Abstract
In the brain, dopamine exerts an important modulatory influence over behaviors such as emotion, cognition, and affect as well as mechanisms of reward and the control of locomotion. The dopamine transporter (DAT), which reuptakes the released neurotransmitter into presynaptic terminals, is a major determinant of the intensity and duration of the dopaminergic signal. Knockout mice lacking the dopamine transporter (DAT-KO mice) display marked changes in dopamine homeostasis that result in elevated dopaminergic tone and pronounced locomotor hyperactivity. A feature of DAT-KO mice is that their hyperactivity can be inhibited by psychostimulants and serotonergic drugs. The pharmacological effect of these drugs occurs without any observable changes in dopaminergic parameters, suggesting that other neurotransmitter systems in addition to dopamine might contribute to the control of locomotion in these mice. We report here that the hyperactivity of DAT-KO mice can be markedly further enhanced when N-methyl-d-aspartate receptor-mediated glutamatergic transmission is blocked. Conversely, drugs that enhance glutamatergic transmission, such as positive modulators of l-α-amino-3-hydroxy-5-methylisoxazole-4-propionate glutamate receptors, suppress the hyperactivity of DAT-KO mice. Interestingly, blockade of N- methyl-d-aspartate receptors prevented the inhibitory effects of both psychostimulant and serotonergic drugs on hyperactivity. These findings support the concept of a reciprocal functional interaction between dopamine and glutamate in the basal ganglia and suggest that agents modulating glutamatergic transmission may represent an approach to manage conditions associated with dopaminergic dysfunction.
Frontostriatal circuitry is one of the most prominent brain pathways involved in the control of locomotion, affect, impulsivity, attention, and emotion (1, 2). One axis of this circuitry involves dopaminergic projections into the striatal and mesolimbic brain areas (1, 3). Dopaminergic transmission has been intensively studied and is relatively well characterized (1, 3), largely because alterations in dopaminergic tone have clear behavioral manifestations such as changes in locomotor activity. In addition to dopaminergic innervation from substantia nigra and ventral tegmental area, the basal ganglia receive dense glutamatergic input predominantly from prefrontal cortical areas, as well as from the hippocampus, periventricular thalamus, and amygdala (1, 4, 5). There is a growing appreciation for the concept that dopaminergic and glutamatergic systems intimately interact at the level of medium-sized spiny neurons in the basal ganglia to control behavior (1, 6, 7). Particularly, an interaction at the levels of receptor signaling and regulation between dopamine D1 and/or D2-like receptors and ionotropic glutamate N-methyl-d-aspartate (NMDA) and l-α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptors, has been put forth (7–9). Recent findings in mice with decreased NMDA receptor expression (10) confirmed and extended previous pharmacological studies (1, 11, 12), suggesting a reciprocal interaction of glutamatergic and dopaminergic transmission in the control of motor behaviors. Several lines of evidence suggest that serotonin (5-HT) also plays an important role in this interaction (13–17), in part by modulating the activity of glutamatergic neurons in the frontal cortex (12–15, 17). Nevertheless, the exact nature of the interplay of these systems within compartments of frontostriatal circuitry is still poorly understood.
By deleting the gene encoding the dopamine transporter (DAT), a strain of mice lacking the mechanism to provide reuptake of extracellular DA has been developed (18). In the absence of DAT, important changes are observed in extracellular dopamine dynamics and presynaptic homeostasis of dopaminergic terminals in the striatum of these mice, suggesting a prominent role of transporter function in the maintenance of normal homeostatic control (19). Because of their characteristics, the DAT-knockout (DAT-KO) mice represent an interesting animal model in which the interplay between various neuronal systems can be examined.
Characterization of Dopamine Homeostasis in DAT-KO Mice
Pharmacological evidence would predict that elimination of the DAT should result in changes in the extracellular dynamics of dopamine (3). Cyclic voltammetry experiments in mouse striatal slices demonstrated a 300-fold increase in the amount of time dopamine spends in the extracellular space (18–20). Moreover, cyclic voltammetry studies revealed that the rate of dopamine clearance was unaltered either by inhibitors of other transporters or by selective inhibitors of the dopamine degradation enzymes (19). Amphetamine, methylphenidate, and cocaine were found to be unable to affect clearance or extracellular dopamine levels in the striatum of DAT-KO mice (18, 19, 21). These observations suggested that over the time it takes to clear dopamine released by a single pulse stimulation, diffusion alone plays the major role in removing dopamine from the extracellular space in the striatum of DAT-KO mice (19). To directly prove that this prolonged clearance could result in alterations in extracellular dopamine concentrations, an alternative approach to assess extracellular dopamine dynamics, a quantitative “no net flux” microdialysis technique in freely moving mice was used (19, 20). These studies revealed a 5-fold elevation in steady-state striatal extracellular dopamine in DAT-KO mice in comparison to wild-type (WT) mice. These simple neurochemical parameters would suggest that the DAT-KO mice could represent a genetic model of persistent functional hyperdopaminergia.
From these initial neurochemical characterization, it became clear that in DAT-KO mice not only changes in extracellular dopamine dynamics but also numerous alterations in both presynaptic and postsynaptic components of dopaminergic transmission might exist. For example, the prolongation of the striatal dopamine clearance rate was associated with a significant (75–95%) decrease in the amount of dopamine released in response to stimulation (18, 19, 22), suggesting that the actual amount of releasable dopamine in the DAT-KO mice was decreased. In fact, total striatal tissue dopamine levels, which mostly reflect the intraneuronal vesicular storage pool of dopamine, were reduced in DAT-KO mice to only 1/20th of that in WT mice (19, 20). Interestingly, these low levels of dopamine in the striatum were extremely sensitive to inhibition of tyrosine hydroxylase (TH) by α-methyl-para-tyrosine, suggesting that they may mostly represent a newly synthesized pool of dopamine (19). These reductions in dopamine tissue levels could not be explained as a consequence of abnormal development or degeneration of dopamine neurons because quantitation of TH-positive neurons in the substantia nigra was not markedly different from that in WT mice. In addition, markers of dopaminergic terminals such as l-aromatic amino acid decarboxylase (AADC) and the neuronal vesicular monoamine transporter (VMAT2) levels were not significantly decreased (20, 23). These findings strongly suggest that depletion of the dopamine storage pools and decreased dopamine release in DAT-KO mice are directly caused by the absence of inward transport of dopamine through the DAT. Consequently, in a normal situation, a tight dependence of dopamine storage on recycled dopamine must exist (20).
In contrast to dopamine levels, the tissue content of dopamine metabolites were unaltered 3,4-dihydroxyphenylacetic acid (DOPAC) or elevated homovanillic acid (HVA) in DAT-KO mice, suggesting that additional parameters of dopamine homeostasis might be affected. Dopamine synthesis rate as assessed in vivo by accumulation of l-3,4-dihydroxyphenylalanine (l-DOPA) after inhibition of l-aromatic amino acid decarboxylase (AADC) by 3-hydroxybenzylhydrazine (NSD-1015), was found to be significantly elevated (about 200% of control) (19). This finding indicates that both dopamine synthesis and turnover are extremely high in the mutant animals. However, the striatal protein levels of TH, the rate-limiting enzyme in the synthesis of dopamine, were reduced by more than 90% of control levels (19, 23). This apparent paradox may be explained by the disinhibition of TH, which under normal conditions is subject to tonic inhibition by both intraneuronal and extraneuronal dopamine (3). In addition, activation of TH might be explained by a loss of autoreceptor function caused by pronounced extracellular dopamine concentrations. Indeed, D2 autoreceptor mRNA and binding were found to be decreased by 50% in the substantia nigra and ventral tegmental area of the DAT-KO mice (18, 24). Moreover, functional studies revealed marked desensitization in the major autoreceptor functions: regulation of neuronal firing rate, nerve terminal dopamine release, and synthesis (24). Altogether, these data, which demonstrate a profound neurochemical plasticity of dopaminergic neurons, illustrate the critical role of DAT in the maintenance of presynaptic functions.
Another consequence of the altered extracellular dopamine dynamics appears to be a dysregulation of postsynaptic dopamine receptor responsiveness. Protein and mRNA levels of the two major postsynaptic dopamine receptors, D1 and D2, are down-regulated by ≈50%, in the striatum of DAT-KO mice (18). Surprisingly, however, in the DAT-KO mice some population of postsynaptic dopamine receptors appear to be supersensitive as DAT-KO mice were hyperresponsive to postsynaptic doses of direct dopamine receptor agonists after depletion of endogenous dopamine by inhibition of TH (25). These observations may correlate with increased expression of certain dopamine receptor subtypes or unaltered electrophysiological responsiveness of postsynaptic receptors to a microiontophoretically applied D1 receptor agonist,† despite the marked decrease in receptor numbers (18). Thus, it appears that different populations of postsynaptic receptors have followed divergent paths in their response to the inactivation of DAT, in directions that would not necessarily have been expected, with some being down-regulated but others becoming supersensitive. All of these findings suggest that the DAT should be considered not only as an important component terminating extracellular dopamine signals, but also as a primary determinant of dopamine system homeostasis (19, 20).
Behavioral Consequences of Hyperdopaminergia
As a result of persistently enhanced dopaminergic tone, DAT-KO mice display significantly elevated locomotor activity especially when exposed to a novel environment (18, 21, 26). In addition, DAT-KO mice show significant impairment in tests of sensorimotor gating (27) as well as spatial learning and memory (21) but display normal social interaction (26). In DAT-KO mice, psychostimulants do not further enhance locomotor activity but paradoxically inhibit it. This effect is independent of measurable changes in striatal dopaminergic parameters but is mimicked by increases in serotonergic transmission (21). In the DAT-KO mice, the inhibitory action of 5-HT on locomotion is not mediated through a direct modulation of the dopaminergic system (21). These findings suggest that the presence of a persistent hyperdopaminergic tone in these mice reveals the contribution of neuronal systems other than dopamine to the control of behavioral activation. The glutamatergic system represent a major regulatory input to the striatal motor complex (1, 4, 5), and 5-HT has important modulatory influence on the glutamate system (15, 17). In the present study we used DAT-KO mice as a test model to evaluate the potential effect of glutamatergic drugs on this hyperactivity phenotype and its modulation by psychostimulants and serotonergic drugs.
Materials and Methods
Mice.
All experiments were conducted in accordance with the National Institutes of Health guidelines for the care and use of animals and with an approved animal protocol from the Duke University Animal Care and Use Committee. WT mice, mice heterozygous for deletion of DAT (DAT+/−), and DAT-KO mice were derived from crossing (over 10 generations) heterozygous DAT C57BL6/129SvJ animals. Mice were housed 4–5/cage, maintained under standard lab conditions (12 h light/dark cycle) with food and water provided ad libitum, and tested at 12–20 weeks of age.
Activity Measurements.
Locomotion was evaluated in an automated Omnitech Digiscan apparatus (AccuScan Instruments, Columbus, OH) under illuminated conditions (21). Animals were tested individually at 5-min intervals. Horizontal activity was measured in terms of the total distance covered, and vertical activity was expressed in terms of the number of beam breaks caused by rearing. All drugs used in in vivo experiments were dissolved in saline with the following exceptions: fluoxetine was dissolved in distilled water, and aniracetam was suspended in a minimal amount of Tween and made up to a volume (10 ml/kg) with saline. Control animals received saline or corresponding vehicle (10 ml/kg). CX516, CX546 (preformulated as 2-hydroxypropyl-β-cyclodextrin complex), CX672, and CX776 were generously donated by Cortex Pharmaceuticals (Irvine, CA). All of the other drugs were from regular commercial sources. All data are presented as means and SEMs. Statistical significance of all data presented in this article was analyzed by two-way ANOVA followed by Student's t tests for individual comparisons. A P < 0.05 was considered significant.
In Vivo Microdialysis.
Microdialysate samples were collected from the right striatum 24 h after surgery, separated, and quantitated by HPLC with electrochemical detection as described for freely moving mice (19, 21).
Tissue Levels of 5-HT and Metabolite Measurements.
Mice were treated with d-amphetamine (1 mg/kg, i.p.), methylphenidate (30 mg/kg, i.p.), or saline (10 ml/kg, i.p.). One hour later mice were killed, brains were removed, and regions were dissected on ice, frozen in liquid nitrogen, and stored at −80°C. Dissected frontal cortex was homogenized, filtered through 0.22-μm filter, and analyzed for levels of 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) by using HPLC with electrochemical detection (19).
c-Fos Expression Measurements.
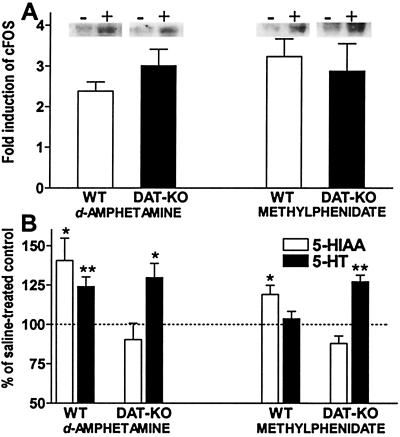
Mice were treated with d-amphetamine, methylphenidate, and saline as described, and 1 h later frontal cortex was harvested and frozen. Tissues were then thawed on ice in lysis buffer [50 mM Tris⋅HCl, pH 8.0/1 mM EDTA/1 mM PMSF/0.1% SDS/1 mM DTT/1 protease inhibitor mixture tablet (Complete, Mini, EDTA-free; Roche) per 10 ml]. Tissue was disrupted via brief polytron homogenization, followed by probe sonication to assure disruption of nuclei. Samples were then passed three times through an insulin syringe to shear DNA. After clearing residual cell debris via centrifugation, protein levels were assessed by Lowry assay. Equivalent levels of protein (50 μg) were loaded per each lane of a 10% Tris-glycine gel. After transfer to membranes, c-Fos was detected by an antibody for c-Fos (Calbiochem, #PC05) and anti-rabbit horseradish peroxidase secondary antibody visualized by chemiluminiscence. Shown (see Fig. 5A) is the average density of 5–6 samples (1 sample/mouse) as quantified by the National Institutes of Health image program. WT and DAT-KO mice treated with saline did not significantly differ in their levels of c-Fos expression in the frontal cortex (data not shown). Equivalent levels of protein in each lane were confirmed by Ponceau S staining of the membranes.
Figure 5.
Effect of d-amphetamine and methylphenidate on the markers of neuronal activity in the frontal cortex of WT and DAT-KO mice. (A) Increased levels of c-Fos in the frontal cortex of DAT-KO and WT mice 1 h after d-amphetamine (1 mg/kg, i.p.) and methylphenidate (30 mg/kg, i.p.) as detected by immunoblot analysis. The data are presented as fold increase over saline-treated controls as compared on the same gels; n = 5–6 per each group. (B) Effect of d-amphetamine (1 mg/kg, i.p.) and methylphenidate (30 mg/kg, i.p.) on the 5-HIAA and 5-HT tissue levels in the frontal cortex of DAT-KO and WT mice 1 h after drug administration. Data presented as percent of saline-treated controls (ng/mg wet tissue: 0.978 ± 0.031 for 5-HT in WT mice and 0.768 ± 0.028 in DAT-KO mice; 0.515 ± 0.072 for 5-HIAA in WT mice and 0.258 ± 0.019 in DAT-KO mice); n = 5–6 per each group. *, P < 0.05; **, P < 0.01 vs. respective saline-treated controls.
Results
Potentiation of Hyperactivity in DAT-KO Mice by MK-801.
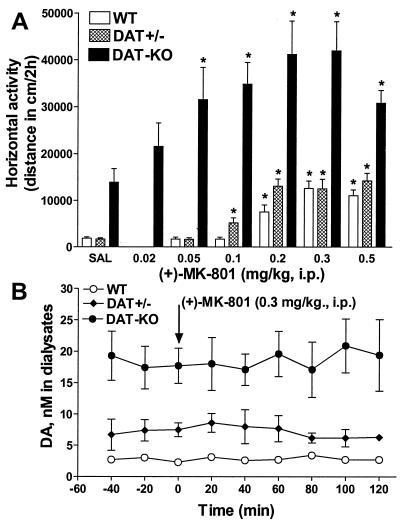
Elevated dopaminergic transmission is commonly associated with behavioral activation (1, 18, 21). Alternatively, hyperactivity can be induced by pharmacological disruption of the glutamatergic transmission by NMDA receptor antagonists (1, 10–13, 28). To assess whether blockade of NMDA receptors could further potentiate the hyperactivity induced by enhanced dopaminergic tone, the effect of the selective NMDA receptor antagonist (+)-MK-801 (10, 29) on the locomotor activity of WT, DAT+/−, and DAT-KO mice was investigated. (+)-MK-801 induced a dose-dependent activation in locomotor activity of all groups of mice. As is characteristic for this drug (28), a pattern of hyperactivity consisting of forward locomotion (Fig. 1A) with reduced incidences of vertical activity (data not shown) was observed in all genotypes. The most robust effect occurred in DAT-KO mice, whereas both WT and DAT+/− mice were activated to a lesser extent and reached comparable levels of maximal activation. The dose response of the effect was significantly altered in mutant mice. Importantly, the amount of (+)-MK-801 required to enhance locomotion was inversely proportional to the level of dopaminergic tone in these mice. For example, (+)-MK-801 exerted significant hyperlocomotor effects at doses as low as 0.05 mg/kg in DAT-KO mice, 0.1 in DAT+/− mice, and 0.2 mg/kg in WT mice (Fig. 1A), which have, respectively, 5- and 2-fold elevation of basal extracellular dopamine levels in comparison to WT controls (19). Interestingly, in rats that have been depleted of dopamine, (+)-MK-801 is able to reverse the inhibition of locomotor activity (11), but this effect is shifted to extremely high doses of the drug (almost 10-fold higher than that required to enhance activity in intact animals). In addition, ligand binding experiments with [3H](+)-MK-801 performed in the frontal cortex, hippocampus, and striatum did not reveal any significant alteration in NMDA receptor number in DAT-KO mice in comparison to WT controls (data not shown). This would suggest that the altered sensitivity of mutant mice to the locomotor effect of (+)-MK-801 may be related to dopamine-dependent modulation of NMDA receptor function and signaling (7–9) rather than just receptor availability.
Figure 1.
Effects of NMDA receptor antagonist (+)-MK-801 on locomotor activity and striatal extracellular dopamine levels in WT, DAT+/−, and DAT-KO mice. (A) Horizontal activity in a novel environment for WT, DAT+/−, and DAT-KO mice in response to (+)-MK-801 administration. Immediately before exposure to the open field, mice were injected with saline (SAL) or (+)-MK-801. Activity was recorded each 5 min and presented as cumulative value for 2 h. Each group consisted of n = 6–8 for WT, n = 6–8 for DAT+/−, and n = 7–15 for DAT-KO animals. *, P < 0.05 vs. respective saline-treated controls. (B) Effect of (+)-MK-801 (0.3 mg/kg, i.p.) on the extracellular dopamine levels monitored by microdialysis in the striatum of freely moving mice. Samples were taken every 20 min for 3 h, reflecting the times before (1 h) and after (2 h) drug administration; n = 5 for WT, n = 4 for DAT+/−, and n = 5 for DAT-KO mice. The data are presented as means and SEMs of dialysate concentration of dopamine. No effect of (+)-MK-801 treatment in any group in comparison to saline-treated controls (data not shown) was noted.
Striatal Extracellular Levels of Dopamine Are Not Affected by MK-801.
One of the hypotheses put forth to explain the stimulatory actions of NMDA receptor antagonists on behavior suggests a direct influence of these drugs on the extracellular dopamine levels in dopaminergic regions of the brain (for review see ref. 12). To assess whether the hyperlocomotor effect of (+)-MK-801 is related to its potential influence on striatal extracellular dopamine, conventional in vivo microdialysis was used in mutant and WT mice. Despite a significant difference in steady-state dialysate content of dopamine in mutant versus WT mice (19) and the degree of activation induced by (+)-MK-801 (0.3 mg/kg) in these mice, neither genotype showed significant overall changes in striatal extracellular dopamine levels in response to this dose of the drug (Fig. 1B). These data confirm the notion that the hyperactivity induced by pharmacological or genetic suppression of NMDA receptor function is not related to the modulation of striatal extracellular dopamine levels (10–12, 30–33).
Positive Modulators of AMPA Glutamate Receptors Counteract Hyperactivity of DAT-KO Mice.
The present data support the concept of a reciprocal interaction between dopaminergic and glutamatergic inputs in the basal ganglia in the control of motor behaviors (1, 10–13). Accordingly, it is reasonable to suggest that drugs enhancing glutamatergic function could counteract dopaminergic hyperactivity (11, 34–36). Several strategies are currently being used to develop drugs that can enhance NMDA receptor-mediated glutamate function (37–40). One such approach has been the development of drugs that could positively modulate AMPA receptors (34–36, 38, 39, 41–46). Recent advances in understanding NMDA receptor physiology have highlighted the critical role of AMPA receptor-mediated events in the NMDA receptor currents and functional output such as long-term potentiation (47, 48). AMPA receptors are known to undergo rapid attenuation of function (43, 47), and drugs interfering with this process (also referred to as AMPAkines) have been shown to regulate synaptic strength at glutamatergic synapses (42–46, 49).
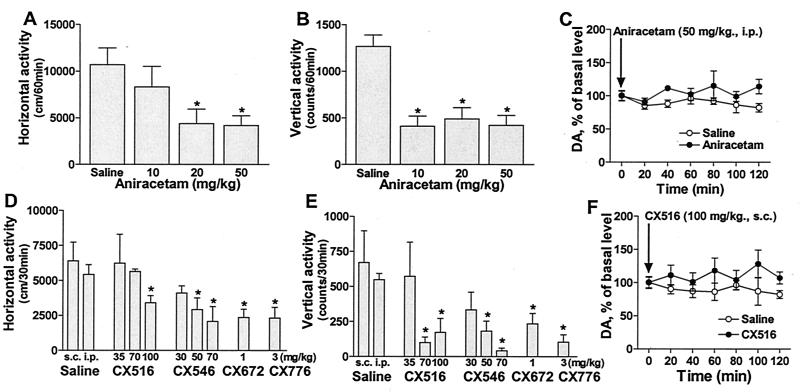
To test this notion, positive modulators of AMPA receptor-dependent glutamatergic transmission, aniracetam (42, 44, 50–52) and the AMPAkines 1-(quinoxalin-6-ylcarbonyl) piperidine, CX516 (34–36, 45, 46), and 1-(1,4-benzodioxan-6-ylcarbonyl)piperidine, CX546 (49, 53) were tested for their effects on hyperactivity in DAT-KO mice (Fig. 2 A, B, D, and E). Each drug dose-dependently decreased both horizontal and vertical activities in DAT-KO mice in a novel environment, thereby suggesting that facilitation of glutamatergic transmission can counteract dopaminergic hyperactivity (11, 36). Similar effects were observed when AMPAkines of a new generation, CX672 (1 mg/kg, i.p.) and CX776 (3 mg/kg, s.c.) were tested (Fig. 2 D and E). Importantly, no corresponding decreases in extracellular dopamine levels after administration of aniracetam (50 mg/kg, i.p.) or CX516 (100 mg/kg, s.c.) were noted in the striatum of freely moving DAT-KO mice in microdialysis experiments (Fig. 2 C and F), once again illustrating a lack of direct involvement of striatal dopaminergic transmission in the modulatory effect of glutamatergic drugs on locomotor activity.
Figure 2.
Effects of positive modulators of AMPA receptors on the locomotor activity and striatal extracellular dopamine levels of DAT-KO mice. DAT-KO mice were placed in the open-field apparatus for an initial period of 30 min and then were injected with aniracetam (10, 20, and 50 mg/kg, i.p.) (A and B) or AMPAkines CX516 (35, 70, and 100 mg/kg, s.c.), CX546 (30, 50, and 70 mg/kg, s.c.), CX672 (1 mg/kg, i.p.), and CX776 (3 mg/kg, s.c.) (D and E). Controls were given corresponding vehicle (10 ml/kg, i.p. or s.c.). Mice were immediately returned to the apparatus and horizontal (A and D) and vertical (B and E) activities in an open-field environment were recorded every 5 min for an additional 60 min after aniracetam and 30 min after AMPAkines; n = 8–10 for aniracetam and n = 6–11 for CX516 and CX546 per each group. *, P < 0.05; vs. respective vehicle-treated controls. (C and F) Effect of aniracetam (50 mg/kg, i.p.) (C) and CX516 (100 mg/kg, s.c.) (F) on the extracellular dopamine levels monitored by microdialysis in the striatum of freely moving DAT-KO mice. The data are expressed as percentage of average values of at least three basal values before the drug or saline administration; n = 5 for saline-treated controls for both groups, n = 5 for aniracetam and n = 4 for CX516 per each group.
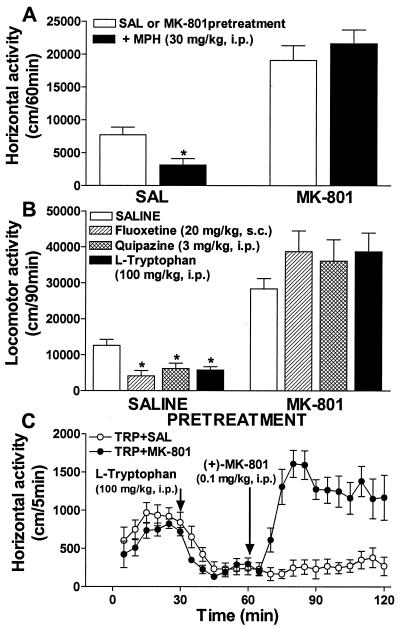
MK-801 Disrupts Inhibitory Effect of Amphetamine on Hyperactivity of DAT-KO Mice.
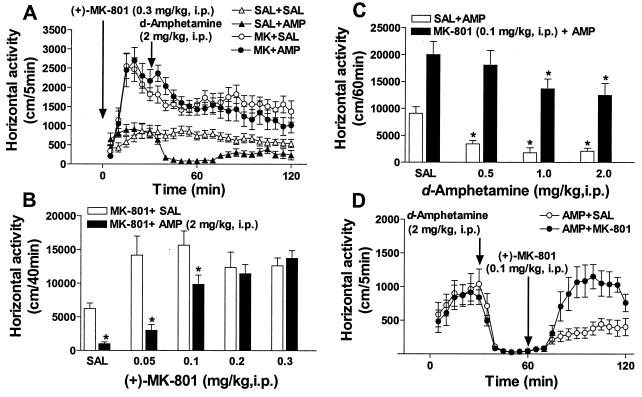
Previously, it has been shown that the inhibitory action of psychostimulants on hyperactivity in DAT-KO mice does not involve modulation of dopaminergic activity but rather reflects the influence of these drugs on 5-HT transmission (21). Moreover, it has been demonstrated that the effect of 5-HT on dopaminergic hyperactivity is likely to occur by modulation of postsynaptic dopaminergic responses (21). One possibility for this effect may involve glutamate transmission as an intermediate in the 5-HT–dopamine interaction (1, 6, 10–13). This hypothesis would suggest that a blockade of glutamatergic transmission by (+)-MK-801 could interfere with the ability of psychostimulants to exert their inhibitory action in these mice. To test this hypothesis, we investigated the ability of (+)-MK-801 to affect the inhibitory action of amphetamine on the hyperactivity in DAT-KO mice. In agreement with a previous report (21), d-amphetamine at a dose of 2 mg/kg attenuated activity of DAT-KO mice (Fig. 3A). Pretreatment with (+)-MK-801 at a dose of 0.3 mg/kg induced potent activation in DAT-KO mice (see also Fig. 1A), and completely disrupted the attenuating effect of d-amphetamine on hyperactivity. (+)-MK-801 administered at lower doses exerted similar potent hyperlocomotor effects but its attenuation of the inhibitory effect of d-amphetamine (2 mg/kg) was dose-dependent with doses 0.1 and 0.2 mg/kg being less effective and a dose of 0.05 mg/kg being virtually ineffective (Fig. 3B). Similarly, the inhibitory effect of a lower dose of d-amphetamine (0.5 mg/kg) was absent when mice were pretreated with a dose of 0.1 mg/kg of (+)-MK-801 (Fig. 3C), but d-amphetamine at higher doses (1 and 2 mg/kg) was still effective to exert inhibitory action in similarly pretreated mice. Persistence of the inhibitory effect of amphetamine in mice activated by low doses of (+)-MK-801 argues against the possibility that the lack of inhibitory effect of amphetamine in mice treated with higher doses of (+)-MK-801 is simply because baseline hyperactivity and MK-801-induced hyperactivity are two separate and unrelated processes. Rather, the dose-dependent attenuating effect of (+)-MK-801 on the inhibitory effect of amphetamine in DAT-KO mice is consistent with the dependence of this inhibitory action of amphetamine on the intensity of glutamatergic transmission.
Figure 3.
Attenuation of the hypolocomotor effect of d-amphetamine in DAT-KO mice by (+)-MK-801. (A) Effect of combined treatment of (+)-MK-801 (0.3 mg/kg, i.p.; administered immediately before exposure to locomotor activity chamber) and d-amphetamine (2 mg/kg, i.p.; 30 min later) on the horizontal activity of DAT-KO mice in a novel environment. Horizontal activity was monitored for 2 h at 5-min intervals; n = 12 for the saline-treated DAT-KO mice (▵), n = 9 for the d-amphetamine-treated DAT-KO (▴), n = 15 for the DAT-KOs given (+)-MK-801 (○), and n = 11 for the DAT-KOs given (+)-MK-801 plus d-amphetamine (●). (+)-MK-801 effectively prevented inhibition of novelty-driven hyperactivity of DAT-KO mice by d-amphetamine. (B) Dose dependence of the effect of (+)-MK-801 on amphetamine induced hypoactivity in DAT-KO mice. Mice were pretreated with saline or different doses of (+)-MK-801 and placed in locomotor activity boxes; 30 min later d-amphetamine (2 mg/kg, i.p.) was administered. Horizontal activity was further monitored every 5 min and presented as cumulative value for 40 min after d-amphetamine administration; n = 7–10 for each group. *, P < 0.05 vs. respective saline- or (+)-MK-801-pretreated controls. (C) Increasing doses of d-amphetamine are able to counteract to effect of (+)-MK-801 in DAT-KO mice. Mice were treated as described in A and B with saline or a single dose of (+)-MK-801 (0.1 mg/kg, i.p.) and 30 min later the effect of saline or various doses of d-amphetamine was tested; n = 6–9 for each group. Data presented as described in B. *, P < 0.05 vs. saline- or (+)-MK-801-pretreated controls, respectively. (D) (+)-MK-801 (0.1 mg/kg, i.p.) reverses hypolocomotor behavior of DAT-KO mice induced by d-amphetamine (2 mg/kg, i.p.). d-Amphetamine was administered 30 min after exposure of mice to the locomotor activity chamber and 30 min later (+)-MK-801 or saline was injected. d-Amphetamine plus saline-treated controls (○), n = 7, and d-amphetamine plus (+)-MK-801-treated mice (●), n = 6. SAL, saline; AMP, amphetamine; MK, (+)-MK-801.
In addition, as shown in Fig. 3D, the attenuating effect of d-amphetamine (2 mg/kg) on hyperactivity of DAT-KO mice could be completely reversed by subsequent administration of a low dose of (+)-MK-801 (0.1 mg/kg). In a separate set of experiments, the hypolocomotor effect of another psychostimulant, methylphenidate (21), also could be completely blocked by (+)-MK-801 pretreatment of DAT-KO mice (Fig. 4A). These data suggest that the hypolocomotor effect of psychostimulants in DAT-KO mice requires intact glutamatergic neurotransmission.
Figure 4.
Blockade of the hypolocomotor effect of methylphenidate and serotonergic drugs in DAT-KO mice by (+)-MK-801. (A) Effect of (+)-MK-801 (0.3 mg/kg, i.p.) pretreatment on the effect of methylphenidate (30 mg/kg, i.p.) on horizontal activity of DAT-KO mice in a novel environment. DAT-KO mice were pretreated with saline or (+)-MK-801 and 30 min later with methylphenidate as described in Fig. 3 A–C. Horizontal activity was monitored for 2 h at 5-min intervals and presented as cumulative value for 1 h after methylphenidate administration; n = 7–15 for each group. *, P < 0.05 vs. saline- or (+)-MK-801-pretreated controls, respectively. (B) Effect of (+)-MK-801 (0.3 mg/kg, i.p.) pretreatment on the effect of serotonergic drugs on horizontal activity of DAT-KO mice. DAT-KO mice were pretreated with saline or (+)-MK-801 and 30 min later were administered a 5-HT transporter inhibitor (fluoxetine; 20 mg/kg, s.c.), a nonselective 5-HT receptor agonist (quipazine; 3 mg/kg, i.p.), or 5-HT precursor (l-tryptophan; 100 mg/kg, i.p.) as described in Fig. 3 A–C. Horizontal activity was monitored for 2 h at 5-min intervals and presented as cumulative value for 90 min after administration of serotonergic drugs; n = 6–15 for each group. *, P < 0.05 vs. saline- or (+)-MK-801-pretreated controls, respectively. (C) (+)-MK-801 (0.1 mg/kg, i.p.) reverses hypolocomotor behavior of DAT-KO mice induced by l-tryptophan (100 mg/kg, i.p.). l-tryptophan was administered 30 min after exposure of mice to locomotor activity chamber and 30 min later (+)-MK-801 or saline was injected. l-tryptophan plus saline-treated controls (○), n = 7, and l-tryptophan plus (+)-MK-801-treated mice (●), n = 8. SAL, saline; MPH, methylphenidate; TRP, tryptophan.
MK-801 Disrupts the Inhibitory Action of Serotonergic Drugs on Hyperactivity.
The inhibitory action of psychostimulants on hyperactivity in the DAT-KO mice has been suggested to involve modulation of 5-HT transmission (21). To test whether the inhibitory action of 5-HT drugs also would be sensitive to a blockade of glutamergic transmission, several serotonergic drugs were evaluated in combination with (+)-MK-801. In agreement with our earlier study (21), fluoxetine (20 mg/kg), quipazine (3 mg/kg), and l-tryptophan (100 mg/kg) significantly inhibited locomotor activity of DAT-KO mice. Again, pretreatment with (+)-MK-801 not only resulted in a further increase in activity but also in the absence of an inhibitory effect of all serotonergic drugs tested (Fig. 4B). In addition, (+)-MK-801 at a low dose (0.1 mg/kg) effectively reversed the reduced locomotor activity of DAT-KO mice pretreated with l-tryptophan (Fig. 4C). Altogether, these findings demonstrate that the inhibitory action of 5-HT on hyperactivity involves glutamatergic transmission and gives further support to the notion that this effect does not directly involve modulation of dopaminergic pathways (21).
Psychostimulants Modulate Serotonergic Transmission in the Frontal Cortex of DAT-KO Mice.
A major glutamatergic input to the basal ganglia derives from frontal cortical areas (1, 4–6, 12). Several lines of evidence indicate that psychostimulants affect not only striatum and nucleus accumbens, but also exert potent effects in other brain areas, particularly in the frontal cortex (41, 54–60). Interestingly, local application of amphetamine into the medial prefrontal cortex counteracts hyperactivity induced by the systemic administration of the same drug (61). Previously, we observed that cocaine causes a significant induction of c-Fos expression in several regions of frontal cortex in DAT-KO mice (62). To assess whether d-amphetamine and methylphenidate also can exert effects in the frontal cortex of DAT-KO mice, levels of c-Fos, a nonspecific marker of neuronal activation and an important intermediate in the cascade of events initiated by psychostimulants (62–64), were determined. Immunoblotting analyses revealed that treatments with d-amphetamine and methylphenidate significantly increased c-Fos levels in the frontal cortex of both WT and DAT-KO mice as compared with saline-treated controls (Fig. 5A). Because d-amphetamine and methylphenidate treatments elevated c-Fos levels in the frontal cortex to a similar extent in both WT and DAT-KO mice, it would appear that in the normal situation psychostimulants exert a significant portion of their effect via interaction with targets other than DAT. When neurochemical parameters of serotonergic transmission in the frontal cortex were examined (Fig. 5B), it was found that d-amphetamine increased cortical tissue levels of both 5-HT and its metabolite 5-HIAA in WT mice, whereas affecting only 5-HT levels in DAT-KO mice. Methylphenidate induced significant elevation of 5-HIAA levels in the frontal cortex of WT mice and 5-HT in DAT-KO mice (Fig. 5B). Similar effects of amphetamine (13, 60) and methylphenidate (59, 65) on c-Fos expression (59, 60) and neurochemical indices of serotonergic transmission (13, 65) in the frontal cortex of rats have been reported. The present data suggest that one of the potential mechanisms of psychostimulant action to affect glutamatergic transmission, and, consequently, dopaminergic hyperactivity, may involve modulation of 5-HT transmission in the frontal cortex. However, the precise cellular and molecular sites of this putative interaction remains to be elucidated.
Discussion
The persistent hyperdopaminergic tone and hyperactivity in the DAT-KO mice provides a model in which the contribution of neuronal systems other than dopamine in regulating locomotion can be more readily assessed than in normal animals. Changes in dopaminergic tone are highly related to alterations in locomotor activity (1, 11–13, 18). At the same time, it is increasingly clear that another major input to the striatum, namely the frontostriatal glutamatergic pathway, which converges with the dopamine system at the level of medium spiny γ-aminobutyric acid neurons in the striatum and nucleus accumbens, plays an important role in the control of locomotion. The reciprocal functional interaction between these systems, which most likely occurs at the level of postsynaptic dopamine and glutamate receptors signaling and regulation (7–9), is now being recognized as critical for the control of motor behavior (1, 10–13, 37, 38). Both the striking potentiation of activity in DAT-KO mice by the blockade of NMDA receptors and the significant inhibitory effect of AMPAkines on locomotor activity in these mice provide further support for this concept. The shift in dose–response curves of the NMDA antagonist in the hyperdopaminergic mice suggests that alterations in dopaminergic signaling can significantly modulate responsiveness to glutamatergic manipulations. Conversely, the potent effect of drugs enhancing or inhibiting glutamatergic transmission on the “high” dopamine-induced hyperactivity illustrates an important modulatory role of glutamate in dopaminergic functional responses. In addition, the present data showing that glutamate antagonists can enhance hyperactivity in already hyperactive DAT-KO mice suggest that combined dysfunctions in glutamate and dopamine systems could potentially result in more pronounced behavioral abnormalities than defects in each of the two systems separately.
A promising direction in the search for pharmacological agents that are able to enhance glutamatergic transmission without inducing side effects characteristic to direct glutamate agonists (39) is the development of compounds positively modulating AMPA receptor function (42–46). One of the first drugs, which was recognized to have significant AMPA receptor-modulating properties, is aniracetam (42–44, 50, 51), a representative of so-called cognitive enhancers or nootropic drugs (52, 66). This and similar drugs, piracetam-like cognitive enhancers (52), were developed in an attempt to enhance cognitive functions and are used clinically in several countries. A new generation of the positive modulators of AMPA-receptor compounds, referred to as AMPAkines, have been shown to enhance excitatory transmission, facilitate long-term potentiation, and enhance learning and memory both in experimental animals and humans (45, 46). AMPAkines have little effect on spontaneous locomotor activity in intact animals but can suppress methamphetamine-induced hyperactivity in rats (34–36). The emerging evidence of the involvement of the glutamatergic system in certain manifestations of schizophrenia (10–13, 37, 38, 57, 67) provides a theoretical basis to explore the potential therapeutical benefit of AMPAkines in this disorder (34–36, 68). The efficacy of these drugs in counteracting dopaminergic hyperactivity suggests that AMPAkines also could potentially be effective in treating dopamine related symptoms of this disorder as well as other conditions resulting primarily from dopaminergic dysfunctions.
The frontal cortex and basal ganglia are considered to be the brain areas primarily responsible for behavioral manifestations observed in subjects with attention deficit hyperactivity disorder (ADHD) (69–72). ADHD is one of the most prevalent childhood disorders manifesting as impulsivity, hyperactivity, and inattention (69). Psychostimulants and some antidepressants are the most effective drugs in the treatment of this condition (69). A significant association of ADHD with a variant allele of the DAT gene has been reported (73). Interestingly, DAT-KO mice are hyperactive and demonstrate significant impairment in cognitive functions, and their hyperactivity can be inhibited by psychostimulants. Although a previous study established the involvement of serotonergic transmission in this inhibitory action of psychostimulants in DAT-KO mice (21), the neuronal circuitry involved has not been investigated. The present study reveals the involvement of glutamatergic transmission as an intermediate in the inhibitory action of psychostimulants and serotonergic drugs on the hyperactivity of DAT-KO mice. Although these data suggest that frontostriatal glutamatergic pathway is involved in this effect, it should be emphasized that the possibility of additional influences from other brain areas (4, 5) cannot be excluded. Several investigators have suggested previously that psychostimulants can affect striatal function both directly via enhanced dopaminergic tone and indirectly through the involvement of the frontostriatal pathway (41, 54–62). Interestingly, the ability of psychostimulants to enhance the inhibitory control of frontal cortex over subcortical brain areas has been hypothesized to be critical for the therapeutic actions of these drugs in ADHD patients; however, the nature of this interaction has remained elusive (70–72). Moreover, perturbations in dopaminergic neurotransmission are believed to be also associated with schizophrenia and several other neuropsychiatric disorders (1, 3). Because suppression of dopaminergic activity has been the major approach used in the management of schizophrenia (1, 10–13) and the modulation of 5-HT–glutamate–dopamine interaction within frontostriatal circuitry is now considered to be a primary determinant of the efficacy of some novel antipsychotics (13–17), our findings may have particular implications in understanding both the etiology and management of this disorder as well.
In conclusion, these findings support the concept of a reciprocal functional interaction between dopamine and glutamate in the basal ganglia and point to agents enhancing glutamatergic transmission as a potential approach to treat conditions associated with hyperdopaminergic function. In addition, these data suggest that glutamate transmission may be part of the neuronal circuitry involved in the inhibitory influence of 5-HT on hyperactivity.
Acknowledgments
This work was supported by Grants DA 06023 (to L.M.B.) and MH-40159 from the National Institutes of Health and unrestricted gifts from Bristol Myers Squibb and Zeneca Pharmaceuticals (to M.G.C.). M.G.C. is an Investigator of the Howard Hughes Medical Institute. R.R.G. is a visiting scientist from the Institute of Pharmacology, Russian Academy of Medical Sciences, Baltiyskaya 8, 125315 Moscow, Russia.
Abbreviations
- 5-HT
serotonin
- DAT
dopamine transporter
- DAT-KO
DAT-knockout
- NMDA
N-methyl-d-aspartate
- AMPA
l-α-amino-3-hydroxy-5-methylisoxazole-4-propionate
- 5-HIAA
5-hydroxyindoleacetic acid
- TH
tyrosine hydroxylase
- WT
wild type
Footnotes
This paper was presented at the Inaugural Arthur M. Sackler Colloquium of the National Academy of Sciences, “Neural Signaling,” held February 15–17, 2001, at the National Academy of Sciences in Washington, DC.
Cooper, D. C., Hu, X.-T., Jones, S. R., Giros, B., Caron, M. G. & White, F. J. (1997) Soc. Neurosci. Abstr. 23, 1210.
References
- 1.Carlsson M L, Carlsson A. Trends Neurosci. 1990;13:272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- 2.Robbins T W. Exp Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- 3.Roth R H, Elsworth J. In: Psychopharmacology: The Fourth Generation of Progress. Bloom F, Kupfer D, editors. New York: Raven; 1995. pp. 227–243. [Google Scholar]
- 4.Phillipson O T, Griffiths A C. Neuroscience. 1985;16:275–296. doi: 10.1016/0306-4522(85)90002-8. [DOI] [PubMed] [Google Scholar]
- 5.Graybiel A M. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 6.Kotter R. Prog Neurobiol. 1994;44:163–196. doi: 10.1016/0301-0082(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 7.Greengard P, Allen P B, Nairn A C. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 8.Leveque J C, Macias W, Rajadhyaksha A, Carlson R R, Barczak A, Kang S, Li X M, Coyle J T, Huganir R L, Heckers S, et al. J Neurosci. 2000;20:4011–4020. doi: 10.1523/JNEUROSCI.20-11-04011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder G L, Allen P B, Fienberg A A, Valle C G, Huganir R L, Nairn A C, Greengard P. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohn A R, Gainetdinov R R, Caron M G, Koller B H. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson M, Carlsson A. J Neural Transm. 1989;75:221–226. doi: 10.1007/BF01258633. [DOI] [PubMed] [Google Scholar]
- 12.Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson M L. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 13.Martin P, Waters N, Schmidt C J, Carlsson A, Carlsson M L. J Neural Transm. 1998;105:365–396. doi: 10.1007/s007020050064. [DOI] [PubMed] [Google Scholar]
- 14.Abi Dargham A, Laruelle M, Aghajanian G K, Charney D, Krystal J. J Neuropsychiatry Clin Neurosci. 1997;9:1–17. doi: 10.1176/jnp.9.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Jakab R L, Goldman-Rakic P S. Proc Natl Acad Sci USA. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meltzer H Y. Neuropsychopharmacology. 1999;21:106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 17.Aghajanian G K, Marek G J. Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 18.Giros B, Jaber M, Jones S R, Wightman R M, Caron M G. Nature (London) 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 19.Jones S R, Gainetdinov R R, Jaber M, Giros B, Wightman R M, Caron M G. Proc Natl Acad Sci USA. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gainetdinov R R, Jones S R, Fumagalli F, Wightman R M, Caron M G. Brain Res Rev. 1998;26:148–153. doi: 10.1016/s0165-0173(97)00063-5. [DOI] [PubMed] [Google Scholar]
- 21.Gainetdinov R R, Wetsel W C, Jones S R, Levin E D, Jaber M, Caron M G. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 22.Benoit-Marand M, Jaber M, Gonon F. Eur J Neurosci. 2000;12:2985–2992. doi: 10.1046/j.1460-9568.2000.00155.x. [DOI] [PubMed] [Google Scholar]
- 23.Jaber M, Dumartin B, Sagne C, Haycock J W, Roubert C, Giros B, Bloch B, Caron M G. Eur J Neurosci. 1999;11:3499–3511. doi: 10.1046/j.1460-9568.1999.00764.x. [DOI] [PubMed] [Google Scholar]
- 24.Jones S R, Gainetdinov R R, Hu X-T, Cooper D C, Wightman R M, White F J, Caron M G. Nat Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- 25.Gainetdinov R R, Jones S R, Caron M G. Biol Psychiatry. 1999;46:303–311. doi: 10.1016/s0006-3223(99)00122-5. [DOI] [PubMed] [Google Scholar]
- 26.Spielewoy C, Roubert C, Hamon M, Nosten M, Betancur C, Giros B. Behav Pharmacol. 2000;11:279–281. doi: 10.1097/00008877-200006000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ralph R J, Paulus M P, Fumagalli F, Caron M G, Geyer M A. J Neurosci. 2001;21:305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehmann-Masten V D, Geyer M A. Neuropharmacology. 1991;30:629–636. doi: 10.1016/0028-3908(91)90083-n. [DOI] [PubMed] [Google Scholar]
- 29.Wong E H, Kemp J A, Priestley T, Knight A R, Woodruff G N, Iversen L L. Proc Natl Acad Sci USA. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kashihara K, Hamamura T, Okumura K, Otsuki S. Brain Res. 1990;528:80–82. doi: 10.1016/0006-8993(90)90197-j. [DOI] [PubMed] [Google Scholar]
- 31.Druhan J P, Rajabi H, Stewart J. Synapse. 1996;24:135–146. doi: 10.1002/(SICI)1098-2396(199610)24:2<135::AID-SYN5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 32.Mele A, Fontana D, Pert A. Synapse. 1997;26:218–224. doi: 10.1002/(SICI)1098-2396(199707)26:3<218::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 33.Callado L F, Hopwood S E, Hancock P J, Stamford J A. NeuroReport. 2000;11:173–176. doi: 10.1097/00001756-200001170-00034. [DOI] [PubMed] [Google Scholar]
- 34.Larson J, Quach C N, LeDuc B Q, Nguyen A, Rogers G A, Lynch G. Brain Res. 1996;738:353–356. doi: 10.1016/s0006-8993(96)01049-9. [DOI] [PubMed] [Google Scholar]
- 35.Vanover K E. Eur J Pharmacol. 1997;332:115–119. doi: 10.1016/s0014-2999(97)01103-5. [DOI] [PubMed] [Google Scholar]
- 36.Johnson S A, Luu N T, Herbst T A, Knapp R, Lutz D, Arai A, Rogers G A, Lynch G. J Pharmacol Exp Ther. 1999;289:392–397. [PubMed] [Google Scholar]
- 37.Coyle J T. Harvard Rev Rsychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 38.Krystal J H, D'Souza D C, Petrakis I L, Belger A, Berman R M, Charney D S, Abi-Saab W, Madonick S. Harvard Rev Psychiatry. 1999;7:125–143. [PubMed] [Google Scholar]
- 39.Parsons C G, Danysz W, Quack G. Drug News Perspect. 1998;11:523–569. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- 40.Snyder S H. Philos Trans R Soc London. 1999;354:1985–1984. doi: 10.1098/rstb.1999.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer L C, Hess U S, Larson J, Rogers G A, Gall C M, Lynch G. Mol Brain Res. 1997;46:127–135. doi: 10.1016/s0169-328x(96)00280-x. [DOI] [PubMed] [Google Scholar]
- 42.Ito I, Tanabe S, Kohda A, Sugiyama H. J Physiol (London) 1990;424:533–543. doi: 10.1113/jphysiol.1990.sp018081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang C M, Shi Q Y, Katchman A, Lynch G. Science. 1991;254:288–290. doi: 10.1126/science.254.5029.288. [DOI] [PubMed] [Google Scholar]
- 44.Nicoletti F, Casabona G, Genazzani A A, Copani A, Aleppo G, Canonico P L, Scapagnini U. Funct Neurol. 1992;7:413–422. [PubMed] [Google Scholar]
- 45.Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers G A, Schehr R S, Lynch G. Exp Neurol. 1997;146:553–559. doi: 10.1006/exnr.1997.6581. [DOI] [PubMed] [Google Scholar]
- 46.Lynch G. Neurobiol Learn Mem. 1998;70:82–100. doi: 10.1006/nlme.1998.3840. [DOI] [PubMed] [Google Scholar]
- 47.Malenka R C, Nicoll R A. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 48.Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser K M, Koster H J, Borchardt T, Worley P, et al. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 49.Holst B D, Vanderklish P W, Krushel L A, Zhou W, Langdon R B, McWhirter J R, Edelman G M, Crossin K L. Proc Natl Acad Sci USA. 1998;95:2597–2602. doi: 10.1073/pnas.95.5.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson D M, Guidotti A, DiBella M, Costa E. Proc Natl Acad Sci USA. 1995;92:7667–7671. doi: 10.1073/pnas.92.17.7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura K, Kurasawa M, Shirane M. Brain Res. 2000;862:266–269. doi: 10.1016/s0006-8993(00)02160-0. [DOI] [PubMed] [Google Scholar]
- 52.Gouliaev A H, Senning A. Brain Res Rev. 1994;19:180–222. doi: 10.1016/0165-0173(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 53.Lauterborn J C, Lynch G, Vanderklish P, Arai A, Gall C M. J Neurosci. 2000;20:8–21. doi: 10.1523/JNEUROSCI.20-01-00008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White F J, Kalivas P W. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 55.Tzschentke T M, Schmidt W J. Eur J Neurosci. 1999;11:4099–4109. doi: 10.1046/j.1460-9568.1999.00834.x. [DOI] [PubMed] [Google Scholar]
- 56.Mehta M A, Owen A M, Sahakian B J, Mavaddat N, Pickard J D, Robbins T W. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyamoto S, Leipzig J N, Lieberman J A, Duncan G E. Neuropsychopharmacology. 2000;22:400–412. doi: 10.1016/S0893-133X(99)00127-X. [DOI] [PubMed] [Google Scholar]
- 58.Volkow N D, Fowler J S. Cerebral Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 59.Lin J-S, Hou Y, Jouvet M. Proc Natl Acad Sci USA. 1996;93:14128–14133. doi: 10.1073/pnas.93.24.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Badiani A, Oates M M, Day H E, Watson S J, Akil H, Robinson T E. J Neurosci. 1998;18:10579–10593. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lacroix L, Broersen L M, Feldon J, Weiner I. Behav Brain Res. 2000;107:111–121. doi: 10.1016/s0166-4328(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 62.Rocha B A, Fumagalli F, Gainetdinov R R, Jones S R, Ator R, Giros B, Miller G W, Caron M G. Nat Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- 63.Morgan J I, Curran T. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 64.Nestler E J. Crit Rev Neurobiol. 1993;7:23–39. [PubMed] [Google Scholar]
- 65.Kuczenski R, Segal D S, Leith N J, Applegate C D. Psychopharmacology. 1987;93:329–335. doi: 10.1007/BF00187252. [DOI] [PubMed] [Google Scholar]
- 66.Mondadori C. Crit Rev Neurobiol. 1996;10:357–370. doi: 10.1615/critrevneurobiol.v10.i3-4.40. [DOI] [PubMed] [Google Scholar]
- 67.Gao X M, Sakai K, Roberts R C, Conley R R, Dean B, Tamminga C A. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- 68.Noorbala A A, Akhonzadeh S, Davari-Ashtiani R, Amini-Nooshabadi H. J Clin Pharm Ther. 1999;24:369–374. doi: 10.1046/j.1365-2710.1999.00238.x. [DOI] [PubMed] [Google Scholar]
- 69.Barkley R A. Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York: Guiford; 1990. [Google Scholar]
- 70.Zametkin A J, Rapoport J L. J Am Acad Child Adolesc Psychiatry. 1987;26:676–686. doi: 10.1097/00004583-198709000-00011. [DOI] [PubMed] [Google Scholar]
- 71.Barkley R A. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 72.Faraone S V, Biederman J. Biol Psychiatry. 1998;44:951–958. doi: 10.1016/s0006-3223(98)00240-6. [DOI] [PubMed] [Google Scholar]
- 73.Cook E H, Jr, Stein M A, Krasowski M D, Cox N J, Olkon D M, Kieffer J E, Leventhal B L. Am J Hum Genet. 1995;56:993–998. [PMC free article] [PubMed] [Google Scholar]