Abstract
Background
End-stage renal disease (ESRD) is a disease with an aging population and a high prevalence of cognitive impairment affecting quality of life, health care costs and mortality. Structural changes in the brain with decreased white matter integrity have been observed in ESRD. Understanding the changes in cognition and associated changes in brain structure after renal transplantation can help define the mechanisms underlying cognitive impairment in ESRD.
Methods
We conducted a prospective, observational cohort study in ESRD patients listed for renal transplantation, and followed them post-transplantation. We assessed their cognitive function with a battery of neuropsychological tests and brain white matter integrity with diffusion tensor imaging (DTI) both pre-transplant and three months post-transplant.
Results
Eleven patients, aged 56.5 ± 10.7 completed the study. Cognitive measures of memory and executive function improved post-transplant, specifically on tests of logical memory I (p=0.004), logical memory II (p=0.003) and digit symbol (p<0.0001). DTI metrics also improved post-transplant with an increase in fractional anisotropy (p=0.01) and decrease in mean diffusivity (p=0.004). These changes were more prominent in tracts associated with memory and executive function.
Conclusions
Cognitive function, particularly memory and executive function, improve post-transplant with concurrent improvements in white matter integrity in tracts associated with memory and executive function. These data suggest that abnormalities in cognition and brain structure seen in the ESRD population are at least partially reversible.
Keywords: Cognitive impairment, renal transplantation, ESRD, brain MRI, brain white matter integrity, diffusion tensor imaging, fractional anisotropy
Introduction
Up to 87% of the patients on hemodialysis have cognitive impairment[1]. Cognitive impairment in end-stage renal disease (ESRD) directly impacts patient compliance, health care costs and clinical outcomes[2, 3].
Brain imaging of patients with ESRD demonstrates cerebral atrophy and cerebral infarcts, which can contribute to cognitive impairment[4, 5]. However, cognition is impaired in younger patients with ESRD even in the absence of these abnormalities[6], implying that factors other than cerebrovascular disease may play a role. Although cognitive impairment associated with uremia improves with dialysis, renal transplantation provides further improvement in cognition beyond that of dialysis[7]. However, detailed characterizations of the domains of cognition most impacted and alterations to the structural integrity of the brain with renal transplantation are lacking.
Diffusion imaging, a magnetic resonance imaging (MRI) technique, evaluates the structural integrity of white matter by mapping diffusion of water molecules restricted to specific tissue. The direction and magnitude of diffusion is expressed as a rotationally invariant tensor known as diffusion tensor imaging (DTI). DTI can detect subtle but functionally significant ultrastructural abnormalities. It is particularly useful in organized tissues such as the white matter in the brain, where water molecules diffuse more freely along an axonal fiber tract than across it. The DTI metrics, fractional anisotropy (FA), a measure of diffusion directionality, and mean diffusivity (MD), a measure of the overall diffusivity, together assess white matter integrity.
Lower FA and higher MD values are associated with impaired cognition[8, 9]. Patients with ESRD have lower FA and higher MD values[6, 10–13]. The effect of transplantation on these DTI parameters remains unknown. In this prospective pilot study, we examined the effect of renal transplantation on different domains of cognition and whether these observed changes are associated with changes in white matter integrity. Understanding the mechanisms underlying cognitive improvement post-transplant will yield useful insights into the mechanisms of cognitive impairment in ESRD patients and may lead to novel therapeutic strategies for treating this condition.
Methods
The primary aims of this study were to compare cognition and white matter integrity pre-transplant and three months post-transplantation and identify specific cognitive domains and white matter tracts, which show change after transplantation. We also wanted to determine temporal trends in cognition post-transplantation.
Participants
Adult patients, (men and women) were included if they were i) listed for renal transplantation at University of Kansas Hospital; ii) were able to sign informed consent; iii) had transportation for study visits; and iv) without history of stroke or brain injury. Patients were excluded if they i) were claustrophobic or had other contra-indication for MRI; ii) had hearing/visual impairment; iii) were unable to read/write/speak/understand English; iv) were using antipsychotics or anti-epileptics; v) had a panel reactive antibody titer of > 20% or vi) were listed for dual organ transplantation. Baseline demographic data, past medical history, laboratory tests results were obtained.
The study was approved by the Institutional Review Board at the University of Kansas Medical Center. Written informed consent was obtained from all subjects before enrollment in compliance with the declaration of Helsinki. The study is registered on the clinical trials website (NCT01883349).
Neuropsychological tests
A battery of standard NP tests (Table S1) was conducted to detect subtle changes in cognition, which can be missed by screening tests like the mini mental state exam. Test selection was based on a broad review of the literature in ESRD. The tests included in the battery were mini mental state exam, digit span, logical memory I and II, category fluency, trail making A and B, digit symbol test, block design, Stroop test, and free and cued recall. The NP tests were performed by trained psychometricians. Patients undergoing hemodialysis three times a week were tested on their non-dialysis days to avoid potential changes in cognition associated with hemodialysis[14]. The test results were compared to normative data from the normative calculator for the Uniform Data Set (UDS) Neuropsychological Test Battery[15].
Depression and physical activity were also evaluated as they can impact cognition[16]. Depression was assessed by the Beck Depression Inventory, which has been validated in CKD[17]. Physical activity was assessed using the rapid assessment of physical activity (RAPA) questionnaire[18].
Diffusion Tensor Imaging (DTI)
Brain MRI was performed with a 3T whole-body scanner (Siemens Skyra, Erlangen, Germany). DTI sequences were collected using a double refocused spin echo sequence with a TE of 90 ms (TR = 10,000 ms, FOV = 300 × 300, matrix 128 × 128, final in-plane resolution = 2.34 × 2.34 mm, 75 2mm-thick contiguous axial slices). The diffusion gradients were applied along 64 directions (diffusion gradient pulse δ = 14 ms and diffusion application time Δ = 53 ms, b- value b = 1,000 s mm2).
DTI analyses were performed with the functional software laboratory (FSL) pipelines for data pre-processing, including diffusion eddy current correction, tensor calculations (DTIFIT), registration (FLIRT), and smoothing (FSLMATHS). To analyze longitudinal data, we first registered each participant’s post-transplant scan to the corresponding pre-transplant scan, and then realigned both pre- and post-transplant scans to the mid-point of the registration matrix. We then normalized all images to a common template (FMRIB_FA_58mm). The normalized data were smoothed with a 2mm Gaussian kernel (FSLMATHS). We employed a tract-of-interest (TOI) approach. Mean FA values for various white matter tracts were extracted from the normalized data within thirteen TOI target masks from the Johns Hopkins University white matter probabilistic atlas. The analyzed tracts included the anterior internal capsule (AntIC), corpus callosum (BodyCC, GenuCC, SpleniumCC), cingulum (CingCG, CingH), cortico-spinal tract (CST), forceps major (FMa) and minor (FMi), inferior fronto-occipital fasciculus (IFOF), uncinate fasciculus (UF), inferior longitudinal fasciculus (ILF), and superior longitudinal fasciculus (SLF). For bilateral tracts (AntIC, CingCG, CingH, CST, IFOF, UF, ILF, SLF) the values were extracted separately for left and right hemisphere, and then averaged to provide a single value. For each TOI, voxels within the mask were included in the mean if the FA at that voxel exceeded 0.2. MD was also extracted from the same regions by applying the thresholded FA TOI masks to the normalized and smoothed MD images.
Statistical analysis
Archived cognitive assessment and MRI evaluations closest to the date of transplantation were used to analyze within-subject change pre- to post-surgery. Two-tailed paired t-tests were used to analyze changes in NP tests pre- and post-transplant. To evaluate practice effects (learning from the repeated exposure to the testing materials), the baseline pre-transplant NP tests were compared with one-year pre-transplant NP tests on the patients (n = 4) who remained on the waitlist for a year without receiving the transplant. The three-month post-transplant NP tests were also compared to the one-year post-transplant NP tests for the patients (n = 8) who were transplanted at least a year ago.
DTI metrics (FA and MD) were entered as the dependent variable into separate repeated measures ANOVAs, with independent variables of Tract (13 levels representing each tract: BodyCC, GenuCC, SpleniumCC, Fma, Fmi, AntIC, CingCG, CingH, CST, IFOF, UF, ILF, SLF) and Scan (2 levels: Pre-transplant, Post-transplant). FA values range from 0 (completely random) to 1 (completely unidirectional) and MD values were measured in m2/s. For significant interactions, post-hoc two-tailed paired t-tests were conducted to determine which tracts had significant change in DTI pre- to post-transplant. For all analyses, p < 0.05 was considered statistically significant. The statistical analysis was performed using SPSS 22 (IBM Corporation, Somers, NY).
Results
Patient characteristics
Out of the 18 patients enrolled, 11patients, (nine men, two women) with mean age of 56.5 ± 10.7 years had renal transplant and completed the study. The remaining seven got baseline testing, and then either transferred to a different transplant center or did not receive a transplant.
Pre-transplant demographics are summarized in Table 1. All patients received standard induction therapy with rabbit antithymocyte globulin or basiliximab along with steroids and a mycophenolic acid compound for induction immunosuppression, and tacrolimus, a calcineurin inhibitor and mycophenolic acid compound with or without steroids for maintenance immunosuppression. The mean trough level of tacrolimus was 9.6 ± 2.9 mg/L. Post-transplant, there was no significant change in blood pressure, body weight or BMI (Table S2). Mean hemoglobin improved from 11.06 ± 1.4 g/dl pre transplant to 13.72 ± 1.5 g/dl post-transplant. The mean serum creatinine post-transplant was 1.6 ± 0.6 mg/dl with an estimated GFR of 45.2 ± 11.2 ml/min. There was no significant difference in other laboratory measures.
Table 1.
Baseline characteristics.
| Age | 56.5 ± 10.7 |
| Race | |
| African American | 1 |
| Caucasian | 10 |
| Marital Status | |
| Single | 1 |
| Married | 9 |
| Engaged | 1 |
| Highest level of Education completed | |
| No high school | 0 |
| High school, no college | 3 |
| Some college | 4 |
| 4 year degree | 2 |
| Attended graduate school | 2 |
| Currently Employed | 8 |
| Annual household income | |
| 20,000–50,000 | 2 |
| 50,000–100,000 | 5 |
| 100,000–150,000 | 3 |
| More than 150,000 | 1 |
| Months on dialysis before transplant | 26.6 ± 22.2 |
| Cause of renal failure | |
| Diabetic nephropathy | 3 |
| Hypertension | 1 |
| ADPKD | 0 |
| Ig A nephropathy | 3 |
| Bladder cancer | 1 |
| Renal cell carcinoma | 1 |
| Coronary artery disease | 3 |
| Atrial fibrillation | 1 |
| History of Smoking | 3 |
The mean time intervals between the pre-transplant brain MRI and NP tests, and the date of transplant were 31 ± 49 and 34 ± 50 days respectively. The mean time intervals between the post-transplant brain MRI and NP tests, and the date of transplant were 89 ± 10 and 90 ± 12 days respectively.
Neuropsychological assessments
The NP test results were compared to normative age adjusted data from the National Alzheimer’s Coordinating Center Uniform Data Set (NACC, UDS)[15]. Table 2 shows that pre-transplant, the participants scored < 25th percentile on MMSE, logical memory I and II, digit span backwards, category fluency of vegetables and digit symbol. Three months post- transplantation, mean performance on NP test improved (Table 3) for logical memory I (p=0.004), logical memory II (p=0.003) and digit symbol (p<0.0001) reflecting improvement in memory and executive function (Figure S1). Follow up evaluation, one year after transplantation showed sustained improvements in cognitive function compared to baseline (Table S3a). Four patients received repeat evaluation one year after their baseline evaluation in the pre-transplant period. These NP tests did not show any significant changes compared to the initial evaluation (Table S3b).
Table 2.
Comparison of pre-transplant and post-transplant neuropsychological test (NP) results of study patients with normative age adjusted data from the National Alzheimer’s Coordinating Center Uniform Data Set (NACC, UDS). MMSE, mini mental state exam.
| Pre-transplant | Post-transplant | |||
|---|---|---|---|---|
|
|
||||
| Z Score | Percentile | Z Score | Percentile | |
|
|
||||
| MMSE | −1.37 | 8.5 | −0.91 | 18 |
| Logical Memory I | −1.1 | 13.6 | −0.17 | 43.1 |
| Logical Memory II | −1.04 | 14.9 | 0.06 | 52.5 |
| Digit Span Forward | −0.26 | 39.9 | −0.49 | 31.1 |
| Digit Span Backward | −0.77 | 22.1 | −0.63 | 26.4 |
| Category Fluency: Animals | −0.27 | 39.4 | −0.2 | 42.2 |
| Category Fluency: Vegetables | −0.92 | 17.9 | −0.59 | 27.8 |
| Trail making A | −0.11 | 45.5 | −0.09 | 46.6 |
| Trail making B | −0.5 | 30.9 | −0.29 | 38.4 |
| Digit Symbol | −1.3 | 10.5 | −0.81 | 20.9 |
Table 3.
Within subject comparison (paired t-test) of neuropsychological (NP) tests pre- and post-renal transplant. MMSE, mini mental state exam.
| Test | Pre-transplant (mean ± Sd dev) |
Post-transplant (mean ± Sd dev) |
p (n=11) |
|---|---|---|---|
| MMSE | 27.6 ± 2.4 | 28.2 ± 2.6 | 0.19 |
| Digit Span | 7.6 ± 2.6 | 9.8 ± 2.5 | 0.007** |
| Logical Memory I | 10.2 ± 4.5 | 13.8 ± 4.3 | 0.004** |
| Logical Memory II | 8.4 ± 5.0 | 13.1 ± 4.6 | 0.003** |
| Category Fluency | 16.7 ± 4.6 | 17.6 ± 4.5 | 0.19 |
| Trail making | 55.4 ± 23.4 | 50.5 ± 22.3 | 0.18 |
| Digit Symbol | 42.8 ± 10.5 | 47.7 ± 10.9 | 0.001** |
| Block Design | 32.3 ± 12.9 | 34.1 ± 14.2 | 0.35 |
| Stroop Test | 37.4 ± 6.2 | 38.3 ± 5.9 | 0.29 |
| Free Recall | 29.1 ± 6.9 | 31.9 ± 6.6 | 0.0 |
p value < 0.01,
p value <0.05
Depression and Physical Activity
Symptoms of depression improved after transplantation (scores 11.1 ± 8.8 pre-transplant versus 4.5 ± 3.1 post-transplant, p=0.02). A trend to increased physical activity was observed (RAPA scores 5.9 ± 2.6 pre-transplant versus 6.9 ± 1.7 post-transplant, p=0.12).
White Matter Integrity
Fractional Anisotropy (FA)
Means and standard deviations for FA values for each tract, pre- and post-transplantation are provided in Table 5a. The Tract (13) by Scan (2) ANOVA for FA revealed a significant interaction of Tract by Scan (F(12, 120) = 2.28, p = 0.01, η2 = 0.19), indicating that the change in FA after transplant was not consistent across all tracts. There was a significant main effect of Tract (F(12, 120) = 510.63, p < 0.001, η2 = 0.98), indicating that FA was not homogenous across tracts. For example, FA in the SpleniumCC was 0.66, but 0.38 in the AntIC (Table 4a). There was also a marginal effect of Scan (F(1, 10) = 4.16, p = 0.07, η2 = 0.29), indicating that there was a trend for increase in FA after transplantation across all tracts. Post-hoc two tailed paired t-tests comparing pre- and post-transplantation values for each tract revealed significant increases in FA in the BodyCC (MDIFF = 0.007, SEMDIFF = 0.002, t(10) = 3.26, p < 0.01), Fmi (MDIFF = 0.004, SEMDIFF = 0.002, t(10) = 2.59, p = 0.03), and CingCG (MDIFF = 0.006, SEMDIFF = 0.002, t(10) = 3.16, p = 0.01) (Table 4a and Figure S2), suggesting improvement in post-transplant white matter integrity (Figure 2A).
Table 4.
Within subject pre- and post-transplant fractional anisotropy (FA) and mean diffusivity (MD) results. BodyCC, body of the corpus callosum; GenuCC,genu of the corpus callosum; SpleniumCC, splenium of the corpus callosum; Fma, forceps major; Fmi, forceps minor, AntIC, anterior limb of the internal capsule; CingCG, cingulate gyrus; CingH, cingulate gyrus, hippocampus; CST, corticospinal tract; IFOF, inferior fronto-occipital fasciculus; UF, uncinated fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus.
4a) Within subject pre- and post-transplant fractional anisotropy (FA) values
4b) Within subject pre- and post-transplant mean diffusivity (MD) values
| a | |||
|---|---|---|---|
| Region | Pre-transplant (M±SD) |
Post-transplant (M±SD) |
p |
| BodyCC | 0.54 ± 0.03 | 0.55 ± 0.03 | .009** |
| FMa | 0.53 ± 0.03 | 0.54 ± 0.03 | .11 |
| FMi | 0.43 ± 0.02 | 0.44 ± 0.02 | .03* |
| GenuCC | 0.52 ± 0.02 | 0.53 ± 0.02 | .06 |
| SpleniumCC | 0.66 ± 0.03 | 0.67 ± 0.03 | .06 |
| AntIC | 0.38 ± 0.01 | 0.38 ± 0.01 | .27 |
| CingCG | 0.44 ± 0.02 | 0.45 ± 0.02 | .01* |
| CingH | 0.36 ± 0.02 | 0.36 ± 0.03 | .21 |
| CST | 0.54 ± 0.02 | 0.54 ± 0.02 | .84 |
| IFOF | 0.41 ± 0.02 | 0.41 ± 0.02 | .24 |
| ILF | 0.42 ± 0.02 | 0.42 ± 0.03 | .06 |
| SLF | 0.41 ± 0.02 | 0.41 ± 0.02 | .37 |
| UF | 0.38 ± 0.01 | 0.38 ± 0.01 | .65 |
| b | |||
|---|---|---|---|
| Region | Pre-transplant (M±SD) *103 m^2/s |
Post-transplant (M±SD) *103 m^2/s |
p |
| BodyCC | 0.99 ± 0.07 | 0.99 ± 0.07 | .34 |
| FMa | 0.94 ± 0.10 | 0.92 ± 0.10 | .008** |
| FMi | 0.85 ± 0.05 | 0.85 ± 0.04 | .24 |
| GenuCC | 1.06 ± 0.10 | 1.06 ± 0.10 | .71 |
| SpleniumCC | 0.88 ± 0.06 | 0.87 ± 0.05 | .02* |
| AntIC | 0.83 ± 0.06 | 0.83 ± 0.06 | .78 |
| CingCG | 0.74 ± 0.04 | 0.74 ± 0.03 | .86 |
| CingH | 0.81 ± 0.04 | 0.81 ± 0.04 | .76 |
| CST | 0.76 ± 0.03 | 0.76 ± 0.03 | .72 |
| IFOF | 0.83 ± 0.04 | 0.83 ± 0.42 | .58 |
| ILF | 0.80 ± 0.04 | 0.80 ± 0.03 | .19 |
| SLF | 0.75 ± 0.03 | 0.74 ± 0.02 | .08 |
| UF | 0.80 ± 0.03 | 0.81 ± 0.02 | .53 |
p value < 0.01,
p value <0.05
Mean Diffusivity (MD)
Means and standard deviations for MD values for each tract, pre- and post-transplantation are provided in Table 4b. The Tract (13) by Scan (2) ANOVA for MD revealed a significant interaction of Tract by Scan (F(12, 120) = 2.61, p = 0.004, η2 = 0.21), indicating that the change in MD after transplant was not consistent across all tracts. As in the case for FA, there was a significant main effect of Tract (F(12, 120) = 67.26, p < 0.001, η2 = 0.87), indicating that MD was not the same in all tracts studied. The main effect of Scan was not significant (F(1, 10) = 1.46, p = 0.25, η2 = 0.13), indicating that the overall MD did not change across the whole brain. Post-hoc two tailed paired t-tests comparing pre- and post-transplantation values for each tract revealed significant decreases in MD in the Fma (MDIFF = 0.017*103, SEMDIFF = 0.017*103, t(10) = 3.28, p < 0.01), and splenium of the corpus callosum (MDIFF = 0.012*103, SEMDIFF = 0.015*103, t(10) = 2.65, p = 0.02) (Table 4b and Figure S3), suggesting improvement in post-transplant white matter integrity (Figure 2B).
Discussion
Our study systematically measured cognitive function and white matter integrity both pre- and post-renal transplant using standard NP tests and brain MRI to assess changes in cognition and white matter integrity. We observed both cognitive function and white matter integrity to improve after renal transplantation. More importantly, we found that the tracts with significant improvement in white matter integrity post-transplant, namely the corpus callosum, cingulate gyrus, forceps minor and forceps major, are the tracts associated with memory and executive function[19–21], the domains of cognition that specifically improved after transplantation. We also found that cognitive improvement seen post-transplant persisted for one year but did not improve further compared to the function at three months post-transplant.
Cognitive impairment in ESRD is associated with decreased white matter integrity[6], indicating that structural changes or white matter integrity may play a role in impairment of cognition in this population. Patients with ESRD have lower FA and higher MD values[6, 10–13], which are generally associated with poorer cognitive function[8, 9, 22, 23]. Moreover, improvement in FA and MD is associated with an improvement in cognition[24]. Other investigators have shown improvements in cognitive function post-transplant[25–30], but to the best of our knowledge, our study is the first to evaluate the effects of renal transplantation on brain structure.
We found an increase in FA and decrease in MD post-transplant suggesting improved white matter integrity. Also cognition, specifically memory (logical memory I; p= 0.004, logical memory II; p=0.003, and digit span, p=0.007) and executive function (digit symbol; p<0.001) improved post-transplant. Prior studies suggest the association of specific cognitive domains with specific white matter tracts[31]. Interestingly, the structural changes observed in the specific white matter tracts corresponded to specific improvements in memory and executive function, the domains of cognition associated with these tracts[19–21]. The forceps minor connects the lateral and medial surfaces of the frontal lobes, which regulate executive function[19]. Abnormal frontal connectivity is present in mental illness with executive dysfunction[32, 33]. The cingulate gyrus is part of the limbic system located immediately above the corpus callosum and plays a role in executive function along with learning, processing and memory[20]. We saw improvement in FA in forceps minor and cingulate gyrus, as well as improvement in executive function. We also saw an improvement in FA and MD values at the corpus callosum post-transplant. The corpus callosum is the largest white matter tract consisting of over 200 million contralateral axonal projections that connects the left and right cerebral hemispheres. The size of the corpus callosum correlates positively with verbal memory capacity and semantic testing[21]. Of note, two tracts of importance did not improve post-transplant (i.e. the corticospinal tracts and the uncinate fasciculus). The corticospinal tracts have not been reported to have a specific role in memory or executive function and control voluntary movement. The uncinate fasciculus control emotions[34]. The degree of change in DTI metrics were consistent with the degree of improvement seen in some other medical conditions[22, 35].
Although we demonstrate improved white matter integrity post-transplant, we cannot rule out the confounding effects of depression and physical activity on cognitive function and DTI measurements. Both depression and physical activity improved post-transplant and are known to affect cognition. Depression is associated with lower FA values[36]. Physical activity positively correlates with white matter integrity[37] with increase in FA values with exercise[38]. Another potential confounder could be reduced cerebral edema post-transplant, which can increase FA values. These factors should be further evaluated in future studies. We did not observe any differences in blood pressure, body weight and electrolytes post-transplant. However, it is possible that lower FA values in ESRD could be due to certain (likely non-dialyzable) uremic toxins and metabolites, and improvement in renal function after transplantation permitted better excretion or tubular secretion of these toxins resulting in an increase in FA post-transplant. All patients in the study were on a calcineurin inhibitor post-transplant. Calcineurin is known to modulate synaptic activity and memory[39]. Specific effects of various immunosuppressive strategies on cognitive function post-transplant remain unclear, requiring further studies.
Our study has several strengths. We were meticulous in testing cognitive function. Trained and experienced psychometrists administered NP tests. Also, we used a detailed battery of NP tests to assess a wider range of cognitive domains than previous studies[26, 28–30]. Dialysis can affect cognition[14]. We assessed patients on their non-dialysis days, when, perhaps their cognitive function is the best. Despite that, we saw further improvements in cognition after transplantation. The timing of dialysis to NP testing is an important factor and should be considered in the design of future studies. We checked for practice effect by comparing the NP tests at two time points pre-transplant in a subgroup of patients. Results remained stable arguing against a practice effect. All of the NP tests were spaced by a minimum of three months to minimize practice effect.
Despite the strengths, there are some limitations. The sample sizes were relatively small. However, this study is the first to evaluate the effects of transplantation on cognition and associated changes in the brain not visible on conventional MRI. Moreover, the within-subject design added sensitivity to detect change. It is encouraging that even with 11 patients, we saw changes in cognition and DTI metrics, which were very consistent among the different subjects. We did not measure correlation coefficients due to the small sample size. This study is hypothesis generating and will lead to other studies evaluating the reversibility of brain alterations seen in ESRD. Identification of specific areas of reversibility in cognition and brain structure will have therapeutic implications. Our patients had variable demographics, which can affect cognition. However, we did paired analysis and each patient was his/her control. Our patient demographics indicate that they were perhaps the healthier ESRD patients. It can be argued that a greater change may be seen in sicker patients. The adherence of the enrolled patients and the follow-up was excellent.
Conclusion
Our study indicates that cognition, particularly memory and executive function, improves post-transplantation with corresponding improvement in white matter integrity. Future studies are needed to establish the role of white matter integrity in cognitive impairment in ESRD.
Supplementary Material
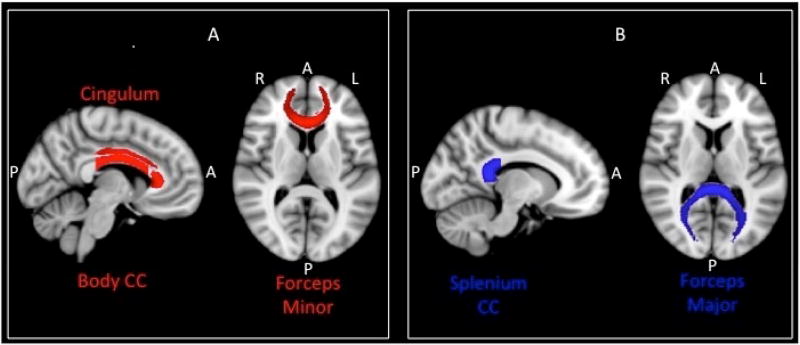
Figure 1.
Tracts with increase in fractional anisotropy (FA) and decrease in mean diffusivity (MD) after renal transplantation.
A) FA increased in cingulum, body of corpus callosum and forceps minor after transplantation (shown in red).
B) MD decreased in splenium of corpus callosum and forceps major after transplantation (shown in blue). Body CC, body of corpus callosum; Splenium CC, splenium of corpus callosum; A, anterior; P, posterior; R, right; L, left.
Acknowledgments
Funded by the KUMC Kidney Institute Pilot award
Abbreviations
- AntIC
anterior limb of the internal capsule
- BodyCC
body of the corpus callosum
- CingCG
cingulate gyrus
- CingH
cingulate gyrus, hippocampus
- CST
corticospinal tract
- DTI
diffusion tensor imaging
- ESRD
end stage renal disease
- FA
fractional anisotropy
- Fma
forceps major
- Fmi
forceps minor
- GenuCC
genu of the corpus callosum
- IFOF
inferior fronto-occipital fasciculus
- ILF
inferior longitudinal fasciculus
- NP
neuropsychological
- RAPA
rapid assessment of physical activity
- SpleniumCC
splenium of the corpus callosum
- SLF
superior longitudinal fasciculus
- TOI
tract-of-interest
- UF
uncinated fasciculus
Footnotes
Registered in Clinicaltrials.gov, NCT01883349
None of the authors have any conflicts of interest to declare
References
- 1.Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 2.Kurella M, Mapes DL, Port FK, Chertow GM. Correlates and outcomes of dementia among dialysis patients: the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2006;21:2543–2548. doi: 10.1093/ndt/gfl275. [DOI] [PubMed] [Google Scholar]
- 3.Drew DA, Weiner DE, Tighiouart H, Scott T, Lou K, Kantor A, et al. Cognitive function and all-cause mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2015;65:303–311. doi: 10.1053/j.ajkd.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikram MA, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Breteler MM. Kidney function is related to cerebral small vessel disease. Stroke. 2008;39:55–61. doi: 10.1161/STROKEAHA.107.493494. [DOI] [PubMed] [Google Scholar]
- 5.Drew DA, Bhadelia R, Tighiouart H, Novak V, Scott TM, Lou KV, et al. Anatomic brain disease in hemodialysis patients: a cross-sectional study. Am J Kidney Dis. 2013;61:271–278. doi: 10.1053/j.ajkd.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh TJ, Chang JM, Chuang HY, Ko CH, Hsieh ML, Liu GC, et al. End-stage renal disease: in vivo diffusion-tensor imaging of silent white matter damage. Radiology. 2009;252:518–525. doi: 10.1148/radiol.2523080484. [DOI] [PubMed] [Google Scholar]
- 7.Ozcan H, Yucel A, Avsar UZ, Cankaya E, Yucel N, Gozubuyuk H, et al. Kidney Transplantation Is Superior to Hemodialysis and Peritoneal Dialysis in Terms of Cognitive Function, Anxiety, and Depression Symptoms in Chronic Kidney Disease. Transplant Proc. 2015;47:1348–1351. doi: 10.1016/j.transproceed.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, et al. Age-related decline in white matter tract integrity and cognitive performance: a DTI tractography and structural equation modeling study. Neurobiol Aging. 2012;33:21–34. doi: 10.1016/j.neurobiolaging.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Wang A, Tang J, Wei D, Li P, Chen K, et al. Association of white matter integrity and cognitive functions in patients with subcortical silent lacunar infarcts. Stroke. 2015;46:1123–1126. doi: 10.1161/STROKEAHA.115.008998. [DOI] [PubMed] [Google Scholar]
- 10.Zhang R, Liu K, Yang L, Zhou T, Qian S, Li B, et al. Reduced white matter integrity and cognitive deficits in maintenance hemodialysis ESRD patients: a diffusion-tensor study. Eur Radiol. 2015;25:661–668. doi: 10.1007/s00330-014-3466-5. [DOI] [PubMed] [Google Scholar]
- 11.Kim HS, Park JW, Bai DS, Jeong JY, Hong JH, Son SM, et al. Diffusion tensor imaging findings in neurologically asymptomatic patients with end stage renal disease. NeuroRehabilitation. 2011;29:111–116. doi: 10.3233/NRE-2011-0684. [DOI] [PubMed] [Google Scholar]
- 12.Chen CL, Lai PH, Chou KJ, Lee PT, Chung HM, Fang HC. A preliminary report of brain edema in patients with uremia at first hemodialysis: evaluation by diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2007;28:68–71. [PMC free article] [PubMed] [Google Scholar]
- 13.Chou MC, Hsieh TJ, Lin YL, Hsieh YT, Li WZ, Chang JM, et al. Widespread white matter alterations in patients with end-stage renal disease: a voxelwise diffusion tensor imaging study. AJNR Am J Neuroradiol. 2013;34:1945–1951. doi: 10.3174/ajnr.A3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray AM, Pederson SL, Tupper DE, Hochhalter AK, Miller WA, Li Q, et al. Acute variation in cognitive function in hemodialysis patients: a cohort study with repeated measures. Am J Kidney Dis. 2007;50:270–278. doi: 10.1053/j.ajkd.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Shirk SD, Mitchell MB, Shaughnessy LW, Sherman JC, Locascio JJ, Weintraub S, et al. A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimers Res Ther. 2011;3:32. doi: 10.1186/alzrt94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agganis BT, Weiner DE, Giang LM, Scott T, Tighiouart H, Griffith JL, et al. Depression and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis. 2010;56:704–712. doi: 10.1053/j.ajkd.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ. Validation of depression screening scales in patients with CKD. Am J Kidney Dis. 2009;54:433–439. doi: 10.1053/j.ajkd.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenter PA, Just MA, Reichle ED. Working memory and executive function: evidence from neuroimaging. Curr Opin Neurobiol. 2000;10:195–199. doi: 10.1016/s0959-4388(00)00074-x. [DOI] [PubMed] [Google Scholar]
- 20.Ohtani T, Nestor PG, Bouix S, Saito Y, Hosokawa T, Kubicki M. Medial frontal white and gray matter contributions to general intelligence. PLoS One. 2014;9:e112691. doi: 10.1371/journal.pone.0112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozlovskiy SAVAV, Pyasik MM, Nikonova EY. Functional role of corpus callosum regions in human memory functioning. International Journal of Psychophysiology. 2012;85:396–397. [Google Scholar]
- 22.Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- 23.Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newcombe VF, Correia MM, Ledig C, Abate MG, Outtrim JG, Chatfield D, et al. Dynamic Changes in White Matter Abnormalities Correlate With Late Improvement and Deterioration Following TBI: A Diffusion Tensor Imaging Study. Neurorehabil Neural Repair. 2015 doi: 10.1177/1545968315584004. [DOI] [PubMed] [Google Scholar]
- 25.Radic J, Ljutic D, Radic M, Kovacic V, Dodig-Curkovic K, Sain M. Kidney transplantation improves cognitive and psychomotor functions in adult hemodialysis patients. Am J Nephrol. 2011;34:399–406. doi: 10.1159/000330849. [DOI] [PubMed] [Google Scholar]
- 26.Kramer L, Madl C, Stockenhuber F, Yeganehfar W, Eisenhuber E, Derfler K, et al. Beneficial effect of renal transplantation on cognitive brain function. Kidney Int. 1996;49:833–838. doi: 10.1038/ki.1996.115. [DOI] [PubMed] [Google Scholar]
- 27.Teschan PE, Ginn HE, Bourne JR, Ward JW. Neurobehavioral responses to "middle molecule" dialysis and transplantation. Trans Am Soc Artif Intern Organs. 1976;22:190–194. [PubMed] [Google Scholar]
- 28.Griva K, Thompson D, Jayasena D, Davenport A, Harrison M, Newman SP. Cognitive functioning pre- to post-kidney transplantation--a prospective study. Nephrol Dial Transplant. 2006;21:3275–3282. doi: 10.1093/ndt/gfl385. [DOI] [PubMed] [Google Scholar]
- 29.Radic J, Ljutic D, Radic M, Kovacic V, Dodig-Curkovic K, Sain M. Kidney Transplantation Improves Cognitive and Psychomotor Functions in Adult Hemodialysis Patients. American Journal of Nephrology. 2011;34:399–406. doi: 10.1159/000330849. [DOI] [PubMed] [Google Scholar]
- 30.Harciarek M, Biedunkiewicz B, Lichodziejewska-Niemierko M, Debska-Slizien A, Rutkowski B. Cognitive performance before and after kidney transplantation: a prospective controlled study of adequately dialyzed patients with end-stage renal disease. J Int Neuropsychol Soc. 2009;15:684–694. doi: 10.1017/S1355617709990221. [DOI] [PubMed] [Google Scholar]
- 31.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fletcher P, McKenna PJ, Friston KJ, Frith CD, Dolan RJ. Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. Neuroimage. 1999;9:337–342. doi: 10.1006/nimg.1998.0411. [DOI] [PubMed] [Google Scholar]
- 33.Spence SA, Liddle PF, Stefan MD, Hellewell JS, Sharma T, Friston KJ, et al. Functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. Focal dysfunction and distributed disconnectivity reappraised. Br J Psychiatry. 2000;176:52–60. doi: 10.1192/bjp.176.1.52. [DOI] [PubMed] [Google Scholar]
- 34.Oishi K, Faria AV, Hsu J, Tippett D, Mori S, Hillis AE. Critical role of the right uncinate fasciculus in emotional empathy. Ann Neurol. 2015;77:68–74. doi: 10.1002/ana.24300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dervan L, Poliakov A, Friedman SD, Shaw D, Pihoker C, Roberts JS, et al. Change in fractional anisotropy during treatment of diabetic ketoacidosis in children. Pediatr Res. 2014;75:62–66. doi: 10.1038/pr.2013.168. [DOI] [PubMed] [Google Scholar]
- 36.Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38:49–56. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gons RA, Tuladhar AM, de Laat KF, van Norden AG, van Dijk EJ, Norris DG, et al. Physical activity is related to the structural integrity of cerebral white matter. Neurology. 2013;81:971–976. doi: 10.1212/WNL.0b013e3182a43e33. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto Y, Kemp GJ, Isobe T, Sato E, Hirano Y, Shoda J, et al. Changes in diffusion tensor imaging (DTI) eigenvalues of skeletal muscle due to hybrid exercise training. Magn Reson Imaging. 2014;32:1297–1300. doi: 10.1016/j.mri.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Dineley KT, Hogan D, Zhang WR, Taglialatela G. Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol Learn Mem. 2007;88:217–224. doi: 10.1016/j.nlm.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.