Abstract
Prevalence of obesity, an established risk factor for many cancers, has increased dramatically over the past 50 years in the United States and across the globe. Relative to normoweight cancer patients, obese cancer patients often have poorer prognoses, resistance to chemotherapies, and are more likely to develop distant metastases. Recent progress on elucidating the mechanisms underlying the obesity-cancer connection suggests that obesity exerts pleomorphic effects on pathways related to tumor development and progression, and thus there are multiple opportunities for primary prevention and treatment of obesity-related cancers. Obesity-associated alterations, including systemic metabolism, adipose inflammation, growth factor signaling and angiogenesis, are emerging as primary drivers of obesity-associated cancer development and progression. These obesity-associated host factors interact with the intrinsic molecular characteristics of cancer cells, facilitating several of the hallmarks of cancer. Each is considered in the context of potential preventive and therapeutic strategies to reduce the burden of obesity-related cancers. Additionally, this review focuses on emerging mechanisms behind the obesity-cancer link as well as relevant dietary interventions, including calorie restriction, intermittent fasting, low-fat diet, and ketogenic diet, that are being implemented in preclinical and clinical trials, with the ultimate goal of reducing incidence and progression of obesity-related cancers.
Keywords: obesity, cancer, risk biomarkers, calorie restriction, ketogenic diet
Introduction
Over the past half century in the United States the prevalence of obesity, defined as body mass index (BMI) of 30 kg/m2 or greater, has tripled with nearly 40% of adults and 20% of children burdened by the disease.1 Not only epidemic to the United states, more than 600 million adults are obese worldwide with and additional 2.1 billion are considered overweight.2 Increasing prevalence of obesity signifies a major public health problem as obesity increases risk of several chronic diseases and comorbidities including type II diabetes (T2DM), cardiovascular disease (CVD), hypertension, chronic inflammation and, as discussed in this review, many types of cancer.3
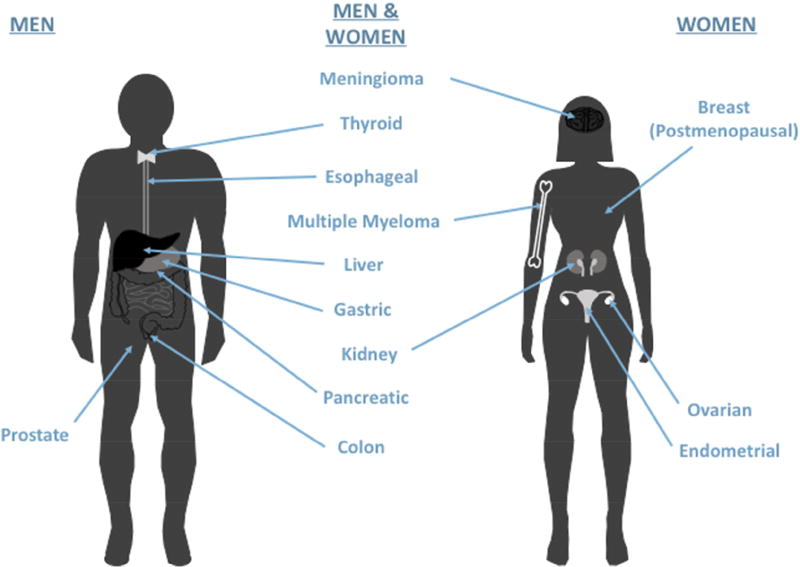
As illustrated in Figure 1, and based on the recent report from the International Agency for Research on Cancer, risk of 13 distinct cancer types is increased with excess body fatness: breast (in postmenopausal women), ovarian, liver, gallbladder, kidney, colon, pancreatic, gastric, esophageal, endometrial, thyroid, multiple myeloma, and meningioma.4 Overall, an estimated 13% of incident cases worldwide, and approximately 20% of incident cases in Europe and North America, are attributable to obesity.5 Aside from higher risk of developing cancer, obese individuals are more likely to have reduced response to anticancer therapies,6 and obesity is implicated in approximately 20% of all cancer-related mortalities.7 This includes prostate cancer, for which obesity increases progression but not incidence.8 Here, we discuss (with a focus on developing mechanism-based intervention strategies) mechanisms through which obesity affects normal tissue homeostasis and cancer development and/or progression, including alterations in systemic metabolism, growth factor signaling, inflammation and angiogenesis.
Figure 1.
Obesity is associated with increased risk of developing and dying from the following cancers: breast (in postmenopausal women), ovarian, liver, gallbladder, kidney (renal cell), colon, pancreatic, gastric, esophageal (adenocarcinoma), endometrial, thyroid, multiple myeloma, and meningioma. In addition, obesity is associated with progression (but not incidence) of prostate cancer.
Methods
A traditional literature review was performed to describe the multiple mechanisms underlying the obesity-cancer link, as well as dietary interventions targeting those mechanisms for primary cancer prevention and treatment. Searches were completed using PubMed and Google Scholar. A variety of key words were searched including obesity, metabolic reprogramming, secretome, cell signaling, inflammation, adipose, angiogenesis, cancer prevention, cancer treatment, calorie restriction, intermittent fasting, low fat diet and ketogenic diet.
Obesity-Associated Systemic Alterations Impact the Hallmarks of Cancer
Hanahan and Weinberg identified essential biological capabilities acquired by all cancer cells during the multistep development of tumors in their classic article titled “The Hallmarks of Cancer”9 first published in 2000 and updated in their 2011 “Hallmarks of Cancer: the Next Generation.”10 These essential aberrations of cancer cells are sustaining proliferative signaling, insensitivity to anti-growth signals, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating processes related to invasions and metastasis. Conceptual progress in the decade between these two articles led to identification of additional hallmarks: deregulation of cellular energy metabolism, evading immune destruction, tumor-promoting inflammation and genomic instability. Extensive research on the obesity-cancer link supports the concept of obesity-associated systemic alterations, including 1) obesity-associated metabolic reprograming, 2) dysregulation of growth factor signaling, 3) adipose tissue inflammation and 4) induction of angiogenesis, facilitating several hallmarks of cancer.11
Obesity-Associated Metabolic Reprogramming
Metabolic reprogramming has long been known as a feature of cancer cells since the ‘Warburg effect,’ whereby cancer cells readily utilize glycolysis under normoxic conditions, was first described in 1924.12 Increased attention in the last two decades has led to inclusion of metabolic reprogramming as a true hallmark of cancer cells.10 Cancer cells can alter their metabolism including glycolytic, mitochondrial, and anapleurotic pathways to adapt to changing environments13,14 and progress to more aggressive disease.15–18 Many of these pathways converge on the tricarboxylic acid(TCA) cycle, with β-oxidation of fatty acids generating acetyl-CoA, glutaminolysis producing α-ketoglutarate and pyruvate being converted to oxaloacetate.13 Selective pressure to proliferate in cancer cells drives these intermediates to be shuttled from the mitochondria and utilized as precursors for synthetic pathways including lipid, nucleotide and amino acid synthesis, rather than ATP production via the electron transport chain.13,19 As a result, cancer cells may become more reliant on glucose for ATP generation, and amino acids and fatty acids for TCA intermediates. Overnutrition increases provisions of - glucose and fat, all of which can feed into this metabolic reprogramming to fuel cancer cell proliferation. Moreover, glycolysis has been shown to be enhanced in cancer cells in the context of obesity.20,21 In addition, obesity is often associated with metabolic syndrome and diabetes,22,23 conditions characterized by hyperglycemia and/or hypertriglyceridemia,24 providing ample circulating nutrients to a developing tumor, even between feeding periods.25 Both elevated serum glucose and triglyceride26,27 levels have been associated with increased cancer risk.
In many cancers autophagy forms part of metabolic reprogramming. Autophagy. the process in which cells digest and recycle their cellular contents during low nutrient availability, can provide cancer cells with lipids, amino acids and nucleotides needed for proliferation.28 Recent studies have shown high dependence on autophagy for tumor microenvironment cells that adopt senescence-associated secretory phenotype, which supports neighboring cancer cells through provision of digested contents, growth factors and inflammatory factors.29,30 Obesity has been shown to induce autophagy, particularly in adipocytes.31–33 Inhibition of autophagy combined with calorie restriction has been shown to reduce tumor growth.34
These obesity-associated metabolic adaptations facilitate the development of cancer hallmarks including insensitivity to anti-growth signals, resistance to cell death, and deregulation of cellular energetics.11 Interactions between cancer cell energetics and systemic metabolism highlight novel therapeutic strategies and interventions, particularly in obese individuals, as cancer cells may be more sensitive to metabolic perturbation, having already committed to a metabolic reprogram.
Dysregulation of Growth Factor Signaling
As depicted in Figure 2, obesity and metabolic syndrome are associated with aberrations in insulin signaling, growth factor signaling, and glucose metabolism.35 One growth factor implicated in cancer risk and progression is insulin-like growth factor (IGF)-1. Produced primarily following growth hormone stimulation in the liver, IGF-1 functions as a regulator of growth and developmental processes.36 IGF binding proteins bind circulating IGF-1 and limit its bioavailability to bind to its receptor and initiate downstream signals promoting growth and/or survival.37 Hyperglycemia and hyperinsulinemia, hallmarks of metabolic syndrome, increase IGF-1 production and bioavailability. Hyperglycemia suppresses IGF-1 binding protein synthesis and hyperinsulinemia promotes expression of growth hormone receptor and subsequent IGF-1 synthesis.35 Growth and survival functions of IGF-1 give it the potential to impact many hallmarks of cancer, including sustained proliferative signaling, insensitivity to anti-growth signals, induction of angiogenesis and metastatic potential.38 As a result, elevated IGF-1 is established as a risk factor for multiple cancer types including breast, prostate, lung and colorectal.37,39
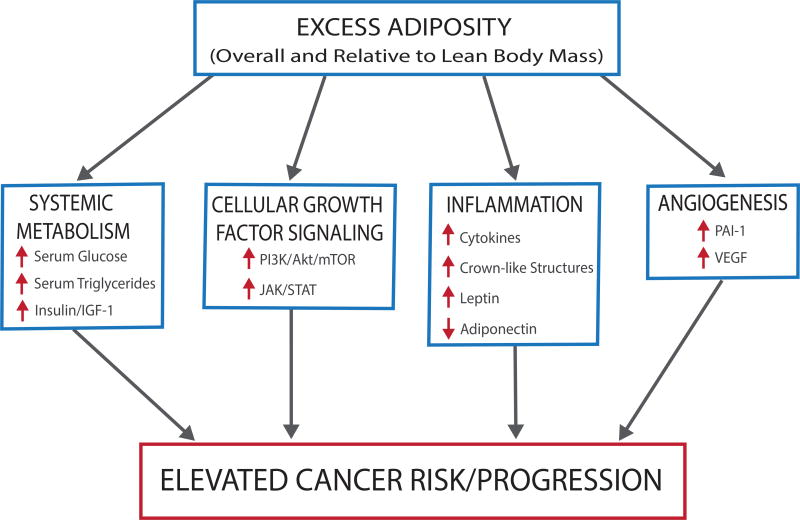
Figure 2.
Expansion of adipose tissue depots characteristic of obesity results in many metabolic disturbances including altered systemic metabolism and increased growth factor, inflammatory and angiogenic signaling. These obesity-associated perturbations foster a microenvironment favorable for tumorigenesis, facilitating many of the hallmarks of cancer and increasing disease risk and progression.
In response to elevated blood glucose levels, pancreatic β-cells release insulin, a peptide hormone that stimulates peripheral uptake of glucose, glucose metabolism, and energy storage pathways. IGF-1 receptor and insulin receptor stimulate the same downstream activation of phosphoinositide 3-kinase (PI3K)/Akt pathway (Figure 2), a pathway frequently altered in epithelial cancers.40 In response to these growth factors and nutrient availability, PI3K/Akt produces lipid messengers that initiate Akt signaling,40 activating mammalian target of rapamycin (mTOR)-associated signaling cascade which promotes cell growth, proliferation and survival.41 Oncogenic signals or loss of tumor suppressors can also activate mTOR signaling, while low nutrient conditions activate AMP-activated protein kinase (AMPK), an energy responsive pathway that inhibits mTOR.42 Obesity-induced activation of mTOR can contribute to several hallmarks of cancer including: sustained proliferative signaling, insensitivity to anti-growth signals, induction of angiogenesis, and activation of processes related to invasion and metastasis.43 In preclinical models, blocking mTOR signaling with drugs such as rapamycin (mTOR inhibitor)44–46 and metformin (AMPK activator),46–48 block tumor-enhancing effects associated with the obese phenotype.49 Interestingly, rapamycin has exhibited anti-inflammatory attributes, attenuating inflammation as well as tumor promotion, suggesting crosstalk between mTOR-related growth and survival signals and inflammatory signals.50
Adipose Tissue Inflammation
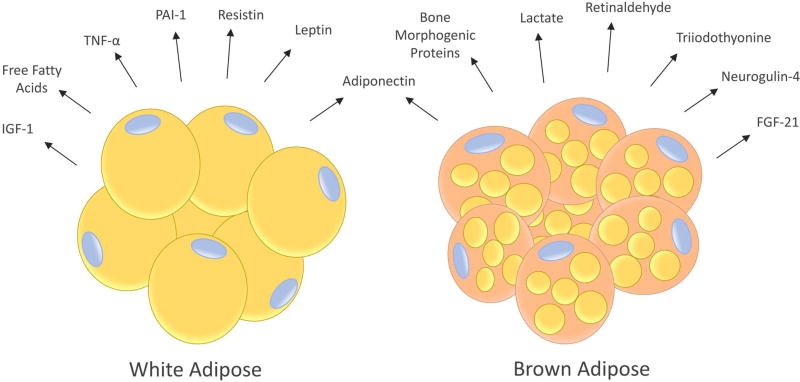
Mammals, including humans, have 2 major fat depots: subcutaneous and visceral (intra-abdominal). These adipose depots contain white adipose tissue (WAT) that stores energy in the form of triacylglycerol and brown adipose tissue (BAT) that dissipates energy by burning fatty acids to generate heat. WAT and BAT have important differences in their morphology, metabolism and transcriptional profiles. White adipocytes have few mitochondria, low oxidative rate, and contain an unilocular lipid droplet comprised primarily of triacylglycerol, while brown adipocytes have a high number of mitochondria (hence the darker appearance), high rate of fatty acid and glucose uptake and oxidation, and possess multilocular lipid droplets.51 Moreover, the secretome of white versus brown adipocytes differs markedly (Figure 3); the former is characterized by secretion of leptin, resistin, PAI-1, inflammatory cytokines, and FFA, while the latter is characterized by secretion of bone morphogenetic proteins, lactate (which induces uncoupling proteins), retinaldehyde, triiodothyonine (T3) and other factors associated with response to cold stress and/or increased energy expenditure.51 Brown adipocytes also produce adiponectin (but not leptin) and fibroblast growth factor-21, which can be anti-inflammatory and insulin sensitizing.51 WAT contains a number of stromal cells including pre-adipocytes, vascular cells, fibroblasts and a host of immune cells such as adipose tissue macrophages.52 Obesity increases WAT mass which drives chronic inflammation through altered adipokine and hormone signaling, generation of crown-like structures, and adipose remodeling and ectopic lipid infiltration to other tissues, as described below. Adipose tissue derived inflammation results in the secretion of a variety of signaling molecules and activation of gene expression programs that facilitate several of the hallmarks of cancer including sustained proliferative signaling, activation of programs related to invasion and metastasis, induction of angiogenesis, promotion of genome instability, and evasion of immune destruction.11
Figure 3.
The human body contains two types of adipocytes: white adipocytes (which have a unilocular lipid droplet) and brown adipocytes (which have many small lipid droplets). When engorged with triglyceride, white adipocytes secrete a number of factors that promote growth factor signaling and inflammation including leptin, resistin, insulin-like growth factor (IGF)-1, free fatty acids, tumor necrosis factor (TNF)-α and interleukin (IL)-6. Additionally, they reduce production of anti-inflammatory adiponectin. Brown adipocytes secrete several factors involved in thermogenesis, decreased inflammation, normalized insulin sensitivity and/or increased energy expenditure such as adiponectin, bone morphogenetic proteins, neuregulin-4, lactate, triiodothyronine (T3), retinaldehyde, and fibroblast growth factor (FGF)-21.
1. Altered Adipose Secretome
Leptin, an energy-responsive peptide hormone produced by adipocytes, is positively correlated with adiposity. Through signaling to the hypothalamus, leptin decreases hunger cues, food intake and weight gain.53 Leptin release from adipocytes is stimulated by a variety of factors including insulin, TNFα, glucocorticoids, and estrogen.53 In obesity, leptin is overproduced by adipocytes, reducing hypothalamic sensitivity to the signal.54 Circulating leptin binds to receptors in central nervous system and peripheral tissues, regulating processes including energy homeostasis, cytokine production, immune function, and carcinogenesis.53,55 The leptin receptor OB-R, classified as a class I cytokine receptor, gives leptin the ability to activate signal transducer and activator of transcription (STAT) family transcription factors, resulting in initiation of STAT-induced transcription programs for proliferation, cell growth and survival, migration and differentiation.56 Deregulation of STATs activity is often observed in cancer.57
Adiponectin, another peptide hormone secreted from adipocytes, functions as an energy sensor that promotes energy intake and insulin sensitivity,58 opposing the functions of leptin. Although the most abundant hormone secreted from the WAT,59 adiponectin levels are negatively correlated with adiposity and release is stimulated during energy deficit.60 Adiponectin opposes obesity-associated metabolic alterations through regulating glucose metabolism, increasing insulin sensitivity and fatty acid oxidation, and reducing IGF-1 signaling through activation of AMPK.61 Adiponectin also attenuates inflammation through inhibition of nuclear factor kappa-light-chain-enhancer of B cells (NF-κB), which reduces expression of proinflammatory cytokines while increasing expression of anti-inflammatory cytokines.62 Due to the anticancer functions of adiponectin, adiponectin agonists are emerging as possible chemotherapeutic agents, particularly for obesity-related cancers.63 While individually these adipokines have established associations with cancer risk, the leptin to adiponectin ratio is increasingly considered a more sensitive measure in evaluating cancer risk.64
Sex hormones, including estrogen, androgens and progestogens, regulate a variety of growth and developmental processes including weight homeostasis.65 Although the ovaries and testes are the major sites for sex hormone production, adipose tissue can significantly impact synthesis as adipose tissue expresses sex-steroid-metabolizing enzymes, including aromatase, which convert androgens into estrogens.65 Excess adipose tissue becomes a major site of estrogen production in obesity.66 In postmenopausal women, BMI is positively correlated with estrone, estradiol, and free estradiol.67 Elevation of estrogens is also detected in obese men;68,69 however, testosterone levels are significantly reduced.70 Circulating estrogens bind to one of two estrogen receptors (ER), ERα or ERβ. Once bound, receptors dimerize and translocate to the nucleus and bind to DNA or other transcription factors, influencing gene expression profiles that regulate growth, proliferation and differentiation.71 In the context of cancer, the two receptors have differing roles. ERα is mitogenic and an established target in treatment of estrogen receptor-positive breast cancer, while ERβ is suggested to be tumor suppressive.72 Obesity and postmenopausal status increase risk of ER-positive breast cancers compared with ER-negative breast cancer.73 Due to the positive association between obesity, circulating estrogen and risk of ER-positive breast cancer, aromatase inhibitors and ER antagonist tamoxifen are effective treatment therapies.74 In addition to breast cancer,67,68,75 elevated estrogen levels are associated with increased risk of ovarian76 and endometrial cancers.77 In prostate cancer, sex hormone levels are associated with disease progression, not disease risk.78 Low levels of circulating testosterone correlates with aggressive disease progression.79 Moreover, sex hormones have been implicated in risk and/or progression of colorectal and lung cancers.80
2. Crown-Like Structures
Obesity drives subclinical inflammation in visceral and subcutaneous WAT, characterized by crown-like structures, or rings of activated macrophages surrounding engorged or necrotic adipocytes. This adipocyte-macrophage interaction results in a proinflammatory secretome from both cell types, activating the cellular transcription factor NF-kB, increasing levels of cytokines and other inflammatory factors, and triggering inflammation.81
3. Adipose Remodeling and Lipid Infiltration in Other Tissues
During low nutrient availability or increased energy needs, glucagon secretion stimulates lipolysis of adipocytes, releasing FFA into the blood stream.82 Circulating FFA can then be utilized by peripheral tissues, providing substrate for β-oxidation and serving as intermediates for energy production through TCA cycle and oxidative phosphorylation. Conversely, overnutrition remodels existing adipose tissue, expanding adipocyte number and size, and altering adipokine secretion, FFA flux, and adipocyte death.83 In response, adipose stromal cells modify their functions to promote clearance of necrotic adipocytes and generation of new adipocytes and vasculature. Chronic overnutrition or obesity-induced tissue remodeling, results in sustained, low-grade inflammation and metabolic alterations.83 As stated above, cancer cells adapt to changing energy needs for proliferation through metabolic reprogramming, increasing anaerobic metabolism and shunting TCA cycle intermediates to synthetic pathways.10,84 Production of daughter cells demands increased levels of FFA for formation of lipid bilayers, thus excess WAT promotes proliferation of tumor cells through provision of circulating FFA85,86
When WAT depots reach capacity, excess lipids are deposited in organs such as the muscle, liver or pancreas, further complicating metabolism.87 Ectopic lipid intermediates exert lipotoxic effects, impairing cellular organelle functions, releasing inflammatory cytokines, and fostering development of insulin resistance.88 Consequently, individuals can develop muscle dysfunction and hepatic and pancreatic steatosis, all of which have been positively correlated with insulin resistance and impaired lipid metabolism.87
Nonalcoholic fatty liver disease, diagnosed as >5–10% liver fat content by weight in the absence of alcohol use or other liver disease, encompasses a variety of liver diseases including simple steatosis, nonalcoholic steatohepatitis (NASH) and cirrhosis.89 One of the most common chronic diseases,90–92 nonalcoholic fatty liver disease is present in 65–85% of obese patients89,93 with rapidly rising incidence among adults and children.91,94 Excess lipid accumulation in the liver, induces production of reactive oxygen species, activation of pro-inflammatory programs, and endoplasmic reticular stress, impairing function of cellular organelles and potentially inducing hepatic cell death.95 Additionally, accumulation of lipids and pro-inflammatory cytokines promotes activation of intracellular kinases, leading to impaired insulin signaling and development of insulin resistance.96 While simple steatosis is benign, NASH is more detrimental, characterized by liver injury, inflammation and/or fibrosis. NASH can further result in the development of cirrhosis, liver failure, and hepatocellular carcinoma.97
Ectopic deposition of adipocytes in the pancreas is hypothesized to be a mechanism behind obesity-associated pancreatic dysfunction.98,99 Infiltrating fat in the pancreas has been associated with increased BMI, visceral WAT mass, insulin resistance and pancreatic exocrine dysfunction.98–101 These endocrine alterations further complicate the complex metabolic and inflammatory perturbations characterized in obesity and metabolic syndrome and can trigger the development of pancreatic steatosis, pancreatitis and/or nonalcoholic fatty pancreatic disease, all established risk factors for pancreatic cancer.100,101
Induction of Angiogenesis
As adipose tissue depots expand in obesity, the existing vasculature must expand to meet demand. This outgrowth of new blood vessels is termed angiogenesis. Key mediators of this process include VEGF and PAI-1. VEGF, a potent angiogenic factor that is produced by adipocytes and tumor cells, acts on endothelial cells stimulating mitogenic and vascular permeability-enhancing activities.102 Obesity is associated with increased circulating VEGF, and elevated VEGF correlates with poor prognosis for many obesity-related cancers.103 PAI-1, another angiogenic factor produced by adipocytes, endothelial cells, and stromal cells in visceral WAT,104 is frequently elevated in obese subjects. Increased circulating PAI-1 is associated with increased risk of other chronic diseases including CVD, T2DM and a number of cancers.104 While interaction of angiogenic factors with proximal endothelial cells induce formation of local blood vessels, providing a route for oxygen and nutrient delivery and waste removal, these factors can also interact with peripheral tissues, facilitating angiogenesis, and potentially promoting progression at tumor sites. These newly formed blood vessels could provide primary tumor mass with oxygen and nutrients to sustain proliferation and survival as well as a route for metastasis to distant sites. PAI-1 functionally inhibits plasminogen activators, thus regulating extracellular matrix integrity.105 Extracellular matrix remodeling is a key feature of invasive disease, and integral in the development of metastatic lesions.106 Due to the antitumorigenic potential of factors that modulate angiogenesis, targeted drugs have been developed. However, caution should be advised in administration of anti-angiogenic treatments in obese patients, as these drugs can induce hypoxia in primary tumors, potentially encouraging metastasis, already a concern in the obese population.106
Elevation of these factors may also impact efficacy of treatment regimens, as excess circulating VEGF in obese patients contributes to reduced efficacy of anti-VEGF therapies (e.g. bevacizumab) compared with non-obese ovarian cancer patients.107
Emerging Mechanisms Linking Obesity and Cancer
Emerging research suggests other mechanisms including circadian rhythm108 and the microbiome109,110 influence the obesity-cancer link. Often referred to as the “body clock,” circadian rhythm regulates multiple physiological processes including secretion of key metabolic hormones leptin, adiponectin and insulin.111 Circadian rhythm has been linked to metabolic homeostasis, particularly lipogenic and adipogenic pathways, as well as cell cycle regulation. Consequently, disrupted circadian rhythm can lead to metabolic disorders, such as obesity,112 and increase cancer risk and progression.108,112 Preclinical models describe circadian rhythm as energy responsive. Consumption of a high fat diet results in disruption of the circadian rhythm, while dietary restriction and fasting can reset the circadian clock and improve metabolic health.111
Gut microbiome, or the community of commensal, symbiotic and pathogenic microorganisms that inhabit an individual’s gut, influences a number of chronic diseases including obesity. Obesity changes composition113 and diversity114 of the microbiota, shifting towards populations with enhanced ability to harvest dietary energy. These alterations have been linked to elevated systemic inflammation.115 Broad spectrum antibiotics, which alter microbiota composition, can completely prevent systemic inflammation resultant from high-fat diet feeding.116 Through this influence on obesity-induced inflammation, microbiota may contribute to the obesity-cancer link. While both of these fields are in their infancy, their existence highlights additional mechanisms that must be delineated to effectively understand and target the obesity-cancer link.
Dietary Interventions Targeting Obesity-Induced Alterations for Primary Cancer Prevention and Therapy
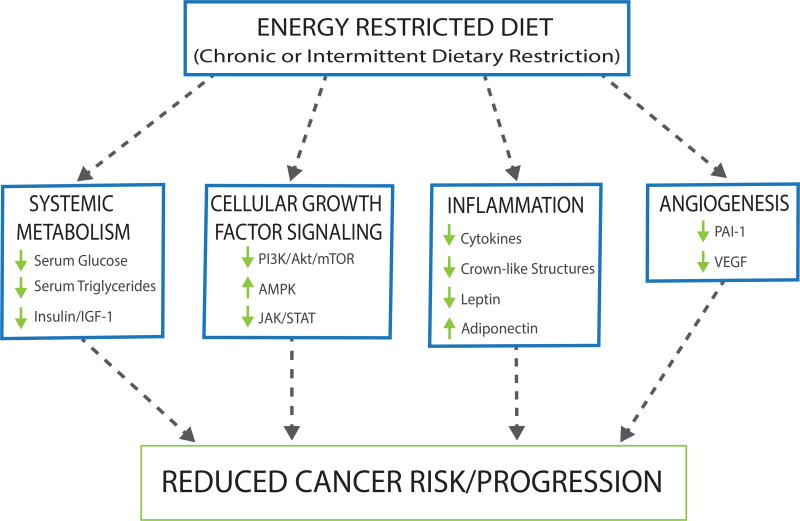
Given the multifaceted role of obesity in promoting a protumorigenic microenvironment that facilitates tumor development and progression, interventions are urgently needed to break the obesity-cancer link. To date, the only weight loss intervention in obese people consistently associated with reduced cancer risk is bariatric surgery.117 In light of the expense and complications inherent in surgical weight loss approaches, current efforts are focusing on reducing adiposity through dietary interventions. To achieve reductions in weight and adiposity these interventions have aimed to 1) promote negative energy balance through reduced energy intake via either calorie restriction (CR) or intermittent fasting (IF) or 2) modulate macronutrient distributions via implementation of low-fat diet (LFD) or a high-fat, ketogenic diet (KD). Preclinical and some clinical studies suggest that these interventions can favorably and inversely modulate cancer risk biomarkers, as discussed below. Modulation of these biomarkers could result in downstream reductions in growth factor signaling, inflammation, and angiogenesis, attenuating cancer risk and progression (Figure 4).
Figure 4.
Reductions in adiposity and weight attained from dietary interventions including caloric restriction (CR), intermittent fasting (IF), low-fat diet (LFD) and ketogenic diet (KD), have been shown to reduce adiposity and inversely modulate many of the same cancer risk biomarkers that are impacted by obesity and excess adiposity. Obesity-associated systemic alterations are reduced through these dietary interventions resulting in decreased circulating glucose, growth factor signaling, inflammation, and angiogenesis, attenuating cancer risk and progression. Metabolic alterations of CR and IF interventions have been associated with reduced cancer risk and progression. KD has not been linked to cancer risk in humans; however, it has been demonstrated that adherence to KD reduces cancer risk and progression in preclinical studies. Evidenced is mixed concerning implementation of a LFD, exhibiting little to no reduction in cancer risk and progression.
Calorie Restriction
Calorie restriction (CR), defined as reduction of dietary energy intake without malnutrition, is broadly effective dietary intervention that significantly decreases adiposity. Preclinical models demonstrate 30% CR, compared with ad libitum-fed control, ameliorates risk factors and delays onset of cancer through metabolic alterations fostering increased insulin sensitivity and decreased serum glucose, serum triglycerides, growth factor signaling, inflammation, oxidative stress and angiogenesis.118–122 These metabolic changes translate into significantly decreased cancer incidence in murine models.123 Due to long latency of cancer in humans, the literature does not have data linking CR directly with cancer incidence in humans. However, randomized control trials implementing long-term 20% CR in overweight human subjects have confirmed reduced adiposity, improved glucose homeostasis, increased adiponectin, and reduced leptin and inflammatory markers TNFα and C-reactive protein.124,125 Substantial weight loss of >10% may be necessary to consistently observe these benefits.126–128
Limited clinical studies exist on CR during cancer treatment. Direct application of CR in cancer patients is complicated by high rates of weight loss associated with cancer cachexia, a condition in which tumor-derived signals degrade muscle and adipose tissue. A recent trial combining CR and physical activity suggests presurgical weight loss is safe and feasible in prostate cancer patients.129 Moreover, preliminary clinical trials suggest that application of CR in combination with chemotherapy and/or radiation has potential to reduce risk biomarkers and increase responsiveness to treatment.130,131
Intermittent Fasting
Preclinical and clinical studies have begun to explore implementation of intermittent fasting (IF), which may be easier for most people to adopt and may have beneficial metabolic effects relative to chronic CR. Human trials most often study one of three IF regimens: alternate day fasting, alternate day energy restriction (~75%) or 2 consecutive days of 65% energy restriction, the latter often referred to as intermittent calorie restriction.132 Periods of IF stimulate reduced insulin and increased glucagon, resulting in increased lipolysis and fatty acid oxidation to provide alternate substrates for energy production. These metabolic alterations are accompanied by reductions in several cancer-related risk factors including reduced serum glucose, insulin resistance, inflammation, and circulating IGF-1.133 The impact of IF on angiogenesis in the context of cancer remains unexplored in currently published research. Preclinical studies with IF consistently exhibit a cancer preventative effect with reduced rates of tumor growth for multiple cancer types including lymphoma,134 breast, lung, ovarian, hepatic, and pancreatic.133,135,136 To our knowledge there is no published data on IF and cancer incidence in human subjects, although there are reports of favorable effects of IF in overweight humans, including improved adipokine ratios and reduced growth factor signaling and inflammatory markers,136,137 suggesting the reported preclinical anticancer effects of IF may be translatable to humans.
One IF regimen being examined as primary prevention strategy for breast cancer is the 5:2 diet which involves 5 days/week of a healthy diet, such as the Mediterranean diet, with two consecutive days of a low calorie, low carbohydrate diet. The Mediterranean diet is primarily a plant-based diet high in fruits, vegetables, whole grains, legumes and nuts. Compared to North American dietary patterns, the Mediterranean diet has been associated with better control of body weight, reduction of cancer risk biomarkers and decreased cancer incidence.138–142 The diet results in favorable modulation of inflammation, oxidative stress, and growth factor signaling. Combining a Mediterranean diet with 2 days of a very low calorie, low carbohydrate diet for one month in 24 obese women at high risk for breast cancer induced changes in breast tissue gene expression and metabolites associated with reduced risk of breast cancer.143
Regarding the effects of IF on cancer prognosis, a study by Safdie, et al suggests IF during cancer therapy may decrease adverse effects of chemotherapy. Ten cancer patients of varying cancer types (four breast, two prostate, one ovarian, one uterine, one small cell carcinoma of the lung, and one esophageal adenocarcinoma) voluntarily fasted prior to (48–140 hours) or following (5–56 hours) chemotherapy treatment. Compared with non-restricted control subjects, fasting reduced chemotherapy-induced side effects including fatigue, weakness and gastrointestinal side effects while exhibiting the same chemotherapy-induced reduction in tumor volume or biomarkers.144 Following this ground breaking study, others have implemented IF in small scale clinical trials including de Groot, S., et al., 2015, where short term IF among stage II/III breast cancer patients was well tolerated, reduced signs of hematological toxicity and stimulated faster recovery from DNA damage in normal host peripheral blood mononuclear cells.145 Limited preclinical findings suggest that IF may selectively protect healthy cells and make cancer cells more vulnerable to chemotherapeutic agents, reducing side-effects and increasing drug efficacy.133 More research is needed to confirm these findings and identify underlying mechanisms.
Low-fat Diet
Numerous clinical studies have examined the effects of low-fat diet (LFD) interventions on cancer risk. Clinical trials with risk biomarker end points demonstrate that adherence to LFD over a two-year period resulted in normalization of glucose metabolism and reduction in growth factor signaling as well as favorable modulation of adipokine levels.146 In the Women’s Health Initiative clinical trial, nearly 50,000 postmenopausal women were followed for an average of 8.1 years after random assignment to a LFD with high fruit, vegetable, and grain intake or a comparison group. Analyses of this study have indicated that a LFD does not decrease risk of invasive cancer.147,148 Further separated by cancer type, no decrease in invasive breast,149 colorectal,150 endometrial,147 or melanoma151 cancer risk was observed, but the diet intervention did decrease ovarian cancer risk.147 Other randomized clinical trials have also demonstrated that LFD intake does not reduce breast cancer risk152 or adenoma recurrence,153,154 though a 35% decrease in the odds ratio was observed for “super compliers” in the latter trial.155
Researchers have also examined the impact of a LFD on cancer outcomes in several clinical trials, including the Women’s Health Initiative trial, which found a reduction in breast cancer mortality in the diet intervention group,156 but no decrease in total cancer mortality.148 The Women’s Intervention Nutrition Study further demonstrated that LFD significantly increases relapse-free survival in early-stage breast cancer patients, with greater effects seen in subjects with estrogen receptor negative tumors.157 However, the Women’s Healthy Eating and Living trial, which also enrolled early stage breast cancer patients, found no reduction in breast cancer recurrence or mortality with LFD,158 unless the analysis was limited to subjects without post-treatment hot flashes.159 Others have examined the effects of short-term low-fat dietary interventions on biomarkers in prostate cancer patients, pre-radical prostatectomy. A low-fat, fish oil-supplemented diet decreased prostate cancer cell proliferation, though fish oil may be the primary mediator of this effect.160 In support of this hypothesis, proliferation rates were also significantly reduced in men on a flaxseed-supplemented diet, but not in those that followed a LFD without flaxseed.161
High-fat, Ketogenic Diet
Ketogenic diet (KD) is a very-low carbohydrate diet with high fat and moderate protein composition. Low carbohydrate consumption reduces available glucose, a cancer cell’s preferred energy source, and increases catabolism of proteins and fats to provide gluconeogenic glucose and ketones. With prolonged consumption of KD, glycogen stores reach critical levels and the body is no longer able to oxidize fats to glucose via gluconeogenesis. This results in a shift to increased ketone production and physiological ketosis. Ketosis is not to be confused with ketoacidosis that is seen with diabetes mellitus. In ketosis there is less accumulation of ketones, as they are being used efficiently by the brain and body as an energy source, and individuals do not experience the adverse side effects associated with ketoacidosis.162 Ketosis from KD has been shown to favorably modulates many cancer risk biomarkers including serum glucose, triglycerides, IGF-1, leptin, adiponectin, inflammatory markers, and angiogenic factors.163–167 Preclinical studies suggest that KD can attenuate these markers without a reduction in caloric intake; however, weight loss may be needed.168,169 KD may induce weight loss via several interrelated mechanisms, including: reduced appetite due to high protein intake, which can induce higher satiety, and high ketones, known to modulate appetite-regulating hormones; reduced caloric intake due to satiety; reduced lipogenesis and increased lipolysis; greater metabolic efficiency; and increased metabolic cost of gluconeogenesis and ketogenesis.162.
Beneficial effects of the ketogenic diet have long been established for epilepsy and T2DM; emerging is its role in primary cancer prevention and adjuvant treatment.162 Early preclinical studies found KD reduced tumor burden and cachexia in a mouse model of colon cancer.170 Further preclinical models have confirmed these findings and extended benefits of decreased tumor growth and increased survival to other cancer types including malignant glioma, gastric and prostate cancers.171 To date results from clinical trials focused on implementation of KD in primary cancer prevention and treatment have been limited, and ongoing clinical trials are beginning to address this gap in the literature with KD as adjuvant therapy in glioblastoma, pancreatic, head and neck, lung, and breast cancer patients.172 It is important to also consider potential adverse effects of KD. Select preclinical studies have found long-term KD to cause dyslipidemia, hepatic steatosis and glucose intolerance.173 More research is needed to evaluate the safety and efficacy of ketogenic diets as primary cancer prevention and adjuvant treatment interventions.
Summary and Conclusions
A strong link between obesity and cancer risk and/or poor prognosis has been established in the epidemiological and preclinical literature. Several of the Hallmarks of Cancer are impacted by obesity-associated systemic alterations including obesity-associated metabolic reprogramming, dysregulated growth factor signaling, adipose tissue inflammation, and induction of angiogenesis. Establishment of this obesity-cancer link has spurred extensive research focused on implementation of different dietary interventions to attain weight loss, attenuate risk biomarkers, and prevent obesity-associated cancers. Preclinical and early clinical work on putative anticancer dietary interventions, including CR, IF, LFD and KD, are being evaluated, some showing promise in reducing cancer risk. Ongoing clinical trials are also evaluating utilization of these dietary interventions as adjuvant therapy (Table 1). Limited evidence from these trials suggests that CR, IF, and KD may improve response and/or reduce side effects of therapy. Future studies will need to focus on the safety and added benefit, beyond that of current therapies, and consider the potential of the dietary interventions to sensitize patients and improve therapeutic response to lower doses chemotherapy or radiation therapy.
Table 1.
| Intervention | Cancer Type (s) | Adjuvant Treatment (s) |
Principle Investigator, Institution, Clinical Trials ID |
Results/ Anticipated Completion Date |
|
|---|---|---|---|---|---|
| Calorie Restriction | Calorie Restriction | Breast, Endometrial, Prostate | Prior to Cancer Surgery | Nicole Simone, MD Thomas Jefferson University NCT02983279 | October 2021 |
| "Fasting-mimicking Diet" Very Low Calorie, low amino acid | Breast | Chemotherapy | Judith R. Kroep, MD PhD Hanno Pijl, MD PhD Koos JM van der Hoeven, MD PhD Leiden University Medical Center NCT02126449 | December 2019 | |
| Fasting | Fasting up to 72 hours | All | Gemcitabine Hydrochloride and Cisplatin | Tanya Dorff, MD University of Southern California NCT00936364 | July 2018 |
| Fasting up to 60 hours | Prostate | Chemotherapy | Andreas Michalsen, Prof. Dr.Kurt Miller, Prof. Dr.Ursula Steiner, Dr.Charite University NCT02710721 | December 2017 | |
| Fasting up to 48 hours | Breast | Chemotherapy | Hanno Pijl, MD PhD Leiden University Medical Center NCT01304251 (Safdie et al, 2009) | Reduced self-reported side effects | |
| Fasting up to 72 hours | Ovarian, Breast | Chemotherapy | Andreas Michalsen, Prof. Dr.Charite University NCT01954836 | Completed, no reported results | |
| Fasting during Ramadan | All | Chemotherapy | Ali SM Al-Shanqeeti, MD King Fahad Medical City NCT00757094 | Completed, no reported results | |
| Ketogenic Diet | Ketogenic Diet | Glioblastoma | n/a | Pavel Klein, MD Mid-Atlantic Epilepsy and Sleep Center NCT01865162 | Completed, no reported results |
| Ketogenic Diet | Glioblastoma | n/a | Pavel Klein, MD Mid-Atlantic Epilepsy and Sleep Center NCT02302235 | August 2017 | |
| Ketogenic Diet | Glioblastoma | Chemotherapy | Song Lin, MD Beijing Tiantan Hospital NCT02939378 | December 2018 | |
| Ketogenic Diet | Glioblastoma | Radiation Therapy, Temozolomide | Adrienne C Scheck, PhD Barrow Neurological Institute NCT02046187 | March 2018 | |
| Ketogenic Diet | Glioblastoma | n/a | Michael D Jenkinson, PhD FRCS University of Liverpool NCT03075514 | March 2019 | |
| Ketogenic Diet | Glioblastoma | Following Standard Treatment | Johannes Rieger, MD Senckenberg Institute of Neurooncology, University of Frankfurt NCT00575146, (Rieger et al,2014) | Feasible and safe, no significant clinical activity as single agent | |
| Ketogenic Diet | Glioblastoma | Radiation Therapy, Chemotherapy | Kenneth Schwartz, MD Michigan State University NCT01535911 | July 2017 | |
| Ketogenic Diet | Glioma | n/a | Nachum Vaisman, Prof.Tel-Aviv Sourasky Medical Center NCT01092247 | Completed, no reported results | |
| Ketogenic Diet | Breast | n/a | Eugene J Fine, MD Albert Einstein College of Medicine, Inc. NCT02744079 | September 2020 | |
| Ketogenic Diet | Pancreaticobiliary | n/a | Chang Moo Kang, MD Yonsei University NCT02964806 | October 2017 | |
| Ketogenic Diet | Bladder | Prescribed Treatment | Eugene Lee, MD University of Kansas Medical Center NCT02716623 | March 2018 | |
| Ketogenic Diet | Colorectal, Prostate, Brain, Breast, Pancreatic, Hepatobiliary, Melanoma, Sarcoma, Lung, Genitourinary | n/a | Jocelyn Tan, MD VA Pittsburgh Health System NCT01716468, (Tan-Shalaby et al, 2016) | Maintained or slightly improved quality of life measures, 10%weight loss resulted in better response | |
| Ketogenic Diet | Breast, Rectum, Head & Neck | Radiation Therapy | Rainer J Klement, PhD MVZ Leopoldina GmbH NCT02516501 | June 2018 | |
| Combination | Calorie-restricted, Ketogenic Diet with Intermittent Fasting | Glioblastoma | During Reirradiation | Johannes Rieger, Dr.Joachim P Steinbach, Prof. Johann Wolfgang Goethe University Hospital NCT01754350 | October 2017 |
| Calorie-restriction with Physical Activity | Prostate | Prior to or Post Prostatectomy | Wendy Demark-Wahnefried, PhD, RD University of Alabama at Birmingham NCT01886677 | Feasible and safe, no serious adverse events reported. | |
| Calorie-restriction with Physical Activity | Breast | Prior to Mastectomy/Lumpectomy | Wendy Demark-Wahnefried, PhD, RD University of Alabama at Birmingham NCT02224807 | December 2017 | |
| Low-carb Atkins diet, Intermittent Fasting | Glioblastoma | Radiation Therapy and/or Temozolomide | Jaishri O. Blakeley, MD Johns Hopkins University Wake Forest School of Medicine NCT02286167 | November2017 |
Research Snapshot.
Research Question
What are the mechanisms through which obesity increases cancer risk and progression? Does implementation of dietary interventions attenuate obesity-associated cancer risk factors?
Key Findings
A traditional literature review revealed that obesity-associated metabolic perturbations are emerging as major drivers of obesity-related cancer including alterations in growth factor signaling, inflammation and angiogenesis. Preclinical evidence suggests that dietary interventions such as calorie restriction, intermittent fasting, and ketogenic diet have the potential to reverse some of these obesity-associated alterations; however, more clinical data is needed to confirm translation to human subjects.
Acknowledgments
Funding/Financial Disclosures: SDH is funded by grants from the Breast Cancer Research Foundation (BCRF 16-075, SDH as PI) and National Cancer Institute/NIH (R35 CA197627; SDH as PI). EHA is funded by a Marilyn Gentry Fellowship from the American Institute for Cancer Research. LWB is supported by a postdoctoral fellowship from the Cancer Control Education Program (R25 CA057726) at the UNC Lineberger Comprehensive Cancer Center.
Abbreviations
- AMPK
AMP kinase
- BMI
body mass index
- BAT
brown adipose tissue
- CR
calorie restriction
- CVD
cardiovascular disease
- ER
estrogen receptor
- FFA
free fatty acids
- IGF-1
insulin-like growth factor-1
- IL
interleukin
- IF
intermittent fasting
- KD
ketogenic diet
- LFD
low-fat diet
- MCP-1
monocyte chemo-attractant protein-1
- mTOR
mammalian target of rapamycin
- NASH
non-alcoholic steatohepatitis
- NFκB
nuclear factor kappa-light-chain-enhancer of B cells
- PAI-1
plasminogen activator inhibitor-1
- PI3K
phospatidylinositol-3 kinase
- PPAR
peroxisome proliferator-activated receptor
- STAT
signal transducer and activator of transcription
- TCA
tricarboxylic acid
- TNF-α
tumor necrosis factor-α
- T2DM
type II Diabetes
- VEGF
vascular endothelial growth factor
- WAT
white adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: LAS wrote the first draft with contributions from COF, LWB, EHA and SDH. All authors reviewed and commented on subsequent drafts of the manuscript.
Conflicts of Interest Disclosures: The authors have no conflicts of interest to report.
Contributor Information
Laura A. Smith, Department of Nutrition, University of North Carolina at Chapel Hill, 135 Dauer Drive, Chapel Hill, NC 27599, 919-843-4983.
Ciara H. O’Flanagan, Department of Nutrition, University of North Carolina at Chapel Hill, 135 Dauer Drive, Chapel Hill, NC 27599, 919-843-4983.
Laura W Bowers, Department of Nutrition, University of North Carolina at Chapel Hill, 135 Dauer Drive, Chapel Hill, NC 27599, 919-843-4983.
Emma H. Allott, Department of Nutrition, University of North Carolina, 135 Dauer Drive, Chapel Hill, NC 27599, (919)966-7230.
Stephen D Hursting, Department of Nutrition, University of North Carolina at Chapel Hill, 135 Dauer Drive, Chapel Hill, NC 27599, (919)966-7346.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khaodhiar L, McCowen KC, Blackburn GL. Obesity and its comorbid conditions. Clin Cornerstone. 1999;2(3):17–31. doi: 10.1016/s1098-3597(99)90002-9. [DOI] [PubMed] [Google Scholar]
- 4.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2014 doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lashinger LM, Rossi EL, Hursting SD. Obesity and resistance to cancer chemotherapy: interacting roles of inflammation and metabolic dysregulation. Clin Pharmacol Ther. 2014;96(4):458–463. doi: 10.1038/clpt.2014.136. [DOI] [PubMed] [Google Scholar]
- 7.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 8.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63(5):800–809. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Donohoe CL, Lysaght J, O'Sullivan J, Reynolds JV. Emerging Concepts Linking Obesity with the Hallmarks of Cancer. Trends Endocrinol Metab. 2017;28(1):46–62. doi: 10.1016/j.tem.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Warburg O, Posener K, Negelein E. Über den Stoffwechsel der Carcinomzelle. Biochem Zeitschr. 1924(152):309–344. [Google Scholar]
- 13.Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23(1):27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayers JR, Vander Heiden MG. Nature and Nurture: What Determines Tumor Metabolic Phenotypes? Cancer Res. 2017;77(12):3131–3134. doi: 10.1158/0008-5472.CAN-17-0165. [DOI] [PubMed] [Google Scholar]
- 15.O'Flanagan CH, Smith LA, McDonell SB, Hursting SD. When less may be more: calorie restriction and response to cancer therapy. BMC Med. 2017;15(1):106. doi: 10.1186/s12916-017-0873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupuy F, Tabaries S, Andrzejewski S, et al. PDK1-Dependent Metabolic Reprogramming Dictates Metastatic Potential in Breast Cancer. Cell Metab. 2015;22(4):577–589. doi: 10.1016/j.cmet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Lehuede C, Dupuy F, Rabinovitch R, Jones RG, Siegel PM. Metabolic Plasticity as a Determinant of Tumor Growth and Metastasis. Cancer Res. 2016;76(18):5201–5208. doi: 10.1158/0008-5472.CAN-16-0266. [DOI] [PubMed] [Google Scholar]
- 18.Pascual G, Avgustinova A, Mejetta S, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541(7635):41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 19.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giles ED, Wellberg EA, Astling DP, et al. Obesity and overfeeding affecting both tumor and systemic metabolism activates the progesterone receptor to contribute to postmenopausal breast cancer. Cancer Res. 2012;72(24):6490–6501. doi: 10.1158/0008-5472.CAN-12-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavazos DA, deGraffenried MJ, Apte SA, Bowers LW, Whelan KA, deGraffenried LA. Obesity promotes aerobic glycolysis in prostate cancer cells. Nutr Cancer. 2014;66(7):1179–1186. doi: 10.1080/01635581.2014.951738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klil-Drori AJ, Azoulay L, Pollak MN. Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing? Nat Rev Clin Oncol. 2017;14(2):85–99. doi: 10.1038/nrclinonc.2016.120. [DOI] [PubMed] [Google Scholar]
- 23.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28(26):4058–4065. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singla P, Bardoloi A, Parkash AA. Metabolic effects of obesity: A review. World J Diabetes. 2010;1(3):76–88. doi: 10.4239/wjd.v1.i3.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Connell TM. The complex role of branched chain amino acids in diabetes and cancer. Metabolites. 2013;3(4):931–945. doi: 10.3390/metabo3040931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulmer H, Borena W, Rapp K, et al. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br J Cancer. 2009;101(7):1202–1206. doi: 10.1038/sj.bjc.6605264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coppola JA, Shrubsole MJ, Cai Q, et al. Plasma lipid levels and colorectal adenoma risk. Cancer Causes Control. 2015;26(4):635–643. doi: 10.1007/s10552-015-0555-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimmelman AC, White E. Autophagy and Tumor Metabolism. Cell Metab. 2017;25(5):1037–1043. doi: 10.1016/j.cmet.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young AR, Narita M, Ferreira M, et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23(7):798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorr JR, Yu Y, Milanovic M, et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013;501(7467):421–425. doi: 10.1038/nature12437. [DOI] [PubMed] [Google Scholar]
- 31.Haim Y, Bluher M, Slutsky N, et al. Elevated autophagy gene expression in adipose tissue of obese humans: A potential non-cell-cycle-dependent function of E2F1. Autophagy. 2015;11(11):2074–2088. doi: 10.1080/15548627.2015.1094597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosacka J, Kern M, Kloting N, et al. Autophagy in adipose tissue of patients with obesity and type 2 diabetes. Mol Cell Endocrinol. 2015;409:21–32. doi: 10.1016/j.mce.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Cairo M, Villarroya J, Cereijo R, Campderros L, Giralt M, Villarroya F. Thermogenic activation represses autophagy in brown adipose tissue. Int J Obes (Lond) 2016;40(10):1591–1599. doi: 10.1038/ijo.2016.115. [DOI] [PubMed] [Google Scholar]
- 34.Lashinger LM, O'Flanagan CH, Dunlap SM, et al. Starving cancer from the outside and inside: separate and combined effects of calorie restriction and autophagy inhibition on Ras-driven tumors. Cancer Metab. 2016;4:18. doi: 10.1186/s40170-016-0158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braun S, Bitton-Worms K, LeRoith D. The link between the metabolic syndrome and cancer. Int J Biol Sci. 2011;7(7):1003–1015. doi: 10.7150/ijbs.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agrogiannis GD, Sifakis S, Patsouris ES, Konstantinidou AE. Insulin-like growth factors in embryonic and fetal growth and skeletal development (Review) Mol Med Rep. 2014;10(2):579–584. doi: 10.3892/mmr.2014.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12(3):159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 38.Brahmkhatri VP, Prasanna C, Atreya HS. Insulin-like growth factor system in cancer: novel targeted therapies. Biomed Res Int. 2015;2015:538019. doi: 10.1155/2015/538019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92(18):1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 40.Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Current opinion in genetics & development. 2010;20(1):87–90. doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Memmott RM, Dennis PA. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal. 2009;21(5):656–664. doi: 10.1016/j.cellsig.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Populo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13(2):1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Athar M, Kopelovich L. Rapamycin and mTORC1 inhibition in the mouse: skin cancer prevention. Cancer Prev Res (Phila) 2011;4(7):957–961. doi: 10.1158/1940-6207.CAPR-11-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nogueira LM, Dunlap SM, Ford NA, Hursting SD. Calorie restriction and rapamycin inhibit MMTV-Wnt-1 mammary tumor growth in a mouse model of postmenopausal obesity. Endocr Relat Cancer. 2012;19(1):57–68. doi: 10.1530/ERC-11-0213. [DOI] [PubMed] [Google Scholar]
- 46.Cifarelli V, Lashinger LM, Devlin KL, et al. Metformin and Rapamycin Reduce Pancreatic Cancer Growth in Obese Prediabetic Mice by Distinct MicroRNA-Regulated Mechanisms. Diabetes. 2015 doi: 10.2337/db14-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomimoto A, Endo H, Sugiyama M, et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 2008;99(11):2136–2141. doi: 10.1111/j.1349-7006.2008.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaudhary SC, Kurundkar D, Elmets CA, Kopelovich L, Athar M. Metformin, an antidiabetic agent reduces growth of cutaneous squamous cell carcinoma by targeting mTOR signaling pathway. Photochem Photobiol. 2012;88(5):1149–1156. doi: 10.1111/j.1751-1097.2012.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Angel RE, Conti CJ, Wheatley KE, et al. The enhancing effects of obesity on mammary tumor growth and Akt/mTOR pathway activation persist after weight loss and are reversed by RAD001. Mol Carcinog. 2013;52(6):446–458. doi: 10.1002/mc.21878. [DOI] [PubMed] [Google Scholar]
- 50.Checkley LA, Rho O, Moore T, Hursting S, DiGiovanni J. Rapamycin is a potent inhibitor of skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Prev Res (Phila) 2011;4(7):1011–1020. doi: 10.1158/1940-6207.CAPR-10-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saely CH, Geiger K, Drexel H. Brown versus white adipose tissue: a mini-review. Gerontology. 2012;58(1):15–23. doi: 10.1159/000321319. [DOI] [PubMed] [Google Scholar]
- 52.Eto H, Suga H, Matsumoto D, et al. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast Reconstr Surg. 2009;124(4):1087–1097. doi: 10.1097/PRS.0b013e3181b5a3f1. [DOI] [PubMed] [Google Scholar]
- 53.Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest. 2011;121(6):2087–2093. doi: 10.1172/JCI45888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedman JM, Mantzoros CS. 20 years of leptin: from the discovery of the leptin gene to leptin in our therapeutic armamentarium. Metabolism. 2015;64(1):1–4. doi: 10.1016/j.metabol.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 55.Park HK, Ahima RS. Leptin signaling. F1000Prime Rep. 2014;6:73. doi: 10.12703/P6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mullen M, Gonzalez-Perez RR. Leptin-Induced JAK/ STAT Signaling and Cancer Growth. Vaccines (Basel) 2016;4(3) doi: 10.3390/vaccines4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14(11):736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 58.Lee B, Shao J. Adiponectin and energy homeostasis. Rev Endocr Metab Disord. 2014;15(2):149–156. doi: 10.1007/s11154-013-9283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14(11–12):741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guenther M, James R, Marks J, Zhao S, Szabo A, Kidambi S. Adiposity distribution influences circulating adiponectin levels. Transl Res. 2014;164(4):270–277. doi: 10.1016/j.trsl.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaiopoulos AG, Marinou K, Christodoulides C, Koutsilieris M. The role of adiponectin in human vascular physiology. Int J Cardiol. 2012;155(2):188–193. doi: 10.1016/j.ijcard.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 62.Fantuzzi G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine. 2013;64(1):1–10. doi: 10.1016/j.cyto.2013.06.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Otvos L, Jr, Haspinger E, La Russa F, et al. Design and development of a peptide-based adiponectin receptor agonist for cancer treatment. BMC Biotechnol. 2011;11:90. doi: 10.1186/1472-6750-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ollberding NJ, Kim Y, Shvetsov YB, et al. Prediagnostic leptin, adiponectin, C-reactive protein, and the risk of postmenopausal breast cancer. Cancer Prev Res (Phila) 2013;6(3):188–195. doi: 10.1158/1940-6207.CAPR-12-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 66.Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86(3–5):225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 67.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150(6):2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kirschner MA, Schneider G, Ertel NH, Worton E. Obesity, androgens, estrogens, and cancer risk. Cancer Res. 1982;42(8 Suppl):3281s–3285s. [PubMed] [Google Scholar]
- 69.Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf) 2011;203(1):259–269. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17(3):224–232. doi: 10.1097/MED.0b013e3283398ee2. [DOI] [PubMed] [Google Scholar]
- 71.Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 72.Huang B, Warner M, Gustafsson JA. Estrogen receptors in breast carcinogenesis and endocrine therapy. Mol Cell Endocrinol. 2014 doi: 10.1016/j.mce.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 73.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1558–1568. [PubMed] [Google Scholar]
- 74.Goodwin PJ. Obesity and endocrine therapy: host factors and breast cancer outcome. Breast. 2013;22(Suppl 2):S44–47. doi: 10.1016/j.breast.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 75.Bernstein L, Ross RK. Endogenous hormones and breast cancer risk. Epidemiol Rev. 1993;15(1):48–65. doi: 10.1093/oxfordjournals.epirev.a036116. [DOI] [PubMed] [Google Scholar]
- 76.Ho SM. Estrogen, progesterone and epithelial ovarian cancer. Reprod Biol Endocrinol. 2003;1:73. doi: 10.1186/1477-7827-1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rizner TL. Estrogen biosynthesis, phase I and phase II metabolism, and action in endometrial cancer. Mol Cell Endocrinol. 2013;381(1–2):124–139. doi: 10.1016/j.mce.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 78.Endogenous H, Prostate Cancer Collaborative G. Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100(3):170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schnoeller T, Jentzmik F, Rinnab L, et al. Circulating free testosterone is an independent predictor of advanced disease in patients with clinically localized prostate cancer. World J Urol. 2013;31(2):253–259. doi: 10.1007/s00345-012-0902-5. [DOI] [PubMed] [Google Scholar]
- 80.Folkerd EJ, Dowsett M. Influence of sex hormones on cancer progression. J Clin Oncol. 2010;28(26):4038–4044. doi: 10.1200/JCO.2009.27.4290. [DOI] [PubMed] [Google Scholar]
- 81.Subbaramaiah K, Howe LR, Bhardwaj P, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4(3):329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front Endocrinol (Lausanne) 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21(3):297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Balaban S, Lee LS, Schreuder M, Hoy AJ. Obesity and Cancer Progression: Is There a Role of Fatty Acid Metabolism? Biomed Res Int. 2015;2015:274585. doi: 10.1155/2015/274585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O'Flanagan CH, Bowers LW, Hursting SD. A weighty problem: metabolic perturbations and the obesity-cancer link. Horm Mol Biol Clin Investig. 2015;23(2):47–57. doi: 10.1515/hmbci-2015-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Henry SL, Bensley JG, Wood-Bradley RJ, Cullen-McEwen LA, Bertram JF, Armitage JA. White adipocytes: more than just fat depots. Int J Biochem Cell Biol. 2012;44(3):435–440. doi: 10.1016/j.biocel.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 88.Suganami T, Tanaka M, Ogawa Y. Adipose tissue inflammation and ectopic lipid accumulation. Endocr J. 2012;59(10):849–857. doi: 10.1507/endocrj.ej12-0271. [DOI] [PubMed] [Google Scholar]
- 89.Geisler CE, Renquist BJ. Hepatic lipid accumulation: cause and consequence of dysregulated glucoregulatory hormones. J Endocrinol. 2017 doi: 10.1530/JOE-16-0513. [DOI] [PubMed] [Google Scholar]
- 90.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 91.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 92.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10(12):1342–1359. e1342. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vanni E, Bugianesi E, Kotronen A, De Minicis S, Yki-Jarvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis. 2010;42(5):320–330. doi: 10.1016/j.dld.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 94.Berardis S, Sokal E. Pediatric non-alcoholic fatty liver disease: an increasing public health issue. Eur J Pediatr. 2014;173(2):131–139. doi: 10.1007/s00431-013-2157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tolman KG, Dalpiaz AS. Treatment of non-alcoholic fatty liver disease. Ther Clin Risk Manag. 2007;3(6):1153–1163. [PMC free article] [PubMed] [Google Scholar]
- 96.Farese RV, Jr, Zechner R, Newgard CB, Walther TC. The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab. 2012;15(5):570–573. doi: 10.1016/j.cmet.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hui JM, Kench JG, Chitturi S, et al. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38(2):420–427. doi: 10.1053/jhep.2003.50320. [DOI] [PubMed] [Google Scholar]
- 98.Tushuizen ME, Bunck MC, Pouwels PJ, et al. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care. 2007;30(11):2916–2921. doi: 10.2337/dc07-0326. [DOI] [PubMed] [Google Scholar]
- 99.Heni M, Machann J, Staiger H, et al. Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: a nuclear magnetic resonance study. Diabetes Metab Res Rev. 2010;26(3):200–205. doi: 10.1002/dmrr.1073. [DOI] [PubMed] [Google Scholar]
- 100.Smits MM, van Geenen EJ. The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol. 2011;8(3):169–177. doi: 10.1038/nrgastro.2011.4. [DOI] [PubMed] [Google Scholar]
- 101.van Geenen EJ, Smits MM, Schreuder TC, van der Peet DL, Bloemena E, Mulder CJ. Nonalcoholic fatty liver disease is related to nonalcoholic fatty pancreas disease. Pancreas. 2010;39(8):1185–1190. doi: 10.1097/MPA.0b013e3181f6fce2. [DOI] [PubMed] [Google Scholar]
- 102.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9(4):777–794. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cottam D, Fisher B, Ziemba A, et al. Tumor growth factor expression in obesity and changes in expression with weight loss: another cause of increased virulence and incidence of cancer in obesity. Surg Obes Relat Dis. 2010;6(5):538–541. doi: 10.1016/j.soard.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 104.Iwaki T, Urano T, Umemura K. PAI-1, progress in understanding the clinical problem and its aetiology. British journal of haematology. 2012;157(3):291–298. doi: 10.1111/j.1365-2141.2012.09074.x. [DOI] [PubMed] [Google Scholar]
- 105.Bauman KA, Wettlaufer SH, Okunishi K, et al. The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice. J Clin Invest. 2010;120(6):1950–1960. doi: 10.1172/JCI38369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Malik R, Lelkes PI, Cukierman E. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015;33(4):230–236. doi: 10.1016/j.tibtech.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Slaughter KN, Thai T, Penaroza S, et al. Measurements of adiposity as clinical biomarkers for first-line bevacizumab-based chemotherapy in epithelial ovarian cancer. Gynecol Oncol. 2014;133(1):11–15. doi: 10.1016/j.ygyno.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 108.Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer. 2009;9(12):886–896. doi: 10.1038/nrc2747. [DOI] [PubMed] [Google Scholar]
- 109.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13(11):800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rogers CJ, Prabhu KS, Vijay-Kumar M. The microbiome and obesity-an established risk for certain types of cancer. Cancer J. 2014;20(3):176–180. doi: 10.1097/PPO.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 111.Froy O. Circadian rhythms and obesity in mammals. ISRN Obes. 2012;2012:437198. doi: 10.5402/2012/437198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Froy O. Metabolism and circadian rhythms--implications for obesity. Endocr Rev. 2010;31(1):1–24. doi: 10.1210/er.2009-0014. [DOI] [PubMed] [Google Scholar]
- 113.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 114.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 117.Casagrande DS, Rosa DD, Umpierre D, Sarmento RA, Rodrigues CG, Schaan BD. Incidence of cancer following bariatric surgery: systematic review and meta-analysis. Obes Surg. 2014;24(9):1499–1509. doi: 10.1007/s11695-014-1276-0. [DOI] [PubMed] [Google Scholar]
- 118.Mahoney LB, Denny CA, Seyfried TN. Caloric restriction in C57BL/6J mice mimics therapeutic fasting in humans. Lipids Health Dis. 2006;5:13. doi: 10.1186/1476-511X-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31(2):89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mattison JA, Roth GS, Beasley TM, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hursting SD, Dunlap SM, Ford NA, Hursting MJ, Lashinger LM. Calorie restriction and cancer prevention: a mechanistic perspective. Cancer Metab. 2013;1(1):10. doi: 10.1186/2049-3002-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lv M, Zhu X, Wang H, Wang F, Guan W. Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: a systematic review and meta-analysis. PLoS One. 2014;9(12):e115147. doi: 10.1371/journal.pone.0115147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weiss EP, Racette SB, Villareal DT, et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84(5):1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: An update. Ageing Res Rev. 2016 doi: 10.1016/j.arr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Byers T, Sedjo RL. Does intentional weight loss reduce cancer risk? Diabetes Obes Metab. 2011;13(12):1063–1072. doi: 10.1111/j.1463-1326.2011.01464.x. [DOI] [PubMed] [Google Scholar]
- 127.Fabian CJ, Kimler BF, Donnelly JE, et al. Favorable modulation of benign breast tissue and serum risk biomarkers is associated with >10 % weight loss in postmenopausal women. Breast Cancer Res Treat. 2013;142(1):119–132. doi: 10.1007/s10549-013-2730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fontana L, Villareal DT, Das SK, et al. Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell. 2016;15(1):22–27. doi: 10.1111/acel.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Demark-Wahnefried W, Nix JW, Hunter GR, et al. Feasibility outcomes of a presurgical randomized controlled trial exploring the impact of caloric restriction and increased physical activity versus a wait-list control on tumor characteristics and circulating biomarkers in men electing prostatectomy for prostate cancer. BMC Cancer. 2016;16:61. doi: 10.1186/s12885-016-2075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saleh AD, Simone BA, Palazzo J, et al. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle. 2013;12(12):1955–1963. doi: 10.4161/cc.25016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brandhorst S, Longo VD. Fasting and Caloric Restriction in Cancer Prevention and Treatment. Recent Results Cancer Res. 2016;207:241–266. doi: 10.1007/978-3-319-42118-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2016 doi: 10.1016/j.arr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19(2):181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Descamps O, Riondel J, Ducros V, Roussel AM. Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: effect of alternate-day fasting. Mech Ageing Dev. 2005;126(11):1185–1191. doi: 10.1016/j.mad.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 135.Harvie M, Howell A. Energy restriction and the prevention of breast cancer. Proc Nutr Soc. 2012;71(2):263–275. doi: 10.1017/S0029665112000195. [DOI] [PubMed] [Google Scholar]
- 136.Harvie MN, Howell T. Could Intermittent Energy Restriction and Intermittent Fasting Reduce Rates of Cancer in Obese, Overweight, and Normal-Weight Subjects? A Summary of Evidence. Adv Nutr. 2016;7(4):690–705. doi: 10.3945/an.115.011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011;35(5):714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Lorgeril M, Salen P, Martin JL, Monjaud I, Boucher P, Mamelle N. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med. 1998;158(11):1181–1187. doi: 10.1001/archinte.158.11.1181. [DOI] [PubMed] [Google Scholar]
- 139.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ. 2008;337:a1344. doi: 10.1136/bmj.a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brown T, Avenell A, Edmunds LD, et al. Systematic review of long-term lifestyle interventions to prevent weight gain and morbidity in adults. Obes Rev. 2009;10(6):627–638. doi: 10.1111/j.1467-789X.2009.00641.x. [DOI] [PubMed] [Google Scholar]
- 141.Romaguera D, Norat T, Mouw T, et al. Adherence to the Mediterranean diet is associated with lower abdominal adiposity in European men and women. J Nutr. 2009;139(9):1728–1737. doi: 10.3945/jn.109.108902. [DOI] [PubMed] [Google Scholar]
- 142.Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med. 2015;4(12):1933–1947. doi: 10.1002/cam4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Harvie MN, Sims AH, Pegington M, et al. Intermittent energy restriction induces changes in breast gene expression and systemic metabolism. Breast Cancer Res. 2016;18(1):57. doi: 10.1186/s13058-016-0714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Safdie FM, Dorff T, Quinn D, et al. Fasting and cancer treatment in humans: A case series report. Aging (Albany NY) 2009;1(12):988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.de Groot S, Vreeswijk MP, Welters MJ, et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC Cancer. 2015;15:652. doi: 10.1186/s12885-015-1663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 147.Prentice RL, Thomson CA, Caan B, et al. Low-fat dietary pattern and cancer incidence in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst. 2007;99(20):1534–1543. doi: 10.1093/jnci/djm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thomson CA, Van Horn L, Caan BJ, et al. Cancer incidence and mortality during the intervention and postintervention periods of the Women's Health Initiative dietary modification trial. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2924–2935. doi: 10.1158/1055-9965.EPI-14-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 150.Beresford SA, Johnson KC, Ritenbaugh C, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):643–654. doi: 10.1001/jama.295.6.643. [DOI] [PubMed] [Google Scholar]
- 151.Gamba CS, Stefanick ML, Shikany JM, et al. Low-fat diet and skin cancer risk: the women's health initiative randomized controlled dietary modification trial. Cancer Epidemiol Biomarkers Prev. 2013;22(9):1509–1519. doi: 10.1158/1055-9965.EPI-13-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Martin LJ, Li Q, Melnichouk O, et al. A randomized trial of dietary intervention for breast cancer prevention. Cancer Res. 2011;71(1):123–133. doi: 10.1158/0008-5472.CAN-10-1436. [DOI] [PubMed] [Google Scholar]
- 153.Schatzkin A, Lanza E, Corle D, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med. 2000;342(16):1149–1155. doi: 10.1056/NEJM200004203421601. [DOI] [PubMed] [Google Scholar]
- 154.Lanza E, Yu B, Murphy G, et al. The polyp prevention trial continued follow-up study: no effect of a low-fat, high-fiber, high-fruit, and -vegetable diet on adenoma recurrence eight years after randomization. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1745–1752. doi: 10.1158/1055-9965.EPI-07-0127. [DOI] [PubMed] [Google Scholar]
- 155.Sansbury LB, Wanke K, Albert PS, et al. The effect of strict adherence to a high-fiber, high-fruit and -vegetable, and low-fat eating pattern on adenoma recurrence. Am J Epidemiol. 2009;170(5):576–584. doi: 10.1093/aje/kwp169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chlebowski RT, Aragaki AK, Anderson GL, et al. Low-Fat Dietary Pattern and Breast Cancer Mortality in the Women's Health Initiative Randomized Controlled Trial. J Clin Oncol. 2017 doi: 10.1200/JCO.2016.72.0326. JCO2016720326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98(24):1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 158.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298(3):289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gold EB, Pierce JP, Natarajan L, et al. Dietary pattern influences breast cancer prognosis in women without hot flashes: the women's healthy eating and living trial. J Clin Oncol. 2009;27(3):352–359. doi: 10.1200/JCO.2008.16.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aronson WJ, Kobayashi N, Barnard RJ, et al. Phase II prospective randomized trial of a low-fat diet with fish oil supplementation in men undergoing radical prostatectomy. Cancer Prev Res (Phila) 2011;4(12):2062–2071. doi: 10.1158/1940-6207.CAPR-11-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Demark-Wahnefried W, Polascik TJ, George SL, et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3577–3587. doi: 10.1158/1055-9965.EPI-08-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789–796. doi: 10.1038/ejcn.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9(3):200–205. [PMC free article] [PubMed] [Google Scholar]
- 164.Fu SP, Li SN, Wang JF, et al. BHBA suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-kappaB activation. Mediators Inflamm. 2014;2014:983401. doi: 10.1155/2014/983401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Woolf EC, Curley KL, Liu Q, et al. The Ketogenic Diet Alters the Hypoxic Response and Affects Expression of Proteins Associated with Angiogenesis, Invasive Potential and Vascular Permeability in a Mouse Glioma Model. PLoS One. 2015;10(6):e0130357. doi: 10.1371/journal.pone.0130357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goldberg EL, Asher JL, Molony RD, et al. beta-Hydroxybutyrate Deactivates Neutrophil NLRP3 Inflammasome to Relieve Gout Flares. Cell Rep. 2017;18(9):2077–2087. doi: 10.1016/j.celrep.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Merra G, Gratteri S, De Lorenzo A, et al. Effects of very-low-calorie diet on body composition, metabolic state, and genes expression: a randomized double-blind placebo-controlled trial. Eur Rev Med Pharmacol Sci. 2017;21(2):329–345. [PubMed] [Google Scholar]
- 168.Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am J Physiol Endocrinol Metab. 2009;297(5):E1197–1204. doi: 10.1152/ajpendo.00357.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nandivada P, Fell GL, Pan AH, et al. Eucaloric Ketogenic Diet Reduces Hypoglycemia and Inflammation in Mice with Endotoxemia. Lipids. 2016;51(6):703–714. doi: 10.1007/s11745-016-4156-7. [DOI] [PubMed] [Google Scholar]
- 170.Tisdale MJ, Brennan RA, Fearon KC. Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br J Cancer. 1987;56(1):39–43. doi: 10.1038/bjc.1987.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox Biol. 2014;2:963–970. doi: 10.1016/j.redox.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Branco AF, Ferreira A, Simoes RF, et al. Ketogenic diets: from cancer to mitochondrial diseases and beyond. Eur J Clin Invest. 2016;46(3):285–298. doi: 10.1111/eci.12591. [DOI] [PubMed] [Google Scholar]
- 173.Ellenbroek JH, van Dijck L, Tons HA, et al. Long-term ketogenic diet causes glucose intolerance and reduced beta- and alpha-cell mass but no weight loss in mice. Am J Physiol Endocrinol Metab. 2014;306(5):E552–558. doi: 10.1152/ajpendo.00453.2013. [DOI] [PubMed] [Google Scholar]