ABSTRACT
Francisella tularensis, the causative agent of tularemia, lacks typical bacterial virulence factors and toxins but still exhibits extreme virulence. The bacterial multidrug efflux systems consist of an inner membrane, a transmembrane membrane fusion protein, and an outer membrane (OM) component that form a contiguous channel for the secretion of a multitude of bacterial products. Francisella contains three orthologs of the OM proteins; two of these, termed TolC and FtlC, are important for tularemia pathogenesis. The third OM protein, SilC, is homologous to the silver cation efflux protein of other bacterial pathogens. The silC gene (FTL_0686) is located on an operon encoding an Emr-type multidrug efflux pump of F. tularensis. The role of SilC in tularemia pathogenesis is not known. In this study, we investigated the role of SilC in secretion and virulence of F. tularensis by generating a silC gene deletion (ΔsilC) mutant and its transcomplemented strain. Our results demonstrate that the ΔsilC mutant exhibits increased sensitivity to antibiotics, oxidants, silver, diminished intramacrophage growth, and attenuated virulence in mice compared to wild-type F. tularensis. However, the secretion of antioxidant enzymes of F. tularensis is not impaired in the ΔsilC mutant. The virulence of the ΔsilC mutant is restored in NADPH oxidase-deficient mice, indicating that SilC resists oxidative stress in vivo. Collectively, this study demonstrates that the OM component SilC serves a specialized role in virulence of F. tularensis by conferring resistance against oxidative stress and silver.
IMPORTANCE Francisella tularensis, the causative agent of a fatal human disease known as tularemia, is a category A select agent and a potential bioterror agent. The virulence mechanisms of Francisella are not completely understood. This study investigated the role of a unique outer membrane protein, SilC, of a multidrug efflux pump in the virulence of F. tularensis. This is the first report demonstrating that the OM component SilC plays an important role in efflux of silver and contributes to the virulence of F. tularensis primarily by providing resistance against oxidative stress. Characterization of these unique virulence mechanisms will provide an understanding of the pathogenesis of tularemia and identification of potential targets for the development of effective therapeutics and prophylactics for protection from this lethal disease.
KEYWORDS: Francisella tularensis, TolC, multidrug efflux pumps, oxidative stress, virulence
INTRODUCTION
The Centers for Disease Control and Prevention has classified Francisella tularensis as a tier 1 category A select agent and a potential bioterror agent due to its extreme virulence, the ease of aerosol dissemination of this organism, and the lack of effective prophylactic measures against it (1–3). F. tularensis is a Gram-negative intracellular bacterial pathogen. The strains responsible for causing a fatal human disease known as tularemia belong to F. tularensis subsp. tularensis and F. tularensis subsp. holarctica. The live vaccine strain (LVS) is a derivative of F. tularensis subsp. holarctica. The LVS is used as a vaccine against tularemia in several countries; however, adverse reactions in immunized individuals and insufficient protection against respiratory tularemia caused by highly virulent F. tularensis strains have prevented its licensure in the United States (4). Due to its attenuated virulence in humans, the LVS serves as an excellent surrogate to highly virulent strains to study tularemia pathogenesis.
F. tularensis virulence is mediated to some extent by the ability of the bacteria to replicate inside macrophages and several other cell types such as neutrophils, dendritic cells, and lung epithelial cells (5–7). The F. tularensis genome encodes components of putative type I, type II, type V, and type VI secretion systems (T1SS, T2SS, T5SS, and T6SS) (8). Components of TAT and Sec secretion systems are also present; however, type III and type IV secretion systems are absent in Francisella (8, 9). Components of the type IV pili and the T6SS are encoded by Francisella pathogenicity island (FPI) (10). T1SS is the simplest of all the secretions systems and is comprised of an inner membrane (IM) component, a membrane fusion protein (MFP) component that spans the inner and the outer membranes, and an outer membrane (OM) component that serves as a porin. These three components form a contiguous channel for the secretion of a multitude of bacterial products, including toxins. The multidrug transporters/efflux pumps have a structure similar to that of a prototypical T1SS (11). These multidrug efflux pumps are used by pathogenic bacteria to confer resistance against antibiotics, dyes, detergents, and antimicrobial agents (12, 13). The multidrug transporters are classified into five families: the ATP binding cassette (ABC) superfamily, the major facilitator superfamily (MFS), the multidrug and toxic-compound extrusion (MATE) family, the small multidrug resistance (SMR) family, and the resistance nodulation division (RND) family. T1SSs are architecturally related to the MFS and the RND family of multidrug efflux pumps due to their tripartite organization. F. tularensis has been predicted to encode 31 MFS transport and 15 functional ABC systems. However, the roles of a majority of these multidrug efflux systems in the resistance to antibiotics, intracellular survival, and virulence of F. tularensis are not known (14).
The RND efflux systems are composed of an inner membrane-associated efflux protein (AcrB), an MFP located in the periplasmic space (AcrA), and an outer membrane protein that is homologous to the TolC protein found in Escherichia coli. The functional roles of the Francisella RND-type AcrAB multidrug efflux pump have been characterized. AcrB of the F. tularensis LVS RND transporter is required for resistance against several antibiotics and antimicrobial compounds and for virulence in mice (15). In contrast, AcrAB components of the RND pump of the virulent F. tularensis strain SchuS4 are required for efflux of antibiotics, dyes, and detergents but not for virulence in mice (16). In addition to the AcrAB-type RND system, Francisella also possesses an Emr-type MFS multidrug efflux system. We have reported that the Emr-type MFS is unique in F. tularensis and is composed of an IM component, EmrB (FTL_0688), an MFP, EmrA1 (FTL_0687), and an OM component, SilC (FTL_0686). Unlike the AcrAB system, in which the genes acrA and acrB are located on the same operon while the OM component genes for tolC and fltC are transcribed at a distant location, all three of the genes encoding the Emr multidrug efflux pump of F. tularensis are positioned adjacently and are transcribed as an operon (17). In our previous study, we characterized the role of EmrA1, the MFP component of the Emr multidrug efflux pump. We have demonstrated that EmrA1 contributes to antibiotic resistance, intramacrophage survival, and virulence in mice. Most importantly, we have demonstrated that the loss of emrA1 is associated with enhanced sensitivity of the emrA1 mutant to oxidants and impaired secretion of antioxidant enzymes catalase (KatG) and superoxide dismutase B (SodB) (17). These results indicate that the Emr multidrug efflux system of F. tularensis is designed to serve a unique role by providing resistance to oxidative stress.
Genomic and bioinformatic analyses of F. tularensis have confirmed the existence of three genes that have a high sequence homology with the OM component TolC of E. coli and other bacterial TolC orthologs. These genes are tolC (FTL_1865), ftlC (FTL_1107), and silC (FTL_0686) (18). The OM components TolC and FtlC of F. tularensis LVS are required for resistance against antibiotics, dyes, and detergents (19). However, the requirement of only TolC but not of FtlC for virulence in mice indicates a nonredundant role of these OM proteins of F. tularensis LVS. Moreover, the tolC gene deletion mutant of F. tularensis SchuS4 is only partially attenuated for virulence in mice. TolC is also required for survival in macrophages, dissemination in mouse infection model, suppression of proinflammatory innate immune response, and apoptotic cell death (20, 21). However, the induction of apoptosis in neutrophils is independent of TolC (22). The virulence factors of F. tularensis that are secreted in a TolC/FtlC-dependent fashion are not known. It has been suggested that the OM components TolC and FtlC act in conjunction with the AcrAB multidrug efflux system (15). The role of the third OM component, SilC, in F. tularensis secretion and virulence is unknown. Based on the unique genomic organization and close proximity of silC to genes of the Emr multidrug efflux system, in this study we investigated if the OM protein SilC is designed to act in conjunction with the MFP EmrA1 and IM protein EmrB of F. tularensis. The results demonstrate that SilC, similar to EmrA1, serves a specialized role by contributing to oxidant and silver resistance of F. tularensis.
RESULTS
Genomic organization of the silC gene and confirmation of ΔsilC mutant and transcomplemented strains.
silC (FTL _0686) of F. tularensis LVS is the last gene of the Emr locus and is cotranscribed with the emrA1 (FTL_0687) and emrB (FTL_0688) genes (17). These three genes encode a tripartite Emr MFS-type multidrug efflux pump (Fig. 1A). The silC gene encodes a 490-amino-acid-long protein annotated as a TolC-like outer membrane protein, SilC. The SilC is highly conserved among F. tularensis strains. Multiple-amino-acid alignment analysis with Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo) revealed 99.59% identity with SilC of F. tularensis SchuS4 (FTT_1257) and 99.39% identity with SilC of Francisella novicida (FTN_1277). Further, the SilC protein sequence alignment using BLAST analysis (https://blast.ncbi.nlm.nih.gov) revealed that the SilC protein of F. tularensis LVS belongs to the TolC family of outer membrane proteins that include NodT family outer membrane lipoproteins and the copper/silver efflux system outer membrane protein CusC. SilC of F. tularensis LVS shares an overall 25% identity with the copper/silver efflux system outer membrane component CusC of Escherichia coli K-12. SilC protein exhibits 21.03% identity with TolC and 22% identity with FtlC of F. tularensis LVS.
FIG 1.
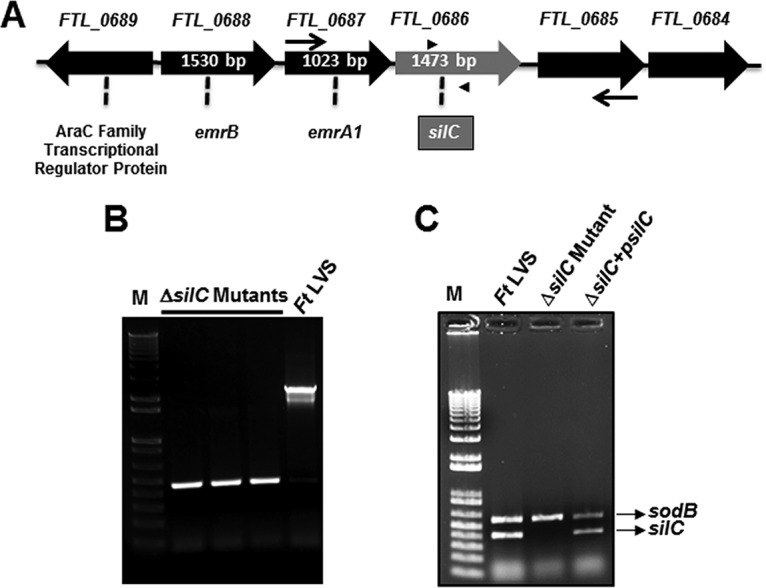
Genomic organization of the silC gene and generation of the ΔsilC mutant of F. tularensis. (A) Genomic organization of the silC gene of F. tularensis LVS. The thin arrows and the arrowheads indicate the location of the flanking primers and primers internal to the silC gene, respectively, used for the confirmation of silC gene deletion. (B and C) Confirmation of silC gene deletion by PCR using flanking primers (B) and primers internal to the silC gene (C). sodB gene-specific primers were used as internal controls.
The deletion of the silC gene in the ΔsilC mutant was confirmed by PCR using the primers flanking the upstream FTL_0685 and the downstream FTL_0687 genes. The deletion of the silC gene was indicated by the presence of an amplification product smaller than that obtained for the wild-type F. tularensis LVS (Fig. 1B). We further confirmed the silC gene deletion using silC gene-specific primers. Both the wild-type F. tularensis LVS and the transcomplemented strain amplified a fragment internal to the silC gene. The absence of an amplification product confirmed the silC gene deletion. sodB gene-specific primers were used as an internal control (Fig. 1C). DNA sequencing of the flanking region confirmed the deletion of the silC gene in the ΔsilC mutant (not shown).
SilC does not contribute to resistance against dyes and detergents but confers resistance to antibiotics.
Since the other two OM components, TolC and FtlC, of F. tularensis have been shown to be required for resistance against detergents, dyes, and antibiotics (19), we investigated if SilC has a similar role. The sensitivity of the ΔsilC mutant to dyes, detergents, and antibiotics was determined by disc diffusion assays. It was observed that the ΔsilC mutant did not show any enhanced sensitivity to detergent such as SDS and dyes such as ethidium bromide and acriflavine compared to the wild-type F. tularensis LVS (Table 1).
TABLE 1.
Sensitivities of F. tularensis LVS, the ΔsilC mutant, and the transcomplemented strain ΔsilC+psilC to antibiotics, detergent, and dyes
| Antibiotic, detergent, or dye | Concn (μg/disc) | Zone of inhibition (mm)c |
||
|---|---|---|---|---|
| F. tularensis LVS | ΔsilC mutant | ΔsilC+psilC | ||
| Streptomycin | 1.25 | 14.667 ± 0.33 | 17 ± 0a | 14 ± 0 |
| 5 | 19.33 ± 0.33 | 23.33 ± 0.33a | 19.33 ± 0.33 | |
| Nalidixic acid | 1.88 | 20.66 ± 0.33 | 20.66 ± 0.33 | 20.66 ± 0.33 |
| 3.75 | 26.33 ± 0.33 | 26 ± 0 | 25.667 ± 0.33 | |
| 30 | 41.50 ± 1.0 | 46.75 ± 4.92 | NDb | |
| Chloramphenicol | 3.75 | 27.5 ± 1.5 | 28 ± 2 | 29 ± 1 |
| 5.0 | 32.5 ± 1.0 | 35.75 ± 2.18 | ND | |
| Novobiocin | 7.5 | 13.5 ± 0.5 | 13.5 ± 0.5 | 13.5 ± 0.5 |
| 15 | 16.5 ± 0.5 | 17.5 ± 0.5 | 18 ± 0 | |
| 30 | 21.75 ± 2.36 | 22.75 ± 3.10 | ND | |
| Tetracycline | 3.75 | 25 ± 0.5 | 25 ± 0.5 | 26 ± 1 |
| 7.5 | 31 ± 1 | 30.5 ± 0.5 | 30 ± 0.5 | |
| Neomycin | 3.75 | 11.33 ± 0.33 | 11 ± 0 | 11.33 ± 0.33 |
| 7.5 | 19.667 ± 0.33 | 19.33 ± 0.33 | 19.33 ± 0.667 | |
| Erythromycin | 15 | 6 ± 0 | 6 ± 0 | 6 ± 0 |
| 45 | 6 ± 0 | 6 ± 0 | 6 ± 0 | |
| Gentamicin | 1.25 | 22 ± 1 | 21.50 ± 1.5 | 21.50 ± 1.5 |
| 2.5 | 26 ± 0 | 25.5 ± 0.50 | 25.5 ± 0.50 | |
| SDS | 750 | 15.53 ± 0.58 | 16.67 ± 0.58 | ND |
| Ethidium bromide | 5 | 15.33 ± 0.58 | 13.67 ± 1.53 | ND |
| Acriflavine | 25 | 20.33 ± 0.58 | 20.33 ± 0.58 | ND |
Significantly higher than in F. tularensis LVS or the transcomplemented strain (P < 0.05) by one-way ANOVA.
ND, not determined.
Boldface indicate enhanced sensitivity of the ΔsilC mutant to these antibiotics.
The antibiotic sensitivity testing revealed that the ΔsilC mutant exhibits slightly enhanced sensitivities to streptomycin, nalidixic acid, and chloramphenicol but not to other antibiotics tested, such as neomycin, novobiocin, erythromycin, tetracycline, and gentamicin, compared to the wild-type F. tularensis LVS or the transcomplemented strain (Table 1). Collectively, these results indicate that SilC does not contribute to resistance against detergent and dyes but strains lacking it exhibit a small increase in susceptibility to some antibiotics.
The ΔsilC mutant of F. tularensis LVS exhibits enhanced sensitivity to oxidants.
Our previous study has shown that the mutant of the emrA1 gene is extremely sensitive to the superoxide-generating compound pyrogallol and H2O2 (17). We next investigated if the ΔsilC mutant of F. tularensis LVS exhibits a similar oxidant-sensitive phenotype. The sensitivity of the ΔsilC mutant to superoxide-generating compounds paraquat and pyrogallol and to peroxides, such as cumene hydroperoxide (CHP), tert-butyl hydroperoxide (TBH), and H2O2, was determined by disc diffusion assay, bacterial killing assay, and growth curve analysis. It was observed that the ΔsilC mutant is sensitive to the superoxide-generating compounds paraquat and pyrogallol, as indicated by larger zones of inhibition for the ΔsilC mutant than those obtained for the wild-type F. tularensis LVS and the transcomplemented strain in disc diffusion assays (Fig. 2A and B) and by enhanced killing in bacterial killing assays following 1 h of exposure to increasing concentrations of both paraquat and pyragallol (Fig. 2C and D).
FIG 2.
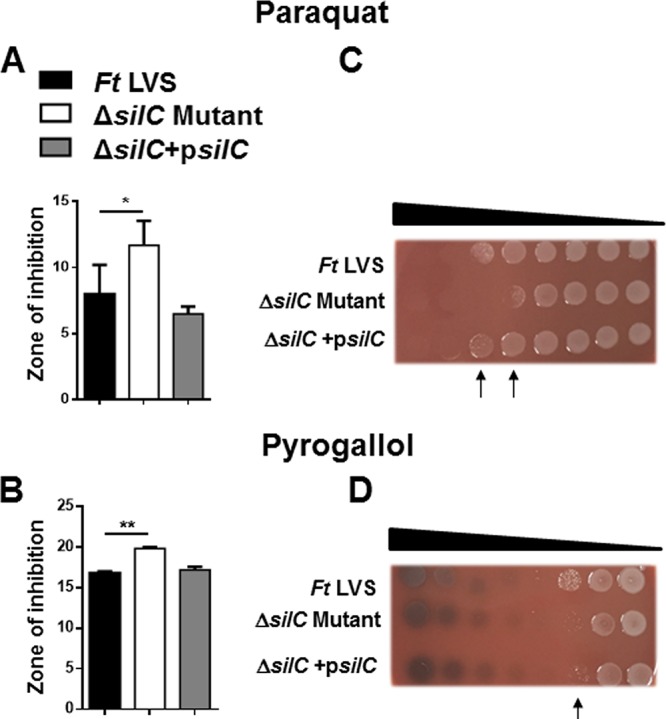
The ΔsilC mutant of F. tularensis LVS exhibits enhanced sensitivity to superoxide-generating compounds. The sensitivities of the wild-type F. tularensis (Ft) LVS, the ΔsilC mutant, and the transcomplemented strain ΔsilC+psilC were determined by disc diffusion and bacterial killing assays against superoxide-generating compounds paraquat (A and C) and pyrogallol (B and D) using the protocols and concentrations described in Materials and Methods. For disc diffusion assays, the results are expressed as the zone of inhibition diameters in millimeters in means ± standard deviations (SD) for triplicate samples. In the bacterial killing assay, the Francisella strains were exposed to serially diluted paraquat and pyrogallol for 1 h and spotted onto MH chocolate agar plates to determine the bacterial killing. The arrows in panels C and D indicate the concentrations of paraquat and pyrogallol that resulted in enhanced killing of the ΔsilC mutant. The results shown are representative of 3 independent experiments, which yielded identical results. The P values were determined by one-way ANOVA, and a P value of <0.05 is considered statistically significant. *, P < 0.05; **, P < 0.01.
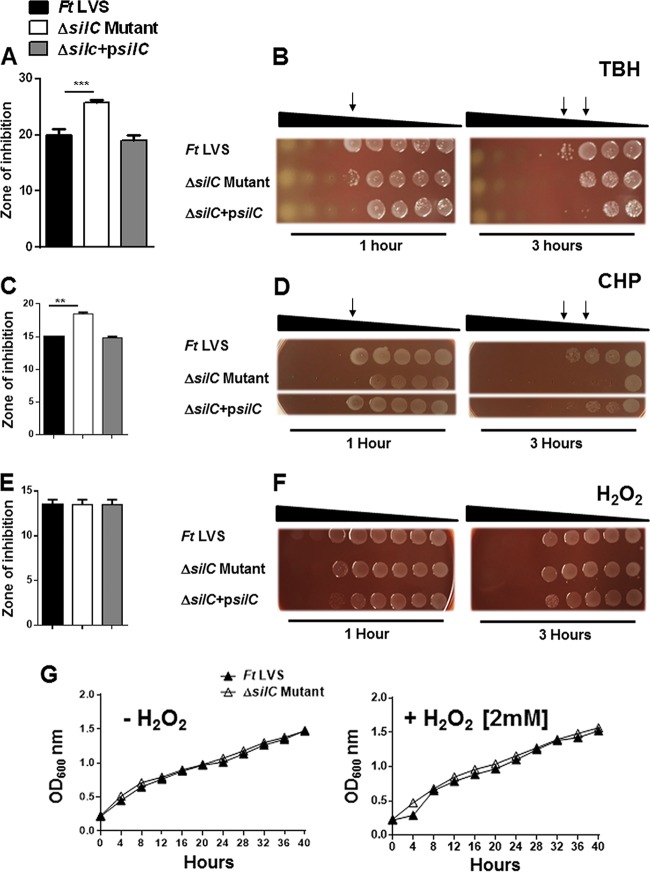
Similar to what was seen with paraquat and pyrogallol, the ΔsilC mutant was also observed to be sensitive to the organic peroxides TBH and CHP compared to wild-type F. tularensis LVS or the transcomplemented strain when tested either by disc diffusion or the bacterial killing assay (Fig. 3A to D). However, unlike the organic peroxides TBH and CHP, the ΔsilC mutant did not exhibit any enhanced sensitivity to H2O2 either by disc diffusion or the bacterial killing assay (Fig. 3E and F). We also confirmed these findings by generating growth curves. The growth of the ΔsilC mutant remained similar to that of the wild-type F. tularensis LVS irrespective of the presence or absence of H2O2 in the growth medium (Fig. 3G).
FIG 3.
The ΔsilC mutant of F. tularensis LVS exhibits enhanced sensitivity to organic peroxides but not to H2O2. (A to F) The sensitivities of the wild-type F. tularensis (Ft) LVS, the ΔsilC mutant, and the transcomplemented strain ΔsilC+psilC to organic peroxides tert-butyl hydroperoxide (TBH) (A and B), cumene hydroperoxide (CHP) (C and D), and H2O2 (E and F) were determined by disc diffusion and bacterial killing assays using the protocols and concentrations described in Materials and Methods. For disc diffusion assays, the results are expressed as the diameters of the zone of inhibition in millimeters in means ± SD for triplicate samples. In bacterial killing assays, the Francisella strains were exposed to serially diluted TBH, CHP, and H2O2 for 1 and 3 h and spotted onto MH chocolate agar plates to determine the bacterial killing. The arrows in panels B and D indicate the concentrations of TBH and CHP, respectively, that resulted in enhanced killing of the ΔsilC mutant. (G) Growth curves of the wild-type F. tularensis and the ΔsilC mutant in the absence or presence of 2 mM H2O2. The results shown are representative of 3 independent experiments, which yielded identical results. The P values were determined by one-way ANOVA, and a P value of <0.05 is considered statistically significant. **, P < 0.01; ***, P < 0.001.
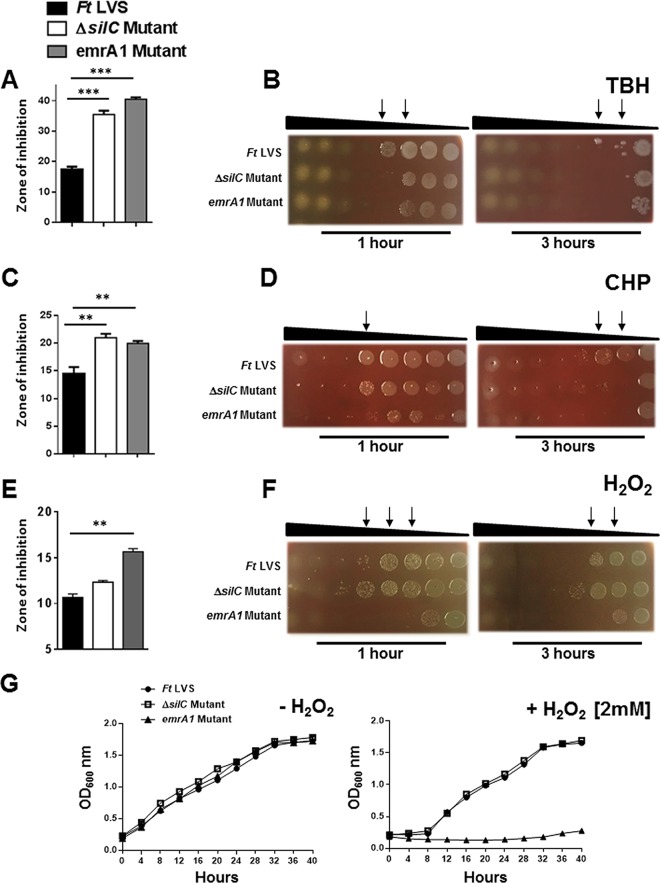
Since the emrA1 mutant of F. tularensis also shows an enhanced sensitivity to oxidants, we next made side-by-side comparisons of the ΔsilC and the emrA1 mutants for their sensitivities to the superoxide-generating compounds paraquat and pyrogallol and the organic peroxides TBH, CHP, and H2O2. The ΔsilC mutant was found to be as sensitive as the emrA1 mutant to both paraquat and pyrogallol (Fig. 4). The emrA1 mutant showed an enhanced sensitivity to TBH and CHP compared to the wild-type F. tularensis LVS. The ΔsilC mutant was also as sensitive as the emrA1 mutant to TBH and CHP (Fig. 5A to D). The emrA1 mutant, as reported earlier (17), was found to be highly sensitive to H2O2, while the ΔsilC mutant did not show any enhanced sensitivity to H2O2 (Fig. 5E and F). Growth curves generated in the presence of H2O2 also supported the results obtained with disc diffusion and bacterial killing assays in that the loss of silC did not enhance the sensitivity of the ΔsilC mutant to H2O2. Collectively, these results demonstrate that the loss of silC enhances the sensitivity of the ΔsilC mutant to oxidants such as superoxide-generating compounds and organic peroxides, a phenotype similar to that observed for the emrA1 mutant. However, interestingly, unlike the emrA1 mutant, the ΔsilC mutant does not exhibit any enhanced sensitivity to H2O2.
FIG 4.
The sensitivity of the ΔsilC mutant of F. tularensis to superoxide-generating compounds is similar to that observed for the emrA1 mutant. The sensitivities of the wild-type F. tularensis (Ft) LVS, the ΔsilC mutant, and the emrA1 mutant to superoxide-generating compounds paraquat (A and B) and pyrogallol (C and D) were determined by disc diffusion and bacterial killing assays using the protocols and concentrations described in Materials and Methods. For disc diffusion assays, the results are expressed as diameters of zone of inhibition in millimeters in means ± SD for triplicate samples. In bacterial killing assays, the Francisella strains were exposed to serially diluted paraquat and pyrogallol for 1 and 3 h and spotted onto MH chocolate agar plates to determine the bacterial killing. The arrows in panels B and D indicate the concentrations of paraquat and pyrogallol, respectively, that resulted in enhanced killing of the ΔsilC and emrA1 mutants. The results shown are representative of 3 independent experiments, which yielded identical results. The P values were determined by one-way ANOVA, and a P value of <0.05 is considered statistically significant. **, P < 0.01; ***, P < 0.001.
FIG 5.
The sensitivity of the ΔsilC mutant of F. tularensis to organic peroxides is similar to that observed for the emrA1 mutant, but the mutants' sensitivities to hydrogen peroxide differ. (A to F) The sensitivities of the wild-type F. tularensis (Ft) LVS, the ΔsilC mutant, and the emrA1 mutant to organic peroxides tert-butyl hydroperoxide (TBH) (A and B), cumene hydroperoxide (CHP) (C and D), and hydrogen peroxide (H2O2) (E and F) were determined by disc diffusion and bacterial killing assays using the concentrations described in Materials and Methods. For disc diffusion assays, the results are expressed as diameters of zone of inhibition in millimeters in means ± SD for triplicate samples. In bacterial killing assays, the Francisella strains were exposed to serially diluted compounds for 1 and 3 h and spotted onto MH chocolate agar plates to determine the bacterial killing. The arrows indicate the concentrations of TBH and CHP that resulted in enhanced killing of the ΔsilC and the emrA1 mutants (B and D) and the emrA1 mutant (F). (G) Growth curves of the wild-type F. tularensis and the ΔsilC and the emrA1 mutants grown in Mueller-Hinton broth (MHB) in the absence or presence of 2 mM H2O2. The results shown are representative of 3 independent experiments, which yielded identical results. The P values were determined by one-way ANOVA, and a P value of <0.05 is considered statistically significant. **, P < 0.01; ***, P < 0.001.
The antioxidant enzymes SodB and KatG are present in the culture supernatants of the ΔsilC mutant.
We have reported earlier that the emrA1 mutant of F. tularensis LVS fails to secrete antioxidant enzymes SodB and KatG in the culture supernatants (17). We next investigated if the ΔsilC mutant is also defective for secretion of these antioxidant enzymes of F. tularensis. Western blot analysis was performed for detection of SodB and KatG in culture supernatants of the ΔsilC mutant or the wild-type F. tularensis LVS after 4, 8, and 12 h of growth in Chamberlain's defined medium (CDM). Bacterial lysates collected at similar time points were also analyzed for the presence of these two antioxidant enzymes. It was observed that similar to the bacterial lysates, both SodB and KatG were also detected in culture supernatants of the wild-type F. tularensis LVS as well as the ΔsilC mutant (Fig. 6A). Since these results were in contrast to what we have reported for the emrA1 mutant in our previous study (17), we made a side-by-side comparison of the ΔsilC and the emrA1 mutants. The mutants were grown in CDM or Mueller-Hinton broth (MHB) for a period of 12 h, and the culture supernatants and the bacterial lysates were analyzed for the presence of SodB and KatG. Both SodB and KatG were absent from the culture supernatants of the emrA1 mutant but were present in those from the ΔsilC mutant (Fig. 6B).
FIG 6.
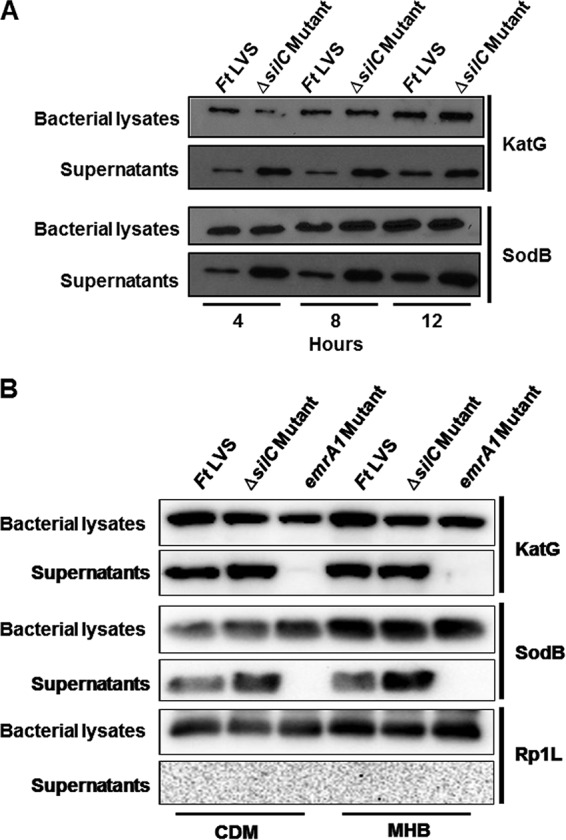
Loss of SilC does not affect the secretion of antioxidant enzymes KatG or SodB. (A) Cultures of F. tularensis (Ft) LVS and the ΔsilC mutant were grown in Chamberlain's defined medium (CDM). The culture filtrates (supernatants) or the lysates of the bacterial pellets were analyzed at the indicated times by Western blot analysis using anti-SodB and anti-KatG antibodies. (B) Cultures of F. tularensis (Ft) LVS and the ΔsilC and emrA1 mutants were grown in CDM or Mueller-Hinton broth (MHB) for 12 h. The culture filtrates (supernatants) or the lysates of the bacterial pellets were analyzed at the indicated times by Western blot analysis using anti-SodB and anti-KatG and anti-Rp1L antibodies.
To ascertain if the presence of SodB and katG in the culture supernatants of the ΔsilC mutant was not due to enhanced lysis of the ΔsilC mutant, we stripped and reprobed the blots from the bacterial lysates and the culture supernatants with antibodies against the ribosomal protein Rp1L, which is present exclusively in the bacterial cytosol and is released in the culture supernatants only upon bacterial lysis. The Western blot analysis with anti-Rp1L antibodies revealed the presence of Rp1L in bacterial lysates but not in the culture supernatants of all the bacterial strains tested (Fig. 6B). Collectively, these results demonstrate that the loss of the OM protein SilC is not associated with impaired secretion of Francisella antioxidant enzymes in the culture supernatants.
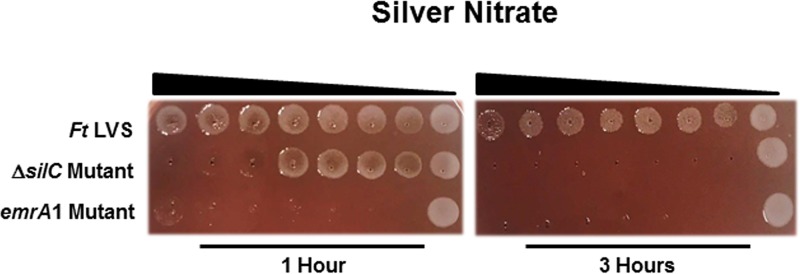
The ΔsilC mutant exhibits enhanced sensitivity to silver.
Since SilC of F. tularensis is homologous to the silver cation efflux protein in other bacterial pathogens (18), a protein that is involved in resistance to silver, we next investigated if the loss of silC results in enhanced sensitivity of the ΔsilC mutant to silver nitrate. The ΔsilC mutant was found to be sensitive to silver nitrate. The emrA1 mutant exhibited even higher sensitivity to silver nitrate than that observed for the ΔsilC mutant after 1 h of exposure to silver nitrate. However, an identical bacterial killing pattern was observed for both the ΔsilC and the emrA1 mutants after 3 h of exposure to silver nitrate (Fig. 7). Collectively, these results demonstrate that the Emr efflux system of F. tularensis is required for the efflux of silver.
FIG 7.
Both the ΔsilC and the emrA1 mutants are highly sensitive to silver. Sensitivities of the wild-type F. tularensis (Ft) LVS, the ΔsilC mutant, and the emrA1 mutant to silver as determined by killing assays against silver nitrate as described in Materials and Methods. The Francisella strains were exposed to serially diluted silver nitrate for 1 and 3 h and spotted onto MH chocolate agar plates to determine the level of bacterial killing.
The ΔsilC mutant is attenuated for intramacrophage growth and virulence in mice.
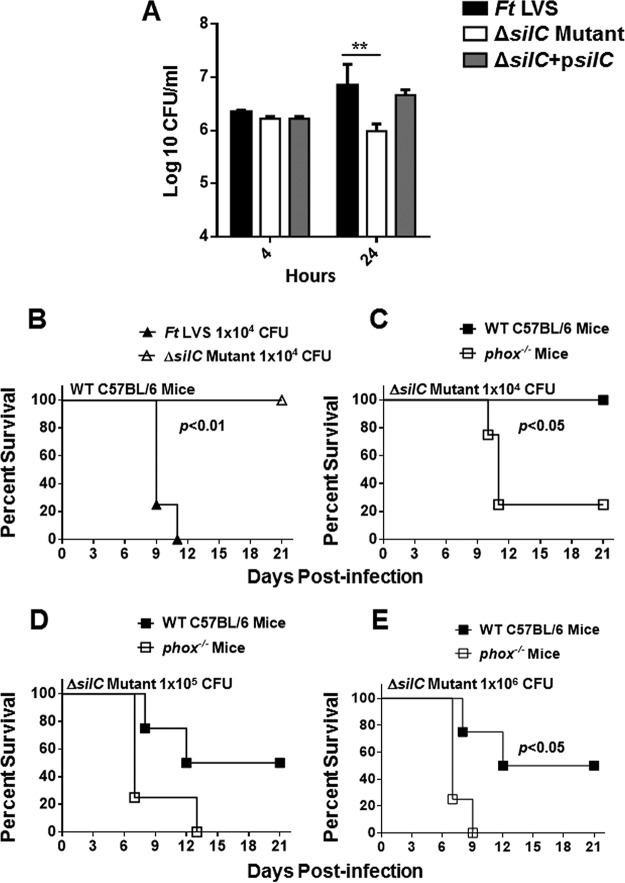
We next investigated the requirement of SilC for survival and growth in macrophages and for virulence in mice. RAW 264.7 macrophages were infected with the wild-type F. tularensis LVS, the ΔsilC mutant, and the transcomplemented strain at a multiplicity of infection (MOI) of 100. The cells were lysed at 4 and 24 h of infection to determine the number of bacteria that entered the macrophages and replicated intracellularly, respectively. It was observed that equal numbers of all three bacterial strains entered the macrophages at 4 h. However, the ΔsilC mutant failed to replicate in macrophages and the number of bacteria recovered after 24 h of infection was similar to that observed after 4 h of infection. In contrast, the numbers of F. tularensis LVS and the transcomplemented strain isolates increased nearly 10- to 15-fold at 24 h postinfection. These results demonstrate that the ΔsilC mutant is attenuated for intramacrophage growth (Fig. 8A).
FIG 8.
The ΔsilC mutant of F. tularensis is attenuated for intramacrophage growth and virulence in mice, and its virulence is restored in phox−/− mice. (A) RAW 264.7 macrophages were infected with the F. tularensis (Ft) LVS, the ΔsilC mutant, or the transcomplemented strain (ΔsilC+psilC) at an MOI of 100 (n = 3 biological replicates). The cells were lysed at 4 and 24 h and plated on MH chocolate agar plates for enumeration of bacterial CFU. The data are representative of three independent experiments, which yielded identical results. The data are expressed as log10 CFU/milliliter. The data were analyzed by ANOVA with a Tukey-Kramer posttest, and a cutoff P value of 0.05 or less was considered significant. Comparisons are shown with Ft LVS. **, P < 0.01. (B, C, D, and E) C57BL/6 and phox−/− mice (n = 4/group) were infected intranasally with the indicated doses of F. tularensis LVS or the ΔsilC mutant and observed for mortality. The data from a single experiment are shown. The mice were observed for the indicated periods for morbidity and mortality. The results are expressed as Kaplan-Meier survival curves, and the P values were determined using the log rank test.
To investigate the role of SilC in virulence, wild-type C57BL/6 and phox−/− mice were infected intranasally (i.n.) with 1 × 104, 1 × 105, or 1 × 106 CFU of the ΔsilC mutant, which represents 1, 10, and 100 100% lethal doses (LD100) of F. tularensis in our hands, respectively. The infected mice were observed for morbidity and mortality. Mice exhibiting signs of immobility and loss of more than 25% body weight were considered moribund and were euthanized. It was observed that 100% of mice infected with F. tularensis LVS succumbed to infection by day 11 postinfection. On the other hand, 100% of mice receiving 1 × 104 CFU of the ΔsilC mutant survived the infection (Fig. 8B).
Oxidant sensitivity of the ΔsilC mutant indicates that SilC may contribute to the virulence by overcoming the oxidative stress. To investigate this notion, wild-type C57BL/6 and phox−/− mice deficient in NADPH oxidase were infected i.n. with 1 × 104, 1 × 105, and 1 × 106 CFU of the ΔsilC mutant and observed for morbidity and mortality. It was observed that 100% of wild-type mice infected with 1 × 104 CFU and 50% of mice infected with 1 × 105 or 1 × 106 CFU of the ΔsilC mutant survived the infection. On the other hand, 80% of phox−/− mice infected with 1 × 104 CFU of the ΔsilC mutant and 100% of the mice receiving 1 × 105 or 1 × 106 CFU of the ΔsilC mutant succumbed to infection (Fig. 8C to E). Collectively, these results indicate that SilC serves as a virulence factor of F. tularensis and contributes to the intramacrophage survival and virulence of F. tularensis. These results also indicate that SilC contributes to the virulence of F. tularensis by resisting oxidative stress.
DISCUSSION
F. tularensis has been predicted to encode 31 major facilitator superfamily (MFS) and 15 functional ATP binding cassette (ABC) transport systems (14). These systems have a very simple architecture and consist of three structural components, IM protein, MFP, and the OM components. These three components form a contiguous channel for the secretion of a multitude of substrates. Francisella contains three orthologs of the OM proteins; two of these, termed TolC and FtlC, act in conjunction with an AcrAB-type multidrug efflux pump and have been reported to be important for tularemia pathogenesis (15, 16, 19–21). The third OM protein, SilC, is named for its homology with the silver cation efflux protein in other bacterial pathogens (18). The contribution of SilC in tularemia pathogenesis is not known. In this study, we investigated the role of SilC in the secretion and virulence of F. tularensis LVS. This is the first report demonstrating that the OM component SilC serves as an important virulence factor of F. tularensis and contributes to virulence primarily by providing resistance against oxidative stress.
The MFS-type Emr multidrug efflux system of F. tularensis appears to have several unique features. In several Gram-negative bacterial pathogens, the emrB and emrA1 genes are cotranscribed while the OM gene encoding the TolC protein is transcribed at a distant location. In E. coli and Salmonella, TolC serves as the functional OM protein for the Emr multidrug efflux pumps (23, 24). In contrast, in F. tularensis the three genes emrB, emrA1, and OM gene silC are adjacently positioned and constitute an operon (17). This specific genomic organization suggests that the OM protein SilC is designed to act in conjunction with the MFP EmrA1 and IM protein EmrB in F. tularensis. The notion that SilC act in conjunction with EmrB and EmrA1 is supported by similarities observed in the phenotypes of the emrA1 and the ΔsilC mutants. Specifically, both EmrA1 and SilC are not required for the efflux of detergents and dyes but are required for efflux of a small subset of antibiotics. Most importantly, loss of either EmrA1 or SilC results in enhanced sensitivity to the superoxide-generating compounds paraquat and pyrogallol, to the organic peroxides TBH and CHP, and to silver, attenuated intramacrophage survival, and attenuated virulence in mice. Moreover, the virulence of both the emrA1 (17) and the silC mutants is restored in NADPH-deficient mice, indicating a unique role of this efflux system in providing resistance against oxidative stress. Taken together, these similarities in the phenotypes of emrA1 and silC mutants indicate that SilC functions as the OM component of the Emr multidrug efflux pump of F. tularensis.
Besides these similarities in the phenotypes of the emrA1 and the ΔsilC mutants, some differences were also observed. Specifically, the ΔsilC mutant was not found to be sensitive to H2O2 and unlike what occurred in the emrA1 mutant, the secretion of the antioxidant enzymes SodB and KatG was not impaired in the ΔsilC mutant. Apparently, both of these antioxidant enzymes were found to be present at higher concentrations in the culture supernatants of the ΔsilC mutant than those observed for the wild-type F. tularensis LVS. The presence of these antioxidant enzymes in the culture supernatant of the ΔsilC mutant was independent of the bacterial cell lysis. These observations are intriguing and indicate that different mechanisms may be responsible for the H2O2 sensitivity of the emrA1 mutant and the ΔsilC mutant. We propose that the oxidant sensitivity of the emrA1 mutant may be attributable to its failure to secrete antioxidant enzymes such as SodB and KatG. It has been demonstrated that in the tolA mutant of Vibrio cholerae and E. coli, the loss of OM protein TolA is associated with elevated oxidative stress due to the enhanced accumulation of reactive oxygen species and changes in iron physiology. Further, the perturbed membrane integrity has been shown to result in the leakage of cellular proteins into the culture supernatants (25). It has also been reported that loss of TolC causes oxidative stress, resulting in elevated activities of antioxidant enzymes glutathione reductase, catalase (KatA), and superoxide dismutase (SodB) in Sinorhizobium meliloti (26). Similarly, the TolC-like protein of Acinetobacter baumannii is also required for antimicrobial and oxidative stress resistance (27). Based on these observations, we speculate that the oxidant sensitivity of the ΔsilC mutant is due to the oxidative stress induced by the loss of OM protein SilC. Another plausible explanation could be that SilC functions with the Emr efflux system for pumping out some but not all the substrates or that other OM proteins participate with the Emr efflux system for the efflux of H2O2. Many efflux systems exhibit interchangeability for the OM proteins. For example, it has been suggested that the AcrAB system of Francisella can work with both TolC and FtlC (15, 19). Similarly, the AcrAB system of E. coli shares TolC with AcrEF and a hemolysin secretion system (28). On the contrary, such a flexibility for the OM proteins is not exhibited by the copper-silver (Cus) efflux system, in which CusC is proposed to work only with the Cus efflux system and is not replaceable by TolC due to its unique secondary structure (29). Since SilC is homologous to the Cus efflux system OM proteins, it may be speculated that SilC may not be interchangeable with TolC. Nevertheless, the results demonstrating the resistance of SilC to H2O2 are intriguing, and understanding the underlying mechanisms of this resistance will require additional studies.
The results from this study demonstrate a novel role of the Emr efflux system of Francisella in providing resistance against silver compounds. Both the ΔsilC and the emrA1 mutants were found to be extremely sensitive to silver. The role of the Emr efflux system in resistance against heavy metal ions is also supported by the unique genomic organization of the Emr locus. Similar to the Francisella Emr locus, the closely related heavy metal cus and sil loci of E. coli and Salmonella, respectively (29, 30), are similarly organized, and the OM protein is also a part of the operon. It has been reported that the fish pathogen Francisella noatunensis is not killed by commercial silver nanoparticles, which are bactericidal for a number of disease-causing bacteria in fish (31), indicating an inherent resistance to silver across the Francisella species.
The results from this study also demonstrated that SilC serves specialized roles that are different from those of the other two OM proteins, TolC and FtlC, of F. tularensis. The primary differences are that unlike TolC and FtlC (19), SilC is not required for efflux of detergent and dyes. Both the tolC and ftlC mutants have been reported to be sensitive to SDS, sodium deoxycholate, and ethidium bromide (19). However, we did not observe any enhanced sensitivity of the ΔsilC mutant to these agents when they were used in concentrations similar to those reported for the tolC or the ftlC mutants. The ΔsilC mutant showed sensitivity only to streptomycin, nalidixic acid, and chloramphenicol, while the tolC and the ftlC mutants, in addition to sensitivity to these antibiotics, also exhibit sensitivities to a multitude of antibiotics, indicating their major role in efflux of antibiotics (19). Neither TolC nor FtlC is required for survival in macrophages; in contrast, the ΔsilC mutant was found to be attenuated for intramacrophage growth. Moreover, TolC has been shown to be required for virulence when administered by the intradermal route, which results in a less fulminate infection than that caused by i.n. administration (19). We report that SilC is required for virulence in mice infected by the i.n. route. The virulence of the tolC or ftlC mutant by the i.n. route is not known.
To conclude, this study demonstrates that the Emr- and AcrAB-type efflux pumps have been designed to serve specific roles in tularemia pathogenesis. The association of TolC/FtlC with the AcrAB RND multidrug efflux system and the similarities in phenotypes of the acrB and tolC mutants indicate that these components act in concert primarily to provide resistance against detergents, dyes, and antibiotics. Unlike the emrA1 or the ΔsilC mutant, the acrB and tolC mutants are not sensitive to oxidants, and the mutation of the latter set of genes does not interfere with the secretion of antioxidant enzymes (17). On the other hand, similarities in the oxidant-sensitive phenotypes of the emrA1 and the ΔsilC mutants indicate that these two components of the Emr multidrug efflux system act in concert to serve a specialized role and contribute to the virulence of F. tularensis by providing resistance against oxidative stress. Given the extreme virulence of F. tularensis, characterization of such unique virulence mechanisms will provide a detailed understanding of the pathogenesis of tularemia and will also result in the identification of potential targets for the development of effective therapeutics and prophylactics for protection from this lethal disease.
MATERIALS AND METHODS
Bacterial strains and media.
The F. tularensis subsp. holarctica LVS (ATCC 29684; American Type Culture Collection, Rockville, MD) used in this work was obtained from BEI Resources, Manassas, VA. The deletion mutant of F. tularensis LVS (ΔsilC) and its transcomplemented strain (ΔsilC+psilC) were generated in this study. The emrA1 mutant available in our lab and reported in a previous study was also used (17). All work with these strains was conducted under biosafety level 2 (BSL2) containment conditions. All bacterial stock cultures were grown at 37°C with 5% CO2 on Mueller-Hinton (MH) chocolate agar plates. After 48 h of growth, individual colonies were inoculated into MH broth (MHB) supplemented with anhydrous calcium chloride, hydrous magnesium chloride, glucose, ferric pyrophosphate, and Isovitalex (BD Biosciences, San Jose, CA). The cultures were grown at 37°C for 12 to 16 h with constant shaking. Aliquots of mid-log-phase bacteria grown in MHB were stored at −80°C, and a frozen vial was thawed in a 37°C water bath before use. The transcomplemented strain (ΔsilC+psilC) was grown on MH chocolate agar or in MHB supplemented with hygromycin (Hygro; 100 μg/ml).
Construction of silC gene deletion mutant (ΔsilC) and transcomplemented strains.
The plasmid vectors and sequences of the primer used are listed in Table 2. A gene deletion mutant (ΔsilC) was generated by the allelic exchange method, while a transcomplemented strain of the ΔsilC mutant was generated by incorporating a copy of the silC gene in trans as previously described with slight modifications (32, 33). Briefly, to generate an in-frame ΔsilC (FTL_0686) mutant of F. tularensis LVS, a 1,078-bp 5′ fragment upstream of the start codon of the silC (FTL_0686) gene was generated by PCR using primers MP129 and MP130 and a 1,139-bp 3′ downstream fragment of FTL_0686 with primers MP133 and MP134. Both of the flanking primers, MP129 and MP134, were engineered with BamHI and SalI restriction sites, respectively, at their 5′ ends. The amplified fragments were fused together using an overlapping extension PCR with primers MP129 and MP134, generating a 2,217-bp fragment. The fused fragment was cloned into pJC84, a suicide vector, at BamHI and SalI restriction sites, resulting in plasmid pMM04. After verification by PCR, the pMM04 was transformed into F. tularensis LVS by electroporation, and colonies were selected on MH chocolate agar plates containing kanamycin (Kan; 25 μg/ml). For sucrose counterselection, kanamycin-resistant clones were grown to an optical density at 600 nm (OD600) of 0.5 in MHB at 37°C with shaking, and sucrose at a final concentration of 5% was then added. The cultures were incubated for an additional 2 h, serially diluted, plated on MH chocolate agar plates containing 8% sucrose, and incubated at 37°C with 5% CO2 for 48 to 72 h. Sucrose-resistant clones were spotted onto MH chocolate agar plates with or without kanamycin (25 μg/ml) to verify the loss of kanamycin resistance. The sucrose-resistant and kanamycin-sensitive clones were screened for the silC gene deletion using colony PCR with primer sets MP037/MP038 (sodB for internal control) and MP202/MP203 for the gene-specific amplification of silC. Genomic DNA sequencing was performed on clones testing positive by PCR to confirm in-frame gene deletion of silC.
TABLE 2.
Bacterial strains, plasmids and sequences of the primers used in this studya
| Strain, plasmid, or primer | Description, relevant genotype, or sequence | Source or reference |
|---|---|---|
| Strains | ||
| F. tularensis LVS | Wild-type strain | ATCC |
| F. tularensis ΔsilC mutant | Deletion mutant of LVS silC gene | This study |
| F. tularensis emrA1 mutant | LVS FTL_0687::Tn5 Kanr | 17 |
| F. tularensis silC transcomplemented strain (ΔsilC+psilC) | LVS ΔsilC pMM010 (pMP822+silC) Hygror | This study |
| E. coli DH5α | F− ϕ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rk− mk+) phoA supE44 λ− thi-1 gyrA96 relA1 | Invitrogen |
| Plasmids | ||
| pMP822 | E. coli-Francisella shuttle vector, Hygror | 36 |
| pJC84 | E. coli-Francisella suicide vector, Kanr | 37 |
| pMM04 | pJC84 + fused flanking fragment of silC gene, Kanr | This study |
| pMM010 | pMP822 + silC, Hygror | This study |
| Primers for silC gene deletion | ||
| MP129 | 5′-CAAggatccTGCGCCTGTTGCAGTAATG-3′ | |
| MP130 | 5′-TGTTGTGACCTATAATGTATCTAC-3′ | |
| MP133 | 5′-GTAGATACATTATAGGTCACAACATTTGATGATTTTCTAGTTTAGTAAGG-3′ | |
| MP134 | 5′-TGATgtcgacTCTTTAGGTAATTCTCCCTTTTC-3′ | |
| Primers for confirmation of silC gene deletion | ||
| MP202 | 5′-TGATCTAGCTAAACCGATATTCGC-3′ | |
| MP203 | 5′-CACGTCTTAGCAAAAAAATGCA-3′ | |
| Primers for transcomplementation | ||
| MP278 | 5′-CAAggatccATGATACGAAATAAAATACTTCCT-3′ | |
| MP279 | 5′-TGATctcgagCTATTTATCAAAAGCTGGGTTATC-3′ |
Bases in lowercase letters represent restriction endonuclease sites: BamHI, ggatcc; SalI, gtcgac; XhoI, ctcgag.
For the transcomplementation of the ΔsilC mutant, the full-length silC gene (FTL_0686) of F. tularensis LVS was amplified by PCR using primers MP278 and MP279 engineered at their 5′ ends with BamH and XhoI restriction sites, respectively. The amplified fragment was digested with BamHI and XhoI restriction enzymes and cloned into the E. coli-Francisella shuttle vector pMP822 digested with same restriction enzymes. The resulting plasmid, pMM010, was verified by PCR and DNA sequencing, electroporated into the ΔsilC mutant, and selected on MH chocolate agar plates supplemented with hygromycin (200 μg/ml). The transcomplementation of the ΔsilC mutant was confirmed by PCR using silC gene-specific primers. The transcomplemented strain was designated the ΔsilC+psilC strain.
Determination of sensitivity of the ΔsilC mutant to antibiotics, dyes, detergents, and oxidants.
The susceptibilities of the wild-type F. tularensis LVS, the ΔsilC mutant, and the transcomplemented ΔsilC+psilC strain to dyes, detergents, antibiotics, and oxidants were determined using disc diffusion assays. The bacterial cultures, adjusted to an OD600 of 2.5, were spread on MH chocolate agar plates using sterile cotton swabs. Different concentrations of antibiotics were dissolved in phosphate-buffered saline (PBS), and 5 μl of the antibiotic mixture was loaded onto the sterile filter paper discs after placing them on these plates. These antibiotics included streptomycin (1.25 and 5 μg/disc), gentamicin (1.25 and 2.5 μg/disc), neomycin (3.75 and 7.5 μg/disc), erythromycin (15 and 45 μg/disc), chloramphenicol (3.75 and 7.5 μg/disc), novobiocin (7.5 and 15 μg/disc), tetracycline (3.75 and 7.5 μg/disc), and nalidixic acid (1.88, 3.75, and 30 μg/disc). Dyes included ethidium bromide (5 μg/disc) and acriflavine (25 μg/disc). The detergent used was sodium dodecyl sulfate (SDS) (750 μg/disc). The plates were incubated at 37°C with 5% CO2 for 48 h, and the diameters of zones of inhibition around the discs were measured.
The susceptibilities of the wild-type F. tularensis LVS, the ΔsilC mutant, and the transcomplemented ΔsilC+psilC strain to oxidants were determined using the disc diffusion assay and the bacterial killing assay and by generating growth curves as previously described (32, 34, 35). Sterile discs were placed firmly on the surface of the inoculated agar plates and impregnated with superoxide-generating compounds, such as paraquat (15.6 μg/disc), pyrogallol (250 μg/disc), and menadione (6.25 μg/disc); with hydrogen peroxide (H2O2) (50 nM/disc); and with organic peroxides such as cumene hydroperoxide (CHP) (500 μg/disc) and tert-butyl hydroperoxide (TBH) (3.5 μg/disc). These assays were also repeated using the emrA1 mutant of F. tularensis LVS. The plates were then incubated at 37°C for 48 h, and the diameters of the zones of inhibition were measured to determine the sensitivities of the tested bacterial strains to these compounds.
The bacterial killing assays were performed as follows. Briefly, oxidants such as paraquat, pyrogallol, TBH, CHP, and H2O2 were diluted 2- to 10-fold in sterile MHB in a 96-well plate. Bacterial cultures were adjusted to an OD600 of 0.2 by resuspending in MHB and were then added onto the oxidant compounds, mixed, and incubated at 37°C with 5% CO2 for 1 and 3 h. Five microliters of cultures was spotted onto MH chocolate agar plates and incubated for 48 h at 37°C with 5% CO2. The plates were observed for differences in bacterial killing.
The growth curves of the wild-type F. tularensis LVS and the ΔsilC mutant were generated to test their susceptibility to H2O2. Bacterial suspensions adjusted to an OD600 of 0.2 were added to 10 ml of Chamberlain's defined medium (CDM) (32) containing 0 and 2 mM H2O2 and incubated with constant shaking at 175 rpm at 37°C for 40 h. The OD600 values were recorded at 4-h intervals and plotted.
Determination of sensitivity of the ΔsilC mutant to silver.
The susceptibilities of the wild-type F. tularensis LVS, the ΔsilC mutant, the transcomplemented ΔsilC+psilC strain, and the emrA1 mutant to silver nitrate (AgNO3) were determined using a bacterial killing assay. Briefly, 25 mM AgNO3 was diluted 2-fold in sterile MHB in a 96-well plate. Bacterial cultures adjusted to an OD600 of 0.2 by resuspending in MHB were then added onto the wells containing AgNO3 and mixed, and the wells were incubated at 37°C with 5% CO2 for 1 and 3 h. Five microliters of cultures was spotted onto MH chocolate agar plates, and the plates were incubated for 48 h at 37°C with 5% CO2. The plates were observed for bacterial killing.
Macrophage cell culture assay.
The capabilities of the wild-type F. tularensis LVS, the ΔsilC mutant, and the ΔsilC+psilC transcomplemented strains to survive and replicate in macrophages were determined using a macrophage cell culture assay. Briefly, RAW 264.7 macrophages cultured in Dulbecco's modified Eagle medium (DMEM) were seeded in 24 well-plates and incubated overnight at 37°C with 5% CO2. The cells were then infected with the wild-type F. tularensis LVS, the ΔsilC mutant, or the transcomplemented ΔsilC+psilC strain at a multiplicity of infection (MOI) of 100:1 (ratio of bacteria to cells). The infections were synchronized by centrifuging the plates at 1,000 rpm for 10 min and then incubated for 2 h at 37°C with 5% CO2. After 2 h, the cell culture medium was replaced with 1 ml of DMEM containing 250 μg/ml of gentamicin to kill extracellular and adherent bacteria. The plate was incubated for 1 h at 37°C with 5% CO2, after which the medium containing gentamicin was replaced with 1 ml of medium without any antibiotics. The cells were lysed at 4 and 24 h postinfection with 0.1% sodium deoxycholate, serially diluted 10-fold in sterile PBS, and plated on MH chocolate agar plates. The plates were incubated at 37°C with 5% CO2 for 48 h. Colonies were counted and expressed as log10 CFU per milliliter.
In vivo studies.
Six- to 8-week-old wild-type (WT) C57BL/6 and gp91phox−/− (phox−/−) mice were purchased from Jackson Laboratories. Mice were maintained in a specific-pathogen-free environment in the Animal Facility of New York Medical College. All animal procedures were conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines approved by New York Medical College. Prior to inoculation, mice were deeply anesthetized by intraperitoneal injection of a cocktail of ketamine (Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (Phoenix Scientific, St. Joseph, MO). Then, mice were challenged intranasally (i.n.) with 1 × 104 CFU of F. tularensis LVS or 1 × 104, 1 × 105, or 1 × 106 CFU of the ΔsilC mutant. Mice were observed for a period of 21 days for morbidity and mortality. Mice that were immobile or lost more than 25% of their body weight were considered moribund and were removed from the study and euthanized. The results were plotted as Kaplan-Meier survival curves, and the data were analyzed by the log rank test.
Western blot analysis.
Wild-type F. tularensis LVS and the ΔsilC mutant and ΔsilC+psilC transcomplemented strains were grown on MH chocolate agar plates at 37°C for 48 h. The bacterial cultures were resuspended in CDM to achieve an OD600 of 0.2 and then were grown with constant shaking at 175 rpm at 37°C. Aliquots of the bacterial cultures were collected at 4, 8, and 12 h and centrifuged at 10,000 rpm for 10 min. The culture supernatants were collected and filtered immediately with 0.22-μm syringe filters to eliminate any residual bacteria. The filtrates were concentrated to 1/10 of their original volume. The cell pellets were lysed in 200 μl of lysis buffer [200 mM Tris-HCl (pH 8.0), 320 mM (NH4)2SO4, 5 mM MgCl2, 10 mM EDTA, 10 mM EGTA, 20% glycerol, 1 mM dithiothreitol (DTT) supplemented with protease and phosphatase inhibitors]. The protein concentrations of both the culture supernatants and the cell lysates were determined using a Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific). Equal amounts of proteins (∼5 μg) were resolved on a 10% SDS-PAGE gel, transferred to a polyvinylidene difluoride (PVDF) membrane, and probed with anti-KatG (1:20,000) or anti-SodB (1:20,000) antibodies and secondary anti-rabbit antibodies (1:5,000) conjugated to horseradish peroxidase as described previously (17). The blots were developed by autoradiography and photographed. The blots were stripped and reprobed with anti-Rp1L antibodies. All the antibodies were kindly provided by Karsten Hazlett, Albany Medical College, Albany, NY.
Statistical analysis.
All data were statistically analyzed using the InStat program (Graph-Pad Software). The data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni corrections. Results were expressed as means ± standard errors of the means (SEM), and differences between the experimental groups were considered statistically significant at P values of <0.05. The survival data were expressed as Kaplan-Meier survival curves, and statistical significance for survival results was evaluated by analyzing the mean time to death by the log rank test.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R56AI101109, P01AI056320 (C.S.B.), and R15AI107698 (M.M.).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No financial conflicts of interest regarding the contents of the manuscript and its authors exist.
REFERENCES
- 1.Bossi P, Bricaire F. 2003. Tularemia, a potential bioterrorism weapon. Presse Med 32:1126–1130. (In French.) [PubMed] [Google Scholar]
- 2.Cronquist SD. 2004. Tularemia: the disease and the weapon. Dermatol Clin 22:313–320, vi-vii. doi: 10.1016/j.det.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Ellis J, Oyston PC, Green M, Titball RW. 2002. Tularemia. Clin Microbiol Rev 15:631–646. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffin KF, Oyston PC, Titball RW. 2007. Francisella tularensis vaccines. FEMS Immunol Med Microbiol 49:315–323. doi: 10.1111/j.1574-695X.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- 5.Sjostedt A. 2006. Intracellular survival mechanisms of Francisella tularensis, a stealth pathogen. Microbes Infect 8:561–567. doi: 10.1016/j.micinf.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Allen LA. 2003. Mechanisms of pathogenesis: evasion of killing by polymorphonuclear leukocytes. Microbes Infect 5:1329–1335. doi: 10.1016/j.micinf.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Bosio CM, Dow SW. 2005. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J Immunol 175:6792–6801. doi: 10.4049/jimmunol.175.10.6792. [DOI] [PubMed] [Google Scholar]
- 8.Champion MD, Zeng Q, Nix EB, Nano FE, Keim P, Kodira CD, Borowsky M, Young S, Koehrsen M, Engels R, Pearson M, Howarth C, Larson L, White J, Alvarado L, Forsman M, Bearden SW, Sjostedt A, Titball R, Michell SL, Birren B, Galagan J. 2009. Comparative genomic characterization of Francisella tularensis strains belonging to low and high virulence subspecies. PLoS Pathog 5:e1000459. doi: 10.1371/journal.ppat.1000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margolis JJ, El-Etr S, Joubert LM, Moore E, Robison R, Rasley A, Spormann AM, Monack DM. 2010. Contributions of Francisella tularensis subsp. novicida chitinases and Sec secretion system to biofilm formation on chitin. Appl Environ Microbiol 76:596–608. doi: 10.1128/AEM.02037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eshraghi A, Kim J, Walls AC, Ledvina HE, Miller CN, Ramsey KM, Whitney JC, Radey MC, Peterson SB, Ruhland BR, Tran BQ, Goo YA, Goodlett DR, Dove SL, Celli J, Veesler D, Mougous JD. 2016. Secreted effectors encoded within and outside of the Francisella pathogenicity island promote intramacrophage growth. Cell Host Microbe 20:573–583. doi: 10.1016/j.chom.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abby SS, Cury J, Guglielmini J, Neron B, Touchon M, Rocha EP. 2016. Identification of protein secretion systems in bacterial genomes. Sci Rep 6:23080. doi: 10.1038/srep23080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piddock LJ. 2006. Multidrug-resistance efflux pumps—not just for resistance. Nat Rev Microbiol 4:629–636. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 13.Piddock LJ. 2006. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev 19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marohn ME, Santiago AE, Shirey KA, Lipsky M, Vogel SN, Barry EM. 2012. Members of the Francisella tularensis phagosomal transporter subfamily of major facilitator superfamily transporters are critical for pathogenesis. Infect Immun 80:2390–2401. doi: 10.1128/IAI.00144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bina XR, Lavine CL, Miller MA, Bina JE. 2008. The AcrAB RND efflux system from the live vaccine strain of Francisella tularensis is a multiple drug efflux system that is required for virulence in mice. FEMS Microbiol Lett 279:226–233. doi: 10.1111/j.1574-6968.2007.01033.x. [DOI] [PubMed] [Google Scholar]
- 16.Qin A, Scott DW, Mann BJ. 2008. Francisella tularensis subsp. tularensis Schu S4 disulfide bond formation protein B, but not an RND-type efflux pump, is required for virulence. Infect Immun 76:3086–3092. doi: 10.1128/IAI.00363-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Z, Banik S, Rane H, Mora VT, Rabadi SM, Doyle CR, Thanassi DG, Bakshi CS, Malik M. 2014. EmrA1 membrane fusion protein of Francisella tularensis LVS is required for resistance to oxidative stress, intramacrophage survival and virulence in mice. Mol Microbiol 91:976–995. doi: 10.1111/mmi.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huntley JF, Conley PG, Hagman KE, Norgard MV. 2007. Characterization of Francisella tularensis outer membrane proteins. J Bacteriol 189:561–574. doi: 10.1128/JB.01505-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil H, Platz GJ, Forestal CA, Monfett M, Bakshi CS, Sellati TJ, Furie MB, Benach JL, Thanassi DG. 2006. Deletion of TolC orthologs in Francisella tularensis identifies roles in multidrug resistance and virulence. Proc Natl Acad Sci U S A 103:12897–12902. doi: 10.1073/pnas.0602582103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doyle CR, Pan JA, Mena P, Zong WX, Thanassi DG. 2014. TolC-dependent modulation of host cell death by the Francisella tularensis live vaccine strain. Infect Immun 82:2068–2078. doi: 10.1128/IAI.00044-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platz GJ, Bublitz DC, Mena P, Benach JL, Furie MB, Thanassi DG. 2010. A tolC mutant of Francisella tularensis is hypercytotoxic compared to the wild type and elicits increased proinflammatory responses from host cells. Infect Immun 78:1022–1031. doi: 10.1128/IAI.00992-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinkead LC, Whitmore LC, McCracken JM, Fletcher JR, Ketelsen BB, Kaufman JW, Jones BD, Weiss DS, Barker JH, Allen LH. 2017. Bacterial lipoproteins and other factors released by Francisella tularensis modulate human neutrophil lifespan: effects of a TLR1 SNP on apoptosis inhibition. Cell Microbiol doi: 10.1111/cmi.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horiyama T, Yamaguchi A, Nishino K. 2010. TolC dependency of multidrug efflux systems in Salmonella enterica serovar Typhimurium. J Antimicrob Chemother 65:1372–1376. doi: 10.1093/jac/dkq160. [DOI] [PubMed] [Google Scholar]
- 24.Daury L, Orange F, Taveau JC, Verchere A, Monlezun L, Gounou C, Marreddy RK, Picard M, Broutin I, Pos KM, Lambert O. 2016. Tripartite assembly of RND multidrug efflux pumps. Nat Commun 7:10731. doi: 10.1038/ncomms10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikora AE, Beyhan S, Bagdasarian M, Yildiz FH, Sandkvist M. 2009. Cell envelope perturbation induces oxidative stress and changes in iron homeostasis in Vibrio cholerae. J Bacteriol 191:5398–5408. doi: 10.1128/JB.00092-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santos MR, Cosme AM, Becker JD, Medeiros JM, Mata MF, Moreira LM. 2010. Absence of functional TolC protein causes increased stress response gene expression in Sinorhizobium meliloti. BMC Microbiol 10:180. doi: 10.1186/1471-2180-10-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan VB, Vaidyanathan V, Rajamohan G. 2015. AbuO, a TolC-like outer membrane protein of Acinetobacter baumannii, is involved in antimicrobial and oxidative stress resistance. Antimicrob Agents Chemother 59:1236–1245. doi: 10.1128/AAC.03626-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau SY, Zgurskaya HI. 2005. Cell division defects in Escherichia coli deficient in the multidrug efflux transporter AcrEF-TolC. J Bacteriol 187:7815–7825. doi: 10.1128/JB.187.22.7815-7825.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delmar JA, Su CC, Yu EW. 2014. Bacterial multidrug efflux transporters. Annu Rev Biophys 43:93–117. doi: 10.1146/annurev-biophys-051013-022855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta A, Matsui K, Lo JF, Silver S. 1999. Molecular basis for resistance to silver cations in Salmonella. Nat Med 5:183–188. doi: 10.1038/5545. [DOI] [PubMed] [Google Scholar]
- 31.Shaalan MI, El-Mahdy MM, Theiner S, El-Matbouli M, Saleh M. 2017. In vitro assessment of the antimicrobial activity of silver and zinc oxide nanoparticles against fish pathogens. Acta Vet Scand 59:49. doi: 10.1186/s13028-017-0317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Z, Russo VC, Rabadi SM, Jen Y, Catlett SV, Bakshi CS, Malik M. 2016. Elucidation of a mechanism of oxidative stress regulation in Francisella tularensis live vaccine strain. Mol Microbiol 101:856–878. doi: 10.1111/mmi.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahawar M, Kirimanjeswara GS, Metzger DW, Bakshi CS. 2009. Contribution of citrulline ureidase to Francisella tularensis strain Schu S4 pathogenesis. J Bacteriol 191:4798–4806. doi: 10.1128/JB.00212-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakshi CS, Malik M, Regan K, Melendez JA, Metzger DW, Pavlov VM, Sellati TJ. 2006. Superoxide dismutase B gene (sodB)-deficient mutants of Francisella tularensis demonstrate hypersensitivity to oxidative stress and attenuated virulence. J Bacteriol 188:6443–6448. doi: 10.1128/JB.00266-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melillo AA, Mahawar M, Sellati TJ, Malik M, Metzger DW, Melendez JA, Bakshi CS. 2009. Identification of Francisella tularensis live vaccine strain CuZn superoxide dismutase as critical for resistance to extracellularly generated reactive oxygen species. J Bacteriol 191:6447–6456. doi: 10.1128/JB.00534-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LoVullo ED, Sherrill LA, Perez LL, Pavelka MS Jr. 2006. Genetic tools for highly pathogenic Francisella tularensis subsp. tularensis. Microbiology 152:3425–3435. doi: 10.1099/mic.0.29121-0. [DOI] [PubMed] [Google Scholar]
- 37.Wehrly TD, Chong A, Virtaneva K, Sturdevant DE, Child R, Edwards JA, Brouwer D, Nair V, Fischer ER, Wicke L, Curda AJ, Kupko JJ III, Martens C, Crane DD, Bosio CM, Porcella SF, Celli J. 2009. Intracellular biology and virulence determinants of Francisella tularensis revealed by transcriptional profiling inside macrophages. Cell Microbiol 11:1128–1150. doi: 10.1111/j.1462-5822.2009.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]