Abstract
Introduction
The aim of our current study was to evaluate cerebrospinal fluid (CSF) and serum CXCL9 concentrations and diagnostic usefulness of this molecule in tick-borne encephalitis (TBE). The study included TBE patients in the acute phase (TBE I) and after 2 weeks of follow-up (TBE II). The control group consisted of patients investigated for suspected central nervous system (CNS) infection, but with normal CSF findings.
Material and methods
Concentrations of CXCL9 were measured using enzyme-linked immunosorbent assay (ELISA).
Results
Cerebrospinal fluid and serum concentrations of CXCL9 in patients with TBE were significantly higher than in controls (p < 0.001). This alteration was also observed in the case of the CXCL9 index (ICXCL9; CSF CXCL9 concentration divided by serum CXCL9 concentration) (p < 0.001); moreover, ICXCL9 significantly decreased after 2 weeks (p < 0.001). This is the first study to evaluate the CSF and serum levels of CXCL9 in subjects with TBE.
Conclusions
CXCL9 is a ligand for CXCR3, which was found on all Th1 memory lymphocytes present in the peripheral blood; therefore the elevated concentrations of CXCL9 in TBE patients as compared to the controls might indicate that this chemokine perhaps takes part in the trafficking of Th1 cells into the CNS. The results presented here support the hypothesis that CXCL9 may play a role in TBE. However, further studies are required to determine whether this protein might be used as a potential tool for the diagnosis and monitoring of inflammation in TBE.
Keywords: chemokines, cerebrospinal fluid, tick-borne encephalitis
Introduction
Tick-borne encephalitis (TBE) is the most common tick-borne disease in Europe, the Far East, and Asia [1, 2]. This zoonotic disease is caused by an RNA virus of the genus Flavivirus within the Flaviviridae family [2–4]. The main vectors of TBE virus (TBEV) are Ixodes ricinus and Ixodes persulcatus [1–3].
According to the literature, one third of TBE cases might be without neurological symptoms [2]. The most common neurological manifestation of TBE is meningitis; meningoencephalomyelitis occurs less frequently [2, 3]. The cerebrospinal fluid (CSF) analysis of patients with diagnosed TBE generally reveals moderate pleocytosis with domination of lymphocytes, which might exist for several weeks after clinical improvement [3]. It is hypothesized that immune-mediated neuronal cell death mediated by the T-cell response in the CSF, rather than direct viral lysis, leads to the brain damage and dysfunction in TBE [4].
The laboratory diagnosis of TBE is defined by the demonstration of specific serum TBEV-immunoglobulin M (IgM) and TBEV-immunoglobulin G (IgG) antibodies in the serum samples taken from the patients when central nervous system (CNS) symptoms manifest. The virus can also be detected by the reverse transcriptase polymerase chain reaction (RT-PCR) from the blood, but only occasionally is detected in the CSF [4]. RT-PCR for detection of TBEV RNA is efficiently applied in animal models, but clinical use of RT-PCR still needs to be validated in humans [5].
Chemokines are small proteins (8–15 kDa) that play a crucial role in the migration of immunocompetent cells to the sites of inflammation [6]. They were discovered by their leukocyte adhesion, chemotaxis, and activation abilities, both in vivo and in vitro [7–9]. Chemokines are also involved in immune surveillance and function to localize B or T lymphocytes with antigen [10, 11]. Under physiological conditions they are constitutively present in the brain on the glial cells and neurons, and are involved in intracellular communication [8, 11–13]. Cells of the CNS are capable of releasing chemokines upon stimulation; moreover, CNS cells are also able to respond to them by their receptors.
Chemokines are classified depending on the position of conserved cysteines (C) in their sequence into four structural subfamilies: CXC, CC, C, and CXC3 [14–16]. The CXC chemokines are further subdivided into those containing a glutamic acid-leucine-arginine (ELR) motif near their N-terminus and those not containing this motif (non-ELR) [11]. ELR-positive CXC chemokines act mostly on neutrophils, while non-ELR chemokines are mainly chemotactic for mononuclear cells [17]. CXCL9/MIG (monokine-induced by γ-interferon), CXCL10/IP-10 (interferon-inducible 10 kDa protein), and CXCL11/I-TAC (inducible T cell-α chemoattractant) are the three ELR-negative chemokines, which are more closely related to each other than to any other chemokine [18]. Moreover, genes for the above-mentioned chemokines are highly inducible by γ-interferon (IFN-γ), whose production is highly restricted to activated T cells and NK cells [11]. CXCL9, CXCL10, and CXCL11 target lymphocytes and signal via the chemokine (C-X-C motif) receptor 3 (CXCR3/CD183) [19]. Higher levels of CXCR3 were found on human T helper type 1 (Th1) cell lines as compared to Th2 cell lines. Additionally, CXCR3 was found on all Th1 memory lymphocytes present in the peripheral blood. Because CXCL9 is a ligand for CXCR3, it may be suggested that the role of CXCL9 is the recruitment of effector T cells, particularly Th1 lymphocytes, to the sites of inflammation [19]. In various diseases CXCL9 expression was accompanied by an increased number of T cells [20–22].
Data concerning the concentrations of non-ELR chemokines in the CSF and serum of patients with TBE are limited [23, 24]. So far, according to our knowledge, the levels and diagnostic usefulness of chosen chemokines that lack the ELR motif in TBE have been assayed only by Lepej et al. and Zajkowska et al., who analyzed CXCL10 and CXCL11 concentrations [23, 24]. It was established that CXCR3–non-ELR chemokine interactions are very important for the pathogenesis of diverse neurological disorders [11]. Because little is known about the non-ELR chemokines and their receptor concentrations in TBE cases, the aim of our current study was to evaluate CXCL9 and CXCR3 levels in serum and CSF of patients with TBE (confirmed by the presence of TBEV-specific IgM and IgG) in the acute phase and after two weeks of follow-up. Gathering more data related to CXCR3-ligand interactions is extremely important because CXCR3 is a critical receptor taking part in the recruitment of T-cells into the CNS.
Material and methods
Patients
The study was approved by the ethical committee of the Medical University of Białystok. All patients gave their written informed consent. The patients and the controls were recruited between 2012 and 2013. The study included 24 TBE patients (aged 18–79 years (mean: 45); 8 male). All patients lived in north-eastern Poland.
Tick-borne encephalitis diagnosis was based on clinical signs/symptoms of meningitis or meningoencephalitis, and detection of specific TBEV IgM and IgG antibodies in the CSF and serum.
Venous blood and CSF were obtained from patients with the diagnosis of TBE, at the time of the patient’s admission to the hospital (TBE I) and during follow-up examinations after 2 weeks (TBE II).
The control group consisted of 13 patients (aged 18–85 years (mean: 51); 5 male) with initially suspected but later, after CSF analysis, excluded CNS infection (negative TBEV antibodies; no pleocytosis, CSF total protein concentrations within the normal range). In controls, CSF and serum analysis was performed only once, at the time of the patient’s admission to the hospital.
Serologic tests for Borrelia burgdorferi, a tick-transmitted bacterium, were negative for all patients included in the study.
Sample preparations
Cerebrospinal fluid was obtained by lumbar puncture. All samples were collected in polypropylene tubes and they were centrifuged (1100 g, 10 min, room temperature), aliquoted into several portions of 200 μl, and frozen at –80°C until assayed.
Blood samples were left for clotting for 30 min before centrifugation (1000 g, 15 min, room temperature), and separated serum was aliquoted into several portions of 200 μl, and frozen at –80°C until further analysis.
Patients’ demographics and laboratory measurements
White blood cell count (WBC), red blood cell count (RBC), hemoglobin concentration (HGB), platelet count (PLT) in the blood, and TBEV IgM and IgG antibodies in the serum and CSF were measured with standard laboratory techniques in the laboratory at the time of the patients’ admission to the hospital (Table I).
Table I.
Laboratory characteristics of the group of patients with TBE at the time of admission to the hospital
| CSF: | |
| TBEV IgM | Positive in 13 of 24 TBE patients |
| TBEV IgG [U/ml] | 5.0 (5.0–8.3) |
| Blood: | |
| TBEV IgM | Positive in 24 TBE patients |
| TBEV IgG [U/ml] | 10.7 (7.9–25.8) |
| WBC [× 103/μl] | 10.5 (8.6–11.9) |
| RBC [× 103/μl] | 4.4 (4.3–4.8) |
| HGB [g/dl] | 13.8 (12.9–14.6) |
| PLT [× 103/μl] | 206 (163–234) |
Results are presented as medians and interquartile ranges. CSF – cerebrospinal fluid, HGB – hemoglobin concentration, IgG – immunoglobulin G, IgM – immunoglobulin M, PLT – platelet count, RBC – red blood cell count, SD – standard deviation, TBE – tick-borne encephalitis, TBEV IgG – TBE virus IgG antibodies, TBEV IgM – TBE virus IgM antibodies, WBC – white blood cell count.
Pleocytosis, albumin, total protein, and glucose concentrations in the CSF as well as serum C-reactive protein (CRP) were measured with standard laboratory techniques in the laboratory at the time of the patient’s admission to the hospital (TBE I) and after 2 weeks of follow-up (TBE II) (Table II). Similarly, CXCL9 and CXCR3 concentrations were measured on both occasions (Table III).
Table II.
Biochemical parameters and pleocytosis in the CSF and serum of TBE patients
| Parameter | TBE I | TBE II | P-value |
|---|---|---|---|
| CSF: | |||
| Albumin [mg/dl] | 46 (37–62) | 43 (33–61) | NS |
| Total protein [mg/dl] | 69 (52–87) | 65 (50–90) | NS |
| Glucose [mg/dl] | 58 (55–63) | 54 (52–58) | NS |
| Pleocytosis [cells/μl] | 140 (83–244) | 52 (35–75) | < 0.001 |
| Serum: | |||
| CRP [mg/dl] | 9.7 (3.0–22.4) | 0.9 (0.7–1.3) | < 0.001 |
Results are presented as medians and interquartile ranges. CRP – C-reactive protein, CSF – cerebrospinal fluid, NS – not statistically significant, TBE – tick-borne encephalitis, TBE I – TBE patients at the time of admission to the hospital, TBE II – TBE patients after 2 weeks of follow-up.
Table III.
CXCL9 concentrations and CXCL9 index (ICXCL9) in the CSF and serum of TBE patients as compared to the control group (C)
| Parameter | I N = 24 | II N = 24 | C N = 13 | P-value |
|---|---|---|---|---|
| CSF CXCL9 [pg/ml] | 215 (100–346) | 166 (119–240) | 6 (5–23) | I vs. II, NS I vs. C, p < 0.001 II vs. C, p < 0.001 |
| Serum CXCL9 [pg/ml] | 111 (91–137) | 138 (113–163) | 58 (51–62) | I vs. II, NS I vs. C, p < 0.001 II vs. C, p < 0.001 |
| ICXCL9 | 2.0 (0.9–3.4) | 1.2 (0.9–1.6) | 0.2 (0.1–0.3) | I vs. II, p < 0.001 I vs. C, p < 0.001 II vs. C, p < 0.001 |
ICXCL9 was determined as follows: CSF CXCL9 concentration divided by serum CXCL9 concentration. Results are presented as medians and interquartile ranges. C – control group, CSF – cerebrospinal fluid, CXCL9 – monokine-induced by γ-interferon, ICXCL9 – CXCL9 index, TBE – tick-borne encephalitis.
The detection of TBEV antibodies in the CSF and serum of TBE patients was performed using the enzyme-linked immunosorbent assay (ELISA) method (SERION ELISA classic TBE IgG/IgM kits, Institut Virion/Serion GmbH, Würzburg, Germany) according to the manufacturer’s instructions.
Analysis of CXCL9 and CXCR3
Cerebrospinal fluid and serum levels of CXCL9 were measured using the ELISA Quantikine Human CXCL9/MIG Immunoassay kit (catalog number: DCX900; R&D Systems Europe Ltd., Abingdon, England) according the manufacturer’s instructions. Samples were not diluted before analysis. The manufacturer of the assay kits refers to the intra-assay coefficient of variation (CV %) as 3.9% at CXCL9 mean concentration of 253 ±9.9 pg/ml.
Cerebrospinal fluid and serum concentrations of CXCR3 were measured with the ELISA Assay kit for chemokine C-X-C-Motif receptor 3 (catalog number: SEA625Hu, USCN Life Science Inc., Wuhan, China) according the manufacturer’s instructions. Samples were not diluted prior to assay. The manufacturer of the assay kits refers to the intra-assay coefficient of variation (CV%) as less than 10%.
Statistical analysis
The preliminary statistical analysis (χ2 test) revealed that the CSF and serum concentrations of all proteins tested did not follow a normal distribution, and hence the non-parametric Kolmogorov-Smirnov test was used to compare the two groups analyzed. The results are presented as medians and interquartile ranges. Differences were considered statistically significant for p < 0.05. The relation between the sensitivity and specificity was illustrated using a receiver operator characteristic (ROC) curve. The ROC curve is a line graph that plots the probability of true positive results – or the sensitivity of the test – against the probability of false positive results for a range of different cut-off points [25]. Statistical analyses and area under the ROC curves (AUCs) were calculated using the Statistica 9.0 PL program (StatSoft Inc., Tulsa, OK, USA).
Results
CXCL9 and CXCR3 results obtained in the CSF and serum of TBE patients and controls
In TBE II patients pleocytosis and CRP concentrations were significantly lower as compared to the TBE I patients (Table II).
In both patient groups analyzed CSF as well as serum CXCL9 concentrations were significantly higher as compared to the controls (Table III). CSF CXCL9 was higher and serum CXCL9 was lower in TBE I in comparison to TBE II, but neither difference was statistically significant (Table III). To exclude possible impairment of the blood-CSF barrier and/or BBB functions as potential sources influencing CXCL9 concentrations, the CSF CXCL9 concentrations were related to the concentrations in the serum by calculating the CXL9 index (ICXCL9). ICXCL9 in TBE I and TBE II were significantly higher as compared to the controls, and significantly decreased after symptoms resolution after 2 weeks of follow-up (Table III).
In TBE I cases CXCL9 concentrations were significantly higher in CSF than in serum (p < 0.05) (Table III). In TBE II cases CXCL9 concentrations were also higher in CSF as compared to the results obtained in serum, but none of the differences were statistically significant (Table III).
All CSF CXCR3 concentrations in TBE I and TBE II were above the upper limit of detection (the assay range of the used kit is 0–10 pg/ml). In case of serum specimens CXCR3 concentrations were detected only in 3 patients (obtained values 6.0, 5.0, and 4.0 pg/ml, respectively). Because of the obtained results in the groups of TBE patients CXCR3 concentrations in the controls were not analyzed.
In TBE I patients CSF CXCL9 significantly correlated with serum CXCL9 (p < 0.05; r = 0.70), and ICXCL9 (p < 0.05; r = 0.91). In TBE II patients CSF CXCL9 was significantly correlated with serum CXCL9 (p < 0.05; r = 0.60); moreover, both CSF and serum CXCL9 were significantly correlated with ICXCL9 (p < 0.05; r = 0.92 and p < 0.05; r = 0.48, respectively). Additionally, in TBE I patients pleocytosis was positively correlated with ICXCL9 (p < 0.05; r = 0.44).
Diagnostic criteria for CXCL9 and ICXCL9
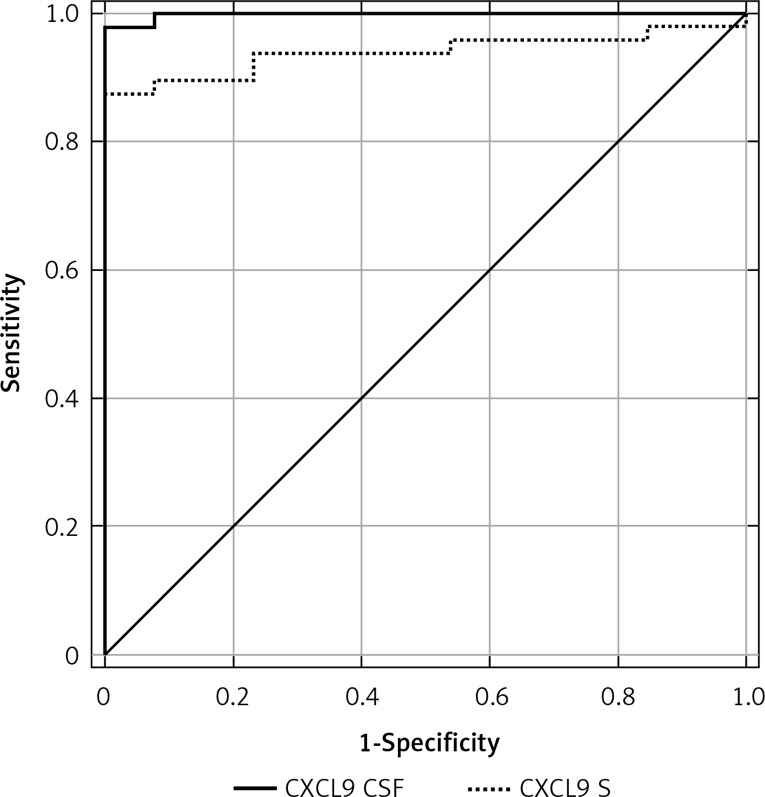
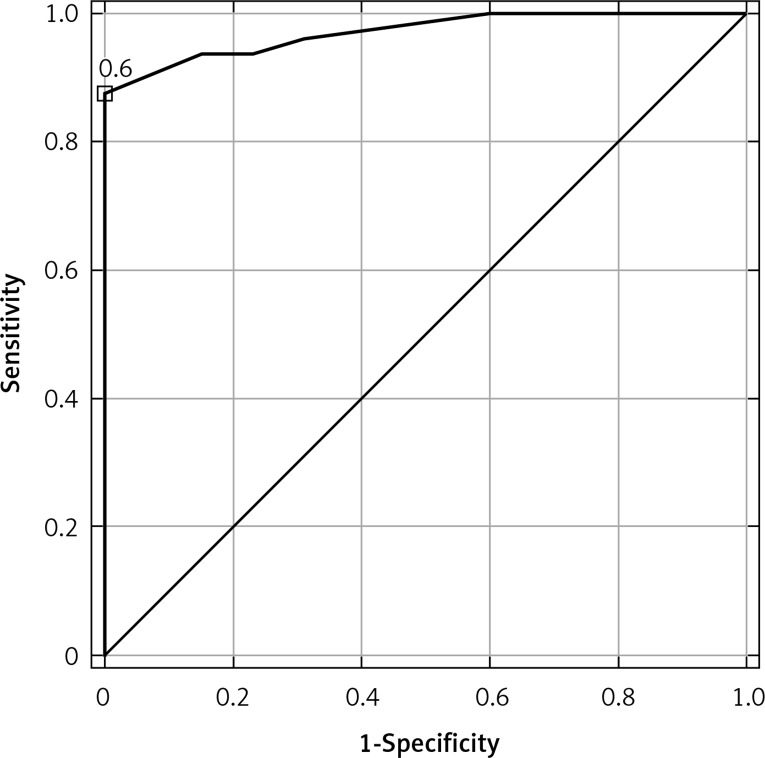
Using ROC curves, sensitivity and specificity were calculated for disease presence for each possible threshold value. The optimal cut-off for CSF CXCL9 application was 42 pg/ml; the sensitivity and specificity were 98% and 100%, respectively. The optimal cut-off for serum CXCL9 application was 81 pg/ml; the sensitivity and specificity were 86% and 100%, respectively. The optimal cut-off for ICXCL9 application was 0.6; the sensitivity and specificity were 88% and 100%, respectively. The CSF CXCL9 ROC AUC (0.998) was higher than the AUCs for serum CXCL9 (0.939) and ICXCL9 (0.970) (Figures 1–2).
Figure 1.
Area under ROC curves for CSF CXCL9 (AUC = 0.998; cut-off: 42 pg/ml) and for serum CXCL9 (AUC = 0.939; cut-off: 81 pg/ml) in differentiation between TBE patients and controls
Figure 2.
Area under ROC curve for ICXCL9 (AUC = 0.970; cut-off: 0.6) in differentiation between TBE patients and subjects and controls
Discussion
This is the first study to evaluate the CSF and serum levels of CXCL9 and CXCR3 in subjects with TBE compared to subjects with no signs of CNS disorder. In this paper, we report a significant increase in CSF and serum concentrations of CXCL9 in patients with TBE as compared to the controls without inflammation in the CNS. Furthermore, this alteration was also observed in the case of ICXCL9; moreover, ICXCL9 significantly decreased after symptoms resolution. CSF CXCR3 concentrations in TBE groups were above the assay upper limit; in serum specimens CXCR3 concentrations were within the assay range only in three TBE cases.
Chemokines and their receptors are constitutively present in the brain in glial cells and neurons, take part in intercellular communication, and have a pivotal role during CNS diseases [8, 11–13]. Physiologically, the CNS is an immune-privileged site because of the highly selective blood-brain barrier (BBB), separating the brain from the circulating blood. The disease state leads to the activation of microglia and consequently to neuronal and glial cell injury as well as death through chemokine signaling. These events lead to migration of immune cells across the BBB [12].
Some authors hypothesize that immune-mediated neuronal cell death mediated by the T-cell response in the CSF leads to the brain damage and dysfunction in TBE [4]. Two-thirds of patients with TBE have moderate pleocytosis (100 leucocytes/μl or fewer) [4, 26–28], which was also confirmed by our study. The study of Tomažič et al. and Holub et al. showed that the majority of cells present in the CSF are T lymphocytes; only a small number are B lymphocytes and natural killer (NK) cells [29, 30]. In the brains of fatal human TBE cases, macrophages, CD8+ cells, and CD4+ cells were present [31–33].
CXCL9 overexpression, confirmed by the measurement of mRNA or concentrations of CXCL9, has been observed in different CNS diseases, both in a rodent model and in humans [34–36]. Moreover, Ochiai et al. observed lower numbers of CD(4+) and CD(8+) T cells isolated from brains of severe combined immunodeficient (SCID) mice infected with Toxoplasma gondii treated with anti-CXCL9 serum as compared to mice treated with control serum. This may indicate a strong role of CXCL9 in recruiting immune T cells into the brain. Accumulation of CD(3+) T cells into the sites of tachyzoite growth was also markedly less in SCID mice treated with anti-CXCL9 serum as compared to mice treated with control sera, which indicates the role of CXCL9 in preventing reactivation of infection [37].
CXCL9 is constitutively expressed on human brain-derived microvascular endothelial cells and astrocytes [11]. In our study, CSF and serum CXCL9 concentrations were significantly higher as compared to the controls without inflammation in the CNS, which indicates that this protein might be involved in TBE pathogenesis. It should be noted that CXCL9 is strongly induced by IFN-γ – the most typical Th1 cytokine [11, 18].
Three chemokines that lack an ELR (Glu-Leu-Arg), and are more closely related to each other than to any other chemokine, are CXCL9, CXCL10, and CXCL11 [18]. Zajkowska et al. reported that CSF CXCL10 and CXCL11 concentrations were significantly higher in the acute phase of TBE as compared to the controls; after 3 weeks of follow-up CSF CXCL10 and CXCL11 concentrations were lower than in TBE patients in the acute phase, but still remained higher than in controls [24]. In our study we observed similar alteration in the case of CXCL9 – the third chemokine that lacks an ELR motif. Modifications in CSF CXCL9 concentrations depending on the CNS disease severity caused by different viruses were also found by other authors [35, 36]. Sato et al. found a strong correlation between CSF CXCL9 concentrations and disease progression in patients infected with human T-lymphotropic virus type 1 (HTLV-1). It should be mentioned that about 4% of HTLV-1 infected individuals develop HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [35]. Also Wang et al. observed different CSF CXCL9 concentrations according to the disease severity in patients with brainstem encephalitis (BE) caused by Enterovirus 71 (EV71). Their study showed increased CSF CXCL9 concentrations in patients with cardiopulmonary complications, including pulmonary edema and autonomic nervous system dysregulation, as compared to the uncomplicated BE [36]. In our study CXCL9 concentrations were also higher in CSF than in serum, which might support the hypothesis that in TBE CXCL9 is synthesized intrathecally and might be recognized as a biomarker of TBE.
To exclude possible impairment of the blood-CSF barrier and/or BBB functions as potential sources of the alterations in CXCL9 concentrations, we related the CSF CXCL9 concentrations to the concentrations in serum. ICXCL9 in TBE I and TBE II were significantly higher as compared to the controls, and – in contrast to the CSF or serum CXCL9 concentrations – significantly decreased after 2 weeks. This might indicate that the CXCL9 index is a better indicator of symptoms resolution in TBE than CSF or serum CXCL9 concentrations. Comparative analysis of CXCL9 concentrations depending on the disease severity in patients with viral BE was performed by Wang et al. The authors observed that the CSF as well as plasma CXCL9 index tended to increase with increasing BE severity [36]. This is in line with our study, in which the CXCL9 ratio was also higher in the acute phase of TBE.
Lepej et al. reported that the percentage of memory CD45RO+CD4+ T-cells expressing CXCR3 was significantly higher in the CSF of patients with acute neuroborreliosis (NB) as compared to the peripheral blood. Additionally, CXCL10 and CXCL11 concentrations in the CSF of NB patients were significantly higher as compared to the corresponding serum samples, which in their opinion suggests that the analyzed proteins create a chemokine gradient between the CSF and serum and recruit CXCR3-expressing memory CD4+ T-cells into the CSF [23]. In our study all CSF CXCR3 concentrations in the groups of patients with viral infection were above the upper limit of detection; in the case of serum CXCR3 concentrations were detected only in three patients. Moreover, CXCL9 concentrations were also higher in CSF than in serum, which might indicate that also in TBE CXCR3-expressing memory CD4+ T-cells are recruited to the CSF. However, this hypothesis requires further, more detailed analyses.
To assess the potential diagnostic significance of CXCL9, evaluation of the diagnostic criteria including the sensitivity, specificity, and the ROC AUCs was performed. We found that the CSF CXCL9 ROC AUC was higher than the AUCs for serum CXCL9 and ICXCL9. However, all AUCs were very high (≥ 0.94). This may suggest that evaluation of CXCL9 in CSF and serum has diagnostic significance in distinguishing patients with TBE from subjects with initially suspected but later, after CSF analysis, excluded CNS infection. The diagnostic usefulness (AUC > 0.8) of CXCL9 in distinguishing diagnosis of patients with HAM/TSP from control subjects (asymptomatic carriers infected with HTLV-1) was also confirmed by Sato et al., who selected CXCL9 as a promising prognostic candidate biomarker for the early identification of patients at increased risk of debilitating disease progression. The authors suggested that the measurement of CXCL9 could lead to more accurate prognoses as well as patient-dedicated treatment plans [35].
Our study has at least two limitations. First, the study group is small, which we tried to compensate by the viral IgM and IgG distinguishing serology between TBE and neuroborreliosis (NB) (north-east Poland is an endemic area of tick-transmitted diseases) [38]. Second, the potential specificity of the findings should be checked by the evaluation of CXCL9 concentrations as compared to the patients with other viral meningitis as well as bacterial meningitis, but this aspect requires further studies.
In conclusion, CXCL9 is a ligand for CXCR3, which was found on all Th1 memory lymphocytes present in the peripheral blood; therefore elevated concentrations of CXCL9 in TBE patients as compared to the controls might indicate that this chemokine perhaps takes part in the trafficking of Th1 cells into the CNS. The evaluation of CXCL9 revealed that it has diagnostic significance and seems to be a good biomarker for acute TBE. The results presented here support the hypothesis that CXCL9 may play a role in TBE. However, further studies are required to explain whether CXCL9 might be used as potential biomarker for the diagnosis and monitoring of inflammation in TBE.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Süss J. Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia – an overview. Ticks Tick Borne Dis. 2011;2:2–15. doi: 10.1016/j.ttbdis.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Gritsun TS, Lashkevich VA, Gould EA. Tick-borne encephalitis. Antivir Res. 2003;57:129–46. doi: 10.1016/s0166-3542(02)00206-1. [DOI] [PubMed] [Google Scholar]
- 3.Bogovic P, Lotric-Furlan S, Strle F. What tick-borne encephalitis may look like: clinical signs and symptoms. Travel Med Infect Dis. 2010;8:246–50. doi: 10.1016/j.tmaid.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Lindquist L, Vapalahti O. Tick-borne encephalitis. Handb Clin Neurol. 2008;371:1861–71. doi: 10.1016/S0140-6736(08)60800-4. [DOI] [PubMed] [Google Scholar]
- 5.Puchhammer-Stöckl E, Kunz C, Mandl CW, Heinz FX. Identification of tick-borne encephalitis virus ribonucleic acid in tick suspensions and in clinical specimens by a reverse transcription-nested polymerase chain reaction assay. Clin Diagn Virol. 1995;4:321–6. doi: 10.1016/0928-0197(95)00022-4. [DOI] [PubMed] [Google Scholar]
- 6.Mazur G, Jaskuła E, Kryczek I, et al. Proinflammatory chemokine gene expression influences survival of patients with non-Hodgkin’s lymphoma. Folia Histochem Cytobiol. 2011;49:240–7. doi: 10.5603/fhc.2011.0033. [DOI] [PubMed] [Google Scholar]
- 7.Bajetto A, Bonavia R, Barbero S, Florio T, Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol. 2001;22:147–84. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- 8.Banisadr G, Rostène W, Kitabgi P, Parsadaniantz SM. Chemokines and brain functions. Curr Drug Targets Inflamm Allergy. 2005;4:387–99. doi: 10.2174/1568010054022097. [DOI] [PubMed] [Google Scholar]
- 9.Guerreiro R, Santos-Costa Q, Azevedo-Pereira JM. The chemokines and their receptors: characteristics and physiological functions. Acta Med Port. 2011;24:967–76. [PubMed] [Google Scholar]
- 10.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002;42:469–99. doi: 10.1146/annurev.pharmtox.42.091901.115838. [DOI] [PubMed] [Google Scholar]
- 11.Müller M, Carter S, Hofer MJ, Campbell IL. Review: the chemokine receptor CXCR3 and its ligands CXCL9, CXCL10, and CXCL11 in neuroimmunity – a tale of conflict and conundrum. Neuropathol Appl Neurobiol. 2010;36:368–87. doi: 10.1111/j.1365-2990.2010.01089.x. [DOI] [PubMed] [Google Scholar]
- 12.Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013;2013:480739. doi: 10.1155/2013/480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rostène W, Kitabgi P, Parsadaniantz SM. Chemokines: a new class of neuromodulator? Nat Rev Neurosci. 2007;8:895–903. doi: 10.1038/nrn2255. [DOI] [PubMed] [Google Scholar]
- 14.Sorce S, Myburgh R, Krause KH. The chemokine receptor CCR5 in the central nervous system. Prog Neurobiol. 2011;93:297–311. doi: 10.1016/j.pneurobio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 16.Bendall L. Chemokines and their receptors in disease. Histol Histopathol. 2005;20:907–26. doi: 10.14670/HH-20.907. [DOI] [PubMed] [Google Scholar]
- 17.Rupprecht TA, Koedel U, Muhlberger B, Wilske B, Fontana A, Pfister HW. CXCL11 is involved in leucocyte recruitment to the central nervous system in neuroborreliosis. J Neurol. 2005;252:820–3. doi: 10.1007/s00415-005-0752-9. [DOI] [PubMed] [Google Scholar]
- 18.Clark-Lewis I, Mattioli I, Gong JH, Loetscher P. Structure-function relationship between the human chemokine receptor CXCR3 and its ligands. J Biol Chem. 2003;278:289–95. doi: 10.1074/jbc.M209470200. [DOI] [PubMed] [Google Scholar]
- 19.Park MK, Amichay D, Love P, et al. The CXC chemokine murine monokine induced by IFN-gamma (CXC chemokine ligand 9) is made by APCs, targets lymphocytes including activated B cells, and supports antibody responses to a bacterial pathogen in vivo. Immunol. 2002;169:1433–43. doi: 10.4049/jimmunol.169.3.1433. [DOI] [PubMed] [Google Scholar]
- 20.Lian JQ, Yang XF, Zhao RR, et al. Expression profiles of circulating cytokines, chemokines and immune cells in patients with hepatitis B virus infection. Hepat Mon. 2014;14:e18892. doi: 10.5812/hepatmon.18892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon KC, Park CS, You IC, et al. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010;51:643–50. doi: 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flier J1, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<397::aid-path899>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 23.Lepej SZ, Rode OD, Jeren T, Vince A, Remenar A, Barsić B. Increased expression of CXCR3 and CCR5 on memory CD4+ T-cells migrating into the cerebrospinal fluid of patients with neuroborreliosis: the role of CXCL10 and CXCL11. J Neuroimmunol. 2005;163:128–34. doi: 10.1016/j.jneuroim.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Zajkowska J, Moniuszko-Malinowska A, Pancewicz SA, et al. Evaluation of CXCL10, CXCL11, CXCL12 and CXCL13 chemokines in serum and cerebrospinal fluid in patients with tick borne encephalitis (TBE) Adv Med Sci. 2011;56:311–7. doi: 10.2478/v10039-011-0033-z. [DOI] [PubMed] [Google Scholar]
- 25.Lalkhen AG, McCluskey A. Clinical tests: sensitivity and specificity. 2014. Downloaded from http://ceaccp.oxfordjournals.org/ at Uniwersytet Medyczny w Bialymstoku on April 16, 2014.
- 26.Kaiser R. The clinical and epidemiological profile of tick-borne encephalitis in southern Germany 1994-98: a prospective study of 656 patients. Brain. 1999;122:2067–78. doi: 10.1093/brain/122.11.2067. [DOI] [PubMed] [Google Scholar]
- 27.Mickiene A, Laiskonis A, Günther G, Vene S, Lundkvist A, Lindquist L. Tick-borne encephalitis in an area of high endemicity in Lithuania: disease severity and long-term prognosis. Clin Infect Dis. 2002;35:650–8. doi: 10.1086/342059. [DOI] [PubMed] [Google Scholar]
- 28.Günther G, Haglund M, Lindquist L, Forsgren M, Sköldenberg B. Tick-bone encephalitis in Sweden in relation to aseptic meningoencephalitis of other etiology: a prospective study of clinical course and outcome. J Neurol. 1997;244:230–8. doi: 10.1007/s004150050077. [DOI] [PubMed] [Google Scholar]
- 29.TomaŽič J, Ihan A. Flow cytometric analysis of lymphocytes in cerebrospinal fluid in patients with tick-borne encephalitis. Acta Neurol Scand. 1997;95:29–33. doi: 10.1111/j.1600-0404.1997.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 30.Holub M, Klucková Z, Beran O, Aster V, Lobovská A. Lymphocyte subset numbers in cerebrospinal fluid: comparison of tick-borne encephalitis and neuroborreliosis. Acta Neurol Scand. 2002;106:302–8. doi: 10.1034/j.1600-0404.2002.01314.x. [DOI] [PubMed] [Google Scholar]
- 31.Bardina SV, Lim JK. The role of chemokines in the pathogenesis of neurotropic flaviviruses. Immunol Res. 2012;54:121–32. doi: 10.1007/s12026-012-8333-3. [DOI] [PubMed] [Google Scholar]
- 32.Gelpi E, Preusser M, Garzuly F, Holzmann H, Heinz FX, Budka H. Visualization of Central European tick-borne encephalitis infection in fatal human cases. J Neuropathol Exp Neurol. 2005;64:506–12. doi: 10.1093/jnen/64.6.506. [DOI] [PubMed] [Google Scholar]
- 33.Gelpi E, Preusser M, Laggner U, et al. Inflammatory response in human tick-borne encephalitis: analysis of postmortem brain tissue. J Neurovirol. 2006;12:322–7. doi: 10.1080/13550280600848746. [DOI] [PubMed] [Google Scholar]
- 34.Wen X, Kudo T, Payne L, Wang X, Rodgers L, Suzuki Y. Predominant interferon-gamma-mediated expression of CXCL9, CXCL10, and CCL5 proteins in the brain during chronic infection with Toxoplasma gondii in BALB/c mice resistant to development of toxoplasmic encephalitis. J Interferon Cytokine Res. 2010;30:653–60. doi: 10.1089/jir.2009.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato T, Coler-Reilly A, Utsunomiya A, et al. CSF CXCL10, CXCL9, and neopterin as candidate prognostic biomarkers for HTLV-1-associated myelopathy/tropical spastic paraparesis. PLoS Negl Trop Dis. 2013;7:e2479. doi: 10.1371/journal.pntd.0002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang SM, Lei HY, Yu CK, Wang JR, Su IJ, Liu CC. Acute chemokine response in the blood and cerebrospinal fluid of children with enterovirus 71-associated brainstem encephalitis. J Infect Dis. 2008;198:1002–6. doi: 10.1086/591462. [DOI] [PubMed] [Google Scholar]
- 37.Ochiai E, Sa Q, Brogli M, et al. CXCL9 is important for recruiting immune T cells into the brain and inducing an accumulation of the T cells to the areas of tachyzoite proliferation to prevent reactivation of chronic cerebral infection with Toxoplasma gondii. Am J Pathol. 2015;185:314–24. doi: 10.1016/j.ajpath.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grygorczuk S, Mierzynska D, Zdrodowska A, et al. Tick-borne encephalitis in north-eastern Poland in 1997-2001: a retrospective study. Scand J Infect Dis. 2002;34:904–9. doi: 10.1080/0036554021000026979. [DOI] [PubMed] [Google Scholar]