Abstract
Background
The causal role of high density lipoprotein (HDL) cholesterol in cardioprotection has been questioned by genetic and randomized studies. Novel measures that relate to HDL function may contribute new information to prediction of cardiovascular risk. Apolipoprotein C-III (apoC-III) is a key regulator of lipoprotein metabolism. We investigated whether subspecies of HDL defined by apoC-III are associated with coronary heart disease (CHD).
Methods
We used immuno-affinity chromatography to measure the apoA-I concentrations of HDL that contains and lacks apoC-III in two prospective studies of adults free of CHD. In the Multi-Ethnic Study of Atherosclerosis (MESA), 5,657 participants (52% women; age 52–72 y) were followed for risk of CHD from 2000-2002 through 2013. In a case-cohort study nested within the Danish Diet, Cancer and Health (DCH) study, 3,642 participants (47% women; age 51-64 y) were followed from 1994-1997 through 2010. Subsequently, we conducted a meta-analysis that combined these results with the previously published findings from two cohort studies that used similar laboratory methodology to measure lipoproteins, totaling 2,997 incident cases.
Results
ApoC-III was found on 6-8% of apoA-I. The two HDL subspecies showed opposing associations with risk of CHD in each of the individual cohorts and in the meta-analysis (p-heterogeneity between the two subspecies <0.01). HDL that contains apoC-III was associated with higher risk of CHD (pooled relative risk [RR] per SD = 1.09, 95% confidence interval [95% CI] = 1.01 to 1.18), whereas HDL that lacks apoC-III was associated with lower risk (RR= 0.76; 95% CI = 0.70 to 0.83). The relative risk for HDL lacking apoC-III was even more negative than the relative risk for total HDL (RR= 0.80; 95% CI = 0.74 to 0.87).
Conclusions
Our findings from four prospective studies support the hypothesis that apoC-III may mark a subfraction of HDL that is associated with higher risk of CHD. New measures reflecting HDL structure and function may provide novel insights for cardiovascular risk that extend beyond traditional plasma HDL cholesterol concentrations.
Keywords: CHD, cohort study, HDL, biomarker
Introduction
High density lipoprotein (HDL) cholesterol is a strong, inverse predictor of coronary heart disease (CHD).1 However, the potential causal role of HDL-cholesterol in cardioprotection has been called into question by randomized studies of potent HDL-cholesterol-raising drugs that failed to lower CHD rates.2–4 Genetic observational studies that do not find fewer cardiovascular events among individuals with genetically determined high HDL-cholesterol levels further highlight the limitations in using HDL-cholesterol to evaluate novel therapeutics aimed at CHD-reduction.5, 6
Emerging measures of key anti-atherogenic properties of HDL, such as its capacity to mediate cholesterol efflux from macrophages, are not captured by average HDL-cholesterol levels.7, 8 The strong inverse association between cholesterol efflux capacity and cardiovascular events suggests that markers reflecting functional properties of HDL, rather than its cholesterol concentration alone, may substantially contribute to our understanding of HDL’s role in atherosclerosis.
The heterogeneous protein composition of HDL led to the proposal that HDL circulates in subtypes based on the presence and absence of certain apolipoproteins that guide their downstream metabolism and function by mediating their interactions with enzymes and receptors.9 Growing evidence suggests apoC-III, a small apolipoprotein present on some but not all VLDL, LDL and HDL particles, may play a key role in lipid metabolism.10–12 Rare mutations in the APOC3 gene that lower circulating apoC-III levels also associate with lower triglycerides and a greatly reduced risk of cardiovascular disease.13, 14 ApoC-III inhibits hepatic clearance from the circulation of apoB-containing lipoproteins by blocking the interaction of apoE and apoB100 with hepatic receptors, and in large amounts, it can inhibit lipases that metabolize the triglyceride in these lipoproteins.12, 15, 16 It is not known if apoC-III affects HDL metabolism. However, apoC-III diminishes the protective functions of HDL that are unrelated to lipoprotein metabolism such as inhibition of monocyte adhesion to endothelial cells, an early event in atherosclerosis.17 In US male health professionals and female nurses, we found that the cholesterol concentrations of HDL defined by the presence or absence of apoC-III have opposite associations with CHD.18 However, this relatively small study was not powered to investigate potential susceptible subgroups and did not include measures of apoA-I, the main structural protein of HDL that is strongly associated with risk of CHD.
To investigate whether subspecies of HDL-apoA-I containing or lacking apoC-III are associated with incident CHD, we prospectively studied this in two large population-based studies with greater racial and ethnic diversity and a large number of well-defined incident events. We further meta-analyzed all current data on HDL subspecies according to apoC-III totaling over 2900 incident events and queried for potential effect modification by important cardiovascular risk factors including obesity, smoking, and clinical HDL-cholesterol measures.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results only with proper IRB approvals and strict adherence to cohort-specific regulations. Requests for data can be directed to senior authors: RMC, KO and EBR.
Study populations and clinical endpoints
Between 2000 and 2002, the Multi-Ethnic Study of Atherosclerosis (MESA) enrolled 6,814 men and women aged 45-84 years from six regions in the U.S. who self-identified as primarily of Caucasian, Chinese, African or Hispanic descent, and were free of clinical CVD.19 Information on demographics, anthropometrics, and medications were obtained at baseline through questionnaires and physical examinations. Incident CHD events (myocardial infarction [MI], resuscitated cardiac arrest, and CHD death) were ascertained and adjudicated as previously reported.20 By the end of follow-up in December 2013, 300 incident CHD events had occurred.
The Diet, Cancer, and Health (DCH) is a population-based study started in 1993-1997 when 57,053 Danish born residents, aged 50 to 65 years and free of cancer, participated in a clinical examination and detailed lifestyle survey.21 All incident CHD events occurring between baseline and May 2008 (MI, resuscitated cardiac arrest, and fatal CHD) were included by linking participants to Danish registers via the unique identification number assigned to all Danish citizens (see online supplement for details on registers).22–24 The total number of confirmed incident cases of CHD between study entry and May 2010 was 2,063.
Study designs and laboratory measurements
Blood was sampled at the baseline study visit in both studies and stored at −70 °C in MESA and −150 °C in the Danish study. Our lipid laboratory at the Harvard T.H. Chan School of Public Health received plasma from all MESA participants except 1000 participants excluded by the study Steering Committee due to extensive laboratory testing on baseline samples, leading to depletion of stored plasma in these randomly chosen individuals (included n=5,796). In the DCH study, a case-cohort was designed for the assessment of blood markers.25 Plasma samples were received from all incident CHD cases and a sub-cohort of randomly sampled participants drawn from the study population who were free of CHD at baseline (n=1,824).
HDL subspecies according to apoC-III were assessed by concentrations of apoA-I with and without apoC-III in both studies. In MESA, a modified sandwich ELISA first fractionates plasma by binding lipoproteins containing apoC-III using affinity-purified anti-human apoC-III and subsequently quantifies the concentration of apoA-I in the apoC-III-bound and unbound fractions by standard ELISA assays. Additionally, the concentration of total plasma apoC-III was measured by ELISA separately. Upon exclusion of 45 participants with implausible apolipoprotein ranges, 27 with missing information on CHD events, 67 with missing covariate data, 5,657 MESA participants remained for our analyses.
In the assessment of DCH samples, we used immuno-affinity chromatography with anti-apoC-III antibody to separate the plasma into fractions with and without apoC-III. Subsequently, concentrations of apoA-I in the apoC-III-bound and unbound fractions were assessed by standard ELISA. To compare with our earlier report where HDL was quantified using measures of HDL-cholesterol concentrations rather than apoA-I concentrations,18 we also assessed the concentrations of cholesterol (in HDL) containing and lacking apoC-III upon precipitation of the apoB-containing lipoproteins from the apoC-III-defined immuno-fractions. Total plasma levels of triglycerides, apoB, apoC-III, and apoC-III in the HDL fraction were also assessed in our lipid laboratory. From the original 3,830 participants in the DCH case-cohort, 161 did not have enough samples left for qualitative analyses in the lipid laboratory. We also excluded 27 samples that did not pass quality control and therefore the final case-cohort set included 1,949 incident CHD cases and 1,693 non-cases. Due to the case-cohort design, 57 incident CHD cases also belonged to the reference sub-cohort (total n=1,750). More details on laboratory methodology are available in online supplement.
Additional Cohorts for Meta-analysis
To provide the most robust estimate of risk, we considered all available studies of incident CHD that incorporated the measures of HDL subspecies according to apoC-III content and conducted a cumulative meta-analysis. The inclusion of our previous investigations of HDL-cholesterol containing and lacking apoC-III in case control studies nested within the Nurses’ Health Study [NHS] and the Health Professionals Follow-Up Study [HPFS] (details in supplement),18 summed to a total of 2,997 incident CHD events.
In a subset of the HPFS controls (n=301), cholesterol efflux from macrophages was also measured using methods previously described.8
Statistical Analyses
We assessed characteristics of the MESA participants and the random sub-cohort of the DCH using means (medians of skewed variables) and proportions of covariates of interest. In both MESA and DCH, risk of incident CHD was analyzed using Cox proportional hazards regression where participants contributed person-time from baseline until the date of an event, death, or end of follow-up, whichever occurred first. In the DCH, Kalbfleisch and Lawless weights and robust variance suitable for the case-cohort data were applied.26 All analyses adjusted for age and sex; multivariable-adjusted models also included all traditional ASCVD (atherosclerotic cardiovascular disease) risk factors except for HDL cholesterol. Thus, prevalent diabetes, treatment with anti-hypertensive medication, race/ethnicity (MESA only), systolic blood pressure, smoking, and total cholesterol were included in multivariable models.27 The two subfractions of HDL-apoA-I were simultaneously included in all analyses of these subspecies as they sum to total HDL-apoA-I. In each study, we assessed the heterogeneity between the two associations of HDL containing and lacking apoC-III with risk of CHD in Cox regression models with both subfractions included as linear terms per study-specific SDs as well as per 5 mg/dL. We then tested the linear hypothesis that the regression coefficients for the two subfractions were equal (using a 1 d.f. Wald test).
Initial evaluations found no statistically significant difference in associations of HDL subspecies with risk of CHD across sex and race/ethnicity (MESA only), and therefore results from the total study population are presented. No evidence for non-proportionality of hazards was found by examining martingale residuals. A subsequent model additionally included log-transformed plasma triglycerides and LDL-cholesterol (available in MESA) or total apolipoprotein B (available in DCH) concentrations to address whether the associations were independent of adjustment for these clinical risk factors.
The results based on the NHS/HPFS data were used as previously reported (also available in supplement),18 and with the additional measures of cholesterol efflux in a subset of HPFS controls, we estimated the age-adjusted spearman correlations with the apoC-III based HDL subspecies.
Meta-analysis
To test relationships with CHD across the diverse measures and study designs, we performed meta-analyses per study-specific SDs, quintiles, and per 5 mg/dL fixed increment of the HDL measures containing or lacking apoC-III. In the absence of between-study heterogeneity, we estimated the fixed-effects.28 We also pooled all estimates from cohort-specific analyses of HDL subspecies with risk of CHD in strata of CVD risk factors; sex, obesity, smoking, diabetes, use of cholesterol-lowering medication, and clinically-defined low HDL-cholesterol (HDL-cholesterol < 40 mg/dL).29
Statistical analyses were performed using SAS 9.4 (SAS Institute; Cary, NC) and STATA 11 (STATA Corp., College Station, TX).
The first author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Written informed consent was obtained from all participants and the protocol was approved by the institution review board at the Harvard T. H. Chan School of Public Health, all MESA study sites, the Danish National Committee on Health Research Ethics (journal nr. (KF) 01-345/93), and the Danish Data Protection Agency.
Results
The mean age at study entry was 63 years in MESA and 57 years in DCH. In MESA, 52% were female, and 37% self-identified as Caucasians, 12% as Chinese, 29% as African-Americans, and 22% as Hispanics, whereas DCH participants were Danish-born residents (Table 1). At baseline, 17% of MESA participants reported use of lipid-lowering medication, whereas this was uncommon in Denmark (<1% reported use). Similarly, despite higher average systolic blood pressure in Danes, fewer reported a physician diagnosis of hypertension, and taking medicine for this was uncommon. The median apoA-I levels were 125 mg/dL in MESA and 136 mg/dL in DCH and, on average, 6-8% of the HDL-apoA-I contained apoC-III.
Table 1.
Baseline characteristics of participants from the Multi-Ethnic Study of Atherosclerosis (MESA) and the randomly selected sub-cohort from Diet, Cancer and Health (DCH) study
| Variables | MESA N=5657 |
DCH sub-cohort N=1750 |
|---|---|---|
| Age, mean years (SD) | 62.7 (10.3) | 56.5 (4.4) |
| Female, No. (%) | 2941 (52.0) | 817 (46.7) |
| Postmenopausal (females only), No. (%) | 2444 (83.2) | 479 (58.6) |
| Race/ethnicity, N0. (%) | ||
| Caucasian | 2102 (37.2) | 1750 (100) |
| Chinese-American | 694 (12.3) | 0 |
| African-American | 1617 (28.6) | 0 |
| Hispanic | 1244 (22.0) | 0 |
| Carotid intima-media thickness, mean um* (SD) | 978 (361) | N/A |
| Diabetes, No. (%) | 721 (12.8) | 31 (1.8) |
| Hypertension, No (%) | 2578 (45.6) | 324 (18.5) |
| Use of blood pressure lowering drugs, No (%) | 2136 (38) | 192 (11) |
| Hypercholesterolemia, No (%) | 542 (9.6) | 140 (8.0) |
| Use of lipid lowering drugs, No (%) | 953 (16.9) | 14 (0.8) |
| Systolic blood pressure, mean mmHg (SD) | 127 (22) | 139 (20) |
| Waist circumference, mean cm (SD) | 98.2 (14.3) | 89.4 (12.8) |
| Body mass index, mean kg/m2 (SD) | 28.3 (5.4) | 26.1 (4.0) |
| Physical activity, median MET-hr/wk (IQR)† | 58 (73) | 60 (49) |
| Current smoker, No (%) | 712 (12.6) | 662 (37.8) |
| No alcohol intake, No (%) | 2547 (45.0) | 39 (2.2) |
| Alcohol intake, mean drinks/wk (SD) | 2.3 (5.3) | 10.7 (11.1) |
| HDL-C, median mg/dL (IQR) | 48 (19) | 52 (25) |
| HDL-C containing apoC-III, median mg/dL (IQR) | N/A | 7.7 (6.7) |
| HDL-C lacking apoC-III, median mg/dL (IQR) | N/A | 43.4 (22.3) |
| Proportion of HDL-C that contains apoC-III, mean % (IQR) | N/A | 14.9 (10.4) |
| ApoA-I, median mg/dL (IQR) | 125 (41) | 135.5 (57.3) |
| ApoA-I containing apoC-III, median mg/dL (IQR) | 7.9 (3.6) | 11.3 (8.2) |
| ApoA-I lacking apoC-III, median mg/dL (IQR) | 118 (38) | 123.4 (52.1) |
| Proportion of apoA-I that contains apoC-III, mean % (IQR) | 6.3 (1.9) | 8.2 (4.8) |
| Total apoC-III, median mg/dL (IQR) | 8.7 (4.1) | 11.4 (7.5) |
| ApoC-III in HDL, median mg/dL (IQR) | N/A | 5.6 (4.9) |
| Triglycerides, median mg/dL (IQR) | 111 (84) | 80.7 (67.4) |
| Total cholesterol, median mg/dL (IQR) | 192 (45) | 235.5 (57.9) |
cIMT only available in 5,494 of the MESA participants.
Physical activity includes moderate and vigorous activity. Participants reporting more than 18 hrs/day of total physical activity excluded (MESA, N=4,894 after exclusions).
In DCH hypertension, diabetes, and hypercholesterolemia were self-reported physician-diagnoses.
To convert apoA-I and apoC-III to g/L multiply by 0.01; HDL-C and total cholesterol to mmol/L multiply by 0.0259; triglycerides to mmol/L multiply by 0.0113.
The age-adjusted spearman correlation (in a subset of the HPFS participants) between cholesterol efflux and the proportion of HDL cholesterol that has apoC-III was 0.12 (p=0.2). Age- and sex-adjusted spearman correlations of the HDL subspecies amongst each other (0.7, p <0.01) and with triglycerides and total apoC-III in Supplemental Table S1 were based on the MESA cohort. While total apoC-III was moderately correlated with triglycerides (0.6, p<0.01), the HDL subspecies containing or lacking apoC-III were weakly or not correlated with triglycerides.
Associations with risk of coronary heart disease in MESA and DCH
The concentration of total HDL-apoA-I was inversely associated with the risk of CHD in both cohorts (Table 2). However, the two HDL subspecies defined by the presence versus absence of apoC-III were differentially associated with the risk of CHD. Only HDL-apoA-I lacking apoC-III was inversely associated with risk of CHD (hazard ratio per SD = 0.83; 95% confidence interval [CI], 0.71 to 0.96 in MESA and 0.75, 95% CI, 0.66 to 0.84 in DCH), whereas the risk associated with each SD increment in HDL-apoA-I containing apoC-III was 1.09 (0.95 to 1.25) in MESA and 1.03 (0.92 to 1.15) in DCH. The relative risks associated with HDL containing and lacking apoC-III were significantly different from each other (p for heterogeneity in multivariable-adjusted models was 0.03 in MESA and 0.01 in DCH).
Table 2.
Apolipoprotein A-I concentrations in total plasma and in subspecies of HDL containing and lacking apoC-III and risk of coronary heart disease. The Multi-Ethnic Study of Atherosclerosis and the Diet, Cancer and Health Study*
| MESA | DCH | |||||
|---|---|---|---|---|---|---|
| Per SD | Q5 vs Q1 | P | Per SD | Q5 vs Q1 | P | |
| Total apoA-I | SD=36.4 mg/dL | SD=45.8 mg/dL | ||||
| Age- and sex-adjusted | 0.85 (0.74, 0.96) | 0.60 (0.41, 0.88) | 0.01 | 0.74 (0.66, 0.83) | 0.33 (0.23, 0.45) | <0.0001 |
| + ASCVD factors | 0.87 (0.76, 1.00) | 0.65 (0.44, 0.95) | 0.04 | 0.76 (0.67, 0.85) | 0.35 (0.25, 0.50) | <0.0001 |
| ApoA-I lacking apoC-III | SD=34.3 mg/dL | SD=41.7 mg/dL | ||||
| Age- and sex-adjusted | 0.80 (0.69, 0.92) | 0.50 (0.32, 0.78) | 0.003 | 0.72 (0.64, 0.81) | 0.36 (0.26, 0.51) | <0.0001 |
| + ASCVD factors | 0.83 (0.71, 0.96) | 0.55 (0.35, 0.87) | 0.01 | 0.75 (0.66, 0.84) | 0.39 (0.28, 0.56) | <0.0001 |
| ApoA-I containing apoC-III | SD=3.3 mg/dL | SD=7.5 mg/dL | ||||
| Age- and sex-adjusted | 1.10 (0.96, 1.26) | 1.26 (0.82, 1.95) | 0.16 | 1.05 (0.95, 1.17) | 1.19 (0.85, 1.65) | 0.3 |
| + ASCVD factors | 1.09 (0.95, 1.25) | 1.18 (0.76, 1.83) | 0.24 | 1.03 (0.92, 1.15) | 1.14 (0.82, 1.59) | 0.6 |
| Proportion of apoA-I that contains apoC-III | SD=3 % | SD=4 % | ||||
| Age- and sex-adjusted | 1.05 (0.95, 1.15) | 1.27 (0.89, 1.83) | 0.34 | 1.11 (1.01, 1.22) | 1.19 (0.87, 1.63) | 0.03 |
| + ASCVD factors | 1.03 (0.94, 1.14) | 1.20 (0.83, 1.74) | 0.49 | 1.08 (0.98, 1.19) | 1.17 (0.85, 1.42) | 0.1 |
| Total apoC-III | SD=3.9 mg/dL | SD=6.4 mg/dL | ||||
| Age- and sex-adjusted | 1.06 (0.94, 1.19) | 1.16 (0.82, 1.65) | 0.33 | 1.12 (1.01, 1.25) | 1.35 (0.97, 1.90) | 0.04 |
| + ASCVD factors | 1.00 (0.88, 1.14) | 0.98 (0.67, 1.44) | 0.97 | 1.00 (0.89, 1.13) | 0.97 (0.67, 1.42) | 0.9 |
| ApoC-III in HDL mg/dL | NA | SD=4.3 mg/dL | ||||
| Age- and sex-adjusted | NA | 1.09 (0.97, 1.22) | 1.00 (0.71, 1.42) | 0.16 | ||
| + ASCVD factors | NA | 1.02 (0.91, 1.15) | 0.80 (0.56, 1.14) | 0.72 | ||
Hazard Ratios and 95% confidence intervals for coronary heart disease per 1 SD (in mg/dL) increments and for comparison of extreme quintiles. Multivariable model adjusted for ASCVD (atherosclerotic cardiovascular disease) risk factors: for age, sex, prevalent diabetes, treatment with anti-hypertensive medication, race/ethnicity (MESA), systolic blood pressure, smoking, and total cholesterol. P-heterogeneity between continuous measures of apoA-I with and without apoC-III: age- and sex-adjusted model, MESA= 0.01, DCH=0.0001; ASCVD factors model, MESA = 0.03; DCH=0.01.
Measures of apoC-III itself, including the absolute concentration of apoC-III within the plasma HDL fraction (only available in DCH) and total plasma apoC-III both showed tendencies for higher risk of CHD with higher levels, but neither of them were statistically significant in the fully-adjusted models (Table 2).
In the DCH, we obtained measures of apoC-III defined HDL subspecies by assessing both HDL-cholesterol concentrations and apoA-I concentrations. Results for HDL-cholesterol containing and lacking apoC-III were similar to those reported for HDL subspecies based on apoA-I measures (Supplemental Table S2). Cholesterol concentrations of the HDL subspecies were not measured in MESA.
Meta-analysis of four studies
The divergent associations observed for HDL-apoA-I containing and lacking apoC-III in MESA and DCH were similar to the results observed using HDL-cholesterol measures in both DCH (Supplemental Table S3) and the previous report from the Nurses’ Health and Health Professionals Follow-Up Studies (results from all four cohorts shown using study-specific quintiles and SDs in fig 1).18 We also repeated the analysis in each cohort using a fixed increment of 5 mg/dL for both HDL subfractions. The results, which were very similar to those shown in fig 1, are included in online supplement Table S4. Thus, we pooled all cohorts with analyses of HDL subspecies according to apoC-III and found the multivariable-adjusted relative risk for CHD per each SD higher HDL lacking apoC-III was 0.76 (0.70 to 0.83) and the relative risk for HDL containing apoC-III was 1.09 (1.01 to 1.18).(fig 2, and study-specific quintile medians presented in supplemental Table S5 and forest plots in figures S1 and S2) Using 5 mg/dL increments, the relative risks were 0.96 (0.93 to 0.98) and 1.13 (0.96 to 1.30), respectively. The association with CHD for HDL containing apoC-III was statistically significantly different than for HDL lacking apoC-III (p for heterogeneity <0.01). Adjustment for triglycerides and LDL cholesterol attenuated the results but HDL lacking apoC-III remained inversely associated with risk of CHD (relative risk per SD =0.84; 0.75 to 0.95) and the relative risk per each SD of HDL containing apoC-III was 1.05 (0.96 to 1.14) (p for heterogeneity=0.04).
Fig 1. Coronary heart disease risk associated with HDL containing (red) and HDL lacking apoC-III (blue) in four prospective studies.
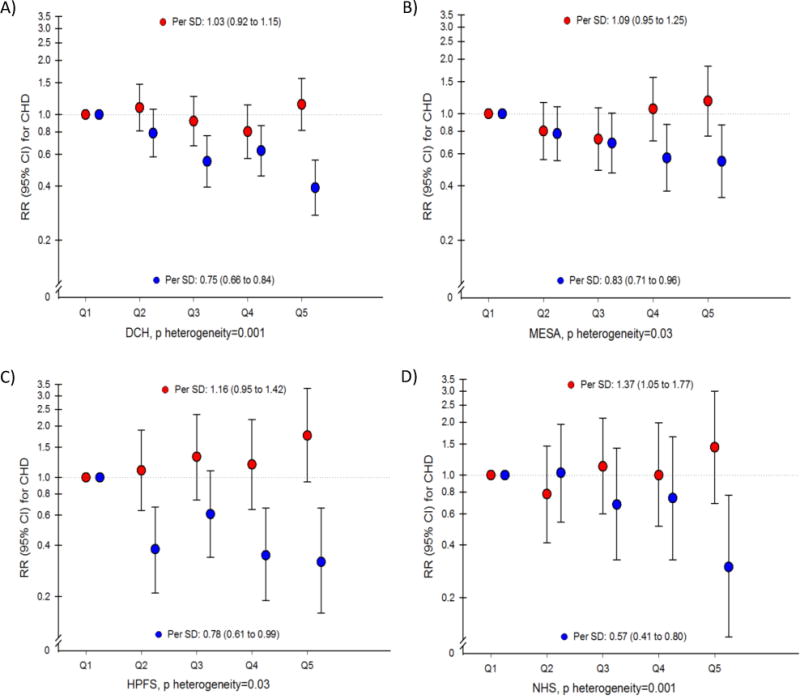
Relative risks with 95% CI for risk of CHD estimated in four prospective studies (A) the Diet, Cancer and Health [DCH] Study, B) the Multi-Ethnic Study of Atherosclerosis [MESA], C) the Health Professionals Follow-Up Study [HPFS], and D) the Nurses’ Health Study [NHS]) per SD and using study-specific quintiles. Adjusted for ASCVD (atherosclerotic cardiovascular disease) risk factors: age, sex, prevalent diabetes, treatment with anti-hypertensive medication, (race/ethnicity in MESA), systolic blood pressure, smoking, and total cholesterol. Both subtypes of HDL (containing and lacking apoC-III) are simultaneously included in all models. P heterogeneity = test of the linear hypothesis that the regression coefficients for the two subfractions were equal (1 d.f. Wald test).
Fig 2. Coronary heart disease risk associated with HDL containing (red dot) and HDL lacking apoC-III (blue dot) in meta-analysis of four prospective studies.
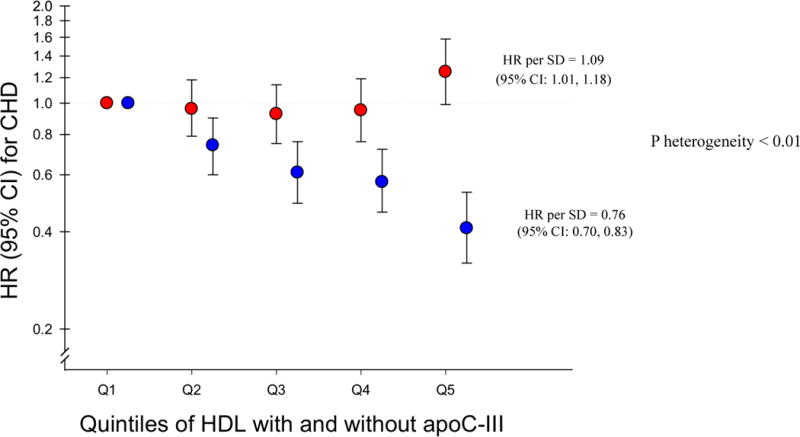
Pooled relative risks with 95% CI for risk of CHD estimated in fixed-effects meta-analysis using study-specific SDs and study-specific quintiles in four prospective studies (the Diet, Cancer and Health [DCH] Study, the Multi-Ethnic Study of Atherosclerosis [MESA], the Health Professionals Follow-Up Study [HPFS], and the Nurses’ Health Study [NHS]). Adjusted for ASCVD (atherosclerotic cardiovascular disease) risk factors: age, sex, prevalent diabetes, treatment with anti-hypertensive medication, (race/ethnicity in MESA), systolic blood pressure, smoking, and total cholesterol. HDL assessed per apoA-I concentrations in MESA and DCH and per HDL-cholesterol concentrations in NHS and HPFS. Both subtypes of HDL (containing and lacking apoC-III) are simultaneously included in all models. P heterogeneity = test of the linear hypothesis that the regression coefficients for the two subfractions were equal (1 d.f. Wald test).
In meta-analyses, the proportion of HDL containing apoC-III was associated with higher risk of CHD (relative risk per SD=1.09; 1.03 to 1.16), while each SD higher total HDL was associated with a relative risk of 0.80 (0.74 to 0.87). (Supplemental fig S3)
Analyses in strata of CVD risk factors
We analyzed the risk of CHD for each SD higher HDL containing and lacking apoC-III according to CVD risk factors in each of the four cohorts and pooled the data using fixed-effects meta-analysis. Shown in fig 3, HDL lacking apoC-III was generally inversely associated with risk of CHD across all participant characteristics, whereas HDL containing apoC-III was associated with higher risk of CHD. As indicated by the confidence intervals, some subgroups had few cases (women, prevalent diabetes, use of cholesterol lowering medication, and low HDL-cholesterol level). Nevertheless, the stratified results suggested that HDL subspecies are differentially associated with CHD risk broadly across several participant characteristics associated with CHD risk. The results appeared to be slightly stronger in groups of participants characterized by lower cardiovascular risk, such as among those who were normal-weight, non-smokers, free of diabetes, who did not use cholesterol-lowering medication, or had HDL-cholesterol greater than 40 mg/dL, but the differences were not significant. Analyses in strata of self-reported race/ethnicity of the MESA participants only were not statistically powered to highlight potential differences across these subgroups (Supplemental Table S6).
Fig 3. Coronary heart disease risk per standard deviation of HDL containing (red) and lacking (blue) apoC-III in strata of CVD-risk factors in four prospective studies.
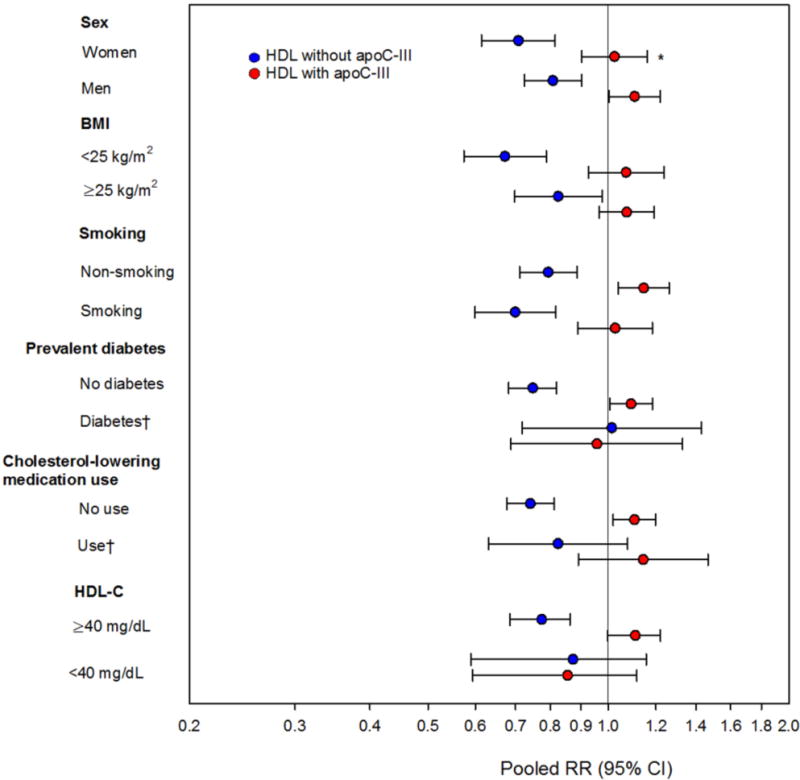
Pooled relative risks with 95% CI for risk of CHD estimated in fixed-effects meta-analysis using study-specific SDs in four prospective studies (the Diet, Cancer and Health [DCH] Study, the Multi-Ethnic Study of Atherosclerosis [MESA], the Health Professionals Follow-Up Study [HPFS], and the Nurses’ Health Study [NHS]). Adjusted for ASCVD (atherosclerotic cardiovascular disease) risk factors: age, sex, prevalent diabetes, treatment with anti-hypertensive medication, (race/ethnicity in MESA), systolic blood pressure, smoking, and total cholesterol.
Both subtypes of HDL (containing and lacking apoC-III) are simultaneously included in all models.
*Between-study heterogeneity, p<0.05.
†Estimates not available in NHS and HPFS.
Discussion
In this investigation of two large prospective studies, separation of HDL-apoA-I according to the presence and absence of apoC-III identified two subtypes of HDL that had qualitatively different associations with risk of CHD. In a cumulative meta-analysis of all current data on these HDL subspecies, the statistically significant direct association of HDL containing apoC-III with risk of CHD was strikingly different from the strong, inverse association for HDL lacking apoC-III, which appeared even stronger than for the association with total HDL.
While the fraction of HDL that contains apoC-III is small (approximately 5-6% of apoA-I or 10-15% of HDL cholesterol has apoC-III), these investigations may shed light on ways in which the HDL proteome could result in HDL particles with more or less cardioprotective functions. Thus far, little is known about the potential effects of apoC-III on important functional aspects of HDL. We have previously demonstrated that apoC-III inhibits the clearance from the circulation of apoB-containing lipoproteins by interfering with the interaction of apoE with hepatic receptors,12 and preliminary data from our laboratory support a similar delayed clearance of HDL containing apoC-III. We observed a weak correlation between the proportion of HDL that contains apoC-III and cholesterol efflux (0.12), consistent with their separate biological roles. As such, the measure of HDL subspecies according to apoC-III may be viewed as complementary to that of cholesterol efflux capacity via ABCA1, which is focused on the initial lipidation of the apoA-I particle, but does not reflect the delivery of cholesterol to the liver. Further, apoC-III may have influence beyond cholesterol transport. Riwanto et al. found that compared to healthy patients, HDL from CHD patients had elevated apoC-III levels and had lost its capacity to inhibit endothelial cell apoptosis.30 Earlier, we found that HDL that lacks apoC-III inhibits the monocyte-endothelial cell interaction, leading to a lowered inflammatory response, whereas HDL with apoC-III did not diminish this interaction.31 Thus, even if total HDL levels are high, this would not translate into a lower risk of CHD in individuals who have a large proportion of HDL containing apoC-III.
We also measured the total plasma concentration of apoC-III and the concentration of apoC-III in HDL in the Danish study. Consistent with the literature, we observed that while apoC-III is directly associated with CHD risk, it is not robust to adjustment for clinical risk factors and other lipids,18, 32 suggesting that the concentrations of lipoproteins (HDL and LDL) that contain any apoC-III may be more relevant cardiovascular risk measures than are total plasma apoC-III or the concentration of apoC-III in LDL and HDL.18, 33
Strengths of this study include its large number of cases and the opportunity to look into potential effect modification by race/ethnicity and sex. However, we had limited statistical power for stratified analyses by prevalent diabetes status and use of lipid-lowering medications. In the meta-analysis, we included two prior publications where the surrogate measure of HDL was HDL-cholesterol concentration, rather than apoA-I. Hence, we used study-specific SDs and quintiles as our primary units of measurement as no universal cutpoints have been identified. Similar results ensued from using fixed increments of 5 mg/dL and overall the two measures of HDL, cholesterol and apoA-I, provided associations of same magnitude and direction. The direct measure of the concentration of apoA-I containing and lacking apoC-III using two antibodies has shortened the process of measuring the HDL subspecies considerably and reduced measurement error, making this biomarker measure well-suited for assessment in future large-scale epidemiologic settings.
We had information on LDL-cholesterol or apoB in both MESA and DCH, but did not measure LDL with and without apoC-III in those studies. However, we previously found in NHS and HPFS that adjustment for LDL subspecies had virtually no effect on estimates for HDL subspecies after adjustment for total LDL-cholesterol.18
While our data suggest that apoC-III may confer pro-atherogenic properties to HDL, further research is necessary to establish how measures of HDL containing and lacking apoC-III relate to functional properties of HDL, such as cholesterol efflux from cells, inflammation, or cholesterol excretion.
Lastly, our HDL measurements from a single time point may not reflect long-term exposure, and does not allow the evaluation of associations between changes in HDL subspecies distribution with the risk of CHD.
In conclusion, our findings from four prospective studies support the hypothesis that apoC-III may mark a subfraction of HDL that is associated with higher risk of CHD. New measures reflecting HDL structure and function may provide novel insights for cardiovascular risk that extend beyond traditional plasma HDL concentrations.
Supplementary Material
Clinical Perspective.
What is new?
We identified a subspecies of HDL that contains apoC-III. HDL containing apoC-III comprises 5-6% of apoA-I or 10-15% of HDL cholesterol.
In four prospective studies, HDL containing apoC-III was associated with a greater risk of coronary heart disease, whereas HDL that lacked apoC-III was inversely associated with risk, more strongly than the total HDL.
What are the clinical implications?
ApoC-III may adversely affect anti-atherogenic properties of HDL.
HDL containing apoC-III may be useful to evaluate response to future HDL-raising therapies.
Measures that reflect function or structure of HDL may provide information on cardiovascular risk beyond traditional HDL concentrations.
Acknowledgments
Sources of Funding: MESA is an NHLBI-funded study supported by grants R01 HL071739 and R21 HL091217 from the National Heart, Lung, and Blood Institute (NHLBI), T32 DK 007703 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources, and by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from NHLBI. HDL apoC-III measurements were supported by an independent research grant from Roche Pharmaceuticals and by the William F. Milton Fund, Harvard Medical School.
The Diet, Cancer and Health Study is supported by the Danish Independent Research Council and the Danish Cancer Society. HDL apoC-III measurements in were funded by a Young Research Award from the Danish Independent Research Council.
The funding sources had no role in the conduct of the study.
Disclosures: Roche Pharmaceuticals provided unrestricted funding for the development of the novel ApoA-I/ApoC-III ELISA and had no role in the design of the study, data collection, analyses or report. Harvard University holds a patent for the apoC-III defined HDL subspecies assay.
References
- 1.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, Ruzyllo W, Bachinsky WB, Lasala GP, Tuzcu EM. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–1316. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 4.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 5.Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, DerOhannessian S, Kontush A, Surendran P, Saleheen D, Trompet S, Jukema JW, De Craen A, Deloukas P, Sattar N, Ford I, Packard C, Majumder A, Alam DS, Di Angelantonio E, Abecasis G, Chowdhury R, Erdmann J, Nordestgaard BG, Nielsen SF, Tybjaerg-Hansen A, Schmidt RF, Kuulasmaa K, Liu DJ, Perola M, Blankenberg S, Salomaa V, Mannisto S, Amouyel P, Arveiler D, Ferrieres J, Muller-Nurasyid M, Ferrario M, Kee F, Willer CJ, Samani N, Schunkert H, Butterworth AS, Howson JM, Peloso GM, Stitziel NO, Danesh J, Kathiresan S, Rader DJ, Consortium CHDE, Consortium CAE and Global Lipids Genetics C Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–1171. doi: 10.1126/science.aad3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, Konig IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schafer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O’Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–513. doi: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alaupovic P. Significance of apolipoproteins for structure, function, and classification of plasma lipoproteins. Methods Enzymol. 1996;263:32–60. doi: 10.1016/s0076-6879(96)63004-3. [DOI] [PubMed] [Google Scholar]
- 10.Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, Geary RS, Baker BF, Graham MJ, Crooke RM, Witztum JL. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med. 2014;371:2200–2206. doi: 10.1056/NEJMoa1400284. [DOI] [PubMed] [Google Scholar]
- 11.Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, Mitchell BD, Miller M, O’Connell JR, Shuldiner AR. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendivil CO, Zheng C, Furtado J, Lel J, Sacks FM. Metabolism of very-low-density lipoprotein and low-density lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler Thromb Vasc Biol. 2010;30:239–245. doi: 10.1161/ATVBAHA.109.197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-Function Mutations in APOC3 and Risk of Ischemic Vascular Disease. N Engl J Med. 2014;371:32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 14.TG, HDL Working Group of the Exome Sequencing Project NHLBI. Loss-of-Function Mutations in APOC3, Triglycerides, and Coronary Disease. N Engl J Med. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsson M, Vorrsjo E, Talmud P, Lookene A, Olivecrona G. Apolipoproteins C-I and C-III inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J Biol Chem. 2013;288:33997–34008. doi: 10.1074/jbc.M113.495366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacks FM. The crucial roles of apolipoproteins E and C-III in apoB lipoprotein metabolism in normolipidemia and hypertriglyceridemia. Curr Opin Lipidol. 2015;26:56–63. doi: 10.1097/MOL.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawakami A, Aikawa M, Libby P, Alcaide P, Luscinskas FW, Sacks FM. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation. 2006;113:691–700. doi: 10.1161/CIRCULATIONAHA.105.591743. [DOI] [PubMed] [Google Scholar]
- 18.Jensen MK, Rimm EB, Furtado JD, Sacks FM. Apolipoprotein C-III as a Potential Modulator of the Association Between HDL-Cholesterol and Incident Coronary Heart Disease. J Am Heart Assoc. 2012;1:jah3–e000232. doi: 10.1161/JAHA.111.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 20.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 21.Tjonneland A, Olsen A, Boll K, Stripp C, Christensen J, Engholm G, Overvad K. Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: a population-based prospective cohort study of 57,053 men and women in Denmark. Scand J Public Health. 2007;35:432–441. doi: 10.1080/14034940601047986. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen CB, Goetzche H, Moeller J, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. DanMed Bull. 2006;53:441–449. [PubMed] [Google Scholar]
- 23.Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. DanMed Bull. 1999;46:263–268. [PubMed] [Google Scholar]
- 24.Joensen AM, Jensen MK, Overvad K, Dethlefsen C, Schmidt E, Rasmussen L, Tjonneland A, Johnsen S. Predictive values of acute coronary syndrome discharge diagnoses differed in the Danish National Patient Registry. J Clin Epidemiol. 2009;62:188–194. doi: 10.1016/j.jclinepi.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–12. [Google Scholar]
- 26.Petersen L, Sorensen TI, Andersen PK. Comparison of case-cohort estimators based on data on premature death of adult adoptees. Stat Med. 2003;22:3795–3803. doi: 10.1002/sim.1672. [DOI] [PubMed] [Google Scholar]
- 27.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC., Jr Tomaselli GF and American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ, Coordinating Committee of the National Cholesterol Education P Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, Perisa D, Heinrich K, Altwegg L, von Eckardstein A, Luscher TF, Landmesser U. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127:891–904. doi: 10.1161/CIRCULATIONAHA.112.108753. [DOI] [PubMed] [Google Scholar]
- 31.Kawakami A, Aikawa M, Alcaide P, Luscinskas FW, Libby P, Sacks FM. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation. 2006;114:681–687. doi: 10.1161/CIRCULATIONAHA.106.622514. [DOI] [PubMed] [Google Scholar]
- 32.Blankenhorn DH, Alaupovic P, Wickam E, Chin HP, Azen SP. Prediction of angiographic change in native human coronary arteries and aortocoronary bypass grafts. Circulation. 1990;81:470–476. doi: 10.1161/01.cir.81.2.470. [DOI] [PubMed] [Google Scholar]
- 33.Mendivil CO, Rimm EB, Furtado J, Chiuve SE, Sacks FM. Low-density lipoproteins containing apolipoprotein C-III and the risk of coronary heart disease. Circulation. 2011;124:2065–2072. doi: 10.1161/CIRCULATIONAHA.111.056986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


