997;18:257–267. doi: 10.1016/s0896-6273(00)80266-4. [DOI] [PubMed] [Google Scholar]
- 56.Martinez A, Alcantara S, Borrell V, Del Rio J A, Blasi J, Otal R, Campos N, Boronat A, Barbacid M, Silos-Santiago I, et al. J Neurosci. 1998;18:7336–7350. doi: 10.1523/JNEUROSCI.18-18-07336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pozzo-Miller L D, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng Z H, Lu B. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodman C S, Shatz C J. Cell. 1993;72:S77–S98. [Google Scholar]
- 59.Katz L C, Shatz C J. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 60.Bi G, Poo M. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- 61.Artola A, Brocher S, Singer W. Nature (London) 1990;347:69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L I, Tao H, Poo M. Nat Neurosci. 2000;3:708–715. doi: 10.1038/76665. [DOI] [PubMed] [Google Scholar]
- 63.Mooney R, Madison D V, Shatz C J. Neuron. 1993;10:815–825. doi: 10.1016/0896-6273(93)90198-z. [DOI] [PubMed] [Google Scholar]
- 64.Kirkwood A, Lee H-K, Bear M F. Nature (London) 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- 65.Dudek S M, Friedlander M J. Neuron. 1996;16:1097–1106. doi: 10.1016/s0896-6273(00)80136-1. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L I, Tao H W, Holt C E, Harris W A, Poo M M. Nature (London) 1998;395:37–44. doi: 10.1038/25665. [DOI] [PubMed] [Google Scholar]
- 67.Feldman D E, Nicoll R A, Malenka R C. J Neurobiol. 1999;41:92–101. [PubMed] [Google Scholar]
- 68.Maletic-Savatic M, Malinow R, Svoboda K. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 69.Engert F, Bonhoeffer T. Nature (London) 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 70.Poo M M. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 71.Kang H J, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 72.Schinder A F, Berninger B, Poo M. Neuron. 2000;25:151–163. doi: 10.1016/s0896-6273(00)80879-x. [DOI] [PubMed] [Google Scholar]
- 73.Lin S Y, Wu K, Levine E S, Mount H T, Suen P C, Black I B. Brain Res Mol Brain Res. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- 74.Wang X H, Poo M M. Neuron. 1997;19:825–835. doi: 10.1016/s0896-6273(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 75.McAllister A K, Lo D C, Katz L C. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 76.Cohen-Cory S, Fraser S E. Nature (London) 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- 77.Gallo G, Letourneau P C. Curr Biol. 1998;8:80–82. doi: 10.1016/s0960-9822(98)70051-x. [DOI] [PubMed] [Google Scholar]
- 78.McAllister A K, Katz L C, Lo D C. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 79.Schuman E M. Curr Opin Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- 80.Schinder A F, Poo M. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- 81.Blochl A, Thoenen H. Eur J Neurosci. 1995;7:1220–1228. doi: 10.1111/j.1460-9568.1995.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 82.Wang X H, Berninger B, Poo M M. J Neurosci. 1998;18:4985–4992. doi: 10.1523/JNEUROSCI.18-13-04985.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boulanger L, Poo M M. Nat Neurosci. 1999;2:346–351. doi: 10.1038/7258. [DOI] [PubMed] [Google Scholar]
- 84.Berardi N, Maffei L. J Neurobiol. 1999;41:119–126. [PubMed] [Google Scholar]
- 85.Cabelli R J, Hohn A, Shatz C J. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 86.Cabelli R J, Shelton D L, Segal R A, Shatz C J. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- 87.Lenz R A, Wagner J J, Alger B E. J Physiol. 1998;512:61–73. doi: 10.1111/j.1469-7793.1998.061bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alger B E, Pitler T A. Trends Neurosci. 1995;18:333–340. doi: 10.1016/0166-2236(95)93923-l. [DOI] [PubMed] [Google Scholar]
- 89.Morishita W, Alger B E. J Physiol. 1997;505:307–317. doi: 10.1111/j.1469-7793.1997.307bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wilson R I, Nicoll R A. Nature (London) 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 91.Kreitzer A C, Regehr W G. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 92.Ohno-Shosaku T, Maejima T, Kano M. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 93.Shi S H, Hayashi Y, Petralia R S, Zaman S H, Wenthold R J, Svoboda K, Malinow R. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 94.Carroll R C, Lissin D V, Von Zastrow M, Nicoll R A, Malenka R C. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- 95.Hayashi Y, Shi S H, Esteban J A, Piccini A, Poncer J C, Malinow R. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 96.Lledo P M, Zhang X Y, Südhof T C, Malenka R C, Nicoll R A. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- 97.Luscher C, Nicoll R A, Malenka R C, Muller D. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- 98.Luscher C, Xia H, Beattie E C, Carroll R C, Von Zastrow M, Malenka R C, Nicoll R A. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 99.Liu G S, Choi S W, Tsien R W. Neuron. 1999;22:395–409. doi: 10.1016/s0896-6273(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 100.Mainen Z F, Malinow R, Svoboda K. Nature (London) 1999;399:151–155. doi: 10.1038/20187. [DOI] [PubMed] [Google Scholar]
- 101.Choi S, Klingauf J, Tsien R W. Nat Neurosci. 2000;3:330–336. doi: 10.1038/73895. [DOI] [PubMed] [Google Scholar]
- 102.Renger J J, Egles C, Liu G. Neuron. 2001;29:469–484. doi: 10.1016/s0896-6273(01)00219-7. [DOI] [PubMed] [Google Scholar]
- 103.Malenka R C, Nicoll R A. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 104.Liao D Z, Hessler N A, Malinow R. Nature (London) 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 105.Durand G M, Kovalchuk Y, Konnerth A. Nature (London) 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 106.Isaac J T R, Nicoll R A, Malenka R C. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 107.Kullmann D M. Nature (London) 1999;399:111–112. doi: 10.1038/20089. [DOI] [PubMed] [Google Scholar]
- 108.Carlin R K, Siekevitz P. Proc Natl Acad Sci USA. 1983;80:3517–3521. doi: 10.1073/pnas.80.11.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Edwards F A. Trends Neurosci. 1995;18:250–255. doi: 10.1016/0166-2236(95)80003-k. [DOI] [PubMed] [Google Scholar]
- 110.Hawkins R D, Son H, Arancio O. Prog Brain Res. 1998;118:155–172. doi: 10.1016/s0079-6123(08)63206-9. [DOI] [PubMed] [Google Scholar]
- 111.Garthwaite J, Charles S L, Chess-Williams R. Nature (London) 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- 112.Garthwaite J, Garthwaite G, Palmer R M, Moncada S. Eur J Pharmacol. 1989;172:413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- 113.Zhuo M, Small S A, Kandel E R, Hawkins R D. Science. 1993;260:1946–1950. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]
- 114.O'Dell T J, Hawkins R D, Kandel E R, Arancio O. Proc Natl Acad Sci USA. 1991;88:11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schuman E M, Madison D V. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- 116.Son H, Hawkins R D, Martin K, Kiebler M, Huang P L, Fishman M C, Kandel E R. Cell. 1996;87:1015–1023. doi: 10.1016/s0092-8674(00)81796-1. [DOI] [PubMed] [Google Scholar]
- 117.Arancio O, Kiebler M, Lee C J, Lev-Ram V, Tsien R Y, Kandel E R, Hawkins R D. Cell. 1996;87:1025–1035. doi: 10.1016/s0092-8674(00)81797-3. [DOI] [PubMed] [Google Scholar]
- 118.Murase S, Schuman E M. Curr Opin Cell Biol. 1999;11:549–553. doi: 10.1016/s0955-0674(99)00019-8. [DOI] [PubMed] [Google Scholar]
- 119.Cremer H, Chazal G, Carleton A, Goridis C, Vincent J D, Lledo P M. Proc Natl Acad Sci USA. 1998;95:13242–13247. doi: 10.1073/pnas.95.22.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss J Z. Neuron. 1996;17:413–422. doi: 10.1016/s0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- 121.Luthl A, Laurent J P, Figurov A, Muller D, Schachner M. Nature (London) 1994;372:777–779. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- 122.Hagler D J, Goda Y. Neuron. 1998;20:1059–1062. doi: 10.1016/s0896-6273(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 123.Goodman L J, Valverde J, Lim F, Geschwind M D, Federoff H J, Geller A I, Hefti F. Mol Cell Neurosci. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- 124.Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H. J Neurosci. 1997;17:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lessmann V, Heumann R. Neuroscience. 1998;86:399–413. doi: 10.1016/s0306-4522(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 126.Jovanovic J N, Czernik A J, Fienberg A A, Greengard P, Sihra T S. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- 127.Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Patterson S L, Abel T, Deuel T A, Martin K C, Rose J C, Kandel E R. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 129.Xu B, Gottschalk W, Chow A, Wilson R I, Schnell E, Zang K, Wang D, Nicoll R A, Lu B, Reichardt L F. J Neurosci. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li Y X, Xu Y, Ju D, Lester H A, Davidson N, Schuman E M. Proc Natl Acad Sci USA. 1998;95:10884–10889. doi: 10.1073/pnas.95.18.10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ohno-Shosaku T, Sawada S, Kano M. Neurosci Res. 2000;36:67–71. doi: 10.1016/s0168-0102(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 132.Bonhoeffer T, Staiger V, Aertsen A. Proc Natl Acad Sci USA. 1989;86:8113–8117. doi: 10.1073/pnas.86.20.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schuman E M, Madison D V. Science. 1994;263:532–536. doi: 10.1126/science.8290963. [DOI] [PubMed] [Google Scholar]
- 134.Engert F, Bonhoeffer T. Nature (London) 1997;388:279–284. doi: 10.1038/40870. [DOI] [PubMed] [Google Scholar]
- 135.Cash S, Zucker R S, Poo M M. Science. 1996;272:998–1001. doi: 10.1126/science.272.5264.998. [DOI] [PubMed] [Google Scholar]
- 136.Fitzsimonds R M, Song H J, Poo M M. Nature (London) 1997;388:439–448. doi: 10.1038/41267. [DOI] [PubMed] [Google Scholar]
- 137.Tao H, Zhang L I, Bi G, Poo M. J Neurosci. 2000;20:3233–3243. doi: 10.1523/JNEUROSCI.20-09-03233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Harris K M. Trends Neurosci. 1995;18:365–369. doi: 10.1016/0166-2236(95)93930-v. [DOI] [PubMed] [Google Scholar]
- 139.Ganguly K, Kiss L, Poo M. Nat Neurosci. 2000;3:1018–1026. doi: 10.1038/79838. [DOI] [PubMed] [Google Scholar]
- 140.Meyer T. Cell. 1991;64:675–678. doi: 10.1016/0092-8674(91)90496-l. [DOI] [PubMed] [Google Scholar]
- 141.Hempel C M, Vincent P, Adams S R, Tsien R Y, Selverston A I. Nature (London) 1996;384:166–169. doi: 10.1038/384166a0. [DOI] [PubMed] [Google Scholar]
- 142.Zheng J Q, Zheng Z, Poo M. J Cell Biol. 1994;127:1693–1701. doi: 10.1083/jcb.127.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lux H D, Veselovsky N S. Neurosci Lett. 1994;178:231–234. doi: 10.1016/0304-3940(94)90766-8. [DOI] [PubMed] [Google Scholar]
- 144.Purves D. J Physiol. 1975;252:429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Purves D. J Physiol. 1976;259:159–175. doi: 10.1113/jphysiol.1976.sp011459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.von Bartheld C S, Williams R, Lefcort F, Clary D O, Reichardt L F, Bothwell M. J Neurosci. 1996;16:2995–3008. doi: 10.1523/JNEUROSCI.16-09-02995.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]