Abstract
The hippocampal formation (HF) and medial prefrontal cortex (mPFC) play critical roles in spatial working memory (SWM). The nucleus reuniens (RE) of the ventral midline thalamus is an important anatomical link between the HF and mPFC, and as such is crucially involved SWM functions that recruit both structures. Little is known, however, regarding the role of RE in other behaviors mediated by this circuit. In the present study, we examined the role of RE in spatial working memory and executive functioning following reversible inactivation of RE with either muscimol or procaine. Rats were implanted with an indwelling cannula targeting RE and trained in a delayed nonmatch to sample spatial alternation T-maze task. For the task, sample and choice runs were separated by moderate or long delays (30, 60, and 120s). Following asymptotic performance, rats were tested following infusions of drug or vehicle. Muscimol infused into RE impaired SWM at all delays, whereby procaine only impaired performance at the longest delays. Furthermore, RE inactivation with muscimol produced a failure in win-shift strategy as well as severe spatial perseveration, whereby rats persistently made re-entries into incorrect arms during correction trials, despite the absence of reward. This demonstrated marked changes in behavioral flexibility and response strategy. These results strengthen the role of nucleus reuniens as a pivotal link between hippocampus and prefrontal cortex in cognitive and executive functions, and suggest that nucleus reuniens may be a potential target in the treatment of CNS disorders such as schizophrenia, attention deficit hyperactivity disorder, addiction, and obsessive-compulsive disorder, whose symptoms are defined by hippocampal-prefrontal dysfunctions.
Keywords: hippocampus, medial prefrontal cortex, orbital cortex, perseverative behavior, goal directed behavior
1 INTRODUCTION
It is well recognized that the hippocampal formation (HF) and the medial prefrontal cortex (mPFC) serve a critical role in spatial working memory (SWM). Lesions or inactivation of either structure in rats produces severe deficits in SWM (Floresco et al., 1997; Lee and Kesner, 2003; Jones and Wilson, 2005; Yoon et al., 2008; Churchwell et al., 2010; Churchwell and Kesner, 2011; Colgin, 2011; Gordon, 2011; O’Neill et al., 2013; Preston and Eichenbaum, 2013; Griffin, 2015; Sapiurka et al., 2016). Evidence suggests that the HF and mPFC may function independently to process spatial information at brief or no delays (seconds) in SWM tasks, but mutually interact at longer delays. In this regard, Kesner and colleagues (Lee and Kesner, 2003; Churchwell and Kesner, 2011) demonstrated that the HF and mPFC act in parallel to process spatial memory at 10 sec delays, but interact at 5 min delays. Specifically, they showed that the bilateral inactivation of the HF or the mPFC, or disconnecting the two structures, produced deficits at 5 min delays on a spatial delay non-match to sample task (DNMTS), but both manipulations were ineffective at 10 sec delays. In effect, although the hippocampus and the mPFC are in constant communication in the ongoing exchange of information at multiple time scales, the mPFC appears to possess all the information needed to make goal directed decisions at no (or short delays), as indicated by its well documented role in delayed response tasks (for review, Goldman-Rakic, 1995; Kesner, 2000; Dalley et al., 2004), but requires stored information from the HF at longer delays.
Accordingly, several studies have described coordinated activity between the HF and mPFC during the performance of working memory tasks (Jones and Wilson, 2005; Siapas et al., 2005; Benchenane et al., 2010; Hyman et al., 2010; Gordon, 2011; O’Neill et al., 2013; Remondes and Wilson, 2013; Shin and Jadhav, 2016). For instance, using a spatial alternation task in rats, Jones and Wilson (2005) demonstrated strong hippocampal theta coherence and phase locking to cells of the mPFC which was associated with successful task performance. Similarly, Benchenane et al. (2010) described increases in hippocampal and prefrontal theta coherence, particularly at the choice point of a Y maze during a working memory task.
With respect to the precise nature of HF-mPFC interactions in delay tasks, Hyman et al. (2011) proposed that the mPFC and HF encode overlapping information about the environment, but from slightly different perspectives. Specifically, the HF is more attuned to the location of an animal within its environment, whereas the mPFC provides a “more holistic and egocentric representation of the entire environment”. As such, appropriate responding in delay tasks, requires the alignment of the egocentric representations provided by the mPFC with the allocentric information conveyed by the HF. According to them, this is primarily accomplished by the entrainment of mPFC cells to hippocampal theta selectively at the choice point in WM tasks (Hyman et al., 2010, 2011).
The nucleus reuniens (RE) of the ventral midline thalamus is strongly reciprocally connected with the HF and the mPFC (Vertes et al., 2006), and contains a population of cells that project via axon collaterals to both sites (Hoover and Vertes, 2012; Varela et al., 2014). Further, in the absence of direct projections from the mPFC to the HF (Laroche et al., 2000; Vertes, 2002, 2004), RE is a major route linking the mPFC to the HF (Vertes et al., 2006, 2007, 2015). Accordingly, RE is ideally suited to coordinate the actions of the HF and the mPFC in SWM, and alterations of RE disrupt performance in SWM tasks involving HF-mPFC interactions (Hembrook and Mair, 2011; Hembrook et al., 2012; Loureiro et al., 2012; Cholvin et al., 2013; Hallock et al., 2013a; Layfield et al., 2015).
For instance, Mair and colleagues (Hembrook and Mair, 2011; Hembrook et al., 2012) reported that suppression of RE (and the dorsally adjacent rhomboid nucleus, RH) impaired performance on a delayed non-match to sample radial arm maze (RAM) task and on a delayed non-match to position task -- both of which are sensitive to disruption of the HF and the mPFC. Performance, however, was unaltered on a variable choice paradigm on the RAM, dependent only on the hippocampus. In addition, Layfield et al. (2015) recently reported that the reversible inactivation of RE via muscimol impaired spatial working memory performance on a delayed alteration task.
It is well recognized that dorsal HF, by contrast with the ventral HF, is primarily involved in spatial behaviors (Moser and Moser, 1998; Fanselow and Dong, 2010; Nadel et al., 2013). Interestingly, however, the dorsal HF does not project to the mPFC, suggesting that dorsal HF effects on the mPFC in spatial processing are mediated by an intervening structure(s) – a likely candidate being RE. Specifically, the dorsal HF (or dorsal subiculum) projects to RE which, in turn, distributes heavily to the mPFC (Jay and Witter, 1991; Carr and Sesack, 1996; Laroche et al., 2000; Hoover and Vertes, 2007). Supporting RE as a link between dorsal HF and the mPFC, Griffin and colleagues (Hallock et al., 2016) recently showed that dorsal HF actions on the mPFC in SWM were routed through RE. Specifically, they demonstrated that: (1) a population of mPFC cells, selectively active during a delayed SWM task, were entrained to hippocampal theta during successful task performance; (2) hippocampal theta was strongly coupled to theta and gamma oscillations of the mPFC under the same conditions; and (3) reversible inactivation of RE with muscimol disrupted HF-mPFC synchronous oscillations and performance on the delayed SWM task. The authors concluded that their findings provide direct evidence that “reuniens contributes to SWM by modulating hippocampal-prefrontal synchrony”.
In addition to its role in SWM, RE has also been shown to be directly involved in “executive functions” such as attention, goal directed behavior, and behavioral flexibility. This is consistent with pronounced RE projections to the medial and orbital prefrontal cortices -- key modulators of behavioral regulation (Bannerman et al., 2004; Dalley et al., 2004; Kehagia et al., 2010; Chudasama et al., 2012; Abela and Chudasama, 2013; Abela et al., 2013; Izquierdo, 2017). In this regard, Dolleman–van der Weel et al. (2009) initially reported that RE lesions did not alter spatial reference memory on a water maze task, but instead led to a maladaptive search strategy on probe trials which was deemed a prefrontal deficit. Along similar lines, Prasad et al. (2013) found that RE lesions produced impairments in behavioral inhibition, as demonstrated by increases in premature (impulsive) responses on a 5-choice serial reaction time task (5-CSRTT).
Premature responding is viewed as a failure of impulse control and is seen with lesions of the infralimbic cortex on the 5-CSRTT task (Chudasama et al., 2003). Finally, we recently described deficits in attention and reversal learning (a measure of behavioral flexibility) on an attentional set shifting task in RE lesioned rats (Linley et al., 2016).
In the present report, then, we sought to further define the role of nucleus reuniens: (1) in spatial working memory, by examining the effects of reversible inactivation of RE on a delayed alternation T-maze task using moderate to long delays (30, 60 or 120s) presented in random order; and (2) in executive functions, by examining the ability of rats to flexibly alter their behavior following incorrect (non-rewarded) responses on the T-maze task. RE was reversibly inactivated using muscimol, a GABA-A agonist, or procaine, a sodium channel blocking blocker. We showed that the inactivation of nucleus reuniens with muscimol impaired spatial working memory at all delays, and further resulted in severe perseverative behavior wherein rats persistently made incorrect choices on the T-maze in the absence of reward.
2 MATERIALS AND METHODS
2.1 Animals
Adult male Long Evans rats (n=18) weighing between 275–350 grams upon arrival (Harlan, Indianapolis, IN; Charles Rivers, Wilmington MA) served as subjects in the experiment. Rats were single housed in a climate-controlled colony on a 12 h light/dark cycle (lights on at 7 AM) and had access to a variety of enriching materials in their homecage throughout the experiment. Food and water were available at libitum at arrival and before and after surgery. After a minimum of 7 days of post-surgical recovery, rats were placed on a food restricted diet and maintained at 85–90% their free feeding weight. The experiment was approved by the Florida Atlantic University Institutional Annual Care and Use Committee and conform to all federal regulations and National Institutes of Health guidelines for the care and use of laboratory animals.
2.2 Surgical procedures
Rats were anesthetized with isoflurane (4–5% induction, 1.5–3% maintenance) and placed in a stereotaxic apparatus, where an incision was made to expose the skull. A burr hole was drilled over the midline thalamus and a 6 mm guide cannula (Plastics One, Roanoke, VA), lowered at an angle of 15°, was implanted above the reuniens and rhomboid nuclei of the ventral midline thalamus (stereotaxic coordinates: −2.0/-2.1 mm AP; +2.0 mm L; 6.5 – 7.0 mm DV). Bone screws were anchored to the skull, and the screws and cannula were affixed to the skull using dental cement. Dummy cannulas (7 mm in length) were inserted to cover the implanted guide. Rats were given 7 days to recuperate before being placed on a food restricted diet and maintained at 85–90% free feeding weight throughout the remainder of the experiment.
2.3 Apparatus
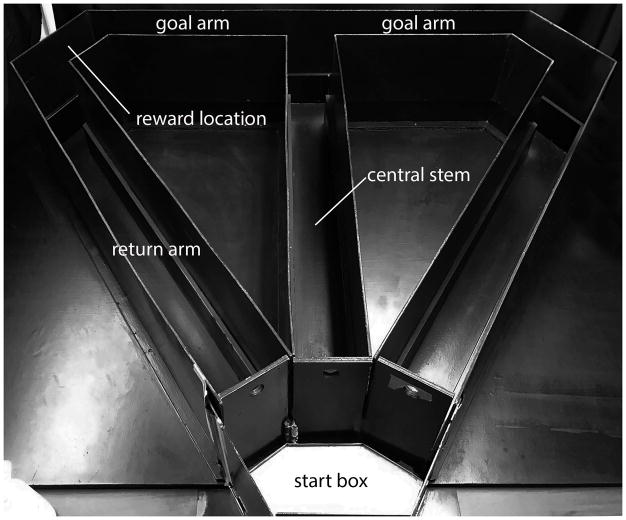
The behavioral apparatus was a black wooden T-maze (Fig. 1). A start box (18 cm in length) opened to the central stem (116 cm length × 10 cm width × 30 cm height) of the maze and extended to the intersection of two (L and R) goal arms (56.5 cm length × 10 cm width × 18 cm height). At the end of each goal arm, a plastic cap contained a food reward (Dustless Precision Pellets – 45 mg chocolate flavor purified rodent diet, BioServe, Flemington, NJ). The goal arms were connected to two return arms (112 cm length × 10 cm width × 30 cm height) to the start box. Removable guillotine doors were positioned at the beginning of the central stem, at the start point of each goal arm, and at the end of each return arm. The maze was fixed to a table which was elevated approximately 60 cm from the floor and was enclosed by a tent frame, surrounded by black curtains, which served as constant contextual cues. A single halogen lamp located on the floor and outside the curtain provided illumination to the room. White noise was generated using the iTunes app White Noise Box (Skunk Brothers GmbH, 2014), which played continuously in the background via 2 audio speakers throughout the training and testing sessions.
Figure 1.
Photograph of the T-maze used in the experiment. Rats were placed in the startbox and required to run down the central stem of the maze to a choice point where they could turn left or right to retrieve a food reward at the goal location. Return arms allowed the rat to go back to the startbox where rats remained during the delay intervals. See experimental procedures for maze dimensions.
2.4 Behavioral testing
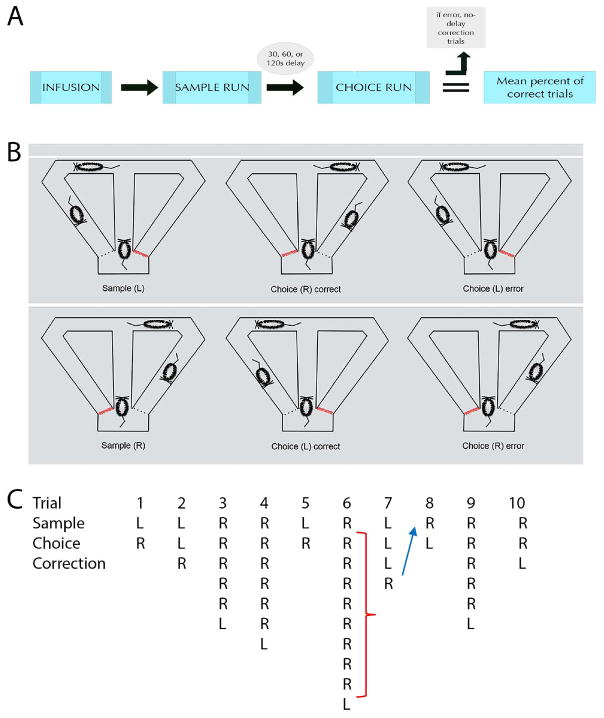
Prior to training, rats were acclimated to the testing room and maze, and then were pretrained using a no-delay spatial alternation task. In this task, both goal arms are baited with a food reward. Rats were given 10 daily trials, each of which consisted of a sample and choice run. During a sample run, rats traversed the central stem and were allowed to freely choose a directional response and retrieve a reward from one of the goal arms. When they returned to the start box, the choice run began immediately, whereby entering the opposite goal arm yielded a reward. Once performance reached a level of 80% correct trials across 3 consecutive testing sessions, delays were interposed between the sample and choice run, and rats were trained to alternate following delays. A delayed nonmatch to sample (DNMS) testing session consisted of 10 trials, beginning with a single no-delay spatial alternation trial, followed by 30s, 60s, or 120s delay trials, presented in a randomized order, wherein each delay was equally represented in each session. The delay period began when the rat re-entered the start box through the return arm and ended when the divider from the central stem was lifted. Following a return into the startbox after a choice run, arms were re-baited and the next trial began – yielding an intertrial interval (ITI) of 10–15 seconds. Figure 2 illustrates the experimental design of the DNMS task.
Figure 2.
The experimental design of the delayed nonmatch to sample (DNMS) task used in the present experiment is shown using a flowchart (A) and schematic illustrations (B). Once trained to asymptotic levels, rats were infused with one of four agents (0.25 mg/mL muscimol, 0.50 mg/mL muscimol, procaine, or PBS vehicle) and following a period of either 30 min (muscimol) or 5 min (procaine), rats were given 10 trials, each of which included a sample and choice run. In the sample run, rats could freely choose between the left or right goal arms to retrieve a food reward. Following a delay of either 30, 60, or 120s, rats were given a choice run, whereby only the opposite arm was baited with a reward. If rats made an error on the choice run, they were given no-delay correction runs, whereby they could make repeated runs down the maze until the correct arm was chosen and reward was retrieved. Red lines indicate closed doors at all times in the startbox prior to sample or choice runs. Dotted lines indicate closed doors, one of which will be opened once rats make a directional choice during sample or choice runs. An example of a testing session following a muscimol injection for a representative case is depicted in (C), showing three distinct types of errors in the study. In trial 2, a failure to alternate following the delay between the sample and choice run is denoted as a working memory error and would be marked as incorrect. Trial 6 depicts examples of perseverative errors (red bracket) following a working memory error, whereby after making an incorrect choice run following a delay, the rat persists in making incorrect runs during no-delay correction trials despite the absence of a reward. Lastly, trial 8 depicts an example of a win-shift failure (blue arrow), which is a failure to alternate in a subsequent sample run after making the correct directional selection on the previous trial.
As described above, delay trials began with a sample run, whereby rats could freely choose which arm to traverse to retrieve a reward. When the rat returned to the startbox (and after set delays) rats were required to alternate to the opposite arm to collect the reward. When an incorrect choice was made during the choice run, rats were given correction runs. Correction runs began immediately (no delay) after the rats returned to the start box following an error. Rats were allowed to make correction runs until the reward was successfully retrieved (i.e., the rat made the correct directional response). If a rat did not correct their behavior after 10 correction runs, the trial was terminated when the rat returned to the startbox. This was followed by the next sample run. This modified version of the DNMS task allowed for the measurement of three distinct types of errors: choice errors, defined as a failure to alternate between the sample and choice runs on a trial; perseverative errors, defined as continued re-entries into the incorrect arm during correction trials; and win-shift failures, defined as the failure to alternate on a subsequent sample run after making the correct directional selection on the previous trial. Figure 2c depicts examples of working memory, win-shift, and perseverative errors for one rat. Once rats reached 80% correct or greater performance across three consecutive days, microinfusion procedures began. The rats were tested in a within subjects design across several infusion days. Following an infusion, rats were given as many non-infusion training sessions necessary to return performance to asymptotic levels. Figure 2a outlines the infusion/test procedures.
2.5 Drug and microinfusion procedures
Rats underwent 4 infusion sessions: phosphate buffered saline (PBS, vehicle), muscimol 0.25 mg/mL, muscimol 0.50 mg/mL and a 20% procaine solution given in randomized but counterbalanced order across subjects. Muscimol (Sigma, St. Louis, MO) and procaine hydrochloride (Sigma, St. Louis, MO) were dissolved in PBS and given in a volume of 0.5 μl into the ventral midline thalamus at a flow rate of 0.25 μl/min. Doses of muscimol were based on previous descriptions of dose dependent behavioral effects of muscimol injected into RE (Hallock et al, 2013; Layfield et al., 2015). Doses of procaine were based on values used in previous studies (Oddie et al., 1994, 1996; Bland et al., 1994). Once rats achieved asymptotic levels of performance (80%), they were tested with a mock infusion, wherein rats were restrained, screwable dummy caps were replaced with a 26 g internal cannula that extended 1 mm past the length of the internal cannula guide, but no infusion was made. Rats were then infused 24 hours later once performance reached 80% accuracy or greater during a mock session. During infusions, the internal cannula was connected to a Hamilton microliter syringe via a polyethylene tube, which was loaded into a syringe pump (Model 22 Syringe Pump, Harvard Apparatus, Holliston, MA). The infusers remained in place for an additional 2 minutes to allow for diffusion and to avoid reflux of the solution. Thirty minutes after muscimol infusions and five minutes after procaine infusions, rats underwent behavioral testing.
2.6 Histological analysis
At the completion of behavioral testing, rats were perfused intracardially with 150 mL heparinized saline followed by 300–400 mL 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were removed and post-fixed for another 24–48 hours in 4% paraformaldehyde, then placed in 30% sucrose solution for another 48 hours. Following sucrose cryoprotection, 50 μm coronal sections were cut on a freezing sliding microtome, and every 6th section throughout the thalamus was collected. Sections were mounted on chrome-alum gelatin slides, stained with cresyl violet, and coverslipped with Permount. The Nissl stained sections were used to identify the nuclear boundaries of thalamic nuclei and the placement of both the indwelling cannula guide and tip of the dummy and infusers cannula. A subset of rats was infused with fluorescent conjugated muscimol (Life Technologies/Thermo Fisher Scientific, Waltham, MA) and perfused 30 minutes later. This series of sections was coverslipped using DPX media (BDH Laboratories, Poole, England) or Permount and the spread of the fluorescent conjugated muscimol was visualized using epi-fluorescent techniques.
2.7 Data analysis
A 3 (delay) × 4 (experimental condition) mixed design analysis of variance was conducted on all measures unless otherwise specified. Post-hoc analysis was done using Bonferroni’s correction to analyze pairwise comparisons. All data in the text of results are represented as mean (M) and standard deviation (SD). Statistics are reported as F(degrees of freedom)= F statistic, p value. Error bars represent standard error of the mean. All calculated measures used an alpha level of < 0.05 to reach statistical significance. Statistics tests were computed using SPSS 23.0 for Mac.
3 RESULTS
3.1 Histological verification of microinfusions into nucleus reuniens
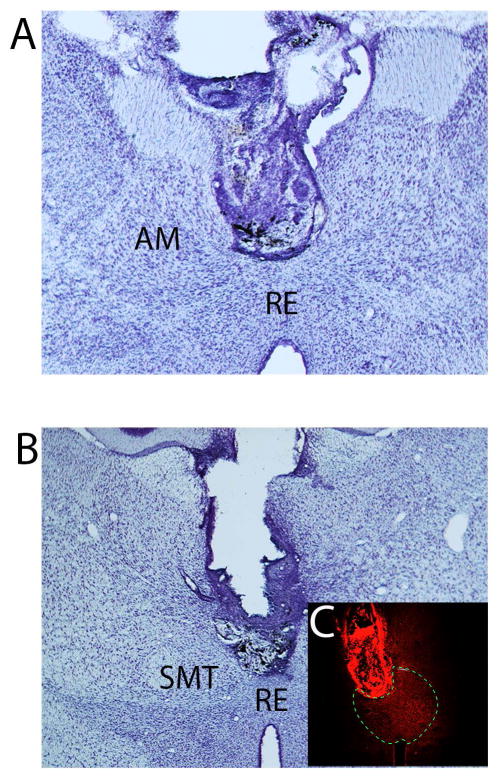
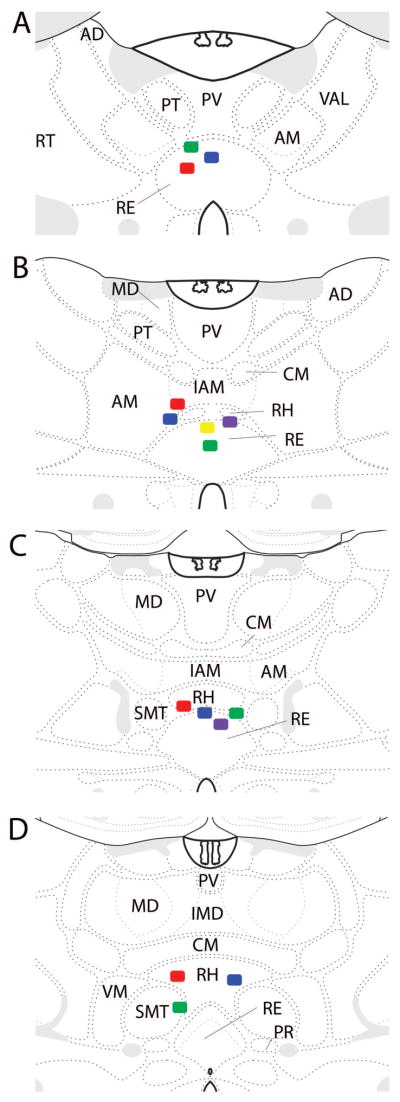
Fifteen rats were included in this study using histological verification that muscimol infusions targeted the ventral midline thalamus. In most of these cases, the cannula guide was positioned just dorsal to the start of the rhomboid or reuniens nuclei, so that the tip of the infuser was centered in RE (n = 11). In five animals, the tip of the infuser extended into dorsal aspects of RE. Figure 3(A, B) shows photomicrographs of Nissl-stained sections of two representative cases demonstrating cannula placements in RE. The photomicrograph of Figure 3C shows the spread of the fluorescent conjugated muscimol was restricted to the ventral midline thalamus (RE/RH) in this representative case. Figure 4 is a series of schematic plates showing the cannula placements for all rats included in the study. Nine of these cases had cannula placements targeting rostral RE, while 6 rats had cannula centered in mid-rostral to mid-levels of RE. Of the original 18 rats, three were excluded from the study due to misplacement of the guide cannula. In these cases, the guide cannulas were positioned in the ventral RE, wherein infusions extended into the hypothalamus (n = 2) or positioned laterally off the midline (n = 1) whereby the injection spread outside of RE.
Figure 3.
Low magnification photomicrographs of Nissl stained transverse sections showing the locations of guide cannula in the rhomboid (A) and reuniens (B) nuclei of the ventral midline thalamus of two representative cases. C: photomicrograph illustrating the spread of the fluorescent conjugated muscimol restricted to RE and RH in this representative case. The borders of RE are indicated with the dashed line. Abbreviations: AM, anterior medial nucleus of the thalamus; RE, nucleus reuniens of thalamus; RH, rhomboid nucleus of thalamus; SMT submedial nucleus of thalamus.
Figure 4.
Schematic diagrams illustrating the infusion sites at four levels (A–D) of the midline thalamus (plates adapted from Swanson, 2003). Colored squares show the position of the tip of the infusion cannula (1 mm below the end of guide cannula). As shown, infusion sites were located within the rhomboid (RH) and reuniens (RE) nuclei of the ventral midline thalamus in each of the included cases. Abbreviations: AD, anterior dorsal nucleus of thalamus; AM, anterior medial nucleus of thalamus; CM, central medial nucleus of thalamus; IAM, interoanteromedial nucleus of thalamus; IMD, interomediodorsal nucleus of thalamus; MD, mediodorsal nucleus of thalamus; PR, perireuniens nucleus of thalamus; PT, parataenial nucleus of thalamus; PV, paraventricular nucleus of thalamus; RT, reticular nucleus of thalamus; SMT, submedial nucleus of thalamus; VAL, ventral anterior lateral nucleus of thalamus; VM, ventromedial nucleus of thalamus.
3.2 Reversible inactivation of RE via muscimol impairs choice accuracy at all delays
In the delayed nonmatch to sample task, rats were given a unforced sample run, wherein they were allowed to select freely from the L or R goal arm of the T-maze. Upon returning to the start box, rats were delayed by 30s, 60s or 120s before making their ‘choice’ run. During the choice run, only the opposite arm contained a food reward and was the correct response. In effect, choice accuracy reflected the ability of rats to successfully alternate between goal arms across the sample and choice runs, as a measure of spatial working memory. Trials were scored as incorrect if rats failed to alternate between sample and choice runs. Infusions began after rats reached a criterion of 80% accuracy for three consecutive days. The mean number of training sessions to reach 80% accuracy or better on no delay training was 6.47 sessions (SD= 2.70), while the mean number of DNMS training to reach criterion was 26.90 sessions (SD = 15.46). Choice accuracy was measured by calculating the mean percentage of correct trials across each delay (30, 60 and 120s) for each of the following conditions: infusions of vehicle (PBS), 0.25 mg/mL muscimol, 0.50 mg/mL muscimol, or 20% procaine solution. The results are illustrated as a bar graph in Figure 5.
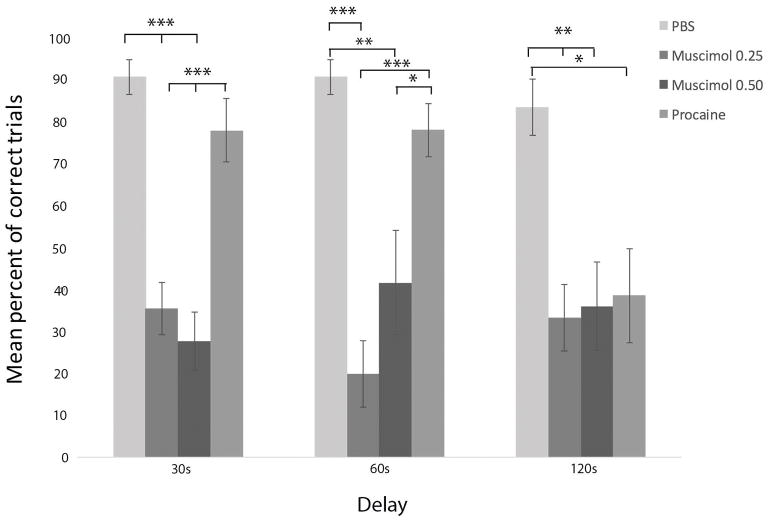
Figure 5.
Infusions of muscimol, at two doses, into RE impaired choice accuracy at each of the three delay times, measured by mean percent of correct trials, demonstrating that the inactivation of RE profoundly disrupts spatial working memory. By contrast, procaine injections in RE impaired choice accuracy only during the longest delay (120s). Error bars represent standard error of the mean. Significance is indicated by asterisks: * p < 0.05; ** p < 0.01; *** p < 0.001.
A repeated measures analysis of variance yielded no significant difference of performance on the initial no delay (0s) trial across any of the experimental conditions (PBS vehicle; muscimol 0.25 mg/mL; muscimol 0.50 mg/mL; procaine), F(3, 27) = 1.64, p > 0.05. By comparison, multivariate analysis of variance yielded a significant effect of experimental condition on choice accuracy across each of the delay intervals, F(3, 49) = 25.31, p < 0.001. Post-hoc analysis of pairwise comparisons using the Bonferroni correction revealed muscimol significantly decreased mean percent of correct trials at the 30s delay condition. Infusions of both 0.25 mg/mL (M = 35.47%; SD = 23.60) and 0.50 mg/mL (M = 27.67%; SD = 23.98) of muscimol into RE significantly decreased mean percent of correct trials compared to PBS vehicle (M = 90.57%; SD = 15.47). Muscimol inactivation of RE reduced performance from a mean of 91% correct responses with PBS infusions for the 30s delay trials to 30–40% after muscimol infusions, diminishing performance to below chance levels. Similarly, significant effects were also found across conditions for 60s delays whereby muscimol infusions reduced performance to approximately 20% correct responses at doses of 0.25 mg/mL (M = 19.93%; SD = 30.34) and to 42% at doses of 0.50 mg/mL (M = 41.67%; SD = 42.98) compared to PBS infusions (M = 90.57%; 15.47). Muscimol also significantly reduced the mean percent of correct trials at 120s delays to 30–40% following infusions of 0.25 mg/mL (M=33.27%; SD= 30.94) and 0.50 mg/mL (M = 36.00%; SD = 36.16) in comparison to vehicle infusions (M = 83.36% mean correct; SD = 25.37). No dose dependent effects of muscimol on mean percent of correct trials were observed between 0.25 mg/mL and 0.50 mg/mL muscimol at any delays (30, 60 or 120s).
No significant within subjects effect in performance was observed across each of the three delays, F(2, 48) = 0.948, p > 0.05, indicating no overall differences in performance across delays. Similarly, there was no significant drug × delay interaction, F (6,98) = 1.92, p > 0.05, demonstrating that performance was similar across all delays for all treatment conditions -- or no significant interactions between drug conditions and delays (30, 60 or 120s).
The order of infusions was counterbalanced across all subjects. To account for the possibility of order effects, or general improvement over time due to over training, we conducted a repeated measures analysis of variance with mean total percent of correct trials across each test session as the within subjects factor (all delay trials), across each of the four ordinal infusion days (regardless of drug condition). No significant differences in mean percent correct number of trials per session were found between the first (M = 52.00%; SD = 34.38), second (M = 53.33%; SD = 33.09), third (M = 67.33%; SD=26.58), or last drug infusion (M = 56.67%; SD = 29.92). This indicated that training, practice effects, or repeated administration (of muscimol) did not affect the outcome of the results.
3.3 Effects of procaine infusions into RE on working memory performance in the DNMS task
While muscimol significantly impaired choice accuracy across all delays, no significant differences in the mean percent of correct trials were found between procaine (M = 77.83%; SD = 26.00) and PBS (M = 90.57%; SD = 15.47) at 30s delay trials or between procaine (M = 77.92%; SD = 21.68) and PBS (M = 90.57%; SD = 15.47) at 60s delay trials. As would be expected, however, since significant differences in choice accuracy were observed between muscimol and PBS injections, choice performance was poorer following muscimol (0.25 and 0.50 mg/mL) infusions in RE, at 30s and 60s delays, compared to procaine infusions. Multivariate analysis of variance found no significant difference on 120 s delay trials between procaine (M = 55.50; SD = 38.58), 0.25 mg/mL muscimol (M = 33.27; SD = 30.94), and 0.50 mg/mL muscimol (M = 36.00; SD= 36.16) and PBS (M = 83.36; SD = 25.37) infusions into RE. Despite this, procaine infusions into RE reduced the mean percent of correct 120 second delay trials to near chance levels (55%) from a mean of 83% following vehicle infusions. Correspondingly, a pairwise independent t-test examining differences between procaine and vehicle infusions at 120s was significant, t(18.51) = 2.14, p < 0.05. In summary, while procaine infusions into RE did not significantly affect choice accuracy performance at 30 and 60s delays, it did at 120s delay compared to vehicle control.
The lack of behavioral effects at the shorter delays following procaine injections into RE was unexpected. Though the task is self-paced, testing sessions can last between 20–30 minutes or up to 1 hour if repeated errors are made. We examined whether the null effects of the drug could be related to the length of the testing sessions as the effects of procaine are fast-acting and short-lasting. We compared the mean percent of correct trials for the first half of the testing session (5 trials) to the mean percent of correct trials for the second half of the testing session (last five trials) for all rats by conducting a paired samples t-test. While mean percent of correct trials was lower in the first five trials (M = 67.14; SD = 26.73) in comparison to the last five trials (M = 75.71; SD = 22.43), these differences were not significant, t(13) = −1.03, p >0.05.
3.4 Behavioral inflexibility following reversible inactivation of RE with muscimol
We examined behavioral flexibility and spatial perseveration using a correction paradigm to determine whether or not rats could correct their response selection following an error. As indicated above, if rats selected the incorrect arm during the choice run (following a delay), they could make up to 10 correction runs by redirecting their response and alternating to the correct goal arm. The correction runs were made without delay intervals. During correction runs, incorrect responses (choosing the same direction as the sample run) were not rewarded. The measure of behavioral flexibility was the number of perseverative responses, or repeated incorrect choices of the unrewarded goal arm.
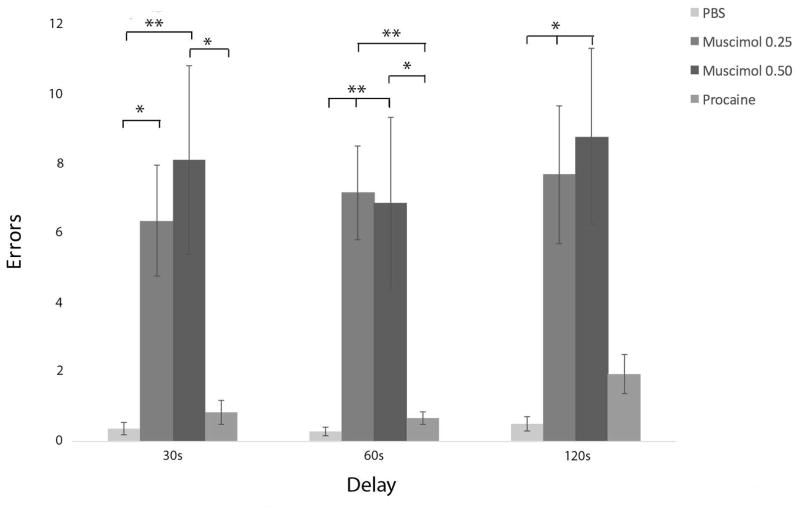
Figure 6 is a bar graph showing mean total perseverations for each delay following PBS, muscimol, and procaine infusions into nucleus reuniens. Multivariate analysis of variance revealed a significant effect of experimental condition on perseverative errors, F(3, 47) = 10.89, p < 0.001. Pairwise comparisons showed that muscimol at a dose of 0.25 mg/mL (M = 6.33; SD = 6.21) and 0.50 mg/mL (M = 8.08; SD = 9.38) produced significantly more perseverative errors following 30s delays compared to PBS vehicle (M = 0.36; SD = 0.63) – however, only the higher dose of muscimol produced significantly more perseverative errors compared to procaine (M = 0.83; SD = 1.19). On 60s delay trials, doses of 0.25 mg/mL muscimol (M = 7.13; SD = 5.26) and 0.50 mg/mL muscimol (M = 6.883; SD = 8.53) significantly increased perseverations compared to PBS (M = 0.29; SD = 0.47) and procaine (M = 0.67; SD = 0.65) infusions. Finally, 0.25 mg/mL muscimol (M = 7.00; SD = 7.66) and 0.50 mg/mL muscimol (M = 7.17; SD = 8.75) significantly increased perseverative responding at 120s delay compared to PBS (M = 0.50; SD = 0.76). There were no dose dependent effects of muscimol on behavioral flexibility; that is, no significant differences in mean total number of perseverations between 0.25 mg/mL and 0.50 mg/mL muscimol across any delay trials. In summary, muscimol infusions into RE significantly increased the number of perseverative responses across all delays (30, 60, and 120s).
Figure 6.
Inactivation of RE with muscimol produced striking spatial perseverative behavior in the DNMS task, whereby rats repeatedly re-entered the incorrect arm on correction runs, despite the absence of reward. Error bars represent standard error of the mean. Significance is indicated by asterisks: * p < 0.05; ** p < 0.01.
Finally, we examined win-shift failures, which were defined as the failure to alternate (or choose the opposite arm) after successfully shifting to the rewarded arm in the preceding choice run. Analysis of variance yielded a significant effect of experimental condition, F(3,49) = 4.21, p = 0.01. Post-hoc pairwise comparisons found that at all delay trials muscimol at doses of 0.25 mg/mL (M = 3.87; SD = 2.23) and 0.50 mg/mL (M= 3.50; SD = 1.78) produced significantly more win shift failures than either PBS (M = 1.50; SD = 1.91) and procaine (M = 2.75; SD = 1.54) trials. In summary, reversible inactivation of RE with muscimol not only impairs choice accuracy following delays (30s, 60s, or 120s), but further disrupts ability of rats to adapt and shift their behavioral response to the correct directional goal arm following repeated incorrect choices (or perseveration). Additionally, RE inactivation via muscimol significantly increased win-shift failures across all delays in the DNMS task.
4 DISCUSSION
We found that reversible inactivation of nucleus reuniens (RE) of the ventral midline thalamus with muscimol impaired working memory performance in a delay nonmatch to sample (DNMS) alternation paradigm using a T-maze task. The task required rats to hold information for moderate to long delay periods (30s, 60s or 120s). Whereas we hypothesized that impairments would be greater for the longer intervals (as cognitive load increased), this was not the case. Muscimol, at doses of 0.25 mg/mL and 0.50 mg/mL, infused directly into RE, impaired choice accuracy and increased errors at each of the delay times -- with performance well below chance levels. Furthermore, we showed that reversible inactivation of RE impaired behavioral flexibility. Following a delay period, if an error was made, rats were given the opportunity to correct their directional response with “correction runs”. In effect, following an error, (and without delay) rats could make repeated runs down the maze to choose the correct arm for reward. Following RE inactivation with muscimol, however, rats exhibited very pronounced spatial perseverative behaviors, repeatedly selecting the incorrect arm, and thus unable to shift their non-rewarded directional responding. These results indicate that RE is not only involved in spatial working memory but guides goal directed behavior and executive functioning.
4.1 Nucleus reuniens as a conduit in the exchange of cognitive information between the hippocampus and medial prefrontal cortex in SWM
As discussed, the hippocampus (HF) and the medial prefrontal cortex (mPFC) play a pivotal role in spatial working memory (SWM), or the ability to hold and manipulate spatially relevant information for brief periods of time. For instance, the disruption of the hippocampus or the medial prefrontal cortex has been shown to impair SWM on various spatial tasks. In alternation paradigms, hippocampal lesions do not affect the ability of rats to alternate continuously in T or Y mazes, but produce delay dependent deficits -- with delays ranging from seconds to several minutes (Rawlins and Olton, 1982; Hock and Bunsey, 1998; Ainge et al., 2007; Yoon et al., 2008; Czerniawski et al., 2009; Sapiurka et al., 2016). By contrast, lesions or inactivation of the medial prefrontal cortex produce delay independent effects on SWM tasks, altering performance on both continuous and delayed versions of spatial and nonspatial working memory tasks (Granon et al., 1994; Sanchez-Santed et al., 1997; Delatour and Gisquet-Verrier, 1996, 2000; Floresco et al., 2008; Kinoshita et al., 2008; Horst and Laubuch, 2009; Rossi et al., 2012; Shaw et al., 2013; Urban et al., 2014; Sapiurka et al., 2016). The essentially equivalent effects of disruption of the HF or the mPFC on SWM are consistent with findings showing that SWM involves the interaction/cooperation of these two structures (Wang and Cai, 2006; Churchwell and Kesner, 2011; Spellman et al., 2015).
For instance, as discussed (see Introduction), Churchwell and Kesner (2011), using a disconnection procedure, showed the HF and mPFC function independently at short (10s) delays, but mutually cooperate at longer (5 min) delays. Similarly, Wang and Cai (2006) reported that bilateral inactivation of the mPFC or the HF, or disconnecting the structures by unilaterally inactivating them in opposite hemispheres, impaired SWM on a delayed alternation t-maze task. By comparison, inactivation of the mPFC and HF on the same side of the brain had no effect on performance. In effect, disruption of the communication between the HF and the mPFC severely impaired SWM.
Consistent with this, several reports have described the dependence of hippocampal-mPFC interactions for successful performance on various SWM tasks (Jones and Wilson, 2005; Siapas et al., 2005; Benchenane et al., 2010; Hyman et al., 2010, 2011; Sigurdsson et al., 2010; O’Neill et al., 2013; Spellman et al., 2015; Hallock et al., 2016; Shin and Jadhav, 2016; Roy et al., 2017). Typically, this involves: (1) the entrainment of mPFC unit activity to hippocampal oscillations (e.g., theta rhythm); and/or (2) the synchronization of oscillatory activity between these structures. For instance, using a DNMS task, Hyman et al. (2010, 2011), reported that mPFC units became entrained to hippocampal theta during correct trials, resulting in successful task performance, whereas entrainment was lost on error trials. They concluded that “mPFC-HPC interactions are the key to successful DNMS performance”.
Whereas early studies largely focused on the influence of the hippocampus on the mPFC in SWM, more recent reports (Spellman et al., 2015; Hallock et al., 2016) have described two-way communication between these structures in SWM – with the direction of influence dependent on specific stages of the task. Specifically, Griffin and colleagues (Hallock et al., 2016), using a delayed T-maze alternation task, showed that hippocampal theta led and organized mPFC unit and theta activity in the delay period between trials, suggesting that during the delay, information about a previous trial (or choice) is transferred from the HF to the mPFC to guide future actions. By comparison, choice behavior was associated with slow gamma activity in the mPFC leading and predicting slow gamma oscillations in the HF suggesting that “trial specific information may be retrieved by the hippocampus from the mPFC when the animal makes a SWM-directed decision.” In effect, successful delayed alternation performance appears to consist of the mPFC informing the hippocampus of the choice that was made (e.g., right turn). The hippocampus then stores this information over the delay period and subsequently presents it to the mPFC to use in making the decision to turn in the opposite direction (e.g., left turn).
With respect to the anatomical substrates for the bidirectional communication between the HF and the mPFC for SWM, it is note-worthy that: (1) the mPFC does not project directly to the hippocampus; and (2) the dorsal HF does not project to the mPFC (Jay and Witter, 1991; Carr and Sesack, 1996; Laroche et al., 2000; Hoover and Vertes, 2007). In lieu of this, the nucleus reuniens, with strong reciprocal connections with the HF and with the mPFC, appears to be an important structure interlinking the hippocampus and the mPFC, thus completing the loop(s) between them (Laroche et al., 2000; Vertes, 2002, 2004; Vertes et al., 2006, 2007; Hoover and Vertes, 2012; Varela et al., 2014). Accordingly, alterations of RE would be expected to significantly disrupt HF-mPFC communication and thereby impair SWM, dependent on coupled activity between these structures (Cassel et al, 2013; Griffin, 2015).
In this regard, as discussed, several reports have shown that RE lesions/inactivation produce deficits on various SWM and mnemonic tasks -- reportedly dependent on HF-mPFC interactions (Hembrook and Mair, 2011; Hembrook et al., 2012; Loureiro et al., 2012; Cholvin et al., 2013; Hallock et al., 2013; Layfield et al., 2015). For instance, Hallock et al. (2013a) compared the effects of RE suppression on two versions of a conditional discrimination T-maze task: one involving a working memory (WM) component and the other not a WM component. RE inactivation severely disrupted performance on the WM, but not on the conditional discrimination, version of the task, leading the authors to conclude that RE is a necessary component of WM performance which is “thought to depend on the hippocampal–prefrontal circuit.” Finally, Hallock et al. (2016) recently showed that the inactivation of RE abolished bidirectional synchronous oscillations between the dorsal HF and the mPFC and disrupted SWM.
Layfield et al. (2015) described SWM deficits on a T-maze task following muscimol infusions into RE. Whereas this report, like ours, demonstrated delay dependent effects on a T-maze alternation task with RE inactivation, there were notable differences in the two studies. First, Layfield et al. (2015) reported that RE inactivation impaired performance following both short (5s) and longer (30s) delays, but for the short delay (5s) only the highest dose of muscimol (0.50 ug/ul) was effective. By contrast, we used longer delay times (30s, 60s and 120s) and found that both low (0.25 ug/ul) and high (0.50 ug/ul) doses of muscimol produced equivalent impairments at each of the delay times. Secondly, for Layfield et al. (2015) “delay” was used as an independent factor, such that groups of rats were only exposed to and tested at one delay time and comparisons were between groups at the different delays. By comparison, we used a within subjects design, wherein all rats were trained and tested at each delay (30s, 60s and 120s), and the order of the delay times was pseudorandomized. Finally, Layfield et al. (2015) used a continuous alternation paradigm wherein, following imposed delays, rats continuously alternated between L and R arms. If errors were made, rats were expected to alternate to the opposite arm on the next trial. By comparison, we used a free choice two run (sample and choice) procedure wherein rats chose one of two baited arms during a sample run and following a delay were required to choose the opposite arm for reward. As part of our paradigm, if rats made an error(s), they could correct them on the next or subsequent no delay runs. This importantly measured the ability of rats to switch their behavior in the absence of reward (behavioral flexibility) -- thereby testing for executive functioning as well as for SWM.
4.2 Procaine injections in RE
Whereas muscimol infusions into RE produced pronounced impairments in SWM, unexpectedly procaine injections into RE were less effective in disrupting SWM than muscimol injections. Specifically, procaine infusions in RE only impaired choice accuracy (mean percent of correct trials) at 120s delay times, but not with delays of 30 or 60s. As well recognized, both agents suppress neural activity by different mechanisms: procaine via blocking sodium voltage-gated channels and hence action potential generation, and muscimol by activation of GABA-A receptors to thereby hyperpolarize neurons containing these receptors (Martin and Ghez, 1999).
It is unclear why procaine was less effective than muscimol in our preparation since procaine has been shown to exert suppressive effects in other systems (Oddie et al., 1994, 1996; Ikemoto and Witkin, 2003). Although speculative, this could be due to the effective diffusion (or spread) of procaine at RE, compared to muscimol, and/or to the time course of action of the two substances. Regarding the latter, procaine is short acting compared to muscimol which is very long acting (Martin and Ghez, 1999). For instance, Tehovnik and Sommer (1997) examined the spread and time course of lidocaine injections in the cortex of monkeys, and showed that a 7 μl injection of lidocaine suppressed unit activity within 1 mm of the injection, but importantly most cells regained activity by 30 min post-injection. By contrast, the effects of muscimol are remarkably long lasting; that is, effects reportedly persist for 12–24 hours post-injection (Hikosaka and Wurtz, 1985; Martin and Ghez, 1999). Finally, it is very possible that a higher concentration of procaine would have been more effective. The present concentration (20% solution) was based on values we previously found to exert a marked suppressive effect on the posterior hypothalamus leading to a disruption of septal pace-making cells and hippocampal theta (Bland et al., 1994; Oddie et al., 1994).
4.3 Role of nucleus reuniens in flexible behavior and spatial perseveration
Reversible RE inactivation with muscimol not only produced impairments in working memory, but also impaired behavioral flexibility during corrections trials. As mentioned, in our DNMS task, if rats made an error during the choice run, they were given no delay correction runs, whereby they could re-enter the central stem and select the correct arm to retrieve food reward. During training sessions and vehicle infusions, errors were easily corrected on the first correction run. By contrast, injections of muscimol (0.25 mg/mL and 0.50 mg/mL) into RE produced pronounced spatial perseveration during correction runs, wherein rats continued to make incorrect choices in the absence of reward. Perseverative responding was so strong for rats in our pilot studies that often 30 or more consecutive incorrect responses were made before trials were terminated (Viena et al., 2016). Accordingly, we capped the correction runs at 10 per trial. The foregoing findings suggest the disruption of RE not only affects mnemonic functioning but also behavioral flexibility: an executive function defined as the ability to adapt one’s behavior to changing environmental and response contingencies. In the present study, rats were unable to shift their response (directional selection) following errors, and persistently made the wrong choice, despite the absence of (food) reward.
While the role of nucleus reuniens in spatial working memory has been well documented, few studies have examined its involvement in executive functions. This is despite strong RE connections with the medial and orbital prefrontal cortex, known to be critical for behavioral regulation (Bannerman et al., 2004; Dalley et al., 2004; Kehagia et al., 2010; Chudasama et al., 2012; Abela and Chudasama, 2013; Abela et al., 2013). The present findings of behavioral inflexibility with the suppression of RE are nonetheless consistent with previous reports describing a role for RE in flexible behavior and executive functions. As discussed, Dolleman–van der Weel et al. (2009) initially reported that RE lesions produced maladaptive search strategies in a standard water maze task, akin to prefrontal deficits. In accord with this, Cassel and colleagues (Cholvin et al., 2013) reported that following inactivation of RE, rats were unable to successfully navigate a ‘double H’ water maze which involved a switch in strategy from response to place responding. Similarly, Prasad et al. (2013) found that RE lesions produced an impairment in behavioral inhibition as shown by the inability to suppress premature (impulsive) responses in a 5-choice serial reaction time task 5-CSRTT). Finally, we recently demonstrated a significant impairment of RE lesioned rats during the initial stages of reversal learning on an odor/texture discrimination task, indicating an inability to shift their response to new stimulus-reward contingencies – or behavioral inflexibility.
The role of the rodent medial prefrontal cortex in executive processes and cognition is well documented (Dalley et al., 2004; Kesner and Churchwell, 2011; Bari and Robbins, 2013). Distinct subregions of the PFC are known to modulate different aspects of behavioral regulation including attention, impulsivity, extinction, behavioral flexibility, and working memory (Floresco et al., 1997; Birrell and Brown, 2000; Chudasama, 2011; Cassaday et al., 2014; Guistino and Maren, 2015; Jin and Maren, 2015). However, the majority of reports which have examined the role of the mPFC in spatial behaviors have failed to find persistent spatial perseverative effects. Specifically, lesions or inactivation of the mPFC produce prominent working memory errors in delayed alternation tasks, but not perseverative errors (Sanchez-Santed et al., 1997; Yoon et al., 2008; Horst and Laubach, 2009; Sapiurka et al., 2016).
By contrast, spatial perseveration has been strongly linked to the dysregulation of hippocampus (Dalland, 1970, 1976; Whishaw and Tomie, 1997; Deacon et al., 2001; Yoon et al., 2008; Kosaki and Watanbe, 2012; Arkhipov et al., 2008). Dalland (1970, 1976) showed early on that lesions of the dorsal HF produced spatial perseveration on a spatial alternation task. More recently, Hallock et al. (2013b) compared the effects of reversible inactivation of the dorsal hippocampus or the striatum on two tasks: a spatial delay alternation task and a visual conditional discrimination task, dependent on the corticostriatal system. They found that hippocampal, but not striatal, inactivation disrupted performance on the delayed alternation task, and notably increased scores on a perseveration index, a measure of revisits to a previous arm divided by the total number of trials (Hallock et al. 2013b). In like manner, using a delayed alternation paradigm with a figure 8 maze, Yoon et al. (2008) compared the effects of reversible inactivation of the mPFC or HF on spatial working memory, and showed that mPFC inactivation increased the number of working memory errors, whereas hippocampal inactivation significantly increased perseverative errors.
Zhang et al. (2013) showed that selective inactivation of NMDA receptors in the dorsal CA1 of HF not only produced working memory deficits, but also win-shift errors, and pronounced perseverative behavior. In a paradigm similar to ours, using sample and choice phases separated by 5 or 30 s delays, NMDA antagonists at CA1 significantly impaired the ability of rats to correct their behavior following errors, termed “lose-shift errors” as well as an ability to alternate following successful choices, or win-shift errors. In addition to marked perseveration, we also found that RE inactivation significantly increased win-shift failures. Specifically, during training and vehicle infusions, rats would continuously alternate across runs, such that following a sample run they would choose the opposite arm on the choice and then the alternate arm on the succeeding sample run. This pattern, however, was significantly altered with muscimol infusions producing increases in win-shift failures. RE inactivation impaired the use of a win-shift strategy such that rats failed to alternate across trials, whether or not they perseverated on the previous trial. In essence, RE inactivation not only altered working memory and spatial perseveration but may have disrupted learned response strategies. In like fashion, we recently found that RE lesions not only altered behavioral flexibility but impaired rule abstraction, or the ability to use previously learned rule/response strategies to guide responses to new sensory stimuli (Linley et al. 2016).
Furthermore, it should be noted that mean total percent of correct responses during the choice phase fell below chance levels (< 50%) for all delays following infusions of muscimol into RE. In this regard, failures to alternate across sample and choice runs may not necessarily be attributed to a deficit in working memory (WM) -- or the inability to hold information during the delay period. Alternatively, the deficit may result from a failure to use a well-learned response strategy (alternation) during the testing session, or in effect an impairment in response strategy. Accordingly, RE may not solely serve a role in WM, but may additionally be a core structure in an extended network mediating executive functioning.
Lastly, whereas the mPFC does not appear to play a major role in perseverative responding (or spatial perseveration), such behaviors are a hallmark of the orbital cortex (OC). Specifically, the OC plays a distinct role in flexible behavior, most notably in adapting behavior to reward contingencies (Kolb et al., 1974; DeBruin et al. 1983; Chudasama and Robbins, 2003; McAlonan et al., 2003; Boulougouris et al. 2007, 2008; Flaisher-Grinberg et al. 2008; Boulougouris and Robbins 2010; Sul et al., 2010; Young and Shapiro, 2011a; Amodeo et al., 2017; Izquierdo, 2017). Recordings of OC cells in rodents during maze tasks found that the orbital cortex: (1) contained populations of neurons which fired on trials in which a reward was given; and (2) these same cells increased their firing at the decision point in a maze leading to the correct rewarded choice (Steiner and Redish, 2012). Similar results were found in a two-choice odor discrimination task; that is, OC neurons fired in response to outcome (reward vs. non-reward) and to choice locations (Feierstein et al., 2006).
Young and Shapiro (2011b) recorded OC neurons in rats performing a plus maze task, dependent on both the OC and hippocampus, that involved spatial navigation, reversal learning, and strategy shifting. They found that the activity of OC neurons corresponded to the reward probabilities of the paths taken on the plus maze. Similar to the above-mentioned studies on mPFC-HF synchrony, they further showed that successful performance on reversal and strategy switching on the task was related to OC-HF theta synchrony. Although it is not entirely clear how the OC acquires and maintains information necessary for evaluative decisions, Wikenheiser and Schoenbaum (2016) recently proposed that spatial and contextual features of the environment, encoded by the hippocampus, are relayed to the orbital cortex and there evaluated for valence and reward properties to determine appropriate behavioral responses. Accordingly, the disruption of communication between the HF and the OC would impair the ability of the OC to distinguish among objects/situations, resulting in an inappropriate assignment of value to them, with consequent effects on behavior.
With respect to anatomical connections between the OC and HF, the OC does not receive projections from the dorsal HF, and the OC does not distribute directly to the HF (Dolleman van-der Weel and Witter, 1996; Reep et al., 1996; Vertes et al., 2006; Vertes et al., 2007; Hoover and Vertes, 2011, 2012; Prasad and Chudasama, 2013). As such, RE is putatively the main relay linking the HF (or dHF) and the OC (Prasad and Chudasama, 2013). Regarding an OC → RE → HF circuitry, the inactivation of RE may disrupt the transfer of information, on reward outcomes and behavioral adaptation, from the OC to the HF. Consequently, the spatial perseveration presently seen with RE inactivation may not necessarily involve incorrect directional responses (or SWM deficits), but may alternatively reflect inappropriate affective or emotional response to non-reward, thus leading to a failure to redirect behavior, via a return circuitry to OC. The spatial perseveration presently shown with inactivation of RE appears very similar to the compulsive-like responding observed following lesions or pharmacological manipulations of the OC (Chudasama and Robbins, 2003; McAlonan et al., 2003; Boulougouris et al. 2007, 2008; Everitt et al., 2007; Flaisher-Grinberg et al. 2008; Boulougouris and Robbins 2010; Sul et al., 2010). Accordingly, RE-associated perseverative behavior may result from an uncoupling of the OC and the HF.
In conclusion, we found that the reversible inactivation of the nucleus reuniens with muscimol produced spatial working memory impairments and perseveration in a delayed nonmatch to sample spatial alternation task. These results further strengthen the role of RE as an essential contributor to cognitive behaviors. RE not only serves as a pivotal link in communication between the mPFC and HF for spatial working memory, but is also instrumental in shaping goal directed behavior as part of a larger thalamocortical circuit mediating executive functions. As such, RE is a potential target in the treatment of CNS disorders such as schizophrenia, attention deficit hyperactivity disorder, addiction, and obsessive compulsive disorder, whose symptoms are defined by hippocampal/PFC dysfunctions.
Acknowledgments
The authors thank the following individuals for their assistance in the collection of the behavioral data: Bruno Dos Santos, Macarena Rey Martinez, Christopher Minnerly, and Max Schreiber. We also thank Michelle Gallo for technical assistance with the surgeries. This research was supported by NIMH grant MH099590 to RPV.
References
- Abela AR, Chudasama Y. Dissociable contributions of the ventral hippocampus and orbitofrontal cortex to decision-making with a delayed or uncertain outcome. European Journal of Neuroscience. 2013;37:640–647. doi: 10.1111/ejn.12071. [DOI] [PubMed] [Google Scholar]
- Abela AR, Dougherty SD, Fagen ED, Hill CJ, Chudasama Y. Inhibitory control deficits in rats with ventral hippocampal lesions. Cerebral Cortex. 2013;23:1396–1409. doi: 10.1093/cercor/bhs121. [DOI] [PubMed] [Google Scholar]
- Ainge JA, van der Meer MA, Langston RF, Wood ER. Exploring the role of context-dependent hippocampal activity in spatial alternation behavior. Hippocampus. 2007;17:988–1002. doi: 10.1002/hipo.20301. [DOI] [PubMed] [Google Scholar]
- Amodeo LR, McMurray MS, Roitman JD. Orbitofrontal cortex reflects changes in response-outcome contingencies during probabilistic reversal learning. Neuroscience. 2017;345:27–37. doi: 10.1016/j.neuroscience.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkhipov V, Kulesskaja N, Lebedev D. Behavioral perseveration and impairment of long-term memory in rats after intrahippocampal injection of kainic acid in subconvulsive dose. Pharmacology, Biochemistry and Behavior. 2008;88:299–305. doi: 10.1016/j.pbb.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HHJ, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neuroscience & Biobehavorial Reviews. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Progress in Neurobiology. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. Journal of Neuroscience. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland BH, Oddie SD, Colom LV, Vertes RP. Extrinsic modulation of medial septal cell discharges by the ascending brainstem hippocampal synchronizing pathway. Hippocampus. 1994;4:649–660. doi: 10.1002/hipo.450040604. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behavioural Brain Research. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 2008;33:2007–2019. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. Journal of Neuroscience. 2010;30:930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. Journal of Comparative Neurology. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cassaday HJ, Nelson AJ, Pezze MA. From attention to memory along the dorsal-ventral axis of the medial prefrontal cortex: some methodological considerations. Frontiers in Systems Neuroscience. 2014;8:160. doi: 10.3389/fnsys.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel JC, Pereira de Vasconcelos A, Loureiro M, Cholvin T, Dalrymple-Alford JC, Vertes RP. The reuniens and rhomboid nuclei: Neuroanatomy, electrophysiological characteristics and behavioral implications. Progress in Neurobiology. 2013;111:34–52. doi: 10.1016/j.pneurobio.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholvin T, Loureiro M, Cassel R, Cosquer B, Geiger K, Nogueira DDS, Raingard H, Robelin L, Kelche C, de Vasconcelos AP, Cassel JC. The ventral midline thalamus contributes to strategy shifting in a memory task requiring both prefrontal cortical and hippocampal functions. Journal of Neuroscience. 2013;33:8772–8783. doi: 10.1523/JNEUROSCI.0771-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y. Animal models of prefrontal-executive function. Behavioral Neuroscience. 2011;125:327–343. doi: 10.1037/a0023766. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. Journal of Neuroscience. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Doobay VM, Liu Y. Hippocampal-prefrontal cortical circuit mediates inhibitory response control in the rat. Journal of Neuroscience. 2012;32:10915–10924. doi: 10.1523/JNEUROSCI.1463-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Kesner RP. Hippocampal-prefrontal dynamics in spatial working memory: interactions and independent parallel processing. Behavioural Brain Research. 2011;225:389–395. doi: 10.1016/j.bbr.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Musso ND, Kesner RP. Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiology of Learning and Memory. 2010;93:415–421. doi: 10.1016/j.nlm.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Colgin LL. Oscillations and hippocampal-prefrontal synchrony. Current Opinion in Neurobiology. 2011;21:467–474. doi: 10.1016/j.conb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Yoon T, Otto T. Dissociating space and trace in dorsal and ventral hippocampus. Hippocampus. 2009;19:20–32. doi: 10.1002/hipo.20469. [DOI] [PubMed] [Google Scholar]
- Dalland T. Response and stimulus perseveration in rats with septal and dorsal hippocampal lesions. Journal of Comparative and Physiological Psychology. 1970;71:114–118. doi: 10.1037/h0028956. [DOI] [PubMed] [Google Scholar]
- Dalland T. Response perseveration of rats with dorsal hippocampal lesions. Behavioral Biology. 1976;17:473–484. doi: 10.1016/s0091-6773(76)90865-8. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience & Biobehavioral Reviews. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- de Bruin JP, van Oyen HG, Van de Poll N. Behavioural changes following lesions of the orbital prefrontal cortex in male rats. Behavioural Brain Research. 1983;10:209–232. doi: 10.1016/0166-4328(83)90032-3. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Bannerman DM, Rawlins NP. Conditional discriminations based on external and internal cues in rats with cytotoxic hippocampal lesions. Behavioral Neuroscience. 2001;115:43–57. doi: 10.1037/0735-7044.115.1.43. [DOI] [PubMed] [Google Scholar]
- Delatour B, Gisquet-Verrier P. Prelimbic cortex specific lesions disrupt delayed-variable response tasks in the rat. Behavioral Neuroscience. 1996;110:1282–1298. doi: 10.1037//0735-7044.110.6.1282. [DOI] [PubMed] [Google Scholar]
- Delatour B, Gisquet-Verrier P. Functional role of rat prelimbic-infralimbic cortices in spatial memory: evidence for their involvement in attention and behavioural flexibility. Behavioural Brain Research. 2000;109:113–128. doi: 10.1016/s0166-4328(99)00168-0. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van Der Weel MJ, Witter MP. Projections from the nucleus reuniens thalami to the entorhinal cortex, hippocampal field CA1, and the subiculum in the rat arise from different populations of neurons. Journal of Comparative Neurology. 1996;364:637–650. doi: 10.1002/(SICI)1096-9861(19960122)364:4<637::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Lopes da Silva FH, Witter MP. Nucleus reuniens thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. Journal of Neuroscience. 1997;17:5640–5650. doi: 10.1523/JNEUROSCI.17-14-05640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, Morris RG, Witter MP. Neurotoxic lesions of the thalamic reuniens or mediodorsal nucleus in rats affect non-mnemonic aspects of watermaze learning. Brain Structure and Function. 2009;213:329–342. doi: 10.1007/s00429-008-0200-6. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER, Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. Journal of Neuroscience. 2000;20:2964–2977. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Annals of the New York Academy of Science. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF. Representation of spatial goals in rat orbitofrontal cortex. Neuron. 2006;51:495–507. doi: 10.1016/j.neuron.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Flaisher-Grinberg S, Klavir O, Joel D. The role of 5-HT2A and 5-HT2C receptors in the signal attenuation rat model of obsessive-compulsive disorder. International Journal of Neuropsychopharmacology. 2008;11:811–825. doi: 10.1017/S146114570800847X. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behavioural Brain Research. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. Journal of Neuroscience. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF, Maren S. The role of the medial prefrontal cortex in the conditioning and extinction of fear. Frontiers in Behavioral Neuroscience. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1987;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Oscillations and hippocampal-prefrontal synchrony. Current Opinion in Neurobiology. 2011;21:486–491. doi: 10.1016/j.conb.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granon S, Vidal C, Thinus-Blanc C, Changeux JP, Poucet B. Working memory, response selection, and effortful processing in rats with medial prefrontal lesions. Behavioural Neuroscience. 1994;108:883–891. doi: 10.1037//0735-7044.108.5.883. [DOI] [PubMed] [Google Scholar]
- Griffin AL. Role of the thalamic nucleus reuniens in mediating interactions between the hippocampus and medial prefrontal cortex during spatial working memory. Frontiers in Systems Neuroscience. 2015;9:29. doi: 10.3389/fnsys.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Wang A, Shaw CL, Griffin AL. Transient inactivation of the thalamic nucleus reuniens and rhomboid nucleus produces deficits of a working-memory dependent tactile-visual conditional discrimination task. Behavioural Neuroscience. 2013a;127:860–866. doi: 10.1037/a0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Arreola AC, Shaw CL, Griffin AL. Dissociable roles of the dorsal striatum and dorsal hippocampus in conditional discrimination and spatial alternation T-maze tasks. Neurobiology of Learning and Memory. 2013b;100:108–116. doi: 10.1016/j.nlm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Hallock HL, Wang A, Griffin AL. Ventral midline thalamus is critical for hippocampal-prefrontal synchrony and spatial working memory. Journal of Neuroscience. 2016;36:8372–8389. doi: 10.1523/JNEUROSCI.0991-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembrook JR, Mair RG. Lesions of reuniens and rhomboid thalamic nuclei impair radial maze win-shift performance. Hippocampus. 2011;21:815–826. doi: 10.1002/hipo.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembrook JR, Onos KD, Mair RG. Inactivation of ventral midline thalamus produces selective spatial delayed conditional discrimination impairment in the rat. Hippocampus. 2012;22:853–860. doi: 10.1002/hipo.20945. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. Journal of Neurophysiology. 1985;53:292–308. doi: 10.1152/jn.1985.53.1.292. [DOI] [PubMed] [Google Scholar]
- Hock BJ, Bunsey MD. Differential effects of dorsal and ventral hippocampal lesions. Journal of Neuroscience. 1998;18:7027–7032. doi: 10.1523/JNEUROSCI.18-17-07027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure and Function. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Projections of the medial orbital and ventral orbital cortex in the rat. Journal of Comparative Neurology. 2011;519:3766–3801. doi: 10.1002/cne.22733. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Collateral projections from nucleus reuniens of thalamus to hippocampus and medial prefrontal cortex in the rat: a single and double retrograde fluorescent labeling study. Brain Structure and Function. 2012;217:191–209. doi: 10.1007/s00429-011-0345-6. [DOI] [PubMed] [Google Scholar]
- Horst NK, Laubach M. The role of rat dorsomedial prefrontal cortex in spatial working memory. Neuroscience. 2009;164:444–456. doi: 10.1016/j.neuroscience.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Frontiers in Integrative Neuroscience. 2010;4:2. doi: 10.3389/neuro.07.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Hasselmo ME, Seamans JK. What is the functional relevance of prefrontal cortex entrainment to hippocampal theta rhythms? Frontiers in Neuroscience. 2011;5:24. doi: 10.3389/fnins.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Witkin BM. Locomotor inhibition induced by procaine injections into the nucleus accumbens core, but not the medial ventral striatum: implication for cocaine-induced locomotion. Synapse. 2003;47:117–122. doi: 10.1002/syn.10151. [DOI] [PubMed] [Google Scholar]
- Izquierdo A. Functional heterogeneity within rat orbitofrontal cortex in reward learning and decision making. Journal of Neuroscience. 2017;37:10529–10540. doi: 10.1523/JNEUROSCI.1678-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. Journal of Comparative Neurology. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jin J, Maren S. Prefrontal-hippocampal interactions in memory and emotion. Frontiers in Systems Neuroscience. 2015;9:170. doi: 10.3389/fnsys.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biology. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Current Opinion in Neurobiology. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Yokoyama C, Masaki D, Yamashita T, Tsuchida H, Nakatomi Y, Fukui K. Effects of rat medial prefrontal cortex lesions on olfactory serial reversal and delayed alternation tasks. Neuroscience Research. 2008;60:213–218. doi: 10.1016/j.neures.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Kolb B, Nonneman AJ, Singh RK. Double dissociation of spatial impairments and perseveration following selective prefrontal lesions in rats. Journal of Comparative and Physiological Psychology. 1974;87:772–780. doi: 10.1037/h0036970. [DOI] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–446. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Layfield DM, Patel M, Hallock H, Griffin AL. Inactivation of the nucleus reuniens/rhomboid causes a delay-dependent impairment of spatial working memory. Neurobiology of Learning and Memory. 2015;125:163–167. doi: 10.1016/j.nlm.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. Journal of Neuroscience. 2003;23:1517–1523. doi: 10.1523/JNEUROSCI.23-04-01517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley SB, Gallo MM, Vertes RP. Lesions of the ventral midline thalamus produce deficits in reversal learning and attention on an odor texture set shifting task. Brain Research. 2016;1649:110–122. doi: 10.1016/j.brainres.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro M, Cholvin T, Lopez J, Merienne N, Latreche A, Cosquer B, Geiger K, Kelche C, Cassel JC, Pereira de Vasconcelos A. The ventral midline thalamus (reuniens and rhomboid nuclei) contributes to the persistence of spatial memory in rats. Journal of Neuroscience. 2012;32:9947–9959. doi: 10.1523/JNEUROSCI.0410-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. Journal of Neuroscience Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural Brain Research. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Nadel L, Hoscheidt S, Ryan LR. Spatial cognition and the hippocampus: the anterior-posterior axis. Journal of Cognitive Neuroscience. 2013;25:22–28. doi: 10.1162/jocn_a_00313. [DOI] [PubMed] [Google Scholar]
- O’Neill PK, Gordon JA, Sigurdsson T. Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. Journal of Neuroscience. 2013;33:14211–14224. doi: 10.1523/JNEUROSCI.2378-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddie SD, Bland BH, Colom LV, Vertes RP. The midline posterior hypothalamic region comprises a critical part of the ascending brainstem hippocampal synchronizing pathway. Hippocampus. 1994;4:454–473. doi: 10.1002/hipo.450040408. [DOI] [PubMed] [Google Scholar]
- Oddie SD, Stefanek W, Kirk IJ, Bland BH. Intraseptal procaine abolishes hypothalamic stimulation-induced wheel-running and hippocampal theta field activity in rats. Journal of Neuroscience. 1996;16:1948–1956. doi: 10.1523/JNEUROSCI.16-05-01948.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad JA, Chudasama Y. Viral tracing identifies parallel disynaptic pathways to the hippocampus. Journal of Neuroscience. 2013;33:8494–8503. doi: 10.1523/JNEUROSCI.5072-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad JA, Macgregor EM, Chudasama Y. Lesions of the thalamic reuniens cause impulsive but not compulsive responses. Brain Structure and Function. 2013;218:85–96. doi: 10.1007/s00429-012-0378-5. [DOI] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Current Biology. 2013;23:R764–773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins JNP, Olton DS. The septo-hippocampal system and cognitive mapping. Behavioural Brain Research. 1982;5:331–358. doi: 10.1016/0166-4328(82)90039-0. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, King V. Neuronal connections of orbital cortex in rats: topography of cortical and thalamic afferents. Experimental Brain Research. 1996;111:215–232. doi: 10.1007/BF00227299. [DOI] [PubMed] [Google Scholar]
- Remondes M, Wilson MA. Cingulate-hippocampus coherence and trajectory coding in a sequential choice task. Neuron. 2013;80:1277–1289. doi: 10.1016/j.neuron.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MA, Hayrapetyan VY, Maimon B, Mak K, Je HS, Yin HH. Prefrontal cortical mechanisms underlying delayed alternation in mice. Journal of Neurophysiology. 2012;108:1211–1222. doi: 10.1152/jn.01060.2011. [DOI] [PubMed] [Google Scholar]
- Roy A, Svensson FP, Mazeh A, Kocsis B. Prefrontal-hippocampal coupling by theta rhythm and by 2–5 Hz oscillation in the delta band: The role of the nucleus reuniens of the thalamus. Brain Structure and Function. 2017;222:2819–2830. doi: 10.1007/s00429-017-1374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Santed F, de Bruin JP, Heinsbroek RP, Verwer RW. Spatial delayed alternation of rats in a T-maze: effects of neurotoxic lesions of the medial prefrontal cortex and of T-maze rotations. Behavioural Brain Research. 1997;84:73–79. doi: 10.1016/s0166-4328(97)83327-x. [DOI] [PubMed] [Google Scholar]
- Sapiurka M, Squire LR, Clark RE. Distinct roles of hippocampus and medial prefrontal cortex in spatial and nonspatial memory. Hippocampus. 2016;26:1515–1524. doi: 10.1002/hipo.22652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw CL, Watson GD, Hallock HL, Cline KM, Griffin AL. The role of the medial prefrontal cortex in the acquisition, retention, and reversal of a tactile visuospatial conditional discrimination task. Behavioural Brain Research. 2013;236:94–101. doi: 10.1016/j.bbr.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JD, Jadhav SP. Multiple modes of hippocampal-prefrontal interactions in memory-guided behavior. Current Opinion in Neurobiology. 2016;40:161–169. doi: 10.1016/j.conb.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015;522:309–314. doi: 10.1038/nature14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AP, Redish AD. The road not taken: neural correlates of decision making in orbitofrontal cortex. Frontiers in Neuroscience. 2012;6:131. doi: 10.3389/fnins.2012.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul JH, Kim H, Huh N, Lee D, Jung MW. Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron. 2010;66:449–460. doi: 10.1016/j.neuron.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. 3. New York: Elsevier; 2003. [Google Scholar]
- Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. Journal of Neuroscience Methods. 1997;74:17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- Urban KR, Layfield DM, Griffin AL. Transient inactivation of the medial prefrontal cortex impairs performance on a working memory-dependent conditional discrimination task. Behavioral Neuroscience. 2014;128:639–643. doi: 10.1037/bne0000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C, Kumar S, Yang JY, Wilson MA. Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Structure and Function. 2014;219:911–929. doi: 10.1007/s00429-013-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. Journal of Comparative Neurology. 2002;442:163–187. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. Journal of Comparative Neurology. 2006;499:768–796. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Research Bulletin. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neuroscience & Biobehavioral Reviews. 2015;54:89–107. doi: 10.1016/j.neubiorev.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viena TD, Linley SB, Vertes RP. Nucleus reuniens infusions of muscimol, but not procaine, produce impairments on choice accuracy and perseverative behavior using a T-maze paradigm. Society for Neuroscience Abstract. 2016 181.08. [Google Scholar]
- Wang GW, Cai JX. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behavioural Brain Research. 2006;175:329–336. doi: 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie J. Perseveration on place reversals in spatial swimming pool tasks: further evidence for place learning in hippocampal rats. Hippocampus. 1997;7:361–370. doi: 10.1002/(SICI)1098-1063(1997)7:4<361::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Wikenheiser AM, Schoenbaum G. Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nature Reviews Neuroscience. 2016;17:513–523. doi: 10.1038/nrn.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Okada J, Jung MW, Kim JJ. Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learning and Memory. 2008;15:97–105. doi: 10.1101/lm.850808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JJ, Shapiro ML. The orbitofrontal cortex and response selection. Annals of the New York Academy of Sciences. 2011a;1239:25–32. doi: 10.1111/j.1749-6632.2011.06279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JJ, Shapiro ML. Dynamic coding of goal-directed paths by orbital prefrontal cortex. Journal of Neuroscience. 2011b;31:5989–6000. doi: 10.1523/JNEUROSCI.5436-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XH, Liu SS, Yi F, Zhuo M, Li BM. Delay-dependent impairment of spatial working memory with inhibition of NR2B-containing NMDA receptors in hippocampal CA1 region of rats. Molecular Brain. 2013;6:13. doi: 10.1186/1756-6606-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]