Abstract
Future deep space missions to Mars and near-Earth asteroids will expose astronauts to chronic solar energetic particles (SEP) and galactic cosmic ray (GCR) radiation, and likely one or more solar particle events (SPEs). Given the inherent radiosensitivity of hematopoietic cells and short latency period of leukemias, space radiation-induced hematopoietic damage poses a particular threat to astronauts on extended missions. We show that exposing human hematopoietic stem/progenitor cells (HSC) to extended mission-relevant doses of accelerated high-energy protons and iron ions leads to the following: (1) introduces mutations that are frequently located within genes involved in hematopoiesis and are distinct from those induced by γ-radiation; (2) markedly reduces in vitro colony formation; (3) markedly alters engraftment and lineage commitment in vivo; and (4) leads to the development, in vivo, of what appears to be T-ALL. Sequential exposure to protons and iron ions (as typically occurs in deep space) proved far more deleterious to HSC genome integrity and function than either particle species alone. Our results represent a critical step for more accurately estimating risks to the human hematopoietic system from space radiation, identifying and better defining molecular mechanisms by which space radiation impairs hematopoiesis and induces leukemogenesis, as well as for developing appropriately targeted countermeasures.
Introduction
A major challenge facing long-duration manned missions beyond low Earth orbit (LEO), such as those planned to Mars and near-Earth asteroids, is the poorly defined health risks associated with the chronic low dose rate exposure of astronauts to space radiation comprised of solar energetic particles (SEPs) and galactic cosmic rays (GCR). SEPs and GCRs include accelerated charged particles ranging from protons to high charge and energy (HZE) ions. The Mars Science Laboratory Radiation Assessment Detector recently measured the typical fluences and energies of charged particles to which astronauts will be exposed during a Mars mission.1–4 Although the relative fluences of HZE ions in the solar and GCR fields are fairly low, their linear energy transfer (LET) values are orders of magnitude higher than those of traditional X-rays and γ-rays. As such, they constitute a significant portion of the total dose equivalent (TDE) received by astronauts.5–7 Nuclear fragmentation resulting from charged particle interactions with the spacecraft hull and contents and the subsequent generation of low-energy secondaries, further contributes to the TDE and complicates risk analysis.5,7–9
The cumulative radiation exposure received from these sources during long-duration spaceflight could have significant short- and long-term untoward effects on human physiology,5,6,9–17 including the potential to increase cancer morbidity/mortality in astronauts. Unfortunately, due to an incomplete understanding of the biological effects during and following exposure to the space radiation environment,17,18 and the paucity of human epidemiological studies for these more exotic radiation types, it is difficult to accurately estimate the risk of carcinogenesis from long-term chronic exposures that astronauts will experience on extended deep space missions.8,18–20 Complicating matters is the additional ∼ 20% probability of short-term solar particle events (SPEs) during the roughly 400- day round trip to and from Mars, which could conceivably impart a relatively high acute dose of predominantly protons and light ions within an hour or less, significantly increasing mission TDE.7,21,22 Despite the clear importance from the standpoint of astronaut safety and mission outcome/success, studies to-date assessing human cancer risks from space radiation have largely been: 1) based on the assumption that protons and HZE ions damage cells similarly to traditional low LET radiation such as X- and γ-rays; and 2) extrapolated from rodent studies involving exposure to single ion species at high dose rates.18,20,23,24 Current NASA probabilistic risk models continue to be refined and improved to incorporate factors to account for dose rate- and LET-dependent quality factors as more data become available.8,9,13,20
Seminal ground-based radiobiology studies at particle accelerators have shown that protons, the primary SEP constituent, are more damaging to human DNA than X-rays or γ-rays despite having nearly identical LET values and producing a greater number of complex DNA ‘clustered’ lesions per unit dose, behaving in a fashion more characteristic of heavier charged particles like HZE ions.25,26 Later work showed that dual mixed-field exposures of relatively low doses of protons and HZE ions produced additive or even synergistic effects on primary human fibroblast killing and transformation, most notably when HZE ion (56Fe ions) irradiation followed within an hour after proton irradiation (an exposure scheme typical in space). More recent murine studies have shown similar effects of sequential irradiation with protons followed by HZE ions in the lung, heart and hippocampus.27‐29 Collectively, these studies suggest that exposure of human cells, notably hematopoietic stem cells (HSC), to SEP and GCR radiation may significantly increase risk of carcinogenesis, and highlights the need for additional research on human cells using combinations of radiation species (ions) and exposure schemes that best approximate the space radiation environment.
Given the inherent sensitivity of the hematopoietic system to ionizing radiation30‐33 and the short latency period of leukemias,19 SEP and GCR-induced hematopoietic damage may pose a particular threat to astronauts during prolonged missions. To begin defining the potential risk to astronaut hematopoietic systems from space travel beyond LEO, we performed in vitro and in vivo studies to define the functional impact of exposing human bone marrow-derived hematopoietic stem/progenitor cells (HSC), isolated from healthy donors of typical astronaut age (30‐55 years), to mission-relevant doses of simulated SEP protons (1 Gy of 50 MeV protons; primary SPE energy34) or GCR (20 cGy of 1 GeV/n 56Fe ions) using sham-irradiated and γ-irradiated same donor HSC as controls.
Materials and Methods
Preparation of human bone marrow-derived HSC and exposure to simulated SEP/GCR radiation
Frozen human CD34+ cells isolated from healthy adult donors of typical astronaut (age 30‐55 years) were obtained commercially. On the day prior to the scheduled irradiations at the NASA Space Radiation Laboratory (NSRL) at Brookhaven National Laboratory (BNL), human CD34+ cells were thawed, packaged and shipped to NSRL. Upon receipt at NSRL, HSC were exposed to mission-relevant doses of simulated SEP and GCR radiation.
Analysis of hematopoietic colony-forming potential by HALO
Upon receipt at WFIRM, cells were washed, counted and viability checked. Their in vitro functionality/colony-forming potential was then assessed by HALO assay.35
Transplantation of immunodeficient mice to assess human HSC functionality in vivo
To test their in vivo potential/functionality, human HSC exposed to each irradiation scheme were used to reconstitute human hematopoiesis in immunodeficient NSG mice,36 in accordance with a Wake Forest University Health Science IACUC-approved protocol.
Histopathology/Immunohistochemistry on enlarged spleens
To define the cause of the marked splenomegaly in the mice transplanted with human HSC exposed to 56Fe ions, the spleen was collected at the time of euthanasia, processed and analyzed by immunohistochemistry with human-specific antibodies.
FACS analysis of human hematopoietic engraftment and differentiation
At euthanasia, all long bones and the spleen were collected from each animal and processed to obtain a single cell suspension. Cells were then stained with a panel of fluorophore-conjugated antibodies to various human markers/antigens, and analyzed on a FACS caliber (BDIS, San Jose, CA, USA) flow cytometer.
Whole exome sequencing of DNA from human HSC exposed to SEP and GCR
Immediately upon receipt at WFIRM (∼18 h post irradiation), total genomic DNA was extracted from aliquots of CD34+ cells exposed to each irradiation scheme, and subjected to whole exome sequencing to identify genetic variants induced by each irradiation scheme.
Results
In vitro studies
Human CD34+ hematopoietic stem/progenitor cells (HSC) were obtained from bone marrow of six healthy adults of typical astronaut age, transported overnight to the NASA Space Radiation Laboratory (NSRL), and exposed to one of the following irradiation schemes:
Sham irradiation
1 Gy of 137Cs γ-irradiation (linear energy transfer (LET) = 0.91 keV/μm)
1 Gy of 50 MeV protons (primary SEP energy; LET = 1.26 keV/μm)
20 cGy of 1 GeV/n 56Fe ions (model GCR radiation; LET = 151 keV/μm)
1 Gy of 50 MeV protons followed 15 min later by 20 cGy of 1 GeV/n 56Fe ions (an exposure scheme previously shown to have synergistic effects on human fibroblasts25,26).
Samples were then analyzed at ∼18 h post irradiation. HSC from each exposure group were enumerated, and an equal number were assessed for functionality (in replicates of 6–8) in vitro using the HALO-96 Human Stem/Progenitor Cell Assay (Hemogenix, Inc., Colorado Springs, CO, USA). This highly sensitive assay simultaneously quantitates seven different stem/progenitor cell populations at varying stages of differentiation: HPP-CFC, CFU-GEMM/ Mix, CFU-GM, BFU-E, CFC-Mk, CFC-T and CFC-B.
The HALO results in Figure 1 show that, in most cases, 1 Gy of cesium-137 γ-rays and 50 MeV protons, both low LET radiations, exert fairly similar effects on HSC function (P > 0.05 comparing effects of protons vs γ-rays), with both significantly reducing (by 50–80%, when comparing either protons or γ-rays to sham-irradiated controls, depending upon colony type) the ability of HSC to form primitive colonies (HPP-CFC and CFU-GEMM; P ≤ 0.01), as well as more differentiated myeloid (CFU-GM; P < 0.01), erythroid (BFU-E; P ≤ 0.03), megakaryocyte/platelet (CFC-Mk; P ≤ 0.01) and lymphoid (CFC-T and CFC-B; P ≤ 0.01) colonies.
Figure 1.
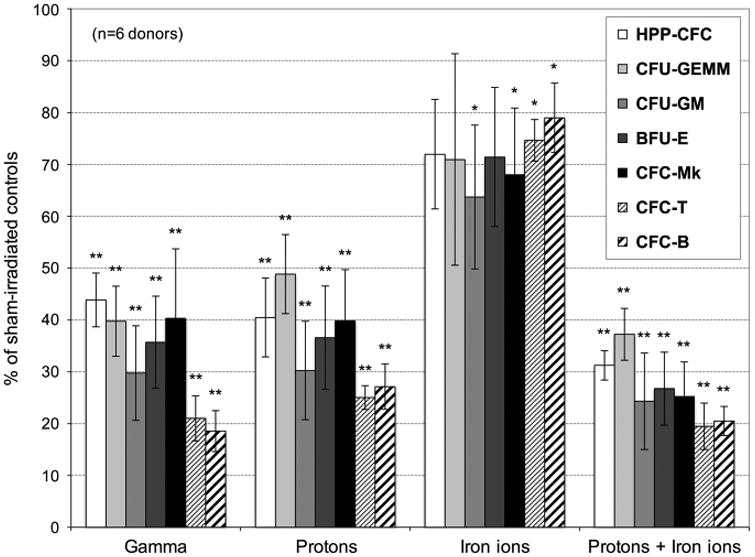
Effect of γ-ray, proton, iron ion and mixed-field proton/iron ion irradiation on HSC function. Human BM-derived CD34+ cells (n = 6 different human donors) were exposed to 137Cs γ-ray irradiation (1 Gy, 662 keV) at BNL; monoenergetic protons (1 Gy, 50 MeV; primary SEP energy) at NSRL; iron ions (20 cGy, 1 GeV/n) at NSRL; or sequential monoenergetic protons (1 Gy, 50 MeV), followed 15 min later by iron ions (20 cGy, 1 GeV/n) at NSRL, to better simulate astronaut radiation exposure in deep space. Upon return to WFIRM (∼18hr post irradiation), all HSC (as well as ‘sham-irradiated’ HSC that were shipped to NSRL and back) were assayed for functionality/colony-forming potential using the HALO-96 Human Stem/Progenitor Cell Assay (HuSPCA; Hemogenix, Inc.). Data are displayed as mean ± s.e.m.
In contrast to γ-rays and protons, 20 cGy of 1 GeV/n 56Fe ions had a much less pronounced effect on the colony-forming potential of HSC, exerting no significant effect (P > 0.05) on their ability to give rise to the most primitive HPP-CFC colonies. Exposure to these high-LET ions did, however, reduce the colony-forming potential in the remaining lineages by 20–40%, depending upon the specific colony type (Figure 1). Furthermore, combining protons with iron ions within a 15-min interval (to mimic a typical deep space exposure scenario) proved to be highly deleterious to HSC function, reducing their ability to produce all colony types by 60–80% (Figure 1). This decrease was, in all cases, statistically different from the effects seen with either 56Fe ions alone (P < 0.01 comparing sequential proton+56Fe ions to iron ions alone) or protons alone (P < 0.05 comparing sequential proton+56Fe ions to protons alone). Of importance is that the effects observed on hematopoietic colony formation following exposure of HSC to sequential proton+56Fe ions often differed markedly from the effects seen with either single ion species; moreover, these effects varied from one lineage (colony type) to another.
The CD34+ cell population used in these studies is heterogeneous, in that it contains cells at multiple stages of differentiation. HALO enables precise quantitation of hematopoietic cells at varying stages of differentiation/lineage commitment, offering a temporal ‘snapshot’ into the presence/ functionality of the different early stem/progenitor populations. Each colony type forms as a result of its ‘parent cell's’ ability to proliferate with a specific cocktail of cytokines and provides a precise readout of the relative radiosensitivity of hematopoietic cells at specific stages of differentiation/lineage commitment. Each colony type is derived exclusively from a specific precursor cell. As such, reduction in formation of specific colony types following exposure of CD34+ cells to GCR/SEP radiation indicates that the stem/progenitor cell that gives rise to that particular colony type is sensitive to the radiation species in question. The observed reduction in HPP-CFC colonies following exposure of CD34+ cells to GCR/SEP radiation indicates that these primitive human HSC are sensitive to these space radiation species. Similarly, the reduction in the formation of BFU-E, CFC-T and CFC-B colonies demonstrates that progenitors of the erythroid, T-lymphoid and B-lymphoid lineages, respectively, are likewise sensitive to GCR/SEP radiation.
Whole exome sequencing on human HSC exposed to proton and iron ion irradiation
To assess whether differences in colony-forming potential from exposure to protons, 56Fe ions, or sequential proton+56Fe ion irradiations might translate to variable leukemogenic potential in vivo, DNA was isolated from HSC from two human donors at 18 h post irradiation, and subjected to unbiased whole-genome amplification followed by whole-exome sequencing (WES), with 100× coverage. Sequence data from HSC exposed to each irradiation scheme were compared with that of sham-irradiated HSC from the same donor, to ensure that any observed alterations were not simply due to genetic variation. As shown in Figure 2a, each irradiation scheme induced a large number of genetic alterations, some of which were common to all irradiation schemes, and some of which were unique to the specific radiation species employed. Of note, the pattern and magnitude of alterations induced in response to each irradiation scheme were almost identical in HSC from the two different human donors.
Figure 2.
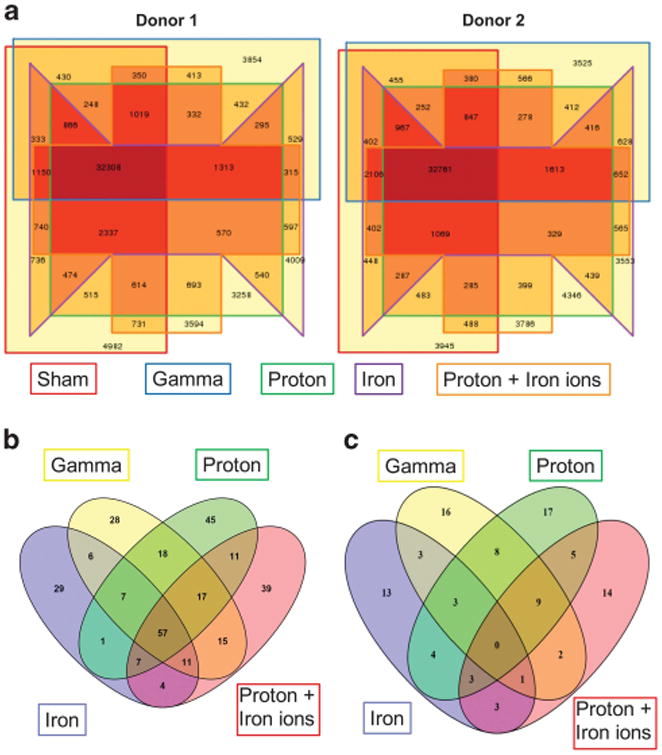
Whole-exome sequencing of HSC following exposure to γ-ray, proton, iron ion and mixed-field proton/iron ion irradiation. Human BM-derived CD34+ cells (from two different donors) were exposed to the following: 1) monoenergetic protons (1 Gy, 50 MeV); 2) iron ions (20 cGy, 1 GeV/n); or 3) sequential protons followed 15 min later by iron ions at NSRL. Upon return to WFIRM, DNA was isolated from each aliquot of cells, subjected to whole genome amplification, and analyzed by Ion Torrent™-based whole exome sequencing. The Venn diagrams in (a) display the data from each individual donor, with the total number of genomic alterations induced by each irradiation scheme bordered with a distinct colored line in the diagram. The Venn diagram in (b) displays the number of single-nucleotide variants (SNV) present within coding regions of known genes in either of the two human donors as a result of exposure to each irradiation scheme shown within each colored oval. The Venn diagram in (c) shows the composite data from the two donors, with the SNV that are present in both donors as a result of exposure to each irradiation scheme shown within each colored oval.
Given the large number of alterations present, data were filtered to identify genomic regions that were potentially enriched for functional mutations in each irradiated sample. The genome was divided into intervals of 50 kb, the number of variants induced by each irradiation scheme was counted, and the top 0.1% of those intervals was extracted. This list was further restricted by only considering the single-nucleotide variants (SNV) that fell within exons. Figure 2b depicts the SNV induced by each irradiation scheme, in HSC from either of the two donors, whereas Figure 2c shows these data after filtering to only show SNV present in HSC from both donors following exposure to each irradiation scheme. It is intriguing that, once data from the 2 donors were pooled, there were no longer any SNV common to all irradiation schemes, yet many SNV unique to each specific irradiation (especially those involving protons or 56Fe ions) scheme were present in the HSC from both donors.
The main graph in Figure 3 depicts the percentage of SNV present within HSC that were unique to the specific irradiation scheme, and shows that a large percentage of SNV induced by protons and 56Fe ions were unique to those radiations. As we observed different effects on HSC functionality when cells were sequentially exposed to protons followed by 56Fe ions (compared to either ion alone), we analyzed the WES data to ascertain whether HSC sequentially exposed to protons and 56Fe ions had unique SNV when compared with the HSC exposed to either ion alone. As can be seen in the smaller graph (inset), 38% of the SNV present in the HSC (from both donors) following sequential exposure to protons and 56Fe ions were unique to this sequential irradiation scheme, and were not present in HSC from these same donors following exposure to either ion species alone.
Figure 3.
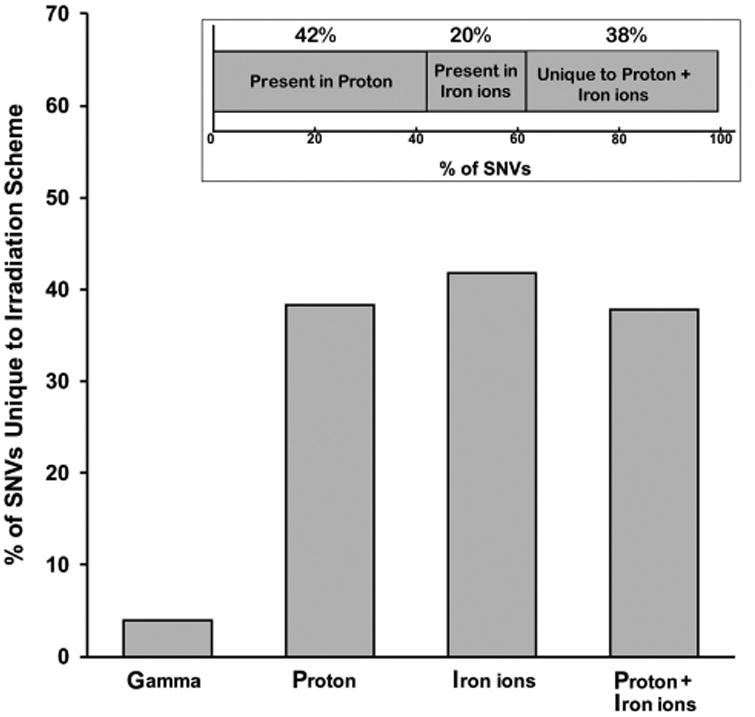
Percentage of SNV unique to each irradiation scheme. The main bar graph shows the percentage of unique SNV occurring in response to each irradiation scheme. The smaller bar graph in the inset shows the relative percentage of SNV present in HSC from both donors in response to sequential proton+iron ion mixed-field irradiation that are also present in HSC from the same donors following exposure to protons alone or iron ions alone, and the percentage of SNV that are unique to sequential proton+iron ion irradiation (and present in both donors).
To assess whether the SNV that occurred in response to each irradiation scheme could account, at least in part, for the observed alterations in functionality/colony-forming potential of the HSC, we interrogated each identified SNV by performing an extensive search of online databases (NCBI Nucleotide, GeneCards, PubMed and Google), to determine whether genes harboring the SNV were related to hematopoiesis, or if alterations in the gene in question had been implicated in any type of leukemia. Figure 4 shows that only ∼ 15% of the genes containing SNV that were common to all irradiation schemes (data from the 2 donors were pooled as there were no SNV common to all irradiation schemes in both donors) have a role in hematopoiesis and/or have been implicated in leukemia. Similarly, only ∼ 20% of SNV present as a result of exposure to γ-radiation occurred in hematopoiesis-related genes. In contrast, nearly 50 and 42% of the SNV present following exposure to monoenergetic protons or 56Fe ions, respectively, were in hematopoiesis and/or leukemia-related genes. Quite strikingly, when HSC were exposed to sequential protons and 56Fe ions, nearly 60% of the unique SNV were within genes related to hematopoiesis and/or implicated in leukemia.
Figure 4.
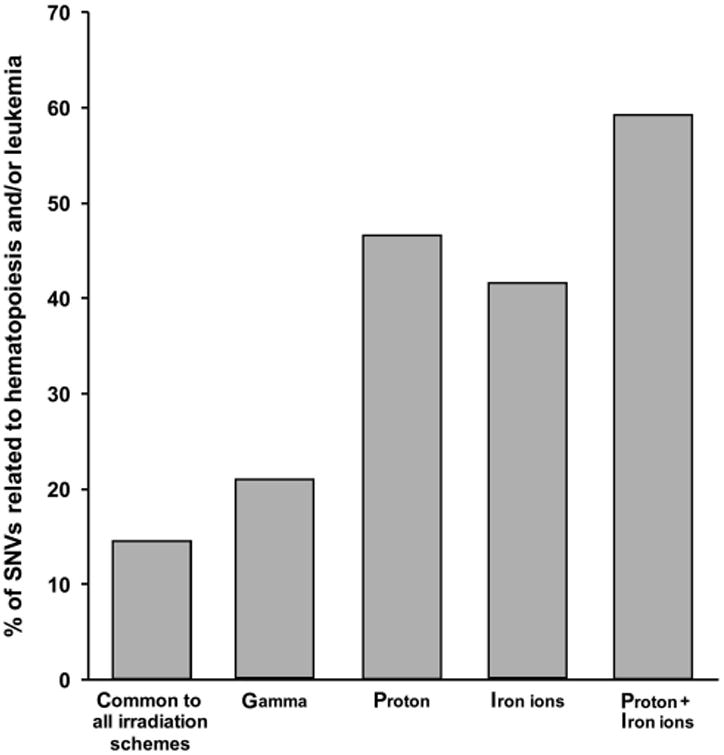
Unique SNV induced by proton, iron ion and mixed-field proton/iron ion irradiation exhibit a predilection for genes related to hematopoiesis and/or implicated in leukemia. SNV that were unique to each irradiation scheme were interrogated by online database searching (GeneCards, PubMed, NCBI Nucleotide and Google) for involvement in normal hematopoiesis and/or having been implicated in hematological malignancies. The bar graph displays the percentage of unique SNV in each irradiation scheme that fit these criteria.
In vivo studies
While in vitro assays provide valuable information regarding short-term functionality of human HSC, the gold standard for assessing true potential/function is testing their ability to repopulate the hematopoietic system following transplantation into conditioned/ablated immunodeficient murine recipients. NSG mice were conditioned with busulfan,36 rather than sublethal γ-irradiation, to avoid additional radiation effects resulting from irradiation of the resident marrow microenvironment/niche within the recipient mice or residual murine hematopoietic cells.
Conditioned NSG mice were transplanted with 2×105 human HSC that had been exposed to the same irradiation schemes as above (n = 9 mice per group): (1) sham irradiation; (2) 1 Gy of 137Cs γ-irradiation; (3) 1 Gy of 50 MeV protons; (4) 20 cGy of 1 GeV/n 56Fe ions; or (5) 1 Gy of 50 MeV protons followed within 15 min by 20 cGy of 1 GeV/n 56Fe ions. Mice were euthanized at 5–9 months post transplant, their spleen and bone marrow harvested, and analyzed by flow cytometry to ascertain whether these irradiation schemes had altered HSC engraftment potential and/or differentiative capacity.
Figure 5 shows that treatment of human HSC with 137Cs γ-rays, 50 MeV protons, 1 GeV/n 56Fe ions, or protons followed 15 min later by iron ions (mix) markedly altered the ability of the human HSC to mediate long-term (5‐6 months) engraftment. Interestingly, the mice transplanted with unirradiated human HSC had the lowest overall engraftment, whereas those transplanted with human HSC exposed to 56Fe ions exhibited the highest engraftment levels.
Figure 5.
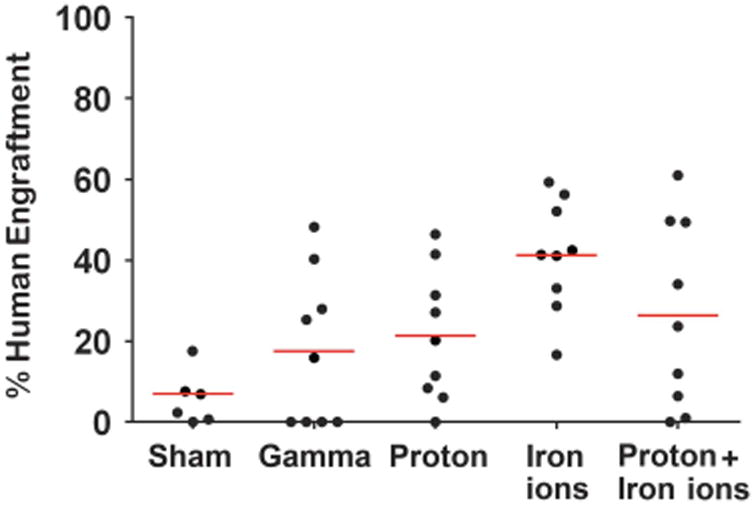
Effect of proton, iron ion and mixed-field proton/iron ion irradiation on long-term engraftment of human HSC in NSG mice: To assess the in vivo functional impact of direct exposure of human HSC to mission-relevant doses of simulated SEP and/or GCR radiation, human BM-derived CD34+ cells were exposed to: (1) monoenergetic protons (1 Gy, 50 MeV); (2) iron ions (20 cGy, 1 GeV/n); or (3) sequential protons followed 15 min later by 56Fe ions at NSRL Upon return to WFIRM, they were transplanted intravenously into busulfan-conditioned NSG mice. Mice were euthanized at 5‐7 months post transplant, and their long bones collected, homogenized and analyzed by flow cytometry with anti-CD59 (which has been shown to be the most sensitive and specific means of detecting human cells in immunodeficient mice86) to assess overall levels of marrow engraftment.
In addition to affecting overall levels of engraftment, irradiation with protons, 56Fe ions or sequential protons+56Fe ions also markedly skewed the human HSC differentiative potential after 5–9 months. The relative lineage distributions of engrafted human cells in the marrow of the transplanted mice appears in Figure 6a. As can be seen, commitment to the erythroid lineage was significantly impaired following exposure to all radiation types tested. Myeloid commitment was largely unaffected, but there was a significant expansion of the T-lymphoid and B-lymphoid lineages. The relative lineage distributions of engrafted human cells in the spleens of transplanted mice are shown in Figure 6b, where we likewise observed a reduction in erythropoiesis and expansion of the T and B lineages. However, there was also a marked expansion of the myeloid lineage as a result of exposing HSC to each radiation scheme prior to transplant. Detailed analyses of these lineage distributions are shown in Figures 6c and d for marrow and spleen, respectively.
Figure 6.
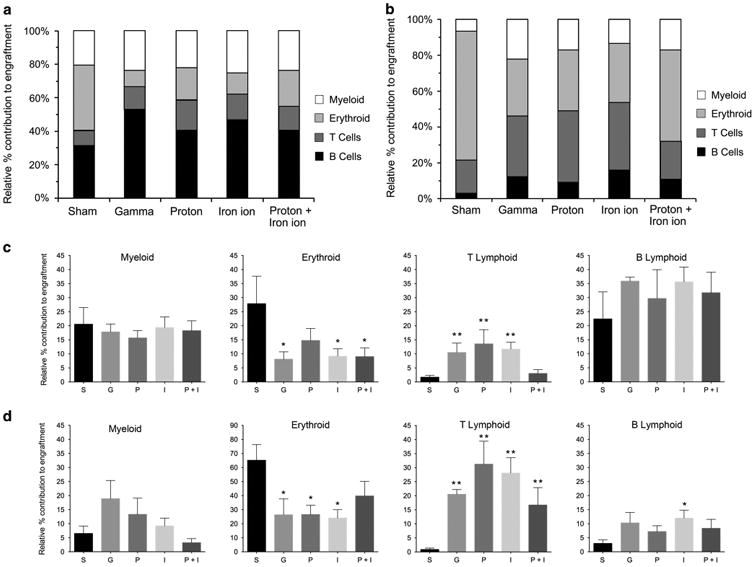
Effect of proton, iron ion and mixed-field proton/iron ion irradiation on differentiation/lineage commitment of human HSC in bone marrow or spleen of NSG mice: To assess the in vivo impact direct exposure of human HSC to mission-relevant doses of simulated SEP and/or GCR radiation has on differentiation/lineage commitment, human BM-derived CD34+ cells were exposed to the following: (1) monoenergetic protons (1 Gy, 50 MeV); (2) 56Fe ions (20 cGy, 1 GeV/n); or (3) sequential protons followed 15 min later by 56Fe ions at NSRL. Upon return to WFIRM, they were transplanted intravenously into busulfan-conditioned NSG mice. Mice were euthanized at 5–7 months post transplant, and then their long bones were collected and homogenized, and their spleens were collected and homogenized. Each were analyzed by flow cytometry with a panel of human-specific antibodies to CD antigens to identify various hematopoietic lineages within the recipient mice. (a) Relative lineage distribution of human cell engraftment in BM of recipient mice. (b) Relative lineage distribution of human cell engraftment in spleen of recipient mice. (c) Detailed analysis of lineage distribution of human cell engraftment in BM in response to each irradiation scheme. Data are displayed as mean ± s.e.m. (d) Detailed analysis of lineage distribution of human cell engraftment in spleen in response to each irradiation scheme. Data are displayed as mean ± s.e.m. For statistical analyses in c and d, lineage engraftment with human HSC exposed to each irradiation scheme was compared with lineage engraftment with sham-irradiated human HSC from the same donor. *P < 0.05; ** P < 0.01. Data outliers were removed for analysis in GraphPad Prism 6 setting Q = 2%.
In addition to the lineage skewing, two of the recipients of 56Fe ion-irradiated human HSC exhibited dramatic splenomegaly with spleens reaching ∼ 30× the normal size (Figure 7a). Unfortunately, the spleens from these mice were homogenized for flow cytometry, precluding us from performing histopathology. To better delineate the cause of the marked splenomegaly, we transplanted an additional cohort of NSG mice with HSC (from a different healthy human donor) exposed to the same irradiation schemes (n = 5 mice/group). Remarkably, as in the first set of transplants, the only mice in this second cohort exhibiting splenomegaly were those transplanted with human HSC exposed to 20 cGy of 56Fe ions, with 3/5 mice developing splenomegaly. As such, the overall incidence of splenomegaly in mice repopulated with 56Fe ion-irradiated human HSC in these studies was 5/14 (∼36%), whereas no mice in any other group developed even mild splenomegaly, including the sequential proton and iron ion irradiation groups.
Figure 7.
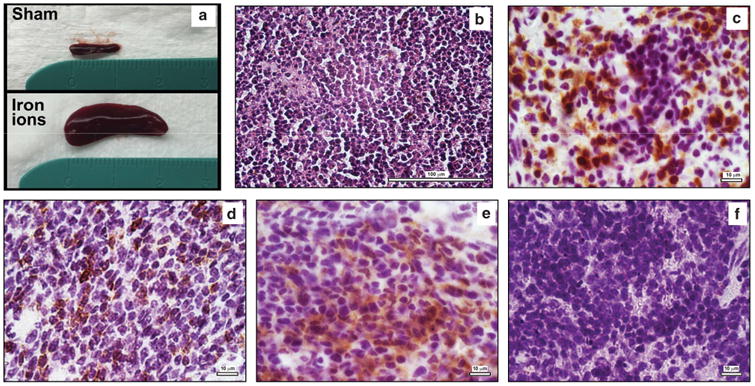
Pronounced splenomegaly and development of human T-ALL in NSG recipients of 56Fe ion-irradiated human HSC: At the time of euthanasia, 5 of the 14 NSG mice that were repopulated with human HSC that had been exposed to a mission-relevant dose of 56Fe ions (20 cGy, 1 GeV/n) exhibited marked splenomegaly. (a) The spleen from one of these mice, being compared with the spleen from a control mouse transplanted with sham-irradiated human HSC. This particular recipient's spleen was ∼ 30 times the normal volume; (b) H&E staining of the spleen from one of the mice transplanted with 56Fe ion-exposed human HSC, displaying overpopulation with a monotonous, rounded, lymphoid-like cell population; (c) Staining of the spleen from this mouse with anti-human CD3. (d) Staining of the spleen from this mouse with anti-human CD7; (e) Staining of the spleen from this mouse with anti-TdT; (f) Absence of any staining in the spleen from an untransplanted (negative control) NSG mouse with anti-human CD3.
To ascertain the cause of splenomegaly, spleens from the second cohort of mice were analyzed at 6–9 months post transplant by histopathology and immunophenotyping. As can be seen in Figure 7, which shows representative sections from the spleen of one of the splenomegalic mice (histology/staining results were similar in all three mice exhibiting splenomegaly), the spleen exhibited an abnormal morphology, populated largely by cells with a rounded, highly monotonous appearance consistent with lymphoid cells (Figure 7b). Immunohistochemistry with human-specific antibodies confirmed that the vast majority of the cells populating the spleen were human, and they expressed CD3 (Figure 7c) and CD7 (Figure 7d). Many of these cells also expressed terminal deoxynucleotidyl transferase (TdT; Figure 7e), a marker frequently associated clinically with human lymphocytic leukemias.37–40 An untransplanted NSG mouse spleen was also stained with the same panel of antibodies to verify their specificity. No staining was seen with anti-human CD3 (Figure 7f) or any of the other antibodies employed (data not shown). On the basis of the observed histopathology and immunophenotyping, a team of clinical hematopathologists concurred that human T-ALL appears to have developed in these mice. To our knowledge, this represents the first report of hematological malignancy as a result of exposing human HSC to mission-relevant doses of HZE ions present in the space radiation environment.
Discussion
The present studies provide compelling in vitro and in vivo evidence that human hematopoiesis is negatively affected by exposure of HSC to simulated SEP (protons) or GCR (HZE iron ions) space radiation. The markedly deleterious effect of a relatively high dose of lower-energy protons typical of a potential short-duration SPE on the immediate ability of human HSC to give rise to cells of the T-lymphoid and B-lymphoid lineages is of particular note, given the large amount of published work showing that astronauts exhibit alterations in immune repertoire and function.41–52 Although it is likely that additional spaceflight stressors (for example, microgravity) have an important role, our in vitro data support the notion that short-term immune alterations observed in astronauts may be due, in part, to radiation induced reductions in the ability of their HSC to differentiate along the lymphoid lineage. By contrast, following 5–9 months of expansion in immunocompromised mice, the lymphoid lineages predominated in the marrow and spleen, with T-cell lineage output specifically becoming significantly increased. However, our data suggest that low-dose HZE ion exposures also significantly increase the long-term risk for HSC-derived hematological malignancy development. A possible mechanistic explanation for these findings comes from studies by DeGregori's group,53–56 who showed that exposure of HSC to radiation reduces their colony-forming potential in vitro, but also selects for clones with mutations that confer enhanced survival/proliferative potential (for example, Tp53 and Notch). Moreover, when transplanted in vivo, these cells repopulate very efficiently and give rise to significantly higher rates of hematological malignancies than unirradiated cells. Other studies demonstrate that IR exposure alters HSC functionality by multiple mechanisms.57 When combined with other confounding space flight conditions, these cells may fail to generate a functionally competent immune system to effectively monitor and fight challenges such as detecting pre-leukemic transformants.58
The pronounced differences in HSC proliferative potential/lineage commitment we observed for the low LET gamma and proton irradiations versus high-LET iron ion irradiations originate from microdosimetric considerations of the numbers of particle traversals per cell nucleus for each irradiation scheme. Using a surface area estimate of ∼30 μm2 for HSC nuclei,59 the mean number of charged particles/nucleus can be calculated from the respective fluences of protons and iron ions (∼5.3 × 106 and 4.4 ×104 particles/cm2•cGy), respectively. For 50 MeV proton irradiations, HSC nuclei receive ∼ 1.6 protons/cGy with the 100 cGy total dose equating to a mean of 160 particles/HSC nucleus. These values are ∼ 620-fold lower for the high-LET 56Fe ion irradiations, considering the ∼ 124-fold difference in LET and fivefold lower dose used, with HSC nuclei receiving ∼ 0.013 ions/cGy and a mean of 0.26 particles/nucleus for the total 20 cGy dose, with the numbers of particle traversals/nucleus following a Poisson distribution (that is, ∼77% of nuclei do not experience any iron ion traversals). It should be noted that whereas only ∼23% of the HSC population are exposed to ≥ 1 iron ions, all cells in these cultures will be exposed to low LET δ-rays in the iron ion's penumbra. With the LET value for 137Cs γ-ray-induced photoelectrons similar to that of 50 MeV protons (0.91 versus 1.22 keV/μm in H2O, respectively), HSC experience similar numbers of nuclear traversals by photoelectrons during γ-ray irradiation.60
In the case of the γ-ray and proton exposures, DNA damage, including DSBs and highly localized clustered lesions, are generated by stochastic energy depositions from the hundreds to thousands of particle traversals that are distributed randomly across the nuclear volume and throughout the genome. These IR-induced DSBs activate ATM and DNA-PK-mediated DNA damage responses (DDR) within seconds to minutes, generating cytologically visible repair foci encompassing hundreds of kilobases of modified chromatin in each direction of the break site that serves to recruit end-processing and DSB repair proteins to restitute the break.61‐64 In the case of ∼ 1‐2 high-LET iron ion traversals, nearly all of the energy deposited by their tracks and associated δ-ray penumbras involve more limited nuclear volumes and genomic regions located directly along the particle track65,66 with much higher local ionization densities and resulting DSBs and clustered lesions.67 HZE ion-induced DSBs have been shown to be more refractory and slower to repair than low LET IR-induced DSBs, with a greater proportion being preferentially repaired by Rad51-mediated homologous recombinational repair (HRR),68,69 yielding much higher levels of simple and complex chromosomal rearrangements post irradiation.70 Interestingly, two recent studies demonstrate that HSC have reduced DSB repair capacities compared with mature T-cells. A study by Vandevoorde et al.71 showed significantly higher residual levels of low-dose X-ray-induced H2AX/53BP1 foci at 24 h and micronuclei in CD34+ HSC. In a study by Kraft et al.,72 non-homologous end-joining (NHEJ) and HRR capacities were shown to be significantly lower in human HSC compared with differentiated PBLs, which the authors attributed to reduced expression of NHEJ and HRR genes in HSC as well as a preference for repairing DSBs via microhomology-mediated end-joining.
Although a previous study by Zhou et al.26 measuring in vitro primary human fibroblast neoplastic transformation following sequential proton and iron ion exposures reported highly synergistic effects for the same mixed-field regimen used herein (protons followed 15 min later by iron ions), we identified this scheme as being partially radioprotective for human HSC by both in vitro and in vivo assays. When considering the less-than-additive effects we observe for the sequential exposures, it is likely that the initial irradiation with ∼ 500–1000 protons/nucleus fully activates the cell's DDR, with many proteins still engaged in DNA damage signaling/repair 15 min post-IR.73 This robust DDR activation readies the cell for subsequent irradiation with 1–2 densely ionizing HZE particles at a time of maximal DDR activation. This phenomenon of an initial ‘priming’ dose reducing the effective ness of a subsequent ‘challenge’ dose of radiation to induce a specific biological effect is termed the radiation ‘adaptive response’ and has been documented extensively for low LET X-rays and γ-rays (reviewed in refs 74–76). Effects of mixed-field proton/iron ion irradiations on cellular adaptive responses were examined by Elmore et al.77 using an in vitro HeLa X human fibroblast hybrid cell transformation assay, but interestingly was only shown to be radioprotective when 10 cGy 1 GeV/n iron ions was delivered prior to 1 Gy of 1 GeV protons, (that is, the reverse order of our irradiations). More recently, Buonanno et al.78 reported significant radioprotection for chromosomal damage (micronuclei) induction in primary human fibroblasts exposed to 20 cGy of 50 MeV or 1 GeV protons followed by 50 cGy of 1 GeV/n iron ions, with the radioprotective effect persisting for 24 h.
Our findings from the WES experiments also raise the troubling possibility that astronauts on more extended deep space missions may be further at risk of developing hematological malignancies, as genetic mutations induced in HSC by exposure to SEP/GCR radiation could potentially lead directly to leukemic transformation. The preponderance of unique SNVs identified in hematopoiesis-related genes we identified in cells exposed to protons, iron ions and the sequential mixed-field regimen suggests that these charged particle radiations induced mutations more effectively than γ-rays, with some likely imparting a selective growth advantage for subsequent enhanced in vivo expansion, similar to DeGregori's work.53‐56 Further studies are needed to clarify what influence HSC chromatin structure, nuclear organization and DNA repair capacities have on the generation of SNVs and chromosomal aberrations along the tracks of these charged particles,79 and identify the HSC mutations responsible for and/or associated with the cases of iron ion-induced T-ALL identified in the current study. This research will be very valuable for gaining molecular insights on HZE ion-induced leukemia initiation and promotion in human cells, such has been done previously for HZE ion-induced murine leukemias and solid cancers,80,81 and also for providing information on potential risks of secondary treatment-related cancers following proton and high-LET carbon ion radiotherapy.82
Another important finding from these studies from the standpoint of astronaut safety is the pronounced effect of single and mixed-field charged particle irradiation on erythroid colony-forming potential. Our in vivo results are intriguing given the wealth of data on so-called ‘spaceflight anemia’.83 In our studies, individual or sequential exposures of HSC to protons and 56Fe ions significantly reduced erythroid output in most cases by ∼50-75%. Our results thus provide a possible mechanism that may contribute to the observed reduction in red cell numbers during manned spaceflight.
Despite the inherent technical limitations of using a xenogeneic transplant system (detailed in Supplementary Materials),84,85 the immunocompromised mouse model enabled us to demonstrate, for the first time, that human HSC can be leukemogenically transformed by HZE ions typical of the space radiation environment, and that this process may be initiated by a single high-LET ion traversal. Furthermore, we establish that prior high-dose proton irradiation is radioprotective for T-ALL development in this scenario.
In conclusion, these studies represent a critical step forward for refining current NASA risk calculations for space radiation-induced human leukemogenesis and carcinogenesis. We provide specific information on the deleterious effects of two typical space radiations on the proliferative and leukemogenic potential of the human hematopoietic system. Additional research is required to identify the duration and extent of HSC radioprotection stimulated by proton irradiation and the dose range required to activate this adaptive response, especially considering the very low fluxes of protons and HZE ions in space and the low likelihood that a given astronaut HSC may likely not be afforded any radioprotective benefit prior to being traversed by a single highly deleterious HZE ion. This work also highlights the need for additional studies examining other ion species present in the space radiation environment, particularly at lower, more space-relevant dose rates, and for further mechanistic studies to define molecular and cellular signaling pathways by which charged particle irradiation impairs human hematopoiesis, the results of which can help guide the development of appropriate physical and biomedical countermeasures.
Supplementary Material
Acknowledgments
This work was supported by grant #NNX13AB67G from National Aeronautics and Space Administration (NASA).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
References
- 1.Guo J, Zeitlin C, Wimmer-Schweingruber RF, Hassler DM, Ehresmann B, Kohler J, et al. MSL-RAD radiation environment measurements. Radiat Prot Dosimetry. 2015;166:290–294. doi: 10.1093/rpd/ncv297. [DOI] [PubMed] [Google Scholar]
- 2.Hassler DM, Zeitlin C, Wimmer-Schweingruber RF, Ehresmann B, Rafkin S, Eigenbrode JL, et al. Mars' surface radiation environment measured with the Mars Science Laboratory's Curiosity rover. Science. 2014;343:1244797. doi: 10.1126/science.1244797. [DOI] [PubMed] [Google Scholar]
- 3.Kohler J, Ehresmann B, Zeitlin C, Wimmer-Schweingruber RF, Hassler DM, Reitz G, et al. Measurements of the neutron spectrum in transit to Mars on the Mars Science Laboratory. Life Sci Space Res (Amst) 2015;5:6–12. doi: 10.1016/j.lssr.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Zeitlin C, Hassler DM, Cucinotta FA, Ehresmann B, Wimmer-Schweingruber RF, Brinza DE, et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science. 2013;340:1080–1084. doi: 10.1126/science.1235989. [DOI] [PubMed] [Google Scholar]
- 5.Durante M, Cucinotta FA. Physical basis of radiation protection in space travel. Rev Mod Phys. 2011;83:1245–1281. [Google Scholar]
- 6.Durante M, Cucinotta FA. Heavy ion carcinogenesis and human space exploration. Nat Rev Cancer. 2008;8:465–472. doi: 10.1038/nrc2391. [DOI] [PubMed] [Google Scholar]
- 7.Cucinotta FA. Review of NASA approach to space radiation risk assessments for Mars exploration. Health Phys. 2015;108:131–142. doi: 10.1097/HP.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 8.Cucinotta FA. Biophysics of NASA radiation quality factors. Radiat Prot Dosimetry. 2015;166:282–289. doi: 10.1093/rpd/ncv144. [DOI] [PubMed] [Google Scholar]
- 9.Cucinotta FA, Schimmerling W, Wilson JW, Peterson LE, Badhwar GD, Saganti PB, et al. Space radiation cancer risks and uncertainties for Mars missions. Radiat Res. 2001;156:682–688. doi: 10.1667/0033-7587(2001)156[0682:srcrau]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Badhwar GD. Shuttle radiation dose measurements in the International Space Station orbits. Radiat Res. 2002;157:69–75. doi: 10.1667/0033-7587(2002)157[0069:srdmit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Badhwar GD, Nachtwey DS, Yang TH. Radiation issues for piloted Mars mission. Adv Space Res. 1992;12:195–200. doi: 10.1016/0273-1177(92)90108-a. [DOI] [PubMed] [Google Scholar]
- 12.Bottollier-Depois JF, Chau Q, Bouisset P, Kerlau G, Plawinski L, Lebaron-Jacobs L. Assessing exposure to cosmic radiation during long-haul flights. Radiat Res. 2000;153:526–532. doi: 10.1667/0033-7587(2000)153[0526:aetcrd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Cucinotta FA. Once we know all the radiobiology we need to know, how can we use it to predict space radiation risks and achieve fame and fortune? Phys Med. 2001;17:5–12. [PubMed] [Google Scholar]
- 14.Nachtwey DS, Yang TC. Radiological health risks for exploratory class missions in space. Acta Astronaut. 1991;23:227–231. doi: 10.1016/0094-5765(91)90122-l. [DOI] [PubMed] [Google Scholar]
- 15.Petrov VM. Radiation risk during long-term spaceflight. Adv Space Res. 2002;30:989–994. doi: 10.1016/s0273-1177(02)00168-0. [DOI] [PubMed] [Google Scholar]
- 16.Todd P. Space radiation health: a brief primer. Gravit Space Biol Bull. 2003;16:1–4. [PubMed] [Google Scholar]
- 17.Townsend LW. Implications of the space radiation environment for human exploration in deep space. Radiat Prot Dosimetry. 2005;115:44–50. doi: 10.1093/rpd/nci141. [DOI] [PubMed] [Google Scholar]
- 18.Barcellos-Hoff MH, Blakely EA, Burma S, Fornace AJ, Jr, Gerson S, Hlatky L, et al. Concepts and challenges in cancer risk prediction for the space radiation environment. Life Sci Space Res (Amst) 2015;6:92–103. doi: 10.1016/j.lssr.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Simonsen LC, Wilson JW, Kim MH, Cucinotta FA. Radiation exposure for human Mars exploration. Health Phys. 2000;79:515–525. doi: 10.1097/00004032-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Cucinotta FA. A new approach to reduce uncertainties in space radiation cancer risk predictions. PLoS One. 2015;10:e0120717. doi: 10.1371/journal.pone.0120717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rapp D. Radiation Effects and Shielding Requirements in Human Missions to the Moon and Mars. MARS. 2006;2:46–71. [Google Scholar]
- 22.Rapp D. Human Missions to Mars: Enabling Technologies for Exploring the Red Planet. Springer Praxis Books; Berlin, Heidelberg: 2008. [Google Scholar]
- 23.Technical Evaluation of the NASA Model for Cancer Risk to Astronauts Due to Space Radiation. Washington (DC): 2012. [PubMed] [Google Scholar]
- 24.Cucinotta FA, Kim MH, Chappell LJ, Huff JL. How safe is safe enough? Radiation risk for a human mission to Mars. PLoS One. 2013;8:e74988. doi: 10.1371/journal.pone.0074988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hada M, Sutherland BM. Spectrum of complex DNA damages depends on the incident radiation. Radiat Res. 2006;165:223–230. doi: 10.1667/rr3498.1. [DOI] [PubMed] [Google Scholar]
- 26.Zhou G, Bennett PV, Cutter NC, Sutherland BM. Proton-HZE-particle sequential dual-beam exposures increase anchorage-independent growth frequencies in primary human fibroblasts. Radiat Res. 2006;166:488–494. doi: 10.1667/RR0596.1. [DOI] [PubMed] [Google Scholar]
- 27.Raber J, Allen AR, Sharma S, Allen B, Rosi S, Olsen RH, et al. Effects of Proton and Combined Proton and (56)Fe Radiation on the Hippocampus. Radiat Res. 2016;185:20–30. doi: 10.1667/RR14222.1. [DOI] [PubMed] [Google Scholar]
- 28.Nzabarushimana E, Prior S, Miousse IR, Pathak R, Allen AR, Latendresse J, et al. Combined exposure to protons and (56)Fe leads to overexpression of Il13 and reactivation of repetitive elements in the mouse lung. Life Sci Space Res (Amst) 2015;7:1–8. doi: 10.1016/j.lssr.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramadan SS, Sridharan V, Koturbash I, Miousse IR, Hauer-Jensen M, Nelson GA, et al. A priming dose of protons alters the early cardiac cellular and molecular response to (56)Fe irradiation. Life Sci Space Res (Amst) 2016;8:8–13. doi: 10.1016/j.lssr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadhim MA, Lorimore SA, Townsend KM, Goodhead DT, Buckle VJ, Wright EG. Radiation-induced genomic instability: delayed cytogenetic aberrations and apoptosis in primary human bone marrow cells. Int J Radiat Biol. 1995;67:287–293. doi: 10.1080/09553009514550341. [DOI] [PubMed] [Google Scholar]
- 31.Kadhim MA, Wright EG. Radiation-induced transmissable chromosomal instability in haemopoietic stem cells. Adv Space Res. 1998;22:587–596. doi: 10.1016/s0273-1177(98)00081-7. [DOI] [PubMed] [Google Scholar]
- 32.Kato K, Omori A, Kashiwakura I. Radiosensitivity of human haematopoietic stem/progenitor cells. J Radiol Prot. 2013;33:71–80. doi: 10.1088/0952-4746/33/1/71. [DOI] [PubMed] [Google Scholar]
- 33.Nagayama H, Misawa K, Tanaka H, Ooi J, Iseki T, Tojo A, et al. Transient hematopoietic stem cell rescue using umbilical cord blood for a lethally irradiated nuclear accident victim. Bone Marrow Transplant. 2002;29:197–204. doi: 10.1038/sj.bmt.1703356. [DOI] [PubMed] [Google Scholar]
- 34.Cengel KA, Diffenderfer ES, Avery S, Kennedy AR, McDonough J. Using electron beam radiation to simulate the dose distribution for whole body solar particle event proton exposure. Radiat Environ Biophys. 2010;49:715–721. doi: 10.1007/s00411-010-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reems JA, Hall KM, Gebru LH, Taber G, Rich IN. Development of a novel assay to evaluate the functional potential of umbilical cord blood progenitors. Transfusion. 2008;48:620–628. doi: 10.1111/j.1537-2995.2007.01586.x. [DOI] [PubMed] [Google Scholar]
- 36.Hayakawa J, Hsieh MM, Uchida N, Phang O, Tisdale JF. Busulfan produces efficient human cell engraftment in NOD/LtSz-Scid IL2Rgamma(null) mice. Stem Cells. 2009;27:175–182. doi: 10.1634/stemcells.2008-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L, Zhang JS, Cui D, Liu DG. Cytological diagnosis of T lymphoblastic leukemia/lymphoma in patients with pleural effusion as an initial clinical presentation: two case reports of an algorithmic approach using complete immunohistochemical phenotyping. Acta Cytol. 2015;59:485–492. doi: 10.1159/000443760. [DOI] [PubMed] [Google Scholar]
- 38.Kim DY, Park HS, Choi EJ, Lee JH, Lee JH, Jeon M, et al. Immunophenotypic markers in adult acute lymphoblastic leukemia: the prognostic significance of CD20 and TdT expression. Blood Res. 2015;50:227–234. doi: 10.5045/br.2015.50.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lahjouji A, Bachir F, Bennani S, Quessar A, Amzazi S. The immunophenotype of adult T acute lymphoblastic leukemia in Morocco. Exp Oncol. 2015;37:64–69. [PubMed] [Google Scholar]
- 40.Subashchandrabose P, Ramiah Madanagopaal L, Subba Rao TM. Diagnosis and classification of acute leukemia in bone marrow trephine biopsies, utility of a selected panel of minimal immunohistochemical markers. Int J Hematol Oncol Stem Cell Res. 2016;10:138–146. [PMC free article] [PubMed] [Google Scholar]
- 41.Cogoli A. The effect of space flight on human cellular immunity. Environ Med. 1993;37:107–116. [PubMed] [Google Scholar]
- 42.Gmunder FK, Konstantinova I, Cogoli A, Lesnyak A, Bogomolov W, Grachov AW. Cellular immunity in cosmonauts during long duration spaceflight on board the orbital MIR station. Aviat Space Environ Med. 1994;65:419–423. [PubMed] [Google Scholar]
- 43.Chapes SK, Simske SJ, Forsman AD, Bateman TA, Zimmerman RJ. Effects of space flight and IGF-1 on immune function. Adv Space Res. 1999;23:1955–1964. doi: 10.1016/s0273-1177(99)00456-1. [DOI] [PubMed] [Google Scholar]
- 44.Sonnenfeld G. Immune responses in space flight. Int J Sports Med. 1998;19:S195–S202. doi: 10.1055/s-2007-971992. discussion S202-194. [DOI] [PubMed] [Google Scholar]
- 45.Sonnenfeld G, Shearer WT. Immune function during space flight. Nutrition. 2002;18:899–903. doi: 10.1016/s0899-9007(02)00903-6. [DOI] [PubMed] [Google Scholar]
- 46.Stowe RP, Sams CF, Pierson DL. Effects of mission duration on neuroimmune responses in astronauts. Aviat Space Environ Med. 2003;74:1281–1284. [PubMed] [Google Scholar]
- 47.Taylor GR. Immune changes in humans concomitant with space flights of up to 10 days duration. Physiologist. 1993;36:S71–S74. [PubMed] [Google Scholar]
- 48.Taylor GR, Janney RP. In vivo testing confirms a blunting of the human cell-mediated immune mechanism during space flight. J Leukoc Biol. 1992;51:129–132. doi: 10.1002/jlb.51.2.129. [DOI] [PubMed] [Google Scholar]
- 49.Walther I, Cogoli A, Pippia P, Meloni MA, Cossu G, Cogoli M, et al. Human immune cells as space travelers. Eur J Med Res. 1999;4:361–363. [PubMed] [Google Scholar]
- 50.Crucian B, Stowe R, Mehta S, Uchakin P, Quiriarte H, Pierson D, et al. Immune system dysregulation occurs during short duration spaceflight on board the space shuttle. J Clin Immunol. 2013;33:456–465. doi: 10.1007/s10875-012-9824-7. [DOI] [PubMed] [Google Scholar]
- 51.Mehta SK, Crucian BE, Stowe RP, Simpson RJ, Ott CM, Sams CF, et al. Reactivation of latent viruses is associated with increased plasma cytokines in astronauts. Cytokine. 2013;61:205–209. doi: 10.1016/j.cyto.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 52.Mehta SK, Laudenslager ML, Stowe RP, Crucian BE, Sams CF, Pierson DL. Multiple latent viruses reactivate in astronauts during Space Shuttle missions. Brain Behav Immun. 2014;41:210–217. doi: 10.1016/j.bbi.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 53.Fleenor CJ, Higa K, Weil MM, DeGregori J. Evolved cellular mechanisms to respond to genotoxic insults: implications for radiation-induced hematologic malignancies. Radiat Res. 2015;184:341–351. doi: 10.1667/RR14147.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fleenor CJ, Marusyk A, DeGregori J. Ionizing radiation and hematopoietic malignancies: altering the adaptive landscape. Cell Cycle. 2010;9:3005–3011. doi: 10.4161/cc.9.15.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marusyk A, Casas-Selves M, Henry CJ, Zaberezhnyy V, Klawitter J, Christians U, et al. Irradiation alters selection for oncogenic mutations in hematopoietic progenitors. Cancer Res. 2009;69:7262–7269. doi: 10.1158/0008-5472.CAN-09-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marusyk A, Porter CC, Zaberezhnyy V, DeGregori J. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol. 2010;8:e1000324. doi: 10.1371/journal.pbio.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shao L, Luo Y, Zhou D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid Redox Signal. 2014;20:1447–1462. doi: 10.1089/ars.2013.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crucian B, Stowe RP, Mehta S, Quiriarte H, Pierson D, Sams C. Alterations in adaptive immunity persist during long-duration spaceflight. Microgravity. 2015;1:1–10. doi: 10.1038/npjmgrav.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng Y, Zhang N, Jacobs KM, Jiang W, Yang LV, Li Z, et al. Polarization imaging and classification of Jurkat T and Ramos B cells using a flow cytometer. Cytometry A. 2014;85:817–826. doi: 10.1002/cyto.a.22504. [DOI] [PubMed] [Google Scholar]
- 60.Task Group on Radiation Quality Effects in Radiological Protection CoREICoRP. Relative biological effectiveness (RBE), quality factor (Q), and radiation weighting factor (w(R)) Ann ICRP. 2003;33:1–117. doi: 10.1016/s0146-6453(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 61.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 62.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 63.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 64.Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- 65.Cucinotta FA, Nikjoo H, Goodhead DT. Model for radial dependence of frequency distributions for energy imparted in nanometer volumes from HZE particles. Radiat Res. 2000;153:459–468. doi: 10.1667/0033-7587(2000)153[0459:mfrdof]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 66.Mirsch J, Tommasino F, Frohns A, Conrad S, Durante M, Scholz M, et al. Direct measurement of the 3-dimensional DNA lesion distribution induced by energetic charged particles in a mouse model tissue. Proc Natl Acad Sci USA. 2015;112:12396–12401. doi: 10.1073/pnas.1508702112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopez Perez R, Best G, Nicolay NH, Greubel C, Rossberger S, Reindl J, et al. Superresolution light microscopy shows nanostructure of carbon ion radiation-induced DNA double-strand break repair foci. FASEB J. 2016;30:2767–2776. doi: 10.1096/fj.201500106R. [DOI] [PubMed] [Google Scholar]
- 68.Rall M, Kraft D, Volcic M, Cucu A, Nasonova E, Taucher-Scholz G, et al. Impact of charged particle exposure on homologous DNA double-strand break repair in human blood-derived cells. Front Oncol. 2015;5:250. doi: 10.3389/fonc.2015.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saha J, Wilson P, Thieberger P, Lowenstein D, Wang M, Cucinotta FA. Biological characterization of low-energy ions with high-energy deposition on human cells. Radiat Res. 2014;182:282–291. doi: 10.1667/RR13747.1. [DOI] [PubMed] [Google Scholar]
- 70.Asaithamby A, Hu B, Chen DJ. Unrepaired clustered DNA lesions induce chromosome breakage in human cells. Proc Natl Acad Sci USA. 2011;108:8293–8298. doi: 10.1073/pnas.1016045108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vandevoorde C, Vral A, Vandekerckhove B, Philippe J, Thierens H. Radiation sensitivity of human CD34(+) cells versus peripheral blood T lymphocytes of newborns and adults: DNA repair and mutagenic effects. Radiat Res. 2016;185:580–590. doi: 10.1667/RR14109.1. [DOI] [PubMed] [Google Scholar]
- 72.Kraft D, Rall M, Volcic M, Metzler E, Groo A, Stahl A, et al. NF-kappaB-dependent DNA damage-signaling differentially regulates DNA double-strand break repair mechanisms in immature and mature human hematopoietic cells. Leukemia. 2015;29:1543–1554. doi: 10.1038/leu.2015.28. [DOI] [PubMed] [Google Scholar]
- 73.Wilson PF, Nham PB, Urbin SS, Hinz JM, Jones IM, Thompson LH. Inter-individual variation in DNA double-strand break repair in human fibroblasts before and after exposure to low doses of ionizing radiation. Mutat Res. 2010;683:91–97. doi: 10.1016/j.mrfmmm.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 74.de Toledo SM, Buonanno M, Li M, Asaad N, Qin Y, Gonon G, et al. The impact of adaptive and non-targeted effects in the biological responses to low dose/low fluence ionizing radiation: the modulating effect of linear energy transfer. Health Phys. 2011;100:290–292. doi: 10.1097/HP.0b013e31820832d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feinendegen LE. Quantification of adaptive protection following low-dose irradiation. Health Phys. 2016;110:276–280. doi: 10.1097/HP.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 76.Wolff S. The adaptive response in radiobiology: evolving insights and implications. Environ Health Perspect. 1998;106:277–283. doi: 10.1289/ehp.98106s1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elmore E, Lao XY, Kapadia R, Swete M, Redpath JL. Neoplastic transformation in vitro by mixed beams of high-energy iron ions and protons. Radiat Res. 2011;176:291–302. doi: 10.1667/rr2646.1. [DOI] [PubMed] [Google Scholar]
- 78.Buonanno M, De Toledo SM, Howell RW, Azzam EI. Low-dose energetic protons induce adaptive and bystander effects that protect human cells against DNA damage caused by a subsequent exposure to energetic iron ions. J Radiat Res. 2015;56:502–508. doi: 10.1093/jrr/rrv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kraft D, Ritter S, Durante M, Seifried E, Fournier C, Tonn T. Transmission of clonal chromosomal abnormalities in human hematopoietic stem and progenitor cells surviving radiation exposure. Mutat Res. 2015;777:43–51. doi: 10.1016/j.mrfmmm.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 80.Steffen LS, Bacher JW, Peng Y, Le PN, Ding LH, Genik PC, et al. Molecular characterisation of murine acute myeloid leukaemia induced by 56Fe ion and 137Cs gamma ray irradiation. Mutagenesis. 2013;28:71–79. doi: 10.1093/mutage/ges055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weil MM, Ray FA, Genik PC, Yu Y, McCarthy M, Fallgren CM, et al. Effects of 28Si ions, 56Fe ions, and protons on the induction of murine acute myeloid leukemia and hepatocellular carcinoma. PLoS One. 2014;9:e104819. doi: 10.1371/journal.pone.0104819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laprie A, Hu Y, Alapetite C, Carrie C, Habrand JL, Bolle S, et al. Paediatric brain tumours: A review of radiotherapy, state of the art and challenges for the future regarding protontherapy and carbontherapy. Cancer Radiother. 2015;19:775–789. doi: 10.1016/j.canrad.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 83.Smith SM. Red blood cell and iron metabolism during space flight. Nutrition. 2002;18:864–866. doi: 10.1016/s0899-9007(02)00912-7. [DOI] [PubMed] [Google Scholar]
- 84.Willey CD, Gilbert AN, Anderson JC, Gillespie GY. Patient-derived xenografts as a model system for radiation research. Semin Radiat Oncol. 2015 Oct;25:273–280. doi: 10.1016/j.semradonc.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Futrega K, Lott WB, Doran MR. Direct bone marrow HSC transplantation enhances local engraftment at the expense of systemic engraftment in NSG mice. Sci Rep. 2016;6:23886. doi: 10.1038/srep23886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Varga NL, Barcena A, Fomin ME, Muench MO. Detection of human hematopoietic stem cell engraftment in the livers of adult immunodeficient mice by an optimized flow cytometric method. Stem Cell Stud. 2010;1 doi: 10.4081/scs.2010.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


