Abstract
This study investigated whether or not vitamin D and calcium supplementation affected bone metabolism and bone mineral density (BMD) over a period of four years of denosumab therapy in patients with primary osteoporosis. Patients were divided into a denosumab monotherapy group (22 cases) or a denosumab plus vitamin D and calcium supplementation group (combination group, 21 cases). We measured serum bone alkaline phosphatase (BAP), tartrate-resistant acid phosphatase (TRACP)-5b, urinary N-terminal telopeptide of type-I collagen (NTX), and BMD of the lumbar 1–4 vertebrae (L-BMD) and bilateral hips (H-BMD) at baseline and at 12, 24, 36, and 48 months of treatment. There were no significant differences in patient background. Serum BAP, TRACP-5b, and urinary NTX were significantly and comparably inhibited in both groups from 12 to 48 months versus baseline values. L-BMD was significantly increased at every time point in both groups, while H-BMD was significantly increased at every time point in the combination group only. There were significant differences between the groups for L-BMD at 24, 36, and 48 months (P < 0.05) and for H-BMD at 12 months (P < 0.05). Compared with denosumab monotherapy, combination therapy of denosumab plus vitamin D and calcium significantly increased H-BMD at 12 months and L-BMD from 24 to 48 months. These findings indicate that continuous vitamin D and calcium supplementation is important, especially for 12 months to improve H-BMD and from 24 to 48 months to improve L-BMD.
Keywords: bone mineral density, bone turnover markers, denosumab, primary osteoporosis, vitamin D
1. Introduction
Osteoporosis is the most common bone disease and affects millions of people worldwide, particularly the elderly and post-menopausal women. Thus, appropriate lifestyle, nutritional supplement, and pharmacological therapeutic choices are required.
The American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology Medical Guidelines for Practice very recently declared alendronate, risedronate, zoledronic acid, and denosumab as suitable initial therapies for most osteoporosis patients, especially those at high risk of fracture [1].
Denosumab is a fully human monoclonal antibody against receptor activator of nuclear factor-kappa B ligand (RANKL) that has been shown to selectively inhibit osteoclastogenesis. Consequently, denosumab abrogates bone resorption, increases bone mineral density (BMD), and prevents fragility fractures [2,3]. We and others have reported that the drug is a good therapeutic option for primary as well as secondary osteoporosis and fracture prevention in young and elderly patients [4,5,6,7,8,9,10,11,12,13]. However, most reports focus on the short-term efficacy of denosumab, i.e., 1 or 2 years, and its efficacy in real-world settings is largely unknown.
Known as calcitriol, active vitamin D (1,25-dihidroxycholecalciferol; 1,25(OH)2D3) regulates calcium metabolism [14]. Native vitamin D (from nutrition) is cholecalciferol. The latter is hydroxylated in the liver to become 25(OH)D3 (calcifediol), which is further hydroxylated in the kidneys to form 1,25(OH)2D3 [15]. Soon after the approval of denosumab use in Japan, calcium and vitamin D supplementation has been advocated to prevent hypocalcemia in osteoporosis treatment using denosumab, such as with the newly developed Denotas Chewable® (vitamin D and calcium supplementation tablets).
In our previous study [9], vitamin D and calcium supplementation was required with denosumab for primary osteoporosis since they improved bone metabolism and BMD to a greater extent after 1 year. To the best of our knowledge, however, there have been no reports on the long-term differences in bone metabolism and BMD between denosumab monotherapy and combination therapy with vitamin D and calcium in the clinical setting.
It is unclear if active vitamin D provides additive BMD and bone turnover marker gains in patients undergoing long-term bisphosphonate (BP) usage. We recently found that the addition of eldecalcitol with BP substantially increased BMD over time [16]. On the other hand, we have also observed that serum 25(OH)D3 concentrations are significantly increased after 3 years of BP therapy, even without vitamin D supplementation [17]. Thus, additional vitamin D may not be required in long-term BP therapy for osteoporosis, but the comparative effects of extended denosumab treatment with or without vitamin D and calcium supplementation on bone metabolism and BMD remain uncertain.
In this study, we examined the 1) efficacy of denosumab therapy, and 2) the transitions in serum calcium, phosphorus, 1,25(OH)2D3, and parathyroid hormone (PTH), which plays important roles in determining bone resorption and bone mass, with or without vitamin D and calcium supplementation over a period of four years in primary Japanese osteoporosis patients.
2. Patients and Methods
Forty-three patients were recruited at Shinshu University School of Medicine and Showa-Inan General Hospital between 2013 and 2017 (Table 1). The inclusion criteria for this investigation were primary treatment-naïve osteoporotic patients with low (T-score less than −2.5 SD) bilateral hip BMD (H-BMD). The exclusion criteria were chronic renal failure (an estimated glomerular filtration rate of < 40 mL/min/1.73 m2) with bone metabolic disorder or diabetes mellitus possibly affecting osteoporosis. Patients were divided into two groups: 22 receiving denosumab alone (denosumab monotherapy group) and 21 receiving denosumab and vitamin D supplementation (combination group) (Table 1). All patients were diagnosed as having primary osteoporosis. No patient had been pretreated with any BP before denosumab treatment (Table 1). The diagnosis of primary osteoporosis was made in accordance with the revised criteria established by the Japanese Society of Bone and Mineral Research [18]. All patients received denosumab (60 mg, s.c.) once every 6 months. Subjects in the combination group were given newly approved vitamin D and calcium supplementation tablets (610 mg of calcium, 400 IU of cholecalciferol, 30 mg of magnesium) daily during the denosumab regime.
Table 1.
Patient characteristics prior to denosumab treatment.
| Characteristic | Denosumab (n = 22) | Combination (n = 21) | p-Value |
|---|---|---|---|
| Age (years) | 72.3 ± 2.0 | 72.1 ± 2.7 | 0.94 |
| Gender (F:M) | 20:2 | 18:3 | |
| BMI (kg/m2) | 21.8 ± 0.9 | 21.5 ± 0.7 | 0.74 |
| Serum-corrected calcium (mg/dL) | 9.2 ± 0.1 | 9.1 ± 0.1 | 0.19 |
| Serum phosphorus (mg/dL) | 3.7 ± 0.1 | 3.5 ± 0.2 | 0.40 |
| Serum BAP (μg/L) | 21.4 ± 2.3 | 22.1 ± 2.5 | 0.85 |
| TRACP-5b (mU/dL) | 595.1 ± 45.7 | 593.4 ± 49.2 | 0.98 |
| Urinary NTX (nmol BCE/mmol CRE) | 52.6 ± 5.6 | 49.7 ± 5.6 | 0.67 |
| 1,25(OH)2D3 (pg/mL) | 57.9 ± 4.7 | 57.6 ± 6.7 | 0.97 |
| Serum whole PTH (pg/mL) | 28.9 ± 2.8 | 30.0 ± 2.8 | 0.77 |
| Lumbar 1–4 BMD (g/cm2) | 0.789 ± 0.02 | 0.798 ± 0.04 | 0.84 |
| Total hip BMD (g/cm2) | 0.657 ± 0.02 | 0.672 ± 0.03 | 0.68 |
BMI: body mass index; BAP: bone specific alkaline phosphatase; TRACP-5b: tartrate-resistant acid phosphatase-5b; NTX: N-terminal telopeptide of type-I collagen; PTH: parathyroid hormone; BMD: bone mineral density. Results are the mean ± standard error of the mean. A P-value of < 0.05 was considered statistically significant.
The percent changes in serum concentrations of calcium, phosphorus, whole PTH, 1,25(OH)2D3, bone alkaline phosphatase (BAP), tartrate-resistant acid phosphatase (TRACP)-5b, and urinary N-terminal telopeptide of type-I collagen (NTX) were measured at baseline and at 12, 24, 36, and 48 months of treatment. BAP was measured as a bone-formation marker using a chemiluminescent enzyme immunoassay. TRACP-5b and urinary NTX (Osteomark®; Ostex International, Seattle, WA, USA) were assessed as markers of bone resorption using an enzyme-linked immunosorbent assay. Whole PTH and 1,25(OH)2D3 were measured by immunoradiometric assays. After an overnight fast, serum and first-void urine samples were collected between 08:30 and 10:00 a.m. Immunoassays were carried out by SRL (Tokyo, Japan).
The percent changes in BMD were calculated using a dual-energy X-ray absorption fan-beam bone densitometer (Lunar Prodigy; GE Healthcare, Waukesha, WI, USA) at the lumbar 1–4 vertebrae of the posteroanterior spine (L-BMD) and the bilateral hips.
Results are expressed as the mean ± standard error of the mean. For both groups, we compared the changes in serum calcium, phosphorus, whole PTH, 1,25(OH)2D3, bone turnover markers, L-BMD, and H-BMD with baseline values at each time point using the Bonferroni correction method for multiple comparisons. Comparisons of the above parameters between the two groups at each measurement point were performed using Welch’s t-test. Differences were considered statistically significant at P < 0.05.
The study protocol was approved by the Ethics Committee of Shinshu University School of Medicine (Matsumoto, Japan) and Showa-Inan General Hospital (Komagane, Japan). This investigation was carried out in accordance with the ethical standards set forth in the Declaration of Helsinki (2014 revision). Written informed consent was obtained from all patients.
3. Results
There were no significant differences in patient background between the denosumab monotherapy group and combination group (Table 1). No serious adverse events, such as hypocalcemia or atypical fracture, occurred during the study.
3.1. Serum Albumin-Corrected Concentrations of Calcium, Phosphorus, Whole PTH, and 1,25(OH)2D3
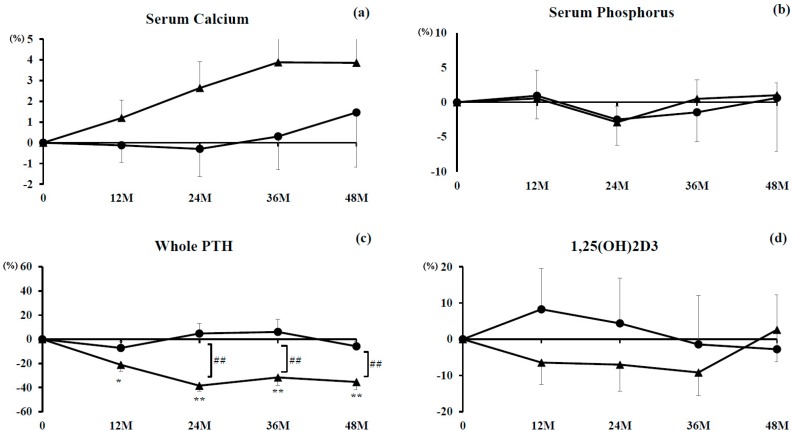
No significant differences in serum calcium concentration were found in either group with their respective baseline or between the groups at any time point (Figure 1a). Percent changes hovered around baseline in the denosumab monotherapy group and were slightly increased in the combination group throughout the study period.
Figure 1.
Percent changes in concentrations of serum albumin-corrected calcium (a); phosphorus (b); whole parathyroid hormone (PTH) (c); and 1,25(OH)2D3 (d). Closed circles indicate the denosumab monotherapy group and closed triangles indicate the combination group. Single (P < 0.05) and double (P < 0.01) asterisks denote significant differences compared with pretreatment values. Double hashtags denote a significant difference (P < 0.01) between the denosumab monotherapy and combination groups at indicated time points.
Similarly for percent changes in serum phosphorus, no significant differences were seen for either group and their respective baseline or between the groups at any time point (Figure 1b). Percent changes transitioned comparably in both groups during the observational period.
The percent changes in serum concentrations of the whole PTH were significantly inhibited at 12 months (P < 0.05) and at 24, 36, and 48 months (P < 0.01) in the combination group. However, they remained around baseline in the denosumab monotherapy group. Significant differences were noted at 24, 36, and 48 months between the groups (P < 0.01) (Figure 1c).
No significant differences were seen for percent changes in serum 1,25(OH)2D3 for either group and their respective baseline or between the groups at any time point (Figure 1d).
3.2. Markers of Bone Turnover
3.2.1. Marker of Bone Formation
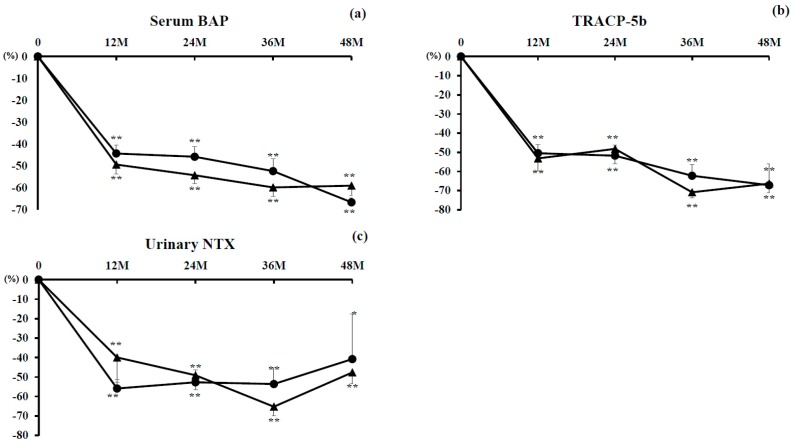
Percent changes in serum concentrations of BAP were significantly decreased from 12 to 48 months in both groups (P < 0.01). There were no significant differences between the groups (Figure 2a).
Figure 2.
Percent changes in concentrations of bone alkaline phosphatase (BAP) (a) and tartrate-resistant acid phosphatase (TRACP)-5b (b) and of urinary N-terminal telopeptide of type-I collagen (NTX) (c). Closed circles indicate the denosumab monotherapy group and closed triangles indicate the combination group. Single (P < 0.05) and double (P < 0.01) asterisks denote significant differences compared with pretreatment values.
3.2.2. Markers of Bone Resorption
Percent changes in serum concentrations of TRACP-5b were significantly suppressed from 12 to 48 months in both groups (P < 0.01). There were no significant differences between the groups (Figure 2b).
Percent changes in concentrations of urinary NTX were also significantly suppressed from 12 to 48 months in both groups (P < 0.01, and P < 0.05 at 48 months only in the denosumab monotherapy group). There were no significant differences between the groups (Figure 2c).
3.2.3. L-BMD and H-BMD
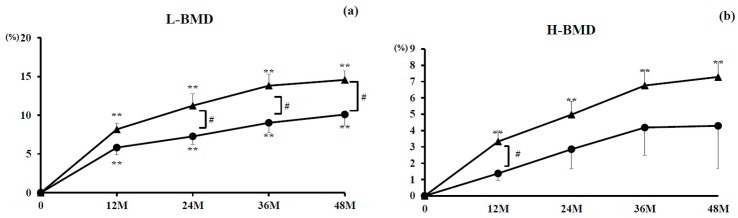
Percent changes in L-BMD were significantly increased throughout treatment in the denosumab monotherapy group (10.1% increase at 48 months) (all P < 0.01) and the combination group (14.6% increase at 48 months) (all P < 0.01) compared with baseline values. There were significant differences at 24, 36, and 48 months (P < 0.05) between the groups (Figure 3a).
Figure 3.
Percent changes in lumbar 1–4 spine bone mineral density (L-BMD) and total hip BMD (H-BMD). Closed circles indicate the denosumab monotherapy group and closed triangles indicate the combination group. Double asterisks denote a significant difference (P < 0.01) compared with pretreatment values. Single hashtag denotes a significant difference (P < 0.05) between the groups at indicated time points.
Percent changes in H-BMD were significantly increased throughout treatment in the combination group (7.3% increase at 48 months) (all P < 0.01) and substantially increased for 48 months in the denosumab monotherapy group (4.3% increase at 48 months). There was a significant difference at 12 months between the groups (P < 0.05) (Figure 3b).
4. Discussion
We report for the first time long-term comparative data for denosumab with or without vitamin D and calcium supplementation in Japanese patients with primary osteoporosis. Compared with denosumab monotherapy, combination therapy significantly inhibited percent changes in PTH and increased percent changes in H-BMD at 12 months and L-BMD from 24 to 48 months.
Denosumab is a potent and effective anti-resorptive compound. We and others have reported a significantly decreased risk of vertebral fracture and absence of hypocalcemia with denosumab for 1 year [9,19]. However, studies on the effectiveness and/or adverse effects of denosumab with or without vitamin D supplementation in osteoporosis patients for longer than 3 years in a real-world setting are lacking. Body et al. observed that denosumab without calcium and vitamin D caused significant hypocalcemia [20], although we earlier showed no hypocalcemia or other serious adverse effects and no significant calcium metabolic change [9], which was consistent with the current data. These results indicate that hypocalcemia can be prevented during denosumab treatment by supplementation with vitamin D and calcium.
Ebina’s group and our own recently reported that denosumab plus active vitamin D combination therapy significantly increased femoral neck BMD values compared with denosumab plus native vitamin D [11,12]. In the present study, serum PTH concentrations were significantly decreased and serum calcium concentrations rose in the combination group over the four years. We earlier described significantly increased serum PTH and significantly decreased serum calcium in a denosumab monotherapy group [7]. Together, these findings suggest that, in the short-term, serum calcium concentrations decrease to thereby increase PTH concentration in denosumab monotherapy patients, while in the long-term, vitamin D and calcium addition increase calcium to decrease PTH. It was noteworthy that serum calcium and PTH concentrations did not change without vitamin D addition during denosumab therapy between 1 and 4 years.
Prior studies have shown that active vitamin D administration decreased serum concentrations of PTH [7,21], which was consistent with the present data. Signaling of PTH receptors in osteoblasts and osteocytes can increase the ratio of RANKL/osteoprotegerin (a decoy receptor of RANKL) to increase the recruitment and activity of osteoclasts and stimulate bone resorption [21]. Thus, the inhibitory effects on PTH caused by vitamin D administration might have resulted in larger gains in BMD in the combination group than in the monotherapy group.
In our cohort, denosumab alone increased L-BMD as much as 10.1% and H-BMD as much as 4.3% at 48 months, while denosumab plus vitamin D and calcium supplementation augmented L-BMD as much as 14.6% and H-BMD as much as 7.3% at 48 months. Moreover, there was a significant difference in H-BMD at 12 months in the combination group over the denosumab monotherapy group and more significant gains in L-BMD from 12 to 48 months in the combination group. Ebina et al. found that PTH values were significantly higher in a native vitamin D group than in an alfacalcidol group, which had shown increased BMD, without differences in bone turnover inhibitory effects between the groups [11]. Taken together, vitamin D addition may significantly increase BMD owing to a decrease in serum PTH.
Heckman et al. described that, in elderly patients with osteoporosis who were unresponsive to BPs, vitamin D addition could improve BMD at the lumbar spine to consequently prevent fracture [22]. Our study showed that H-BMD at 12 months and L-BMD at 24, 36, and 48 months were significantly improved by the addition of vitamin D and calcium during denosumab treatment in primary osteoporosis in the absence of fractures during the observation period. The above evidence confirms that an increase in BMD reduces the risk of fracture and that combination therapy of denosumab with vitamin D may be optimal.
Lastly, Holick reported that serum 25(OH)D3 was the only barometer for vitamin D status in osteoporosis patients and that serum 1,25(OH)2D3 provided limited information about vitamin D status [23]. Since we did not examine serum 25(OH)D3 concentrations in this study, the precise vitamin D status was unknown in the combination group.
The main limitations of this investigation were its relatively small sample size and that serum 25(OH)D3 status was not measured. Further studies are required to ascertain to what extent fractures can be prevented over longer periods.
5. Conclusions
No adverse effects, such as atypical fracture or hypocalcemia, occurred in either the denosumab monotherapy group or the combination group. H-BMD at 12 months and L-BMD from 24 to 48 months were significantly improved in combination therapy of denosumab with vitamin D and calcium. Thus, it is highly recommended that these supplements are included with denosumab administration for primary osteoporosis carrying a high risk of hip and lumbar fracture.
Acknowledgments
We thank Mr. Yuji Takanashi for collecting patient data at Showa-Inan General Hospital and Shinshu University School of Medicine. We also thank Mr. Trevor Ralph for editing the manuscript.
Author Contributions
Y.N. directed the study; Y.N. and H.K. conceived and designed the experiments; T.S. analyzed the data; T.S. and Y.N. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Camacho P.M., Petak S.M., Binkley N., Clarke B.L., Harris S.T., Hurley D.L., Kleerekoper M., Lewiecki E.M., Miller P.D., Narula H.S., et al. American association of clinical endocrinologists and American college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr. Pract. 2016;22:1–42. doi: 10.4158/EP161435.GL. [DOI] [PubMed] [Google Scholar]
- 2.McClung M.R., Lewiecki E.M., Cohen S.B., Bolognese M.A., Woodson G.C., Moffett A.H., Peacock M., Miller P.D., Lederman S.N., Chesnut C.H., et al. Denosumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 3.Silva I., Branco J.C. Denosumab: recent update in postmenopausal osteoporosis. Acta Reumatol. Port. 2012;37:302–313. [PubMed] [Google Scholar]
- 4.Uehara M., Nakamura Y., Takahashi J., Kamimura M., Ikegami S., Suzuki T., Uchiyama S., Yamaguchi T., Kosho T., Kato H. Efficacy of denosumab for osteoporosis in three female patients with osteogenesis imperfecta. Tohoku J. Exp. Med. 2017;242:115–120. doi: 10.1620/tjem.242.115. [DOI] [PubMed] [Google Scholar]
- 5.Isobe F., Nakamura Y., Suzuki T., Kato H. Effects of denosumab on osteoporosis in three cases with anorexia nervosa and a review of the literature. Mod. Rheumatol. Case Rep. 2017;2:104–116. doi: 10.1080/24725625.2017.1370784. [DOI] [Google Scholar]
- 6.Kumaki D., Nakamura Y., Sakai N., Kosho T., Nakamura A., Hirabayashi S., Suzuki T., Kamimura M., Kato H. Efficacy of denosumab for glucocorticoid-induced osteoporosis with duchenne muscular dystrophy. JBJS Case Connect. 2018 doi: 10.2106/JBJS.CC.17.00190. in press. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura Y., Kamimura M., Ikegami S., Mukaiyama K., Uchiyama S., Taguchi A., Kato H. Changes in serum vitamin D and PTH values using denosumab with or without bisphosphonate pre-treatment in osteoporotic patients: a short-term study. BMC Endocr. Disord. 2015;15:81. doi: 10.1186/s12902-015-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamimura M., Nakamura Y., Ikegami S., Uchiyama S., Kato H., Taguchi A. Significant improvement of bone mineral density and bone turnover markers by denosumab therapy in bisphosphonate-unresponsive patients. Osteoporos Int. 2017;28:559–566. doi: 10.1007/s00198-016-3764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura Y., Suzuki T., Kamimura M., Murakami K., Ikegami S., Uchiyama S., Kato H. Vitamin D and calcium are required at the time of denosumab administration during osteoporosis treatment. Bone Res. 2017;5:17021. doi: 10.1038/boneres.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki T., Nakamura Y., Kato H. Significant improvement of bone mineral density by denosumab without bisphosphonate pre-treatment in glucocorticoid-induced osteoporosis. Mod. Rheumatol. 2017 doi: 10.1080/14397595.2017.1416919. [DOI] [PubMed] [Google Scholar]
- 11.Ebina K., Kashii M., Hirao M., Hashimoto J., Noguchi T., Koizumi K., Kitaguchi K., Matsuoka H., Iwahashi T., Tsukamoto Y., et al. Comparison of the effects of denosumab between a native vitamin D combination and an active vitamin D combination in patients with postmenopausal osteoporosis. J. Bone Miner. Metab. 2017;35:571–580. doi: 10.1007/s00774-016-0792-5. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T., Nakamura Y., Tanaka M., Kamimura M., Ikegami S., Uchiyama S., Kato H. Comparison of the effects of denosumab with either active vitamin D or native vitamin D on bone mineral density and bone turnover markers in postmenopausal osteoporosis. Mod. Rheumatol. 2017 doi: 10.1080/14397595.2017.1308454. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki T., Nakamura Y., Kamimura M., Ikegami S., Uchiyama S., Kato H. Compliance and discontinuation of denosumab treatment in postmenopausal Japanese women with primary osteoporosis or rheumatoid arthritis and osteoporosis. Osteoporos. Sarcopenia. 2017;3:108–111. doi: 10.1016/j.afos.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeLuca H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 15.Bouillon R., Okamura W.H., Norman A.W. Structure-function relationships in the vitamin D endocrine system. Endocr Rev. 1995;16:200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- 16.Kamimura M., Mukaiyama K., Koiwai H., Ikegami S., Nakamura Y., Kato H. Additive effects of eldecalcitol for poorly responding long-term bisphosphonate treatment for osteoporosis. Ther. Clin. Risk Manag. 2018 doi: 10.1016/j.afos.2019.06.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura Y., Uchiyama S., Kamimura M., Ikegami S., Komatsu M., Kato H. Increased serum 25(OH)D3 levels in post-menopausal Japanese women with osteoporosis after 3-year bisphosphonate treatment. Tohoku J. Exp. Med. 2017;242:241–246. doi: 10.1620/tjem.242.241. [DOI] [PubMed] [Google Scholar]
- 18.Soen S. New Diagnostic criteria and guidelines on osteoporosis. Diagnostic criteria for primary osteoporosis: Year 2012 revision. Clin. Calcium. 2014;24:323–329. (In Japanese) [PubMed] [Google Scholar]
- 19.Sugimoto T., Matsumoto T., Hosoi T., Miki T., Gorai I., Yoshikawa H., Tanaka Y., Tanaka S., Fukunaga M., Sone T., et al. Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: Results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT) Osteoporos Int. 2015;26:765–774. doi: 10.1007/s00198-014-2964-2. [DOI] [PubMed] [Google Scholar]
- 20.Body J.J., Bone H.G., de Boer R.H., Stopeck A., Van Poznak C., Damião R., Fizazi K., Henry D.H., Ibrahim T., Lipton A., et al. Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur. J. Cancer. 2015;5:1812–1821. doi: 10.1016/j.ejca.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Olmos J.M., Hernández J.L., Llorca J., Nan D., Valero C., González-Macías J. Effects of 25-hydroxyvitamin D3 therapy on bone turnover markers and PTH levels in postmenopausal osteoporotic women treated with alendronate. J. Clin. Endocrinol. Metab. 2012;97:4491–4497. doi: 10.1210/jc.2012-2999. [DOI] [PubMed] [Google Scholar]
- 22.Heckman G.A., Papaioannou A., Sebaldt R.J., Ioannidis G., Petrie A., Goldsmith C., Adachi J.D. Effect of vitamin D on bone mineral density of elderly patients with osteoporosis responding poorly to bisphosphonates. BMC Musculoskel. Dis. 2002;3:6. doi: 10.1186/1471-2474-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holick M.F. Vitamin D status: Measurement, interpretation and clinical application. Ann. Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]