Abstract
Prostate cancer (PCa) is the most common non-skin cancer and the second leading cause of cancer-related deaths in American men. Due to its long latency period, PCa is considered as an ideal cancer type for chemopreventive interventions. Chemopreventive agents include various natural or synthetic agents that prevent or delay cancer development, progression and/or recurrence. Pre-clinical studies suggest that many natural products and dietary agents have chemopreventive properties. However, a limited number of these agents have been tested in clinical trials, with varying success. In this review, we have discussed the available clinical studies regarding the efficacy of natural chemopreventive agents against PCa, including tea polyphenols, selenium, soy proteins, vitamins and resveratrol. We have also provided a discussion on the clinical challenges and opportunities for the potential use of chemopreventive agents against PCa. Based on available literature, it appears that the variable outcomes of the chemopreventive clinical studies necessitate a need for additional studies with more rigorous designs and methodical interpretations in order to measure the potential of the natural agents against PCa.
Keywords: Prostate cancer, chemoprevention, natural agents, clinical trials
1. Introduction
Despite the advances in prostate cancer (PCa) biology and therapeutics, according to the American Cancer Society’s estimates, about 164,690 new cases of PCa will be diagnosed and about 29,430 deaths will occur in 2018, in the United States alone [1]. Traditionally, PCa development and progression is thought to be motivated by androgen and androgen receptor (AR), and therefore first-line of treatment androgen deprivation therapy (ADT) is used. However, the “saturation model” proposed by Morgentaler and Traish suggests that there is a limit to the capability of androgens to promote PCa growth, and this limit is based on maximal androgen-AR binding, which is achieved at well below the physiologic concentrations of serum testosterone [2]. Nevertheless, after initial ADT success, most patients with advanced disease eventually develop resistance and progress to castrate-resistant PCa (CRPC), which remains an incurable disease [3]. Low survival and high mortality of PCa are associated with the emergence of CRPC and subsequent metastatic disease. To advance the battle against PCa, it is necessary to continue both basic and clinical research to improve detection, prevention and treatment practices. However, the preventive approach could be considered as a fundamental strategy to reduce the incidence as well as mortality associated with PCa. To this end, novel strategies are required to be added to existing regimens and standards of care. Epidemiological studies have shown that healthy diet and exercise may significantly influence the pervasiveness of several cancers [4]. Several natural compounds, following promising preclinical testing, have been evaluated in clinics. In this review, we have discussed the data available from the clinical trials regarding several natural chemopreventive agents that have been tested for PCa chemoprevention. Additionally, we also present our thoughts on challenges and opportunities regarding the clinical use of chemopreventive agents for PCa.
2. Methodology
In this narrative review, we have discussed the PCa chemoprevention by selected natural agents based on available clinical evidence. We searched the NCBI’s PubMed database for clinical trials with the key phrases “chemopreventive agents and prostate cancer”, “natural agents and prostate cancer”, and “chemoprevention and prostate cancer”. From these results, we selected all the published clinical studies in which natural agents were used for PCa management. Then we shortlisted those studies which used a chemoprevention regimen for PCa. In order to stay within the scope of a mini-review, we have limited our discussion to only selected agents viz. tea polyphenols, vitamins, selenium, soy, and resveratrol. We are unable to provide an exhaustive review of some important natural agents e.g. lycopene, pomegranate extract, and curcumin, due to the limited scope of our mini-review.
3. Prostate cancer
According to the most recent estimates by the American Cancer Society, in the United States, PCa is the most common non-skin malignancy and the second leading cause of cancer death in American men, after lung cancer [1]. PCa pathogenesis is highly dependent upon signaling through the androgen receptor (AR), a ligand-dependent transcription factor. Binding of AR with androgen ligands, such as testosterone and dihydrotestosterone (DHT), produces a conformational change in AR to undergo homodimerization, allowing its nuclear translocation where AR binds to androgen response elements (ARE) [5]. AR binding to the AREs induces androgen-responsive gene expression that supports survival and growth of prostatic cells and further progression of the PCa (Fig. 1). AR-dependent signaling is involved in all stages of PCa development including initiation, progression, and resistance to available therapies [6]. In fact, one of the downstream AR signaling genes, prostate-specific antigen (PSA), is an established clinical biomarker used for PCa diagnosis, prognosis, progression and response to treatment [7]. These pathways have been depicted in Fig. 1, along with the targets at which many of the chemopreventive agents are reported to act. Although traditional intervention with androgen suppression therapy provides moderate success in treating PCa, it would be useful to find ways either to prevent its occurrence or to delay the progression, which may substantially reduce the costs to the healthcare system, due to this rampant disease.
Figure 1. Schematic representation of PCa pathogenesis showing androgen receptor (AR) dependent and independent signaling as well the potential targets of the chemopreventive agents.
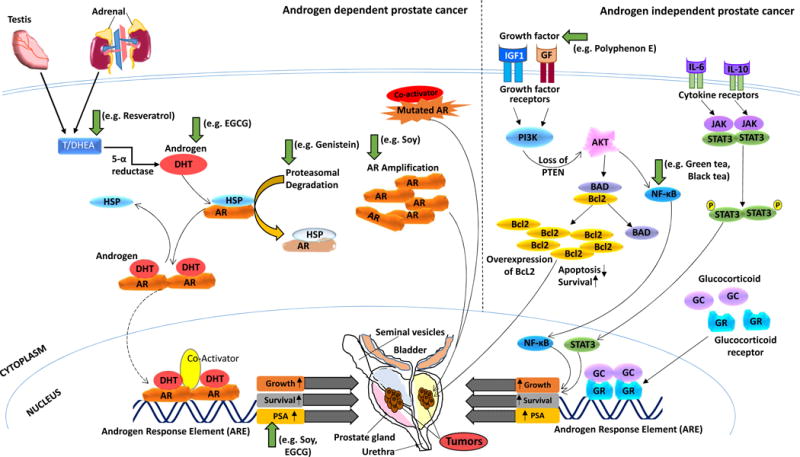
Androgen ligands bind to AR and produce a conformational change in AR to undergo homodimerization that allows for nuclear translocation where AR binds to androgen response elements (ARE). AR binding to the AREs induces androgen-responsive gene expression that supports growth and survival of prostate cells and further progression of the PCa. PSA is one of the downstream AR signaling genes, and a well-known PCa clinical biomarker. AR-independent PCa mechanisms include overexpression of the glucocorticoid receptor (GR) which can substitute AR in binding to AREs, and activation of alternative signaling pathways via cytokines and growth factors. Various chemopreventive agents that are in clinical trials against PCa have been shown to inhibit PCa development and progression through the regulation of major cellular signaling pathways such as the androgen, AR and PSA inhibition as well as affecting AR independent pathways such as inhibiting growth factor and NF-κB. These targets are pointed out in the figure with green arrows.
4. Chemoprevention for PCa management
PCa, like other cancers, can arise due to mutation(s) in important regulatory genes, most commonly through a carcinogenic or mutagenic agent or because of impaired DNA repair [8], as well as epigenetic modulations such as histone modifications, DNA methylation, and noncoding RNA (reviewed in [9]). These mutations can result in an unchecked growth of the affected cells through the three steps of cancer development: initiation, promotion, and progression. Cancer chemoprevention involves the use of natural or synthetic agents to prevent, delay or suppress the process of carcinogenesis [10]. Natural agents for chemoprevention are naturally occurring compounds which possess cancer preventive activities and are present in vegetables, fruits, herbs, fermented products or other dietary sources. Three main categories of chemopreventive agents have been defined. The first category of agents is aimed at preventing the occurrence of primary cancer, either through prevention of mutations or growth of mutated cells. The second category consists of those agents that can inhibit pre-malignant lesions from developing into malignant cancer. The third category of agents is those which may prevent or reduce the risk of reoccurrence of cancer in patients who have undergone successful primary cancer treatment and are in remission. The one caveat of every category of the chemopreventive agent is that the agent must be taken for long periods of time, which makes safety and accessibility key factors when testing chemopreventive agents.
Currently, both naturally occurring and synthetic compounds are being investigated for their potential use against PCa. Although some natural chemopreventive compounds can exhibit harmful toxic effects, including certain vitamins, many have low toxicity and high tolerability. In addition to this, a major benefit of using these agents is that natural compounds found in foods tend to be easily accessible on a daily basis for the general population. Many in vitro and in vivo studies have revealed that natural compounds are able to affect cellular proliferation via modulating a myriad of important cellular signaling pathways, which are frequently disrupted in cancer (reviewed in [11]). Additionally, many epidemiological and pre-clinical studies strongly suggest that various naturally available phytochemicals and dietary agents possess cancer chemopreventive properties and can even enhance the host immune system against tumor cells or sensitize malignant cells to cytotoxic agents (reviewed in [11]). Because of its long latency period, PCa is believed to be an ideal disease for the chemopreventive interventions. Indeed, age is the main risk factor for PCa, as it is primarily diagnosed in men >50 years of age, and patients who are not diagnosed until >70 years of age generally possess less aggressive cancer [12]. PCa chemoprevention studies must be aimed to prevent its initiation and progression to clinically aggressive cancer, to maintain an androgen-responsive clinical state, and to prevent and/or delay the onset of androgen-independent state. Therefore, agents that target multiple aspects of AR signaling are desirable for PCa chemoprevention. There are numerous targets for chemopreventive agents to act upon in the AR-dependent pathways, however, AR-independent signaling molecules are mostly therapeutic targets once the PCa is clinically aggressive. Potential mechanistic targets of various chemopreventive agents that are reported in PCa clinical studies are shown in Fig. 1.
5. PCa clinical trials with natural chemopreventive agents
Though a number of pre-clinical studies have shown the benefits of chemopreventive agents in the prevention of prostate tumor formation and progression, a limited number of agents have been tested in clinical trials. Out of these, many clinical trials have been conducted to evaluate the safety, tolerability, pharmacokinetics and bioavailability of chemopreventive agents. Although this is a necessary step in testing new chemotherapeutic agents, these studies are not the focus of our review. We have specifically focused on studies that have evaluated the efficacy of selected and scientifically popular chemopreventive agents against PCa, and our review is by no means a comprehensive account of all the agents that may have been studied. A limited number of clinical trials with chemopreventive agents have been conducted on healthy men and on patients with high-grade intraepithelial neoplasia (HGPIN) and/or atypical small acinar proliferation (ASAP), as they are high-risk subjects for PCa and intermediate predictive stages of progression. HGPIN is the most established precursor of PCa that develops many months (or years) before becoming clinically evident PCa. Men with HGPIN have a 30% chance of developing PCa within a year of detection (reviewed in [13]). The presence of HGPIN is easily identifiable and exhibits similar cytological features to PCa, making it a valuable candidate for chemoprevention studies. Presently, ASAP is considered as a diagnosis of exclusion as it shows a greater association to PCa than HGPIN [14]. Table 1 summarizes the published human clinical studies we have focused on, that were conducted with the selected chemopreventive agents against PCa, and includes tea polyphenols, vitamins, selenium, soy proteins and resveratrol. Individual discussion on these agents is provided below.
Table 1.
Selected published human clinical trials using natural chemopreventive agents against PCa.
| Chemopreventive Agent | Study design | Study Outcome | Ref | |
|---|---|---|---|---|
| Dose and Duration | Subjects/Sample size/Phase | |||
| Tea polyphenols | ||||
| Green tea, black tea or water | 6 cups/day for 3 to 8 weeks | PCa intervention, N=113; Phase II | NF-κB staining in radical prostatectomy tissue, urinary 8-OHdG and serum PSA levels were significantly decreased in green tea, but not in black tea group. | [18] |
| Green tea or water | 6 cups/day for 3 to 8 weeks | Clinically localized PCa, N=17 | Methylated and nonmethylated forms of EGCG were detectable in prostatectomy tissue. | [19] |
| Green tea, black tea or a caffeine-matched soda control | 1.42 L daily for 5 day | Prostatectomy scheduled Patients, N=20 | Tea polyphenols and theaflavins were found bioavailable in the prostate, and therefore may be effective in the management of PCa. | [17] |
| Green tea catechins (GTC) | 3 × 200 mg capsules; total 600 mg) or placebo control daily for 1 year | HGPIN volunteers, N=60 | Decrease in the tumor incidence and PSA level, improved quality of life, and reduced lower urinary tract symptoms in GTC-supplemented men. In a follow-up study, further reduction in PCa were noticed suggesting long-lasting effect of GTC. | [20, 21] |
| Polyphenon E | 400 mg EGCG/day or placebo for 1 year | HGPIN and/or ASAP N=97 | No significant difference in the number of PCa cases, however, a decrease in PSA was reported in EGCG group. | [22] |
| Polyphenon E | 1.3 g of tea polyphenols including 800 mg EGCG or placebo daily for around 6 weeks | Prostatectomy scheduled Patients, N=26; Phase II | Significant decrease in serum levels of PSA, HGF, IGF-I, IGFBP-3, and VEGF in men with PCa. | [23] |
| Polyphenon E | 800 mg EGCG or placebo daily for 3–6 weeks | Prostatectomy scheduled Patients, N=50; Phase II | Favorable but statistically insignificant changes in systemic and tissue biomarkers including PSA. | [24] |
| Vitamins and Minerals | ||||
| Vitamin D | Calcitriol 0.5 μg/kg) or placebo weekly for 4 weeks | Histologically confirmed PCa, N=39 | Calcitriol was found to downregulate vitamin D receptor expression in human PCa. | [82] |
| Vitamin D analog | 1α-hydroxyvitamin D2; 10 μg orally or placebo daily for 28 days | Clinically localized PCa and HGPIN, N=60, Phase II | Biologic activity of 1α-hydroxyvitamin D2 was minimal in both serum and tissue, and TGF-ß2 was the only biomarker found significantly reduced. | [28] |
| Vitamin E | 400 IU/day of all rac-α-tocopheryl acetate or placebo over 5 years (planned for 7–12 years). | Healthy with negative digital rectal exam, N=35,533, Phase III | There were non-significant increased risks of PCa in the vitamin E group. In subsequent follow-up study, vitamin E supplementation was found to be associated with significant increase in the risk of PCa. | [35, 36] |
| Vitamin E 2,2,5,7,8-pentamethyl-6-chromanol | APC-100, 900–2400 mg orally daily for 28 days | Castrate-resistant PCa (CRPC), N=20, Phase I/IIa | APC-100, which act as both antioxidant as well as antiandrogen, was found to have a slightly better response, as 5 out of 20 patients had stable disease. However, APC-100 was not detectable in plasma. | [39] |
| Calcium carbonate | 3 g; 1,200 mg of calcium or placebo, daily for 4 years | Colorectal adenoma chemoprevention trial, N=673 | There were 33 PCa cases in the calcium-treated group vs 37 in the placebo group after a mean follow-up of 10.3 years, suggesting protective effects of calcium. | [83] |
| Selenium | ||||
| Selenium | As selenized yeast; 200 μg/day) or placebo for a mean of 4.5 years | Men with history of non-melanoma skin cancer, N=974 | Selenium was found to be associated with a significant reduction in the PCa incidence (secondary endpoint), in patients with lower baseline PSA and plasma selenium levels. | [52, 84–86] |
| Selenium | As L-selenomethionin e; 200 μg/day) over 5 years (planned for 7-years) 12 | Healthy men with negative digital rectal exam, N=35,533, Phase III | No preventive effects against PCa were found. However, supplementation in men with high Se status increased the risk of high-grade PCa. | [35, 87] |
| Selenium | As selenized yeast; years 200 or 400 μg/day up to 5 | Men with ≥1 negative sextant prostate biopsy, N=699, Phase III | No effect on incidence of PCa or PSA velocity in men at high risk was found. | [55, 88] |
| Selenium | As selenized yeast; years 200 or 800 μg/day up to 5 | Localized nonmetastatic PCa patients, N=140, Phase II | No significant effect of selenium on PSA velocity was seen. Men in the highest quartile supplemented with the highest dose showed an increase in PSA velocity. | [57, 89] |
| Selenium | As selenomethionin e; 200 μg/day) over a 3-year period. | Men diagnosed with HGPIN, N=423, Phase III | Selenium had no effect on PCa risk, although a subset analysis found a trend of reduced PCa in Se vs placebo patients in the lowest quartile of baseline plasma Se levels. | [56, 90] |
| Soy and isoflavones | ||||
| Dietary soy | High (2 servings of soy foods/day) and low (no added soy) via diet for 3 months | Healthy men aged 58.7+/−7.2 years, N=24 | The study found 14% decline in serum PSA levels, though statistically not significant, in the high soy diet in contrast to the low soy diet. | [63] |
| Soy beverage | 500 mL daily for 6 month | Men with rising PSA after radical radiation, N=29 | A declining trend in PSA levels and a trend towards >2 times prolongation of PSA doubling time in 41% of subjects. | [64] |
| Isoflavone+ | As soy protein drink; 83 mg/day, or isoflavone drink for 12 months | Healthy men aged 50–80 years, N=112 | No significant change in serum PSA level, velocity, or PCa incidence in isoflavone treatment group, or in other prostate conditions that affect serum PSA levels. | [65] |
| Isoflavone | 60 mg/day or placebo for 12 months | Men with rising PSA, N= 158, Phase II | No significant change in PSA levels after isoflavone treatment. However, 53 patients aged ≥65 years, showed significantly lower PCa incidence in the isoflavone group. | [67] |
| Soy protein isolate | 107 or <6 or 0 mg/day isoflavones for 6 months. | High risk PCa patients, N=58 | Isoflavone-rich soy protein significantly reduced AR expression, but no change in estrogen receptor-β or circulating hormones in men at high risk of PCa. | [68] |
| Isoflavones | 5g/day including 450 mg genistein, 300 mg daidzein, or placebo for 6 months | Low-volume PCa patients, N=53 | No significant reduction in PSA levels were found in men with low-volume PCa. | [69] |
| Isoflavones | 80 mg/day for up to 6 week | Patients with localized PCa, N=86, Phase II | No significant change in serum hormone levels, total cholesterol, or PSA after short-term intake of soy isoflavones. | [66] |
| Genistein | 30 mg/day or placebo for 3–6 weeks | Patients with localized PCa, N=47, Phase II | Significant reduction in the androgen-related biomarker KLK4, but no significant changes in proliferation-, cell cycle-, apoptosis-related biomarkers. | [70] |
| Resveratrol and Grapes | ||||
| Resveratrol | 150 or 1,000 mg or placebo daily for 4 months | Men suffering from the metabolic syndrome, N=66 | High dose of resveratrol was associated with lower serum levels of androstenedione, dehydroepiandrosterone-sulphate, and dehydroepiandrosterone. However, there was no effect on prostate volume. | [72] |
| Muscadine grape skin extract | (500–4,000 mg) for 28 days | Recurrent PCa N=14; phase I/II | No patients exhibited a maintained fall in serum PSA from baseline. | [75] |
5.1. Tea polyphenols
Natural agents and antioxidants have long been marketed as supplements to consumers with claims of better health and disease prevention, even without clinical trials to back-up the claims. One such class of antioxidants, the polyphenols, are found in a variety of foods including fruits, wine, chocolate and teas, and can be classified further into the categories of phenolic acids, flavonoids, stilbenes, and lignans [15]. Research investigating polyphenols, particularly those found in green and black tea, and the role they may play in the prevention and progression of certain diseases and cancers is a growing field. Tea contains several types of polyphenols, with the catechins, including (−)-epicatechin, (−)-epicatechin-3-gallate, (−)-epigallocatechin, and (−)-epigallocatechin-3-gallate (EGCG) being the most common in green tea, and the polyphenols theaflavin and thearubgin being most prominent in black tea [16]. Several clinical trials determined that after administration to patients, these antioxidants were detectable in prostate tissue, indicating they are bioavailable in the relevant tissue and therefore may be good candidates for further chemoprevention studies in humans [17–19].
The cancer preventive effects of green tea catechins (GTCs) were examined in a study by Bettuzzi et al. [20]. Volunteers with HGPIN were given 200 mg of GTC three times daily for a total of 600 mg/d. Follow-up after one year found that there was only one instance of PCa in the treatment group (incidence 3%), while the placebo group had 9 cases of PCa (incidence 30%). Additionally, there was a trend of lower PSA levels in the GTC group, although not significant. There was, however, a significant decrease in International Prostate Symptom Score. Further, a two-year follow-up found that 2 of the 9 placebo men followed and 1 of the 13 GTC patients followed were diagnosed with PCa, indicating an 80% reduction in PCa diagnosis in patients with HGPIN [21]. Recently in 2015, Kumar et al, published results of a placebo-controlled, randomized clinical trial in which polyphenon E (PolyE), a mix of GTCs, containing 400 mg of EGCG was given daily for one year to men with HGPIN and/or ASAP. This study reported a decrease in serum PSA levels and ASAP in the Poly E group, however, a significant reduction was not seen in the number of PCa cases in Poly E supplemented men with baseline HGPIN or ASAP [22]. These results reflect potential chemopreventive properties of tea polyphenols, however further studies are required to confirm its efficacy in preventing the transformation of HGPIN into PCa.
The use of tea and the role it may play in malignant PCa was looked at in several studies using men diagnosed with PCa who were scheduled to receive surgical prostatectomies. One study looked at prostatectomy tissues of men who consumed six cups of green tea or water daily for 3–6 weeks prior [19]. This study found that 50% to 60% of the (−)-epigallocatechin and (−)-epicatechin in tissue samples were methylated, with 4″-O-MeEGCG being the most common methylated catechin. In vitro studies found that EGCG methylation was associated with decreased proliferation and increased apoptosis, suggesting that the methylation status of EGCG may alter the effectiveness of green tea intervention on treating PCa. Another study was conducted by McLarty et al, involving a total of 1.3 g of PolyE containing 800 mg of EGCG daily supplementation to men with PCa scheduled for radical prostatectomy. Significant decreases were reported in serum levels of PSA, HGF, and VEGF at the time of prostatectomy (after 3–6 weeks) suggesting a potential of EGCG in the form of Poly E against PCa [23]. Although in a similar patient study, supplementation with PolyE containing 800 mg of EGCG for 3 to 6 weeks resulted in favorable but non-significant changes in serum PSA [24]. More recently, Henning et al, conducted another trial where patients either drank 6 cups of green tea, black tea or water daily [18]. However, no significant change was observed in markers of proliferation, apoptosis, or oxidation (as measured by 8-hydroxydeoxy-guanosine) between the groups, but the tea groups had significantly decreased nuclear factor kappa B (NF-κB) staining, urinary oxidation, and PSA levels [18]. Although the preclinical and preliminary clinical studies are promising (also discussed in [25]), the role of tea polyphenols in PCa needs more long-term and adequately powered studies, in order to determine if they have the potential to be an effective agent against PCa.
5.2. Vitamins
A number of studies have evaluated vitamins as potential chemopreventive agents against PCa, both in pre-clinical and clinical settings. Two of the most well-studied vitamins for chemoprevention and intervention of PCa are vitamins D and E. It has been suggested that reduced levels of active vitamin D are associated with a higher PCa incidence and mortality [26]. However, the results of PCa chemoprevention trials with vitamin D are conflicting, suggesting complexities in vitamin D signaling in PCa. According to a PCa prevention trial, vitamin D may have differential effects depending on PCa stage [27]. A phase II, multi-center clinical trial evaluated the effect of a 28-day supplementation of the vitamin D analog, 1α-hydroxyvitamin D2 (1α-OH-D2), in patients with PCa and HGPIN [28]. Several biomarkers were tested in serum and tissues, but the only biomarker that showed significant alteration was TGF-ß2, suggesting that this vitamin D analog does not have therapeutic or chemopreventive effects, at least in the short term. However, as this trial only assessed the effects of vitamin D on patients with HGPIN or already developed organ-confined PCa, it may be worthwhile to study the effects of this less calcemic vitamin D analog in a prevention setting.
Another vitamin that has shown strong preclinical efficacy and early clinical success against PCa is vitamin E. An effect of vitamin E on PCa risk and mortality was originally found during the Alpha-Tocopherol, Beta-Carotene (ATBC) clinical trial designed to study the effects of α-tocopherol (a form of vitamin E; 50 mg/d) and β-carotene (20 mg/d) on prevention of lung cancer [29]. This was then followed up by several prospective studies, and some of them found inverse relationships between vitamin E supplementation and advanced PCa [30–32]. These findings led to one of the largest chemoprevention clinical trials ever undertaken, the Selenium and Vitamin E Cancer Prevention Trial (SELECT) [33, 34]. This trial enrolled 35,533 men and randomly assigned them to groups of either placebo, vitamin E (400 IU/d), selenium (200 μg/d), or combination of vitamin E and selenium. Despite the hope that this combination would be ideal for PCa chemoprevention, it was stopped early due to no protective effect of either agent, and a non-significant increase in PCa risk in men in the vitamin E group [35]. Further analysis and follow-up confirmed a significant increase in PCa risk, in the vitamin E alone group [36]. The reason for the disparity between the success of the ATBC study and SELECT is unclear, although many believe that it is due to the different formulations and dose regimen of tests agents used as well as the patient populations (ATBC enrolled more smokers) [36, 37]. Several preclinical studies further supported this belief including the one from our lab, where we have demonstrated that in combination with methaneseleninic acid, a lower dose of vitamin E in the form of γ-tocopherol was most effective in imparting antitumor response in 22Rν1 implanted Nu/J mice [38]. In subsequent studies, researchers have explored using other forms of vitamin E against PCa. An example of this is a phase 1/2a dose escalation study using the antioxidant moiety of vitamin E (2,2,5,7,8-Pentamethyl-6-chromanol) [39]. This study was undertaken to determine, in part, the maximum tolerated dose (MTD) and efficacy in men with CRPC. To this end, twenty patients with CRPC were administered between 900–2400 mg of vitamin E orally once daily continuously for 28 days. The study found that five of the 20 patients had stable disease as their best response, and median progression free survival (PFS) for the cohort was 2.8 months [39]. Combined with previous vitamin E successes, these results give renewed hope for using vitamin E for PCa chemoprevention, both alone and in combination with other agents. In fact, the combinatorial approach can be easily seen in the Supplementation en Vitamines et Mineraux Antioxydants (SU.VI.MAX) trial, in which 5,141 French men were enrolled to determine the effects of daily administration of low doses of several common vitamins and minerals, including vitamins C (120 mg/d) and E (30 mg/d), as well as β-carotene (6000 μg/d), zinc (as gluconate, 20 mg/d), and selenium (as selenium-enriched yeast) [40]. Although no significant change was seen in PSA levels or overall PCa incidence, men with normal baseline PSA levels had a significantly reduced risk in developing PCa, while men with a higher initial PSA were found to have a borderline significant increase in PCa incidence [41]. This suggests that formulations of vitamins and patient population need to be carefully considered for future clinical trials with these vitamins as well as other potential chemopreventive agents.
5.3. Selenium
Although toxic in large doses, selenium (Se) is an essential nutrient that is required for many cellular processes, including as a constituent of the amino acids selenocysteine and selenomethionine as well as components or cofactors of antioxidant enzymes including the glutathione peroxidases [42]. While a myriad of different forms of this micronutrient exist and their bioavailability can vary widely, it is the organic Se compounds that are mostly used for chemoprevention studies [42, 43]. The most commonly used form of Se in clinical trials to date is SeMet, both by itself and as the major component of selenized yeast (Se-yeast, consisting of ~85% SeMet [44]). In addition to this form, there are numerous in vitro and rodent experiments using Se-methylselenocysteine (MSeC), and methylseleninic acid (MSeA), as well as explorations of other organoselenium compounds [45]. Se has been explored as a potential chemotherapeutic agent since early studies suggesting that a diet high in Se may be protective against cancer death [46]. Even though numerous epidemiological studies have found inverse relationships between serum and/or toenail Se levels and prostate disease [47, 48], meta-analyses suggested that the relationship was not as clear. Two recent studies by Cui et al, [49] and Cai et al, [50] found an inverse relationship between PCa risk and serum Se levels. In another analysis where collaborating investigators from multiple prospective studies provided individual-patient records on blood or toenail Se concentrations and PCa risk, toe nail (but not blood) Se concentration was found to be inversely associated with risk of total PCa [51]. These studies have provided a strong basis for studying the effects of Se on PCa, but have failed to afford solid conclusion as to whether Se supplementation is useful in PCa prevention, leading to a need for further well-controlled clinical trials.
One of the first clinical studies to enumerate the effects of Se supplementation on PCa incidence was actually begun in order to explore the effects of selenized yeast (Se-yeast) on skin cancer [52]. Interestingly, the Nutritional Prevention of Cancer (NPC) trial did not find any significant effects of Se on skin cancer incidence, but did find a greater than 60% reduction in the incidence of PCa in participants in the 200 g Se group as compared to placebo. The success of this trial, combined with preclinical and clinical data [53], prompted the use of Se in SELECT [31, 35]. However, as discussed above, this trial was terminated early and no protective effects were seen with Se supplementation [36]. At the time when SELECT was initiated, three other studies were undertaken by the Southwest Oncology Group (SWOG) to study the ability of Se to prevent and delay the growth of PCa (reviewed in [54]). The first study, the Negative Biopsy Trial (NBT), examined the effect of Se supplementation (200 or 400 μg/d Se, as Se-yeast) in men with elevated PSA but at least one negative sextant prostate biopsy within 1 year of enrollment [55]. Like SELECT, this study also found that Se had no effect on PCa incidence or PSA velocity. The second trial studied the effects of Se supplementation (200 μg/d Se, as SeMet) on men diagnosed with HGPIN but no other signs of PCa, and found no significant differences in PCa risk after 3 years, although they reported that there was a non-significant reduced risk of PCa in patients in the lowest quartile of baseline Se (relative risk = 0.82; 95% CI: 0.40–1.69; baseline Se <103 ng/mL) [56]. The third study, named the Watchful Waiting study, placed men with non-metastatic PCa on Se (as Se-yeast; 200 or 800 μg/d) for up to 5 years [57]. This study did not observe any protective effect of Se, and in fact found that individuals with higher baseline Se had a higher risk for increased PSA velocity. These studies left researchers scrambling to explain why the preclinical success of Se did not translate to clinical situations.
Based on these negative studies, many researchers started to believe that Se may not be suitable for PCa chemoprevention [58]. However, others began to explore the reasons for the failure of these trials and put forward hypotheses, including improper dosing, formulation and/or patient population chosen, and incomplete biochemical understanding of the mechanism of action of the agents [59, 60]. A recent trial determined the effects of Se-yeast and SeMet on PSA, blood glucose and glutathione, as well as oxidative stress biomarkers, in healthy men [61]. The study did not find significant effects in PSA, glucose, or glutathione. However, the authors observed significant differences in oxidative stress markers, especially in individuals with low baseline levels of Se, but only in the Se-yeast group. This suggests that other components of the Se-yeast (potentially the other organoselenium compounds contained therein) may have stronger anti-oxidative and potentially chemopreventive effects. Indeed, further multi-directional research is needed to define the potential usefulness of Se for PCa chemoprevention.
5.4. Soy
PCa incidence has traditionally been relatively low in many Asian countries, and many postulate that this is due to the drastic differences in diet, among others especially the consumption of soy [62]. Preclinical research has shown a beneficial role for soy isoflavone consumption in the management of PCa, and soy isoflavones have been shown to improve PSA levels in several clinical trials. A randomized 3-month intervention study noted a 14% reduction in circulating serum PSA levels while on a high soy diet, but no change in testosterone levels. This shows the feasibility of a randomized soy intervention trial among men against PCa [63]. The high tolerability and beneficial effects of dietary soy were further explored in a six-month phase II trial of a soy beverage for patients with rising PSA after radical radiation to prevent PCa recurrence. Although the results did not reach significance, almost 14% of patients showed decreasing PSA levels, and 41% of subjects had more than 2 times prolongation of PSA doubling time [64]. However, no effect of soy isoflavones on serum PSA was observed in a 12-month double-blinded, randomized trial, where participants were assigned to consume either a soy protein drink containing 83 mg/d isoflavones (+ISO) or a drink without isoflavones (−ISO) [65]. In a short-term intervention study on patients with localized prostate cancer, the soy isoflavone (80 mg/day in form of capsules) supplementation for up to six weeks did not show any changes in PSA, serum hormone levels, and total cholesterol [66]. Another phase II trial determine the effects of an oral isoflavone (60 mg/d) or a placebo supplementation in men with increasing PSA for 12 months. The incidence of biopsy-detectable PCa showed no statistically significant difference between the isoflavone and placebo groups (21.4% vs 34.0%, P = 0.140). However, for patients aged 65 years or more, the incidence of cancer in the isoflavone group was significantly lower than that in the placebo group (28.0% vs 57.1%) [67]. These results support the value of isoflavone for PCa risk reduction, but further research must occur before more definitive conclusions can be obtained. It is also possible that the effects of isoflavones may depend on the stage of cancer development. Further, isoflavone may have other effects on tumor biology, which are not revealed via serum PSA concentration.
In addition to PSA, androgen receptors (AR) are also used as a marker for PCa prognosis. During a 6-month intervention study, consumption of isoflavone-containing soy protein isolate (SPI+) (107 mg/d isoflavones) significantly suppressed AR expression without altering estrogen receptor-beta expression or circulating hormones in prostate biopsies, compared to milk protein isolate (MPI) (0 mg/d isoflavones) consumption [68]. In addition, consumption of soy protein isolate (SPI−) (<6 mg/d isoflavones) significantly increased estradiol and androstenedione concentrations, and lowered AR expression compared with the MPI group (P = 0.09). While the SPI− results are difficult to interpret, the clinical significance of soy consumption and its effect on the downregulation of AR expression has the potential to be advantageous in PCa prevention.
Nutritionally relevant levels of genistein and daidzein, the predominant isoflavones in soy, may have an inhibitory effect on androgen-related biomarkers and modulate the progression of existing PCa. A two-part study was conducted consisting of a 6-month double-blind, placebo-controlled, randomized trial that involved the treatment group to consume a daily supplement containing 450 mg genistein, 300 mg daidzein, and other isoflavones, followed by a 6-month open label trial with the same isoflavone-rich supplement [69]. Although the PSA concentrations did not change in either group after the double-blind trial or the open-label trial, the 6-month serum concentrations of genistein and daidzein (39.85 and 45.59 mol/l) were significantly greater than baseline values. Because high isoflavone concentrations in prostate tissue may influence PSA levels or other molecular mechanisms, these heightened serum concentrations of genistein and daidzein provide a starting place from which further studies can be planned.
Despite the potential additive effects of using soy protein isolates containing multiple isoflavones, it is important to study the effects of the individual components, including genistein. Lazarevic et al, investigated the effects of genistein on PCa in a phase II, placebo-controlled, randomized double-blind clinical trial with patients before radical prostatectomy [70]. An intervention was given daily as 30 mg genistein or placebo capsules for 3–6 weeks, and various biochemical markers were tested in the PCa tissue that was removed during the prostatectomy. Genistein intervention provided no significant effects on proliferation, cell cycle, apoptosis and neuroendocrine biomarkers. However, in tumor cells, it significantly downregulated the mRNA level of KLK4, which is implicated in cancer progression. This was associated with a non-significant reduction in androgen and cell cycle-related biomarkers. While this study shows that genistein has potential biochemical effects on PCa, the ability of genistein to modulate the expression of prostate tissue biomarkers associated with PCa prediction and progression remains unclear. Overall, although the magnitude of the chemopreventive effect of soy and its constituent isoflavones remains fairly uncertain, it is necessary to further investigate the effects of consistent and prolonged soy consumption for PCa chemoprevention.
5.5. Resveratrol
Resveratrol (trans-3,5,4′-trihydroxystilbene), a naturally occurring polyphenol and an antioxidant found in many plants, including grapes, has been shown to exert chemopreventive as well as therapeutic effects against several cancers. A number of preclinical studies have demonstrated that resveratrol can reduce prostate growth in vitro and in animal models (reviewed in [71]). Thus far, no human clinical trial has been conducted to evaluate the effects of resveratrol specifically on PCa, either in the prevention or in treatment settings. However, recently in 2015, a randomized placebo-controlled clinical human study was undertaken to evaluate the effects of resveratrol on middle-aged men with metabolic syndrome [72]. In this trial, the authors of the study included measurements of prostate size, PSA and sex steroid hormones in the same cohort. This study demonstrated that a high dose of resveratrol (1,000 mg/d) administration for 4 months significantly lowered serum levels of the androgen precursors androstenedione, dehydroepiandrosterone and dehydroepiandrosterone sulfate, although the prostate size and circulating levels of PSA, testosterone, free testosterone, and dihydrotestosterone were unaffected [72]. This suggests that longer-term supplementation may have greater effects, and should be studied further. Also, it may be helpful to test chemopreventive properties of resveratrol in combination with other antioxidant agents that are found together naturally, such as in grape [73, 74]. A phase I/II study published in 2015 evaluated the safety and tolerability of a pulverized muscadine grape skin extract (MPX), which contains resveratrol, ellagic acid, quercetin, and several other grape antioxidants, to determine a dosing regimen to test against biochemically recurrent PCa [75]. The phase I portion of the study found that their maximum dose tested, 4,000 mg/d, was safe with only grade 1 adverse events reported that were related to the study. This dose was then used for the phase II study, which is still under way. Although, the phase I population size was very small and there was no maintained decline in serum PSA from baseline, the results from the study suggest that 4,000 mg/d of muscadine grape skin extract is safe and it is further being investigated in a randomized, multicenter, placebo-controlled, dose-evaluating phase II trial. Thus, resveratrol alone or in combination with other agents merit further investigation for their potential efficacy against PCa in humans.
6. Challenges and opportunities with PCa chemopreventive agents in clinical trials
There are a number of challenges in the translational development of natural chemopreventive agents. Some of these are, i) the lack of immediate effects that are required when chemopreventive agents are used in intervention settings, ii) fear of unexpected toxicity when chemopreventive agents are used in primary prevention settings (long-term supplementation), and iii) low target-organ and/or serum bioavailability of a number of chemopreventive agents. It can be extremely difficult to determine the time needed for a chemopreventive regimen in order to obtain an acceptable response. The failure of many chemopreventive studies, including SELECT, has made researchers take a step back and determine ways to better plan studies and evaluate risk factors [76]. Furthermore, to avoid future failures and to determine whether or not an agent of interest is an appropriate candidate, the FDA has introduced ‘Phase 0’ clinical trials. These studies are often referred as “human microdosing studies,” and are conducted with a limited number of subjects in order to establish whether the drug/chemopreventive agent of interest behaves same in human subjects as expected from preclinical studies [77].
The issue of limited bioavailability at the target organ is very important in secondary or tertiary chemoprevention settings. Efforts have been made towards the targeted slow delivery of chemopreventive agents. For example, Siddiqui et al have introduced the concept of ‘nanochemoprevention’ and showed that encapsulating EGCG in polylactic acid–polyethylene glycol nanoparticles provided a 10-fold dose advantage of nono-EGCG against PCa in both in vitro and in vivo [78]. In a follow-up study, these investigators developed and evaluated the efficacy of polymeric EGCG-encapsulated nanoparticles targeted to prostate-specific membrane antigen (PSMA, a transmembrane protein known to be overexpressed in PCa), and found an enhanced anticancer potential of EGCG against PCa in preclinical studies [79]. Several other similar studies are ongoing at the preclinical levels with multiple chemopreventive agents. However, a clinical translation of these studies does not seem to an easy task.
Another strategy of improving bioavailability or obtain potentially synergistic or additive chemopreventive response, is via a combinatorial approach. Recently, we have discussed challenges associated with resveratrol and other grape antioxidants in its clinical translation, and suggested that resveratrol may be very useful when given in combination with other agents or other antioxidants present in grapes [73, 74, 80, 81]. The effective combination may improve the bioavailability related issues, may target multiple deregulated genes/pathways, and therefore, may exert additive or synergistic responses to improve the biological outcome. Despite these challenges, the available data is promising and concerted efforts are required from researchers to overcome these obstacles.
7. Conclusions
The available literature on clinical studies using chemopreventive agents against PCa suggests variable outcomes postulating some discrepancies, with favorable, null, and unfavorable results. Some chemopreventive agents that target androgen-signaling have been shown to reduce PCa incidence, but it remains to be investigated if these agents can also reduce PCa mortality. As a concluding remark, we would like to point out that many chemopreventive agents have shown potential for PCa chemoprevention, however, further well-designed, rigorous clinical studies are required to optimize dose, formulations, and appropriate target population.
Highlights.
➢ Long latency period makes prostate cancer an ideal target for chemoprevention.
➢ Natural agents have shown potential in clinical trials against prostate cancer.
➢ The hurdles in the clinical chemoprevention needs to be carefully identified.
➢ Future well-designed studies are required to optimize dose and formulations.
Acknowledgments
This work was partially supported by funding from the National Institutes of Health (R01AR059130 and R01CA176748) and the Department of Veterans Affairs (VA Merit Review Awards I01BX001008 and I01CX001441; and a Research Career Scientist Award IK6BX003780 to NA).
Abbreviations
- PCa
prostate cancer
- ADT
androgen deprivation therapy
- CRPC
castrate-resistant Prostate Cancer
- HGPIN
high-grade prostatic intraepithelial neoplasia
- AR
androgen receptor
- DHT
dihydrotestosterone
- ARE
androgen response elements
- PSA
prostate specific antigen
- GR
glucocorticoid receptor
- EGCG
(−)-epigallocatechin-3-gallate
- GTCs
green tea catechins
- 1α-OH-D2
1α-hydroxyvitamin D2
- SELECT
selenium and vitamin E cancer prevention trial
- ATBC
alpha-tocopherol, beta-carotene
- MTD
maximum tolerated dose
- PFS
progression free survival
- SeMet
selenomethionine
- MPX
muscadine grape skin extract
- PSMA
prostate specific membrane antigen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
There is no conflict of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55:310–320. doi: 10.1016/j.eururo.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Tilki D, Schaeffer EM, Evans CP. Understanding Mechanisms of Resistance in Metastatic Castration-resistant Prostate Cancer: The Role of the Androgen Receptor. Eur Urol Focus. 2016;2:499–505. doi: 10.1016/j.euf.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Shephard RJ. Physical Activity and Prostate Cancer: An Updated Review. Sports Med. 2017;47:1055–1073. doi: 10.1007/s40279-016-0648-0. [DOI] [PubMed] [Google Scholar]
- 5.Hoang DT, Iczkowski KA, Kilari D, See W, Nevalainen MT. Androgen receptor-dependent and -independent mechanisms driving prostate cancer progression: Opportunities for therapeutic targeting from multiple angles. Oncotarget. 2017;8:3724–3745. doi: 10.18632/oncotarget.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montgomery JS, Price DK, Figg WD. The androgen receptor gene and its influence on the development and progression of prostate cancer. J Pathol. 2001;195:138–146. doi: 10.1002/1096-9896(200109)195:2<138::AID-PATH961>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 7.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M, G Prostate Cancer Clinical Trials Working Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kgatle MM, Kalla AA, Islam MM, Sathekge M, Moorad R. Prostate Cancer: Epigenetic Alterations, Risk Factors, and Therapy. Prostate Cancer. 2016;2016:5653862. doi: 10.1155/2016/5653862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chhabra G, Ndiaye MA, Garcia-Peterson LM, Ahmad N. Melanoma Chemoprevention: Current Status and Future Prospects. Photochem Photobiol. 2017;93:975–989. doi: 10.1111/php.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotecha R, Takami A, Espinoza JL. Dietary phytochemicals and cancer chemoprevention: a review of the clinical evidence. Oncotarget. 2016;7:52517–52529. doi: 10.18632/oncotarget.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adhami VM, Mukhtar H. Anti-oxidants from green tea and pomegranate for chemoprevention of prostate cancer. Mol Biotechnol. 2007;37:52–57. doi: 10.1007/s12033-007-0047-8. [DOI] [PubMed] [Google Scholar]
- 13.Cui K, Li X, Du Y, Tang X, Arai S, Geng Y, Xi Y, Xu H, Zhou Y, Ma W, Zhang T. Chemoprevention of prostate cancer in men with high-grade prostatic intraepithelial neoplasia (HGPIN): a systematic review and adjusted indirect treatment comparison. Oncotarget. 2017;8:36674–36684. doi: 10.18632/oncotarget.16230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar N, Crocker T, Smith T, Connors S, Pow-Sang J, Spiess PE, Egan K, Quinn G, Schell M, Sebti S, Kazi A, Chuang T, Salup R, Helal M, Zagaja G, Trabulsi E, McLarty J, Fazili T, Williams CR, Schreiber F, Anderson K. Prostate Cancer Chemoprevention Targeting Men with High-Grade Prostatic Intraepithelial Neoplasia (HGPIN) and Atypical Small Acinar Proliferation (ASAP): Model for Trial Design and Outcome Measures. J Clin Trials. 2012;2 doi: 10.4172/jctr.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 16.Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000;71:1698S–1702S. doi: 10.1093/ajcn/71.6.1698S. discussion 1703S–1694S. [DOI] [PubMed] [Google Scholar]
- 17.Henning SM, Aronson W, Niu Y, Conde F, Lee NH, Seeram NP, Lee RP, Lu J, Harris DM, Moro A, Hong J, Pak-Shan L, Barnard RJ, Ziaee HG, Csathy G, Go VL, Wang H, Heber D. Tea polyphenols and theaflavins are present in prostate tissue of humans and mice after green and black tea consumption. J Nutr. 2006;136:1839–1843. doi: 10.1093/jn/136.7.1839. [DOI] [PubMed] [Google Scholar]
- 18.Henning SM, Wang P, Said JW, Huang M, Grogan T, Elashoff D, Carpenter CL, Heber D, Aronson WJ. Randomized clinical trial of brewed green and black tea in men with prostate cancer prior to prostatectomy. Prostate. 2015;75:550–559. doi: 10.1002/pros.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P, Aronson WJ, Huang M, Zhang Y, Lee RP, Heber D, Henning SM. Green tea polyphenols and metabolites in prostatectomy tissue: implications for cancer prevention. Cancer Prev Res (Phila) 2010;3:985–993. doi: 10.1158/1940-6207.CAPR-09-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 21.Brausi M, Rizzi F, Bettuzzi S. Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update. Eur Urol. 2008;54:472–473. doi: 10.1016/j.eururo.2008.03.100. [DOI] [PubMed] [Google Scholar]
- 22.Kumar NB, Pow-Sang J, Egan KM, Spiess PE, Dickinson S, Salup R, Helal M, McLarty J, Williams CR, Schreiber F, Parnes HL, Sebti S, Kazi A, Kang L, Quinn G, Smith T, Yue B, Diaz K, Chornokur G, Crocker T, Schell MJ. Randomized, Placebo-Controlled Trial of Green Tea Catechins for Prostate Cancer Prevention. Cancer Prev Res (Phila) 2015;8:879–887. doi: 10.1158/1940-6207.CAPR-14-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila) 2009;2:673–682. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen MM, Ahmann FR, Nagle RB, Hsu CH, Tangrea JA, Parnes HL, Sokoloff MH, Gretzer MB, Chow HH. Randomized, double-blind, placebo-controlled trial of polyphenon E in prostate cancer patients before prostatectomy: evaluation of potential chemopreventive activities. Cancer Prev Res (Phila) 2012;5:290–298. doi: 10.1158/1940-6207.CAPR-11-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan N, Adhami VM, Mukhtar H. Review: green tea polyphenols in chemoprevention of prostate cancer: preclinical and clinical studies. Nutr Cancer. 2009;61:836–841. doi: 10.1080/01635580903285056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenk JM, Till CA, Tangen CM, Goodman PJ, Song X, Torkko KC, Kristal AR, Peters U, Neuhouser ML. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2014;23:1484–1493. doi: 10.1158/1055-9965.EPI-13-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gee J, Bailey H, Kim K, Kolesar J, Havighurst T, Tutsch KD, See W, Cohen MB, Street N, Levan L, Jarrard D, Wilding G. Phase II open label, multi-center clinical trial of modulation of intermediate endpoint biomarkers by 1alpha-hydroxyvitamin D2 in patients with clinically localized prostate cancer and high grade pin. Prostate. 2013;73:970–978. doi: 10.1002/pros.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 30.Peters U, Littman AJ, Kristal AR, Patterson RE, Potter JD, White E. Vitamin E and selenium supplementation and risk of prostate cancer in the Vitamins and lifestyle (VITAL) study cohort. Cancer Causes Control. 2008;19:75–87. doi: 10.1007/s10552-007-9072-y. [DOI] [PubMed] [Google Scholar]
- 31.Chan JM, Stampfer MJ, Ma J, Rimm EB, Willett WC, Giovannucci EL. Supplemental vitamin E intake and prostate cancer risk in a large cohort of men in the United States. Cancer Epidemiol Biomarkers Prev. 1999;8:893–899. [PubMed] [Google Scholar]
- 32.Rodriguez C, Jacobs EJ, Mondul AM, Calle EE, McCullough ML, Thun MJ. Vitamin E supplements and risk of prostate cancer in U.S. men. Cancer Epidemiol Biomarkers Prev. 2004;13:378–382. [PubMed] [Google Scholar]
- 33.Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, Coltman C. SELECT: the Selenium and Vitamin E Cancer Prevention Trial: rationale and design. Prostate Cancer Prostatic Dis. 2000;3:145–151. doi: 10.1038/sj.pcan.4500412. [DOI] [PubMed] [Google Scholar]
- 34.Lippman SM, Goodman PJ, Klein EA, Parnes HL, Thompson IM, Jr, Kristal AR, Santella RM, Probstfield JL, Moinpour CM, Albanes D, Taylor PR, Minasian LM, Hoque A, Thomas SM, Crowley JJ, Gaziano JM, Stanford JL, Cook ED, Fleshner NE, Lieber MM, Walther PJ, Khuri FR, Karp DD, Schwartz GG, Ford LG, Coltman CA., Jr Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97:94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 35.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL, Jr, Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardenas E, Ghosh R. Vitamin E: a dark horse at the crossroad of cancer management. Biochem Pharmacol. 2013;86:845–852. doi: 10.1016/j.bcp.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh CK, Ndiaye MA, Siddiqui IA, Nihal M, Havighurst T, Kim K, Zhong W, Mukhtar H, Ahmad N. Methaneseleninic acid and gamma-Tocopherol combination inhibits prostate tumor growth in Vivo in a xenograft mouse model. Oncotarget. 2014;5:3651–3661. doi: 10.18632/oncotarget.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyriakopoulos CE, Heath EI, Eickhoff JC, Kolesar J, Yayehyirad M, Moll T, Wilding G, Liu G. A multicenter phase 1/2a dose-escalation study of the antioxidant moiety of vitamin E 2,2,5,7,8-pentamethyl-6-chromanol (APC-100) in men with advanced prostate cancer. Invest New Drugs. 2016;34:225–230. doi: 10.1007/s10637-016-0334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hercberg S, Preziosi P, Briancon S, Galan P, Triol I, Malvy D, Roussel AM, Favier A. A primary prevention trial using nutritional doses of antioxidant vitamins and minerals in cardiovascular diseases and cancers in a general population: the SU.VI.MAX study–design, methods, and participant characteristics. SUpplementation en VItamines et Mineraux AntioXydants. Control Clin Trials. 1998;19:336–351. doi: 10.1016/s0197-2456(98)00015-4. [DOI] [PubMed] [Google Scholar]
- 41.Meyer F, Galan P, Douville P, Bairati I, Kegle P, Bertrais S, Estaquio C, Hercberg S. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. Int J Cancer. 2005;116:182–186. doi: 10.1002/ijc.21058. [DOI] [PubMed] [Google Scholar]
- 42.Abdulah R, Miyazaki K, Nakazawa M, Koyama H. Chemical forms of selenium for cancer prevention. J Trace Elem Med Biol. 2005;19:141–150. doi: 10.1016/j.jtemb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi K, Suzuki N, Ogra Y. Bioavailability Comparison of Nine Bioselenocompounds In Vitro and In Vivo. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ip C, Birringer M, Block E, Kotrebai M, Tyson JF, Uden PC, Lisk DJ. Chemical speciation influences comparative activity of selenium-enriched garlic and yeast in mammary cancer prevention. J Agric Food Chem. 2000;48:4452. doi: 10.1021/jf000932m. [DOI] [PubMed] [Google Scholar]
- 45.Plano D, Baquedano Y, Ibanez E, Jimenez I, Palop JA, Spallholz JE, Sanmartin C. Antioxidant-prooxidant properties of a new organoselenium compound library. Molecules. 2010;15:7292–7312. doi: 10.3390/molecules15107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shamberger RJ, Frost DV. Possible protective effect of selenium against human cancer. Can Med Assoc J. 1969;100:682. [PMC free article] [PubMed] [Google Scholar]
- 47.Geybels MS, Verhage BA, van Schooten FJ, Goldbohm RA, van den Brandt PA. Advanced prostate cancer risk in relation to toenail selenium levels. J Natl Cancer Inst. 2013;105:1394–1401. doi: 10.1093/jnci/djt186. [DOI] [PubMed] [Google Scholar]
- 48.Yoshizawa K, Willett WC, Morris SJ, Stampfer MJ, Spiegelman D, Rimm EB, Giovannucci E. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. J Natl Cancer Inst. 1998;90:1219–1224. doi: 10.1093/jnci/90.16.1219. [DOI] [PubMed] [Google Scholar]
- 49.Cui Z, Liu D, Liu C, Liu G. Serum selenium levels and prostate cancer risk: A MOOSE-compliant meta-analysis. Medicine (Baltimore) 2017;96:e5944. doi: 10.1097/MD.0000000000005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai X, Wang C, Yu W, Fan W, Wang S, Shen N, Wu P, Li X, Wang F. Selenium Exposure and Cancer Risk: an Updated Meta-analysis and Meta-regression. Sci Rep. 2016;6:19213. doi: 10.1038/srep19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allen NE, Travis RC, Appleby PN, Albanes D, Barnett MJ, Black A, Bueno-de-Mesquita HB, Deschasaux M, Galan P, Goodman GE, Goodman PJ, Gunter MJ, Heliovaara M, Helzlsouer KJ, Henderson BE, Hercberg S, Knekt P, Kolonel LN, Lasheras C, Linseisen J, Metter EJ, Neuhouser ML, Olsen A, Pala V, Platz EA, Rissanen H, Reid ME, Schenk JM, Stampfer MJ, Stattin P, Tangen CM, Touvier M, Trichopoulou A, van den Brandt PA, Key TJ, N.B. Endogenous Hormones, G. Prostate Cancer Collaborative Selenium and Prostate Cancer: Analysis of Individual Participant Data From Fifteen Prospective Studies. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL, Jr, Park HK, Sanders BB, Jr, Smith CL, Taylor JR. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 53.Sabichi AL, Lee JJ, Taylor RJ, Thompson IM, Miles BJ, Tangen CM, Minasian LM, Pisters LL, Caton JR, Basler JW, Lerner SP, Menter DG, Marshall JR, Crawford ED, Lippman SM. Selenium accumulation in prostate tissue during a randomized, controlled short-term trial of l-selenomethionine: a Southwest Oncology Group Study. Clin Cancer Res. 2006;12:2178–2184. doi: 10.1158/1078-0432.CCR-05-0937. [DOI] [PubMed] [Google Scholar]
- 54.Marshall JR. Larry Clark’s legacy: randomized controlled, selenium-based prostate cancer chemoprevention trials. Nutr Cancer. 2001;40:74–77. doi: 10.1207/S15327914NC401_13. [DOI] [PubMed] [Google Scholar]
- 55.Algotar AM, Stratton MS, Ahmann FR, Ranger-Moore J, Nagle RB, Thompson PA, Slate E, Hsu CH, Dalkin BL, Sindhwani P, Holmes MA, Tuckey JA, Graham DL, Parnes HL, Clark LC, Stratton SP. Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer. Prostate. 2013;73:328–335. doi: 10.1002/pros.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marshall JR, Tangen CM, Sakr WA, Wood DP, Jr, Berry DL, Klein EA, Lippman SM, Parnes HL, Alberts DS, Jarrard DF, Lee WR, Gaziano JM, Crawford ED, Ely B, Ray M, Davis W, Minasian LM, Thompson IM., Jr Phase III trial of selenium to prevent prostate cancer in men with high-grade prostatic intraepithelial neoplasia: SWOG S9917. Cancer Prev Res (Phila) 2011;4:1761–1769. doi: 10.1158/1940-6207.CAPR-10-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stratton MS, Algotar AM, Ranger-Moore J, Stratton SP, Slate EH, Hsu CH, Thompson PA, Clark LC, Ahmann FR. Oral selenium supplementation has no effect on prostate-specific antigen velocity in men undergoing active surveillance for localized prostate cancer. Cancer Prev Res (Phila) 2010;3:1035–1043. doi: 10.1158/1940-6207.CAPR-09-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Potter JD. The failure of cancer chemoprevention. Carcinogenesis. 2014;35:974–982. doi: 10.1093/carcin/bgu063. [DOI] [PubMed] [Google Scholar]
- 59.Ledesma MC, Jung-Hynes B, Schmit TL, Kumar R, Mukhtar H, Ahmad N. Selenium and vitamin E for prostate cancer: post-SELECT (Selenium and Vitamin E Cancer Prevention Trial) status. Mol Med. 2011;17:134–143. doi: 10.2119/molmed.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frankel PH, Parker RS, Madsen FC, Whanger PD. Baseline selenium and prostate cancer risk: comments and open questions. J Natl Cancer Inst, United States. 2014;106:djuoo5. doi: 10.1093/jnci/dju005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richie JP, Jr, Das A, Calcagnotto AM, Sinha R, Neidig W, Liao J, Lengerich EJ, Berg A, Hartman TJ, Ciccarella A, Baker A, Kaag MG, Goodin S, DiPaola RS, El-Bayoumy K. Comparative effects of two different forms of selenium on oxidative stress biomarkers in healthy men: a randomized clinical trial. Cancer Prev Res (Phila) 2014;7:796–804. doi: 10.1158/1940-6207.CAPR-14-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–864. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 63.Maskarinec G, Morimoto Y, Hebshi S, Sharma S, Franke AA, Stanczyk FZ. Serum prostate-specific antigen but not testosterone levels decrease in a randomized soy intervention among men. Eur J Clin Nutr. 2006;60:1423–1429. doi: 10.1038/sj.ejcn.1602473. [DOI] [PubMed] [Google Scholar]
- 64.Kwan W, Duncan G, Van Patten C, Liu M, Lim J. A phase II trial of a soy beverage for subjects without clinical disease with rising prostate-specific antigen after radical radiation for prostate cancer. Nutr Cancer. 2010;62:198–207. doi: 10.1080/01635580903305318. [DOI] [PubMed] [Google Scholar]
- 65.Adams KF, Chen C, Newton KM, Potter JD, Lampe JW. Soy isoflavones do not modulate prostate-specific antigen concentrations in older men in a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2004;13:644–648. [PubMed] [Google Scholar]
- 66.Hamilton-Reeves JM, Banerjee S, Banerjee SK, Holzbeierlein JM, Thrasher JB, Kambhampati S, Keighley J, Van Veldhuizen P. Short-term soy isoflavone intervention in patients with localized prostate cancer: a randomized, double-blind, placebo-controlled trial. PLoS One. 2013;8:e68331. doi: 10.1371/journal.pone.0068331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyanaga N, Akaza H, Hinotsu S, Fujioka T, Naito S, Namiki M, Takahashi S, Hirao Y, Horie S, Tsukamoto T, Mori M, Tsuji H. Prostate cancer chemoprevention study: an investigative randomized control study using purified isoflavones in men with rising prostate-specific antigen. Cancer Sci. 2012;103:125–130. doi: 10.1111/j.1349-7006.2011.02120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamilton-Reeves JM, Rebello SA, Thomas W, Slaton JW, Kurzer MS. Isoflavone-rich soy protein isolate suppresses androgen receptor expression without altering estrogen receptor-beta expression or serum hormonal profiles in men at high risk of prostate cancer. J Nutr. 2007;137:1769–1775. doi: 10.1093/jn/137.7.1769. [DOI] [PubMed] [Google Scholar]
- 69.deVere White RW, Tsodikov A, Stapp EC, Soares SE, Fujii H, Hackman RM. Effects of a high dose, aglycone-rich soy extract on prostate-specific antigen and serum isoflavone concentrations in men with localized prostate cancer. Nutr Cancer. 2010;62:1036–1043. doi: 10.1080/01635581.2010.492085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lazarevic B, Hammarstrom C, Yang J, Ramberg H, Diep LM, Karlsen SJ, Kucuk O, Saatcioglu F, Tasken KA, Svindland A. The effects of short-term genistein intervention on prostate biomarker expression in patients with localised prostate cancer before radical prostatectomy. Br J Nutr. 2012;108:2138–2147. doi: 10.1017/S0007114512000384. [DOI] [PubMed] [Google Scholar]
- 71.Thompson IM, Jr, Cabang AB, Wargovich MJ. Future directions in the prevention of prostate cancer. Nat Rev Clin Oncol. 2014;11:49–60. doi: 10.1038/nrclinonc.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kjaer TN, Ornstrup MJ, Poulsen MM, Jorgensen JO, Hougaard DM, Cohen AS, Neghabat S, Richelsen B, Pedersen SB. Resveratrol reduces the levels of circulating androgen precursors but has no effect on, testosterone, dihydrotestosterone, PSA levels or prostate volume. A 4-month randomised trial in middle-aged men. Prostate. 2015;75:1255–1263. doi: 10.1002/pros.23006. [DOI] [PubMed] [Google Scholar]
- 73.Singh CK, Siddiqui IA, El-Abd S, Mukhtar H, Ahmad N. Combination chemoprevention with grape antioxidants. Mol Nutr Food Res. 2016;60:1406–1415. doi: 10.1002/mnfr.201500945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh CK, Liu X, Ahmad N. Resveratrol, in its natural combination in whole grape, for health promotion and disease management. Ann N Y Acad Sci. 2015;1348:150–160. doi: 10.1111/nyas.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paller CJ, Rudek MA, Zhou XC, Wagner WD, Hudson TS, Anders N, Hammers HJ, Dowling D, King S, Antonarakis ES, Drake CG, Eisenberger MA, Denmeade SR, Rosner GL, Carducci MA. A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: Safety, tolerability, and dose determination. Prostate. 2015;75:1518–1525. doi: 10.1002/pros.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyskens FL, Jr, Mukhtar H, Rock CL, Cuzick J, Kensler TW, Yang CS, Ramsey SD, Lippman SM, Alberts DS. Cancer Prevention: Obstacles, Challenges and the Road Ahead. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murgo AJ, Kummar S, Rubinstein L, Gutierrez M, Collins J, Kinders R, Parchment RE, Ji J, Steinberg SM, Yang SX, Hollingshead M, Chen A, Helman L, Wiltrout R, Tomaszewski JE, Doroshow JH. Designing phase 0 cancer clinical trials. Clin Cancer Res. 2008;14:3675–3682. doi: 10.1158/1078-0432.CCR-07-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI, Ahmad N, Cui H, Mousa SA, Mukhtar H. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69:1712–1716. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanna V, Singh CK, Jashari R, Adhami VM, Chamcheu JC, Rady I, Sechi M, Mukhtar H, Siddiqui IA. Targeted nanoparticles encapsulating (−)-epigallocatechin-3-gallate for prostate cancer prevention and therapy. Sci Rep. 2017;7:41573. doi: 10.1038/srep41573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh CK, Ndiaye MA, Ahmad N. Resveratrol and cancer: Challenges for clinical translation. Biochim Biophys Acta. 2015;1852:1178–1185. doi: 10.1016/j.bbadis.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh CK, George J, Ahmad N. Resveratrol-based combinatorial strategies for cancer management. Ann N Y Acad Sci. 2013;1290:113–121. doi: 10.1111/nyas.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beer TM, Myrthue A, Garzotto M, O’Hara MF, Chin R, Lowe BA, Montalto MA, Corless CL, Henner WD. Randomized study of high-dose pulse calcitriol or placebo prior to radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2004;13:2225–2232. [PubMed] [Google Scholar]
- 83.Baron JA, Beach M, Wallace K, Grau MV, Sandler RS, Mandel JS, Heber D, Greenberg ER. Risk of prostate cancer in a randomized clinical trial of calcium supplementation. Cancer Epidemiol Biomarkers Prev. 2005;14:586–589. doi: 10.1158/1055-9965.EPI-04-0319. [DOI] [PubMed] [Google Scholar]
- 84.Clark LC, Dalkin B, Krongrad A, Combs GF, Jr, Turnbull BW, Slate EH, Witherington R, Herlong JH, Janosko E, Carpenter D, Borosso C, Falk S, Rounder J. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998;81:730–734. doi: 10.1046/j.1464-410x.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 85.Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF, Jr, Slate EH, Fischbach LA, Marshall JR, Clark LC. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- 86.Duffield-Lillico AJ, Dalkin BL, Reid ME, Turnbull BW, Slate EH, Jacobs ET, Marshall JR, Clark LC, G Nutritional Prevention of Cancer Study Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91:608–612. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 87.Kristal AR, Darke AK, Morris JS, Tangen CM, Goodman PJ, Thompson IM, Meyskens FL, Jr, Goodman GE, Minasian LM, Parnes HL, Lippman SM, Klein EA. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/djt456. djt456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stratton MS, Reid ME, Schwartzberg G, Minter FE, Monroe BK, Alberts DS, Marshall JR, Ahmann FR. Selenium and prevention of prostate cancer in high-risk men: the Negative Biopsy Study. Anticancer Drugs. 2003;14:589–594. doi: 10.1097/00001813-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Stratton MS, Reid ME, Schwartzberg G, Minter FE, Monroe BK, Alberts DS, Marshall JR, Ahmann FR. Selenium and inhibition of disease progression in men diagnosed with prostate carcinoma: study design and baseline characteristics of the ‘Watchful Waiting’ Study. Anticancer Drugs. 2003;14:595–600. doi: 10.1097/00001813-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 90.Marshall JR, Sakr W, Wood D, Berry D, Tangen C, Parker F, Thompson I, Lippman SM, Lieberman R, Alberts D, Jarrard D, Coltman C, Greenwald P, Minasian L, Crawford ED. Design and progress of a trial of selenium to prevent prostate cancer among men with high-grade prostatic intraepithelial neoplasia. Cancer Epidemiol Biomarkers Prev. 2006;15:1479–1484. doi: 10.1158/1055-9965.EPI-05-0585. [DOI] [PubMed] [Google Scholar]


