Abstract
Cell based treatments for myocardial infarction have demonstrated efficacy in the laboratory and in phase I clinical trials, but the understanding of such therapies remains incomplete. Mesenchymal stem cells (MSCs) are classically defined as maintaining the ability to generate mesenchyme-derived cell types, namely adipocytes, chondrocytes and osteocytes. Recent evidence suggests these cells may in fact harbor much greater potency than originally realized, as several groups have found that MSCs can form cardiac lineage cells in vitro. Additionally, experimental coculture of MSCs with cardiomyocytes appears to improve contractile function of the latter. Bolstered by such findings, several clinical trials have begun to test MSC transplantation for improving post-infarct cardiac function in human patients. The results of these trials have been mixed, underscoring the need to develop a deeper understanding of the underlying stem cell biology. To help synthesize the breadth of studies on the topic, this paper discusses current challenges in the field of MSC cellular therapies for cardiac repair, including methods of cell delivery and the identification of molecular markers that accurately specify the therapeutically relevant mesenchymal cell types. The various possible mechanisms of MSC mediated cardiac improvement, including somatic reprogramming, transdifferentiation, paracrine signaling, and direct electrophysiological coupling are also reviewed. Finally, we consider the traditional cell culture microenvironment, and the promise of cardiac tissue engineering to provide biomimetic in vitro model systems to more faithfully investigate MSC biology, helping to safely and effectively translate exciting discoveries in the laboratory to meaningful therapies in the clinic.
Keywords: mesenchymal stem cell, marrow stromal cell, cardiac repair, cellular therapy, mechanisms, molecular markers, reprogramming, differentiation, paracrine signaling, tissue engineering
Introduction
Since mesenchymal stem cells (MSCs) were first definitively isolated in 1999 [1] they have been a source of excitement for their clinical potential. Not only are they present in adults – so that a patient’s own MSCs could be used therapeutically – but they also maintain the potential to become cells of mesenchymal origin, including osteogenic, adipogenic, and chondrogenic lineages [2, 3]. In addition to regenerating bone, fat and cartilage, recent transplant studies have reported that MSCs can also localize to the site of cardiac injury or ischemia [4, 5] and improve cardiac function [6–9], suggesting that MSCs retain an extensive plasticity governed by their environment. In vitro work (Table 1) has shown that coculture of human MSCs and rat neonatal cardiomyocytes (CMs) leads to MSC expression of two markers of cardiac lineage, troponin T and GATA4, although no sarcomeric organization has been observed [10]. Not only does this finding suggest that the cardiac microenvironment enhances the maturation of MSC-derived cardiomyocytes [10] but the formation of a cardiac progenitor-like cell from an MSC suggests that MSC transplantation may be a viable clinical treatment for repopulating damaged myocardium. However, given that early studies used bone marrow derived mononuclear cells (BMMNCs) that contained a mixed cell population, the ability of MSCs to improve cardiac function in vivo (Table 2) remains controversial since it is uncertain whether the beneficial effect of these earlier studies was actually due to the MSCs within the unpurified population or possibly due to another cell type. In this review we will focus primarily on MSCs, but will address relevant studies using whole BMMNCs when the results of such experiments provide possible insight into MSC biology. Despite our limited understanding of MSC-CM interactions, clinical trials utilizing MSCs in the treatment of heart failure have begun, 4 as recently reviewed in Ranganath et al. [11]. Initial results have been mixed (Table 3), with some groups finding a small but significant benefit with MSCs [4, 6, 12–13], and others finding no effect [14] or an effect that only lasts a few months with BMMNCs [15–18]. While it is possible the lack of a sustained benefit may reflect poor cell retention at the graft site, work in non-human animal models suggests that MSCs are stably engrafted six months after injection for small animals [19] and at least three months for large animals [8]. This implies the benefit of MSCs may depend on a transient paracrine signaling mechanism rather than the MSCs themselves.[19]. Despite differences in cell preparation and method of delivery to the patient, a recent meta-analysis of currently running clinical trials identified a small but significant benefit of autologous bone marrow cell transplant for the treatment of myocardial infarcts (MIs) [20].
Table 1.
Immunophenotyping and major outcomes of representative in vitro studies of mesenchymal stem cells for cardiac enhancement, organized chronologically. Bolded entries follow the ISCT standard definition of a MSC.
| Source | Markers | Cell Phenotype | Principal Observation | References |
|---|---|---|---|---|
| 2D In Vitro | ||||
| Human bone marrow | CD105+, CD73+, CD29+, CD44+, CD71+, CD90+, CD106+, CD120a+, CD124+; CD14−, CD34−, CD45− | MSC | Multi-lineage potential of isolated MSC population | [1] |
| Mouse and rat bone marrow | SSEA-1+, Sca-1+, CD90+, CD13+, Flk-1+; Gr-1−, CD11b/CD18−, CD19−, CD3−, CD117−, CD34−, MHC I & II−, CD44−, CD45− | MSC | A subset of MSCs are pluripotent beyond the mesenchymal lineage | [3] |
| Human bone marrow | Not specified | MSC | MSCs exhibit extensive transdifferentiation potential | [2] |
| Human bone marrow | CD105+, CD166+, CD29+, CD44+; CD14−, CD45−, CD34− | MSC | MSC coculture with neonatal rat ventricular myocytes decreased the conduction velocity and increased reentrant phenomena in the culture | [49] |
| Human bone marrow |
MSC: CD105+, CD73+, CD49c+, CD90+; CD45−, CD34−, CD184−, CD106− HSC: CD133+, CD34+ |
MSC & HSC | MSCs and HSCs display limited cardiomyogenic plasticity | [10] |
| Human bone marrow and adipose tissue |
Bone marrow: Not specified Adipose tissue: CD44+, CD49b+, CD105+, CD90+, CD13+; Stro-1−, CD34−, CD15−, CD117−, Flk-1−, Gly-A−, CD133−, HLA-DR−, HLA-Ilow |
MSC | Partial cell fusion & mitochondrial transfer | [22] |
| Human Wharton’s Jelly | CD105+, CD73+, CD44+; CD34− | MSC | Transdifferentiation of MSCs to cardiac phenotype when treated with cardiomyocyte conditioned media | [25] |
| Rat bone marrow | CD29+, CD44+, CD90+, CD105+; CD34−, CD45− | MSC | A subpopulation of MSCs express cardiac progenitor markers | [34] |
| Mouse bone marrow | CD90+, CD73+; CD45−, CD34− | MSC | MSCs enhance CM growth through notch/jagged signaling | [38] |
| hESC, fetal amniotic membrane, umbilical cord, adult human bone marrow, adipose tissue | CD105+, CD90+, CD73+; CD45−, CD34−, CD31−, CD24−, SSEA-4− | MSC | MSCs of embryonic or fetal but not adult origin can differentiate into the three cardiac lineages. Cx-43 is important for cardiogenic differentiation of embryonic and fetal cells | [51] |
| Human and mouse bone marrow | Human: CD10+, CD29+, CD44+, CD71+, CD106+, CD243+, CD309+, GD2+, CD73+, CD90+, CD105+; CD14−, CD34−, CD45− Mouse: Sca1+, rest not specified |
MSC | MSCs fail to differentiate into fully functional CMs despite expression of early CM markers | [37] |
| 3D In Vitro | ||||
| Rat bone marrow | Sca-1+, CD44+; CD45−, CD90− | MSC | WT MSCs improve cardiac function after ischemia/reperfusion in a Langendorff setup. TLR2 dependent. | [40] |
|
| ||||
| Rat bone marrow | CD90+, CD45− | MSC | MSC co-culture enhanced contractile force of CMs in engineered cardiac tissue | [48] |
MSC: Mesenchymal stem cells; HSC: Hematopoietic stem cell; CM: Cardiomyocyte; LV: Left-ventricle; Cx-43: connexin-43; WT: wild-type
Table 2.
Immunophenotyping and major outcomes of representative in vivo studies of mesenchymal stem cells for cardiac repair, organized chronologically. Bolded entries follow the ISCT standard definition of a MSC.
| Source | Markers | Mode of Administration | Cell Phenotype | Principal Observation | References |
|---|---|---|---|---|---|
| Injected Cells | |||||
| Mouse bone marrow | Not specified | Mobilization of endogenous bone marrow following irradiation and transplant of clonally isolated MSC cell line | MSC | Restore cardiac function post-MI in mouse. Found evidence that majority of bone marrow derived CMs are from MSCs | [30] |
| Rat bone marrow | Not specified* | Intramyocardial | MSC | Initial improvement in LV function to 4 wks, 6 mos no longer different from control post-MI in rat | [19] |
| Porcine bone marrow | Not specified* | Intramyocardial | MSC | Improved cardiac function post-MI in pig | [8] |
| Rat bone marrow | CD45−, CD34− | Intravenous | MSC | MSCs decreased the arrhythmogenicity of infarcted rat hearts as determined via optical mapping of the explanted heart | [47] |
| Porcine bone marrow | Not specified, selected for plastic adherence | Transendocardial | MSC | Restore cardiac function post-MI via MSC differentiation in pig | [7] |
| Human adipose tissue | CD105+, CD44+, CD166+, CD29+, CD90+; CD106−, CD45−, CD14− | Intramyocardial | MSC | Up-regulation of cardiac markers in MSCs, reduce infarct size and increase LVEF & FS post-MI in rat | [24] |
| Rat amniotic membrane | CD10+, CD29+, CD44+, CD105+, SSEA-4+; CD14−, CD34−, CD45−, CD117−, CD309−, HLA-ABC−, HLA-D−, HLA−DR− | Intramyocardial | MSC | MSCs engraft and transdifferentiate post-MI in rat | [28] |
| Porcine bone marrow | Gata-4−, CD309−, CD68−, MDR-1−, CD117 | Transendocardial | MSC | MSCs differentiated into CMs and also activated an endogenous cardiac progenitor pool post-MI in pig | [35] |
| Human adipose tissue | CD29+, CD44+, CD73+, CD105+; CD45− | Intramyocardial | MSC | Less impact on CM survivability compared to BM-derived MSCs post-MI in mouse | [23] |
| Human bone marrow | CD29+, CD44+, CD73+, CD105+; CD45− | Intramyocardial | MSC | Increases CM survival post-MI in mouse | [23] |
| Human umbilical cord blood | CD29+, CD44+, CD73+, CD105lo; CD45− | Intramyocardial | MSC | MSCs were lower in CD105 expression and less effective in cardiac repair than bone marrow or adipose-derived MSCs. When sorted for >90% CD105 expression, however, therapeutic potential equaled that of bone marrow-derived MSCs in mouse | [23] |
| Human bone marrow | Not specified, commercial cell line | Intramyocardial | MSC | Cardiomyogenic transdifferentiation & angiogenesis post-MI in rat | [36] |
| Rat bone marrow | Not specified* | Intramyocardial | MSC | Improve LV function post-MI in rat. Activation of the JAK-STAT pathway improved cardiac recovery | [43] |
| Mouse bone marrow |
BMSCs: CD117+; lin− (CD5−, C45R−, CD11b−, Gr-1−, Ter-119−, 7-4−) MSCs: Sca1+, CD105+, CD166+, CD44+; Cd45−, CD117−, CD31− |
Intramyocardial | BMSC and MSC | Increased cardiomyocyte proliferation and activation of a progenitor pool with BMSCs, but not MSCs, post-MI in mouse | [44] |
| Rat bone marrow | Not specified | Intramyocardial and intravenous | MSC | Engrafted and improved cardiac function (Intramyocardial only) post-MI in rat | [5] |
| Human bone marrow | CD73+, CD90+, CD105+; CD14−, CD34−, CD45− | Intramyocardial | MSC | Transplanted MSCs fail to fully differentiate into CMs despite early expression of cardiac markers prior to transplantation in post-MI in mouse | [37] |
|
| |||||
| Rat bone marrow | CD45−, CD34− | Intramyocardial | MSC | Passive electrophysiological coupling of MSCs to CMs in cryoinjured rat myocardium | [50] |
| Engineered Grafts | |||||
| Rat adipose tissue | CD29+, CD90+; CD34−, CD45−, CD31−, αSMA−** | Epicardial graft over infarct | MSC | Cell sheets repair myocardium of infarcted rat hearts | [9] |
| Rat bone marrow | CD29+, CD44+, CD90+; CD11b−, CD34−, CD45− | Graft replacement of excised infarct | MSC | Porous tissue grafts decreased LV remodeling and improved cardiac function post-MI in rat | [59] |
|
| |||||
| hESC-derived | CD105+, CD73+, | Epicardial graft over infarct | MSC | Cell seeded collagen patches lead to neovascularization and decreased remodeling post-MI in rats | [60] |
MSC: Mesenchymal stem cells; BMSC: Bone marrow stem cell; MI: myocardial infarction; BM: Bone marrow; CM: Cardiomyocyte; LV: Left-ventricle; WT: wild-type; LVEF: Left-ventricular ejection fraction; FS: Fractional shortening; HSC: hematopoietic stem cell; TLR2: Toll-like receptor 2; CD117 = c-kit
While surface marker profiles are not directly given in these references, they cite characterization according to Pittenger et al (reference 1).
This was a mixed population with approximately 30% also positive for CD71, CD106, CD117
Table 3.
Immunophenotyping and major outcomes of representative published clinical trials of bone marrow cells and bone marrow derived mesenchymal stem cells for cardiac repair.
| Source | Markers | Mode of administration | Cell Phenotype | Principal Observation | Trial Initiation Date | Trial Name |
|---|---|---|---|---|---|---|
| Autologous bone marrow | Not specified | Intracoronary | BMMNCs | Transient increase in LVEF that is not sustained past 18 months | 2002 | BOOST [15–18] |
| Autologous bone marrow | Not specified | Intracoronary | BMMNCs | No effect on LVEF | 2003 | Autologous Stem Cell Transplantation in Acute Myocardial Infarction [14] |
| Autologous bone marrow | CD105+, CD73+, CD13+, CD90+; CD45−, CD34−, | Intramyocardial | MSC | Improvement in left ventricular function, exercise capacity and clinical symptoms in patients with coronary artery disease | 2005 | Stem Cell Therapy for Vasculogenesis in Patients with Severe Myocardial Ischemia [12] |
| Allogenic bone marrow | CD105+, CD166+; CD45− | Intravenous delivery | MSC | Decreased arrhythmias (Decreased VTs, PVCs) with MSCs, improved LVEF | 2005 | Safety Study of Adult Mesenchymal Stem Cells to Treat Acute Myocardial Infarction [4] |
| Autologous bone marrow | Not specified | Transendocardial, Intramyocardial delivery | BMMNCs or MSC | Improved contractility & recovery at 6 months | 2008 | TAF-HFT [6] |
| Allogenic bone marrow | Not specified* | Adventita of coronary artery | MSC | Improved LVEF and LVSV | 2008 | Safety Study of AMI MultiStem® to Treat Heart Attacks [13] |
BMMNC: Bone marrow mononuclear cell; MSC: Mesenchymal stem cell; LVEF: Left-ventricular ejection fraction; LVSV = Left-ventricular stroke volume; PVC = Premature ventricular contraction; AE = Adverse event
MultiStem derived from the subset of MSCs identified by Jiang et al (reference 3)
Why the success of MSCs cultured with cardiomyocytes in the laboratory, both in vitro and in animal models of MI, has not consistently translated to the clinical setting remains unclear. Disparities in cell preparation and delivery methods are likely to impact the effectiveness of treatment. Underlying these differences is an incomplete comprehension of MSC-CM interactions, limited by inadequate in vitro cell culture platforms. To effectively treat individuals with MSC-based therapies, a stronger mechanistic understanding of MSC biology must be achieved. Toward such an understanding, this review will discuss proper characterization of mesenchymal stem cells and alternative methods of therapeutic cell administration, it will assess evidence of the various mechanisms that MSCs may employ to improve cardiac function, and it will argue in favor of the need to develop biomimetic engineered cardiac tissue models to complement the traditional Petri dish and expand the biological relevance of what can be learned from in vitro cell culture studies.
Identification of MSCs
The first and most fundamental step in successful MSC therapy is proper identification and isolation of the desired mesenchymal stem cells. As proposed by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT), the minimal criteria for defining MSCs include that the cells must be plastic-adherent when maintained in standard culture conditions and they must be capable of differentiating into osteoblast, adipocyte and chondroblast lineages in vitro [21]. Beyond these functional criteria, further specification is complicated by the ignorance of a unique molecular marker of the MSC. Given the diversity of surface markers on cells consistent with this MSC phenotype, it is likely that the resulting cells comprise a heterogeneous population with subtle phenotypic distinctions and correspondingly subtle patterns of cell surface marker expression.
Tissue Sources
Since adult MSCs were first identified in human bone marrow [1], they have also been putatively obtained from adipose tissue [9, 22, 23, 24], umbilical cord endothelium [23, 25], peripheral blood [26], synovial membrane [27], amniotic membrane [28], and lung [29]. While the diversity of tissues from which MSCs can be isolated is impressive, it does emphasize the potential heterogeneity of the resulting cell populations. Indeed, as recently demonstrated by Gaebel and coworkers, when human MSCs were derived from adipose tissue, bone marrow, or umbilical cord blood, and each resulting cell population was used separately for treatment in a mouse model of myocardial infarction (MI), the resulting effects on cardiac structure and performance were strongly dependent on the MSC tissue of origin [23]. The variability of such a cell population underscores the need to selectively identify a known subset of MSCs that are capable of predictable and tissue-specific regeneration. Selection based on cell-surface markers is among the most reliable methods of identifying specific cell populations.
Cell Markers
There is currently no universally accepted repertoire of cell surface markers that distinguishes the MSC population. The lack of definitive identifiers has resulted in an amalgam of different markers reported in the literature to identify MSCs in vitro (Table 1), in vivo (Table 2), and in clinical trials (Table 3). In addition to the functional criteria described above, the ISCT recommends that MSCs must also express the cluster of differentiation (CD) molecules CD105, CD73 and CD90 [21]. Notably, MSCs must also lack markers of the hematopoietic lineage, in particular CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR surface molecules [21]. However, few in vivo studies to date, and no published clinical trials, have used MSCs based on the ISCT criteria. Many of the early clinical trials utilized relatively unpurified BMMNCs as their source of stem cells (Table 3). Considering the innate heterogeneity of unsorted bone marrow stem cells, this has probably contributed to inconsistent clinical outcomes. If, however, the bone marrow cells that home to the myocardium after infarction are first separated from harvested marrow via isolation and cell sorting, it is found that they are nonhematopoeitic in lineage and, thus, likely to be MSCs [30]. One of the most recent clinical trials utilizing such purified MSCs delivered intravenously to patients with myocardial infarction has reported generally positive trends with increased ejection fraction, reduced numbers of arrhythmias and improved pulmonary function, though follow-up has been limited to a relatively short time frame of 6 months [4]. This is a very important finding since it suggests that advancing the field of MSC-based cellular therapies is critically linked to identification and delivery of the specific subtype(s) of MSCs responsible for the functional recovery that is desired. Unfortunately, characterization is further complicated by the finding that MSCs exhibit species-specific marker expression and functional characteristics [31], warranting caution when data collected from animal models is extrapolated to humans.
Administration
In addition to the challenges presented by the lack of definitive markers of the MSC, another question that remains to be answered concerns the most efficacious method for the delivery of MSCs to infarcted myocardium. The route of cell administration can have a substantial impact on cell retention, viability, and homing [32]. Several strategies have been employed for MSC delivery including intramyocardial, intravenous, endocardial, adventitial and intracoronary injections (see Table 2 and Table 3). To date, direct open-chest intramyocardial (IM) injection is the most common mode of delivery in animal models, while catheter-based intracoronary or intravenous delivery is more typical in clinical trials. This disparity between animal and human studies may contribute to the differences observed between the lab and the clinic. Intravascular delivery, while attractive due to the ease of administration, may yield poorer outcomes than IM injection, related to inefficient cardiac homing and off-target cell distribution within the lung [5]. Although the mode of MSC administration clearly requires further optimization for cardiac applications, the benefits and disadvantages of each method of delivery will not be addressed herein, as the topic has been recently reviewed by Dib et al. [33].
Mechanisms of MSC-Enhanced Cardiac Function
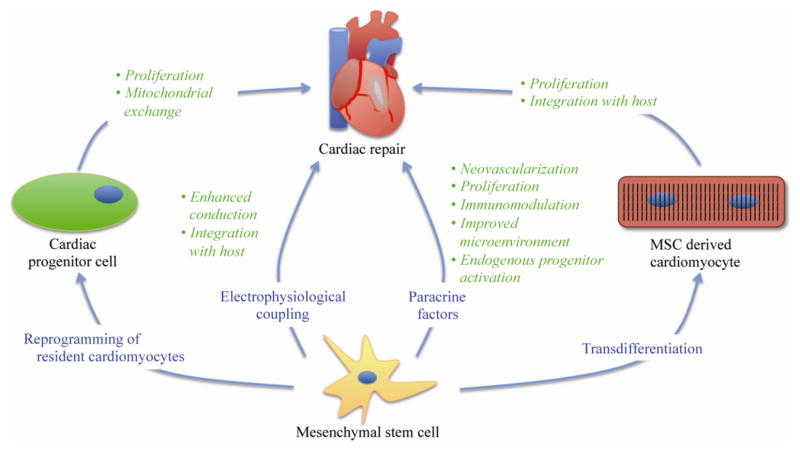
Four primary mechanisms have been proposed to explain the reported benefits of MSC treatment on cardiac function (Figure 1). One possibility is that the MSCs directly reprogram terminally differentiated CMs to a cardiac progenitor cell state that can repair the damaged myocardium. Another is that the MSCs transdifferentiate into cardiomyocytes and thus improve cardiac function via increased cell number. A third mechanism is that the MSCs release paracrine factors that improve function of the CMs or activate a dormant resident cardiac progenitor cell population and limit cardiac remodeling following an infarct. A fourth possibility involves direct electrophysiological coupling of MSCs with cardiomyocytes via gap junctions; this is the least well-studied mechanism of MSC-CM interaction and is only briefly addressed below.
Figure 1.
Schematic illustrating four primary mechanisms for mesenchymal stem cell (MSC)-based repair of injured myocardium. These include reprogramming of differentiated cardiomyocytes into cardiac progenitor cells, transdifferentiation of MSCs into cardiomyocytes, MSC secretion of paracrine factors, and direct electrophysiological coupling of MSCs with host myocardium.
Reprogramming
One possible mechanism by which MSCs could support cardiac regeneration after an insult would be to stimulate a latent regenerative capability of the heart. This might occur by reprogramming fully differentiated adult cardiomyocytes to a cardiac progenitor state, also known as de-differentiation.
Recently, Acquistapace and colleagues found that coculture experiments of human MSCs (of both bone-marrow and adipose origin) with adult mouse cardiomyocytes lead to a reprogramming of the CMs to a progenitor-like state [22]. This finding was supported by an increase in the cardiac progenitor markers GATA-4, Nkx-2.5, and Mef-2C, as well as increased expression of the proliferation marker Ki-67. Concomitantly, the group found with qPCR that as the progenitor markers were increased, expression of the adult cardiac markers desmin, α-sarcomeric actinin, and cardiac Tn-I all decreased, consistent with the occurrence of cardiomyocyte de-differentiation.
Furthermore, this reprogramming was reported to be independent of transdifferentiation, but rather was dependent on a transient cell fusion process that used tunneling membrane nanotubes between the two cell types to exchange cellular material. Cellular exchange was explored with species-specific markers of genomic DNA and a Cre-Lox fate mapping system that was only activated with exchange of cellular proteins between the MSCs and the CMs [22]. No evidence of human DNA was ever found in the reprogrammed mouse cardiomyocytes, suggesting that permanent cell fusion did not occur [22]. Inhibition of tubulin polymerization prevented the reprogramming event from occurring, supporting the hypothesis that active trafficking of cellular material through this transient nanotube structure is responsible for the observed changes in phenotype. In addition to the transfer of cellular proteins, Acquistapace found evidence that mitochondrial exchange may play a key role in reprogramming and/or restoring cardiac function. The mechanism by which mitochondrial transfer may lead to somatic reprogramming is not yet understood.
While Acquistapace’s findings of somatic reprogramming and increased proliferation are fascinating, the group also found some evidence that the MSCs, particularly when nanotube formation was inhibited, could partially transdifferentiate to a cardiac progenitor state via increased expression of GATA-4. Importantly, when transdifferentiation did occur, the reprogramming was incomplete so that the cells were neither functional cardiomyocytes nor proliferative progenitors but rather a nonfunctioning hybrid. Similar observations were made by Wei and colleagues that MSCs that improve cardiac function appear to express limited cardiac progenitor markers themselves [34]. This observation does complicate our understanding, however, since if increased cell number is observed after MSC treatment it is important to identify these cells as either of the mesenchymal or cardiac lineage. In fact, Hatzistergos et al. found evidence that MSCs are capable of both transdifferentiation to form new myocytes directly, and also stimulation of endogenous cardiac progenitors to form new myocytes [35]. Therefore, the ability of MSCs to transdifferentiate in vivo suggests that it may be possible to introduce an exogenous source of stem cells that can differentiate into cardiomyocytes and stimulate a resident progenitor pool even if the surrounding differentiated cells cannot be reprogrammed.
Transdifferentiation
Transdifferentiation occurs when a stem cell differentiates into a cell type beyond its committed lineage. MSCs exhibit extensive ability to transdifferentiate into many different lineages [2, 3] including cardiac lineage cells in culture [30] and in vivo [7, 28, 35]. Since transdifferentiation could lead to a greater number of CMs following cell transplantation, it is one mechanism by which MSCs could improve cardiac function. Several groups have found that MSCs retain the capacity to form cardiomyocytes in the infarct zone in animal models of MI [7, 24, 36]. The molecular mechanisms controlling transdifferentiation of MSCs are not well understood, but one recent study found that pre-incubating MSCs with an angiotensin receptor blocker (ARB) significantly increased the ability of MSCs to transdifferentiate into CMs and lead to a greater improvement in cardiac function in MI models than untreated MSCs [36]. Using ARBs of different specificities, the group found that blockade of the angiotensin 1 receptor (AT1R) and stimulation of the angiotensin 2 receptor (AT2R) improves MSC transdifferentiation potential. This finding implicates angiotensin signaling as having an important role in affecting MSC cardiac transdifferentiation.
One difficulty in studying the capacity of MSCs to transdifferentiate, however, is the possibility for MSCs to fuse with CMs rather than directly transdifferentiating. Tsuji’s study, which used MSCs isolated from human amniotic membrane, has produced the most compelling evidence against permanent fusion by tagging the mitochondria of cells transplanted into GFP expressing host [28]. Following implantation, Tsuji and coworkers found that no cardiomyocytes carrying the tagged mitochondria also expressed GFP. This suggests that the cardiomyocytes that formed from the MSCs were formed via transdifferentiation and not cell fusion. Taken together with the aforementioned fate-mapping experiment by Acquistapace and coworkers [22], evidence indicates that the possible mechanism of MSC-CM cell fusion is unlikely to occur.
While the capacity of MSCs to form the cardiac lineage has been well documented, Koninckx and colleagues argue that the markers of cardiac differentiation in bone marrow transdifferentiated cells fail to show a mature phenotype, suggesting that terminal differentiation is incomplete [10]. A similar observation was made by Acquistapace and colleagues with MSCs when nanotube formation was inhibited, suggesting that transdifferentiation in particular, and not reprogramming to a progenitor state, is incomplete [22]. Thus, Koninckx concludes that while transdifferentiation might occur, paracrine effects probably play a more important role. The basis for this conclusion is that immature CMs produced from MSC transdifferentiation would likely not have an appreciable impact on cardiac function, while cytokines released from the stem cells may have a beneficial effect on the mature cardiomyocytes already present in the tissue. Recent findings that bone marrow derived MSCs expressed cardiogenic mRNA and protein but rarely differentiated into fully functional myocytes, even in the presence of cardiogenic media or direct cell-cell contact with cardiomyocytes, is further evidence that transdifferentiation may not be a predominant mechanism of MSC-mediated cardiac repair
Paracrine Factors
The importance of paracrine factors in MSC-mediated cardiac repair may be inferred from the outcomes of related clinical trials. For instance, while the BOOST trial did find a beneficial increase in left ventricular systolic and diastolic function following autologous BMMNC transplantation, the effect was only evident for six months [15] – at eighteen month and five year follow-ups the beneficial effect was no longer significant [16–18]. The factors responsible for this transient effect are not known, but it is possible MSCs may have undergone apoptosis over the course of the study, reducing their efficacy either directly or through the loss of associated paracrine signaling. A similar effect was seen in rat studies where the control group displayed a catch-up phenomenon and no significant effect of MSC transplantation was seen after 6 months – despite some MSCs still being detectable in the graft at this time [19]. Since large animal studies demonstrate that MSCs can engraft in the heart [8], a transient benefit may also reflect the release of paracrine factors from undifferentiated stem cells that decrease in concentration as the stem cells differentiate or become quiescent. Thus, if this is the case, an understanding of the impact of the microenvironment on preservation of MSC stemness will be critical to translating MSC-based therapies to the clinic for sustained functional enhancement.
Molecular studies of MSC-CM interaction have identified several key pathways that may be responsible for the presumptive paracrine effect. Sassoli and colleagues found that neonatal cardiomyocytes exhibited increased proliferation when cultured with MSCs, and they also up regulated Notch-1 [38], an important receptor in the activation of cardiac progenitor cells [39]. Interestingly, levels of Notch-1 on the cardiomyocytes increased during initial dedifferentiation from 0–24 hours and then sharply decreased, suggesting the cardiomyocytes rapidly return to a more mature phenotype. The level of intracellular domain Notch-1 (Notch-ICD) in the cytoplasm of cardiomyocytes was also significantly increased, particularly in CMs closely associated with MSCs, which coincided with increased levels of Jagged on the MSCs, implicating Jagged-Notch-1 interaction between MSCs and CMs as being responsible for the increase in Notch signaling. This is an important finding since it suggests that either reactivation of a latent cardiac progenitor population, or reprogramming of adult cells to an immature state may be a product of Notch signaling. When Jagged-1 expression was silenced in the MSCs, the expression of Notch-ICD in the CMs was also significantly decreased, supporting the concept that Notch signaling is activated between MSCs and CMs. However, the silencing experiments revealed that levels of Notch-ICD did not return to baseline, suggesting other signaling pathways may also impact the MSC-CM interaction. This interpretation was further supported by the observation that MSC conditioned media contains many cardiotropic cytokines and growth factors such as VEGF and FGF [38].
Several groups have noted that VEGF and neovascularization may be important factors contributing to improved cardiac function with MSC treatment. Meldrum’s group found that in a murine isolated, perfused heart model of ischemia-reperfusion injury, infusion of wild-type MSCs significantly improved cardiac function, whereas when toll-like receptor 2 (TLR2) knockout MSCs were used, levels of VEGF were substantially decreased and cardiac function did not improve [40]. Importantly, the improvements in left ventricular function with wild-type MSCs were observed within approximately one hour of cell administration, suggesting that VEGF may play an additional role in improving short-term cardiac function independent of long-term effects such as neovascularization. Furthermore, the observation that TLR knock-out MSCs do not improve cardiac function suggests that, in addition to Notch signaling, TLR2 signaling may also be important for improving cardiac function. The authors note that while a direct pathway between TLR2 and VEGF expression in MSCs is not known, a link has been found in the cartilage of gram-positive septic joints, where inhibition of TLR2 significantly decreased levels of VEGF [41]. These findings suggest TLR2 may play a critical role in increasing vascularization in the setting of ischemia. Additionally, TLR2 is classically associated with the innate immune response, so the finding that it plays a role in ischemia and cardiac repair is both intriguing and unexpected, and certainly warrants further investigation, particularly in light of the ability of MSCs to significantly modulate the activity of the immune system as reviewed by Williams and Hare [42].
Interestingly, activation of the JAK-STAT pathway increases not only MSC viability but also capillary density in infarcted hearts [43]. However, the downstream effectors of the JAK-STAT pathway responsible for this signaling have not been investigated. Together, the current data suggest that VEGF may play a critical role in improving cardiac function via MSC directed neovascularization of the infarct. Associated with neovascularization may be an increase in cardiomyocyte proliferation via Notch signaling and an as of yet unidentified role of TLR2 in promoting VEGF release in MSCs.
The role of paracrine factors in cell-mediated cardiac repair is also supported by recent findings utilizing c-kit+ lin− bone marrow stem cells (BMSCs). Studies by Loffredo and colleagues in the mouse found that BMSCs, but not mesenchymal stem cells, activate a latent regenerative cardiac progenitor pool in vivo via a paracrine mechanism [44]. However, studies identifying and isolating this cell pool, and explaining how the BMSCs mediate this response, were not completed. In contrast to Loffredo and colleagues’ conclusions, Hatzistergos et al. also observed the activation of an endogenous stem cell pool within the myocardium [35], but using an enriched porcine MSC population. In addition to possible species-specific differences, the contradicting outcomes are likely related to the fact that each group utilized different criteria to identify their MSC population (see Table 2), which underscores the need to establish a definitive repertoire of mesenchymal stem cell markers. It is now clear that MSCs can secrete a variety of known paracrine factors (as recently reviewed by Williams and Hare [42] and Ranganath et al. [11]). In the context of cardiac repair, however, VEGF appears to be the most beneficial of the known paracrine factors that have been tested [45].
Electrophysiological Coupling
Perhaps the least well-studied mechanism of MSC-CM interaction involves electrophysiological coupling between the two cell types. Formation of functional gap junctions between MSCs and cardiomyocytes has been documented in cultured cells [46]. While some studies claim this may improve conduction [47] and tissue excitability [48], others have cautioned about possible arrhythmogenic sodium channel inactivation in cardiomyocytes coupled to MSCs due to the mismatched resting membrane potential [49]. Optical mapping of engrafted MSCs in a rat cryoinjury model showed evidence of electrical coupling and action potential activity; however, this was apparently not MSC-generated but rather a passive effect of coupling to adjacent viable myocardium [50]. Although the gap junction protein connexin-43 appears to be important for the cardiogenic differentiation of human embryonic and fetal mesenchymal stem cells, the same was not true for adult MSCs [51]. Further studies will be necessary to clarify the electrophysiological basis of MSC-CM interactions.
In summary, the current data suggest multiple possible mechanisms by which MSCs may improve cardiac function: reprogramming of adult CMs to a progenitor like state [22] and Notch signaling [38] may both increase proliferation of resident CMs, or progenitors may be stimulated directly by the MSCs [35]. Similarly, the angiotensin receptor [36] may facilitate transdifferentiation of the transplanted MSCs to CMs. Finally, neovascularization of the infarct via increased levels of VEGF would not only lead to increased performance of the resident CMs – and also improve mitochondrial performance by accommodating metabolic demand – but would also permit the delivery of MSCs deeper into the infarct where they can then trigger CM production, transdifferentiate into CMs, form direct intercellular junctions with CMs, or further increase vascular depth. Therefore, it seems likely that one pathway will not be responsible for the observed increase in cardiac function post-MI, but rather it will require a complex interplay of multiple different pathways involving both developmental and immune processes (Figure 1).
Role of the Cell Culture Microenvironment
The three-dimensional structure of a tissue can have a substantial effect on the gene expression and signaling profile of a cell [52]; paracrine effects, therefore, may be influenced by the environment in which the MSC-CM interaction is examined. Substrate micromechanics is another factor shown to directly impact MSC differentiation [53] and cardiomyocyte function [54, 55]. Since tissue culture plastic poorly represents such aspects of the myocardial microenvironment, traditional cell culture studies may be intrinsically limited in their ability to inform certain aspects of MSC mechanobiology. In this context, the field of cardiac tissue engineering, which utilizes soft, anisotropic, three-dimensional, dynamic culture substrates [56], offers more biomimetic model systems to study MSC-CM interactions. Engineered cardiac tissues can be created using controlled types and proportions of cells, defined biochemical constituents can be added to study their effects, functional and structural properties can be measured, and media can be readily collected to study cytokines of interest.
For example, our lab recently reported three-dimensional engineered cardiac tissues in which MSC co-culture increased the force generation capacity of neonatal rat cardiomyocytes and effectively compensated for a 50% reduction in myocyte content [48], with a transient benefit that mimicked animal studies and clinical trials described above. Engineered cardiac tissues using human-derived cell sources [57, 58] offer additional species-specific advantages as in vitro preclinical models of human myocardium.
Cardiac tissue engineering has also been applied to the study of MSC-mediated cardiac repair. Increased capillary density was found with the addition of bioengineered tissue grafts containing MSCs implanted on infarcts in rats [59], again suggesting the important role that MSC-induced neovascularization may play in improving cardiac function post-MI. Similar results were seen with hES-derived MSCs in a collagen matrix applied to infarctions in rats [60]. As illustrated by these studies, cardiac tissue engineering promises to play an increasingly important role for developing and applying stem cell therapies for treating injured myocardium.
Conclusions
Our understanding of mesenchymal stem cell biology has advanced greatly over the last decade. The MSC population offers an exciting possibility for cellular based therapies due to their unique attributes of immune tolerance, multipotency and presence in the adult. While recent findings of MSC-mediated cardiac repair are very encouraging, our understanding of MSC biology remains incomplete, partly reflecting limitations of available in vitro culture systems. It is crucial that prior to applying MSC based therapies on a large scale in the clinic that we address many of the issues raised in this review including: the ideal cell source, proper cell markers, method of delivery, and mechanisms of MSC repair. Once we understand such fundamental aspects of MSC biology, then we can more confidently and safely apply these therapies to the clinic. If MSCs can deliver on the therapeutic promise that has been observed in the laboratory, the future of cell therapies for cardiac repair appears very bright indeed.
Acknowledgments
The authors acknowledge financial support by the Mount Sinai Medical Scientist Training Program Grant T32-GM007280 (TJC) and NIH/NHLBI R21-HL095980 (KDC).
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest
References
- 1.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. The FASEB Journal. 2004;18:980–982. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 4.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. Journal of the American College of Cardiology. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Yao Y, Sheng Z, Yang Y, Ma G. Dual-modal tracking of transplanted mesenchymal stem cells after myocardial infarction. International Journal of Nanomedicine. 2011;6:815–823. doi: 10.2147/IJN.S17611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circulation Research. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nature Medicine. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 10.Koninckx R, Hensen K, Daniëls A, et al. Human bone marrow stem cells co-cultured with neonatal rat cardiomyocytes display limited cardiomyogenic plasticity. Cytotherapy. 2009;11:778–792. doi: 10.3109/14653240902988818. [DOI] [PubMed] [Google Scholar]
- 11.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the Mesenchymal Stem Cell Secretome for the Treatment of Cardiovascular Disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friis T, Haack-Sørensen M, Mathiasen AB, et al. Mesenchymal stromal cell derived endothelial progenitor treatment in patients with refractory angina. Scandinavian Cardiovascular Journal. 2011;45:161–168. doi: 10.3109/14017431.2011.569571. [DOI] [PubMed] [Google Scholar]
- 13.Penn MS, Ellis S, Gandhi S, et al. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: phase I clinical study. Circulation Research. 2012;110:304–311. doi: 10.1161/CIRCRESAHA.111.253427. [DOI] [PubMed] [Google Scholar]
- 14.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. The New England Journal of Medicine. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 15.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer A, Zwadlo C, Fuchs M, et al. Long-term effects of intracoronary bone marrow cell transfer on diastolic function in patients after acute myocardial infarction: 5-year results from the randomized-controlled BOOST trial--an echocardiographic study. European Journal of Echocardiography. 2010;11:165–171. doi: 10.1093/ejechocard/jep191. [DOI] [PubMed] [Google Scholar]
- 17.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. European Heart Journal. 2009;30:2978–2984. doi: 10.1093/eurheartj/ehp374. [DOI] [PubMed] [Google Scholar]
- 18.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 19.Dai W, Hale SL, Martin BJ, et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 20.Wen Y, Meng L, Xie J, Ouyang J. Direct autologous bone marrow-derived stem cell transplantation for ischemic heart disease: a meta-analysis. Expert Opinion on Biological Therapy. 2011;11:559–567. doi: 10.1517/14712598.2011.560567. [DOI] [PubMed] [Google Scholar]
- 21.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 22.Acquistapace A, Bru T, Lesault P-F, et al. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells. 2011;29:812–824. doi: 10.1002/stem.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaebel R, Furlani D, Sorg H, et al. Cell origin of human mesenchymal stem cells determines a different healing performance in cardiac regeneration. PloS One. 2011;6:e15652. doi: 10.1371/journal.pone.0015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayes-Genis A, Soler-Botija C, Farré J, et al. Human progenitor cells derived from cardiac adipose tissue ameliorate myocardial infarction in rodents. Journal of Molecular and Cellular Cardiology. 2010;49:771–780. doi: 10.1016/j.yjmcc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Z, Chen Z, Zhao X, et al. Sphingosine-1-phosphate promotes the differentiation of human umbilical cord mesenchymal stem cells into cardiomyocytes under the designated culturing conditions. Journal of Biomedical Science. 2011;18:37. doi: 10.1186/1423-0127-18-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Research. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurth TB, Dell’accio F, Crouch V, Augello A, Sharpe PT, De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis and Rheumatism. 2011;63:1289–1300. doi: 10.1002/art.30234. [DOI] [PubMed] [Google Scholar]
- 28.Tsuji H, Miyoshi S, Ikegami Y, et al. Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circulation Research. 2010;106:1613–1623. doi: 10.1161/CIRCRESAHA.109.205260. [DOI] [PubMed] [Google Scholar]
- 29.Sabatini F, Petecchia L, Tavian M, Jodon de Villeroché V, Rossi GA, Brouty-Boyé D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Laboratory Investigation. 2005;85:962–971. doi: 10.1038/labinvest.3700300. [DOI] [PubMed] [Google Scholar]
- 30.Kawada H, Fujita J, Kinjo K, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 31.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 32.Bonios M, Terrovitis J, Chang CY, et al. Myocardial substrate and route of administration determine acute cardiac retention and lung bio-distribution of cardiosphere-derived cells. Journal of Nuclear Cardiology. 2011;18:443–450. doi: 10.1007/s12350-011-9369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dib N, Khawaja H, Varner S, McCarthy M, Campbell A. Cell therapy for cardiovascular disease: a comparison of methods of delivery. Journal of Cardiovascular Translational Research. 2011;4:177–181. doi: 10.1007/s12265-010-9253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei F, Wang T, Liu J, Du Y, Ma A. The subpopulation of mesenchymal stem cells that differentiate toward cardiomyocytes is cardiac progenitor cells. Experimental Cell Research. 2011;317:2661–2670. doi: 10.1016/j.yexcr.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circulation Research. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Numasawa Y, Kimura T, Miyoshi S, et al. Treatment of human mesenchymal stem cells with angiotensin receptor blocker improved efficiency of cardiomyogenic transdifferentiation and improved cardiac function via angiogenesis. Stem Cells. 2011;29:1405–1414. doi: 10.1002/stem.691. [DOI] [PubMed] [Google Scholar]
- 37.Siegel G, Krause P, Wöhrle S, et al. Bone marrow-derived human mesenchymal stem cells express cardiomyogenic proteins but do not exhibit functional cardiomyogenic differentiation potential. Stem Cells and Development 2012 doi: 10.1089/scd.2011.0626. -not available-, ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sassoli C, Pini A, Mazzanti B, et al. Mesenchymal stromal cells affect cardiomyocyte growth through juxtacrine Notch-1/Jagged-1 signaling and paracrine mechanisms: clues for cardiac regeneration. Journal of Molecular and Cellular Cardiology. 2011;51:399–408. doi: 10.1016/j.yjmcc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Boni A, Urbanek K, Nascimbene A, et al. Notch1 regulates the fate of cardiac progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15529–15534. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Abarbanell AM, Wang Y, Herrmann JL, et al. Toll-like receptor 2 mediates mesenchymal stem cell-associated myocardial recovery and VEGF production following acute ischemia-reperfusion injury. American Journal of Physiology: Heart and Circulatory Physiology. 2010;298:H1529–36. doi: 10.1152/ajpheart.01087.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varoga D, Paulsen F, Mentlein R, et al. TLR-2-mediated induction of vascular endothelial growth factor (VEGF) in cartilage in septic joint disease. Journal of Pathology. 2006;210:315–324. doi: 10.1002/path.2059. [DOI] [PubMed] [Google Scholar]
- 42.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circulation Research. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu H, Yang Y-J, Qian H-Y, Tang Y-D, Wang H, Zhang Q. Rosuvastatin Treatment Activates JAK-STAT Pathway and Increases Efficacy of Allogeneic Mesenchymal Stem Cell Transplantation in Infarcted Hearts. Circulation Journal. 2011;75:1476–1485. doi: 10.1253/circj.cj-10-1275. [DOI] [PubMed] [Google Scholar]
- 44.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zisa D, Shabbir A, Suzuki G, Lee T. Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair. Biochemical and Biophysical Research Communications. 2009;390:834–838. doi: 10.1016/j.bbrc.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valiunas V, Doronin S, Valiuniene L, et al. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. The Journal of Physiology. 2004;555:617–626. doi: 10.1113/jphysiol.2003.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mills WR, Mal N, Kiedrowski MJ, et al. Stem cell therapy enhances electrical viability in myocardial infarction. Journal of Molecular and Cellular Cardiology. 2007;42:304–314. doi: 10.1016/j.yjmcc.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Serrao GS, Turnbull IC, Ancukiewicz D, et al. Myocyte-Depleted Engineered Cardiac Tissue Support Therapeutic Potential of Mesenchymal Stem Cells. Tissue Engineering Part A. doi: 10.1089/ten.tea.2011.0278. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang MG, Tung L, Sekar RB, et al. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation. 2006;113:1832–1841. doi: 10.1161/CIRCULATIONAHA.105.593038. [DOI] [PubMed] [Google Scholar]
- 50.Costa AR, Panda NC, Yong S, et al. Optical mapping of cryoinjured rat myocardium grafted with mesenchymal stem cells. American Journal of Physiology: Heart and Circulatory Physiology. 2012;302:H270–7. doi: 10.1152/ajpheart.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramkisoensing AA, Pijnappels DA, Askar SFA, et al. Human embryonic and fetal mesenchymal stem cells differentiate toward three different cardiac lineages in contrast to their adult counterparts. PloS One. 2011;6:e24164. doi: 10.1371/journal.pone.0024164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hwang NS, Kim MS, Sampattavanich S, Baek JH, Zhang Z, Elisseeff J. Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem Cells. 2006;24:284–291. doi: 10.1634/stemcells.2005-0024. [DOI] [PubMed] [Google Scholar]
- 53.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 54.Engler AJ, Carag-Krieger C, Johnson CP, et al. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. Journal of Cell Science. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophysical Journal. 2008;95:3479–3487. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Domian IJ, Chiravuri M, van der Meer P, et al. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326:426–429. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tulloch NL, Muskheli V, Razumova MV, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circulation research. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schaaf S, Shibamiya A, Mewe M, et al. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PloS One. 2011;6:e26397. doi: 10.1371/journal.pone.0026397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen C-H, Wei H-J, Lin W-W, et al. Porous tissue grafts sandwiched with multilayered mesenchymal stromal cell sheets induce tissue regeneration for cardiac repair. Cardiovascular Research. 2008;80:88–95. doi: 10.1093/cvr/cvn149. [DOI] [PubMed] [Google Scholar]
- 60.Simpson DL, Boyd NL, Kaushal S, Stice SL, Dudley SC. Use of human embryonic stem cell derived-mesenchymal cells for cardiac repair. Biotechnology and Bioengineering. 2012;109:274–283. doi: 10.1002/bit.23301. [DOI] [PMC free article] [PubMed] [Google Scholar]