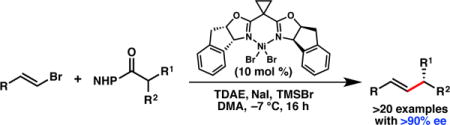
Abstract
An enantioselective Ni-catalyzed cross-coupling of N-hydroxyphthalimide esters with vinyl bromides is reported. The reaction proceeds under mild conditions and uses tetrakis(N,N-dimethylamino)ethylene as a terminal organic reductant. Good functional group tolerance is demonstrated, with over twenty examples of reactions that proceed in greater than 90% ee.
Graphical abstract
Nickel catalyzed cross-coupling reactions have emerged as powerful methods to form C(sp3)–C(sp2) and C(sp3)–C(sp3) bonds.1 Whereas pioneering investigations focused on the canonical cross-coupling of C(sp3) electrophiles with organometallic reagents—variants of the venerable Negishi,2 Kumada,3 and Suzuki,4 reactions, among others—additional modes of alkyl cross-coupling using nickel catalysis have recently been disclosed. These include cross-electrophile “reductive” couplings that use an exogenous, stoichiometric reductant to shuttle electrons to the nickel catalyst,5 as well as cross-coupling reactions that rely on synergistic reactivity between nickel and photoredox co-catalysts.6 Taken together, these reactions enable the cross-coupling of a broad range of C(sp3) substrates, providing access to a variety of products.
Ni-catalyzed reductive cross-coupling reactions have proven particularly useful for the cross-coupling of secondary alkyl electrophiles, often providing chiral products as racemic mixtures.5 Recognizing that the ability to render these transformations enantioselective would enhance their utility,7 our laboratory has recently developed enantioselective Ni-catalyzed reductive cross-coupling reactions of both benzylic chlorides8 and a-chloronitriles.9 An important objective for further improving the synthetic usefulness of asymmetric reductive cross-coupling reactions is to develop reactions of new electrophile classes. Just as in conventional cross-coupling reactions, where different organometallic reagents (e.g. organozinc, organomagnesium, organoboron reagents, etc.) bring unique advantages to a specific synthetic scenario, the ability to cross-couple new electrophile classes broadens the tool box for strategic C–C bond formation. However, it can be challenging to apply conditions from previously developed reductive cross-coupling reactions to new electrophile classes, especially if there are changes to the mechanism by which the coupling partner undergoes oxidative addition; in particular, it can require tuning of either the ligand structure or the stoichiometric reductant (or both) in order to develop reactions that proceed both in good yield and enantioselectivity.
As part of our efforts to develop asymmetric cross-coupling reactions that employ a variety of C(sp3) electrophiles, we became interested in the coupling of redox-active N-hydroxyphthalimide (NHP) esters.10 These electrophiles are prepared from the corresponding carboxylic acids, and have been recently demonstrated as C(sp3) substrates for Ni-catalyzed Negishi,11a,b Suzuki,11c and reductive12 cross-coupling reactions to generate racemic products (Scheme 1).13,14 However, NHP esters have not been demonstrated as competent coupling partners in Ni-catalyzed enantioselective cross-coupling reactions. We recognized that the use of NHP esters might be advantageous for substrates in which the corresponding alkyl chlorides are unstable or challenging to prepare. In this communication, we report the first Ni-catalyzed asymmetric cross-coupling reactions of NHP esters. These reactions proceed under mild conditions using tetrakis(N,N-dimethylamino)ethylene (TDAE) as a homogenous, stoichiometric reductant, and exhibit high functional group tolerance.
Scheme 1.
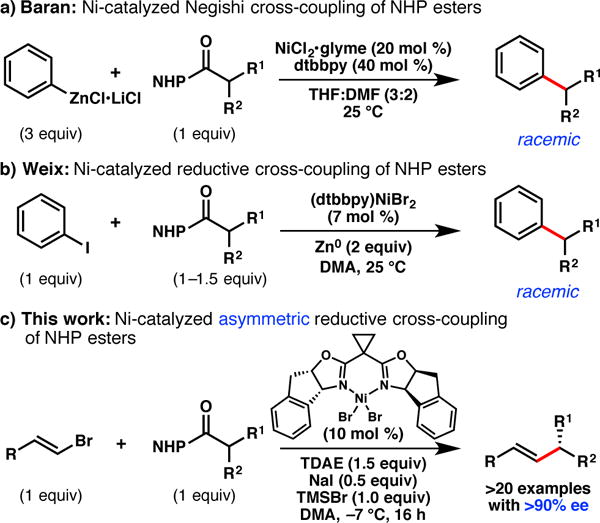
Ni-catalyzed reactions of NHP esters.
Our efforts began with ester 2, which is prepared in one step from commercially available 2-phenylpropanoic acid. Subjection of 2 to our optimal conditions developed for the reductive cross-coupling of vinyl bromides and benzyl chlorides provided only trace quantities of product (Table 1, entry 1),8b highlighting the challenges presented by this new C(sp3)-electrophile. Slightly improved results could be obtained under the same conditions by adding 1.0 equiv TMSCl (entry 2). An investigation of alternative reductants revealed that TDAE substantially increased the reactivity, delivering 3a in 61% yield and 95% ee (entries 2–4).15
Table 1.
Reaction optimization.a
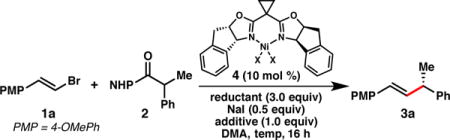
| ||||||
|---|---|---|---|---|---|---|
| entry | X | reductant | additive | temp (°C) |
yield (%)b |
ee (%)c |
| 1 | Cl (4a) | Mn | none | 0 | trace | – |
| 2 | Cl | Mn | TMSCl | 0 | 27 | 90 |
| 3 | Cl | Zn | TMSCl | 0 | 38 | 64 |
| 4 | Cl | TDAE | TMSCl | 0 | 61 | 95 |
| 5 | Br (4b) | TDAE | TMSBr | 0 | 75 | 92 |
| 6 | Br | TDAE | TMSBr | −7 | 79 | 93 |
| 7d | Br | TDAE | TMSBr | −7 | 80e | 96 |
| 8d | Br | TDAE | none | −7 | 19 | 88 |
Reactions conducted on 0.2 mmol scale under N2.
Determined by 1H NMR versus an internal standard.
Determined by SFC using a chiral stationary phase.
1.5 equiv TDAE.
Isolated yield.
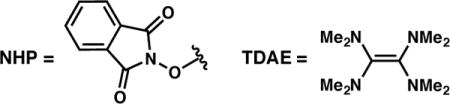
Monitoring the reaction progress at room temperature determined that (E)-1-(2-chlorovinyl)-4-methoxybenzene (5) was forming and accumulating under the reaction conditions, presumably through a Ni-catalyzed halide exchange process.16 Since vinyl chloride 5 does not readily engage in the cross-coupling reaction, we hypothesized that the yield of 3a could be improved by removing chloride from the reaction and thus preventing formation of this unproductive side product. Indeed, the use of TMSBr instead of TMSCl, and the use of L·NiBr2 (4b) as the catalyst, furnished product 3a in 75% yield and 92% ee (entry 5). By decreasing the reaction temperature to −7 °C, and using only 1.5 equivalents of TDAE, 3a could be obtained in 80% yield and 96% ee (entry 7).17,18 Running the reaction under the optimal conditions, but omitting TMSBr confirms that this additive is critical for obtaining high yields of 3a (entry 8).19,20 We note that these optimal reaction conditions employ a 1:1 stoichiometry of the two electrophiles and only 10 mol % catalyst loading.
To demonstrate the scope of the reaction, a series of (E)-vinyl bromides was cross-coupled with NHP ester 2, providing the corresponding products (3) in uniformly good yield and high ee (Figure 1). The reaction exhibits good tolerance of Lewis basic functional groups: for example, anilines (3c), nitriles (3e), and esters (3f, 3j) can be incorporated into the substrate without detriment to the yield or enantioselectivity. A pyridine-containing vinyl bromide also performs well, providing 3i in 67% yield and 95% ee. In addition, alkyl-substituted vinyl bromides react smoothly, providing the corresponding products in good yield and ee (3j–3o). A vinyl bromide possessing a free alcohol couples efficiently, although silylation occurs under the reaction conditions to give silyl ether 3m. The silyl ether can easily be cleaved with a mild acid workup; in this case it was preserved in order to facilitate purification. It is notable that alkenyl MIDA-boronate 3n and alkenyl silane 3o can be prepared in 97% ee from commercially available vinyl bromides. To demonstrate that this method can be used preparatively, the coupling between 1a and 2 was conducted on 5.0 mmol scale, which delivered 918 mg (77% yield) of 3a in 91% ee.
Figure 1. Scope of the vinyl bromide coupling partner.
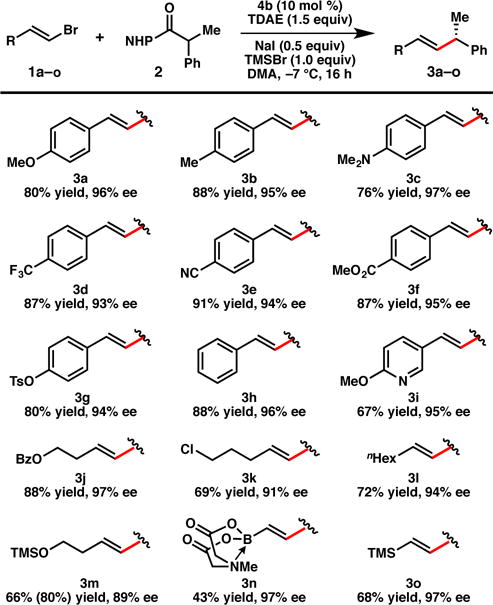
Reactions are conducted on 0.2 mmol scale under N2. Isolated yields are provided; ee is determined by SFC using a chiral stationary phase. For 3g, 1.5 equiv NHP ester was used. For 3m, 2.0 equiv TMSBr was used; the alcohol is silylated under the reaction conditions. NMR yield of 3m versus an internal standard is provided in parenthesis.
The reaction also exhibits broad scope for the NHP ester coupling partner, delivering good yields and high enantioselectivities for a range substrates bearing substitution on the arene or at the benzylic position (Figure 2). In certain cases (e.g. 7a–7c), the NHP esters cross-couple with improved yield relative to the corresponding benzyl chlorides (under the previously reported conditions).8b Moreover, aniline 7e can be prepared in 66% yield and 94% ee; this compound could not be accessed via our previously reported benzylic chloride coupling8b due challenges in preparing and handling 4-(chloro(phenyl)methyl)-N,N-dimethylaniline under standard conditions.21 Higher substitution at the benzylic position is also tolerated (7g–l), although the yield begins to decrease with larger groups (e.g. iPr, 7i). Notable products include 7j, which incorporates a siloxy group, 7k, containing an alkene, and 7l, which has an alkyl chloride. Perfect chemoselectivity for cross-coupling of the NHP ester over the primary alkyl chloride is observed.
Figure 2. Scope of the NHP ester coupling partner.
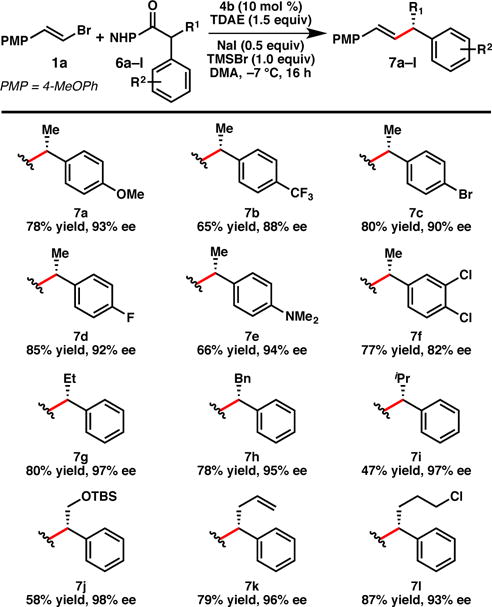
Reactions are conducted on 0.2 mmol scale under N2. Isolated yields are provided; ee is determined by SFC using a chiral stationary phase.
Although the primary focus of this study was the cross-coupling of NHP esters with alkyl substituents at the benzylic position, we have also have begun to investigate substrates containing heteroatom substitution (Scheme 2a). Reaction of α-methoxy ester 8 proceeds smoothly, furnishing allylic ether 9 in good yield and ee. This highlights an advantage of the NHP ester for certain C(sp3) electrophiles, as the corresponding α-chloroether substrate is unstable and difficult to work with.
Scheme 2.
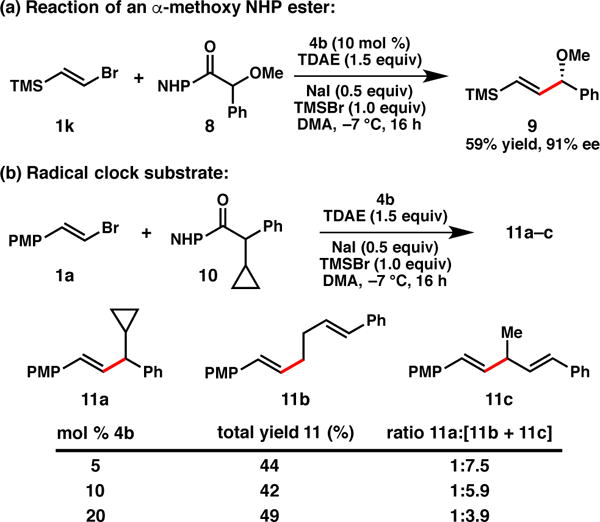
Additional experiments.
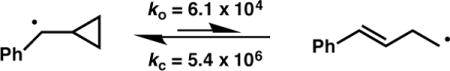
To probe for the intermediacy of a radical species, NHP ester 10 was prepared and subjected to the standard cross-coupling conditions (Scheme 2b, 10 mol % 4b). A 42% combined yield of the coupled products 11a–c was obtained. It has been shown that for phenyl substituted cyclopropyl carbonyl radicals, the ring opening is reversible and that the cyclopropane species is favored at lower temperatures.22 The fact that 11b and 11c predominate, even though they derive from the minor equilibrium species, perhaps indicates that the rate of radical recombination with nickel is sensitive to the steric profile of the radical. When the catalyst loading of 4b was varied, the ratio of 11a to total ring opened product (11b + 11c) was found to increase at higher nickel concentrations. This Ni-dependent behavior suggests that the mechanism proceeds through a cage-escaped radical, which at higher concentrations of 4b, can competitively recombine with nickel before undergoing ring scission.23 Further studies of the mechanism are ongoing; it is unclear at this time whether the absolute stereochemistry is set during the oxidative addition or reductive elimination steps.24 We do note, however, that the products are formed in similar ee when using either the NHP esters under the conditions reported here or the benzylic chloride using the conditions reported previously,8b which suggests that the reactions proceed through the same stereochemistry-determining step.25
In summary, these results demonstrate that Ni-catalyzed reductive cross-coupling reactions of NHP esters can be rendered highly enantioselective, thus broadening the scope of C(sp3) electrophiles available for asymmetric C–C bond formation. In contrast to the related reductive cross-couplings of benzyl chlorides,8b optimal results are obtained when TDAE is used as the terminal reductant. A preliminary result demonstrates that these conditions can be used to cross-couple α-alkoxy NHP esters and other substrates for which the corresponding benzylic chloride could be difficult to prepare or unstable. The ability to use both NHP esters (this study) and benzylic chlorides in asymmetric reductive alkenylation reactions allows users to select from either electrophile depending on factors such as commercial availability of the corresponding carboxylic acid or benzylic chloride starting material, and improves the overall scope of this transformation. The further development of asymmetric cross-electrophile coupling reactions of NHP esters and other C(sp3) electrophiles is the focus of ongoing work in our laboratory.
Supplementary Material
Acknowledgments
We thank the following Caltech staff for their help: Dr. Scott Virgil of the Caltech Center for Catalysis and Chemical Synthesis for access to experimental and analytical equipment; Dr. Michael K. Takase and Larry M. Henling for assistance in collecting X-ray diffraction data; and Dr. Mona Shahgholi and Naseem Torian for assistance with mass spectrometry measurements. We also thank Kevin Sokol and Dr. Alan Cherney (both of Caltech) for preparing NHP esters 6g and 6h, and vinyl bromides 1g, 1h, and 1l, respectively. Fellowship support was provided by the National Science Foundation (graduate research fellowship to J. L. H. and K. E. P., Grant No. DGE-1144469), and Shionogi & Co., Ltd. (postdoctoral fellowship to N. S.). S. E. R. is an American Cancer Society Research Scholar and Heritage Medical Research Institute Investigator. Financial support from the NIH (NIGMS RGM097582-01, R35GM118191) is gratefully acknowledged.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI:
Experimental procedures, characterization, and spectral data (PDF)
Crystallographic data (CIF)
References
- 1.For recent reviews, see:; (a) Jana R, Pathak TP, Sigman MS. Chem Rev. 2011;111:1417. doi: 10.1021/cr100327p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hu X. Chem Sci. 2011;2:1867. [Google Scholar]; (c) Tasker SZ, Standley EA, Jamison TF. Nature. 2014;509:299. doi: 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ananikov VP. ACS Catal. 2015;5:1964. [Google Scholar]; (e) Netherton MR, Fu GC. Adv Synth Catal. 2004;346:1525. [Google Scholar]
- 2.Seminal reports of Ni-catalyzed Negishi cross-couplings with C(sp3) electrophiles:; (a) Park K, Yuan K, Scott WJ. J Org Chem. 1993;58:4866. [Google Scholar]; (b) Devasagayaraj A, Stüdemann T, Knochel P. Angew Chem Int Ed. 1996;34:2723. [Google Scholar]; (c) Giovannini R, Stüdemann T, Dussin G, Knochel P. Angew Chem Int Ed. 1998;37:2387. doi: 10.1002/(SICI)1521-3773(19980918)37:17<2387::AID-ANIE2387>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]; (d) Giovannini R, Knochel P. J Am Chem Soc. 1998;120:11186. [Google Scholar]; (e) Zhou J, Fu GC. J Am Chem Soc. 2003;125:14726. doi: 10.1021/ja0389366. [DOI] [PubMed] [Google Scholar]; (f) Terao J, Todo H, Watanabe H, Ikumi A, Kambe N. Angew Chem Int Ed. 2004;43:6180. doi: 10.1002/anie.200460246. [DOI] [PubMed] [Google Scholar]; (g) Fischer C, Fu GC. J Am Chem Soc. 2005;127:4594. doi: 10.1021/ja0506509. [DOI] [PubMed] [Google Scholar]; (h) Arp FO, Fu GC. J Am Chem Soc. 2005;127:10482. doi: 10.1021/ja053751f. [DOI] [PubMed] [Google Scholar]
- 3.Seminal reports of Ni-catalyzed Kumada cross-couplings with C(sp3) electrophiles:; (a) Tereo J, Watanabe H, Ikumi A, Kuniyasu H, Kambe N. J Am Chem Soc. 2002;124:4222. doi: 10.1021/ja025828v. [DOI] [PubMed] [Google Scholar]; (b) Terao J, Ikumi A, Kuniyasu H, Kambe N. J Am Chem Soc. 2003;125:5646. doi: 10.1021/ja034201p. [DOI] [PubMed] [Google Scholar]; (c) Vechorkin O, Hu X. Angew Chem Int Ed. 2009;48:2937. doi: 10.1002/anie.200806138. [DOI] [PubMed] [Google Scholar]; (d) Vechorkin O, Proust V, Hu X. J Am Chem Soc. 2009;131:9756. doi: 10.1021/ja9027378. [DOI] [PubMed] [Google Scholar]; (e) Lou S, Fu GC. J Am Chem Soc. 2010;132:1264. doi: 10.1021/ja909689t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seminal reports of Ni-catalyzed Suzuki cross-couplings with C(sp3) electrophiles:; (a) Zhou J, Fu GC. J Am Chem Soc. 2004;126:1340. doi: 10.1021/ja039889k. [DOI] [PubMed] [Google Scholar]; (b) González-Bobes F, Fu GC. J Am Chem Soc. 2006;128:5360. doi: 10.1021/ja0613761. [DOI] [PubMed] [Google Scholar]; (c) Saito B, Fu GC. J Am Chem Soc. 2007;129:9602. doi: 10.1021/ja074008l. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Saito B, Fu GC. J Am Chem Soc. 2008;130:6694. doi: 10.1021/ja8013677. [DOI] [PubMed] [Google Scholar]; (e) Lundin PM, Fu GC. J Am Chem Soc. 2010;132:11027. doi: 10.1021/ja105148g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seminal reports of Ni-catalyzed cross-electrophile couplings:; (a) Durandetti M, Gosmini C, Perichon J. Tetrahedron. 2007;63:1146. [Google Scholar]; (b) Everson DA, Shrestha R, Weix DJ. J Am Chem Soc. 2010;132:920. doi: 10.1021/ja9093956. [DOI] [PubMed] [Google Scholar]; (c) Yu X, Yang T, Wang S, Xu H, Gong H. Org Lett. 2011;13:2138. doi: 10.1021/ol200617f. [DOI] [PubMed] [Google Scholar]; For reviews see:; (d) Everson DA, Weix DJ. J Org Chem. 2014;79:4793. doi: 10.1021/jo500507s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Knappke CEI, Grupe S, Gartner D, Corpet M, Gosmini C, Jacobi von Wangelin A. Chem Eur J. 2014;20:6828. doi: 10.1002/chem.201402302. [DOI] [PubMed] [Google Scholar]; (f) Moragas T, Correa A, Martin R. Chem Eur J. 2014;20:8242. doi: 10.1002/chem.201402509. [DOI] [PubMed] [Google Scholar]; (g) Weix DJ. Acc Chem Res. 2015;48:1767. doi: 10.1021/acs.accounts.5b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Gu J, Wang X, Xue W, Gong H. Org Chem Front. 2015;2:1411. [Google Scholar]; (i) Wang X, Dai Y, Gong H. Top Curr Chem. 2016;374:43. doi: 10.1007/s41061-016-0042-2. [DOI] [PubMed] [Google Scholar]
- 6.Selected examples of dual photoredox nickel-catalysis:; (a) Tellis JC, Primer DN, Molander GA. Science. 2014;345:433. doi: 10.1126/science.1253647. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DWC. Science. 2014;345:437. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Noble A, McCarver SJ, MacMillan DWC. J Am Chem Soc. 2015;137:624. doi: 10.1021/ja511913h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Primer DN, Karakaya I, Tellis JC, Molander GA. J Am Chem Soc. 2015;137:2195. doi: 10.1021/ja512946e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Jouffroy M, Primer DN, Molander GA. J Am Chem Soc. 2016;138:475. doi: 10.1021/jacs.5b10963. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Joe CL, Doyle AG. Angew Chem Int Ed. 2016;55:4040. doi: 10.1002/anie.201511438. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Zuo Z, Cong H, Li W, Choi J, Fu GC, MacMillan DWC. J Am Chem Soc. 2016;138:1832. doi: 10.1021/jacs.5b13211. [DOI] [PMC free article] [PubMed] [Google Scholar]; For a review, see:; (h) Cavalcanti LN, Molander GA. Top Curr Chem. 2016;374:39. doi: 10.1007/s41061-016-0037-z. [DOI] [PubMed] [Google Scholar]
- 7.(a) Swift EC, Jarvo ER. Tetrahedron. 2013;69:5799. doi: 10.1016/j.tet.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cherney AH, Kadunce NT, Reisman SE. Chem Rev. 2015;115:9587. doi: 10.1021/acs.chemrev.5b00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Cherney AH, Kadunce NT, Reisman SE. J Am Chem Soc. 2013;153:7442. doi: 10.1021/ja402922w. [DOI] [PubMed] [Google Scholar]; (b) Cherney AH, Reisman SE. J Am Chem Soc. 2014;136:14365. doi: 10.1021/ja508067c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadunce NT, Reisman SE. J Am Chem Soc. 2015;137:10480. doi: 10.1021/jacs.5b06466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Okada K, Okamoto K, Oda M. J Am Chem Soc. 1988;110:8736. [Google Scholar]; (b) Okada K, Okamoto K, Morita N, Okubo K, Oda M. J Am Chem Soc. 1991;113:9401. [Google Scholar]
- 11.(a) Cornella J, Edwards JT, Qin T, Kawamura S, Wang J, Pan CM, Gianatassio R, Schmidt M, Eastgate MD, Baran PS. J Am Chem Soc. 2016;138:2174. doi: 10.1021/jacs.6b00250. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Qin T, Cornella J, Li C, Malins LR, Edwards JT, Kawamura S, Maxwell BD, Eastgate MD, Baran PS. Science. 2016;352:801. doi: 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wang J, Qin T, Chen TG, Wimmer L, Edwards JT, Cornella J, Vokits B, Shaw SA, Baran PS. Angew Chem, Int Ed. 2016;55:9676. doi: 10.1002/anie.201605463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huihui KMM, Caputo JA, Melchor Z, Olivares AM, Spiewak AM, Johnson KA, DiBenedetto TA, Kim S, Ackerman LKG, Weix DJ. J Am Chem Soc. 2016;138:5016. doi: 10.1021/jacs.6b01533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The hydroalkylation of alkenes using NHP esters was also recently reported:; Liu X, Xiao B, Liu L, Fu Y. Chem Eur J. 2016;22:11161. doi: 10.1002/chem.201602486. [DOI] [PubMed] [Google Scholar]
- 14.Review on recent Ni-catalyzed cross-couplings with NHP esters:; Konev MO, Jarvo ER. Angew Chem Int Ed. 2016;55:11340. doi: 10.1002/anie.201605593. [DOI] [PubMed] [Google Scholar]
- 15.Weix and coworkers recently reported the use of TDAE as an organic reductant for the reductive cross-coupling of benzyl chlorides and aryl iodides:; Anka-Lufford LL, Huihui KMM, Gower NJ, Ackerman LKG, Weix DJ. Chem Eur J. 2016;22:11564. doi: 10.1002/chem.201602668. [DOI] [PubMed] [Google Scholar]
- 16.(a) Takagi K, Hayama N, Inokawa S. Chem Lett. 1978:1435. [Google Scholar]; (b) Tsou TT, Kochi JK. J Org Chem. 1980;45:1930. [Google Scholar]
- 17.The reaction mixture freezes at temperatures lower than −8 °C. See Supporting Information for details on reaction setup.
- 18.In the coupling of (1-chloroethyl)-benzene, TDAE provides low yield of the cross-coupled product (23% yield and 94% ee).
- 19.See Supporting Information for additional control experiments.
- 20.The role of TMSBr under the optimal conditions, which do not employ a metal reductant, is unclear. TMSCl has been proposed to activate Mn or Zn:; (a) Everson DA, Jones BA, Weix DJ. J Am Chem Soc. 2012;134:6146. doi: 10.1021/ja301769r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Johnson KA, Biswas S, Weix DJ. Chem Eur J. 2016;22:7399. doi: 10.1002/chem.201601320. Also see ref 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Example conditions: (a) -(4-(dimethylamino)phenyl)ethan-1-ol (1 equiv), SOCl2 (1.2 equiv), CH2Cl2, 0 °C, 15 min. (b) 1-(4-(dimethylamino)phenyl)ethan-1-ol (1 equiv), CCl4 (1.5 equiv), PPh3 (1.4 equiv), CH2Cl2, rt, 12 h.
-
22.(a) Beckwith ALJ, Bowry VW. J Am Chem Soc. 1994;116:2710. [Google Scholar]; (b) Halgren TA, Roberts JD, Horner JH, Martinez FN, Tronche C, Newcomb M. J Am Chem Soc. 2000;122:2988. [Google Scholar]; The rate constants for ring opening (ko) and ring closing (kc) at 20 °C are given below.
- 23.For a related study, see:; Biswas S, Weix DJ. J Am Chem Soc. 2013;135:16192–16197. doi: 10.1021/ja407589e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molander and Kozlowski have calculated that the reductive elimination is the stereochemistry determining step in a related Ni-catalyzed cross-coupling reaction of aryl halides with benzyl trifluoroborates.
- 25.See Supporting Information for ee comparison data.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


