Lay Summary
Research of causes and consequences of animal personality promises exciting insights, yet widely used tests can lead to spurious results: when predictions of individual-level random effects are used in secondary analyses, their error is not carried forward, leading to increased likelihood of ‘false positive’ errors. We demonstrate how alternative approaches enable behavioural ecologists to test hypotheses about the causes and consequences of individual behavioural variation while accounting for the uncertainty inherent in the random effects.
Keywords: animal personality, behavioural plasticity, behavioural syndromes, cognition, physiology
Abstract
Having recognized that variation around the population-level “Golden Mean” of labile traits contains biologically meaningful information, behavioural ecologists have focused increasingly on exploring the causes and consequences of individual variation in behaviour. These are exciting new directions for the field, assisted in no small part by the adoption of mixed-effects modelling techniques that enable the partitioning of among- and within-individual behavioural variation. It has become commonplace to extract predictions of individual random effects from such models for use in subsequent analyses (for example, between a personality trait and other individual traits such as cognition, physiology, or fitness-related measures). However, these predictions are made with large amounts of error that is not carried forward, rendering further tests susceptible to spurious P values from these individual-level point estimates. We briefly summarize the problems with such statistical methods that are used regularly by behavioural ecologists, and highlight the robust solutions that exist within the mixed model framework, providing tutorials to aid in their implementation.
Characterizing individual variation in behaviour is an exciting research area in behavioural ecology, with great interest in the fields of “animal personality” and individual differences in behavioural plasticity (Réale et al. 2010a; Japyassú and Malange 2014). This research is predicated on exploring previously ignored phenotypic variation: behavioural ecologists have escaped the “tyranny of the Golden Mean” in labile traits (Bennett 1987; Wilson 1998; Williams 2008), and are increasingly finding meaningful biology in what was formerly considered residual variation (Cleasby and Nakagawa 2011; Stamps et al. 2012; Brommer 2013a). Progress in these fields has been boosted by the adoption of mixed-effects modelling techniques, particularly the use of quantitative genetics-style approaches for partitioning phenotypic variation into its “between-individual” and “within-individual” components (Nussey et al. 2007; Smiseth et al. 2008; van de Pol and Wright 2009; Dingemanse et al. 2012; Dingemanse and Dochtermann 2013; Royle et al. 2014; Allegue et al. 2016). Behavioural ecologists are also increasingly interested in extending these analyses of individual behavioural variation for new avenues and purposes (Sih et al. 2004; Dall et al. 2012; Japyassú and Malange 2014; Roche et al. 2016; Stamps and Biro 2016). These typically involve exploration of the causes and consequences of individual variation in behaviour (and/or behavioural plasticity), by testing for their association with variation in other individual traits (e.g., physiological, cognitive, social, or fitness-related) or environmental variables. However, the use of anticonservative methods has become pervasive in this field. Here, we highlight not only the problems with a widely-used approach in the study of individual behavioural variation, but also the straightforward statistical solutions to these problems that should thereby hasten progress.
Specifically, it has become common practice to extract predictions of individual random effects from fitted mixed models and to use these in subsequent analyses, such as correlation tests or linear regression models (Table 1). Problems arise from this approach because individual point estimates from random effects in mixed models (sometimes known as conditional modes, or best linear unbiased predictors, BLUPs) are predicted with large amounts of error. Their use in secondary analyses can therefore lead to highly anticonservative tests of biological hypotheses, because the error inherent in their prediction is excluded from these further tests (Hadfield et al. 2010). We stress that BLUP is an incredibly useful technique that should not be dismissed in any way as inherently “bad” (Robinson 1991). Indeed, it is entirely appropriate to use individual-level predictions to say something about individuals (or genotypes, or specific levels of some other random effect). For example, scrutiny of BLUPs could be used to identify which individuals are the “boldest”, or to select individuals for groups to be used in further experimental study. However, when the objective is to say something about population-level processes or relationships then analysing sets of model predictions while ignoring their associated error is not statistically correct. This has been recognized in other fields (notably ecological and evolutionary quantitative genetics), but less so in behavioural ecology, where these improper analyses persist. As detailed by Hadfield et al. (2010), such analyses can therefore result in spuriously narrow confidence intervals and/or spuriously low P values that are interpreted as indicators of biological significance. While the qualitative conclusions of individual papers employing these methods may prove robust in many cases, failure to properly account for uncertainty will increase Type I errors (false positives) across the field. In short, published P values are systematically anticonservative and should not be taken at face value.
Table 1.
Examples in the behavioural literature of questions regarding individual variation in behaviour (“personality”) and behavioural plasticity, using best linear unbiased predictors (BLUPs) in secondary analyses rather than multivariate models
| Test | Species | Reference |
|---|---|---|
| Behavioural syndromes | Microtus arvalis | (Lantová et al. 2011) |
| Taeniopygia guttata | (Wuerz and Krüger 2015) | |
| Latrodectus hesperus | (Montiglio and DiRienzo 2016) | |
| Personality across life stages | Tamiasciurus hudsonicus | (Kelley et al. 2015) |
| Different measures of a single personality trait | Bitis arietans | (Carter et al. 2012c) |
| Pomacentrus wardi, P. amboinensis | (Beckmann and Biro 2013) | |
| Personality and sampling bias | Agama planiceps | (Carter et al. 2012b) |
| Personality and hormones | Tamias striatus | (Montiglio et al. 2012) |
| Canis latrans | (Schell et al. 2016) | |
| Personality and physiology | Cavia aperea | (Guenther and Trillmich 2015) |
| C. aperea | (Finkemeier et al. 2016) | |
| Personality and telomere length | Salmo trutta | (Adriaenssens et al. 2016) |
| Personality and cognition | C. aperea | (Guenther et al. 2014) |
| C. aperea, C. porcellus | (Brust and Guenther 2015) | |
| Personality and social network attributes | Anguilla anguilla | (Geffroy et al. 2014) |
| Marmota flaviventris | (Fuong et al. 2015) | |
| Personality and local density | T. hudsonicus | (Shonfield et al. 2012) |
| Personality and social niche specialisation | Suricata suricatta | (Carter et al. 2014) |
| Personality and group-size preference | Perca fluviatilis | (Hellström et al. 2016) |
| Personality and predation risk | P. fluviatilis | (Magnhagen et al. 2012) |
| (Heynen et al. 2016) | ||
| Personality and mating behaviour | Aquarius remigis | (Wey et al. 2014; Wey et al. 2015) |
| Gerris buenoi | (Pineaux and Turgeon 2017) | |
| Personality and breeding performance | Circus pygargus | (Arroyo et al. 2017) |
| Personality and survival | T. striatus | (Bergeron et al. 2013) |
| Personality and fitness-related traits | S. trutta | (Adriaenssens and Johnsson 2011) |
| Personality and individual variation in behavioural plasticity | A. planiceps | (Carter et al. 2012a) |
| Microcebus murinus | (Dammhahn and Almeling 2012) | |
| T. guttata | (Gibelli and Dubois 2016) | |
| Personality, behavioural plasticity, and reproductive success | Tachycineta bicolor | (Betini and Norris 2012) |
| Personality, behavioural plasticity, and mating | A. remigis | (Montiglio et al. 2016a; Montiglio et al. 2016b) |
| Personality, behavioural plasticity, and fitness | Tenagogerris euphrosyne | (Han and Brooks 2014) |
All were published after the publication of Hadfield et al (2010).
Recent examples of publications (mis-)using BLUPs include tests of associations between personality (and/or individual variation in behavioural plasticity) and a wide range of traits, including physiology, cognition, social networks, niche specialization, and fitness (Table 1). In many cases, authors have explicitly acknowledged the potential for problems as outlined by Hadfield et al. (2010). Nonetheless, use of these “stats on stats” approaches that are known to be inappropriate for hypothesis testing (see Brommer 2013b for further discussion) continues unabated. This is presumably because researchers are not aware of how to implement more robust analytical strategies, and/or because of a misconception that problems are restricted to quantitative genetic models. On the latter point we note that predictions from mixed models in which random effects are assumed to covary between individuals (through e.g., genetic relatedness, spatial/temporal autocorrelation, or social processes) cannot be treated as independent “data points”. However, this in no way justifies ignoring uncertainty when random effects are predicted from a model that assumes no among-individual covariance.
Fortunately, the mixed-effects model framework does offer a way to test hypotheses such as those listed above while fully accounting for the uncertainty inherent in the random effects. An overreliance on the (otherwise excellent) lme4 package for mixed models in R (Bates et al. 2015) may have held many behavioural ecologists in the “Flatland” of univariate modelling (Walsh 2007). In the majority of cases, questions that are multivariate in nature are best answered using a multivariate framework. That is, a modelling framework containing multiple response variables, enabling 1) testing of how explanatory variables (“fixed effects”) predict these responses, as in standard univariate models, and 2) the simultaneous estimation of the variance of each response and the covariance between them, at group levels specified within the random effects structure. It is relatively straightforward to rephrase these multivariate questions in terms of variances and covariances (or derived correlations and regressions), and to fit multivariate models accordingly (some examples include Ferrari et al. 2013; Kluen et al. 2013; Royauté et al. 2013; Boulton et al. 2014; Careau et al. 2015; Niemelä et al. 2015; Petelle et al. 2015; Sanderson et al. 2015; Santostefano et al. 2016; Vallon et al. 2016; White et al. 2016). For instance, we might hypothesize a behavioural syndrome in which positive correlations are predicted between the (repeatable) tendencies of individuals to exhibit 3 behaviours. Having assayed each of these behaviours on multiple occasions for a set of individuals, the correct approach would be to estimate—and test the significance of—those among-individual correlations directly in a trivariate mixed model incorporating all of the behavioural data. This method yields correlation estimates with valid measures of uncertainty (SE or CI). This is not the case when generating individual-level random effect predictions from 3 separate univariate models (one for each behaviour) and then testing whether they are correlated. In the latter approach, uncertainty will be underestimated and thus Type I error is more likely to occur (Figure 1).
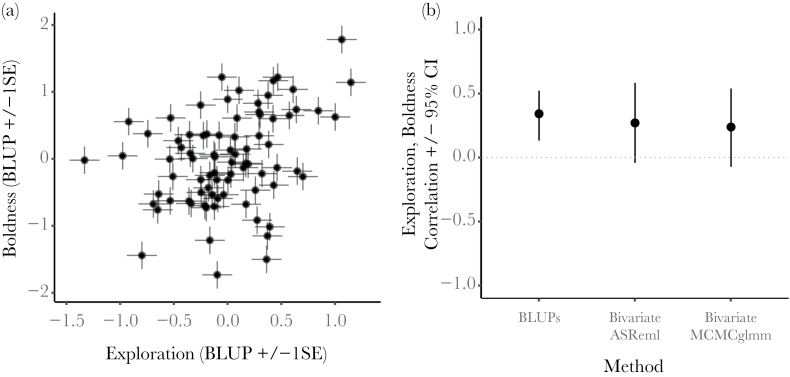
Figure 1.
Taken from a worked example provided in the Supplementary Information, (a) shows a scatterplot of individual-level estimates (BLUPs) of 2 personality traits, extracted from separate univariate models. Bars around each point show the standard error of the estimate for both traits, which is ignored by subsequent analyses of these BLUPs. Testing a correlation using only BLUPs and ignoring their error results in an anticonservative test, as illustrated in (b). The correlation test using BLUPs produces narrow confidence intervals, and a correspondingly small P value of 0.0019, indicating statistical significance (“BLUP” on x axis). However, testing the correlation directly in a bivariate model using REML and retaining all data returns larger (approximate) confidence intervals which straddle zero (95% CI approximated as r ± 1.96SE) and a P value (based on a likelihood ratio test) of 0.12, such that the correlation is not statistically significant (“Bivariate ASReml” on x axis). Using the same data, Bayesian 95% credible intervals also cross zero, which indicates a lack of statistical significance (“Bivariate MCMCglmm").
On a pragmatic point, we note that it is not required that each variable of interest be a repeated measure in these models—for example, it is perfectly feasible to test for the existence of an among-individual correlation between a personality trait (with repeated measures) and some other variable with only one observation per individual, such as an estimate of its lifetime reproductive success. In the Supplementary Material, we provide worked examples of how to set up multivariate statistical models to address these (and several similar) questions using the R packages ASReml-R (Butler 2009) and MCMCglmm (Hadfield 2010). These examples provide users with the tools to test their hypotheses in a multivariate framework, incorporating all of their data and avoiding potentially spurious results.
We also note that multivariate mixed models may often provide a more appropriate route to testing hypotheses about multivariate phenotypes in other contexts. For instance, one approach to exploring behavioural syndromes has been to reduce the dimensionality of observed behaviours by performing principal components analysis (PCA) on multivariate data, and then to use univariate mixed models to calculate repeatability on individual scores for each component (e.g., Edenbrow and Croft 2013; Le Galliard et al. 2013; Brent et al. 2014; Patrick and Weimerskirch 2014; Sussman et al. 2014; Rangassamy et al. 2015). This allows us to ask whether, for instance, the major axis of observed behavioural (co)variation is repeatable. This is a valid question but in many cases perhaps not the most pertinent one, since the first principal component of observed variation includes both among- and within-individual trait variation. For studies of individual differences in behaviour, the more relevant question might be better focused at the among-individual level—that is, what does the major axis of among-individual variation look like? If so, then isolating the among-individual (co)variance matrix (sometimes denoted I; Wilson et al. 2011) by applying a multivariate mixed model to a set of traits is the proper first step. Principal components (or eigenvectors) of I can then be examined directly. This strategy is probably more appropriate for testing models such as “pace of life syndrome” or stress coping styles that posit trait correlations at the among-individual level—i.e., that these correlations are due to consistent differences among individuals, and not because of some temporary aspect of environmental variation (Koolhaas et al. 2007; Carere et al. 2010; Coppens et al. 2010; Réale et al. 2010b; Carter et al. 2013). The value of partitioning individual (co)variances has been discussed in more detail by Brommer (2013a), and illustrations exist in the literature of the use of multivariate mixed models for studying pace of life syndrome (White et al. 2016) and stress coping styles (Boulton et al. 2015).
We fully acknowledge that multivariate mixed models are data hungry. However, a failure of these multivariate models to converge to sensible and/or precise solutions does not mean that we can retreat to the relative comfort of previous methods: in fact, this is likely to indicate a lack of power to answer the question at hand (see Martin et al. 2011; Wolak et al. 2012). In cases where logistical constraints prevent there being enough measurements to partition out the among-individual behavioural (co)variation, a preferable method may sometimes be to work with observed phenotypic (co)variance while acknowledging this and the assumptions that underpin conclusions drawn. Indeed, much of behavioural ecology is predicated on the “phenotypic gambit”, the assumption that phenotypic patterns of trait (co)variation (denoted P) provide a workable proxy for patterns of genetic (co)variance (G). If P can be used (with caveats) in place of G where estimation of genetic parameters is not feasible, then it can also be used (with caveats) in place of I where partitioning of among-individual covariation from within-individual covariation is not feasible.
To conclude, we absolutely wish to encourage more studies that further our understanding of the causes and consequences of individual differences in behaviour. However, we also make a plea to the community to avoid inappropriate methods of analysis that lead to spurious precision and increased Type I errors. This field depends upon embracing the power of previously ignored phenotypic variation, and it is flourishing because of the exciting questions we can now address—but we must ensure that we use the right tools when doing so.
SUPPLEMENTARY MATERIAL
Supplementary data are available at Behavioral Ecology online.
FUNDING
This work was supported by a Biotechnology and Biological Sciences Research Council grant (BB/L022656/1) awarded to A.J.W.
Supplementary Material
Acknowledgments
We are grateful to members of the Behaviour and Evolution research groups at the University of Exeter’s Penryn campus for discussion and for comments on earlier drafts (in particular, T. Bodey, H. Marshall, N. Royle, A. Thornton, T. Tregenza, S. White, and A. Young), and to 2 anonymous reviewers. Special thanks to E. Wood for illustrating our “study species” in the tutorials.
REFERENCES
- Adriaenssens B, Johnsson JI. 2011. Shy trout grow faster: Exploring links between personality and fitness-related traits in the wild. Behav Ecol. 22:135–143. [Google Scholar]
- Adriaenssens B, Pauliny A, Blomqvist D, Johnsson JI. 2016. Telomere length covaries with personality in wild brown trout. Physiol Behav. 165:217–222. [DOI] [PubMed] [Google Scholar]
- Allegue H, Araya-Ajoy YG, Dingemanse NJ, Dochtermann NA, Garamszegi LZ, Nakagawa S, Réale D, Schielzeth H, Westneat DF. 2016. SQuID - Statistical Quantification of Individual Differences: an educational and statistical tool for understanding multi-level phenotypic data in linear mixed models. Methods Ecol Evol. doi:10.1111/2041-210X.12659. [Google Scholar]
- Arroyo B, Mougeot F, Bretagnolle V. 2017. Individual variation in behavioural responsiveness to humans leads to differences in breeding success and long-term population phenotypic changes. Ecol Lett. 20. doi:10.1111/ele.12729. [DOI] [PubMed] [Google Scholar]
- Bates DM, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw. 67:arXiv:1406.5823. [Google Scholar]
- Beckmann C, Biro PA. 2013. On the validity of a single (boldness) assay in personality research. Ethology. 119:937–947. [Google Scholar]
- Bennett AF. 1987. Interindividual variability: an underutilized resource. New Dir Ecol Physiol. 15:147–169. [Google Scholar]
- Bergeron P, Montiglio PO, Réale D, Humphries MM, Gimenez O, Garant D. 2013. Disruptive viability selection on adult exploratory behaviour in eastern chipmunks. J Evol Biol. 26:766–774. [DOI] [PubMed] [Google Scholar]
- Betini GS, Norris DR. 2012. The relationship between personality and plasticity in tree swallow aggression and the consequences for reproductive success. Anim Behav. 83:137–143. [Google Scholar]
- Boulton K, Couto E, Grimmer AJ, Earley RL, Canario AV, Wilson AJ, Walling CA. 2015. How integrated are behavioral and endocrine stress response traits? A repeated measures approach to testing the stress-coping style model. Ecol Evol. 5:618–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton K, Grimmer AJ, Rosenthal GG, Walling CA, Wilson AJ. 2014. How stable are personalities? A multivariate view of behavioural variation over long and short timescales in the sheepshead swordtail, Xiphophorus birchmanni. Behav Ecol Sociobiol. 68:791–803. [Google Scholar]
- Brent LJ, Semple S, Maclarnon A, Ruiz-Lambides A, Gonzalez-Martinez J, Platt ML. 2014. Personality traits in Rhesus macaques (Macaca mulatta) are heritable but do not predict reproductive output. Int J Primatol. 35:188–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brommer JE. 2013a. On between-individual and residual (co)variances in the study of animal personality: Are you willing to take the “individual gambit”? Behav Ecol Sociobiol. 67:1027–1032. [Google Scholar]
- Brommer JE. 2013b. Phenotypic plasticity of labile traits in the wild. Curr Zool. 59:485–505. [Google Scholar]
- Brust V, Guenther A. 2015. Domestication effects on behavioural traits and learning performance: comparing wild cavies to guinea pigs. Anim Cogn. 18:99–109. [DOI] [PubMed] [Google Scholar]
- Butler D. 2009. asreml: asreml() fits the linear mixed model [cited 2017 January 23]. http://www.vsni.co.uk. [Google Scholar]
- Careau V, Montiglio PO, Garant D, Pelletier F, Speakman JR, Humphries MM, Réale D. 2015. Energy expenditure and personality in wild chipmunks. Behav Ecol Sociobiol. 69:653–661. [Google Scholar]
- Carere C, Caramaschi D, Fawcett TW. 2010. Covariation between personalities and individual differences in coping with stress: Converging evidence and hypotheses. Curr Zool. 56:728–741. [Google Scholar]
- Carter AJ, English S, Clutton-Brock TH. 2014. Cooperative personalities and social niche specialization in female meerkats. J Evol Biol. 27:815–825. [DOI] [PubMed] [Google Scholar]
- Carter AJ, Feeney WE, Marshall HH, Cowlishaw G, Heinsohn R. 2013. Animal personality: what are behavioural ecologists measuring? Biol Rev Camb Philos Soc. 88:465–475. [DOI] [PubMed] [Google Scholar]
- Carter AJ, Goldizen A, Heinsohn R. 2012a. Personality and plasticity: Temporal behavioural reaction norms in a lizard, the Namibian rock agama. Anim Behav. 84:471–477. [Google Scholar]
- Carter AJ, Heinsohn R, Goldizen AW, Biro PA. 2012b. Boldness, trappability and sampling bias in wild lizards. Anim Behav. 83:1051–1058. [Google Scholar]
- Carter AJ, Marshall HH, Heinsohn R, Cowlishaw G. 2012c. How not to measure boldness: Novel object and antipredator responses are not the same in wild baboons. Anim Behav. 84:603–609. [Google Scholar]
- Cleasby IR, Nakagawa S. 2011. Neglected biological patterns in the residuals. Behav Ecol Sociobiol. 65:2361–2372. [Google Scholar]
- Coppens CM, de Boer SF, Koolhaas JM. 2010. Coping styles and behavioural flexibility: towards underlying mechanisms. Philos Trans R Soc Lond B Biol Sci. 365:4021–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall SR, Bell AM, Bolnick DI, Ratnieks FL. 2012. An evolutionary ecology of individual differences. Ecol Lett. 15:1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammhahn M, Almeling L. 2012. Is risk taking during foraging a personality trait? A field test for cross-context consistency in boldness. Anim Behav. 84:1131–1139. [Google Scholar]
- Dingemanse NJ, Dochtermann NA. 2013. Quantifying individual variation in behaviour: mixed-effect modelling approaches. J Anim Ecol. 82:39–54. [DOI] [PubMed] [Google Scholar]
- Dingemanse NJ, Dochtermann NA, Nakagawa S. 2012. Defining behavioural syndromes and the role of “syndrome deviation” in understanding their evolution. Behav Ecol Sociobiol. 66:1543–1548. [Google Scholar]
- Edenbrow M, Croft DP. 2013. Environmental and genetic effects shape the development of personality traits in the mangrove killifish Kryptolebias marmoratus. Oikos. 122:667–681. [Google Scholar]
- Ferrari C, Pasquaretta C, Carere C, Cavallone E, von Hardenberg A, Réale D. 2013. Testing for the presence of coping styles in a wild mammal. Anim Behav. 85:1385–1396. [Google Scholar]
- Finkemeier MA, Trillmich F, Guenther A. 2016. Match-mismatch experiments using photoperiod expose developmental plasticity of personality traits. Ethology. 122:80–93. [Google Scholar]
- Fuong H, Maldonado-Chaparro A, Blumstein DT. 2015. Are social attributes associated with alarm calling propensity? Behav Ecol. 26:587–592. [Google Scholar]
- Le Galliard JF, Paquet M, Cisel M, Montes-Poloni L. 2013. Personality and the pace-of-life syndrome: Variation and selection on exploration, metabolism and locomotor performances. Funct Ecol. 27:136–144. [Google Scholar]
- Geffroy B, Bru N, Dossou-Gbété S, Tentelier C, Bardonnet A. 2014. The link between social network density and rank-order consistency of aggressiveness in juvenile eels. Behav Ecol Sociobiol. 68:1073–1083. [Google Scholar]
- Gibelli J, Dubois F. 2016. Does personality affect the ability of individuals to track and respond to changing conditions? Behav Ecol. 28:101–107. [Google Scholar]
- Guenther A, Brust V, Dersen M, Trillmich F. 2014. Learning and personality types are related in cavies (Cavia aperea). J Comp Psychol. 128:74–81. [DOI] [PubMed] [Google Scholar]
- Guenther A, Trillmich F. 2015. Within-litter differences in personality and physiology relate to size differences among siblings in cavies. Physiol Behav. 145:22–28. [DOI] [PubMed] [Google Scholar]
- Hadfield JD. 2010. MCMC Methods for Multi-Response Generalized Mixed Models: The MCMCglmm R Package. J Stat Softw. 33:1–25.20808728 [Google Scholar]
- Hadfield JD, Wilson AJ, Garant D, Sheldon BC, Kruuk LE. 2010. The misuse of BLUP in ecology and evolution. Am Nat. 175:116–125. [DOI] [PubMed] [Google Scholar]
- Han CS, Brooks RC. 2014. Long-term effect of social interactions on behavioral plasticity and lifetime mating success. Am Nat. 183:431–444. [DOI] [PubMed] [Google Scholar]
- Hellström G, Heynen M, Borcherding J, Magnhagen C. 2016. Individual consistency and context dependence in group-size preference of Eurasian perch. Behav Processes. 133:6–11. [DOI] [PubMed] [Google Scholar]
- Heynen M, Borcherding J, Bunnefeld N, Magnhagen C. 2016. Plasticity and consistency of behavioural responses to predation risk in laboratory environments. J Zool. 300:228–235. [Google Scholar]
- Japyassú HF, Malange J. 2014. Plasticity, stereotypy, intra-individual variability and personality: handle with care. Behav Processes. 109(Pt A):40–47. [DOI] [PubMed] [Google Scholar]
- Kelley AD, Humphries MM, McAdam AG, Boutin S. 2015. Changes in wild red squirrel personality across ontogeny: activity and aggression regress towards the mean. Behaviour. 152:1291–1306. [Google Scholar]
- Kluen E, Siitari H, Brommer JE. 2013. Testing for between individual correlations of personality and physiological traits in a wild bird. Behav Ecol. Sociobiol. 68:205–213. [Google Scholar]
- Koolhaas JM, de Boer SF, Buwalda B, van Reenen K. 2007. Individual variation in coping with stress: a multidimensional approach of ultimate and proximate mechanisms. Brain Behav Evol. 70:218–226. [DOI] [PubMed] [Google Scholar]
- Lantová P, Šíchová K, Sedláček F, Lanta V. 2011. Determining behavioural syndromes in voles - the effects of social environment. Ethology. 117:124–132. [Google Scholar]
- Magnhagen C, Hellström G, Borcherding J, Heynen M, Genner M. 2012. Boldness in two perch populations - long-term differences and the effect of predation pressure. J Anim Ecol. 81:1311–1318. [DOI] [PubMed] [Google Scholar]
- Martin JGA, Nussey DH, Wilson AJ, Réale D. 2011. Measuring individual differences in reaction norms in field and experimental studies: a power analysis of random regression models. Methods Ecol Evol. 2:362–374. [Google Scholar]
- Montiglio P-O, DiRienzo N. 2016. There’s no place like home: the contribution of direct and extended phenotypes on the expression of spider aggressiveness. Behav Ecol. 27:1880–1888. [Google Scholar]
- Montiglio P-O, Wey TW, Chang AT, Fogarty S, Sih A. 2016a. Multiple mating reveals complex patterns of assortative mating by personality and body size. J Anim Ecol. 85:125–135. [DOI] [PubMed] [Google Scholar]
- Montiglio P-O, Wey TW, Chang AT, Fogarty S, Sih A. 2016b. Correlational selection on personality and social plasticity: morphology and social context determine behavioural effects on mating success. J Anim Ecol. 86:213–226. [DOI] [PubMed] [Google Scholar]
- Montiglio P-O, Garant D, Pelletier F, Réale D. 2012. Personality differences are related to long-term stress reactivity in a population of wild eastern chipmunks, Tamias striatus. Anim Behav. 84:1071–1079. [Google Scholar]
- Niemelä PT, Lattenkamp EZ, Dingemanse NJ. 2015. Personality-related survival and sampling bias in wild cricket nymphs. Behav Ecol. 26:936–946. [Google Scholar]
- Nussey DH, Wilson AJ, Brommer JE. 2007. The evolutionary ecology of individual phenotypic plasticity in wild populations. J Evol Biol. 20:831–844. [DOI] [PubMed] [Google Scholar]
- Patrick SC, Weimerskirch H. 2014. Personality, foraging and fitness consequences in a long lived seabird. PLoS One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petelle MB, Martin JG, Blumstein DT. 2015. Heritability and genetic correlations of personality traits in a wild population of yellow-bellied marmots (Marmota flaviventris). J Evol Biol. 28:1840–1848. [DOI] [PubMed] [Google Scholar]
- Pineaux M, Turgeon J. 2017. Behavioural Consistency in Female Resistance to Male Harassment in a Water Strider Species. Ethology. 123:83–93. [Google Scholar]
- van de Pol M, Wright J. 2009. A simple method for distinguishing within- versus between-subject effects using mixed models. Anim Behav. 77:753–758. [Google Scholar]
- Rangassamy M, Dalmas M, Féron C, Gouat P, Rödel HG. 2015. Similarity of personalities speeds up reproduction in pairs of a monogamous rodent. Anim Behav. 103:7–15. [Google Scholar]
- Réale D, Dingemanse NJ, Kazem AJ, Wright J. 2010a. Evolutionary and ecological approaches to the study of personality. Philos Trans R Soc Lond B Biol Sci. 365:3937–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio PO. 2010b. Personality and the emergence of the pace-of-life syndrome concept at the population level. Philos Trans R Soc Lond B Biol Sci. 365:4051–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GK. 1991. That BLUP is a good thing: The estimation of random effects. Stat Sci. 6:15–32. [Google Scholar]
- Roche DG, Careau V, Binning SA. 2016. Demystifying animal “personality” (or not): why individual variation matters to experimental biologists. J Exp Biol. 219:3832–3843. [DOI] [PubMed] [Google Scholar]
- Royauté R, Buddle CM, Vincent C. 2013. Interpopulation Variations in Behavioral Syndromes of a Jumping Spider from Insecticide-Treated and Insecticide-Free Orchards. Ethology. 119:1–13. [Google Scholar]
- Royle NJ, Russell AF, Wilson AJ. 2014. The evolution of flexible parenting. Science. 345:776–781. [DOI] [PubMed] [Google Scholar]
- Sanderson JL, Stott I, Young AJ, Vitikainen EIK, Hodge SJ, Cant MA. 2015. The origins of consistent individual differences in cooperation in wild banded mongooses, Mungos mungo. Anim Behav. 107:193–200. [Google Scholar]
- Santostefano F, Wilson AJ, Araya-Ajoy YG, Dingemanse NJ. 2016. Interacting with the enemy: indirect effects of personality on conspecific aggression in crickets. Behav Ecol. 27:1235–1246. [Google Scholar]
- Schell CJ, Young JK, Lonsdorf EV, Mateo JM, Santymire RM. 2016. Olfactory attractants and parity affect prenatal androgens and territoriality of coyote breeding pairs. Physiol Behav. 165:43–54. [DOI] [PubMed] [Google Scholar]
- Shonfield J, Taylor RW, Boutin S, Humphries MM, McAdam AG. 2012. Territorial defence behaviour in red squirrels is influenced by local density. Behaviour 149:369–390. [Google Scholar]
- Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol. 19:372–378. [DOI] [PubMed] [Google Scholar]
- Smiseth PT, Wright J, Kölliker M. 2008. Parent-offspring conflict and co-adaptation: behavioural ecology meets quantitative genetics. Proc Biol Sci. 275:1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamps JA, Biro PA. 2016. Personality and individual differences in plasticity. Curr Opin Behav Sci. 12:18–23. [Google Scholar]
- Stamps JA, Briffa M, Biro PA. 2012. Unpredictable animals: Individual differences in intraindividual variability (IIV). Anim Behav. 83:1325–1334. [Google Scholar]
- Sussman AF, Mates EA, Ha JC, Bentson KL, Crockett CM. 2014. Tenure in current captive setting and age predict personality changes in adult pigtailed macaques. Anim Behav. 89:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon M, Grom C, Kalb N, Sprenger D, Anthes N, Lindström K, Heubel KU. 2016. You eat what you are: personality-dependent filial cannibalism in a fish with paternal care. Ecol Evol. 6:1340–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B. 2007. Escape from flatland. J Evol Biol. 20:36–38; discussion 39. [DOI] [PubMed] [Google Scholar]
- Wey TW, Chang AT, Fogarty S, Sih A. 2014. Personalities and presence of hyperaggressive males influence male mating exclusivity and effective mating in stream water striders. Behav Ecol Sociobiol. 69:27–37. [Google Scholar]
- Wey TW, Chang AT, Montiglio P-O, Fogarty S, Sih A. 2015. Linking short-term behavior and personalities to feeding and mating rates in female water striders. Behav Ecol. 26:1196–1202. [Google Scholar]
- White SJ, Kells TJ, Wilson AJ. 2016. Metabolism, personality and pace of life in the Trinidadian guppy, Poecilia reticulata. Behaviour. 153:1517–1543. [Google Scholar]
- Williams TD. 2008. Individual variation in endocrine systems: moving beyond the “tyranny of the Golden Mean”. Philos Trans R Soc Lond B Biol Sci. 363:1687–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AJ, de Boer M, Arnott G, Grimmer A. 2011. Integrating personality research and animal contest theory: aggressiveness in the green swordtail Xiphophorus helleri. PLoS One. 6:e28024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DS. 1998. Adaptive individual differences within single populations. Philos Trans R Soc B Biol Sci. 353:199–205. [Google Scholar]
- Wolak ME, Fairbairn DJ, Paulsen YR. 2012. Guidelines for estimating repeatability. Methods Ecol Evol. 3:129–137. [Google Scholar]
- Wuerz Y, Krüger O. 2015. Personality over ontogeny in zebra finches: long-term repeatable traits but unstable behavioural syndromes. Front Zool. 12 (suppl 1):S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.