Abstract
Rationale
Preclinical studies consistently report that aerobic exercise decreases drug self-administration and other forms of drug-seeking behavior; however, relatively few studies have examined other types of physical activity.
Objectives
The purpose of the present study was to examine the effects of resistance exercise (i.e., strength training) on heroin self-administration and mRNA expression of genes known to mediate opioid reinforcement and addictive behavior in the nucleus accumbens (NAc) of heroin-exposed rats.
Methods
Female rats were obtained during late adolescence and divided into two groups. Resistance exercise rats were trained to climb a vertical ladder wearing a weighted vest; sedentary control rats were placed repeatedly on the ladder oriented horizontally on its side. All rats were implanted with intravenous catheters and trained to self-administer heroin on a fixed ratio (FR1) schedule of reinforcement. mRNA expression in the NAc core and shell was examined following behavioral testing.
Results
Resistance exercise significantly decreased heroin self-administration, resulting in a downward shift in the dose-effect curve. Resistance exercise also reduced mRNA expression for mu opioid receptors and dopamine D1, D2, and D3 receptors in the NAc core. Resistance exercise increased mRNA expression of dopamine D5 receptors in the NAc shell and increased mRNA expression of brain-derived neurotrophic factor (exons I, IIB, IIC, IV, VI, IX) in the NAc core.
Conclusions
These data indicate that resistance exercise decreases the positive reinforcing effects of heroin and produces changes in opioid and dopamine systems in the NAc of heroin-exposed rats.
Keywords: heroin, resistance exercise, strength training, rats, dopamine, nucleus accumbens
Introduction
Preclinical studies report that physical activity in the form of aerobic exercise decreases drug self-administration and other measures of drug-seeking behavior. For instance, running in an activity wheel or on a treadmill reliably decreases the self-administration of nicotine, cocaine, methamphetamine, morphine, and heroin (Hosseini et al. 2009; Lacy et al. 2014; Miller et al. 2012; Sanchez et al. 2015; Smith and Pitts 2012; Smith and Witte 2012) and reliably decreases the resumption of drug seeking after a period of abstinence (Lynch et al. 2010; Ogbonmwan et al. 2015; Sanchez et al. 2013; Smith et al. 2012; Sobieraj et al. 2016; Zlebnik et al. 2010). Moreover, aerobic exercise produces changes in central dopamine and opioid systems, which may explain these running-induced reductions in drug seeking and drug intake (de Oliveira et al. 2010; Droste et al. 2006; Greenwood et al. 2011; Houghten et al. 1986; Lynch et al. 2013; Sobieraj et al. 2016).
Relatively few studies have examined the effects of non-aerobic forms of physical activity on measures relevant to drug intake. We recently developed a model of resistance exercise (i.e., strength training) in which rats repeatedly climb a vertical ladder wearing a weighted vest. Using this model, we reported that resistance exercise decreases cocaine self-administration and reduces Bdnf mRNA expression in the nucleus accumbens (NAc), suggesting that strength training may serve as an alternative to aerobic exercise in activity-based interventions targeting substance abuse (Strickland et al. 2016).
The purpose of the present study was to examine the effects of resistance exercise on heroin self-administration and to examine associated changes in mRNA expression in the NAc of heroin-exposed rats. To this end, rats were trained to climb a vertical ladder wearing a weighted vest according to a 3-set pyramid regimen: eight climbs carrying 70% of their body weight (BW), six climbs carrying 85% of their BW, and four climbs carrying 100% of their BW. Control rats were repeatedly placed on the ladder oriented horizontally on its side to control for handling and exposure to the apparatus. Rats in both groups were implanted with intravenous catheters and trained to self-administer heroin on a fixed ratio (FR1) schedule of reinforcement. Following determination of the heroin dose-effect curve, rats were sacrificed and mRNA expression was measured in the NAc via quantitative real-time polymerase chain reaction (qRT-PCR). Genes coding for opioid receptors (mu, kappa, delta), dopamine receptors (D1, D2, D3, D4, D5), BDNF (exons I, IIA, IIB, IIC, IV, VI, IX), and the tyrosine receptor kinase B receptor were targeted for analysis due to their role in drug reinforcement and addiction (Collo et al. 2014; Contet et al. 2004; Ghitza et al. 2010; Li and Wolf 2015; Self 2004; Shippenberg et al. 2008). mRNA expression was examined in both the core and shell of the NAc because of the functional and anatomical differences between the two regions, particularly as they relate to behavior and addiction (Di Chiara, 2002; Jacobs et al. 2005; Zahm, 1999).
Materials and Methods
Animals
Female, Long-Evans rats were obtained from Charles River Laboratories (Raleigh, NC, USA) at 42 days of age and housed individually in a colony room on a 12-hr light/dark cycle (lights on: 0500). Females were selected because their slow growth rate limited the range and variability of the resistance loads needed for the multi-week study (determined as a percentage of body weight), and because of their underrepresentation in preclinical research (Clayton and Collins 2014; Klein et al. 2015). Food and drinking water were freely available in the home cage, except during a brief period of lever-press training (see below). All rats were maintained in accordance with the guidelines of the Animal Care and Use Committee of Davidson College.
Apparatus
Rats were trained to climb a vertical ladder, 60 cm tall with rungs spaced 2 cm apart. The apparatus was placed on a countertop approximately 80 cm above the floor. A three-sided enclosure (21 cm × 29 cm × 26 cm) painted black was located at the top of the apparatus. Climbing was encouraged by placing a 60-watt desk lamp below the apparatus and projecting it onto the ladder. Rats could escape the light by entering the enclosure at the top of the apparatus. Towels were placed on the floor beneath the apparatus to cushion the impact of falls, which occurred on less than 2% of climbs. The length of the ladder was such that six to eight dynamic movements of the hindlimbs were required to reach the top (see Strickland et al, 2016 for further description and photographs).
Heroin self-administration training and testing took place in operant conditioning chambers from Med Associates, Inc. (St. Albans, VT, USA). Each chamber contained a houselight, two response levers, two white stimulus lights located above the two levers, a food receptacle located between the two levers, a pellet dispenser located behind the forward wall, and an infusion pump located outside the chamber.
Resistance Exercise Training
A timeline of all experimental events is shown in Figure 1. One week after arrival, rats were randomly assigned to either the resistance exercise group (n = 16) or the sedentary control group (n = 16). Exercising rats were trained daily to climb the ladder while wearing weighted vests according to a 3-set pyramid regimen: eight climbs carrying 70% of their BW, six climbs carrying 85% of their BW, and four climbs carrying 100% of their BW. Rats rested 120 s between sets, but there was no delay between climbs within a set. If a rat failed to climb, a 0.5-s burst of compressed air was directed toward their hindquarters. Compressed air was needed to motivate climbing in all rats during the initial training sessions but was rarely used after the initial 2-3 days of training. One rat from the resistance exercise group was removed from the study because it failed to consistently climb; all other rats completed all climbs in every set in every session throughout the study. Resistance training occurred each evening (1700), approximately 16 hr prior to self-administration testing the following morning (0900). Sedentary rats were placed repeatedly on the ladder oriented on its side to control for enrichment- and handling-related effects. The 60-watt light was projected onto the apparatus during these control trials, but sedentary rats were not required to walk across the flat surface. Sedentary rats followed the same schedule and training parameters as the exercising rats to ensure they received the same number of trials and the same cumulative duration of exposure to the apparatus/light. Training continued in this manner six days per week for the duration of the five-week study.
Fig. 1. Timeline of experimental events.

Solid black line indicates resistance training
Lever-Press Training
Two weeks after arrival and one week after the beginning of resistance exercise training, rats were restricted to ~90% of their free-feeding body weight and trained to press a response lever using food reinforcement. In these sessions, each lever press was reinforced on a fixed ratio (FR1) schedule. Sessions terminated once 40 reinforcers were delivered or 2 hr elapsed, whichever occurred first. Training continued in this matter until five sessions were completed in which 40 reinforcers were obtained in each session. Rats were placed back on unrestricted feed immediately after lever-press training, such that food was continuously available in the home cages for the remainder of the study. Food consumption was not monitored.
Catheter Surgery
Three weeks after arrival and two weeks after the beginning of resistance exercise training, rats were anesthetized with a combination of ketamine (100 mg/kg, ip) and xylazine (8.0 mg/kg, ip) and implanted with intravenous catheters as described previously (Smith et al. 2008). Rats were given Ketoprofen (3.0 mg/kg, sc) after surgery as a post-operative analgesic and wounds were treated with a topical antibiotic for two days. Catheters were flushed daily with ticarcillin (20 mg/kg, iv) and heparinized saline to prevent infection and maintain patency, respectively. After seven days, ticarcillin was discontinued and only heparinized saline was used to maintain patency. Exercise training was suspended for three days following surgery.
Self-Administration Training and Testing
Three days after surgery, resistance exercise training resumed and self-administration training commenced. During training, rats were placed in operant conditioning chambers (different from those used during lever-press training with food) and trained to self-administer heroin on an FR1 schedule of reinforcement. Each session began with illumination of a houselight, illumination of the stimulus light above the active lever, and a noncontingent infusion of heroin. Each lever press produced an infusion of heroin (0.005 mg/kg/infusion) that was followed by a 20-s timeout in which the stimulus light was turned off and responding had no programmed consequences. The 20-s timeout was included to allow time for heroin distribution to brain and lessen the likelihood of unintended overdose. All sessions were 2 hr in duration and no limit was placed on the maximum number of infusions that could be obtained. Training continued in this manner for three consecutive days. Heroin self-administration testing was conducted in an identical manner, with the exception that the dose of heroin changed during each daily test session. Tests were conducted with four doses of heroin (0.001, 0.003, 0.01, and 0.03 mg/kg/infusion) and saline across five daily test sessions. Doses were tested in an irregular order with the stipulation that no more than two ascending or descending doses could be tested in a row. No decrements in climbing performance were observed during heroin self-administration (as would be evidenced by an increase in the use of compressed air to motivate climbing). Rats that lost catheter patency were removed from the study and did not contribute to the data analysis, resulting in the loss of two rats from the sedentary group.
Approximately 24 hr following the last self-administration session, and approximately 40 hr after the last resistance session, subjects were sacrificed via rapid decapitation. Brains were removed, flash frozen in isopentane (C5H12), and placed in a −80°C freezer for storage.
Quantitative real-time PCR (qRT-PCR)
mRNA expression was measured from the NAc core and shell using qRT-PCR. Tissue was collected via gross dissection and based on boundaries defined previously (Paxinos and Watson, 2013; see Online Resource 1). An RNeasy(R) Lipid Tissue Mini Kit (Qiagen, Valencia, CA) was used to isolate total RNA per the manufacturer’s protocol. The quantity and quality of the RNA were determined using a NanoDrop™ Spectrophotometer. cDNA templates were prepared using High-Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Applied Biosystems, Carlsbad, CA) according to the manufacturer’s protocol. The Applied Biosystem StepOnePlus™ real-time PCR system was used to perform qRT-PCR. Either Applied Biosystems TaqMan™ Gene Expression assays or SYBR™Green-Based Detection was used to detect PCR products of interest. The following TaqMan™ Gene Expression Assays were used: brain derived neurotrophic factor (Bdnf) I (Assay ID Rn01484924_m1); Bdnf IIA (Assay ID: Rn00560868_m1); Bdnf IIB (Assay ID: Rn01484926_m1); Bdnf IIC (Assay ID: Rn01484925_m1); dopamine receptor D2 (Drd-2) (AssayID: Rn00561126_m1); opioid receptor, delta 1 (Oprd1) (Assay ID: Rn00561699_m1); opioid receptor, kappa 1 (Oprk1) (Assay ID: Rn01448892_m1); opioid receptor, mu 1 (Oprm1) (Assay ID: Rn01430371_m1). All samples were normalized to either beta-2 microglobulin (B2m) (Assay ID: Rn00560865_m1) or Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) (Assay ID: Rn01775763_g1). Each specific TaqMan primer assay used only one housekeeping gene for normalization, and the same housekeeping gene was used for all samples tested across assays for a specific primer. Applied Biosystems validates and recommends a series of endogenous controls (or housekeeping genes), including B2m and Gapdh, that show constant RNA transcription level under different experimental conditions and are sufficiently abundant across different tissues and cell types. In addition, we prescreen housekeeping primers on our cDNA of interest to assure that expression levels do not vary across experimental conditions. For SYBR™Green-Based detection the following oligonucleotide primers were synthesized by Invitrogen (Carlsbad, CA): Bdnf exon IV, Bdnf exon VI and GAPDH (Schmidt et al. 2012); Bdnf exon IX (Koo et al. 2015); Drd-1, Drd-3, Drd-4, Drd-5 (Wingo et al. 2016); Trkb-1 (Li et al. 2015); and B2m (Walder et al. 2014). For TaqMan and SYBR Green based detection, target and endogenous control genes were measured in triplicate for each cDNA sample during each real-time run to avoid inter-sample variance. Each SYBR Green based qRT-PCR reaction was verified for a single PCR product of expected size with the disassociation melting curve stage. All genes of interest were analyzed with StepOne™ software using the comparative cycle thresholds (CT) method.
Online Resource 1. Boundaries of nucleus accumbens core and shell.
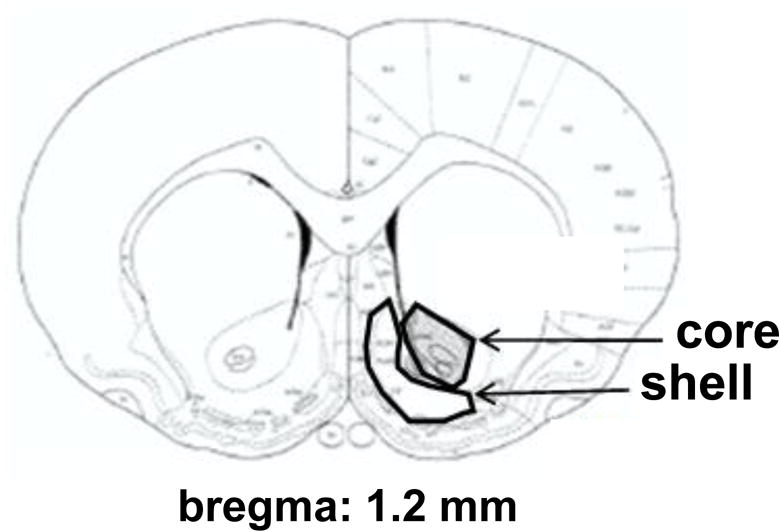
Tissue was collected via gross dissection and based on boundaries described by Paxinos and Watson (2013).
All primers used in SYBR™Green-Based Assays were prescreened in standard curve assays, using rat brain cDNA as a template to measure the amplification efficiency of a primer in a qPCR reaction. All primers tested showed 90-100% efficiency and melt-curve analysis, performed in conjunction with the amplification protocol, and showed only one product demonstrating that only specific products were amplified during the reaction. TaqMan® Gene Expression Assays, are commercial primers (Applied Biosystem) designed for gene expression analysis by RT-PCR and have been extensively validated before release.
Data Analysis
Heroin self-administration data were analyzed via two-way, mixed-factor ANOVA, with group (exercise vs. sedentary) serving as the between-subjects factor and dose serving as the repeated measure. Area under the curve (AUC) estimates were determined from the dose-response data using the Trapezoidal Rule; these estimates were then compared between groups using an independent-samples t-test.
Gene data were clustered by family and region and examined using independent-samples t-tests. For each sample, mRNA expression was first normalized to a reference sample. The reference sample was equal to 1 and defined as the lowest value observed in each region analyzed. The remaining samples in the core and shell were calculated as mRNA expression levels scaled relative to their respective reference sample. Values greater than two standard deviations from the mean were considered outliers and removed from the analysis. No more than one outlier was ever removed from any group in any analysis. To control for Type 1 error, adjusted p values were calculated within each cluster using the False Discovery Rate (FDR) method of correction. Low levels of mRNA expression for the delta opioid receptor (Oprd1), the dopamine D4 receptor (Drd-4), and Bdnf-IIA prevented quantitative comparisons between groups.
All statistical tests were two-tailed and the alpha level was set at .05. Effect sizes were calculated as partial eta-squared (ηp2) for the dose-response data and as Cohen’s d for all other comparisons. Effect sizes were considered large if ηp2 ≥ .14 or d ≥ 0.8 according to standard statistical definitions (Cohen 1988). Pearson product-moment correlations were used to correlate AUC estimates and mRNA expression in each group.
Results
Body weights were similar between groups throughout the study. Mean (SEM) body weights (g) of sedentary and exercising rats at the end of testing were 265.2 (4.5) and 262.0 (3.9), respectively; a difference that was not statistically significant (p = .587). All rats acquired the lever-press response on the first day of training using food reinforcement, and all rats received the maximum number of food pellets on all five days of training (data not shown). Similarly, all rats acquired heroin self-administration on the first day of heroin exposure, receiving at least 20 infusions on the first day of training. No differences were observed between the two groups over the three days of training (Online Resource 2).
Online Resource 2. Heroin intake during training.

Rats were trained on an FR1 schedule of heroin (0.005 mg/kg/infusion) reinforcement over 3 days. The number of infusions was limited to 21 on the first day of training. Heroin intake did not differ significantly between groups during training (no main effect of group or group × session intaction)
Figure 2 shows that responding maintained by heroin generally decreased as a function of dose [main effect of dose: F(3,81) = 13.218, p < 0.001, ηp2 = 0.329]. Exercising rats self-administered significantly less heroin than sedentary rats [main effect of group: F(1,27) = 4.991, p = .034, ηp2 = 0.156], and heroin-maintained responding never significantly exceeded saline control values in exercising rats. Consistent with these findings, AUC estimates obtained from the dose-response data were lower in exercising rats than sedentary rats, and this effect was statistically significant [t(27) = 2.270; p = .031] and characterized by a large effect size (d = .831). Importantly, no differences were observed between the two groups in the saline substitution test or in responding on the inactive response lever (Online Resource 3).
Fig. 2. Resistance exercise decreases heroin self-administration.
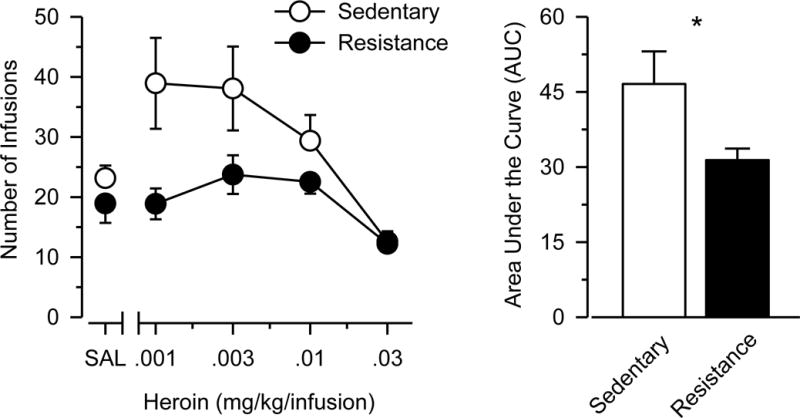
Left Panel: Dose-response data of heroin self-administration in sedentary (n = 14; open circles) and resistance exercise (n = 15; filled circles) rats. Vertical axis depicts number of infusions during a 2-hr session. Horizontal axis depicts doses of heroin in mg/kg/infusion. Points above “SAL” depict the effects of saline. Right Panel: Area under the curve (AUC) estimates of the dose-response data. Vertical axis depicts AUC estimates obtained from four doses of cocaine in sedentary (white bar) and resistance exercise (black bar) rats. Vertical lines surrounding data points represent the SEM; where not indicated, the SEM fell within the data point. Asterisk (*) indicates significant difference
Online Resource 3. Inactive lever responding.
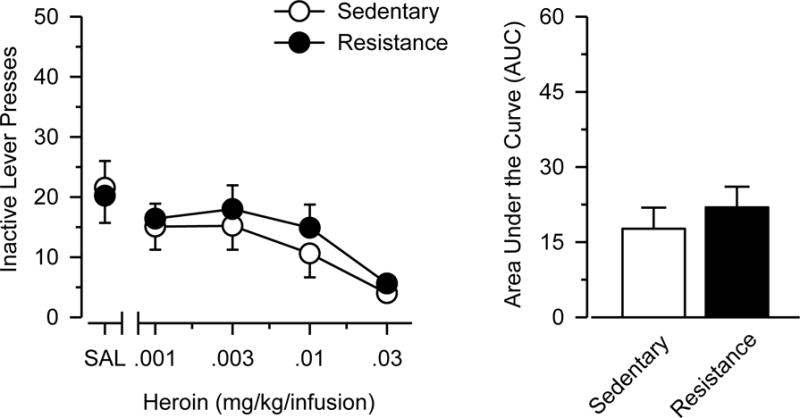
Left Panel: Dose-response data of inactive lever presses in sedentary and resistance exercise rats. Right Panel: Area under the curve (AUC) estimates of the dose-response data. Resistance exercise did not influece inactive lever responding (no main effects of group or group × dose interaction).
Figure 3 shows that mRNA expression for the mu opioid receptor (Oprm1) was significantly lower in the NAc core in exercising rats than sedentary rats [t(27) = 2.659; p = .026], and this effect was characterized by a large effect size (d = .985). Similar but non-significant effects were observed for Oprm1 in the NAc shell [t(26) = 1.954; p = .062; d = .738]. mRNA expression for the kappa (Oprk1) opioid receptor did not differ between groups in either the core or shell.
Fig. 3. Resistance exercise reduces mu opioid mRNA expression in the NAc core.
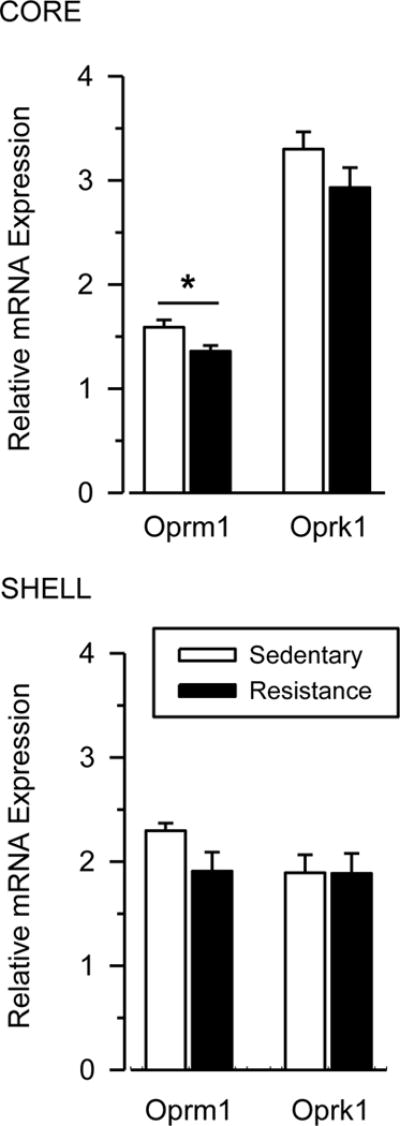
Relative mRNA expression in the NAc core (top) and shell (bottom) for sedentary (white bars) and resistance exercise (black bars) rats. mRNA data are shown for mu (Oprm1) and kappa (Oprk1) opioid receptors. Vertical lines represent the SEM. Asterisk (*) indicates significant difference
Figure 4 shows that mRNA expression for dopamine D1 (Drd-1), D2 (Drd-2), and D3 (Drd-3) receptors differed significantly between exercising and sedentary rats in the core but not the shell of the NAc. Expression was significantly lower for D1 [t(26) = 2.309; p = .041; d = .873], D2 [t(26) = 2.438; p = .041; d = .921], and D3 [t(26) = 2.284; p = .041; d = .803] receptors in exercising rats compared to sedentary rats, with all differences characterized by large effect sizes. In contrast, mRNA expression for the dopamine D5 (Drd-5) receptor was significantly and markedly greater in exercising rats than sedentary rats [t(27) = 3.039; p = .020; d = 1.134] in the shell but not the core.
Fig. 4. Resistance exercise reduces dopamine D1, D2, D3 mRNA expression in the NAc core and increases dopamine D5 mRNA expression in the NAc shell.

Relative mRNA expression in the NAc core (top) and shell (bottom) for sedentary (white bars) and resistance exercise (black bars) rats. mRNA data are shown for dopamine D1 (Drd-1), D2 (Drd-2), D3 (Drd-3), and D5 (Drd-5) receptors. Vertical lines represent the SEM. Asterisks (*) indicate significant difference
Figure 5 shows that mRNA expression for Bdnf consistently differed between groups in the NAc core but not the shell. Indeed, mRNA expression for Bdnf-I [t(26) = 2.164; p = .047; d = .842], Bdnf-IIB [t(25) = 2.767; p = .019; d = 1.077], Bdnf-IIC [t(25) = 2.858; p = .019; d = 1.113], Bdnf-IV [t(27) = 2.733; p = .019; d = 1.031], Bdnf-VI [t(26) = 2.440; p = .031; d = .947], and Bdnf-IX [t(25) = 2.846; p = .019; d = 1.113] was significantly greater in exercising rats than sedentary rats in the NAc core, and all differences were characterized by large effect sizes. Figure 5 also shows that mRNA expression of the tyrosine receptor kinase B receptor (Trkb-1) did not differ between groups in either the core or shell.
Fig. 5. Resistance exercise reduces BDNF mRNA expression in the NAc.
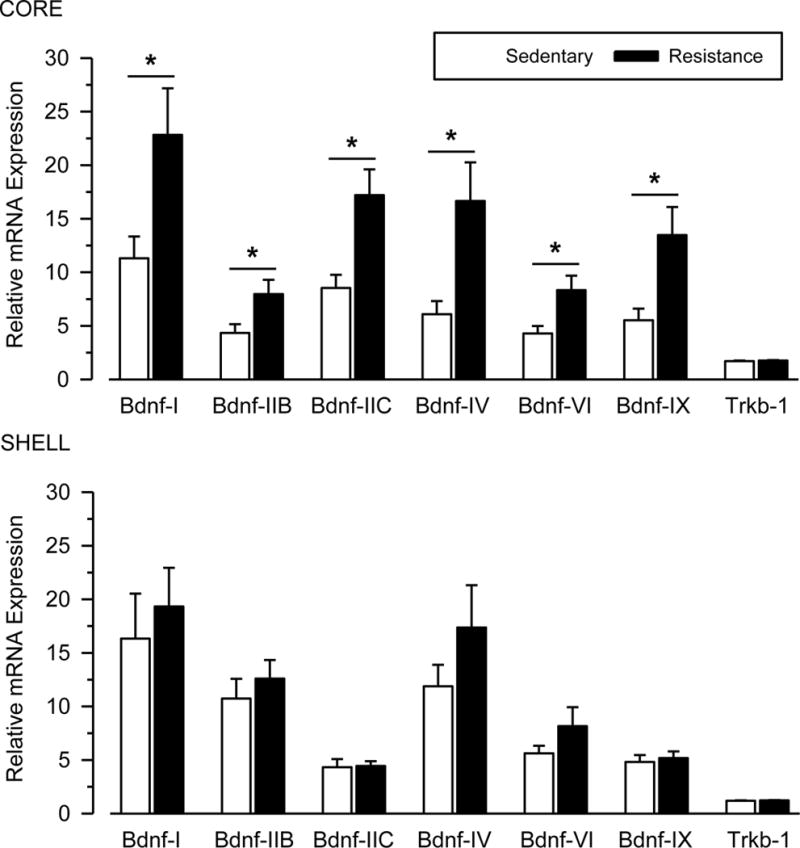
Relative mRNA expression in the NAc core (top) and shell (bottom) for sedentary (white bars) and resistance exercise (black bars) rats. mRNA data are shown for Bdnf-I, Bdnf-IIB, Bdnf-IIC, Bdnf- IV, Bdnf-VI, and Bdnf-IX exons and the tyrosine receptor kinase B receptor (Trkb-1). Asterisks (*) indicate significant difference
No significant correlations were observed between AUC values and mRNA expression for any gene in either group.
Discussion
The purpose of this study was to examine the effects of resistance exercise on heroin self-administration and mRNA expression of several molecular targets known to mediate opioid reinforcement and addictive behavior. The primary finding of this study is that resistance exercise produced large and significant decreases in heroin self-administration and concomitant changes in mRNA expression for genes encoding mu opioid receptors, dopamine receptors, and BDNF in the NAc of heroin-exposed rats. To our knowledge, these are the first data demonstrating that resistance exercise reduces the positive reinforcing effects of heroin and identifies candidate genes that may mediate these effects.
In our model, rats repeatedly climb a vertical ladder wearing a weighted vest. To control for enrichment-related effects due to handling and novelty, sedentary control rats are placed repeatedly on the ladder oriented horizontally on its side according to the same schedule as exercising rats. We previously validated this procedure as a model of resistance exercise by showing that exercising rats exhibit greater gastrocnemius mass (the large muscle of the calf) and greater hindlimb grip strength relative to sedentary rats (Strickland et al. 2016). The primary advantages of this model over other models of resistance exercise are that it avoids the use of potentially painful and stressful stimuli to motivate climbing (e.g., electric shock, water submersion) and minimizes the influence of aerobic factors that could otherwise influence measures of drug-seeking behavior (for review, see Strickland and Smith 2016). One limitation of our model is that our sedentary control group is not exposed to potentially relevant features of the exercise manipulation, such as wearing the weighted vest, navigating the ladder, and avoiding the light. Consequently, additional elements of stress may have been present in the exercising group that are not present in the control group. Although we cannot rule out this possibility, we note that stress-inducing events in the exercising group would be expected to increase (not decrease) drug intake (see Piazza and Le Moal 1998; Logrip et al. 2012).
Resistance exercise produced large decreases in heroin intake, resulting in a significant downward shift in the dose-effect curve. The downward shift in the dose-effect curve suggests that differences in heroin intake between sedentary and exercising subjects were due primarily to differences in the efficacy (rather than the potency) of heroin to serve as a reinforcer. This is pharmacologically relevant because it indicates that exercise-induced decreases in heroin intake are not simply due to changes in the functional dose of heroin, and it suggests that resistance exercise can reduce heroin intake under conditions in which dose is free to vary, as is the case in naturalistic settings. It is also notable that resistance exercise reduced responding to saline control values across all doses examined, suggesting that heroin failed to function as a positive reinforcer in this group. Finally, we note that simple FR schedules are limited in their ability to measure motivational aspects of drug consumption, so future studies should expand this research to incorporate other schedules of reinforcement (e.g., progressive ratio).
We do not know whether resistance exercise would decrease responding maintained by other (i.e., nondrug) stimuli. Unlike aerobic exercise, which generally increases food intake to compensate for increased caloric expenditure (Novak et al. 2012), resistance exercise typically decreases (Aguiar et al. 2010; Aparicio et al. 2011) or does not alter (Duarte et al. 2017; Ebal et al. 2007; Haraguchi et al. 2014) food intake. Resistance exercise did not decrease responding maintained by food during lever-press training, but all subjects were food restricted during this time and the maximal number of food reinforcers was limited to 40. Importantly, no differences in responding were observed between exercising and sedentary rats in a saline substitution test, and no differences were observed in responding on an inactive lever under any dose condition. These findings indicate that the effects of resistance exercise are not due to nonspecific effects on motor performance (e.g., muscle fatigue) but rather are specific to the heroin stimulus.
Heroin produces its positive reinforcing effects through its deacetylation to morphine and subsequent activation of mu opioid receptors. Acute bouts of aerobic exercise increase central concentrations of endogenous opioid peptides, including the mu- and delta-receptor ligands, beta-endorphin, leu-enkephalin, and met-enkephalin (Art et al. 1994; Debruille et al. 1999; Chen et al. 2007), and the kappa-receptor ligand, dynorphin (Aravich et al. 1993; Fontana et al. 1994). Chronic aerobic exercise reduces opioid receptor availability (Houghten et al. 1986; de Oliveira et al. 2010) and decreases sensitivity to exogenous opioid agonists (Mathes and Kanarek 2001; Smith and Lyle 2006). These effects are similar to those observed after chronic opioid administration (Bernstein and Welch 1998; Tao et al. 1998), suggesting that physical activity may down-regulate the number of opioid receptors, thereby producing “tolerance” to the effects of opioids with extended training. Consistent with this possibility, resistance exercise reduced the expression of mRNA encoding mu opioid receptors in heroin-exposed rats in the present study, which may explain its ability to selectively reduce the positive reinforcing effects of heroin without altering nonspecific measures of responding.
Both exercise and heroin increase dopamine in the NAc (Wilson and Marsden 1995; Wise et al. 1995), raising the possibility that exercise can serve as a substitute for heroin use via activation of the dopamine reward system. Consistent with this idea, we found that exercising rats self-administered less heroin and showed significant reductions in D1, D2 and D3 mRNA expression in the NAc core compared to sedentary rats. Interestingly, we observed downregulation of the D1 receptor subtype, as well as two D2-like receptor subtypes, and these changes would be expected to produce opposing neural adaptations. The D1 receptor subtype is a member of the D1-like family of receptors that couple to the Gsα protein and increases cAMP through activation of adenylyl cyclase, whereas the D2 and D3 receptor subtypes are members of the D2-like family of receptors that couple to the Giα protein and suppress cAMP activity through direct inhibition of adenylyl cyclase (Terwilliger et al. 1991). Although evidence suggests that a downregulation of D1 versus D2 receptors produces opposing effects on vulnerability to drug use and addiction (Lynch et al. 2007; Holroyd et al. 2015), there is also evidence suggesting that it is the ratio of D1-D2 activity that is critical (Self 2010). Taken collectively, these findings suggest that resistance exercise may reduce the positive reinforcing effects of heroin by decreasing dopamine receptor gene expression in heroin-exposed rats, which in turn balances adenylyl cyclase and cAMP-dependent protein kinase activity in the NAc.
In contrast to the findings in the NAc core, no significant differences in dopamine receptor mRNA expression were observed between exercising and sedentary heroin-exposed rats in the NAc shell. We did, however, find a significant upregulation of D5 gene expression in the shell. The D5 receptor subtype is a member of the D1 family of receptors, and thus, the ability of exercise to decrease heroin self-administration may occur via enhanced cAMP production. This seems unlikely given that enhanced D1 signaling is typically predictive of an enhanced, rather than reduced, vulnerability to drug use and addiction. A more likely mechanism is through a D5-BDNF interaction. D5 activation is known to enhance BDNF signaling in the reward pathway (e.g., PFC, Perreault et al. 2013). Thus, the upregulation that we see in the NAc shell may be a compensatory mechanism to normalize Bdnf levels in the NAc of sedentary rats exposed to heroin. Although speculative, this interpretation is also consistent with the robust changes that we observed in Bdnf expression (see below).
Opioid administration alters BDNF signaling, and chronic opioid use suppresses BDNF-Trkb signaling in the VTA (Koo et al 2015, 2012). Less established are the effects of opioids on BDNF expression and signaling in the NAc, a major target of the VTA. Koo and colleagues (2012) found that BDNF infusion into the NAc or genetic deletion of TrkB from this region did not influence morphine reward following chronic morphine administration. However, a more recent study showed that knockout of TrkB from DI-type GABAergic medium spiny neurons in the NAc enhanced morphine reward (Koo et al 2014). In the present study, we found that heroin-exposed exercising rats showed a significant increase in levels of Bdnf exon IX mRNA in the NAc core compared to heroin-exposed sedentary rats. This mRNA isoform represents the protein-coding region of Bdnf that is common to all Bdnf transcripts. A recent study showed opioid-induced downregulation of specific Bdnf exons, including II, IV, and VI in rat VTA (Koo et al 2015); consequently, we used primers targeting these exons plus Bdnf exon I to examine alternative transcripts of Bdnf in heroin-exposed rats with and without resistance training. Under these conditions, we found that resistance training significantly increased the expression of Bdnf exons I, IIb, IIc, IV, and VI mRNA in the NAc core compared to heroin-exposed rats that were sedentary. Hence, the exercise-induced increases of Bdnf exon IX mRNA most likely reflect contributions of exon I, II, IV, and VI. In contrast to the core, Bdnf mRNA and associated transcripts from the NAc shell did not significantly change in response to resistance training. Cocaine exposure increases BDNF signaling in the nucleus accumbens (Lynch et al. 2013), and both aerobic (Peterson et al. 2014) and resistance (Strickland et al. 2016) exercise decrease Bdnf gene expression in cocaine-exposed rats. In contrast, heroin exposure decreases BDNF signaling in central dopamine and opioid systems, and here we show that resistance exercise increases Bdnf gene expression in heroin-exposed rats. Thus, resistance training may also exert its effects on drug self-administration by normalizing Bdnf gene expression in drug-exposed subjects. This hypothesis could be tested by administering a TrkB receptor antagonist directly into the core of the NAc and measuring its ability to prevent/reverse the effects of resistance training on heroin self-administration.
It is notable that most of the significant differences in mRNA expression were observed in the NAc core relative to the shell. Heroin and other addictive drugs preferentially increase dopamine concentrations in the shell (Pontieri et al. 1995; Lecca et al. 2007), and repeated stimulation of dopamine transmission in the shell preferentially strengthens stimulus-drug associations, which is believed to contribute to compulsive drug use and addiction (Di Chiara et al. 2004; Di Chiara and Bassareo 2007). Regardless, most of the available evidence suggests that the acute reinforcing effects of heroin are mediated primarily at the level of the NAc core. For instance, excitotoxic lesions of the NAc core (but not the shell) reduce the acquisition and maintenance of heroin self-administration on a FR1 schedule (Alderson et al. 2001) and impair the acquisition of heroin-maintained responding on a second-order schedule (Hutcheson et al. 2001). Furthermore, intra-NAc core (but not shell) infusion of a dopamine D1 receptor antagonist attenuates the augmentation of heroin seeking induced by chronic food restriction (D’Cunha et al. 2016). The relative involvement of the NAc shell in other transitional stages of heroin addiction (e.g., escalation of use following extended access) remains to be determined.
The current study was conducted to demonstrate that resistance exercise reduces heroin self-administration and produces associated changes in mRNA expression in heroin-exposed rats. We note several limitations to guide future research in this area. First, the molecular data are limited to mRNA, and future studies will be necessary to determine how these changes in mRNA expression influence the expression of relevant proteins. Moreover, the molecular data are only descriptive, and mechanistic studies (via the use of site-specific agonists/antagonists) will be needed to determine their causal role in reducing heroin intake. Importantly, we did not include drug-naïve control subjects, and we do not know how resistance exercise influences mRNA expression in the absence of heroin exposure. The use of additional control groups that include heroin-naïve subjects will be needed to advance our understanding on the neurobiology of resistance exercise in the absence of drug exposure (see Strickland and Smith 2016). Similarly, heroin intake was not under direct experimenter control, and we do not know whether differences in mRNA expression are a cause or consequence of differences in heroin intake. Furthermore, a no-weight control group that climbed the ladder could control for nonspecific factors (e.g., entering a dark enclosure) and provide “dose-response” data regarding the effects of different loads. Additional control groups that measure the frequency (e.g., acute vs. chronic) and timing (e.g., before vs. after drug exposure) of resistance exercise would provide valuable information needed for the translation of these findings to clinical trials. Finally, females were chosen for this study, in part, because of slower growth rates between PND 42 and PND 77 (i.e., females gain ~100 g; males gain ~200 g). Future studies should include both male and females and examine sex as a biological variable.
Finally, we note that previous studies have reported that aerobic exercise decreases the positive reinforcing effects of morphine (Hosseini et al. 2009) and heroin (Smith and Pitts 2012), and that resistance exercise decreases the positive reinforcing effects of cocaine (Strickland et al. 2016). Although important methodological differences exist across these studies, these findings support a growing body of literature suggesting that exercise may reduce substance use in human populations (for reviews, see Lynch et al. 2013; Smith and Lynch, 2012).
Supplementary Material
Acknowledgments
The authors thank the National Institute on Drug Abuse for supplying the study drug.
Role of Funding Sources: This study was funded by NIH Grants DA027485 (MAS), DA031725 (MAS), and DA039093 (WJL). The NIH had no role in the design of the study; in the collection, analysis, and interpretation of the data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Contributors
M.A. Smith developed the model and conceived the project. G.E. Fronk, S.E. Bills, and R.T. Lacy collected the behavioral data and assisted in data analysis and interpretation. J.M. Abel and W.J. Lynch collected the molecular data and assisted in data analysis and interpretation. M.A. Smith, G.E. Fronk, and W.J. Lynch drafted the manuscript. All authors approved the manuscript for submission.
Conflict of Interest: No conflict declared.
References
- Aguiar AF, Aguiar DH, Felisberto AD, Carani FR, Milanezi RC, Padovani CR, Dal-Pai-Silva M. Effects of creatine supplementation during resistance training on myosin heavy chain (MHC) expression in rat skeletal muscle fibers. J Strength Cond Res. 2010;24(1):88–96. doi: 10.1519/JSC.0b013e3181aeb103. [DOI] [PubMed] [Google Scholar]
- Alderson HL, Parkinson JA, Robbins TW, Everitt BJ. The effects of excitotoxic lesions of the nucleus accumbens core or shell regions on intravenous heroin self-administration in rats. Psychopharmacology (Berl) 2001;153(4):455–63. doi: 10.1007/s002130000634. [DOI] [PubMed] [Google Scholar]
- Aparicio VA, Nebot E, Porres JM, Ortega FB, Heredia JM, López-Jurado M, Ramírez PA. Effects of high-whey-protein intake and resistance training on renal, bone and metabolic parameters in rats. Br J Nutr. 2011;105(6):836–845. doi: 10.1017/S0007114510004393. [DOI] [PubMed] [Google Scholar]
- Aravich PF, Rieg TS, Lauterio TJ, Doerries LE. Beta-endorphin and dynorphin abnormalities in rats subjected to exercise and restricted feeding: relationship to anorexia nervosa? Brain Research. 1993;622(1–2):1–8. doi: 10.1016/0006-8993(93)90794-n. [DOI] [PubMed] [Google Scholar]
- Art T, Franchioment P, Leukeux P. Plasma beta-endorphin response of thoroughbred horses to maximal exercise. The Veterinary Record. 1994;135(21):499–503. doi: 10.1136/vr.135.21.499. [DOI] [PubMed] [Google Scholar]
- Bernstein MA, Welch SP. mu-Opioid receptor down-regulation and cAMP-dependent protein kinase phosphorylation in a mouse model of chronic morphine tolerance. Brain Research Molecular Brain Research. 1998;55(2):237–42. doi: 10.1016/s0169-328x(98)00005-9. [DOI] [PubMed] [Google Scholar]
- Chen JX, Zhao X, Yue GX, Wang ZF. Influence of acute and chronic treadmill exercise on rat plasma lactate and brain NPY, L-ENK, DYN A1-13. Cellular and Molecular Neurobiology. 2007;27:1–10. doi: 10.1007/s10571-006-9110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Collo G, Cavalleri L, Spano P. Structural plasticity in mesencephalic dopaminergic neurons produced by drugs of abuse: critical role of BDNF and dopamine. Frontiers in Pharmacology. 2014;5:259. doi: 10.3389/fphar.2014.00259. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Current Opinion in Neurobiology. 2004;14(3):370–8. doi: 10.1016/j.conb.2004.05.005. Review. [DOI] [PubMed] [Google Scholar]
- D’Cunha TM, Daoud E, Rizzo D, Bishop AB, Russo M, Mourra G, et al. Augmentation of Heroin Seeking Following Chronic Food Restriction in the Rat: Differential Role for Dopamine Transmission in the Nucleus Accumbens Shell and Core. Neuropsychopharmacology. 2016;42(5):1136–1145. doi: 10.1038/npp.2016.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruille C, Luyckx M, Ballester L, Brunet C, Odou P, Dine T, et al. Serum opioid activity after physical exercise in rats. Physiological Research. 1999;48(2):129–133. [PubMed] [Google Scholar]
- de Oliveira MS, da Silva Fernandes MJ, Scorza FA, Persike DS, Scorza CA, da Ponte JB, et al. Acute and chronic exercise modulates the expression of MOR opioid receptors in the hippocampal formation of rats. Brain Research Bulletin. 2010;83(5):278–83. doi: 10.1016/j.brainresbull.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural Brain Research. 2002;137(1–2):75–114. doi: 10.1016/s0166-4328(02)00286-3. Review. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Current Opinion in Pharmacology. 2007;7(1):69–76. doi: 10.1016/j.coph.2006.11.003. Review. [DOI] [PubMed] [Google Scholar]; Erratum in: Current Opinion in Pharmacology. 7(2):233. [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–41. doi: 10.1016/j.neuropharm.2004.06.032. Review. [DOI] [PubMed] [Google Scholar]
- Droste SK, Schweizer MC, Ulbricht S, Reul JM. Long-term voluntary exercise and the mouse hypothalamic-pituitary-adrenocortical axis: impact of concurrent treatment with the antidepressant drug tianeptine. Journal of Neuroendocrinology. 2006;18(12):915–25. doi: 10.1111/j.1365-2826.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- Duarte FO, Gomes-Gatto CDV, Oishi JC, Lino ADS, Stotzer US, Rodrigues MFC, Gatti da Silva GH, Selistre-de-Araújo HS. Physical training improves visceral adipose tissue health by remodelling extracellular matrix in rats with estrogen absence: a gene expression analysis. Int J Exp Pathol. 2017;98(4):203–213. doi: 10.1111/iep.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebal E, Cavalie H, Michaux O, Lac G. Effect of a moderate exercise on the regulatory hormones of food intake in rats. Appetite. 2007;49(2):521–524. doi: 10.1016/j.appet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Fontana F, Bernardi P, Merlo Pich E, Boschi S, De Iasio R, Capelli M, et al. Endogenous opioid system and atrial natriuretic factor in normotensive offspring of hypertensive parents at rest and during exercise test. Journal of Hypertension. 1994;12(11):1285–1290. [PubMed] [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: a review. Neuroscience & Biobehavioral Reviews. 2010;35(2):157–71. doi: 10.1016/j.neubiorev.2009.11.009. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, et al. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behavioural Brain Research. 2011;217(2):354–62. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi FK, de Brito Magalhães CL, Neves LX, dos Santos RC, Pedrosa ML, Silva ME. Whey protein modifies gene expression related to protein metabolism affecting muscle weight in resistance-exercised rats. Nutrition. 2014;30(7–8):876–881. doi: 10.1016/j.nut.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Holroyd KB, Adrover MF, Fuino RL, Bock R, Kaplan AR, Gremel CM, et al. Loss of Feedback Inhibition via D2 Autoreceptors Enhances Acquisition of Cocaine Taking and Reactivity to Drug-Paired Cues. Neuropsychopharmacology. 2015;40(6):1495–1509. doi: 10.1038/npp.2014.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini M, Alaei HA, Naderi A, Sharifi MR, Zahed R. Treadmill exercise reduces self-administration of morphine in male rats. Pathophysiology. 2009;16(1):3–7. doi: 10.1016/j.pathophys.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Houghten RA, Pratt SM, Young EA, Brown H, Spann DR. Effect of chronic exercise on beta-endorphin receptor levels in rats. NIDA Research Monographs. 1986;75:505–8. [PubMed] [Google Scholar]
- Hutcheson DM, Parkinson JA, Robbins TW, Everitt BJ. The effects of nucleus accumbens core and shell lesions on intravenous heroin self-administration and the acquisition of drug-seeking behaviour under a second-order schedule of heroin reinforcement. Psychopharmacology (Berl) 2001;153(4):464–72. doi: 10.1007/s002130000635. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Long-term gene expression in the nucleus accumbens following heroin administration is subregion-specific and depends on the nature of drug administration. Addiction Biology. 2005;10(1):91–100. doi: 10.1080/13556210412331284748. Review. [DOI] [PubMed] [Google Scholar]
- Klein SL, Schiebinger L, Stefanick ML, Cahill L, Danska J, de Vries GJ, et al. Opinion: Sex inclusion in basic research drives discovery. Proceedings of the National Academy of Science. 2015;112:5257–5258. doi: 10.1073/pnas.1502843112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annual Review of Public Health. 2015;36:559–74. doi: 10.1146/annurev-publhealth-031914-122957. Review. [DOI] [PubMed] [Google Scholar]
- Koo JW, Mazei-Robison MS, Chaudhury D, Juarez B, LaPlant Q, Ferguson D, et al. BDNF is a negative modulator of morphine action. Science. 2012;338(6103):124–8. doi: 10.1126/science.1222265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Lobo MK, Chaudhury D, Labonté B, Friedman A, Heller E, et al. Loss of BDNF signaling in D1R-expressing NAc neurons enhances morphine reward by reducing GABA inhibition. Neuropsychopharmacology. 2014;39(11):2646–53. doi: 10.1038/npp.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Mazei-Robison MS, LaPlant Q, Egervari G, Braunscheidel KM, Adank DN, et al. Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area. Nature Neuroscience. 2015;18(3):415–22. doi: 10.1038/nn.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Brophy MK, Witte MA, Smith MA. Exercise decreases speedball self-administration. Life Sciences. 2014;114(2):86–92. doi: 10.1016/j.lfs.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, et al. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos expressing dorsal striatal neurons. Journal of Neuroscience. 2015;35(21):8232–44. doi: 10.1523/JNEUROSCI.1022-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wolf ME. Multiple faces of BDNF in cocaine addiction. Behavioural Brain Research. 2015;279:240–54. doi: 10.1016/j.bbr.2014.11.018. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Valentini V, Cacciapaglia F, Acquas E, Di Chiara G. Reciprocal effects of response contingent and noncontingent intravenous heroin on in vivo nucleus accumbens shell versus core dopamine in the rat: a repeated sampling microdialysis study. Psychopharmacology (Berl) 2007;194(1):103–16. doi: 10.1007/s00213-007-0815-y. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP, Koob GF. Stress modulation of drug self-administration: Implications for addiction comorbidity with post-traumatic stress disorder. Neuropharmacology. 2012;62(2):552–64. doi: 10.1016/j.neuropharm.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neuroscience & Biobehavioral Reviews. 2013;37(8):1622–44. doi: 10.1016/j.neubiorev.2013.06.011. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biological Psychiatry. 2010;68(8):774–7. doi: 10.1016/j.biopsych.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Kiraly DD, Caldarone BJ, Picciotto MR, Taylor JR. Effect of cocaine self-administration on striatal PKA-regulated signaling in male and female rats. Psychopharmacology. 2007;191(2):263–71. doi: 10.1007/s00213-006-0656-0. [DOI] [PubMed] [Google Scholar]
- Mathes WF, Kanarek RB. Wheel running attenuates the antinociceptive properties of morphine and its metabolite, morphine-6-glucuronide, in rats. Physiology & Behavior. 2001;74(1–2):245–251. doi: 10.1016/s0031-9384(01)00577-7. [DOI] [PubMed] [Google Scholar]
- Miller ML, Vaillancourt BD, Wright MJ, Jr, Aarde SM, Vandewater SA, Creehan KM, et al. Reciprocal inhibitory effects of intravenous d-methamphetamine self-administration and wheel activity in rats. Drug & Alcohol Dependence. 2012;121(1–2):90–6. doi: 10.1016/j.drugalcdep.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: Relationship to energy balance, general activity, and reward. Neuroscience and Biobehavioral Reviews. 2012;36(3):1001–1014. doi: 10.1016/j.neubiorev.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbonmwan YE, Schroeder JP, Holmes PV, Weinshenker D. The effects of post-extinction exercise on cocaine-primed and stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2015;232(8):1395–403. doi: 10.1007/s00213-014-3778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinate. 7th. Academic Press; London: 2013. [Google Scholar]
- Perreault ML, Jones-Tabah J, O’Dowd BF, George SR. A physiological role for the dopamine D5 receptor as a regulator of BDNF and Akt signaling in rodent prefrontal cortex. International Journal of Neuropsychopharmacology. 2013;16(2):477–83. doi: 10.1017/S1461145712000685. Epub 2012 Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AB, Abel JM, Lynch WJ. Dose-dependent effects of wheel running on cocaine-seeking and prefrontal cortex Bdnf exon IV expression in rats. Psychopharmacology (Berl) 2014;231(7):1305–14. doi: 10.1007/s00213-013-3321-4. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19(2):67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proceedings of the National Academy of Science. 1995;92(26):12304–8. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Lycas MD, Lynch WJ, Brunzell DH. Wheel running exercise attenuates vulnerability to self-administer nicotine in rats. Drug & Alcohol Dependence. 2015;156:193–8. doi: 10.1016/j.drugalcdep.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, Lynch WJ. Effect of wheel-running during abstinence on subsequent nicotine-seeking in rats. Psychopharmacology (Berl) 2013;227(3):403–11. doi: 10.1007/s00213-012-2964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Sangrey GR, Darnell SB, Schassburger RL, Cha JH, Pierce RC, et al. Increased brain-derived neurotrophic factor (BDNF) expression in the ventral tegmental area during cocaine abstinence is associated with increased histone acetylation at BDNF exon I-containing promoters. Journal of Neurochemistry. 2012;120(2):202–9. doi: 10.1111/j.1471-4159.2011.07571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW. Dopamine receptor subtypes in reward and relapse. In: Neve KA, editor. The Dopamine Receptors. Second. Humana Press; 2010. pp. 479–524. [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47(Suppl 1):242–55. doi: 10.1016/j.neuropharm.2004.07.005. Review. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Chefer VI. Targeting endogenous mu- and delta-opioid receptor systems for the treatment of drug addiction. CNS & Neurological Disorders - Drug Targets. 2008;7(5):442–53. doi: 10.2174/187152708786927813. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Lyle MA. Chronic exercise decreases sensitivity to mu opioids in female rats: correlation with exercise output. Pharmacology, Biochemistry & Behavior. 2006;85(1):12–22. doi: 10.1016/j.pbb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Smith MA, Lynch WJ. Exercise as a potential treatment for drug abuse: evidence from preclinical studies. Frontiers in Psychiatry. 2012;2:82. doi: 10.3389/fpsyt.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pennock MM, Walker KL, Lang KC. Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats. Drug & Alcohol Dependence. 2012;121(1–2):54–61. doi: 10.1016/j.drugalcdep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pitts EG. Wheel running decreases the positive reinforcing effects of heroin. Pharmacological Reports. 2012;64(4):960–4. doi: 10.1016/s1734-1140(12)70891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug & Alcohol Dependence. 2008;98(1–2):129–35. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Witte MA. The effects of exercise on cocaine self-administration, food-maintained responding, and locomotor activity in female rats: importance of the temporal relationship between physical activity and initial drug exposure. Experimental and Clinical Psychopharmacology. 2012;20(6):437–46. doi: 10.1037/a0029724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieraj JC, Kim A, Fannon MJ, Mandyam CD. Chronic wheel running-induced reduction of extinction and reinstatement of methamphetamine seeking in methamphetamine dependent rats is associated with reduced number of periaqueductal gray dopamine neurons. Brain Structure and Function. 2016;221(1):261–76. doi: 10.1007/s00429-014-0905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Abel JM, Lacy RT, Beckmann JS, Witte MA, Lynch WJ, et al. The effects of resistance exercise on cocaine self-administration, muscle hypertrophy, and BDNF expression in the nucleus accumbens. Drug & Alcohol Dependence. 2016;163:186–94. doi: 10.1016/j.drugalcdep.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Smith MA. Animal models of resistance exercise and their application to neuroscience research. Journal of Neuroscience Methods. 2016;273:191–200. doi: 10.1016/j.jneumeth.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao PL, Han KF, Wang SD, Lue WM, Elde R, Law PY. Immunohistochemical evidence of down-regulation of mu-opioid receptor after chronic PL-017 in rats. European Journal of Pharmacology. 1998;344(2–3):137–42. doi: 10.1016/s0014-2999(97)01596-3. [DOI] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Research. 1991;548(1–2):100–10. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies–tackling the opioid-overdose epidemic. New England Journal of Medicine. 2014;370(22):2063–6. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- Walder RY, Wattiez AS, White SR, Marquez de Prado B, Hamity MV, Hammond DL. Validation of four reference genes for quantitative mRNA expression studies in a rat model of inflammatory injury. Molecular Pain. 2014;10:55. doi: 10.1186/1744-8069-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W, Marsden C. Extracellular dopamine in the nucleus accumbens of the rat during treadmill running. Acta Physiologica (Scandinavia) 1995;155:465–466. doi: 10.1111/j.1748-1716.1995.tb09997.x. [DOI] [PubMed] [Google Scholar]
- Wingo T, Nesil T, Chang SL, Li MD. Interactive Effects of Ethanol and HIV-1 Proteins on Novelty Seeking Behaviors and Addiction-Related Gene Expression. Alcoholism: Clinical and Experimental Research. 2016;40(10):2102–2113. doi: 10.1111/acer.13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Leone P, Rivest R, Leeb K. Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse. 1995;21(2):140–8. doi: 10.1002/syn.890210207. [DOI] [PubMed] [Google Scholar]
- Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Annals of the New York Academy of Sciences. 1999;877:113–28. doi: 10.1111/j.1749-6632.1999.tb09264.x. Review. [DOI] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME. Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology (Berl) 2010;209(1):113–25. doi: 10.1007/s00213-010-1776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


