Given the urgent need to understand the safety of e-cigarettes in adolescents, we sought to identify exposure to toxicants associated with e-cigarette use among adolescents.
Abstract
BACKGROUND:
There is an urgent need to understand the safety of e-cigarettes with adolescents. We sought to identify the presence of chemical toxicants associated with e-cigarette use among adolescents.
METHODS:
Adolescent e-cigarette users (≥1 use within the past 30 days, ≥10 lifetime e-cigarette use episodes) were divided into e-cigarette–only users (no cigarettes in the past 30 days, urine 4-[methylnitrosamino]-1-[3-pyridyl]-1-butanol [NNAL] level <1 pg/mL of creatinine; n = 67), dual users (use of cigarettes in the past 30 days in addition to e-cigarettes, NNAL level >30 pg/mL; n = 16), and never-using controls (N = 20). Saliva was collected within 24 hours of the last e-cigarette use for analysis of cotinine and urine for analysis of NNAL and levels of 8 volatile organic chemical compounds. Bivariate analyses compared e-cigarette–only users with dual users, and regression analyses compared e-cigarette–only users with dual users and controls on levels of toxicants.
RESULTS:
The participants were 16.4 years old on average. Urine excretion of metabolites of benzene, ethylene oxide, acrylonitrile, acrolein, and acrylamide was significantly higher in dual users versus e-cigarette–only users (all P < .05). Excretion of metabolites of acrylonitrile, acrolein, propylene oxide, acrylamide, and crotonaldehyde were significantly higher in e-cigarette–only users compared with controls (all P < .05).
CONCLUSIONS:
Although e-cigarette vapor may be less hazardous than tobacco smoke, our findings can be used to challenge the idea that e-cigarette vapor is safe, because many of the volatile organic compounds we identified are carcinogenic. Messaging to teenagers should include warnings about the potential risk from toxic exposure to carcinogenic compounds generated by these products.
What’s Known on This Subject:
The presence of harmful ingredients in electronic cigarette vapor has been established.
What This Study Adds:
We have demonstrated that at least 5 potentially harmful toxicants are found in the body of human adolescents who use electronic cigarettes.
Electronic cigarettes (e-cigarettes) are marketed to promote smoking cessation or reduced cigarette smoking in adults.1 However, social influence and marketing strategies for these products have clearly had an effect on children as well, because more teenagers now use e-cigarettes than traditional cigarettes.2 In 2016, e-cigarette use in the past 30 days among 10th-graders was more than twice that of cigarette use (11.0% vs 4.9%).3 Reasons for the dramatic increase in adolescent e-cigarette use include peer influence, enticing flavors,4 and extensive marketing presenting e-cigarettes as safer.5,6 Common messages found on product Web sites are that e-cigarettes do not produce the same cancer-causing agents as traditional cigarettes.1
Despite advertising claims, there is uncertainty about the safety of e-cigarettes. By using aerosolized nicotine rather than combusting tobacco, e-cigarettes do produce fewer toxins than smoking cigarettes.7 However, e-cigarettes contain additives and solvents, including propylene glycol and/or glycerol, which can form carcinogenic compounds when heated.8–11 These and other toxic chemicals12 may be inhaled through the vapor produced. Although there is some controversy on how use patterns may affect exposure, some data from adults reveal that these toxicants can be detected in the urine of e-cigarette users.13,14 Importantly, these studies did not exclude participants with a possible exposure to secondhand smoke.
To our knowledge, there are no data on toxicant exposure in adolescent e-cigarette users. However, there is great concern because exposure to toxicants during adolescence may result in greater harm than exposure in adulthood, given vulnerability to the acute and chronic effects of toxicants in general and from their cumulative exposure if started early.15
Given the rapid uptake of e-cigarettes among teenagers, there is an urgent need to understand the safety of these products in adolescents, including how use contributes to toxicant exposure. In this study, we sought to assess in adolescents the presence of certain carcinogenic toxicants linked to e-cigarette use and examine how specific behavioral patterns of use may influence exposure to toxicants.
Methods
Participants and Procedures
As part of an ongoing longitudinal study of the effects of e-cigarettes on adolescents, adolescent (aged 13–18 years) e-cigarette users (used an e-cigarette product on ≥1 day in the past 30 days and had at least 10 lifetime use episodes) were recruited from the San Francisco Bay area by using fliers and online advertising. The research design and procedures were reviewed and approved by the University of California Institutional Review Board.
To capture nicotine exposure and investigate the presence of toxicants, participants were instructed to schedule their baseline appointments in temporal proximity (ie, past 24 hours) to use of their e-cigarettes. Adolescents were never pressured or instructed to use e-cigarettes, and in the cases in which no use occurred, appointments were rescheduled. After signing consents, participants completed a baseline survey including questions about demographics and e-cigarette use behaviors. Participants then provided saliva samples for cotinine measurement and urine for the measurement of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and levels of metabolites of 8 volatile organic compounds (VOCs). Participants received $30.
Specimens were also collected from 20 age-matched control adolescents attending pediatric clinics at a Bay area public hospital with undetectable cotinine and NNAL, confirming no e-cigarette or nicotine use. These adolescents were part of another study on secondhand smoke exposure for which urine was collected and analyzed for NNAL and cotinine.
Measures
Biological
Saliva and urine samples were analyzed at the Clinical Pharmacology Laboratory at the University of California, San Francisco. Salivary specimens were analyzed for cotinine, the main proximate metabolite of nicotine, by using liquid chromatography–tandem mass spectrometry.16,17 Urine was analyzed for NNAL, a metabolite of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco-specific nitrosamine that is a potent carcinogen,13,18 by liquid chromatography–tandem mass spectrometry.18 This was done as an adjunct to self-reported tobacco smoking and to rule out significant secondhand tobacco smoke exposure (or exposure from marijuana blunts), because NNAL is detectable in urine for 6 to 12 weeks after exposure.19 Urine was analyzed for metabolites of a panel of 8 VOCs that are toxic environmental or tobacco smoke constituents, including benzene (phenylmercapturic acid [PMA]), 1,3-butadiene (4-hydroxy-2-buten-1-yl-mercapturic acid), ethylene oxide (2-hydroxyethylmercapturic acid [HEMA]), acrylonitrile (2-cyanoethylmercapturic acid [CNEMA]), acrolein (3-hydroxypropylmercapturic acid [3-HPMA]), propylene oxide (2-hydroxypropylmercapturic acid [2-HPMA]), acrylamide (2-carbamoylethylmercapturic acid [AAMA]), and crotonaldehyde (3-hydroxy-1-methyl-propylmercapturic acid [HMPMA]).20 Both NNAL and VOC concentrations were normalized for creatinine.21
Demographic, E-Cigarette, and Smoking Characteristics
Demographic variables included race and/or ethnicity, sex, and age. Individuals who identified as Hispanic were classified as such, regardless of race. A measure of e-cigarette use was designed for this study that included the time of last use (used to calculate hours since last use), frequency of use (days used in the past month), quantity of use (average sessions per day on using days, calculated by asking how many times they used their devices on each weekday and weekend day and then dividing by 7), usual number of puffs per session in 4 categories (1–4, 5–10, 10–15, or >15), length of each session in 4 categories (1–2, 3–5, 6–10, or >10 minutes), main type of e-cigarette used in 4 categories (vape pen, modified, Juul, other), whether e-cigarettes contained nicotine (always, sometimes, unsure, or never), and the flavors consumed in the past month (fruit, candy, menthol, or tobacco; yes or no). Tobacco use was assessed by asking if participants smoked a cigarette in the past 30 days (yes or no).
Data Analyses
Three categories were developed on the basis of the combination of reported e-cigarette and cigarette use and urine NNAL levels. E-cigarette–only users had used no traditional combustion cigarettes in the past 30 days and had levels of urine NNAL <1 pg/mL of creatinine. We used 1 pg per milliliter of creatinine to exclude smokers on the basis of our data to distinguish adolescents who were smokers from those who were nonsmokers in San Francisco.22 Values between 0 and 1 pg/mg indicate no recent active smoking and either past smoking or light secondhand smoke exposure, neither of which would be expected to substantially increase VOC exposure. Dual users reported use of traditional cigarettes in the past 30 days in addition to e-cigarettes and had to have NNAL levels >30 pg/mL of creatinine. We chose a cutoff of 30 pg/mL of creatinine to ensure primary exposure to combusted tobacco. To ensure no exposure to combusted tobacco or nicotine from other sources (including e-cigarettes), controls had to have levels of NNAL and cotinine below the limit of quantitation (ie, 0.25 and 1 ng/mL respectively). We excluded from analyses participants who did not use an e-cigarette in the previous 24 hours because most VOCs in smokers, including those tested here, decline to baseline levels within 24 hours.23 Finally, for the purposes of creating well-differentiated comparison groups, we also set an a priori exclusion from analyses for those participants who had intermediate levels of NNAL (ie, 1–29 pg/mL of creatinine), because the true source of exposure would be unclear. Conservative criteria for group definitions meant that the e-cigarette–only group was clearly differentiated from the dual user group, and any VOCs found in the e-cigarette–only group could be clearly attributed to e-cigarette use.
Descriptive statistics were used to characterize sociodemographic and e-cigarette use, t tests were used for continuous variables, and Pearson’s χ2 tests were used for categorical variables. Because of skew, the nonparametric Mann–Whitney U test was used to compare the distributions on hours since last use between e-cigarette–only and dual users.
Medians were reported for cotinine, NNAL, and all 8 VOCs because of non-normal distribution. Regression models including planned covariates (sex, race and/or ethnicity) compared e-cigarette–only users (reference group) with dual users and controls on log-transformed levels of VOCs (8 models). Among e-cigarette–only users, Pearson’s r was used to calculate associations between levels of VOCs and e-cigarette use characteristics. For any models revealing significant differences in levels of VOCs between e-cigarette–only users and controls, analysis of variance was used to examine VOCs by type of product used, and t tests were used to compare VOCs by the presence or absence of flavors used in the past month.
Although we tried to eliminate exposure to blunts (tobacco mixed with marijuana) using NNAL, we could not exclude the potential contribution of VOC exposure from marijuana smoking on the day of the study.24 Consequently, we estimated and tested regression models of log-transformed VOC values that were significant in the first set of analyses, including planned covariates (sex, race and/or ethnicity), with the additional covariate of self-reported frequency of marijuana use.
Results
Three hundred eighty-six adolescents were screened, 229 were found to be eligible, and 180 agreed to participate. After verbally reporting use within 24 hours, 29 participants admitted on their surveys to not using an e-cigarette product in the previous 24 hours and thus were excluded from analyses. An additional 48 adolescents had levels of NNAL that might be consistent with substantial secondhand exposure or occasional cigarette smoking (ie, 1–29 pg per milligram of creatinine) and, as per our a priori criteria described above, were excluded from analyses. The final sample consisted of 67 e-cigarette–only users, 16 dual users, and 20 controls.
E-Cigarette Use Behaviors
E-cigarette–only users reported using their e-cigarettes a mean of 12.8 days (SD = 8.9) a month compared with 25.5 days (SD = 6.6) for dual users (P < .001) (Table 1). There was no difference in time since the last use of e-cigarettes between e-cigarette–only (mean: 2:02 hours) and dual users (mean: 1:58 hours; P > .91). Among e-cigarette–only users, the level of salivary cotinine was significantly associated with both the number of days using an e-cigarette in the past 30 days (r = 0.34; P < .01) and the mean number of use sessions a day (r = 0.75; P < .001).
TABLE 1.
E-Cigarette Use Characteristics
| Characteristic | E-Cigarette–Only Users,a n = 67, Mean (SD) or No. (%) | Dual Users,b n = 16, Mean (SD) or No. (%) | Controls, n = 20, Mean (SD) or No. (%) | Pc |
|---|---|---|---|---|
| Age | 16.3 (1.2) | 17.1 (0.96) | 16.0 (1.8) | .06 |
| Sex (male) | 49 (73%) | 12 (80%) | 7 (35%) | <.01 |
| Race and/or ethnicity | <.01 | |||
| Non-Hispanic white | 36 (54%) | 9 (67%) | 0 | |
| Asian American or Pacific Islander | 12 (19%) | 2 (12%) | 2 (10%) | |
| Multiracial | 10 (15%) | 3 (19%) | 0 | |
| Hispanic | 7 (10%) | 2 (12%) | 18 (90%) | |
| Hours since last e-cigaretted | 1:58 (6:29) | 2:02 (7:17) | N/A | >.91 |
| Days used in past 30 d | 12.8 (8.9) | 25.5 (6.6) | N/A | <.001 |
| Sessions per day | 2.0 (3.6) | 8.4 (11.6) | N/A | .05 |
| Usual puffs per session | N/A | .49 | ||
| 1–4 | 14 (21%) | 1 (7%) | ||
| 5–10 | 21 (31%) | 4 (27%) | ||
| 10–15 | 11 (16%) | 4 (27%) | ||
| >15 | 21 (31%) | 6 (40%) | ||
| Usual length of session | N/A | .97 | ||
| 1–2 min | 8 (12%) | 2 (13%) | ||
| 3–5 min | 16 (24%) | 4 (27%) | ||
| 6–10 min | 16 (24%) | 4 (27%) | ||
| >10 min | 27 (40%) | 5 (33%) | ||
| Usual type of device | N/A | .82 | ||
| Vape pen | 24 (36%) | 6 (40%) | ||
| Modified | 17 (25%) | 4 (27%) | ||
| Juul | 18 (27%) | 4 (27%) | ||
| Other or unsure | 8 (12%) | 1 (7%) | ||
| E-cigarettes contain nicotine | N/A | .06 | ||
| Always | 21 (31%) | 9 (60%) | ||
| Sometimes | 26 (39%) | 6 (40%) | ||
| Unsure | 10 (15%) | 0 (0%) | ||
| Never | 10 (15%) | 0 (0%) | ||
| Usual flavor of e-cigarettee | N/A | |||
| Fruit | 37 (55%) | 10 (67%) | .42 | |
| Candy | 11 (16%) | 2 (13%) | .77 | |
| Menthol | 12 (18%) | 2 (13%) | .67 | |
| Tobacco | 5 (8%) | 2 (13%) | .46 |
N/A, not applicable.
Used an e-cigarette product in the past 24 h and had NNAL levels <1 ppm of creatinine.
Used an e-cigarette product in the past 24 h, smoked a cigarette in the past 30 d, and had NNAL levels ≥30 ppm of creatinine.
P values are the result of comparing 3 groups on age (analysis of variance), sex, and ethnicity (χ2); all e-cigarette characteristics are the result of comparing e-cigarette–only use to dual-use groups (t tests for continuous variables and χ2 analyses for categorical variables).
The median was reported because of non-normal distribution.
Participants could select >1.
E-cigarette–only participants who reported using nicotine containing products “all” or “some” of the time had significantly higher levels of saliva cotinine compared with those who “never” used or were “unsure” if there was nicotine in their e-cigarettes (31 ng/mL [SD = 130.8] versus 0.08 ng/mL [SD = 0.38]; P < .001). E-cigarette–only participants who used nicotine in their e-cigarettes also reported using their e-cigarettes more frequently, with an average use of 15.1 (SD = 9.2) days per month compared with 7.6 (SD = 5.6) days (P < .001) and an average of 2.5 (SD = 4.0) sessions per day on days they used versus 0.65 (SD = 0.61) sessions (P < .01).
Presence and Comparison of VOCs
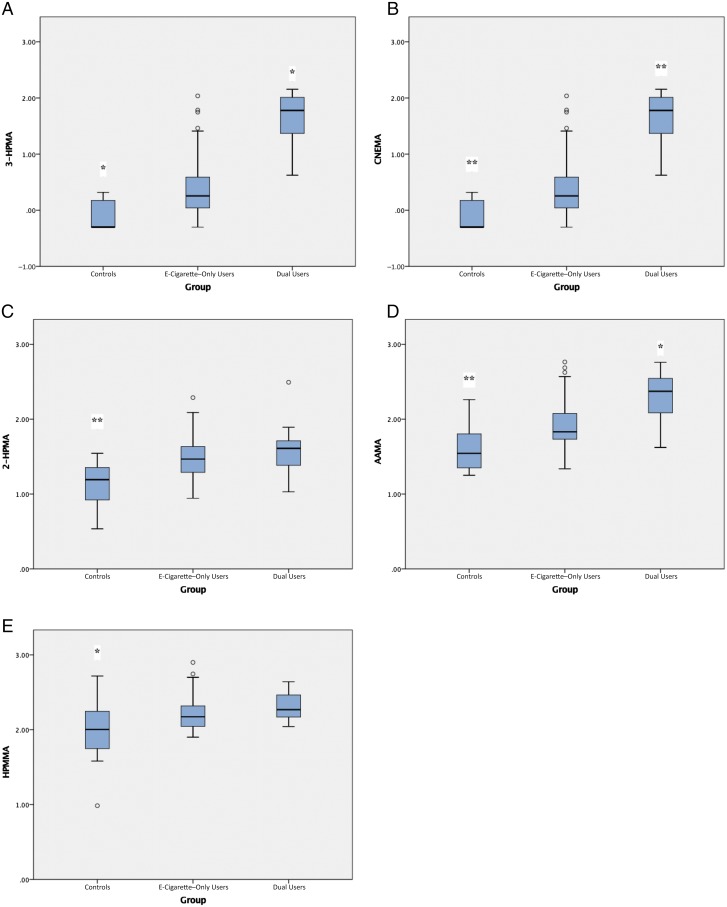
Urine excretion of metabolites of benzene (PMA), ethylene oxide (HEMA), acrylonitrile (CNEMA), acrolein (3-HPMA), and acrylamide (AAMA) was significantly higher in dual users versus e-cigarette–only users and controls (all P < .05; see Table 2; Fig 1). Excretion of metabolites of 5 VOCs was significantly higher in e-cigarette–only users compared with controls (all P < .05): acrylonitrile (341% higher than in controls but 327% lower than in dual users), acrolein (20% higher than in controls but 11% lower than in dual users), propylene oxide (51% higher than in controls but 8% lower than in dual users; 2-HPMA), acrylamide (30% higher than in controls but 23% lower than in dual users), and crotonaldehyde (20% higher than in controls but 7% lower than in dual users; HMPMA).
TABLE 2.
Biomarkers of Nicotine, Tobacco-Specific Nitrosamine, and Volatile Organic Toxicants in Exclusive E-Cigarette–Only Users Versus Dual Users and Controls
| Variable | E-Cigarette–Only Users,a n = 67 | Dual Users,b n = 16 | Controls,c n = 20 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mediand | IQR | Range | Mediane | IQR | Range | Mediane | IQR | Range | |
| Saliva cotinine (ng/mL) | 0 | 3.8 | 0–864.6 | 99.4** | 139.0 | 36.2–302.8 | 0* | 0 | 0 |
| Urine NNAL (pg/mL of creatinine) | 0.3 | 0.7 | 0–0.9 | 68.1** | 68.7 | 32.7–299.3 | 0** | 0 | 0 |
| PMA (ng/mg of creatinine; benzene) | 0 | 0.1 | 0–2.0 | 0.2** | 0.7 | 0–2.4 | 0 | 0 | 0–0.1 |
| MHBMA (ng/mg of creatinine; 1,3-butadiene) | 0 | 0 | 0–2.2 | 0 | 0.1 | 0–0.2 | 0* | 0.5 | 0–1.1 |
| HEMA (ng/mg of creatinine; ethylene oxide) | 0.5 | 1.1 | 0–7.6 | 1.0* | 1.4 | 0–8.2 | 1.3 | 2.3 | 0–4.0 |
| CNEMA (ng/mg of creatinine; acrylonitrile) | 1.3 | 3.2 | 0–108.4 | 59.4** | 81.3 | 3.7–142.6 | 0** | 1.1 | 0–1.6 |
| 3-HPMA (ng/mg of creatinine; acrolein) | 254.3 | 191.4 | 0–2311.6 | 439.7* | 224.1 | 153.6–814.4 | 192.8* | 261.6 | 0–1416.4 |
| 2-HPMA (ng/mg of creatinine; propylene oxide) | 28.8 | 25 | 0–1382.6 | 40.2 | 27.9 | 10.2–310.9 | 15.2** | 14.4 | 0–34.5 |
| AAMA (ng/mg of creatinine; acrylamide) | 67.3 | 69 | 0–581.2 | 235.6** | 239.8 | 41.4–574.7 | 34.5** | 41.6 | 0–182.0 |
| HMPMA (ng/mg of creatinine; crotonaldehyde) | 148.7 | 99 | 0–793.4 | 185.4 | 156.6 | 110.0–437.9 | 100.4* | 129.9 | 0–522.1 |
All comparisons were made with e-cigarette–only users as a comparison group. IQR, interquartile range; MHBMA, 4-hydroxy-2-buten-1-yl-mercapturic acid.
Used an e-cigarette product in the past 24 h and had NNAL levels <1 pg/mL of creatinine.
Used an e-cigarette product in the past 24 h, smoked a cigarette in the past 30 d, and had NNAL levels ≥30 pg/mL of creatinine.
No use of tobacco or e-cigarette in the past 30 d, with NNAL levels <1 pg/mL of creatinine and cotinine levels <1 ng/mL.
The median (IQR) was reported for cotinine, NNAL (pg/mL of creatinine), and VOCs (ng/mg of creatinine) because of non-normal distribution.
Tests were based on regression models of log-transformed values, including planned covariates (sex and race and/or ethnicity) with contrasts for e-cigarette–only users versus controls and for e-cigarette–only users versus dual users.
P < .05; ** P < .001.
FIGURE 1.
Significant VOC exposure in e-cigarette–only users versus controls and e-cigarette–only users versus dual users. A, Acrolein. B, Acrylonitrile. C, Propylene oxide. D, Acrylamide. E, Crotonaldehyde. Tests were based on regression models of shifted log-transformed values, including planned covariates (sex, race and/or ethnicity), with contrasts for e-cigarette–only users versus controls and e-cigarette–only users versus dual users. All comparisons are made with e-cigarette–only users as the comparison group. * P < .05; ** P < .001.
We reran the 5 regression models used to predict the 5 log-transformed VOC values that were significant in the first set of analyses, including predictors of planned covariates (sex, race and/or ethnicity) and contrasts between e-cigarette–only users and dual users, with the additional covariate of self-reported frequency of marijuana use. In all models, group membership remained a statically significant predictor of VOC value (dual users > e-cigarette–only users), accounting for variance independent of marijuana use frequency.
Associations Between VOCs and E-Cigarette Use
Among e-cigarette–only users, levels of the 5 VOCs (ie, CNEMA, 3-HPMA, 2-HPMA, AAMA, HMPMA) that were significantly greater than the levels found in controls were not associated with time since last e-cigarette use (P values ranged from .53 to .92). Compared with those who never used nicotine in their e-cigarettes or were unsure, participants who reported using nicotine in their e-cigarettes all or some of the time had significantly higher median levels of urinary CNEMA (1.50 vs 0.88 ng/mL creatinine; P = .05) and AAMA (71.5 vs 60.4 ng/mL creatinine, P = .05). The average number of sessions of e-cigarette use per day was associated with increased levels of CNEMA (r = 0.36, P = .003). Days of use in the past month was not associated with any increases in urinary VOC levels (P values raged from .21 to .72) among e-cigarette–only users.
There were no differences in levels of the 5 significant VOCs that were based on that type of product used (F test scores ranged from 0.51 to 2.3; P values ranged from .09 [for 2-HPMA] to .67). Participants who reported using fruit flavors in the past month had higher CNEMA levels than those who did not (yes: mean = 10.4 ng/mL creatinine [SD = 21.7]; no: mean 2.1 ng/mL creatinine [SD = 3.4]; P = .03). There were no differences in VOC levels among those who favored candy (P values ranged from .33 to .87), tobacco (P values ranged from .42 to .87), or menthol flavors (P values ranged from .09 [for 2-HPMA] to .95) compared with those who did not.
Discussion
To the best of our knowledge, this is the first study to report on the presence of VOC toxicants in adolescent e-cigarette users. Overall results reveal significantly greater toxicant exposure in adolescent e-cigarette users compared with their nonusing peers. Adolescent e-cigarette–only users had levels of 5 VOC toxicants detected in their urine in quantities up to 3 times greater than in matched controls, including metabolites of acrylonitrile, acrolein, propylene oxide, acrylamide, and crotonaldehyde. Levels of toxicant exposure in dual users were up to 3 times higher than in those who used only e-cigarettes. Post hoc analyses revealed that, among dual users, levels of VOCs were not associated with NNAL (P values ranged from .17 to .81), suggesting that the higher VOCs were not only due to exposure to traditional cigarettes.
The presence of harmful ingredients in e-cigarette vapor has been established25; we can now say that these chemicals are found in the body of human adolescents who use these products. A risk analysis of lifelong exposure to even low-level VOCs, derived using data from secondhand tobacco smoke exposure, indicated an increased cancer risk, which could be applicable to exposure in the current study.26 Of course, this assumes that the exposures will be ongoing, which has not yet been established. It is worth noting that although e-cigarette–only users had significantly higher exposure to 5 VOCs, controls also had detectable levels of these chemicals. In fact, human exposure to VOCs from environmental sources is ubiquitous.27 It is also worth noting that levels of VOCs detected in e-cigarette–only users were on average lower than has been reported among adults.13,14,28 For example, using a similar methodology, Pulvers et al14 reported the following median levels among exclusive e-cigarette users: CNEMA of 20.3 ng/mg of creatinine (versus 1.3 ng/mg in our sample), 3-HPMA of 370.3 ng/mg (versus 254.3 ng/mg), 2-HPMA of 38.0 ng/mg (versus 28.8 ng/mg), AAMA of 96.5 ng/mg (versus 67.3 ng/mg), and HMPMA of 251.6 ng/mg (versus 148.7 ng/mg). However, participants reported more frequent use of e-cigarettes in that study (ie, 24.7 days in the past 30 days and an average of 11.8 times per day on use days), and exclusive use of e-cigarettes was based on self-report only, because this was a switching study in which NNAL levels would not have had time to decline to nonexposed levels. Thus, the increase in VOCs among adults might be reflective of greater exposure to e-cigarettes and/or combustion products. Moreover, unlike our study, none of the authors of these studies employed a control group to account for baseline levels of environmental VOCs.
Nicotine
Not surprisingly, e-cigarette–only participants who reported using nicotine-containing products all or some of the time had significantly higher levels of cotinine compared with those who never used or were unsure if there was nicotine in their e-cigarettes. To the best of our knowledge, this is the first study to report cotinine levels in adolescent e-cigarette–only users. Among e-cigarette–only users, only the VOCs CNEMA and AAMA were higher in users of nicotine containing e-cigarettes. Levels of the 3 other significant and likely toxic VOCs were just as high in users of nonnicotine products as in those using nicotine. This is particularly important because many teenagers initiate e-cigarette use with nicotine-free products,4 in part because they feel that they are safer.29
Type of Product
There were no significant differences found in levels of toxicants by type of product used. Despite a small number of subjects using each type of product, there was great variability among the 3 main types of e-cigarette products used by our participants. Given the results of studies of emissions among adult users of e-cigarettes, which revealed significant differences by brand and type of product,25,30–32 the small numbers of users and variable use patterns among products may have limited our ability to detect small exposure-related differences among products.
Flavorings
There are researchers who suggests that certain flavorings may generate higher levels of toxic chemicals than others.32–35 Among our e-cigarette–only participants, the use of fruit-flavored products produced significantly higher levels of the metabolites of acrylonitrile. This is of particular interest to adolescent e-cigarette use, because 1 of the main reasons teenagers report using e-cigarettes is the appealing flavors.4 Moreover, for various reasons, including the stigma associated with tobacco, some may also feel that the fruit-flavored products are safer than tobacco-flavored products. In fact, fruit flavors were the most popular choice among our e-cigarette users with roughly 55% of e-cigarette–only users and 67% of dual users reporting using fruit flavors most often.
In addition to being the first to report toxicant levels in the urine of adolescent e-cigarette users, we used strict criteria based on objective biomarkers to avoid secondary sources of VOCs by excluding participants with any evidence of exposure to combustion products from tobacco from our e-cigarette–only group. Another strength of this study is the use of age-matched controls to account for the underlying rate of environmental exposures to 8 toxicants. We did not specifically test for marijuana exposure, a task which is fraught with difficulty, given the limitations of the testing itself, which are due to the long half-life of δ-9-tetrahydrocannabinol.36,37 Despite this, our analyses revealed that it is unlikely that the variance in VOCs explained by our e-cigarette use group was accounted for by marijuana use instead of e-cigarette use.
Other limitations of this study include the fact that a wide range of e-cigarette products were used among participants, and thus, it may be difficult to pinpoint variability in toxicant exposure on the basis of the self-reported product used. However, this strengthens the external validity of the study because it gives a more real-world view of the toxicants found from the e-cigarette products commonly used by adolescents. We also only tested 8 likely toxic VOCs, but there may be other significant toxicants, including formaldehyde, which can be produced by e-cigarettes and which could pose a threat to adolescent users of these products; however, formaldehyde exposure is difficult to assess in vivo.25 Although the focus of this study was on e-cigarette–only users, we also had a relatively small number of confirmed (ie, using NNAL) dual users. Lastly, controls were on average more likely to be female and Hispanic compared with e-cigarette–only and dual users. However, we do not feel that this played a role in our VOC findings because the analyses accounted for both sex and race and/or ethnicity. There may be other factors that could have influenced VOC levels, but given the sample size, we limited the number of covariates we included in any analysis. Larger prospective studies are needed to confirm the findings reported here to test for recent marijuana use and examine changes over time, perhaps with more complex matching.
Conclusions
Although e-cigarette vapor may be less dangerous than combustible cigarettes, with lower overall exposure to VOC toxicants, with our findings, we challenge the idea that e-cigarette vapor is safe. Many of the VOCs we identified among e-cigarette users are carcinogenic, including propylene oxide, acrylamide, acrylonitrile, and crotonaldehyde.13 With few exceptions, these toxicants were present whether the product contained nicotine or flavorings. Consequently, as with traditional cigarettes, messaging to teenagers must include warnings about the potential risk from toxic exposure to carcinogenic compounds generated by these products.
Acknowledgments
We thank Mr Richard Ceballos III, Mrs Judy Gonzalez-Vargas, Dr Karma McKelvey, Mr Michael Berry, Mr Mark Thomas, and Mr Jerome Andres for their assistance with data collection and participant recruitment. We are also grateful to Lisa Yu and Peyton Jacob III for assistance with biomarker assays. We thank Trisha Mao, Ethan Yip, Lawrence Chan, and Kristina Bello for performing analytical chemistry. We thank Dr Judith Prochaska for feedback on an earlier draft of the manuscript.
Glossary
- AAMA
2-carbamoylethylmercapturic acid
- CNEMA
2-cyanoethylmercapturic acid
- e-cigarette
electronic cigarette
- HEMA
2-hydroxyethylmercapturic acid
- HMPMA
3-hydroxy-1-methyl-propylmercapturic acid
- NNAL
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
- PMA
phenylmercapturic acid
- VOC
volatile organic compound
- 2-HPMA
2-hydroxypropylmercapturic acid
- 3-HPMA
3-hydroxypropylmercapturic acid
Footnotes
Drs Rubinstein, Delucchi, Ramo, and Benowitz made substantial contributions to the conception and design of the study and to the analysis and interpretation of data, participated in and made substantial contributions to the drafting of the manuscript for important intellectual content, and are in agreement to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: Dr Benowitz is a consultant to several pharmaceutical companies that market medications to aid smoking cessation and has served as a paid expert witness in litigation against tobacco companies. Drs Ramo and Rubinstein have consulted for Carrot Inc, which makes a tobacco cessation device; and Dr Delucchi has indicated he has no financial relationships relevant to this article to disclose.
FUNDING: Funded by National Institutes of Health (NIH) grants R21DA040718, P50 CA180890, P30 DA012393, S10 RR026437, and TRDRP 24XT-0007. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Benowitz is a consultant to several pharmaceutical companies that market medications to aid smoking cessation and has served as a paid expert witness in litigation against tobacco companies. Drs Ramo and Rubinstein have consulted for Carrot Inc, which makes a tobacco cessation device; and Dr Delucchi has indicated he has no potential conflicts of interest to disclose.
References
- 1.Grana RA, Ling PM. “Smoking revolution”: a content analysis of electronic cigarette retail Websites. Am J Prev Med. 2014;46(4):395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE Use of alcohol, cigarettes, and number of illicit drugs declines among U.S. teens. 2014. Available at: http://www.monitoringthefuture.org/pressreleases/14cigpr.pdf. Accessed January 6, 2015
- 3.US Department of Health and Human Services E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General—Executive Summary. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2016 [Google Scholar]
- 4.Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob Res. 2015;17(7):847–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll Chapman SL, Wu LT. E-cigarette prevalence and correlates of use among adolescents versus adults: a review and comparison. J Psychiatr Res. 2014;54:43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wills TA, Knight R, Williams RJ, Pagano I, Sargent JD. Risk factors for exclusive e-cigarette use and dual e-cigarette use and tobacco use in adolescents. Pediatrics. 2015;135(1). Available at: www.pediatrics.org/cgi/content/full/135/1/e43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laino T, Tuma C, Moor P, Martin E, Stolz S, Curioni A. Mechanisms of propylene glycol and triacetin pyrolysis. J Phys Chem A. 2012;116(18):4602–4609 [DOI] [PubMed] [Google Scholar]
- 9.Henderson TR, Clark CR, Marshall TC, Hanson RL, Hobbs CH. Heat degradation studies of solar heat transfer fluids. Sol Energy. 1981;27(2):121–128 [Google Scholar]
- 10.Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci USA. 2006;103(42):15404–15409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosmider L, Sobczak A, Fik M, et al. . Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16(10):1319–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372(4):392–394 [DOI] [PubMed] [Google Scholar]
- 13.Hecht SS, Carmella SG, Kotandeniya D, et al. . Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res. 2015;17(6):704–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pulvers K, Emami AS, Nollen NL, et al. . Tobacco consumption and toxicant exposure of cigarette smokers using electronic cigarettes. Nicotine Tob Res. 2018;20(2):206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wild CP, Kleinjans J. Children and increased susceptibility to environmental carcinogens: evidence or empathy? Cancer Epidemiol Biomarkers Prev. 2003;12(12):1389–1394 [PubMed] [Google Scholar]
- 16.Jacob P III, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(3–4):267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob P III, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr A. 1981;222(1):61–70 [DOI] [PubMed] [Google Scholar]
- 18.Hecht SS, Stepanov I, Carmella SG. Exposure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Acc Chem Res. 2016;49(1):106–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goniewicz ML, Havel CM, Peng MW, et al. . Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3421–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob P III, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem. 2008;80(21):8115–8121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregg EO, Minet E, McEwan M. Urinary biomarkers of smokers’ exposure to tobacco smoke constituents in tobacco products assessment: a fit for purpose approach. Biomarkers. 2013;18(6):467–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benowitz NL, Nardone N, Jain S, et al. . Comparison of urine 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol and cotinine for assessment of active and passive smoke exposure in urban adolescents. Cancer Epidemiol Biomarkers Prev. 2017, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmella SG, Chen M, Han S, et al. . Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22(4):734–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei B, Alwis KU, Li Z, et al. . Urinary concentrations of PAH and VOC metabolites in marijuana users. Environ Int. 2016;88:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goniewicz ML, Knysak J, Gawron M, et al. . Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St Helen G, Jacob P III, Peng M, Dempsey DA, Hammond SK, Benowitz NL. Intake of toxic and carcinogenic volatile organic compounds from secondhand smoke in motor vehicles. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2774–2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain RB. Distributions of selected urinary metabolites of volatile organic compounds by age, gender, race/ethnicity, and smoking status in a representative sample of U.S. adults. Environ Toxicol Pharmacol. 2015;40(2):471–479 [DOI] [PubMed] [Google Scholar]
- 28.Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P III, Benowitz NL. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine Tob Res. 2017;19(2):160–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorukanti A, Delucchi K, Ling P, Fisher-Travis R, Halpern-Felsher B. Adolescents’ attitudes towards e-cigarette ingredients, safety, addictive properties, social norms, and regulation. Prev Med. 2017;94:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N. Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health. 2014;11(11):11192–11200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uchiyama S, Ohta K, Inaba Y, Kunugita N. Determination of carbonyl compounds generated from the e-cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine, followed by high-performance liquid chromatography. Anal Sci. 2013;29(12):1219–1222 [DOI] [PubMed] [Google Scholar]
- 32.Benowitz NL, Fraiman JB. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol. 2017;14(8):447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romagna G, Allifranchini E, Bocchietto E, Todeschi S, Esposito M, Farsalinos KE. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol. 2013;25(6):354–361 [DOI] [PubMed] [Google Scholar]
- 34.Farsalinos KE, Romagna G, Allifranchini E, et al. . Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health. 2013;10(10):5146–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen JG, Flanigan SS, LeBlanc M, et al. . Flavoring chemicals in e-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environ Health Perspect. 2016;124(6):733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol In: Pertwee RG, ed. Handbook of Experimental Pharmacology. Vol 168 New York, NY: Springer; 2005:657–690 [DOI] [PubMed] [Google Scholar]
- 37.Buchan BJ, L Dennis M, Tims FM, Diamond GS. Cannabis use: consistency and validity of self-report, on-site urine testing and laboratory testing. Addiction. 2002;97(suppl 1):98–108 [DOI] [PubMed] [Google Scholar]