Abstract
Objective
Kaposi sarcoma (KS) is an HIV-associated malignancy caused by human herpesvirus-8 that occurs at highest incidence in sub-Saharan Africa. KS patients often present with inflammatory symptoms associated with higher mortality.
Design
We conducted a double-blind, randomized, placebo-controlled study in Uganda to test whether omega-3 (ω-3) supplementation could reduce inflammation in HIV and HHV-8 co-infected adults ≥18 years of age. Patients with acute illness, AIDS, or advanced KS were ineligible, as were pregnant women. Participant IDs were pre-randomized, blocked by KS status, to either the ω-3 or placebo arm.
Methods
ω-3 participants received a 3-gram pill dose daily for 12 weeks (1.8g eicosapentaenoic acid, 1.2mg docosapentaenoic acid); placebo participants received 44.8mg of high oleic safflower oil that appeared indistinguishable from the active supplement. Intervention effects were evaluated as the baseline-adjusted mean differences after 12 weeks between ω-3 and placebo participants in concentrations of fatty acids, inflammatory cytokines, and immune cells.
Results
The final study population was comprised of 56 KS patients and 11 KS-negative, HHV-8 positive participants randomized to receive either ω-3 (N=33) or placebo (N=34). Inflammatory cytokine interleukin-6 (IL-6) concentrations decreased in ω-3 participants (−0.78 pg/mL) but increased in placebo participants (+3.2 pg/mL;P− =0.04). We observed a trend towards decreased IL-6 after ω-3 supplementation specific to KS patients (N=58;P=0.08). CD8+ counts tended to increase in the ω-3 arm KS patients (+60 cells/mm3), while decreasing (−47 cells/mm3) with placebo (P=0.11).
Conclusion
ω-3 supplementation decreased Il-6 concentrations among HIV and HHV-8 co-infected Ugandans, which may have clinical benefit for KS patients.
Keywords: Kaposi sarcoma, human herpes virus-8, inflammation, omega-3 supplementation, Uganda
INTRODUCTION
Cancer increasingly contributes to mortality among HIV-infected persons worldwide.(1, 2) Kaposi sarcoma (KS) is an HIV-associated malignancy that is a leading cause of cancer morbidity and mortality in sub-Saharan Africa.(3, 4) Inflammation is a prominent feature of KS pathogenesis: KS tumors are characterized histologically by a pronounced inflammatory infiltrate,(5, 6) and patients often present clinically with inflammatory symptoms, including fever, cachexia, and edema. Human herpesvirus-8 (HHV-8), the causative agent of KS, encodes several viral gene products believed to promote inflammatory dysregulation, including a viral interleukin-6 (vIL-6) that induces production of human IL-6, IL-10 and other cytokines.(7, 8) Recent studies have described an inflammatory syndrome (KSHV inflammatory cytokine syndrome [KICS]) in patients with HIV and HHV-8 co-infection and report that patients levels of human IL-6 and IL-10 are associated with KS severity.(9) Importantly, this inflammatory syndrome is associated with higher mortality among KS patients, suggesting that inflammation and cytokine overproduction may be an important factor in KS treatment response and survival.
Single-agent clinical trials of omega-3 (ω-3) supplementation in HIV-infected patients in resource-rich settings have demonstrated significant shifts in leukotriene composition and decreases in inflammatory molecules such as IL-6, TNF-α, IL-1β.(10–12) In a preliminary, observational study of HIV-infected Ugandan adults, we noted that the ω-6: ω-3 fatty acid ratio was elevated among adults who developed KS and was also associated with the level of HHV-8 shedding.(13) The traditional diet in Uganda is largely based on maize (>25% of energy) and is low in fish (<6% of energy)(14), which could potentially play a role in poor control of HHV8 replication in this setting. Approximately 40 to 60% of the Ugandan population is infected with HHV-8, and of one third exhibit HHV-8 replication in peripheral blood (viremia), a strong risk factor for progression to KS (15–19).
Given the anti-inflammatory properties of ω-3, as well as the potential link between ω-3 and HHV-8, we hypothesized that altering the fatty acid ratio through supplementation could modify the inflammatory milieu, ultimately impacting KS disease progression. Accordingly, we conducted a double-blind, randomized, placebo-controlled study to evaluate the effect of ω-3 fatty acid supplementation on changes in the plasma fatty acid ratio, systemic inflammation, and immunological status of HIV and HHV-8 co-infected adults.
METHODS
HIV and HHV-8 co-infected individuals were recruited between May and November 2012 from the Uganda Cancer Institute (UCI)/Hutchinson Center Cancer Alliance in Kampala, Uganda. Participants of former studies with known HIV and HHV-8 status were identified through the UCI/Hutchinson Center Cancer Alliance database and were invited to the clinic. HIV-infected patients with KS were also recruited during routine clinic visits by collaborating physicians. In addition to evidence of HIV and HHV-8 infection, eligible participants had to be at least 18 years of age could not be pregnant.
Additional eligibility criteria to avoid enrolling individuals with acute illness, AIDS, or advanced KS into the trial were applied. Specifically, to identify participants who had stable KS disease, KS-positive individuals had to have early stage (T0) by AIDS Clinical Trials Group (ACTG) staging criteria, be receiving ART for at least 2 months, and should not have received chemotherapy for at least 2 weeks. HIV-infected individuals without KS could not have advanced HIV disease. At the time the study was conducted in Uganda, patients with CD4 T-cell counts >500 cells/mm3 were not eligible for antiretroviral therapy (ART). As a proxy for advanced immunosuppression, persons receiving ART were excluded from the study.
Study Procedures
At the screening visit, participants provided informed consent, and HIV antibody and pregnancy tests were conducted. Participants who were confirmed eligible were invited back for an enrollment visit. Participant IDs were pre-randomized, blocked by KS status, via a computer program to either the omega-3 (ω-3) or placebo arm. At the baseline enrollment visit, the study coordinator opened a sequentially numbered, opaque envelope containing a pre-randomized participant ID, which was then linked to a corresponding pill packet (ω-3 or placebo).
At enrollment, study staff measured height and weight and collected data on demographic and health-related characteristics, including usual fish consumption and medication/supplement use. Participants were given their first study pill allotment and were instructed to consume the study dose (8 pills) daily. Participants returned for interim visits every 3 weeks (e.g. weeks 4, 7, 10, and 13) to bring back unused study pills and receive a new pill allotment (in weeks 4, 7, and 10). At each visit, participants provided data on use of ART, chemotherapy, other medications, and supplement use, answered questions regarding adherence to the study dose (8 pills per day), and study staff monitored adverse events and conducted pill counts to assess compliance. Compliance rates were calculated as the difference between the study dose participants were instructed to consume (8 pills per day) and the dose actually taken (determined via pill counts). Non-fasting blood draws (10mL) were collected at the baseline (week 1) and final (week 13) visits, as well as weeks 11 and 12. The institutional review board at the Fred Hutchinson Cancer Research Center, the Makerere University School of Medicine Research and Ethics Committee, the Ugandan National Council for Science and Technology of Science and National Drug Agency approved all study procedures.
12-week treatment regimen
ω-3 arm participants received a daily fatty acid dose of approximately 3 grams (8 pills; 231mg eicosapentaenoic acid [EPA] and 154mg docosapentaenoic acid [DPA] per pill). Placebo arm participants received a daily dose of safflower oil (8 pills: 5.6mg high-oleic safflower oil per pill). Packaging and appearance of ω-3 fatty acid and placebo pills was identical, and neither study participants nor researchers were informed of participants’ study arm assignment.
Study Endpoint Assessment
Plasma concentrations of omega-3 fatty acids (EPA, 18:3n-3, decosahexaenoic acid [DHA], 22:6n-3), DPA, 22:5n-3), inflammatory cytokines (C-reactive protein (CRP), interleukin-6 (IL-6)), and immune cells (CD4+ and CD8+ T-cells) were measured in blood samples collected at baseline (week 1) and final (week 13) visits.
Assays were performed by the Fred Hutchinson Cancer Research Center Public Health Sciences Biomarkers laboratory. Detailed methods for the phospholipid fatty acid assay have been published elsewhere.(13) Briefly, total lipids were extracted from plasma, and phospholipids were separated from other lipids by one-dimensional thin-layer chromatography. Fatty acid methyl ester (FAME) samples were prepared by direct transesterification and separated using gas chromatography. Fatty acid concentrations are expressed in relative terms as the weight percentage of total phospholipid fatty acids. Quality control (QC) samples were run with each batch of 10 study samples, and the laboratory personnel were blinded to participant treatment arm. The inter-batch coefficients of variation (CVs) were: EPA:1.73%; DHA: 0.87%; DPA:0.70%
Plasma CRP was measured using the Wide Range CRP reagent (CRP (3) reagent; Kamiya Biochemical Company, Seattle, WA) on a Roche Cobas Mira chemistry analyzer. Samples were run in duplicate with a median duplicate CV of 2.47%. IL-6 was measured using the Quantikine Human IL-6 Elisa kit (D6050; R&D Systems, Minneapolis, MN). Samples were run in duplicate with a median duplicate CV of 3.60%. A pooled plasma sample was included as a lab QC with every batch of study samples and had an inter-batch CV of 3.91% and 3.10% for CRP and IL-6, respectively.
The Makerere University-Johns Hopkins University (MU-JHU) Core laboratory in Kampala, Uganda measured immune cells (CD4/8 T-cell counts) within 48 hours of non-fasting blood draw. In addition, post-treatment HHV-8 DNA was measured quantitatively at the Hutchinson Center Research Institute-Uganda in Kampala in post-treatment plasma collected at weeks 11, 12, and 13 with real-time polymerase chain reaction (PCR) as previously described;(20) samples with >=50 copies HHV-8 DNA/ml were considered positive.
Statistical Analyses
For concentrations of fatty acids, inflammatory cytokines, and immune cells, intervention effects were defined as the difference in the mean change from baseline to final visit between ω-3 and placebo study arm participants. Linear regression models evaluating these intervention effects adjusted for the respective measure’s baseline concentration. For post-treatment HHV-8 viral load, we evaluated the difference in the mean HHV-8 viral load (mean across the week 11, 12, and 13 measures) between ω-3 and placebo study arm participants. HHV-8 was not measured in baseline blood specimens, so linear regression models included no baseline adjustment.
For all primary analyses outlined above, exploratory models were additionally adjusted for covariates potentially unbalanced by randomization (age, gender, and BMI at enrollment); however, results were similar, so covariates were not included in the final intent-to-treat models. In order to evaluate whether intervention effects were greater among individuals with a history of clinical disease, additional analyses were conducted by KS status. All analyses were performed using SAS version 9.3. Study sample size was determined by setting the statistical power (1-beta error) to 80%, the minimal detectable intervention effect to −3mg/L, and the alpha error rate to 5% for a one-tailed test designed to evaluate whether there was a significant increase in inflammatory cytokine (e.g., CRP) in the placebo versus ω-3 study arm. Calculations indicated that 34 individuals were needed per study arm.
RESULTS
We contacted 109 individuals to determine eligibility for this study. Seventy-one of these HIV and HHV-8 co-infected Ugandan adults were deemed eligible and provided informed consent to participate, of whom 69 were successfully enrolled and randomized to receive either omega-3 (ω-3) fish oil or placebo for 12 weeks (Figure 1; Table 1). The majority of participants (N=58; 84%) had a history of KS, approximately half (54%) were under 40 years of age at enrollment (median=38; range 18–64). One participant was withdrawn at the week-4 clinic visit due to initiation of chemotherapy for KS, and 1 participant died of non-study-related causes 28 days after enrollment. The remaining 33 ω-3 fish oil arm and 34 placebo arm participants completed all study visits and provided samples for endpoint assessment.
Figure 1.
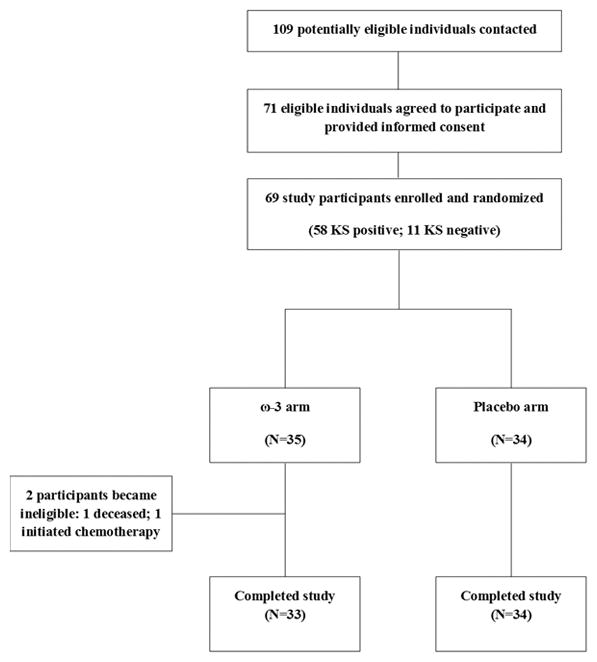
ω-3 Fish Oil Supplementation Trial
TABLE 1.
PATIENT CHARACTERISTICS AT BASELINE ACCORDING TO STUDY ARM
| Omega-3 (N=35) | Placebo Arm (N=34) | Exact P-value | |
|---|---|---|---|
|
| |||
| KS Status | |||
| KS Positive | 29 (82.9%) | 29 (85.3%) | |
| KS Negative | 6 (17.1%) | 5 (14.7%) | P=0.99 |
| Age | |||
| 18–32 years | 9 (25.7%) | 8 (23.5%) | |
| 33–38 years | 12 (34.3%) | 8 (23.5%) | |
| 39–45 years | 7 (20.0%) | 9 (26.5%) | |
| 46–64 years | 7 (20.0%) | 9 (26.5%) | P=0.73 |
| Sex | |||
| Female | 17 (48.6%) | 14 (41.2%) | |
| Male | 18 (51.4%) | 20 (58.5%) | P=0.63 |
| BMI (kg/m2) | |||
| ≤ 20.0 | 6 (17.1%) | 11 (32.4%) | |
| 20.1–23.0 | 10 (28.6%) | 7 (20.6%) | |
| 23.1–25.0 | 11 (31.4%) | 9 (26.5%) | |
| > 25.0 | 8 (22.9%) | 7 (20.6%) | P=0.57 |
| Mean baseline CD4+ T-cell count, mm3 (95% CI) | 439 (366,513) | 423 (359,487) | P=0.74 |
| Mean baseline CD8+ T-cell count, mm3 (95% CI) | 915 (769,1061) | 901 (733,1069) | P=0.90 |
Adherence and Adverse Events
The majority of participants in both the ω-3 and placebo study arms were compliant with the 8-pill daily study dose over the course of the 12-week intervention, demonstrating the feasibility of daily supplementation in this patient setting. According to pill counts conducted by study staff, 78% of study participants took ≥95% of the study dose. Compliance did not substantively differ by study arm, with ≥95% compliance among 79% and 76% of the ω-3 and placebo arms, respectively. Participants experienced few adverse events (ω-3 arm: N=3; placebo arm: N=2), and only one event was classified as possibly related to ω-3 fish oil supplementation; one participant in the ω-3 arm complained of moderate diarrhea (grade 2) that resolved 3 days after initial report with oral rehydration solution.
Fatty Acids after ω-3 treatment
Baseline EPA, DHA, and DPA omega-3 fatty acid concentrations were similar between ω-3 and placebo study arms, with slightly higher DHA levels observed in participants in the ω-3 arm at baseline (P =0.01). We observed stable plasma fatty acid concentrations among placebo arm participants. (Table 2) In contrast, fatty acid concentrations increased in the ω-3 arm: EPA (+4.0), DHA (+2.8), and DPA (+1.3). The resulting intervention effects on plasma fatty acid concentrations at 12 weeks were all statistically significant, with the following differences between ω-3 and placebo study arms after adjustment for baseline measures: EPA (4.3), DHA (3.2), and DPA (1.3), all P <0.001.
TABLE 2.
INTERVENTION EFFECT OF ω-3 FISH OIL ON FATTY ACID CONCENTRATIONS
| Baseline Mean (95% CI) | 12-week Change Mean (95% CI) | Effect (95% CI) and p-value | Effect in KS (N=56) | Effect in KS-negative (N=11) | |
|---|---|---|---|---|---|
| Eicosapentaenoic acid (EPA) | |||||
| Placebo | 0.74 (0.63,0.85) | −0.09 (−0.18,−0.01) | + 4.3 (3.3,5.2) | + 4.7 (3.7,5.8) | + 2.0 (−0.21,4.3) |
| ω-3 | 0.85 (0.68,1.0) | 4.0 (3.0,5.0) | P< 0.001 | P< 0.001 | P=0.07 |
| Docosahexaenoic acid (DHA) | |||||
| Placebo | 3.8 (3.5,4.1) | 0.08 (−0.23,0.40) | + 3.2 (2.5,3.8) | + 3.5 (2.8,4.2) | + 1.3 (−0.13,2.8) |
| ω-3 | 4.5 (4.0,4.9) | 2.8 (1.2,3.5) | P< 0.001 | P< 0.001 | P=0.07 |
| Docosapentaenoic acid (DPA) | |||||
| Placebo | 1.0 (0.94,1.1) | 0.02 (−0.06,0.10) | + 1.3 (0.95,1.6) | + 1.5 (1.1,1.8) | + 0.37 (−0.09,0.83) |
| ω-3 | 1.1 (1.0,1.2) | 1.3 (0.93,1.6) | P< 0.001 | P< 0.001 | P=0.10 |
Compared to the participants who were ≥95% compliant with the ω-3 study dose (N=50), participants who were <95% compliant (N=17) had smaller post-treatment increases in the fatty acid concentration: EPA (+4.5 vs. +2.3), DHA (+3.0 vs. +2.2), and DPA (+1.4 vs. +0.70), although the intervention effects remained statistically significant (data not shown).
Anti-inflammatory effect of ω-3 treatment
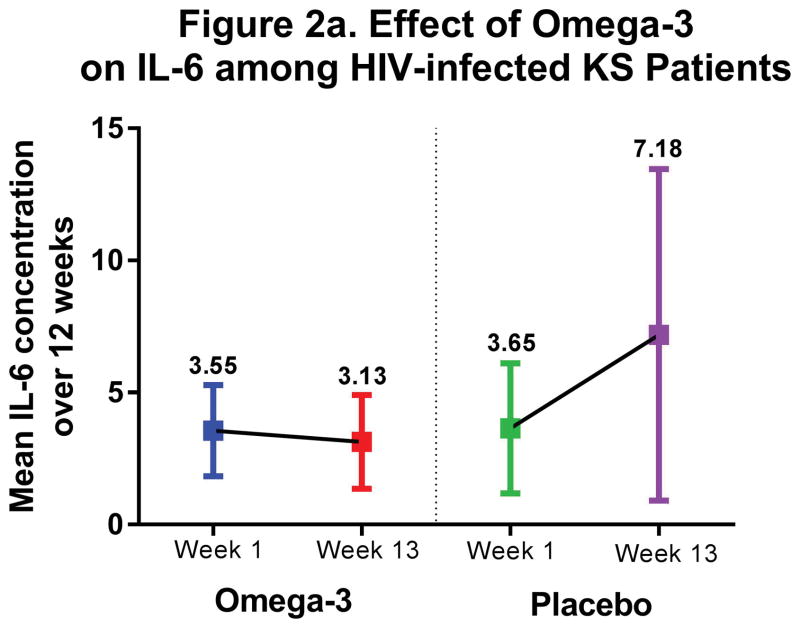
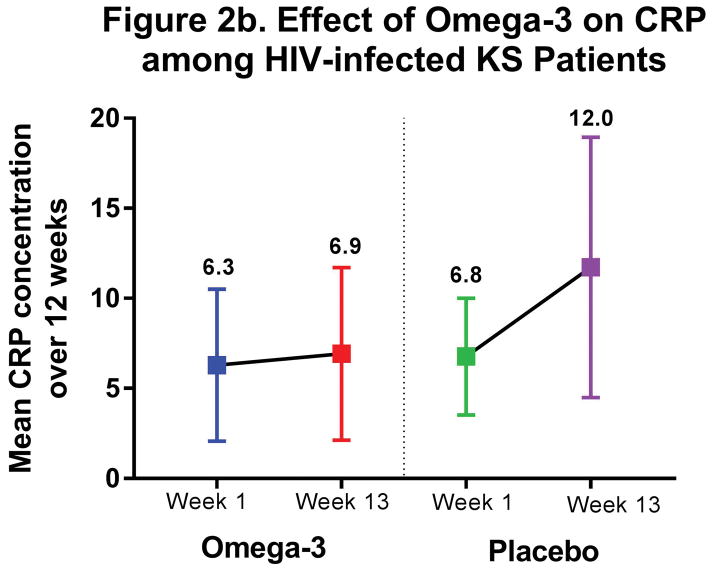
CRP and IL-6 concentrations were similar across study arms at baseline. (Table 3) CRP concentrations increased (+4.2 mg/L) in the placebo arm compared to fairly stable levels (+0.18 mg/L) in the ω-3 arm (P=0.23). IL-6 concentrations also increased in the placebo arm, nearly doubling over 12 weeks (+3.2 pg/mL), and this increase was statistically significantly different than the slight decrease observed in the ω-3 arm (−0.78 pg/mL; P=0.04). We observed the same suggestive trend of stable inflammatory cytokine concentrations over 12 weeks in those receiving ω-3 supplementation, as opposed to increasing inflammation in placebo arm participants, when restricting to KS patients. (Figure 2)
TABLE 3.
INTERVENTION EFFECT OF ω-3 FISH OIL ON IMMUNE AND INFLAMMATORY BIOMARKERS
| Baseline Mean (95% CI) | 12-week Change Mean (95% CI) | Effect (95% CI) and p-value | Effect in KS (N=56) | Effect in KS-negative (N=11) | |
|---|---|---|---|---|---|
| CD4+ T-cell Count, mm3 | |||||
| Placebo | 423 (359,487) | 32 (−9.52,72.9) | + 33.2 (−32.0,98.5) | + 19.4 (−49.2,87.9) | + 81.2 (−142,305) |
| ω-3 | 439 (366,513) | 66 (13.2,118) | P= 0.31 | P= 0.57 | P= 0.43 |
| CD8+ T-cell Count, mm3 | |||||
| Placebo | 901 (733,1069) | −19 (−133,95.2) | + 77.7 (−50.0,205) | + 104 (−23.5,232) | −17.4 (−497,463) |
| ω-3 | 915 (769,1061) | 42 (−63.7,148) | P= 0.23 | P= 0.11 | P= 0.94 |
| C-reactive Protein (CRP), mg/L | |||||
| Placebo | 6.0 (3.2,8.8) | 4.2 (−1.7,10.2) | −4.0 (−10.6,2.6) | −4.4 (−12.2,3.3) | 1.8 (−2.2,5.7) |
| ω-3 | 6.1 (2.3,9.9) | 0.18 (−3.0,3.3) | P= 0.23 | P= 0.26 | P= 0.33 |
| Interleukin-6 (IL-6), pg/mL | |||||
| Placebo | 3.3 (1.2,5.4) | 3.2 (−0.38,6.7) | −4.2 (−8.2,−0.17) | −3.9 (−8.3,0.55) | −0.13 (−2.6,2.3) |
| ω-3 | 3.8 (2.0,5.7) | −0.78 (−3.0,1.4) | P= 0.04 | P= 0.08 | P= 0.90 |
Figure 2.
Anti-inflammatory Effect of ω-3 among HIV-infected KS patients
Immune Cells
Baseline CD4+ and CD8+ T-cell counts were similar between ω-3 and placebo study arms, and after 12 weeks, median CD4+ counts increased in both the ω-3 (+66 cells/mm3) and placebo (+32 cells/mm3) arms. Although the increase in CD4+ count was larger in the ω-3 arm, the intervention effect was not statistically significant (P=0.31). CD8+ counts decreased slightly (−19 cells/mm3) in the placebo arm, but this was not statistically different from the change observed in the ω-3 arm (+42 cells/mm3; P=0.23). The CD8+ intervention effect was more pronounced among the KS patient subgroup (ω-3: +60 cells/mm3; placebo: −47 cells/mm3; P=0.11).
HHV-8 DNA
There was no difference in mean post-treatment HHV-8 viral load between ω-3 and placebo arms (1.4 log cml ± 1.7 vs. 1.4 log cml ± 1.4; P=0.94). The proportion of individuals with no detectable viral load in all 3 blood samples collected at weeks 11, 12, and 13 was similar in the ω-3 arm and placebo arms (26% vs. 31%), both overall (P=0.51) and within the KS patient subgroup (P=0.43).
DISCUSSION
Omega-3 (ω-3) fatty acid supplementation of HIV and HHV-8 co-infected Ugandan adults significantly increased plasma concentrations of ω-3 long-chain polyunsaturated fatty acids (LCPFA) DHA, DPA, and EPA. Supplementation also significantly altered circulating inflammatory cytokine levels. Compared to substantial increases in IL-6 among untreated patients, patients administered ω-3 experienced small decreases in IL-6 levels during treatment. We also observed evidence that ω-3 supplementation over 12 weeks increased CD8 T-cell counts among KS patients, but the intervention effect was not statistically significant.
Patients self-reported high adherence to the daily pill regimen, which was supported by the observations of large, statistically significant increases in fatty acid concentrations in the ω-3 arm during the 12-week trial. We found that patients receiving ω-3 supplementation maintained stable CRP levels and actually had lower IL-6 levels during the 12 weeks of treatment, which stands in contrast to the increases in circulating inflammatory markers observed for untreated HIV-infected participants. Consistent with our findings, randomized trials in HIV-infected patients from non-endemic regions have demonstrated significant effects of ω-3 LCPFA supplementation on inflammatory biomarkers, including IL-6.(10–12, 21, 22) A recent randomized ω-3 supplementation trial of similar length to ours (i.e.,12-weeks) conducted among HIV-infected participants in the United States (N=48) also reported a significant decrease in IL-6 among treated patients, in parallel to an increase in untreated patients.(12) Notably, our results from Uganda are unique because they are specific to a region where both HIV and HHV-8 are endemic viruses, and the data provides preliminary evidence that ω-3 LCPFAs supplementation could alter IL-6 levels in HIV-infected KS patients in this resource-poor setting.
Three previous randomized trials have reported no statistically significant effect of ω-3 supplementation on levels of CRP in HIV-infected patients, (12, 21, 22) consistent with our data. Notably, in the absence of ω-3 supplementation (i.e., in the placebo arm), both CRP and IL-6 levels increased during the study. Because baseline and cell count changes during the study for both CD4 and CD8 T-cells were similar between the two study arms, this observation is likely independent of immune cell function. Instead, we suspect that increased inflammatory markers in the placebo group could reflect the natural history of uncontrolled HHV-8 infection in this population. Our study was the first to evaluate the effect of ω-3 supplementation on HHV-8 viral load. Although we found no significant difference in post-treatment viral load between the ω-3 and placebo arm, our study did not compare changes in pre- and post-treatment HHV-8 copy number. Future studies with longitudinal HHV-8 measurement may provide further insight into the role LCPFAs could play in HHV-8 kinetics and inflammation.
In this trial, we found that ω-3 supplementation was associated with an increase in circulating CD4 T-cell counts by an average of 66 cells/mm3 over 12 weeks; however, this was not significantly different than the increase of 32 cells/mm3 observed in the placebo group. We are not aware of any similar clinical trials conducted to date in an HIV-endemic setting, but our findings are consistent with results from a European clinical trial of similar size (N=74) and immunocompetence (average CD4 >450 cells/mm3). This study reported increases of 61 cells/mm3 and 36 cells/mm3 in the treatment and placebo arms after 12 weeks, respectively (P intervention effect<0.05). (23) Importantly, ω-3 was only one component of the nutritional supplementation administered in this trial, making direct comparison to our data difficult.
Strengths of this study included the double blinding and randomized nature of the intervention. In addition, this was a single agent study directly relevant to the effect of ω-3 LCPFAs as opposed to many of the prior studies, which included nutritional counseling or additional nutritional supplementation. Furthermore, the administered dose of ω-3 in our trial (3g daily) exceeded that of many former single-agent ω-3 trials. The substantial increase in fatty acid concentration in the ω-3 arm provides strong biological evidence of high adherence to study protocol. However, this study is not without limitations, including the small sample size. Only post-treatment HHV-8 replication data were available, and thus we could not evaluate the treatment effects of ω-3 LCPFA supplementation for this endpoint.
The limitation of eligibility to KS patients who were in clinical remission may have diminished our ability to detect effects of omega-3 supplementation on immune measures. For example, the average CD4 T-cell count of our participants was > 400 cells/mm3, reflecting an immunocompetent population without active KS disease. All KS patients also received ART, limiting our ability to assess the potential anti-inflammatory effect of ART; however, because KS participants were on stable ART during the intervention, we do not believe this would have altered our observations. This patient population also did not appear to have high levels of systemic inflammation at baseline, with CRP values similar to that reported among black adults in the US.(24) Finally, the relatively short time that the study drug was administered (12 weeks) may have precluded more substantial changes in T-cell counts or HHV-8 quantities.
In summary, ω-3 LCPFA supplementation among HIV and HHV-8 co-infected Ugandan adults is feasible, and yielded significant increases in plasma DHA, DPA and EPA concentrations. In addition, supplementation significantly decreased Il-6 concentrations, which could have clinical benefit among those KS patients with high IL-6 levels and inflammatory symptoms. Future studies are warranted to further evaluate the potential role of this safe and relatively inexpensive nutritional supplement in the management of KS or other HIV-associated inflammatory conditions.
Acknowledgments
Sources of Funding. This research was supported by the National Institutes of Health T32 CA09168.
Footnotes
Conflicts of Interest. No authors report any conflicts of interest.
References
- 1.Casper C. The increasing burden of HIV-associated malignancies in resource-limited regions. Annu Rev Med. 2011;62:157–70. doi: 10.1146/annurev-med-050409-103711. [DOI] [PubMed] [Google Scholar]
- 2.Coleman MP, Quaresma M, Berrino F, Lutz JM, De Angelis R, Capocaccia R, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9(8):730–56. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–45. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 4.Global Burden of Disease Cancer C. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA oncology. 2016 doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boshoff C, Chang Y. Kaposi’s sarcoma-associated herpesvirus: a new DNA tumor virus. Annu Rev Med. 2001;52:453–70. doi: 10.1146/annurev.med.52.1.453. [DOI] [PubMed] [Google Scholar]
- 6.Boshoff C, Weiss RA. Epidemiology and pathogenesis of Kaposi’s sarcoma-associated herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356(1408):517–34. doi: 10.1098/rstb.2000.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoki Y, Yarchoan R, Wyvill K, Okamoto S, Little RF, Tosato G. Detection of viral interleukin-6 in Kaposi sarcoma-associated herpesvirus-linked disorders. Blood. 2001;97(7):2173–6. doi: 10.1182/blood.v97.7.2173. [DOI] [PubMed] [Google Scholar]
- 8.Powles T, Stebbing J, Bazeos A, Hatzimichael E, Mandalia S, Nelson M, et al. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castleman’s disease. Ann Oncol. 2009;20(4):775–9. doi: 10.1093/annonc/mdn697. [DOI] [PubMed] [Google Scholar]
- 9.Uldrick TS, Wang V, O’Mahony D, Aleman K, Wyvill KM, Marshall V, et al. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without Multicentric Castleman disease. Clin Infect Dis. 2010;51(3):350–8. doi: 10.1086/654798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S–19S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 11.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320(5):265–71. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 12.Metkus TS, Timpone J, Leaf D, Bidwell Goetz M, Harris WS, Brown TT. Omega-3 fatty acid therapy reduces triglycerides and interleukin-6 in hypertriglyeridemic HIV patients. HIV Med. 2013;14(9):530–9. doi: 10.1111/hiv.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song X, Schenk JM, Diep P, Murphy RA, Harris TB, Eiriksdottir G, et al. Measurement of Circulating Phospholipid Fatty Acids: Association between Relative Weight Percentage and Absolute Concentrations. J Am Coll Nutr. 2016;35(7):647–56. doi: 10.1080/07315724.2015.1116417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uganda Bureau of Statistics (UBOS) Uganda National Household Survey. Report on the Crop Survey Module. 2002 [Google Scholar]
- 15.Whitby D, Howard MR, Tenant-Flowers M, Brink NS, Copas A, Boshoff C, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet. 1995;346(8978):799. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]
- 16.Campbell TB, Borok M, Gwanzura L, MaWhinney S, White IE, Ndemera B, et al. Relationship of human herpesvirus 8 peripheral blood virus load and Kaposi’s sarcoma clinical stage. Aids. 2000;14(14):2109. doi: 10.1097/00002030-200009290-00006. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzen T, Albrecht D, Paech V, Meyer T, Hoffmann C, Stoehr A, et al. HHV-8 DNA in blood and the development of HIV-associated Kaposi’s sarcoma in the era of HAART--a prospective evaluation. European journal of medical research. 2002;7(6):283. [PubMed] [Google Scholar]
- 18.Nsubuga MM, Biggar RJ, Combs S, Marshall V, Mbisa G, Kambugu F, et al. Human herpesvirus 8 load and progression of AIDS-related Kaposi sarcoma lesions. Cancer Lett. 2008;263(2):182–88. doi: 10.1016/j.canlet.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston C, Orem J, Okuku F, Kalinaki M, Saracino M, Katongole-Mbidde E, et al. Impact of HIV infection and Kaposi sarcoma on human herpesvirus-8 mucosal replication and dissemination in Uganda. PLoS One. 2009;4(1):1–9. doi: 10.1371/journal.pone.0004222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casper C, Krantz E, Selke S, Kuntz SR, Wang J, Huang ML, et al. Frequent and asymptomatic oropharyngeal shedding of human herpesvirus 8 among immunocompetent men. J Infect Dis. 2007;195(1):30–6. doi: 10.1086/509621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thusgaard M, Christensen JH, Morn B, Andersen TS, Vige R, Arildsen H, et al. Effect of fish oil (n-3 polyunsaturated fatty acids) on plasma lipids, lipoproteins and inflammatory markers in HIV-infected patients treated with antiretroviral therapy: a randomized, double-blind, placebo-controlled study. Scandinavian journal of infectious diseases. 2009;41(10):760–6. doi: 10.1080/00365540903168056. [DOI] [PubMed] [Google Scholar]
- 22.Hileman CO, Carman TL, Storer NJ, Labbato DE, White CA, McComsey GA. Omega-3 fatty acids do not improve endothelial function in virologically suppressed HIV-infected men: a randomized placebo-controlled trial. AIDS Res Hum Retroviruses. 2012;28(7):649–55. doi: 10.1089/aid.2011.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Luis Roman DA, Bachiller P, Izaola O, Romero E, Martin J, Arranz M, et al. Nutritional treatment for acquired immunodeficiency virus infection using an enterotropic peptide-based formula enriched with n-3 fatty acids: a randomized prospective trial. Eur J Clin Nutr. 2001;55(12):1048–52. doi: 10.1038/sj.ejcn.1601276. [DOI] [PubMed] [Google Scholar]
- 24.Woloshin S, Schwartz LM. Distribution of C-reactive protein values in the United States. N Engl J Med. 2005;352(15):1611–3. doi: 10.1056/NEJM200504143521525. [DOI] [PubMed] [Google Scholar]