Abstract
AIM
To explore the correlation between serum vitamin B12 level and peripheral neuropathy in patients with chronic atrophic gastritis (CAG).
METHODS
A total of 593 patients diagnosed with chronic gastritis by gastroscopy and pathological examination from September 2013 to September 2016 were selected for this study. The age of these patients ranged within 18- to 75-years-old. Blood pressure, height and weight were measured in each patient, and the body mass index value was calculated. Furthermore, gastric acid, serum gastrin, serum vitamin and serum creatinine tests were performed, and peripheral nerve conduction velocity and Helicobacter pylori (H. pylori) were detected. In addition, the type of gastritis was determined by gastroscopy. The above factors were used as independent variables to analyze chronic gastritis with peripheral neuropathy and vitamin B12 deficiency risk factors, and to analyze the relationship between vitamin B12 levels and peripheral nerve conduction velocity. In addition, in the treatment of CAG on the basis of vitamin B12, patients with peripheral neuropathy were observed.
RESULTS
Age, H. pylori infection, CAG, vitamin B9 and vitamin B12 were risk factors for the occurrence of peripheral nerve degeneration. Furthermore, CAG and H. pylori infection were risk factors for chronic gastritis associated with vitamin B12 deficiency. Serum vitamin B12 level was positively correlated with sensory nerve conduction velocity in the tibial nerve (R = 0.463). After vitamin B12 supplementation, patients with peripheral neuropathy improved.
CONCLUSION
Serum vitamin B12 levels in patients with chronic gastritis significantly decreased, and the occurrence of peripheral neuropathy had a certain correlation. CAG and H. pylori infection are risk factors for vitamin B12 deficiency and peripheral neuropathy. When treating CAG, vitamin B12 supplementation can significantly reduce peripheral nervous system lesions. Therefore, the occurrence of peripheral neuropathy associated with vitamin B12 deficiency may be considered in patients with CAG. Furthermore, the timely supplementation of vitamin B12 during the clinical treatment of CAG can reduce or prevent peripheral nervous system lesions.
Keywords: Chronic gastritis, Chronic atrophic gastritis, Vitamin B12, Peripheral neuropathy
Core tip: The general situation and peripheral nerve conduction velocity of 593 patients with chronic gastritis were compared. We found that serum vitamin B12 levels in patients with chronic gastritis significantly decreased, and the occurrence of peripheral neuropathy had a certain correlation. Vitamin B12 supplementation can significantly reduce peripheral nervous system lesions. The occurrence of peripheral neuropathy associated with vitamin B12 deficiency may be considered in patients with chronic atrophic gastritis. Timely supplementation of vitamin B12 during the clinical treatment of chronic atrophic gastritis can reduce or prevent peripheral nervous system lesions.
INTRODUCTION
Chronic atrophic gastritis (CAG) is a common chronic digestive system disease, and its main clinical manifestations include excessive abdominal pain, bloating and abdominal discomfort. Some patients may develop numbness and other neurological disease symptoms, and its pathological features are gastric mucosal and inherent glandular atrophy[1,2]. In addition, gastric mucosal and inherent glandular atrophy lead to gastric acid. Furthermore, internal factors, such as the insufficient secretion of substances, affect vitamin B12 (VitB12) absorption[3,4], which in turn leads to lack of VitB12 in vivo[5-9]. Related studies have shown that VitB12 and folic acid deficiency can affect homocysteine metabolism, which leads to impaired neurons, causing peripheral neuropathy[10-12]. Therefore, numbness and other neurological symptoms that may be related to VitB12 and folic acid deficiency should be considered in patients with CAG.
No clinical evidence published to date has confirmed the relationship between these two. Furthermore, no study has reported the supplementation of VitB12 during the occurrence and prognosis of peripheral neuropathy in patients with CAG. Moreover, the association of patients with CAG and peripheral neuropathy remains unclear. Therefore, in the present study, through the treatment of peripheral neuropathy in patients with chronic gastritis, the clinical characteristics were analyzed and the possible risk factors were screened out to identify viable preventive measures and interventions, thereby playing a guiding role in the clinical treatment of CAG. The details are reported as follows.
MATERIALS AND METHODS
Object of study
Outpatients diagnosed with chronic gastritis by gastroscopy and pathological examination in our hospital from September 2013 to September 2016 were selected for this study. Exclusion criteria: (1) patients < 18-years-old or > 75-years-old; (2) patients who received drugs to treat gastritis within the past 2 wk; (3) patients who received VitB12 supplements and folic acid drugs within the past 2 wk; (4) patients whose other systems or organs are good, patients with malignant neoplasms, severe cardiovascular, cerebrovascular, liver or kidney disease, patients with primary disease of the hematopoietic system and patients with mental illness; (5) or patients who are pregnant and lactating. Finally, a total of 593 patients were included in the study. Among these patients, 295 were male and 298 were female. The average age of these patients was 46.5 ± 12.8 years, their mean blood pressure was 130.54 ± 19.96 mmHg/96.56 ± 9.70 mmHg, and their average body mass index (BMI) value was 21.16 ± 2.34. This research program and its experimental design were approved by the Ethics Committee of our institute. All patients provided signed informed consent.
Detection methods and groupings
Measurement of nerve conduction velocity: The Dantec Keypoint EMG/evoked potential (Denmark) was used at room temperature (25 °C). The median nerve, ulnar nerve, tibial nerve and sural nerve sensory and motor nerve conduction velocity of patients were routinely detected. Nerve conduction velocity was lower than the average conduction velocity in healthy young people, and was less than three times the standard deviation, or the same nerve conduction velocity difference of > 10%; that is, peripheral nerve conduction velocity abnormality. These abnormalities were measured again. Hence, there were two results for the abnormal diagnosis for peripheral neuropathy. In our hospital, the motor nerve conduction velocity reference value was as follows: median nerve: 57.8 ± 6.2; ulnar nerve: 55.36 ± 4.65; tibial nerve: 44.96 ± 2.57; and sural nerve: 50.17 ± 3.62. The sensory nerve conduction velocity reference value was as follows: median nerve: 55.18 ± 4.26; ulnar nerve: 50.27 ± 4.53; tibial nerve: 52.43 ± 3.62; and sural nerve: 47.65 ± 6.47. These patients were divided into two groups, according to these results: peripheral neuropathy group, and no peripheral neuropathy group.
Determination of serum creatinine, serum gastrin and vitamin levels: 5 mL of venous blood was collected from all patients after 1 d of fasting. After anticoagulation, the collected samples were centrifuged. Then, after the serum was separated, the sample was frozen and stored in aliquots at -20 °C for testing. Serum creatinine and serum gastrin were detected using a Hitachi 7060 automatic biochemical analyzer (Japan), and serum vitamin was detected by immunoenzyme analysis. All related operations were performed by highly experienced personnel, in strict accordance with instrument instructions. The VitB12 normal reference value in our hospital was > 160 ng/L.
Gastric juice analysis: Patients were instructed to fast for 8-12 hr prior to their examination in the morning. The nasogastric tube was placed into the stomach through the nose, and overnight net pumping of fasting gastric juice was performed. Pentagastrin was subcutaneously injected to stimulate gastric acid secretion, and gastric juice suction was continued for 1 hr. Then, the maximum amount of gastric acid secreted by the patient was recorded.
Gastroscopy: Patients were instructed to fast for 6-8 hr prior to the examination. After the antifoaming agent was administered and pharyngeal anesthesia was performed, an Olympus GIF-XQ230 gastroscope (Japan) was used for the examination. For the endoscopy of each patient, two tissue samples were collected from the antrum and curvature of the gastric body, respectively. The specimens were immediately fixed in methanol after collection. After the specimens were conventionally fixed, the tissues were embedded, sliced, dyed and microscopically observed by experienced hospital laboratory personnel to identify the type of chronic gastritis.
H. pylori detection: Each patient underwent the following tests for H. pylori detection: (1) rapid urease test; (2) 13C urea breath test; and (3) pathological examination. If the results revealed two or more signs of H. pylori positivity, the patient was diagnosed with H. pylori infection.
Intervention method
In addition to the conventional treatment of chronic gastritis, each patient was supplemented for vitamin deficiency according to their condition. The supplementation of VitB12 for CAG patients with peripheral neuropathy was based on the primary disease treatment and control of risk factors that lead to VitB12 deficiency.
Specific methods: In the treatment of CAG or the radical treatment of H. pylori on the basis of conventional medication, patients were intramuscularly injected with 0.5 mg of VitB12 once a week. Then, the VitB12 level and peripheral nerve conduction velocity (tibial nerve sensory nerve) of each patient were determined in vivo after diagnosis; that is, at the start of the medication, before the start of the medication, 1-3 mo after the medication, and 6 mo after the medication, respectively. The data were recorded and compared.
Statistical analysis
SPSS 19.0 was used for statistical analysis. The age and incidence of peripheral neuropathy in each group was used for count data, and analyzed by χ2-test. Age, blood pressure, serum creatinine, gastric acid, serum gastrin and serum vitamin levels, and nerve conduction velocity measurement data were expressed as mean ± SD. T-test was used to compare between groups. The multivariate regression analysis of chronic gastritis with peripheral neuropathy was performed by logistic regression analysis. The correlation analysis between VitB12 and peripheral nerve conduction velocity was analyzed by Pearson analysis. The multivariate regression analysis of chronic gastritis with VitB12 deficiency was performed using logistic regression analysis. The level of serum VitB12 and folic acid were compared using one-way ANOVA after 1-3 mo and 6 mo. P < 0.05 was considered statistically significant.
RESULTS
Groupings and the comparison of peripheral nerve conduction velocity between the two groups
A total of 593 patients with chronic gastritis were included in the present study. Among these patients, 162 had peripheral neuropathy (peripheral neuropathy group) and 431 had no peripheral neuropathy (no peripheral neuropathy group). The peripheral nerve conduction velocity in these two groups was compared. The ulnar-median nerve, tibial nerve and sural nerve sensory and motor nerve conduction velocity, and ulnar nerve sensory nerve conduction velocity were lower in patients with peripheral neuropathy, compared to patients without peripheral neuropathy, and the difference was statistically significant (P < 0.05). There was no significant difference in nerve conduction velocity between these two groups (P > 0.05; Table 1).
Table 1.
Comparison of the peripheral nerve conduction velocity of patients with or without peripheral neuropathy
| Item |
Sensory nerve conduction velocity |
Motor nerve conduction velocity |
||||||
| With peripheral nerve damage | Without peripheral nerve damage | t | P value | With peripheral nerve damage | Without peripheral nerve damage | t | P value | |
| Median nerve | 50.10 ± 7.80 | 52.30 ± 8.90 | -2.733 | 0.006 | 54.20 ± 8.70 | 56.20 ± 10.70 | -2.129 | 0.034 |
| Ulnar nerve | 49.40 ± 8.10 | 51.50 ± 9.20 | -2.556 | 0.011 | 50.30 ± 9.40 | 51.30 ± 8.60 | -1.230 | 0.219 |
| Tibial nerve | 38.30 ± 3.20 | 44.20 ± 7.60 | -9.563 | 0.000 | 50.4 ± 8.70 | 55.60 ± 9.80 | -5.931 | 0.000 |
| Sural nerve | 45.40 ± 5.00 | 50.80 ± 8.30 | -7.622 | 0.000 | 46.70 ± 7.90 | 51.10 ± 9.00 | -5.479 | 0.000 |
Comparison of the general situation of patients in the peripheral neuropathy and no peripheral neuropathy groups
In comparing the general information of patients in these two groups, it was revealed that age, H. pylori infection rate and the prevalence of CAG were higher in patients in the peripheral neuropathy group than in patients in the no peripheral neuropathy group, while BMI, serum vitamin A, vitamin B9 (folic acid) and VitB12 were lower than in patients in the no peripheral neuropathy group, and the differences were statistically significant (P < 0.05). Moreover, the difference in sex, blood pressure, serum creatinine, VitB1, VitB6 and VitE between these two groups were not statistically significant (P > 0.05; Table 2).
Table 2.
Comparison of the general situation of patients in the peripheral neuropathy and no peripheral neuropathy groups
| Item | Peripheral neuropathy group | No peripheral neuropathy group | t/c2 | P value |
| Age in yr | 50.50 ± 13.90 | 45.00 ± 12.40 | 4.653 | 0.000 |
| Sex, % male | 50.60 | 49.50 | 0.068 | 0.795 |
| Systolic blood pressure in mmHg | 130.17 ± 18.98 | 128.35 ± 20.32 | 0.989 | 0.323 |
| Diastolic blood pressure in mmHg | 75.34 ± 10.32 | 77.02 ± 9.45 | -1.880 | 0.061 |
| BMI in kg/m2 | 19.26 ± 2.15 | 21.88 ± 2.27 | -12.703 | 0.000 |
| Gastroscopy results, % prevalence of chronic atrophic gastritis | 76.50% | 59.20% | 15.418 | 0.000 |
| Helicobacter pylori infection, % | 86.40 | 56.40 | 46.452 | 0.000 |
| Gastric acid in mmol | 6.80 ± 3.70 | 17.80 ± 3.50 | -33.570 | 0.000 |
| Serum gastrin in pg/mL | 532.42 ± 167.33 | 208.43 ± 44.12 | 36.968 | 0.000 |
| Serum creatinine in μmol/L | 78.60 ± 17.20 | 76.50 ± 12.40 | 1.643 | 0.101 |
| VitA in ng/mL | 0.267 ± 0.269 | 0.383 ± 0.336 | -3.944 | 0.000 |
| VitB1 in nmol/L | 79.40 ± 20.70 | 82.60 ± 17.50 | -1.884 | 0.060 |
| VitB6 in mmol/L | 30.90 ± 14.80 | 32.70 ± 15.60 | -1.269 | 0.205 |
| VitB9 in ng/mL | 9.06 ± 3.81 | 10.60 ± 3.27 | -2.495 | 0.013 |
| VitB12 in pg/mL | 170.20 ± 111.20 | 216.40 ± 149.80 | -2.731 | 0.007 |
| VitE in μmol/L | 31.60 ± 5.48 | 33.20 ± 6.37 | -1.346 | 0.181 |
BMI: Body mass index; Vit: Vitamin.
Peripheral neuropathy multivariate logistic regression analysis results
A further factorial analysis was performed on factors that were statistically significant in the univariate analysis. Age, BMI, H. pylori infection, endoscopic results (CAG), vitamin A, VitB9 (folic acid) and VitB12 were included in the analysis. The logistic regression analysis results revealed that BMI, gastric acid, serum gastrin and vitamin A had no significant effect on peripheral neuropathy, and the difference was not statistically significant (P > 0.05). On the contrary, age (P =0.037), H. pylori infection (P = 0.000), CAG (P = 0.000), VitB9 (P = 0.034) and VitB12 (P = 0.000) had a significant effect on peripheral neuropathy. Further analysis revealed that based on odds ratio (OR) values, the following factors effected peripheral neuropathy (arranged in descending order according to occurrence): VitB12, CAG, H. pylori infection, VitB9 and age (Table 3).
Table 3.
Peripheral neuropathy multivariate logistic regression analysis results
| Influencing factor | β | SE | Wald value | OR | 95%CI | P value | |
| Age | 0.140 | 0.056 | 4.658 | 1.150 | 1.030 | 1.283 | 0.034 |
| BMI | -0.139 | 2.321 | 3.097 | 0.871 | 0.009 | 82.261 | 0.089 |
| Helicobacter pylori infection, infected = 1; uninfected = 0 | 1.541 | 0.124 | 7.816 | 4.670 | 3.662 | 5.955 | 0.000 |
| Gastric acid | 1.332 | 1.469 | 1.158 | 3.790 | 0.213 | 67.465 | 0.886 |
| Serum gastrin | 1.545 | 2.497 | 1.796 | 4.690 | 0.035 | 626.127 | 0.375 |
| Endoscopy results, atrophic gastritis = 1; nonatrophic gastritis = 0 | 1.663 | 0.197 | 8.562 | 5.276 | 3.586 | 7.762 | 0.000 |
| VitA | 0.039 | 0.127 | 1.562 | 1.041 | 0.811 | 1.334 | 0.645 |
| VitB9 | 0.871 | 0.359 | 4.162 | 2.390 | 1.183 | 4.830 | 0.037 |
| VitB12 | 1.883 | 0.236 | 9.364 | 6.571 | 4.137 | 10.434 | 0.000 |
BMI: Body mass index; CI: Confidence interval; OR: Odds ratio; SE: Standard error; Vit: Vitamin.
Correlation analysis of serum VitB12 levels and sensory nerve conduction velocity in the tibial nerve for patients with chronic gastritis
The correlation between serum VitB12 and peripheral nerve conduction velocity in patients with chronic gastritis was analyzed. Results are shown in Figure 1. There was a positive correlation between serum VitB12 levels and peripheral nerve conduction velocity (r = 0.631, P = 0.000).
Figure 1.
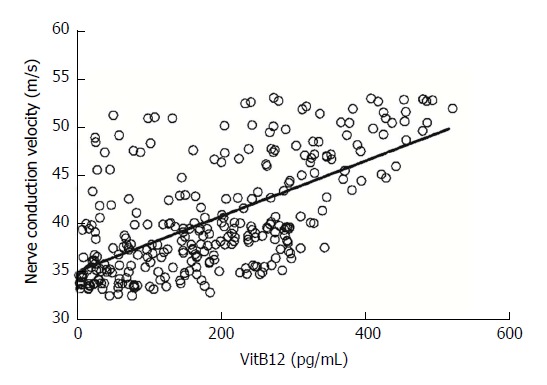
Correlation analysis of serum VitB12 level and sensory nerve conduction velocity in the tibial nerve of patients with chronic gastritis. Vit: Vitamin.
Comparison of the general situation of patients with or without VitB12 deficiency
In comparing the general situation of chronic gastritis patients with VitB12 deficiency and normal VitB12 levels, it was found that age, H. pylori infection rate, the prevalence of CAG and serum gastrin levels were significantly higher in patients with VitB12 deficiency than in patients with normal VitB12 levels (P < 0.05), while BMI values and folic acid levels were low in patients with normal VitB12 levels; and, the difference was statistically significant (P < 0.05). However, the difference in sex, blood pressure and serum creatinine levels between both groups of patients was not statistically significant (P > 0.05; Table 4).
Table 4.
Comparison of the general situation of patients with or without vitamin B12 deficiency
| Item | VitB12 deficiency, n = 207 | Normal VitB12 level, n = 386 | t/c2 | P value |
| Age in yr | 51.70 ± 14.70 | 44.3 ± 11.80 | 6.666 | 0.000 |
| Sex, % male | 51.60 | 48.80 | 0.481 | 0.488 |
| Systolic blood pressure in mmHg | 132.13 ± 19.37 | 129.35 ± 20.06 | 1.628 | 0.104 |
| Diastolic blood pressure in mmHg | 75.26 ± 11.44 | 76.31 ± 9.37 | -1.202 | 0.230 |
| BMI in kg/m2 | 18.36 ± 3.22 | 22.45 ± 2.39 | -17.529 | 0.000 |
| Helicobacter pylori infection, % | 87.6 | 74.8 | 12.949 | 0.000 |
| Gastroscopy results, % prevalence of chronic atrophic gastritis | 86.50 | 51.80 | 70.180 | 0.000 |
| Serum creatinine in μmol/L | 78.60 ± 17.20 | 76.5 ± 12.40 | 1.709 | 0.088 |
| Gastric acid in mmol | 7.90 ± 4.20 | 17.60 ± 3.50 | -29.955 | 0.000 |
| Serum gastrin in pg/mL | 432.85 ± 137.62 | 219.49 ± 47.98 | 27.516 | 0.000 |
BMI: Body mass index; Vit: Vitamin.
Multivariate logistic regression analysis results for chronic gastritis patients with VitB12 deficiency
Further factorial analysis was performed on factors that were statistically significant for the univariate analysis. Age, BMI value, H. pylori infection, endoscopy results (CAG), gastric acid and serum gastrin were included in the analysis. The logistic regression analysis results revealed that BMI values, gastric acid and serum gastrin had no significant effect on VitB12 deficiency, and the difference was not statistically significant (P > 0.05); while age (P = 0.037), H. pylori infection (P = 0.000) and CAG (P = 0.000) had a significant effect on VitB12 deficiency. Further analysis revealed that based on OR values, the following factors affected VitB12 deficiency (in descending order): CAG, H. pylori infection and age (Table 5).
Table 5.
Multivariate logistic regression analysis of VitB12 deficiency
| Influencing factor | β | SE | Wald value | OR | 95%CI | P value | |
| Age | 0.519 | 0.149 | 4.865 | 1.680 | 1.255 | 2.250 | 0.023 |
| BMI | 1.477 | 1.325 | 0.004 | 4.380 | 0.326 | 58.795 | 0.957 |
| Helicobacter pylori infection, positive = 1; negative = 0 | 1.730 | 0.279 | 7.218 | 5.640 | 3.264 | 9.745 | 0.000 |
| Endoscopy results, atrophic gastritis = 1; nonatrophic gastritis = 0 | 2.145 | 0.364 | 9.645 | 8.546 | 4.187 | 17.442 | 0.000 |
| Gastric acid | 0.948 | 1.269 | 1.024 | 2.580 | 0.214 | 31.032 | 0.762 |
| Serum gastrin | 1.479 | 2.226 | 2.549 | 4.390 | 0.056 | 344.567 | 0.267 |
BMI: Body mass index; CI: Confidence interval; OR: Odds ratio; SE: Standard error; Vit: Vitamin.
Changes of serum VitB12 levels and nerve conduction velocity in patients after supplementation with VitB12
Chronic gastritis in patients with VitB12 deficiency occurred mainly due to slow atrophic gastritis and H. pylori infection. In the present study, atrophic gastritis and radical H. pylori infection were treated based on the supplementation of VitB12 in patients. These results revealed that compared with untreated patients, serum VitB12 levels gradually increased (F = 5.241, P < 0.05); and after 1 mo of treatment, the differences were statistically significant (T = 4.647, P = 0.000). Furthermore, nerve conduction velocity gradually accelerated (F = 3.172, P < 0.05; Table 6, Figures 2 and 3).
Table 6.
Changes in serum VitB12 levels and nerve conduction velocity in patients after half a year of VitB12 supplementation
| Item | 0 mo | 1 mo | 2 mo | 3 mo | 6 mo |
| VitB12 in pg/mL | 158.70 ± 104.50 | 237.20 ± 156.40 | 481.50 ± 164.60 | 614.80 ± 186.70 | 635.20 ± 174.80 |
| Nerve conduction velocity in m/s at 0 mo | 40.10 ± 5.50 | 40.30 ± 4.70 | 41.60 ± 7.40 | 42.70 ± 5.90 | 45.80 ± 5.80 |
Vit: Vitamin.
Figure 2.
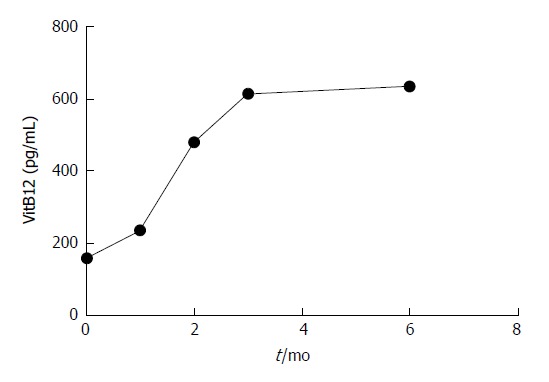
Trend changes in serum VitB12 level. Vit: Vitamin.
Figure 3.
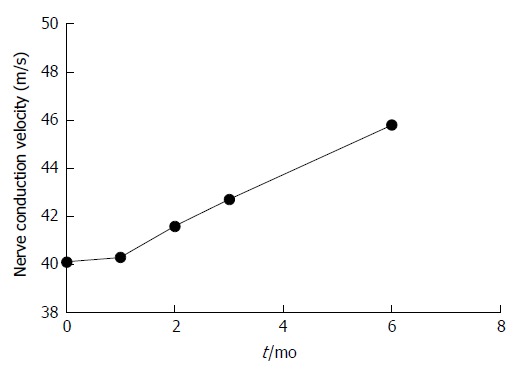
Trend changes in nerve conduction velocity.
DISCUSSION
CAG is a common digestive system disease, which commonly causes H. pylori infection, bile reflux, vasoactive factors and cytokine changes. It has been generally accepted that CAG occurs under the joint action of various factors, and its development process is caused by the long evolution of multiple genes. Its main clinical manifestations include stomach pain, fullness, ruffian nausea, belching and acid reflux. Some patients may also experience numbness and present other nervous system symptoms. In the course of disease development, gastric mucosal and inherent gland atrophy, decreased gastric acid secretion and other serious effects may disrupt the absorption of nutrients[13-15].
VitB12 is one of the essential vitamins that can improve folic acid utilization, and in turn promote homocysteine metabolism[16-18]. Studies have shown that VitB12 and folic acid deficiency lead to homocysteine metabolism, and is inhibited by the role of axons and myelin in Schwann cells, leading to neuronal damage and peripheral neuropathy[19]. Another study revealed that VitB12 deficiency can lead to neuronal myelination[20-22]. However, at present, the relationship between these two has not been clinically confirmed. Furthermore, the effect of VitB12 on the occurrence and outcome of peripheral neuropathy in patients with CAG remains unclear.
In the present study, by comparing the effects of different factors on peripheral nerve conduction velocity and serum VitB12 levels, it was found that VitB12 deficiency may be a major risk factor for CAG patients with peripheral neuropathy, while CAG and H. pylori infection may be risk factors for chronic gastritis patients with VitB12 deficiency. Simultaneously, this study confirmed that treating the primary disease with the supplementation of VitB12 can significantly improve peripheral neuropathy symptoms, suggesting that the timely supplementation of VitB12 can prevent or improve CAG in patients with peripheral neuropathy symptoms.
Analysis of the influencing factors of peripheral neuropathy
In comparing the general situation of patients with or without peripheral neuropathy, it was found that age, H. pylori infection rate, CAG, BMI, serum vitamin A, vitamin B9 (folic acid) and VitB12 were the possible risk factors for peripheral neuropathy in patients with chronic gastritis. Based on further logistic multivariate regression analysis, it was found that age, H. pylori infection, CAG, VitB9 and VitB12 were risk factors for peripheral neurodegeneration. Among these factors, VitB12, H. pylori infection and CAG exhibited a higher relative risk. In addition, age is also one of the risk factors for CAG[23-28], which may be due to its long course; that is, pathogenic factors take a long period of time to become a risk factor for peripheral neuropathy.
Correlation between peripheral nerve conduction velocity and serum VitB12 level
In the assessment of peripheral nerve conduction velocity, it was found that this was more obvious in lower limb peripheral neuropathy, and was particularly evident in tibial nerve sensory nerves in the lower limb. Therefore, the correlation between serum VitB12 level and peripheral conduction velocity was analyzed by tibial nerve sensory nerve conduction velocity. The correlation analysis revealed that peripheral nerve conduction velocity was positively correlated with serum VitB12 levels (R = 0.463); that is, as serum VitB12 levels decreased, the degree of peripheral neuropathy gradually increased.
Analysis of influencing factors for VitB12 deficiency
The above studies show that serum VitB12 levels in patients with chronic gastritis were associated with the risk factors for peripheral neuropathy. In order to explore the etiology of VitB12 in patients with chronic gastritis in the present study, the general situations of chronic gastritis patients associated with VitB12 deficiency were compared. Based on further logistic multivariate regression analysis, it was found that H. pylori infection and CAG were independent risk factors for chronic gastritis with VitB12 deficiency. Among these factors, H. pylori infection can lead to VitB12 deficiency[29-31]. Furthermore, H. pylori infection is one of the common causes of CAG[32-40]. The possible cause for VitB12 deficiency is the damage induced by H. pylori infection on gastric mucosal cells[41,42], which reduces gastric acid secretion and affects the separation of VitB12 from food[43]. At the same time, the reduction in gastric mucosal secretion of vitamin C and stomach pH value is affected by the increased absorption of vitamin B[44,45].
Effects of VitB12 supplementation on peripheral neuropathy
Peripheral neuropathy can be treated by VitB12 supplementation. A large number of studies have shown that VitB12 can significantly improve nervous system diseases in patients, such as spinal cord subacute combined disease and reversible myelopathy[46-50]. In the present study, the management of VitB12 deficiency may be a risk factor (CAG and H. pylori infection). On this basis, by comparing patients with CAG on the basis of conventional treatment without VitB12 supplementation (0 mo), and after 1-3 mo and 6 mo of treatment, the serum VitB12 level and peripheral nerve conduction velocity trend revealed that serum VitB12 level and nerve conduction velocity gradually increased after treatment. As shown in Figures 2 and 3, it can be observed that the increase in peripheral nerve conduction velocity was faster than that of serum VitB12 levels. It can be speculated that the speed of peripheral nerve conduction was accelerated due to elevated serum VitB12 levels. Hence, VitB12 supplementation can improve peripheral neuropathy.
Limitations and outlook
In the present study, the subjects collected for the experiment all came from our hospital, which may give rise to some limitations. However, there were significant differences in VitB12 and nerve conduction velocity between these two groups. Hence, there can still be a certain degree of response to the relationship between these two. In subsequent studies, a multi-center and multi-region joint cooperation should be conducted to expand the sample size and improve the sample representation, in order to provide results with a higher degree of confidence.
Summary
In summary, in the present study, we analyzed the risk factors of chronic gastritis with peripheral neuropathy. Furthermore, the correlation between serum VitB12 level and peripheral neuropathy was analyzed. The level of serum VitB12 in patients with chronic gastritis was a risk factor for peripheral neuropathy, and serum VitB12 levels and the severity of peripheral neuropathy were positively correlated. In addition, CAG and H. pylori infection were the major risk factors for VitB12 deficiency in patients with chronic gastritis.
By comparing the peripheral nerve conduction velocity after VitB12 supplementation, it was found that the treatment of CAG and the control of H. pylori infection while supplementing with VitB12 can significantly reduce peripheral neuropathy. This suggests that the timely supplementation of VitB12 may become a treatment or even prevent the occurrence of CAG in patients or the occurrence of peripheral neuropathy. However, it remains to be further studied whether this can be applied to this population.
ARTICLE HIGHLIGHTS
Research background
The main clinical manifestations of chronic atrophic gastritis are excessive abdominal pain, bloating and abdominal discomfort. It is known that the insufficient secretion of substances would affect vitamin B12 (VitB12) absorption. VitB12 and folic acid deficiency can affect homocysteine metabolism, which leads to peripheral neuropathy. Therefore, the occurrence of patients with chronic atrophic gastritis numbness and other nervous system symptoms may be related to VitB12 and folic acid deficiency
Research motivation
At present, there are no studies reporting the effect of VitB12 supplementation on the occurrence and outcome of peripheral neuropathy in patients with chronic atrophic gastritis. The causes of peripheral neuropathy in patients with chronic atrophic gastritis are also not clear. Therefore, it is necessary to explore the risk factors of peripheral neuropathy in patients with chronic atrophic gastritis.
Research objectives
This study aimed to explore the clinical features of peripheral neuropathy in patients with chronic atrophic gastritis and to screen out the possible risk factors in order to find out the feasible prevention and intervention measures for the clinical treatment of chronic atrophic gastritis
Research methods
In total, 593 patients diagnosed with chronic gastritis were involved and their gastric acid, serum gastrin, serum vitamin and serum creatinine tests, peripheral nerve conduction velocity and Helicobacter pylori (H. pylori) were detected. In addition, the type of gastritis was determined by gastroscopy. All detected results were used to analyze the relationship between VitB12 levels and peripheral nerve conduction velocity.
Research results
H. pylori infection and chronic atrophic gastritis were independent risk factors for chronic gastritis associated with VitB12 deficiency. The separation of VitB12 from food was affected because H. pylori infection in gastric mucosal cells damage gastric acid secretion (reducing it). This study also found that the serum VitB12 and nerve conduction velocity gradually increased after VitB12 supplement treatment, suggesting that VitB12 supplementation can improve peripheral neuropathy.
Research conclusions
This study found that serum VitB12 is a risk factor for peripheral neuropathy in patients with chronic gastritis, and serum vitamin B12 is positively correlated with the severity of peripheral neuropathy. Chronic atrophic gastritis and H. pylori infection are the main risk factors of VitB12 deficiency in patients with chronic gastritis. In addition, timely VitB12 supplementation may be an effective treatment and even a prevention method of peripheral neuropathy in patients with chronic atrophic gastritis.
Research perspectives
Although this study has demonstrated serum VitB12 level is related to peripheral neuropathy in patients with chronic atrophic gastritis, it is still limited since it’s a single center study. Future research should be designed as a multicenter study, and a large sample size is needed to make the findings more credible.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Supported by Cangzhou City Science and Technology Plan Projects, No. 151302138.
Institutional review board statement: The study was reviewed and approved by the Cangzhou Central Hospital, Cangzhou Clinical Medical School of Hebei Medical University Institutional Review Board.
Clinical trial registration statement: This study is registered with the Chinese Clinical Trial Registry, No. ChiCTR-ROC-17014051.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: We declare that there are no conflicts of interest to disclose.
Peer-review started: December 20, 2017
First decision: January 4, 2018
Article in press: February 26, 2018
P- Reviewer: Gobejishvili L, Osuga T, Penaranda G S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Huang Y
Contributor Information
Guo-Tao Yang, Department of Third Neurology, Cangzhou Central Hospital, Cangzhou Clinical Medical School of Hebei Medical University, Cangzhou 061001, Hebei Province, China. yangguotao3815@cz_hospital.ac.cn.
Hong-Ying Zhao, Department of Third Neurology, Cangzhou Central Hospital, Cangzhou Clinical Medical School of Hebei Medical University, Cangzhou 061001, Hebei Province, China; Department of Elderly Internal Medicine, Cangzhou Central Hospital, Cangzhou Clinical Medical School of Hebei Medical University, Cangzhou 061001, Hebei Province, China.
Yu Kong, Department of Third Neurology, Cangzhou Central Hospital, Cangzhou Clinical Medical School of Hebei Medical University, Cangzhou 061001, Hebei Province, China; Department of Second Digestion, Cangzhou Central Hospital, Cangzhou Clinical Medical School of Hebei Medical University, Cangzhou 061001, Hebei Province, China.
Ning-Ning Sun, Department of Third Neurology, Cangzhou Central Hospital, Cangzhou Clinical Medical School of Hebei Medical University, Cangzhou 061001, Hebei Province, China; Department of First Digestion, Cangzhou Central Hospital, Cangzhou Clinical Medical School of Hebei Medical University, Cangzhou 061001, Hebei Province, China.
Ai-Qin Dong, Department of Third Neurology, Cangzhou Central Hospital, Cangzhou Clinical Medical School of Hebei Medical University, Cangzhou 061001, Hebei Province, China.
References
- 1.Wei Y, Ma LX, Yin SJ, An J, Wei Q, Yang JX. Huangqi Jianzhong Tang for Treatment of Chronic Gastritis: A Systematic Review of Randomized Clinical Trials. Evid Based Complement Alternat Med. 2015;2015:878164. doi: 10.1155/2015/878164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mescoli C, Gallo Lopez A, Taxa Rojas L, Jove Oblitas W, Fassan M, Rugge M. Gastritis staging as a clinical priority. Eur J Gastroenterol Hepatol. 2018;30:125–129. doi: 10.1097/MEG.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 3.Nomura S, Ida K, Terao S, Adachi K, Kato T, Watanabe H, Shimbo T; Research Group for Establishment of Endoscopic Diagnosis of Chronic Gastritis. Endoscopic diagnosis of gastric mucosal atrophy: multicenter prospective study. Dig Endosc. 2014;26:709–719. doi: 10.1111/den.12286. [DOI] [PubMed] [Google Scholar]
- 4.Cavalcoli F, Zilli A, Conte D, Massironi S. Micronutrient deficiencies in patients with chronic atrophic autoimmune gastritis: A review. World J Gastroenterol. 2017;23:563–572. doi: 10.3748/wjg.v23.i4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toh BH, Chan J, Kyaw T, Alderuccio F. Cutting edge issues in autoimmune gastritis. Clin Rev Allergy Immunol. 2012;42:269–278. doi: 10.1007/s12016-010-8218-y. [DOI] [PubMed] [Google Scholar]
- 6.Lahner E, Gentile G, Purchiaroni F, Mora B, Simmaco M, Annibale B. Single nucleotide polymorphisms related to vitamin B12 serum levels in autoimmune gastritis patients with or without pernicious anaemia. Dig Liver Dis. 2015;47:285–290. doi: 10.1016/j.dld.2015.01.147. [DOI] [PubMed] [Google Scholar]
- 7.Harakal J, Rival C, Qiao H, Tung KS. Regulatory T Cells Control Th2-Dominant Murine Autoimmune Gastritis. J Immunol. 2016;197:27–41. doi: 10.4049/jimmunol.1502344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsons BN, Ijaz UZ, D’Amore R, Burkitt MD, Eccles R, Lenzi L, Duckworth CA, Moore AR, Tiszlavicz L, Varro A, et al. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog. 2017;13:e1006653. doi: 10.1371/journal.ppat.1006653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulnigg-Dabsch S. Autoimmune gastritis. Wien Med Wochenschr. 2016;166:424–430. doi: 10.1007/s10354-016-0515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varbanova M, Frauenschläger K, Malfertheiner P. Chronic gastritis - an update. Best Pract Res Clin Gastroenterol. 2014;28:1031–1042. doi: 10.1016/j.bpg.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Dobson R, Alvares D. The difficulties with vitamin B12. Pract Neurol. 2016;16:308–311. doi: 10.1136/practneurol-2015-001344. [DOI] [PubMed] [Google Scholar]
- 12.Stredny CM, Frosch O, Singhi S, Furutani E, Durbin AD, Grace RF, Ullrich NJ. Vitamin B12 Deficiency Presenting with Neurological Dysfunction in an Adolescent. Pediatr Neurol. 2016;62:66–70. doi: 10.1016/j.pediatrneurol.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Wilmshurst JM, Ouvrier R. Hereditary peripheral neuropathies of childhood: an overview for clinicians. Neuromuscul Disord. 2011;21:763–775. doi: 10.1016/j.nmd.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Rohde C, von Teeffelen-Heithoff A, Thiele AG, Arelin M, Mütze U, Kiener C, Gerloff J, Baerwald C, Schultz S, Heller C, et al. PKU patients on a relaxed diet may be at risk for micronutrient deficiencies. Eur J Clin Nutr. 2014;68:119–124. doi: 10.1038/ejcn.2013.218. [DOI] [PubMed] [Google Scholar]
- 15.Betesh AL, Santa Ana CA, Cole JA, Fordtran JS. Is achlorhydria a cause of iron deficiency anemia? Am J Clin Nutr. 2015;102:9–19. doi: 10.3945/ajcn.114.097394. [DOI] [PubMed] [Google Scholar]
- 16.Sipponen P, Maaroos HI. Chronic gastritis. Scand J Gastroenterol. 2015;50:657–667. doi: 10.3109/00365521.2015.1019918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni J, Zhang L, Zhou T, Xu WJ, Xue JL, Cao N, Wang X. Association between the MTHFR C677T polymorphism, blood folate and vitamin B12 deficiency, and elevated serum total homocysteine in healthy individuals in Yunnan Province, China. J Chin Med Assoc. 2017;80:147–153. doi: 10.1016/j.jcma.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Katsiki N, Perez-Martinez P, Mikhailidis DP. Homocysteine and Non-Cardiac Vascular Disease. Curr Pharm Des. 2017;23:3224–3232. doi: 10.2174/1381612823666170317124913. [DOI] [PubMed] [Google Scholar]
- 19.Shiran A, Remer E, Asmer I, Karkabi B, Zittan E, Cassel A, Barak M, Rozenberg O, Karkabi K, Flugelman MY. Association of Vitamin B12 Deficiency with Homozygosity of the TT MTHFR C677T Genotype, Hyperhomocysteinemia, and Endothelial Cell Dysfunction. Isr Med Assoc J. 2015;17:288–292. [PubMed] [Google Scholar]
- 20.Schroecksnadel K, Leblhuber F, Fuchs D. Effect of L-dopa on plasma homocysteine in PD patients: relationship to B-vitamin status. Neurology. 2004;62:676; author reply 676–676; author reply 677. [PubMed] [Google Scholar]
- 21.Hedera P. Hereditary and metabolic myelopathies. Handb Clin Neurol. 2016;136:769–785. doi: 10.1016/B978-0-444-53486-6.00038-7. [DOI] [PubMed] [Google Scholar]
- 22.Keenan A, Whittam B, Rink R. Vitamin B12 deficiency in patients after enterocystoplasty. J Pediatr Urol. 2015;11:273.e1–273.e5. doi: 10.1016/j.jpurol.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Lindenbaum J, Healton EB, Savage DG, Brust JC, Garrett TJ, Podell ER, Marcell PD, Stabler SP, Allen RH. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318:1720–1728. doi: 10.1056/NEJM198806303182604. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Weck MN, Schöttker B, Rothenbacher D, Brenner H. Gastric parietal cell antibodies, Helicobacter pylori infection, and chronic atrophic gastritis: evidence from a large population-based study in Germany. Cancer Epidemiol Biomarkers Prev. 2013;22:821–826. doi: 10.1158/1055-9965.EPI-12-1343. [DOI] [PubMed] [Google Scholar]
- 25.Namekata T, Miki K, Kimmey M, Fritsche T, Hughes D, Moore D, Suzuki K. Chronic atrophic gastritis and Helicobacter pylori infection among Japanese Americans in Seattle. Am J Epidemiol. 2000;151:820–830. doi: 10.1093/oxfordjournals.aje.a010282. [DOI] [PubMed] [Google Scholar]
- 26.Brenner H, Rothenbacher D, Weck MN. Epidemiologic findings on serologically defined chronic atrophic gastritis strongly depend on the choice of the cutoff-value. Int J Cancer. 2007;121:2782–2786. doi: 10.1002/ijc.22992. [DOI] [PubMed] [Google Scholar]
- 27.Kohli Y, Kato T, Suzuki K, Tada T, Fujiki N. Incidence of atrophic gastritis with age in Japan and Canada. Jpn J Med. 1987;26:158–161. doi: 10.2169/internalmedicine1962.26.158. [DOI] [PubMed] [Google Scholar]
- 28.Takase Y. An endoscopie and bioptic Study on Chronic Gastrits (I) Atrophic Gastritis. Nihon Shokakibyo Gakkai Zasshi. 2007:99–106. [PubMed] [Google Scholar]
- 29.Neumann WL, Coss E, Rugge M, Genta RM. Autoimmune atrophic gastritis--pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol. 2013;10:529–541. doi: 10.1038/nrgastro.2013.101. [DOI] [PubMed] [Google Scholar]
- 30.Stopeck A. Links between Helicobacter pylori infection, cobalamin deficiency, and pernicious anemia. Arch Intern Med. 2000;160:1229–1230. doi: 10.1001/archinte.160.9.1229. [DOI] [PubMed] [Google Scholar]
- 31.Kaptan K, Beyan C, Ural AU, Cetin T, Avcu F, Gülşen M, Finci R, Yalçín A. Helicobacter pylori--is it a novel causative agent in Vitamin B12 deficiency? Arch Intern Med. 2000;160:1349–1353. doi: 10.1001/archinte.160.9.1349. [DOI] [PubMed] [Google Scholar]
- 32.Franceschi F, Annalisa T, Teresa DR, Giovanna D, Ianiro G, Franco S, Viviana G, Valentina T, Riccardo LL, Antonio G. Role of Helicobacter pylori infection on nutrition and metabolism. World J Gastroenterol. 2014;20:12809–12817. doi: 10.3748/wjg.v20.i36.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sipponen P. Chronic gastritis in former times and now. Helicobacter. 2007;12 Suppl 2:16–21. doi: 10.1111/j.1523-5378.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 34.Weck MN, Brenner H. Association of Helicobacter pylori infection with chronic atrophic gastritis: Meta-analyses according to type of disease definition. Int J Cancer. 2008;123:874–881. doi: 10.1002/ijc.23539. [DOI] [PubMed] [Google Scholar]
- 35.Chen XZ, Schöttker B, Castro FA, Chen H, Zhang Y, Holleczek B, Brenner H. Association of helicobacter pylori infection and chronic atrophic gastritis with risk of colonic, pancreatic and gastric cancer: A ten-year follow-up of the ESTHER cohort study. Oncotarget. 2016;7:17182–17193. doi: 10.18632/oncotarget.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi S, Ogura M, Suzawa N, Horiki N, Katsurahara M, Ogura T, Sakuma H. 18F-FDG uptake in the stomach on screening PET/CT: value for predicting Helicobacter pylori infection and chronic atrophic gastritis. BMC Med Imaging. 2016;16:58. doi: 10.1186/s12880-016-0161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roesler BM, Rabelo-Gonçalves EM, Zeitune JM. Virulence Factors of Helicobacter pylori: A Review. Clin Med Insights Gastroenterol. 2014;7:9–17. doi: 10.4137/CGast.S13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adamu MA, Weck MN, Gao L, Brenner H. Incidence of chronic atrophic gastritis: systematic review and meta-analysis of follow-up studies. Eur J Epidemiol. 2010;25:439–448. doi: 10.1007/s10654-010-9482-0. [DOI] [PubMed] [Google Scholar]
- 39.Reshetnyak VI, Reshetnyak TM. Significance of dormant forms of Helicobacter pylori in ulcerogenesis. World J Gastroenterol. 2017;23:4867–4878. doi: 10.3748/wjg.v23.i27.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watari J, Chen N, Amenta PS, Fukui H, Oshima T, Tomita T, Miwa H, Lim KJ, Das KM. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol. 2014;20:5461–5473. doi: 10.3748/wjg.v20.i18.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee SY. Endoscopic gastritis, serum pepsinogen assay, and Helicobacter pylori infection. Korean J Intern Med. 2016;31:835–844. doi: 10.3904/kjim.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang YJ, Sheu BS. Metabolic Interaction of Helicobacter pylori Infection and Gut Microbiota. Microorganisms. 2016;4 doi: 10.3390/microorganisms4010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Annibale B, Capurso G, Delle Fave G. Consequences of Helicobacter pylori infection on the absorption of micronutrients. Dig Liver Dis. 2002;34 Suppl 2:S72–S77. doi: 10.1016/s1590-8658(02)80170-0. [DOI] [PubMed] [Google Scholar]
- 44.Claeys D, Faller G, Appelmelk BJ, Negrini R, Kirchner T. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology. 1998;115:340–347. doi: 10.1016/s0016-5085(98)70200-8. [DOI] [PubMed] [Google Scholar]
- 45.Lahner E, Persechino S, Annibale B. Micronutrients (Other than iron) and Helicobacter pylori infection: a systematic review. Helicobacter. 2012;17:1–15. doi: 10.1111/j.1523-5378.2011.00892.x. [DOI] [PubMed] [Google Scholar]
- 46.Duque MA, Kresak JL, Falchook A, Harris NS. Nitrous Oxide Abuse and Vitamin B12 Action in a 20-Year-Old Woman: A Case Report. Lab Med. 2015;46:312–315. doi: 10.1309/LM0L9HAVXCHF1UQM. [DOI] [PubMed] [Google Scholar]
- 47.Chaugny C, Simon J, Collin-Masson H, De Beauchêne M, Cabral D, Fagniez O, Veyssier-Belot C. [Vitamin B12 deficiency due to nitrous oxide use: unrecognized cause of combined spinal cord degeneration] Rev Med Interne. 2014;35:328–332. doi: 10.1016/j.revmed.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 48.Pugliese RS, Slagle EJ, Oettinger GR, Neuburger KJ, Ambrose TM. Subacute combined degeneration of the spinal cord in a patient abusing nitrous oxide and self-medicating with cyanocobalamin. Am J Health Syst Pharm. 2015;72:952–957. doi: 10.2146/ajhp140583. [DOI] [PubMed] [Google Scholar]
- 49.Kiasari AZ, Firouzian A, Baradari AG, Nia HS, Kiasari SH. The Effect of Vitamin B12 Infusion on Prevention of Nitrous Oxide-induced Homocysteine Increase: A Double-blind Randomized Controlled Trial. Oman Med J. 2014;29:194–197. doi: 10.5001/omj.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Safari A, Emadi F, Jamali E, Borhani-Haghighi A. Clinical and MRI manifestations of nitrous oxide induced vitamin B12 deficiency: A case report. Iran J Neurol. 2013;12:111–113. [PMC free article] [PubMed] [Google Scholar]


