SUMMARY
The hypothalamus has been implicated in skeletal metabolism (Ducy et al., 2000; Sato et al., 2007; Yadav et al., 2009). Whether hunger-promoting neurons of the arcuate nucleus impact the bone is not known. We generated multiple lines of mice to affect AgRP neuronal circuit integrity. We found that mice with Ucp2 gene deletion, in which AgRP neuronal function was impaired, were osteopenic. This phenotype was rescued by cell-selective reactivation of Ucp2 in AgRP neurons. When the AgRP circuitry was impaired by early postnatal deletion of AgRP neurons or by cell autonomous deletion of Sirt1 (AgRP-Sirt1−/−), mice also developed reduced bone mass. No impact of leptin receptor deletion in AgRP neurons was found on bone homeostasis. Suppression of sympathetic tone in AgRP-Sirt1−/− mice reversed osteopenia in transgenic animals. Taken together, these observations establish a significant regulatory role for AgRP neurons in skeletal bone metabolism independent of leptin action.
Graphical abstract
INTRODUCTION
The skeleton provides physical support and protection of soft organ, houses the hematopoietic system and allows for locomotion in support of survival. In vertebrates, bone is the principal reservoir of calcium, which is essential for cellular metabolism in all tissues. Thus, it is not surprising that evidence has emerged linking skeletal metabolism and whole body metabolic needs.
The central nervous system, and more specifically, the hypothalamus has a major regulatory role in peripheral tissue functions in health and disease (Dietrich et al., 2012; Matarese et al., 2013; Ruan et al., 2014; Warne et al., 2013). A growing body of evidence indicates that the hypothalamus also affects bone homeostasis mediated, at least in part, by the autonomic nervous system and endocrine organs (Ohlsson et al., 2012; Sato et al., 2007; Yadav et al., 2009). Hypothalamic neurons expressing agouti-related peptide (AgRP) drive hunger and have also been implicated in controlling peripheral tissues (Joly-Amado et al., 2012; Matarese et al., 2013), but to date, have not been directly tied to the regulation of bone homeostasis. Using different lines of transgenic mice with altered AgRP neuronal function, the present study was undertaken to determine whether the AgRP circuit influences skeletal metabolism.
RESULTS
Ucp2 impacts bone mass
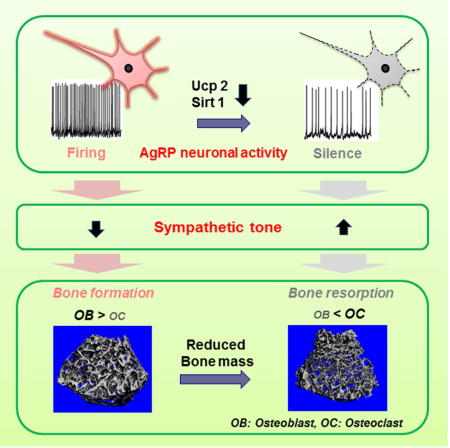
Uncoupling protein 2 (Ucp2) is expressed in AgRP neurons (Coppola et al., 2007; Horvath et al., 1999) and AgRP neuronal activity is impaired in mice with global deletion of the Ucp2 (Andrews et al., 2008). Structural analysis by microcomputed tomography (micro-CT) showed that three-month-old male Ucp2−/− mice exhibited significantly reduced trabecular bone volume (BV/TV, Fig. 1b) and trabecular thickness (Fig. 1c). To clarify the cellular basis for these changes, histomorphometric analysis of trabecular bone from Ucp2−/− mice was performed and demonstrated a reduction in osteoblast number with no change in osteoclast number (Fig. 1d) suggesting that Ucp2-regulated AgRP neuronal activity might affect bone remodeling processes by regulation of osteoblast formation and/or function. Since Ucp2 is widely expressed (Diano and Horvath, 2012), and hence the bone phenotype of UCP2−/− mice might be the consequence of multiple mechanisms, we next generated mice in which Ucp2 is selectively overexpressed in AgRP neurons (Agrp-Ucp2Tg mice) using cre-lox technology (Supplementary Fig. 1a). We confirmed successful cre-mediated recombination in AgRP neurons by detecting EGFP positive cells in the hypothalamic arcuate nucleus (ARC) (Supplementary Fig. 1b). Bone mineral density (BMD) was significantly increased in three-month-old male Agrp-Ucp2Tg mice when analyzed by DXA (Fig. 1e). To further test whether the osteopenia seen in global Ucp2-deficient mice could be due to altered AgRP neuronal activity, we crossed Ucp−/− mice with Agrp-Ucp2Tg mice to generate mice with specific reactivation of Ucp2 in AgRP neurons (Ucp2−/−:Agrp-Ucp2Tg mice). Micro-CT analysis revealed that the reduction in trabecular bone volume (Fig. 1g) and thickness (Fig. 1h) seen in global Ucp2-deficient mice was absent in three-month-old male mice with AgRP-specific expression of Ucp2. These observations indicate that AgRP neurons are involved in bone metabolism, and that the effect of Ucp2 on bone metabolism is mediated, at least in part by these hypothalamic neurons.
Figure 1. Altering AgRP neuronal activity by deletion or overexpression of Ucp2 affects bone mass.
(a) Representative micro-CT images of femoral trabecular bone from three-month-old male Ucp+/+ and Ucp−/− mice. Scale bar = 500 μm. Three-month-old male Ucp2−/− mice exhibited reduced (b) trabecular bone volume (BV/TV), and (c) trabecular thickness (b: n=10 for Ucp2+/+, n=12 for Ucp2−/−, p<0.001; c: n=10 for Ucp2+/+, n=12 for Ucp2−/−, p<0.001). (d) Histomorphometric analysis of trabecular bone from three-month-old male Ucp2−/− mice revealed a reduced bone mass with a reduction in trabecular thickness and osteoblast number (n=5 for Ucp2+/+, n=6 for Ucp2−/−). (e) DXA analysis demonstrated an increase in femoral BMD in three-month-old male Agrp-Ucp2Tg mice (n=6 for CT, n=9 for Agrp-Ucp2Tg, p<0.05). (f) Representative micro-CT images of femoral trabecular bone from three-month-old male Ucp2−/− and Ucp2−/−:AgRP-Ucp2 Tg mice. Scale bar = 500 μm. (g, h) Micro-CT analysis revealed that the reduction in trabecular BV/TV and trabecular thickness seen in three-month-old male Ucp2−/− mice is reversed by reactivating Ucp2 in AgRP-expressing neurons (Ucp2−/−: Agrp-Ucp2Tg). (g: n=3 for CT, n=3 for Ucp2−/−, n=4 for Ucp2−/−: Agrp-Ucp2Tg; p<0.05 for CT versus Ucp2−/−, p<0.05 for Ucp2−/− versus Ucp2−/−: Agrp-Ucp2Tg h: n=3 for CT, n=3 for Ucp2−/−, n=4 for Ucp2−/−: Agrp-Ucp2Tg; p<0.05 for CT versus Ucp2−/−, p=0.0523 for Ucp2−/− versus Ucp2−/−: Agrp-Ucp2Tg). * p<0.05 and *** p<0.001. Data are presented as means ± s.e.m. P values for unpaired comparisons were analyzed by Student’s t test.
Neonatal ablation of AgRP neurons results in reduced bone mass
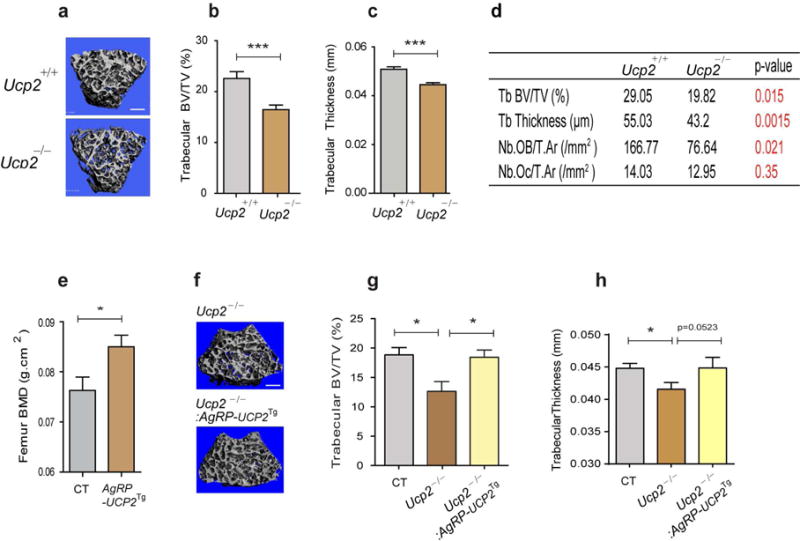
Next, we determined the skeletal phenotype of animals in which AgRP neurons are ablated perinatally using mice with AgRP neuron-specific diphtheria toxin receptors (AgRPDTR). AgRP neurons can be experimentally ablated in these animals by diphtheria toxin injection (Luquet et al., 2005). While ablation of AgRP neurons in the adult results in rapid death due to aphagya, neonatal ablation of these cells is not lethal and does not result in altered feeding behavior (Luquet et al., 2005). We treated transgenic and control mice with diphtheria toxin at postnatal day 5 and analyzed the skeletal phenotype of adult animals. Micro-CT analysis showed that three-month-old male AgRPDTR mice had significantly lower bone mass in both the trabecular and cortical compartments of the femora when compared to control mice (Fig. 2a–e). These data indicate that AgRP neurons are important for CNS-mediated modulation of bone metabolism during growth and development.
Figure 2. Early postnatal ablation of AgRP neurons results in reduced bone mass.
(a) Representative micro-CT images of femoral trabecular bone from three-month-old male control (CT) and AgRPDTR mice. Scale bar = 500 μm. (b–e) Micro-CT analysis demonstrated a reduction in trabecular bone volume (BV/TV), trabecular thickness, cortical BV/TV and cortical thickness in three-month-old male AgRPDTR mice (b: n=10 for CT, n=20 for AgRPDTR, p<0.05; c: n=10 for CT, n=20 for AgRPDTR, p<0.001; d: n=10 for CT, n=20 for AgRPDTR, p<0.05; e: n=10 for CT, n=20 for AgRPDTR, p<0.001). * p<0.05, ** p<0.001 and *** p<0.001. Data are presented as means ± s.e.m. P values for unpaired comparisons were analyzed by Student’s t test.
Impairment of AgRP neuronal excitability by cell autonomous deletion of Sirt1 results in osteopenia
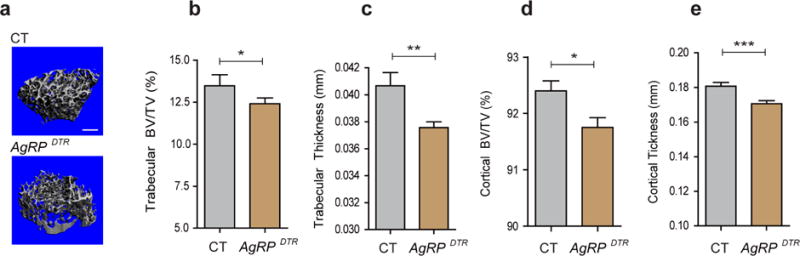
AgRP neurons in which the histone deacetylase, sirtuin 1 (Sirt1) was selectively deleted in vivo (AgrpSirt1−/− mice) were neurobiologically active but exhibited impaired excitation in response to metabolic cues, such as elevated ghrelin levels (Dietrich et al., 2010). We investigated the skeletal phenotype of AgrpSirt1−/− mice. DXA scans demonstrated that three-month-old male AgrpSirt1−/− mice had reduced femoral BMD (Fig. 3a). Micro-CT analysis revealed that there was a 30% decrease in trabecular bone volume in the femur (Fig. 3c) accompanied by a reduction in trabecular thickness (Fig. 3d), trabecular number and connectivity density (data not shown) in three-month-old male AgrpSirt1−/− mice. Additionally, these mice displayed a significantly reduced cortical bone volume (Fig. 3e) and thickness (Fig. 3f). Consistent with the micro-CT findings, histomorphometric analysis of trabecular bone from three-month-old male AgrpSirt1−/− mice demonstrated a tendency towards reduced bone volume (Fig. 3g) and trabecular thickness (Fig. 3g). Histomorphometric analysis also indicated a trend towards reduced osteoblast numbers, (Fig. 3g) and an increase in osteoclast number (Fig. 3g) albeit without statistical significance. However, entirely consistent with the trend towards an increase in osteoclast number, serum levels of carboxy-terminal collagen crosslinks (CTX), a marker of bone resorption, were significantly elevated in three-month-old male AgrpSirt1−/− mice (Fig. 3h). These results suggest that a reduction in bone formation and an increase in bone resorption may contribute to the skeletal phenotype in AgRP circuit-impaired animals.
Figure 3. Impairing AgRP neuronal excitability by deletion of Sirt1 in AgRP neurons results in reduced bone mass in vivo.
(a) DXA analysis demonstrated a reduced femoral bone density in three-month-old male AgrpSirt1−/− mice (n=9 for AgrpSirt +/+, n=9 for AgrpSirt1−/−, p<0.05). (b) Representative micro-CT images of femoral trabecular bone in three-month-old male AgrpSirt +/+ and AgrpSirt1−/− mice. Scale bar = 500 μm. (c–f) Micro-CT analysis shows reduced trabecular BV/TV, trabecular thickness, cortical BV/TV and cortical thickness in three-month-old male AgrpSirt1−/− mice (c: n=14 for AgrpSirt +/+, n=12 for AgrpSirt1−/−, p<0.05; d: n=14 for AgrpSirt +/+, n=12 for AgrpSirt1−/−, p<0.05; e: n=14 for AgrpSirt +/+, n=12 for AgrpSirt1−/−, p<0.01; f: n=14 for AgrpSirt +/+, n=12 for AgrpSirt1−/−, p<0.01). (g) Histomorphometric analysis of trabecular bone from three-month-old male AgrpSirt1−/− mice showed a trend towards a reduction in bone volume with lower numbers of osteoblasts and higher numbers of osteoclasts (n=5 for AgrpSirt +/+, n=5 for AgrpSirt1−/−). (h) Serum levels of CTX were elevated in three-month-old male AgrpSirt1−/− mice (n=6 for AgrpSirt +/+, n=7 for AgrpSirt1−/−, p<0.05). * p<0.05 and ** p<0.01. Data are presented as means ± s.e.m. P values for unpaired comparisons were analyzed by Student’s t test.
AgRP-regulated bone metabolism involves the sympathetic nervous system
The sympathetic nervous system (SNS) is an important mediator of CNS outputs to peripheral tissues. Multiple lines of evidence support a relationship between brain-regulated bone metabolism and centrally-regulated peripheral sympathetic activity (Elefteriou et al., 2005; Yadav et al., 2009). Therefore, we analyzed whether sympathetic activity may be a mediator of AgRP neurons’ effect on bone metabolism. Since Ucp1 mediates β-adrenergic receptor-regulated thermogenesis in brown adipose tissue (BAT), we first tested Ucp1 mRNA levels in BAT isolated form three-month-old male Ucp2−/− mice, Agrp-Ucp2Tg mice and AgrpSirt1−/− mice. We observed that Ucp1 mRNA levels were significantly elevated in BAT isolated from osteopenic Ucp2−/− mice (Supplementary Fig. 2a) and AgrpSirt1−/− mice (Fig. 4a). Whereas Agrp-Ucp2Tg mice demonstrated a reduction in BAT Ucp1 mRNA levels (Supplementary Fig. 2b). In addition, we found that the norepinephrine content in bone was higher in AgrpSirt1−/− mice compared to controls (Fig. 4b). To test whether elevated sympathetic outflow contributes to the osteopenia induced by impaired excitability of AgRP neurons, we evaluated bone mass in three-month-old male control and AgrpSirt1−/− mice treated with propranolol, a sympatholytic beta-blocker. Blockade of β-adrenergic receptors rendered BMD (Fig. 4c) and trabecular bone mass (Fig. 4d) indistinguishable in control and AgrpSirt1−/− mice. These data indicate that attenuating the excitability of AgRP neurons, results in increased sympathetic outflow, which in turn contributes to bone loss.
Figure 4. Osteopenia seen in AgrpSirt1−/− mice is rescued by -adrenergic blockade.
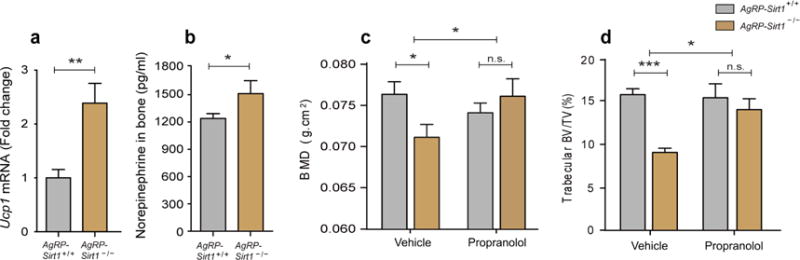
(a, b) three-month-old male AgrpSirt1−/− mice had increased Ucp1 transcript expression in brown adipose tissue and norepinephrine content in bone (a: n=6 for AgrpSirt +/+, n=8 for AgrpSirt1−/−, p<0.01; b: n=5 for AgrpSirt+/+, n=4 for AgrpSirt1−/−, p<0.05). (c) DXA analysis demonstrated that the reduced femoral BMD seen in three-month-old male AgrpSirt1−/− mice was reversed by treatment with propranolol (n=3 for AgrpSirt +/++vehicle, n=4 for AgrpSirt1−/− +vehicle, n=3 for AgrpSirt +/+ +propranolol, n=4 for AgrpSirt1−/− +propranolol; p<0.05 for Student’s t test and two-way ANOVA). (d) Micro-CT analysis demonstrated that the reduction in femoral trabecular BV/TV of three-month-old male AgrpSirt1−/− mice was rescued by treatment with propranolol (n=9 for AgrpSirt +/++vehicle, n=9 for AgrpSirt1−/−+vehicle, n=7 for AgrpSirt +/++propranolol, n=7 for AgrpSirt1−/−+propranolol, p<0.001 for Student’s t test; p<0.05 for Two-way ANOVA). * p<0.05, ** p<0.01 and *** p<0.001. Data are presented as means ± s.e.m. P values for unpaired comparisons were analyzed by Student’s t test. Two-way ANOVA was performed to detect significant interaction between genotype and treatment (propranolol).
Leptin receptors in AgRP do not mediate leptin’s action on skeleton
The metabolic hormone, leptin, was shown to regulate bone metabolism by a central nervous system circuit (Ducy et al., 2000; Takeda et al., 2002). However, the exact cellular targets for leptin in the brain that mediate leptin’s actions on the skeleton remain controversial. AgRP neurons are direct targets of leptin (Cowley et al., 2001; Heisler et al., 2006; Pinto et al., 2004). To test the role of leptin receptors in AgRP neurons in control of bone metabolism, we generated mice in which leptin receptors were cell-selectively ablated in AgRP neurons (AgrpLepr−/− mice). Two-month-old male AgrpLepr−/− mice displayed increased body weight and fat mass and lower lean mass (Supplementary Fig. 3a–c). However, BMD (Supplementary Fig. 3d), as well as trabecular and cortical BV/TV (Supplementary Fig. 3 e,f) were not altered in these animals. These data indicate that leptin signaling in AgRP neurons is relevant to whole body energy metabolism but appears to have less of an impact on bone homeostasis in healthy young mice. Whether leptin-regulated AgRP function plays a role in disease states, such as anorexia nervosa and lipodystrophy where hypoleptinemia is associated with impaired skeletal health (Misra and Klibanski, 2014; Moran et al., 2004), needs further investigation.
DISCUSSION
In this study we explored the involvement of AgRP neurons in the control of skeletal homeostasis. By using a variety of models, we observed that impaired AgRP circuit function leads to an osteopenic phenotype. The effect of AgRP neurons on bone metabolism in the adult is likely mediated, at least in part, by the sympathetic nervous system, because suppression of beta adrenergic system abolished phenotype differences between AgRPSirt1−/− and control mice. We found that global Ucp2-deficient mice displayed a significant reduction of bone mass accompanied by a reduction in bone formation without a change in bone resorprion. Selective re-expression of UCP2 in AgRP neurons in Ucp2−/− animals reversed the osteopenic phenotype suggesting that AgRP neurons may be an important site where UCP2 exerts its effect on bone. However, Ucp2 is widely expressed and it is not unlikely that the skeletal phenotype of Ucp2−/− mice is due to multiple mechanisms. In line with this notion, histomorphometric analyses in AgrpSirt1−/− mice revealed a trend towards reduced osteoblast number and an increase in osteoclast number, changes that were not observed in Ucp2−/− mice. This difference in the cellular changes in bone observed in Ucp−/− mice and AgrpSirt1−/− mice could reflect a cell autonomous effect of global Ucp2 deletion in bone cells.
Although more than one pathway likely mediates AgRP neuron-controlled bone mass, we did find evidence for an effector role of the sympathetic nervous system in the skeletal phenotype of the AgRPSirt1−/− animals. We also found that a surrogate marker of sympathetic activation (brown fat UCP1 mRNA levels) were elevated in mice with AgRP circuit impairment and down regulated in mice with AgRP circuit enhancement. There are many other mechanisms by which AgRP system can affect bone mass, including actions on the thyroid, adrenal and gonadal axes. Further studies are needed to assess humoral control of bone metabolism as a pathway modulated AgRP neurons. Nonetheless, because at least in adult mice interference with the sympathetic tone reversed the bone phenotype of AgRPSirt1−/− mice, it is reasonable to conclude that AgRP neuronal function in adult mice controls bone mass. Finally since the models we employed alter AgRP function during development it may be that changing AgRP signaling during skeletogenesis and modeling also contributed to the adult bone phenotype of the engineered lines that we studied.
Bone metabolism is tightly connected to nutrient availability. However the genetic models we employed did not display significant differences in metabolic phenotypes or body length at the ages they were studied when fed normal murine chow (Andrews et al., 2010; Dietrich et al., 2010; Luquet et al., 2005). These observations suggest that alternation of AgRP neuronal activities affects bone homeostasis independent of metabolic shifts. In further support of this notion, deletion of leptin receptors from AgRP neurons did alter the metabolic phenotype of mice without affecting bone mass. However, leptin signaling (or the lack there of) in AgRP neurons may be relevant to disease states associated with impaired bone metabolism, such as anorexia nervosa or lipodistrophy.
Collectively, our findings demonstrate that hypothalamic AgRP neuronal circuit integrity is a regulator of bone mass and that this effect is mediated, at least in part, by the sympathetic nervous system. These results provide novel insights into the central regulatory component of bone metabolism and offer new strategies to consider in addressing skeletal dysregulation in various disease conditions.
EXPERIMENTAL PROCEDURES
Animals
The following transgenic mice were used in this study: AgrpSirt1−/− mice were generated as described previously (Dietrich et al., 2010). AgrpDTR mice were provided by Dr. R.D. Palmiter (University of Washington, USA) and have been described previously (Luquet et al., 2005). Ucp2−/− mice were provided by Dr. B.B. Lowell (Harvard University, USA) and have been used previously (Andrews et al., 2008). Agrp-Ucp2Tg mice overexpressing Ucp2 in AgRP neurons were generated by cre-lox knock in technology as described in Supplementary Figure 1. In brief, a transgene (lower panel of a) was engineered in which expression of the murine cDNA for Ucp2 is controlled by a CMV promoter when a transcriptional stop cassette is removed by cre recombination. (The Ucp2 cDNA was introduced into the construct using the Asc I restriction enzyme) This transgene was then used to generate transgenic mice (Ucp2Tg mice). To generate AgRP neurons-specific overexpression of Ucp2 gene, Ucp2Tg mice were crossed with AgRP-Ires-cre mice (Agrptm1(cre)Lowl, Jax #012899). We controlled for ectopic expression of Cre in this AgRP line as described earlier (Dietrich et al., 2010; Dietrich et al., 2012), and those with ectopic expression were excluded from further studies. To generate AgRP neuron-specific Ucp2-reactivated Ucp2−/− mice (Ucp2−/−:Agrp-Ucp2Tg mice), Ucp2−/− mice were mated with Agrp-Ucp2Tg mice. To generate a mouse line in which leptin receptor signaling in AgRP neurons is impaired, AgRP-Ires-cre mice were mated with Lepr flox/flox mice (McMinn et al., 2005) (Generated by Streamson Chua, Albert Einstein College of Medicine, USA), and breeding cages maintained by mating Leprflox/flox and Leprflox/flox:AgRP Cre mice. AgrpDTR or wild-type mice from the same litter received an injection of diphtheria toxin at postnatal day 5 (Luquet et al., 2005). All animals were kept in temperature- and humidity-controlled rooms on a 12-h:12-h light:dark cycle, with lights on from 7:00 a.m. to 7:00 p.m. Mice were group housed (3–5 mice per cage) and food and water were provided ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of Yale University.
Histomorphometric analyses
Static histomorphometry was performed as previously reported (Knopp et al., 2005). Analyses were performed on 5 μM thick sections of distal femur stained with toluidine blue, pH 3.7 using a Nikon microscope interfaced with the Osteomeasure system software and hardware (Osteometrics, Atlanta, GA). Measurements were obtained in an area of cancellous bone that measures approximately 2.5 mm2, containing only secondary spongiosa, and located 0.5–2.5 mm proximal to the epiphyseal growth cartilage. Longitudinal sections (5 μm thick) taken in the frontal plane through the cancellous bone of the femora are prepared with a Leica RM2165 microtome, mounted on chrom-alum coated glass slides, and stained with toluidine blue, pH 3.7. All indices are defined according to the American Society of Bone and Mineral Research histomorphometry nomenclature. We analyzed trabecular bone volume, trabecular thickness, the number of osteoblasts and osteoclasts per trabecular area in three-month-old male Ucp2−/− or AgrpSirt1−/− mice compared to littermate control mice.
Bone Densitometry and ultrastructural analyses
Dual-energy X-ray absorptiometry (PIXImus) was used to determine fat mass, lean mass and bone mineral density of two or three-month-old male Ucp2−/−, Agrp-Ucp2Tg, AgrpSirt1−/− and AgrpLepr−/− mice. μCT (Scanco microCT35 machine) was used to separately assess the cortical and trabecular skeletal envelopes as well as microarchitectural features such as trabecular thickness for two or three-month-old male Ucp2−/−, Agrp-Ucp2Tg, Ucp2−/−:Agrp-Ucp2Tg, AgRPDTR, AgrpSirt1−/− and AgrpLepr−/− mice. All experiments were performed in the Yale Core Center for Musculoskeletal Disorders.
Propranolol treatment
Propranolol (Sigma) was administered at a concentration of 0.5 mg/ml in the drinking water of AgrpSirt1−/− and control mice for 4 weeks, from 10 weeks of age. Propranolol-containing water was refreshed three times per week. Control mice had normal drinking water. Mice were scanned by PIXImus to determine bone mineral density and sacrificed at the end of treatment followed by harvesting femora for micro-CT analysis.
Real-time PCR
RNA from brown adipose tissue was isolated with the RNeasy Micro Kit (Qiagen) and reverse transcribed to cDNA using MultiScribe Reverse Transcriptase (Applied Biosystems). Quantitative PCR was performed with the Light Cycler 480 Real-time PCR system (Roche) using TaqMan® probe [Ucp1 (Mm01244861_m1), Applied Biosystems]. The data were normalized with Glyceraldehyde-3-phosphate dehydrogenase [Gapdh (Mm99999915_g1, Applied Biosystems)].
Measurement of norepinephrine in bone
Soft tissue was rapidly removed from the femur and tibia and the both bones snap frozen in liquid nitrogen. The bone was then pulverized in 10 ml of freshly prepared 0.4 N perchloric acid containing 5 nM reduced glutathione and then centrifuged for 15 min at 1300 × G to produce a protein-free supernatant. The entire supernatant was then adjusted to pH 7 and extracted on alumina columns. Catecholamines were analyzed in the extract by high-performance liquid chromatography using electrochemical detection (ESA, Acton, MA).
Measurement of serum CTX
Serum CTX was measured using the RatLaps ELISA kit (Nordic Bioscience Diagnostics A/S, Herlev, Denmark).
Statistical Analyses
Statistical analyses were performed by use of Prism 6.0 software (Graph Pad, San Diego, CA, USA). Data distribution was assumed to be normal but this was not formally tested. No statistical methods were used to predetermine sample sizes but our sample sizes are similar to those reported in previous publication (Dietrich et al., 2012). All analyses were performed in a blinded manner. No randomization was used to assign experimental groups or to collect data but mice were assigned to specific experimental groups without bias. Unpaired t-test was performed to analyze significance between two experimental groups. Two-way ANOVA analysis was performed to detect interaction between treatment and genotype. Significance was taken at p<0.05.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 AG040236, DP1 DK098058, R01 DK097566 and P30 Core Center award AR46032, and an ADA Mentored Fellowship Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
J.G.K., M.O.D., K.I. and T.L.H. designed the study. J.G.K., KI and T.L.H. interpreted the results. J.G.K., M.K., G.Y. and B.S. performed experiments and analyzed the data. J.G.K. contributed to all figures. B.S. contributed to Figure 1–4. G.Y. contributed to Figure 1d and Figure 3g. M.K. contributed to Figure 1f–h and Figure 3b–f. J.G.K., M.O.D. and S.D. contributed to the generation of animal model. J.G.K., K.I. and T.L.H. wrote the paper with input from the other authors.
References
- Andrews ZB, Erion DM, Beiler R, Choi CS, Shulman GI, Horvath TL. Uncoupling protein-2 decreases the lipogenic actions of ghrelin. Endocrinology. 2010;151:2078–2086. doi: 10.1210/en.2009-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschop MH, Shanabrough M, Cline G, Shulman GI, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, Friedman JM, Ricquier D, Richard D, Horvath TL, Gao XB, et al. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell metabolism. 2007;5:21–33. doi: 10.1016/j.cmet.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Diano S, Horvath TL. Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends in molecular medicine. 2012;18:52–58. doi: 10.1016/j.molmed.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Antunes C, Geliang G, Liu ZW, Borok E, Nie Y, Xu AW, Souza DO, Gao Q, Diano S, et al. Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:11815–11825. doi: 10.1523/JNEUROSCI.2234-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Bober J, Ferreira JG, Tellez LA, Mineur YS, Souza DO, Gao XB, Picciotto MR, Araujo I, Liu ZW, et al. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nature neuroscience. 2012;15:1108–1110. doi: 10.1038/nn.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Warden CH, Hajos M, Lombardi A, Goglia F, Diano S. Brain uncoupling protein 2: uncoupled neuronal mitochondria predict thermal synapses in homeostatic centers. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:10417–10427. doi: 10.1523/JNEUROSCI.19-23-10417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly-Amado A, Denis RG, Castel J, Lacombe A, Cansell C, Rouch C, Kassis N, Dairou J, Cani PD, Ventura-Clapier R, et al. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. The EMBO journal. 2012;31:4276–4288. doi: 10.1038/emboj.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp E, Troiano N, Bouxsein M, Sun BH, Lostritto K, Gundberg C, Dziura J, Insogna K. The effect of aging on the skeletal response to intermittent treatment with parathyroid hormone. Endocrinology. 2005;146:1983–1990. doi: 10.1210/en.2004-0770. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Matarese G, Procaccini C, Menale C, Kim JG, Kim JD, Diano S, Diano N, De Rosa V, Dietrich MO, Horvath TL. Hunger-promoting hypothalamic neurons modulate effector and regulatory T-cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6193–6198. doi: 10.1073/pnas.1210644110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn JE, Liu SM, Liu H, Dragatsis I, Dietrich P, Ludwig T, Boozer CN, Chua SC., Jr Neuronal deletion of Lepr elicits diabesity in mice without affecting cold tolerance or fertility. American journal of physiology. Endocrinology and metabolism. 2005;289:E403–411. doi: 10.1152/ajpendo.00535.2004. [DOI] [PubMed] [Google Scholar]
- Misra M, Klibanski A. Anorexia nervosa and bone. The Journal of endocrinology. 2014;221:R163–176. doi: 10.1530/JOE-14-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran SA, Patten N, Young JR, Cochran E, Sebring N, Reynolds J, Premkumar A, Depaoli AM, Skarulis MC, Oral EA, et al. Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metabolism: clinical and experimental. 2004;53:513–519. doi: 10.1016/j.metabol.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Engdahl C, Borjesson AE, Windahl SH, Studer E, Westberg L, Eriksson E, Koskela A, Tuukkanen J, Krust A, et al. Estrogen receptor-alpha expression in neuronal cells affects bone mass. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:983–988. doi: 10.1073/pnas.1111436109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Ruan HB, Dietrich MO, Liu ZW, Zimmer MR, Li MD, Singh JP, Zhang K, Yin R, Wu J, Horvath TL, et al. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell. 2014;159:306–317. doi: 10.1016/j.cell.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Hanada R, Kimura A, Abe T, Matsumoto T, Iwasaki M, Inose H, Ida T, Mieda M, Takeuchi Y, et al. Central control of bone remodeling by neuromedin U. Nature medicine. 2007;13:1234–1240. doi: 10.1038/nm1640. [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Warne JP, Varonin JM, Nielsen SS, Olofsson LE, Kaelin CB, Chua S, Jr, Barsh GS, Koliwad SK, Xu AW. Coordinated regulation of hepatic energy stores by leptin and hypothalamic agouti-related protein. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:11972–11985. doi: 10.1523/JNEUROSCI.0830-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


