Abstract
Conflicting roles for protein kinase C (PKC) isozymes in cardiac disease have been reported. Here, δPKC-selective activator and inhibitor peptides were designed rationally, based on molecular modeling and structural homology analyses. Together with previously identified activator and inhibitor peptides of ɛPKC, δPKC peptides were used to identify cardiac functions of these isozymes. In isolated cardiomyocytes, perfused hearts, and transgenic mice, δPKC and ɛPKC had opposing actions on protection from ischemia-induced damage. Specifically, activation of ɛPKC caused cardioprotection whereas activation of δPKC increased damage induced by ischemia in vitro and in vivo. In contrast, δPKC and ɛPKC caused identical nonpathological cardiac hypertrophy; activation of either isozyme caused nonpathological hypertrophy of the heart. These results demonstrate that two related PKC isozymes have both parallel and opposing effects in the heart, indicating the danger in the use of therapeutics with nonselective isozyme inhibitors and activators. Moreover, reduction in cardiac damage caused by ischemia by perfusion of selective regulator peptides of PKC through the coronary arteries constitutes a major step toward developing a therapeutic agent for acute cardiac ischemia.
There are conflicting reports on the role of protein kinase C (PKC) in mediating myocardial protection from ischemia (1). Moreover, changes in the expression of some PKC isozymes in cardiac hypertrophy and heart failure (2–4) could reflect either their active role or a consequence of these diseases. Current approaches include the use of isozyme nonselective tools and overexpression of individual PKC isozymes, which prevent a meaningful interpretation of the data.
Each of the six PKC isozymes in cardiac myocytes translocates to different subcellular sites upon activation (5–7). We proposed a mechanism for isozyme-specific translocation involving binding of activated PKC to isozyme-specific anchoring proteins termed RACKs [receptors for activated C kinase (7)]. Binding of a specific PKC isozyme to its RACK occurs after phospholipid-induced allosteric conformational changes that activate the enzyme and expose its RACK-binding domain. This leads to PKC translocation and binding to isozyme-specific RACKs at different subcellular sites, which is thought to determine the function of each isozyme (5, 6). Based on this hypothesis, we developed several peptide translocation inhibitors and activators, which when introduced into cells, cause selective regulation of translocation and function of the corresponding PKC isozyme (8).
Translocation inhibitor peptides correspond to specific RACK-binding sites in the C2/V1 domain (9–12) of each isozyme and act as isozyme-selective competitors of PKC-RACK binding and function (10, 12). Peptide activators, on the other hand, are derived from a pseudoRACK (ψRACK) sequence in each PKC isozyme that is similar to a sequence in its corresponding RACK (8, 10, 13). These ψRACK or RACK-like PKC sequences are thought to engage in intramolecular interactions with the RACK-binding site in PKC, thus stabilizing PKC in its inactive “closed” conformation (8, 10, 13). Therefore, interference with this intramolecular interaction and destabilization of the inactive enzyme should enhance PKC translocation and binding to its RACK, thus stabilizing the activated state of that PKC isozyme. Using this rationale, we identified isozyme-selective activator peptides for βPKC and ɛPKC (10, 13). Relevant to this study, these activator peptides were used to show that ɛPKC confers cardiac protection from transient ischemic insult (13) and normal postnatal cardiac development (14). In this study, we used a rational approach to identify δPKC inhibitor and activator peptides and used them to determine the role of δPKC in cardiac functions.
Materials and Methods
Peptide Synthesis.
We synthesized δV1–1, amino acids 8–17 [SFNSYELGSL]; ψδRACK, amino acids 74–81 [MRAAEDPM]; and ψɛRACK, amino acids 85–92 [HDAPIGYD] peptides at Stanford's Protein and Nucleic Acid facility and conjugated them either to Antennapedia, amino acids 43–58 [RQIKIWFQRRMKKWK] (15) or Tat, amino acids 47–57 [YGRKKRRQRRR] (16) via a cysteine-cysteine bond at their N termini.
ɛV1 Structure Prediction.
We used the sequence alignment of the C2/V1 (17) and then modeled ɛV1 according to δV1 (Protein Data Bank accession no. 1BDY), the C2 domain in phospholipase δ1 (Protein Data Bank accession no. 2ISD), and 115–132 of the carboxypeptidase (Protein Data Bank accession no. 1LBU), which has sequence homology to a long gap in the alignment corresponding to residues 16–30 of ɛV1.
Isolation of Adult Rat Cardiac Myocytes and Simulated Ischemia.
Cardiac myocytes from 12-week-old male Wistar rats were isolated as described (13), treated with peptide conjugates in the presence or absence of phorbol 12-myristate 13-acetate (PMA), and subjected to Western blot analysis (13, 18) or simulated ischemia (13, 18). To simulate ischemia, we kept cardiac myocyte pellets for either 90 or 180 min in sealed test tubes in a small volume of buffer, saturated in N2 and devoid of oxygen (<0.5%), glucose, and nutrients (19). We assessed damage to cardiac myocytes by trypan blue dye exclusion assay (19, 20). The staining correlates with increased myocyte rounding, propidium iodide staining, membrane blebbing, and leak of cytosolic enzymes into the cell medium (18).
Simulated Ischemia in Intact Rat Hearts.
We simulated ischemia for 45 min in isolated hearts from 12- to 20-week-old rats by using Langendorff apparatus (18) and determined damage by creatine kinase (CK; used also as a diagnostic marker for cardiac damage in patients) activity (Sigma) in perfusate collected during 30 min of reperfusion. Peptide delivery conjugates were added to perfusion buffer 20 min before simulated ischemia. Assays were always performed within 24 h of the experiment. There was no change in CK activity over this period.
Hemodynamic Measurements of Transgenic Mice.
Hemodynamic parameters were monitored in 12-week-old transgenic mice and their littermates. Mice were anesthetized with avertin i.p., and hearts were rapidly removed and cannulated via the aorta for retrograde perfusion with Kreb–Henseleit buffer on a Langendorff apparatus. Left ventricular pressure and real-time derivative (dP/dt) was monitored via a catheter placed in the ventricular apex. These parameters were measured for 20 min until equilibration. Simulated ischemia was induced by interruption of buffer perfusion for 35 min. Hemodynamic measurements were taken every 20 sec throughout 30-min reperfusion (13).
Dry Heart Weights and Fractional Shortening.
Left ventricular fractional shortening was measured in transgenic mice and their littermates as described (13, 21). Hearts from 12-week-old transgenic and nontransgenic animals were desiccated, and dry weight was determined to demonstrate hypertrophy in transgenic mice.
Dot Blot Analysis of mRNA.
We determined cardiac gene expression from transgenic mice and their littermates by RNA dot Northern blot analysis of total ventricular RNA (3 μg/dot) by using 32P-labeled oligonucleotide probes as described (21).
Simulated Ischemia in Vivo.
Using open chest coronary occlusion and a nontraumatic balloon inflation, we induced ischemia at 37°C (22, 23) for 30 min. To identify the infarcted myocardium, 24 h after occlusion, we perfused the hearts on Langendorff apparatus with a 1% solution of 2,3,5-triphenyltetrazolium chloride (22, 23). Region at risk was determined by tying the coronary artery at the site of the previous occlusion and perfusing hearts with a 5% solution of phthalo blue dye (Heucotech, Fairless Hill, PA). Using computerized video planimetry of transverse slices, we calculated infarct size as a percentage of the region at risk (22, 23).
Hemodynamic Measurements, Dry Heart Weights, and Dot Blot Analysis of mRNA.
We measured left ventricular fractional shortening, changes in cardiac weight, and gene expression as described (13, 21).
Results
Rational Design of δPKC Selective Inhibitor and Activator Peptides.
In the absence of any information about the δRACK sequence, we used a rational design to identify δPKC-selective activator and inhibitor peptides. We had previously observed that the V1 domain of δPKC contains the RACK-binding site (12). We then considered that of the three other novel PKC isozymes (ɛ, θ, and η), δPKC is most similar to θPKC (52% amino acid identity in the first variable or V1 domain) (24, 25). Because each PKC isozyme should interact with a different RACK, we predicted that the sequences that are the least similar between δPKC and θPKC were likely to mediate RACK binding. Based on this assumption, we identified three regions in the V1 domain of δPKC with only ≈10% identity to θPKC (colored bars in Fig. 1A).
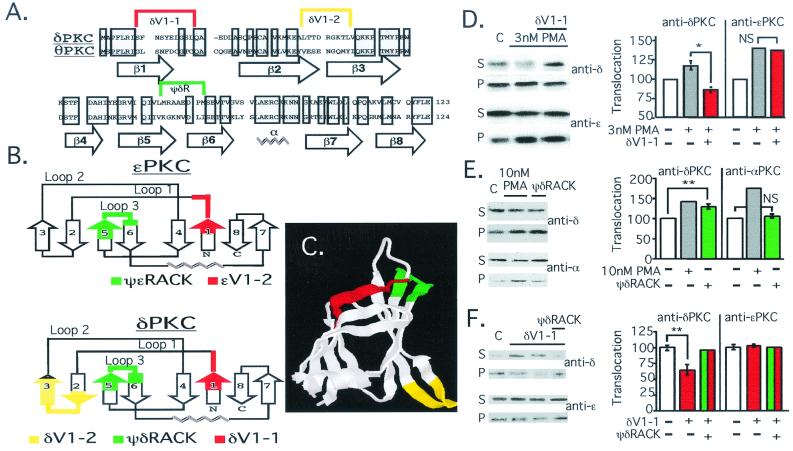
Figure 1.
Rational design of δPKC translocation inhibitor and activator. (A) Alignment of the primary sequence of rat δPKC and mouse θPKC V1 domains (Protein Data Bank accession nos. KIRTCD and NP_032885, respectively); shadowed boxes indicate identity. Location of β-strands and the α-helix based on δV1 structure analysis (26) are indicated below the sequence; sequences most different between the two isozymes are marked above with a color bracket. (B) The secondary structure of δV1 (26) (Lower) and a modeled secondary structure of ɛV1 (G.C., D.M.-R., and L.B., unpublished work; Upper) are schematically depicted, according to ref. 32. Numbering of β-strands in δV1 and ɛV1 domains are marked as in A for δV1. The sequence corresponding to δV1–1, amino acids 8–17 [SFNSYELGSL], δV1–2, amino acids 35–45 [ALTTDRGKTLV], and ψδRACK, amino acids 74–81 [MRAAEDPM], are marked as in A, in red, yellow, and green, respectively. The sequence corresponding to the ɛPKC-selective inhibitor peptide, ɛV1–2, amino acids 14–21 [EAVSLKPT] (12), and activator peptide, ψɛRACK, amino acids 85–92 [HDAPIGYD] (13), are marked in red and green, respectively (Upper). (C) Crystal structure of the δV1 domain (Protein Data Bank ID no. 1BDY; ref. 26) is depicted with areas marked in colors corresponding to those in A and B. (D) Western blot analysis of cytosolic and particulate fractions from adult rat cardiac myocytes was carried out as described (13) to demonstrate isozyme-selective effects on δPKC translocation. Cells were treated with PMA in the presence and absence of δV1–1. (Left) Autoradiogram of soluble (S) and particulate (P) fractions probed with anti-δPKC (Upper) and the same blot probed with anti-ɛPKC antibodies (Lower). (Right) Mean ± SEM of data from three experiments; translocation is expressed as the amount of each isozyme in the particulate fraction over the amount of that isozyme in nontreated cells. *, P < 0.05; NS, not significant; n = 3. (E) Same as in D, except cells were treated with PMA or ψδRACK and translocation of δPKC and αPKC is shown. **, P < 0.01. (F) Same as D, except cells were treated with δV1–1 in the presence and absence of ψδRACK. **, P < 0.01.
To determine which of the peptides corresponding to these sequences was most likely to be a δPKC inhibitor or activator, we compared the tertiary structure of δV1 (previously solved in ref. 26) to the structure of ɛV1 that we modeled (Fig. 1B; (G.C., D.M.-R., and L.B., unpublished work). Both the δV1 domain and the modeled ɛV1 domain are composed of two β-sheets (Fig. 1C and schematically presented in Fig. 1B). We selected the first (δV1–1) and the third (ψδRACK) unique δV1 sequences as potential δPKC-selective inhibitor and activator peptides, because their positions in the structure overlap that of ɛV1–2, the ɛPKC-selective inhibitor peptide (12), and ψɛRACK (13), the ɛPKC-selective activator peptide, respectively (red and green in Fig. 1 B and C).
To determine whether the δPKC peptides had the anticipated activities, we delivered the peptides conjugated to Antennapedia carrier peptide into isolated adult rat cardiac myocytes (13). As predicted, δV1–1 inhibited PMA-induced δPKC translocation, but not the translocation of ɛPKC (Fig. 1D) or αPKC (not shown). ψδRACK had an opposite effect to that of δV1–1; i.e., it selectively induced δPKC translocation in cardiac myocytes, without affecting the translocation of αPKC (Fig. 1E) or ɛPKC (not shown). Furthermore, basal partitioning of δPKC in the particulate fraction was inhibited by δV1–1, and ψδRACK reversed this δV1–1 effect (Fig. 1F). Similar to our data on the ɛPKC activator and inhibitor peptides (13, 27), scrambled peptides cross-linked to Antennapedia, or Antennapedia peptide alone had no effect on δPKC translocation (not shown). In addition, δV1–1 and ψδRACK also caused the same selective effect on hormone-induced translocation of δPKC as determined by immunofluorescence and Western blot analysis (not shown). Furthermore, peptides applied to δPKC or ɛPKC in vitro had no effect on enzymatic activity (not shown), demonstrating their specific effects on translocation. Therefore, δV1–1 is a selective translocation inhibitor and ψδRACK is a selective translocation activator of δPKC.
Role of δPKC in Simulated Cardiac Ischemia in Isolated Adult Cardiac Myocytes.
Using the ɛPKC translocation activator, ψɛRACK, we recently observed that activation of ɛPKC is necessary and sufficient to confer protection from ischemic injury in isolated cardiomyocytes and transgenic mice (13). Because δPKC is also translocated during ischemia (13, 27), we used the δPKC activator peptide, ψδRACK, and the δPKC inhibitor peptide, δV1–1, to determine the role of δPKC in protection from ischemia. We added δV1–1 conjugated to Antennapedia to cardiomyocytes 10 min before 180-min simulated ischemia and found a concentration-dependent protection of cardiac myocytes from ischemic damage (Fig. 2A). Moreover, δV1–1 protection was reversed by coincubation with the δPKC activator, ψδRACK, but not with βPKC-selective translocation activators (28) or other control peptides (Fig. 2A and not shown).
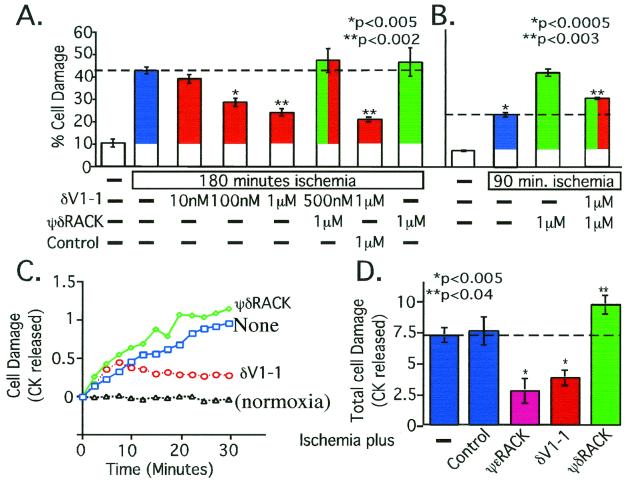
Figure 2.
Ischemic damage is due, in part, to δPKC activation. (A) Isolated rat cardiac myocytes from adult male rats (13, 18) were pretreated with the δPKC-selective inhibitor peptide, δV1–1, and/or activator peptide, ψδRACK (green), before simulated ischemia. Control is βPKC-selective activator (10). Statistical analysis compares all data to those obtained from cells treated with no peptide (blue). (B) As in A, but ischemic period was shortened to illustrate an additive effect between ψδRACK and ischemia in inducing damage. Statistical analysis compares data from cells treated with no peptide (blue; *) and with ψδRACK in the presence of δV1–1 (red plus green; **) to cells treated with ψδRACK alone (green). (C) Whole hearts from adult male rats were perfused with the δPKC-selective inhibitor peptide δV1–1 or activator peptide ψδRACK before global ischemia followed by reperfusion using the Langendorff preparation (13, 18). Shown is average levels over time. (D) Total CK released over the 30-min reperfusion (13, 18) are shown and include the effect of infusion of the ɛPKC-selective activator, ψɛRACK (magenta). Controls (second blue bar) include peptides coupled to Tat-carrier peptide with a scrambled sequence or a nonrelevant sequence and Tat-carrier peptide alone. All measurements were carried out in triplicate, using 3–4 animals per condition.
In addition to the opposing protective effect induced by δV1–1, ψδRACK caused a slight increase in myocyte damage after an ischemic insult (Fig. 2A). When we reduced the time of ischemia from 180 to 90 min, the ψδRACK-induced increase in cell damage became significant and was partially reversed by cotreatment with the δPKC inhibitor, δV1–1 (Fig. 2B). Therefore, cell damage induced by simulated ischemia is caused, at least in part, by activation of δPKC.
Role of δPKC and ɛPKC in Simulated Ischemia in Intact Heart.
We recently found that conjugation of PKC inhibitor or activator peptides to a Tat-derived peptide [Tat 47–57 (16)] enables their delivery through the coronary arteries into intact heart (34). Therefore, to determine the role of δPKC and ɛPKC on cardiac damage caused by 45 min of no-flow myocardial ischemia, we perfused Tat-conjugated peptide regulators of these isozymes into isolated rat hearts. As predicted from our previous cellular and transgenic studies (13), acute activation of ɛPKC by infusion of ψɛRACK through the coronary arteries protected the heart from ischemia by more than 60% (Fig. 2 C and D). In contrast, activation of δPKC with ψδRACK increased cardiac damage by ≈30% (Fig. 2 C and D). Moreover, acute inhibition of δPKC by infusion of δV1–1 protected isolated hearts from ischemic damage as shown by decreased release of CK (Fig. 2 C and D). Scrambled peptides cross-linked to Tat-derived peptide, peptides not linked to Tat-derived carrier peptide, or Tat-derived peptide alone had no effect on the response of the hearts to ischemic insult (Fig. 2D). Together, these data indicate that in the intact heart as well as in cardiac myocytes, these two closely related PKC isozymes have opposing effects; inhibition of δPKC or activation of ɛPKC both conferred at least 50% protection against ischemic damage (Fig. 2D).
Our data demonstrating that δPKC activity increases damage by cardiac ischemia may be in contrast to the study of Kihara and collaborators (29), using a compound that alters the subcellular location of δPKC (JTV). They suggested that δPKC protects isolated rat cardiomyocytes from ischemic damage. However, the drug, which was initially designed to affect annexin, a phospholipid-binding protein, may in fact sequester δPKC and thus inhibit its normal activity.
Role of δPKC and ɛPKC in Simulated Ischemia in Transgenic Mice.
To determine the consequences of sustained activation of δPKC by expression of ψδRACK, we created transgenic mice in which the α-myosin heavy chain promoter drove expression of ψδRACK, as described for ψɛRACK (13). Multiple independent lines of ψδRACK mice exhibited identical phenotypes, including increased association of δPKC with the subcellular particulate fraction (Fig. 3A Upper). We found no changes in overall δPKC levels or in the levels and subcellular association of other PKC isozymes (Fig. 3A and not shown.) For comparison, parallel experiments were carried out by using ψɛRACK transgenic mouse hearts (Fig. 3A Lower). We next determined functional recovery and cardiomyocyte damage in ψδRACK and ψɛRACK mouse hearts after transient (35 min) no-flow global ischemia. Consistent with the results obtained when peptides were acutely introduced into isolated cardiomyocytes (Fig. 2 A and B) or perfused rat hearts (Fig. 2 C and D), the ψδRACK mouse hearts showed significantly impaired recovery of systolic (contraction, +dP/dt) and diastolic (relaxation, −dP/dt) functions from ischemia (Fig. 3B; P < 0.05 and Table 1). In contrast, we previously found that ψɛRACK mouse hearts had significantly improved recovery of cardiac functions after ischemia (13). Furthermore, a significant decrease in cellular damage (CK release) during reperfusion was observed in ψɛRACK-expressing mice (Fig. 3C, magenta), but not in ψδRACK-expressing mice (Fig. 3C, green bar).
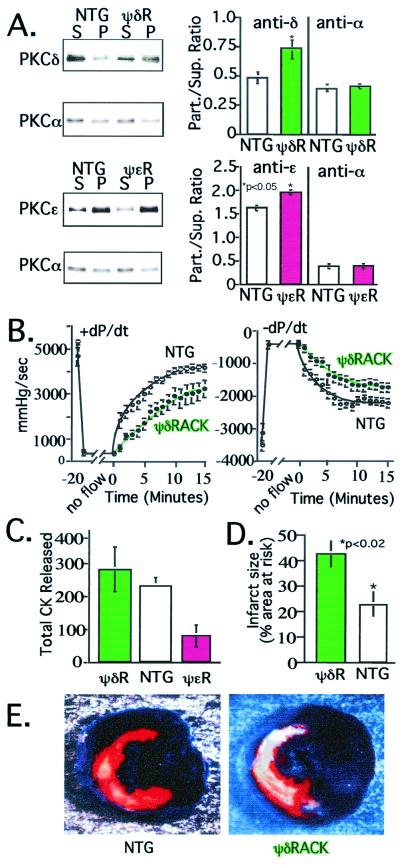
Figure 3.
ψδRACK transgenic mice exhibit increased damage by cardiac ischemia. (A) Western blot and analysis of PKC distribution in cytosolic and particulate fractions from ψδRACK mice (ψδR, Upper; green bars) and ψɛRACK transgenic mice (ψɛR, Lower; magenta bars) and their nontransgenic littermates (NTG; white bars) was carried out as described (13) to demonstrate isozyme-selective translocation of PKC. (Right) Histogram shows mean ± SEM of data from eight mice for each group. (B) Hemodynamic parameters were monitored in hearts from transgenic ψδRACK transgenic mice (green symbols) and their nontransgenic littermates (white symbols) after global ischemia. Left ventricular pressure and real time derivative was monitored via a catheter placed in the ventricular apex (13). Hemodynamic measurements were recorded every 20 sec throughout reperfusion. Data are mean ± SEM of 12 ψδRACK and 11 nontransgenic mice. (C) Fractions of perfusate from mice used in B were collected throughout reperfusion and CK activity was assessed to determine cell damage. For comparison, data from ψɛRACK mice are also included. Data are mean ± SEM of six ψδRACK mice (green bar), six nontransgenic mice (white bar), and seven ψɛRACK mice (magenta bar). (D) Infarct size as a percent of the region of risk in mice with sustained δPKC activation (ψδRACK mice; green bar) and nontransgenic littermates (white bar) was determined in vivo after coronary occlusion followed by 24 h of reperfusion as described (mean ± SEM) (22, 23). The area at risk was not significantly different between the nontransgenic and ψδRACK mice (36 ± 3% and 41 ± 5% of left ventricle for nontransgenic and ψδRACK mice, respectively). Data are from eight 30- to 34-week-old transgenic females and five nontransgenic female littermates. So far only three males were available for analysis and therefore they are not included in the analysis; in all of the other studies, equal numbers of male and female transgenic and nontransgenic mice were used. (E) Example of infarcts in a ψδRACK transgenic mouse (Right) and a littermate (Left) subjected to coronary occlusion and 24 h of reperfusion in vivo. The portion of the left ventricle supplied by the occluded coronary artery (region of risk) was identified by the absence of Phthalo blue dye, which was perfused only through the nonoccluded vascular bed (22, 23). The infarcted area was identified by perfusion with 2,3,5-triphenyltetrazolium chloride, which stains viable tissue bright red, whereas infarcted tissue is light yellow (22, 23).
Table 1.
Left ventricular developed pressure of ψδRACK transgenic mice
| Measurement | Nontransgenic, mmHg/sec | ψδRACK,* mmHg/sec |
|---|---|---|
| +dP/dt | 3,652 ± 167 | 2,582 ± 373 |
| −dP/dt | −2,248 ± 140 | −1,572 ± 169 |
Positive developed pressure over time (+dP/dt) and negative developed pressure over time (−dP/dt) were recorded throughout reperfusion (13). Data were taken 7 min after reperfusion and are mean ± SEM of 12 ψδRACK and 11 nontransgenic mice of 12 weeks of age.
, P < 0.05.
Role of δPKC in Simulated Ischemia in Vivo.
Next, we used a physiologically relevant mouse model of acute ischemic injury induced by a 30-min in vivo coronary occlusion (22, 23) and measured infarct size 24 h after reperfusion. Although the area at risk for infarction was similar in the two groups, ψδRACK mice exhibited over a 2-fold increase in infarct size as compared with nontransgenic littermates (Fig. 3D). The marked in vivo increase in myocyte death in the transgenic mice with sustained δPKC activation (ψδRACK) is illustrated in Fig. 3E, where a much larger light yellow area (infarcted area) is seen in the ψδRACK heart than in the nontransgenic heart. Therefore, activation of δPKC exacerbates damage during ischemia in three different models: isolated myocytes, intact hearts ex vivo, and intact hearts in vivo, which is in contrast to the protective role of ɛPKC in ischemia (here and ref. 13).
Role of δPKC and ɛPKC in Cardiac Hypertrophy.
We previously observed that older transgenic mouse hearts with sustained ɛPKC activation (ψɛRACK mice) developed increased myocardial mass (hypertrophy) with normal contractile function, whereas mice with sustained inhibition of ɛPKC activation developed lethal dilated cardiomyopathy (21). Given the opposing effects of δPKC and ɛPKC on cardiac injury in response to simulated ischemia shown here, we anticipated that transgenic mice with sustained activation of δPKC (ψδRACK mice) would exhibit an effect on myocardial hypertrophy opposite to that of ψɛRACK. Unexpectedly, the ψδRACK mice also developed an increase in cardiac mass similar to age-matched ψɛRACK mice (Fig. 4A). Note, however, that these mice had normal basal and β-adrenergic-stimulated contractile function (Fig. 4B and Table 2; ref. 21) and a normal histological appearance (ref. 21 for ɛPKC, and data not shown for δPKC). Furthermore, both transgenic lines of mice developed identical perturbations of gene expression; a selective increase in expression of the βmyosin heavy chain gene, a known marker for cardiac hypertrophy in mice (Fig. 4C) (30, 31). Therefore, the molecular and functional characteristics of cardiac hypertrophy in the ψδRACK and the ψɛRACK mice appear identical, indicating a parallel role for δPKC and ɛPKC in regulation of cardiac hypertrophy.
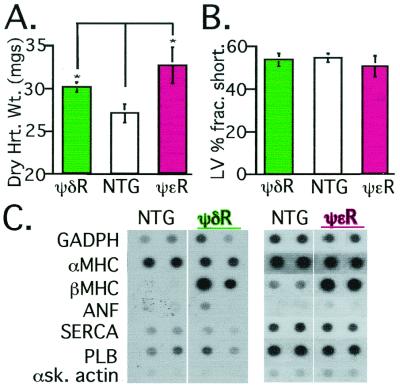
Figure 4.
ψδRACK transgenic mice exhibit hypertrophy similar to ψɛRACK mice. (A) Dry heart weights of nontransgenic and ψδRACK-expressing transgenic mice were measured as in ref. 13, demonstrating hypertrophy in hearts of ψδRACK and ψɛRACK mice. Data are mean ± SEM from 10 ψδRACK mice (green bar), five nontransgenic mice (white bar), and three ψɛRACK mice (magenta bar). (B) Left ventricular fractional shortening was measured in the nontransgenic and transgenic mice as described (13, 21). Data are mean ± SEM obtained from nine ψδRACK mice (green bar), 10 nontransgenic mice (white bar), and four ψɛRACK mice (magenta bar). (C) Dot blot analysis of mRNA from hearts of transgenic and nontransgenic mice showing increased expression of βMHC, a marker for hypertrophy (33). GADPH, glyceraldehyde-3-phosphate dehydrogenase; αMHC, α-myosin heavy chain; βMHC, β-myosin heavy chain; ANF, atrial natriuritic factor; SERCA, sarcoplasmic reticular ATPase; PLB, phospholamban; αSK actin, α skeletal actin.
Table 2.
Basal and β-adrenergic-stimulated contractile function in ψδRACK transgenic mice
| Measurement | Nontransgenic | ψδRACK* |
|---|---|---|
| Fractional shortening | 56 + 3% | 55 + 3% |
| Baseline +dP/dt | 6,315 ± 474 mmHg/sec | 8,386 ± 373 mmHg/sec |
| Peak +dP/Dt | 19,020 ± 387 mmHg/sec | 18,257 ± 476 mmHg/sec |
| Baseline −dP/dt | −7,773 ± 306 mmHg/sec | −9,049 ± 621 mmHg/sec |
| Peak −dP/Dt | −10,858 ± 461 mmHg/sec | −11,751 ± 995 mmHg/sec |
Echocardiogram measurements were taken as described (13, 21). In vivo catheterization measurements shown for baseline and β-adrenergic-stimulated contractile function (dobutamine, 1 ng/g per min for 3 min; dose was continuously doubled up to 32 ng/g per min). Data are mean ± SEM of nine ψδRACK and nine nontransgenic mice of 12 weeks of age.
, P = not significant for all measurements.
Discussion
We have used selective inhibitor and activator peptides of δPKC and ɛPKC translocation to show opposing roles in response to ischemia and similar roles in cardiac hypertrophy for these two isozymes. We presented data demonstrating the role of these isozymes in cardiac protection from ischemia by using two species (rat and mouse), three model systems for simulated ischemia (isolated myocytes, intact heart, and in vivo), three methods of peptide delivery (transgene and acute peptide delivery using Antennapedia- or Tat-carrier peptides), and at least four methods of damage assessment (CK release, dye exclusion, functional recovery of contraction, and infarct size). Therefore, multiple independent methods used to assess the role of δPKC and ɛPKC yielded the same conclusion: inhibiting δPKC or activating ɛPKC reduce damage from simulated ischemia. Similarly, using multiple methods we found that activation and translocation of δPKC and ɛPKC isozymes in transgenic mice cause cardiac hypertrophy.
These data raise four important points. First, it is possible to identify highly efficacious activator and inhibitor peptides of δPKC translocation, using a structure-based rational design. [These peptides were active at intracellular concentrations of ≈25–50 nM, about 5–10% of the applied concentration (8).] To our knowledge, there are no examples of rationally designed activators of signaling enzymes that act intracellularly except for our own. The development of these selective regulators of δPKC relied not only on the assumption that PKC-RACK interactions could be regulated as a means to selectively modify δPKC activity, but was achieved without any knowledge of the δPKC binding protein, δRACK. This method provides an alternative to mutational mapping of protein–protein interactions or random peptide library searches requiring the presence of both proteins and should be generally applicable to research on other protein–protein interactions.
Second, our findings that PKC isozymes can exert an opposing effect on one function and the same effect on another function in a single cell can explain contradictory findings when isozyme nonselective drugs are used. We suggest that previous conflicting reports on the role of PKC in ischemic preconditioning (1), for example, may reflect such use of drugs that do not distinguish between δPKC and ɛPKC.
Third, our observation that δPKC and ɛPKC have opposing effects on ischemic injury was particularly unexpected, because in rats and mice both isozymes are activated by simulated ischemia as well as by stimuli that lead to cardioprotection from ischemia (18, 27). These opposing forces (“yin yang”) illustrate the need for selective therapeutic agents to treat ischemic heart disease.
Finally, we show that these biologically active peptides can be effectively delivered into organs via the blood vessels by using Tat-conjugated peptides. Moreover, this mode of peptide delivery also should be useful for the study of other diseases in intact animals. Because delivery is immediate, it is superior to gene delivery, as adaptations to the changes induced by a signal transduction modulator are less likely to occur. Because we found a greater than 50% reduction in cardiac damage caused by ischemia by coronary perfusion of δPKC translocation inhibitor peptide or ɛPKC activator peptide, we believe that these peptides may be useful therapeutic agents for acute cardiac ischemia.
Acknowledgments
This work was supported, in part, by National Institutes of Health Grants HL52141 (to D.M.-R.), HL43151 and HL55757 (to R.B.), and HL52318 (to G.W.D.). D.S. is supported by the Ares Serano Foundation.
Abbreviations
- PKC
protein kinase C
- RACK
receptor for activated C kinase E
- ψRACK
pseudoRACK
- PMA
phorbol 12-myristate 13-acetate
- CK
creatine kinase
References
- 1.Brooks G, Hearse D J. Circ Res. 1996;79:627–630. doi: 10.1161/01.res.79.3.628. [DOI] [PubMed] [Google Scholar]
- 2.Gu X, Bishop S P. Circ Res. 1994;75:926–931. doi: 10.1161/01.res.75.5.926. [DOI] [PubMed] [Google Scholar]
- 3.Bowling N, Walsh R A, Song G, Estridge T, Sandusky G E, Fouts R L, Mintze K, Pickard T, Roden R, Bristow M R, et al. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 4.Jalili T, Takeishi Y, Song G, Ball N A, Howles G, Walsh R A. Am J Physiol. 1999;277:H2298–H2304. doi: 10.1152/ajpheart.1999.277.6.H2298. [DOI] [PubMed] [Google Scholar]
- 5.Mochly-Rosen D, Henrich C J, Cheever L, Khaner H, Simpson P C. Mol Biol Cell. 1990;1:693–706. doi: 10.1091/mbc.1.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Disatnik M-H, Buraggi G, Mochly-Rosen D. Exp Cell Res. 1994;210:287–297. doi: 10.1006/excr.1994.1041. [DOI] [PubMed] [Google Scholar]
- 7.Mochly-Rosen D. Science. 1995;268:247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- 8.Souroujon M, Mochly-Rosen D. Nat Biotechnol. 1998;16:919–924. doi: 10.1038/nbt1098-919. [DOI] [PubMed] [Google Scholar]
- 9.Mochly-Rosen D, Miller K G, Scheller R H, Khaner H, Lopez J, Smith B L. Biochemistry. 1992;31:8120–8124. doi: 10.1021/bi00150a003. [DOI] [PubMed] [Google Scholar]
- 10.Ron D, Luo J, Mochly-Rosen D. J Biol Chem. 1995;270:24180–24187. doi: 10.1074/jbc.270.41.24180. [DOI] [PubMed] [Google Scholar]
- 11.Sossin W S, Schwartz J H. Trends Biochem Sci. 1993;18:207–208. doi: 10.1016/0968-0004(93)90189-t. [DOI] [PubMed] [Google Scholar]
- 12.Johnson J A, Gray M O, Chen C-H, Mochly-Rosen D. J Biol Chem. 1996;271:24962–24966. doi: 10.1074/jbc.271.40.24962. [DOI] [PubMed] [Google Scholar]
- 13.Dorn G W, II, Souroujon M C, Liron T, Chen C H, Gray M O, Zhou H Z, Csukai M, Wu G, Lorenz J N, Mochly-Rosen D. Proc Natl Acad Sci USA. 1999;96:12798–12803. doi: 10.1073/pnas.96.22.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ping P, Zhang J, Pierce W M, Bolli R. Circ Res. 2001;88:59–62. doi: 10.1161/01.res.88.1.59. [DOI] [PubMed] [Google Scholar]
- 15.Derossi D, Joliot A H, Chassaing G, Prochiantz A. J Biol Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 16.Schwarze S R, Ho A, Vocero-Akbani A, Dowdy S F. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 17.Nalefski E A, Falke J J. Protein Sci. 1996;5:2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C H, Gray M O, Mochly-Rosen D. Proc Natl Acad Sci USA. 1999;96:12784–12789. doi: 10.1073/pnas.96.22.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong S, Downey J M, Ganote C E. Cardiovasc Res. 1994;28:72–77. doi: 10.1093/cvr/28.1.72. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong S C, Kao R, Gao W, Shivell L C, Downey J M, Honkanen R E, Ganote C E. J Mol Cell Cardiol. 1997;29:3009–3024. doi: 10.1006/jmcc.1997.0507. [DOI] [PubMed] [Google Scholar]
- 21.Mochly-Rosen D, Wu G, Hahn H, Osinska H, Liron T, Lorenz J N, Yatani A, Robbins J, Dorn G W, II. Circ Res. 2000;86:1173–1179. doi: 10.1161/01.res.86.11.1173. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Wu W J, Qiu Y, Tang X L, Yang Z, Bolli R. Am J Physiol. 1998;275:H1375–H1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y, Jones W K, Xuan Y T, Tang X L, Bao W, Wu W J, Han H, Laubach V E, Ping P, Yang Z, et al. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osada S-I, Mizuno K, Saido T C, Suzuki K, Kuroki T, Ohno S. Mol Cell Biol. 1992;12:3930–3938. doi: 10.1128/mcb.12.9.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baier G, Telford D, Giampa L, Coggeshall K M, Baier-Bitterlich G. J Biol Chem. 1993;268:4997–5004. [PubMed] [Google Scholar]
- 26.Pappa H, Murray-Rust J, Dekker L V, Parker P J, McDonald N Q. Structure (London) 1998;6:885–894. doi: 10.1016/s0969-2126(98)00090-2. [DOI] [PubMed] [Google Scholar]
- 27.Gray M O, Karliner J S, Mochly-Rosen D. J Biol Chem. 1997;272:30945–30951. doi: 10.1074/jbc.272.49.30945. [DOI] [PubMed] [Google Scholar]
- 28.Ron D, Mochly-Rosen D. Proc Natl Acad Sci USA. 1995;92:492–496. doi: 10.1073/pnas.92.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inagaki K, Kihara Y, Hayashida W, Izumi T, Iwanaga Y, Yoneda T, Takeuchi Y, Suyama K, Muso E, Sasayama S. Circulation. 2000;101:797–809. doi: 10.1161/01.cir.101.7.797. [DOI] [PubMed] [Google Scholar]
- 30.Sakata Y, Hoit B D, Liggett S B, Walsh R A, Dorn G W, II. Circulation. 1998;97:1488–1495. doi: 10.1161/01.cir.97.15.1488. [DOI] [PubMed] [Google Scholar]
- 31.Adams J W, Sakata Y, Davis M G, Sah V P, Wang Y, Liggett S B, Chien K R, Brown J H, Dorn G W, II. Proc Natl Acad Sci USA. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizo J, Sudhof T C. J Biol Chem. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- 33.Dorn G W, II, Tepe N M, Lorenz J N, Koch W J, Liggett S B. Proc Natl Acad Sci USA. 1999;96:6400–6405. doi: 10.1073/pnas.96.11.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen, L., Wright, L., Chen, C. H., Oliver, S., Wender, P. & Mochly-Rosen, D. (2001) Chem. Biol., in press. [DOI] [PubMed]