Abstract
The prevalence of Staphylococcus aureus worldwide as a nosocomial infectious agent is recognized but the reason behind the spread of this bacterium has remained elusive. Here, we hypothesized that the communication of S. aureus might benefit from it blocking other bacteria from establishing themselves on the surface. This was found to be the case for several pathogens as the S. aureus supernatant curtailed their ability to form biofilms. Subsequent analyses using Acinetobacter baumannii as a model found this effect is primarily mediated by S. aureus’ extracellular vesicles (EVs), which bound to the polystyrene surface. We found the EV-treated surfaces were significantly more hydrophilic after EV treatment, a condition that made it difficult for A. baumannii to initially adhere to the polystyrene surface and reduced its resulting biofilm by up to 93%. Subsequent tests found this also extended to several other bacterial pathogens, with a 40-70% decrease in their biofilm mass. The S. aureus EVs and their activity still remained after the surface was washed with 10% bleach, while the use of ethylenediaminetetraacetic acid (EDTA) removed both the EVs from the surface and their activity.
Keywords: Staphylococcus aureus, Membrane vesicles, Extracellular Vesicles, Biofilms, Acinetobacter baumannii
Introduction
The prevalence of Staphylococcus aureus as both a nosocomially- and community-acquired pathogen is well known but not necessarily well understood.1-3 Although it is a commensal bacterium and can be found within the oral cavities of ~30% of the human population,4 S. aureus is also an opportunistic pathogen that is transmitted from one person to another through direct contact5, 6 or the usage of common everyday items, such as cell phones7 and exercise equipment,8, 9 and even hot tubs.9 When an analysis of the bacterial species associated with exercise equipment at a fitness center was performed, 17 different bacterial families and 25 genera were identified, but the most prevalent genus was Staphylococcus.8
Within hospital settings, this bacterium is a particular concern as contamination of surfaces and equipment is related with outbreaks9, 10 and may be a source of continued infections.11, 12 The sources of these contaminating pathogens may be patients themselves.13 In fact, for patients not known to be methicillin resistant S. aureus (MRSA) positive, nearly half of their beds (43%) contained this pathogen.14
When bacteria adhere to a surface, they grow and form complex communities referred to as biofilms, which are regarded as the most common and stable form of bacterial incidence in nature.15 The bacteria present within the biofilm are attached to the surface through a matrix of self-produced extracellular polymeric substances (EPS) containing extracellular DNA,16 protein and polysaccharides.17-19 Possibly the best studied mechanism regulating bacterial biofilm development involves quorum sensing.20, 21 For example, quorum sensing has been shown to control expression of the extracellular polysaccharides19 and DNA.22 For S. aureus, quorum sensing regulates both the development and dispersion of its biofilms, most notably through the accessory gene regulator (agr) system.23
Recently, researchers have found bacteria also secrete extracellular vesicles (EVs), i.e., spherical membrane-bound structures that typically range in size from 20 to 300 nm in diameter. The production of EVs is widespread, with nearly all strains characterized thus far, including Gram-negative and Gram-positive, producing them.24-26 The functions of EVs are as diverse as the hosts producing them, with them being used by different strains to transport proteins, including virulence factors,27, 28 or DNA to other hosts.29, 30 Recent studies have also found the production of EVs aid in the development of biofilms by some strains.31, 32 Despite the widespread production and activity of EVs, much still remains to be learned about the mechanisms controlling their biogenesis.25, 28, 30
Although S. aureus also produces EVs,33, 34 no mention has been made regarding their importance to S. aureus biofilm formation nor with respect to their role in interspecies interactions. Accordingly, we demonstrate in this study a new function of S. aureus EVs and their influence on mixed bacterial communities.
Materials and Methods
Bacterial Strains and Biofilm Assays
All strains used in this study are listed in Table S1 and were stored at −80°C in 25% glycerol stocks. Each strain was streaked onto tryptic soy agar (BD Bacto) and cultivated before every experiment. All strains were inoculated using a single colony and cultured overnight at 37°C in tryptic soy broth (TSB, BD Bacto) at 250 rpm. The S. aureus supernatant was prepared by inoculating a single S. aureus colony into sterile TSB media and growing it overnight (24 h) at 37°C and 250 rpm. After growth, the bacterial cells were removed by centrifuging the sample for 30 min at 4,000g and the resulting supernatant sterilized using a 0.22 μm syringe filter. To evaluate the effects of S. aureus supernatants on the growth of A. baumannii, an overnight A. baumannii culture was diluted into fresh media (1:100) and sub-cultured to an optical density (OD600nm) of 0.05, which was measured with an Eppendorf Biophotometer (Germany). At this point, the culture had the cell-free S. aureus supernatant (0.22 μm filter-sterilized) added to it, to a final concentration of 10%, and was grown for an additional 24 h.
Biofilm formation was performed in 96-well clear plates (COSTAR, Corning, USA) as previously described.28 Briefly, an overnight culture of the test strain was diluted to an OD of 0.05 and 200 μl aliquots were added to the wells of a sterile 96-well plate. The plates were then incubated for 24 h at 37°C without disturbing them. Empty wells were filled with sterile media to prevent evaporation. To evaluate the S. aureus supernatant inhibition for each strain, 20 μl of 0.22 μm filter-sterilized S. aureus supernatant (24 h culture) was added (10% of the total volume). The degree of biofilm formation was determined after washing the wells 3-times with tap water to remove any unattached planktonic cells and air drying. The biofilms were then stained with a 0.1% crystal-violet (CV) solution (250 μl) for 1 h.35 Once more, the biofilms were washed 3-times with tap water and air dried, after which the CV bound to the biofilms and was dissolved with 95% EtOH. The amount of CV present in each sample was measured using a Glomax Multi+ plate reader (Promega, USA) set to read the OD at 560 nm.35
The co-culture experiment was performed by adding overnight cultures of both S. aureus and A. baumannii to fresh TSB media at an OD of 0.05. This mixed culture was cultivated in a 96-well plate as described above. Afterwards, samples of the planktonic and the resuspended biofilm populations were spread on TSB agar plates and the colony forming units (CFU) per well of each strain determined.
Preparation of the Cell-Free S. aureus Supernatants
S. aureus ATCC 25923 was grown as above in TSB media and at set times samples of the culture were taken. The samples were centrifuged at 4000g for 30 min at 4°C to pellet the bacterial cells before being filter-sterilized using a 0.22 μm pore size syringe filter (Millipore). The sterility of the samples was confirmed by spreading a 50 μl aliquot of the filtrate on an TSB agar plate and checking for bacterial growth after 24 hours at 37°C.
Impact of the Cell-Free Supernatants on the A. baumannii Planktonic and Biofilm Populations
Using the cell-free supernatants prepared above, we tested their impact on A. baumannii. For this, the biofilm assays were performed as above but after 24 h, the viable A. baumannii populations within the media and the biofilm were determined. To measure the number within the media, the contents of the well were gently mixed as to not disturb the biofilm and an aliquot of the grown culture was serially diluted into fresh TSB media before being plated on TSB agar plates. Likewise, for the biofilms, after being washed as described above, the biofilm was dispersed using harsh washing steps, i.e., pipetting and vortexing the sample. After the cells were dispersed, the sample was serially diluted and plated in the same manner as the planktonic cultures. The plates from both samples were incubated at 37°C for 24 h and then the number of colonies on each were enumerated.
Confocal Microscope Imaging of the Biofilms and EVs
Imaging of the EVs and biofilms was obtained with an ELYRA S.1 SR-SIM (Super Resolution-Structured Illumination Microscope, Carl Zeiss) and a confocal microscope (IX71 FV1000 SPD, Olympus). After the formation of the biofilms on a polystyrene slide (Electron Microscopy Sciences), the bacterial DNA was stained using DAPI (Invitrogen, USA) and the membranes of both the cells and EVs were stained Rhodamine BR-18 (Invitrogen, USA). For this, both DAPI and Rhodamine BR-18 were diluted 1000-fold from their commercial stocks into sterile water and added to the sample. The slide was then incubated at 37°C for 30 min in the dark to prevent photobleaching36 before being gently rinsed with sterile water to remove any unbound dye. The cells were then imaged using the microscopes mentioned above using 405 nm and 561 nm lasers to excite DAPI and rhodamine BR18, respectively. The images obtained were then analyzed with Metaphor software (Carl Zeiss).
Isolation and Characterization of the S. aureus EVs and Their Activity
S. aureus EVs were purified as described previously.33, 36 Briefly, overnight cultures of S. aureus were pooled and centrifuged at 4000g for 30 min at 4°C. The supernatant was then sterilized using a 0.22 μm pore size syringe filter (Millipore) and concentrated 30-times with a 100 kDa ultracentrifugal filter (Millipore, filter size ~20 nm).33 The residue on the 100 KDa ultracentrifugal filter was collected and further centrifuged at 130,000g for 2 h at 4°C (Optima L-100 XP, Beckman, USA). The pellet was resuspended in sterile HEPES (pH 7.0) and the protein concentration was measured via a Bradford Assay (Promega, USA) and the concentration of EVs was selected based upon their activity (Fig. 2c).
Figure 2.
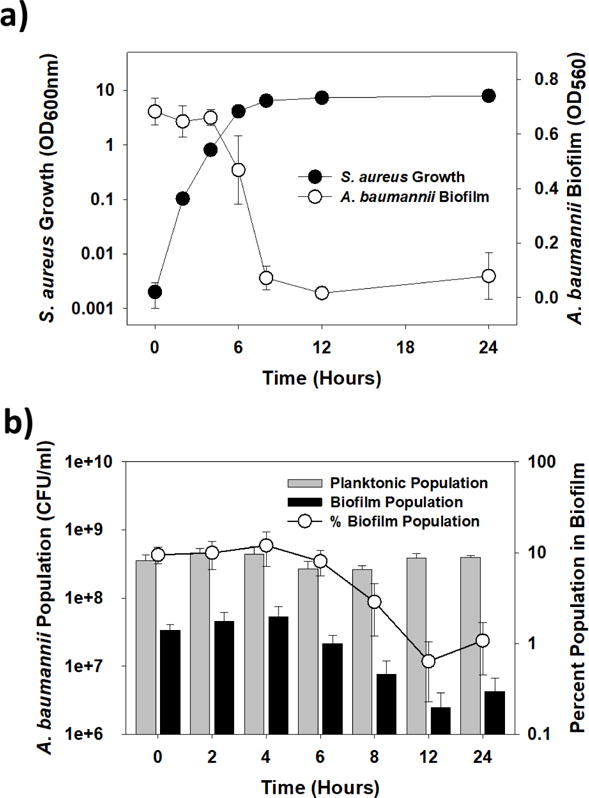
The inhibitor is most active during the stationary phase a) Growth of S. aureus ATCC 25923 and activity of its cell-free supernatants (10% final concentration) at the different points of growth. The results show inhibition was first evident as S. aureus ATCC 25923 entered the stationary phase. b) A. baumannii planktonic and biofilm populations within the cultures in (a). These results show the biofilm cultures alone were impacted by the addition of the supernatant, decreasing by as much as 1-log, while the planktonic populations were steady.
Surface Coating Assay
To test if the EVs bind to a polystyrene surface, a surface coating assay was used. Briefly, in 96-well plates, 200 μl of the cell-free, S. aureus supernatant or purified vesicles were added and the plate was incubated at 37°C for a predetermined time period (0 min, 10 min, 30 min, 60 min, 120 min or 360 min). At these times, the samples were removed and the wells washed gently with sterile water and air dried. To prepare the microscopic samples, polystyrene slides were coated with the EVs by submersing them in the S. aureus EV solution or supernatant for 2 h. The slide was then washed with sterile water and air-dried. After drying briefly, an A. baumannii culture was added to the wells or the slide as described above and the degree of biofilm formation was determined after 24 h.
Subsequent addition of EVs to A. baumannii cultures
S. aureus EV were added to growing cultures of A. baumannii within 96-well plates at different time points to determine if they could still inhibit biofilm formation. Overnight cultures of A. baumannii were diluted to an OD of 0.05 and 180 μl was added to the wells and incubated at 37°C. Samples of the purified EV solution (20 μl) were subsequently added at 0, 2, 6 and 12 h. The plate was incubated at 37°C for a total 24 h.
Water contact angle (WCA) measurements
WCAs were measured with S. aureus EV or supernatant coated or uncoated polystyrene slides (Electron Microscopy Sciences, 71888). Treatment of the substrate with sterile TSB media or the S. aureus EV or supernatant was performed for 1 h, after which the substrate was washed gently with sterile water and air-dried before measuring the sessile WCA. Onto the substrate surface, a water droplet (20 μl) was gently pipetted and the WCA of the droplet was measured using a contact angle meter (KRUSS, DSA100).
Oxygen plasma treatment of the substrate
The surface of polystyrene was treated using an oxygen plasma. Briefly, the oxygen plasma exposure was done inside a CUTE-MPR plasma cleaning system (FEMTO Science, Korea) for up to 1 min using a 50 W power setting and 30 sccm oxygen gas. After the treatment, a bacterial culture was added within 10 min and the degree of biofilm formation was determined as described above.
Bleach or EDTA treatment of the substrates
The polystyrene substrate was coated with EVs using filter-sterilized S. aureus ATCC 25923 spent media as described above. The coating was performed for 1 h. Afterwards, the substrate was rinsed with sterile HEPES and incubated in either HEPES buffer or a 10% bleach solution prepared in HEPES for 1 h and at room temperature. In addition, we tested the impact 0.1 M ethylenediaminetetraacetic acid (EDTA) on the purified EVs (pEVs) as described previously34 with slight changes. Briefly, the pEVs (surface-bound and free) were treated in HEPES at 37°C for one hour. The substrates were then washed in HEPES and air dried under sterile conditions. After drying, the ability of A. baumannii to form biofilms on these substrates was performed as described above.
Statistical analysis
Experiments were conducted and analyzed using 3 independent samples. For the biofilm analyses, at least 3 wells were analyzed for each test and this was performed independently 3-times on different days with all the results being used to perform the statistical analysis. The statistical analysis were based on the Student t-Test using the Graphpad Prism program (version 5.01). Statistical differences were indicated on the graphs using asterisks (** or ***), which corresponded to p values of < 0.01 and <0.001, respectively.
Results
S. aureus produces an effector that curtails biofilm formation by several other bacteria
Addition of cell-free S. aureus ATCC 25923 supernatants (10% v:v) to a clinical multi-drug resistant isolate of Acinetobacter baumannii37 inhibited the latter from forming biofilms on polystyrene surfaces (Figure 1a). The supernatant, however, did not negatively impact the growth of A. baumannii, showing that it is not toxic to this strain (Figure 1a). Similar results were seen with other bacterial cultures, including members of the ESKAPE grouping of pathogens (e.g., Enterococcus faecium and Klebsiella pneumonia), which saw their biofilms inhibited by as much as 57% (p<0.001) (Figure 1c). However, the best activity was against A. baumannii biofilms, with 75% or greater inhibition typically seen. Thus, this strain was selected for all further experiments unless noted otherwise. Although the S. aureus supernatant effectively blocked de novo biofilm formation by this strain, pre-formed A. baumannii biofilms were not removed (Figure S1), showing the activity of the supernatant is only preventative.
Figure 1.
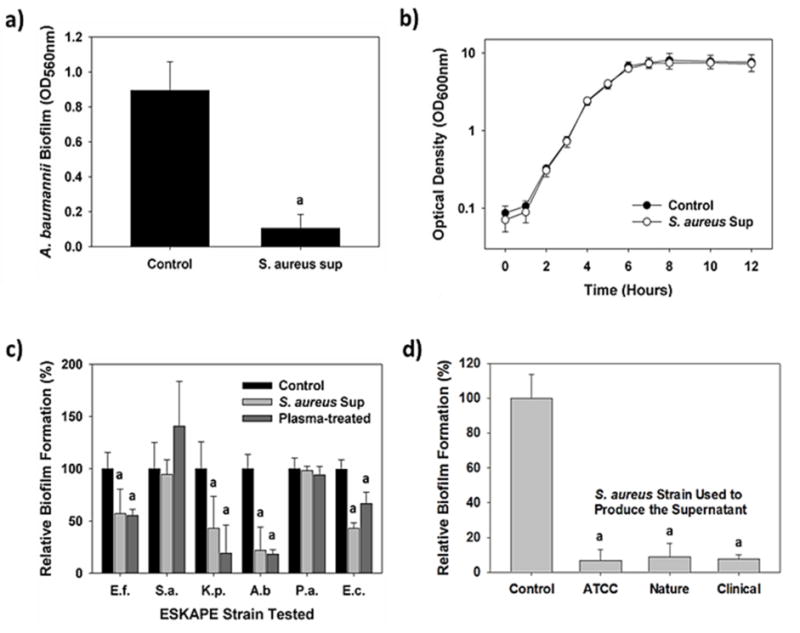
S. aureus inhibits biofilm generation by several different pathogens and E. coli. a) A 10% addition (v:v) of S. aureus supernatant significantly inhibited A. baumannii biofilm formation (n = 12; p <0.001). b) Addition of the S. aureus supernatant did not negatively impact growth of A. baumannii (n = 3; a = p<0.001). c) Relative biofilm formation by the bacteria tested in the presence of 10% S. aureus supernatant showing several strains were negatively impacted. Similar results were obtained when the polystyrene substrate was pretreated with oxygen plasma for 1 min to convert it to a more hydrophilic state (n = 12; a = p <0.001). d) Supernatants from several different S. aureus isolates showed similar inhibitory activities (n = 12; p <0.001).
Tests with supernatants from two additional S. aureus strains, one isolated from soil (Nature) and one from a patient with a nosocomial infection (Clinical), found them to be just as active against A. baumannii biofilm formation as S. aureus ATCC 25923 (Figure 1d). This shows the inhibitory activity is inherent to many, if not all, S. aureus.
S. aureus EVs are responsible for the inhibitory activities
We next performed a set of experiments to determine when the inhibitor was produced by S. aureus. At set times, samples of the S. aureus ATCC 25923 culture were taken and the impact of the cell-free supernatant tested against A. baumannii cultures. As shown in Figure 2a, inhibition was first seen as the S. aureus culture entered the stationary phase. Once more, the supernatants did not negatively impact the planktonic growth of the A. baumannii culture (Figure 2b). However, supernatants taken from S. aureus cultures after 6 hours reduced the number of A. baumannii within the biofilms by as much as 90%, i.e., from 10% of the total population to less than 1% (Figure 2b).
Using the S. aureus ATCC 25923 supernatants, therefore, we sought to identify the effector responsible. Based upon size exclusion analysis shown in Figure 3a, the effector is between 10 nm (100 kDa filter) and 220 nm (0.22 μm) in diameter, a size that suggested EVs might be responsible. Although the production of EVs by S. aureus has been reported before, this was the first indication that they influence biofilms formed by other bacteria. Consequently, EVs from S. aureus ATCC 25923 were purified, imaged using transmission electron microscopy (Figure 3b) and their sizes determined (Figure 3c). Their diameters ranged from 20 to 120 nm (Figure 3c), a value that is in agreement both with a previous report33 and the perceived effector size seen in Figure 3a. S. aureus EVs in the cell-free supernatants were also imaged using rhodamine BR-18, a red-fluorescing membrane incorporating dye (Figure 3d). Using the same technique, we found the number of EVs in the supernatant was minimal during exponential growth and increased dramatically as the culture entered the stationary phase (Figure S2), substantiating the results given in Figure 2.
Figure 3.
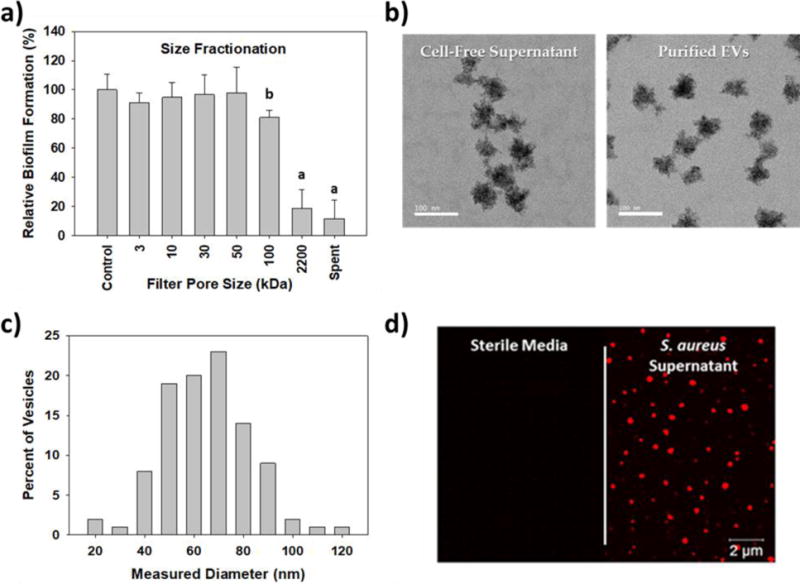
Identifying the inhibitor produced by S. aureus. a) Size fractionation tests show the inhibitor is greater than 10 nm in size but less than 0.22 μm. The impact of S. aureus supernatant (Sa sup) was included for comparison (a = p <0.001, b = p<0.05). b) TEM images of the S. aureus ATCC 25923 EVs within the cell-free supernatant and after purification. c) Size distribution of the pEVs, which were measured using the TEM images. In total, 100 pEVs were measured and their diameters are plotted. d) Super resolution fluorescence images of the polystyrene surfaces after exposure to sterile media or S. aureus supernatant. The EVs, which were labeled using the membrane incorporating fluorescent dye, rhodamine BR-18, are visibly red.
To clearly demonstrate that EVs were responsible for the activity seen, they were purified from the S. aureus supernatants and tested at different concentrations (Figure 4a). A clear dose-dependent inhibition of A. baumannii biofilm development was seen, with pEV concentrations of 12.5 μg/ml or greater leading to inhibitory levels similar with those of the supernatant. Given the similar levels of inhibition seen with the 24 h supernatants and 12.5 μg/ml pEVs, this concentration was used in subsequent experiments.
Figure 4.
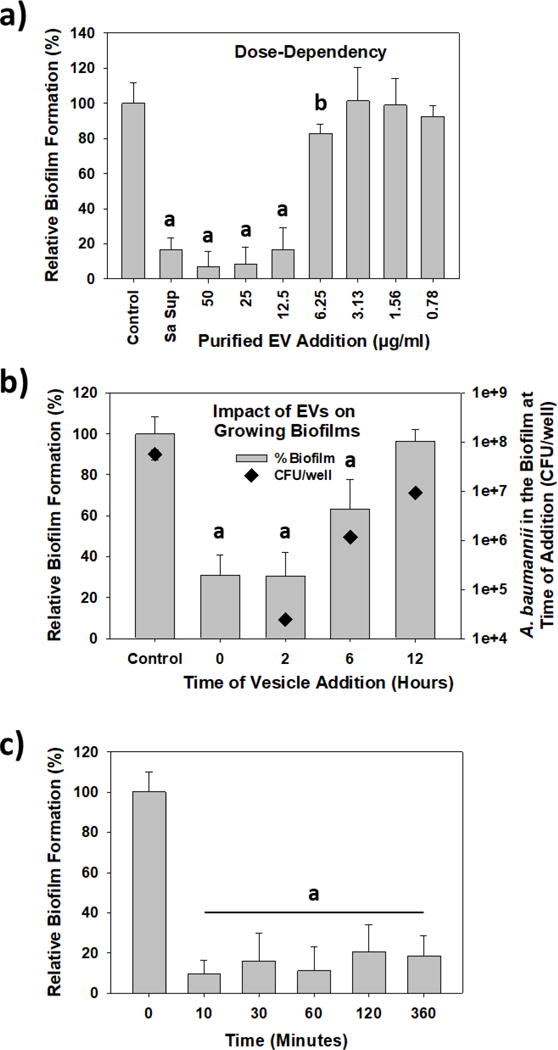
S. aureus pEV activity against A. baumannii biofilm generation. a) the pEV inhibitory activity is dose-dependent. Varying amounts of pEVs were added to A. baumannii cultures and the resulting biofilm biomass after 24 h was determined. Note the similar activity of the S. aureus supernatant (Sa sup) and when pEVs were added to a final concentration of 12.5 μg/ml (a = p <0.001, b = p<0.05). b) S. aureus EVs are capable of blocking A. baumannii biofilm formation even after A. baumannii has grown for several hours. The “Control” sample was not treated while pEVs (12.5 μg/ml final concentration) were added to the growing A. baumannii cultures at the time specified. The amount of biofilm formation was determined after a total of 24 h. In parallel samples, the A. baumannii biofilm populations were determined at each time point (n = 3; a = p <0.001). c) S. aureus EVs rapidly bind to the surface. pEVs (12.5 μg/ml) were added to polystyrene wells and at set times, the supernatant was removed. Afterwards, A. baumannii was added to the wells and cultured for 24 h to permit its biofilm to develop. The results show that pEVs bind to the surface and are active against A. baumannii in less than 10 min (a = p <0.001).
As noted above, the EVs served only a preventative role in biofilm formation (Figure S1). To evaluate this further, pEVs (12.5 μg/ml) were added to growing cultures of A. baumannii at different time points to see how they affected the developing biofilms. As shown in Figure 4b, they were most effective when added during the early stages of A. baumannii growth (0 and 2 h), leading to a 70% reduction in the final biofilm biomass. At these times, the A. baumannii biofilms were still early in their development and had, at most, slightly more than 104 CFU per well.
In addition, we measured the number of A. baumannii present in the biofilm after pEVs were added at 0 h (Figure S3). After 2 hours, both biofilms had similar A. baumannii populations. In the control wells, the biofilm populations increased nearly 100-fold over the next 4 h while those in the pEV-treated samples remained steady, hinting that the pEVs are inhibiting A. baumannii from attaching to the surface. Between 6 and 12 h, both A. baumannii biofilm populations increased but the pEV-treated biofilms were consistently lower, a result which also mirrors that seen with the 24 h S. aureus supernatant (Figure 2b). The impact of S. aureus pEVs on A. baumannii surface adherence was further confirmed in Figure S4, where the polystyrene surface was imaged after exposure to the cell-free S. aureus supernatants or pEVs. In both cases, EVs were dispersed across the surface while the number of A. baumannii attached to the surface were significantly lower. All of these results verify S. aureus EVs are the inhibitory effector and that they work by preventing A. baumannii from adhering to the substrate.
In Figure 4b, when A. baumannii was grown for 6 h, the biofilm populations were nearly 100-fold higher when compared with the 2 h biofilms and had an average of 1.2 × 106 CFU/well. This higher population was more resistant to the S. aureus pEVs and saw a decrease in the final biofilm biomass of only 37% (Figure 4b). Similar tests with 12 h A. baumannii cultures found their biofilms were not affected by the S. aureus pEVs and that they reached a biomass similar with that of the controls. Likewise, treatment of the 24 h A. baumannii biofilms did not impact their biomass (Figure S1) nor their morphologies (Figure S5). As shown in Figure S5, addition of the pEVs did not lead to a significant loss or change in the A. baumannii biofilm structure.
Figure 4c shows the pEVs bind to the polystyrene surface and do so rapidly. In this experiment, the HEPES solution containing the pEVs was incubated in the wells for set times, after which it was removed and the well was washed gently with sterile HEPES buffer before introducing the A. baumannii culture. As shown in Figure 4c, 10 min was sufficient for the EVs to bind the surface and inhibit A. baumannii biofilm formation, while longer incubations (of up to 6 h) did not improve upon their activity.
EV production by S. aureus is mediated by AgrA
Within S. aureus, the global regulon agr locus regulates the expression of numerous surface proteins, such as hemolysins,38 through AgrA-mediated quorum sensing. Given EVs and their activities were most evident during the stationary phase (Figures 2 and S2), this suggested that they may also be regulated by AgrA. To test this, we characterized two additional S. aureus strains, i.e., S. aureus JE2 and its isogenic ΔagrA mutant. In agreement with previous studies,39, 40 biofilms formed by the wild-type S. aureus JE2 were less robust that those formed by its isogenic ΔagrA mutant (Figure 5a).41, 42 When the supernatants from these two S, aureus strains were tested, we found the activity of the Whereas EVs were readily isolated and imaged from cultures of S. aureus JE2, all attempts to purify EVs from the S. aureus JE2 ΔagrA cultures or image them were unsuccessful (Figures 5b and c). For S. aureus JE2, the sizes of the EVs were typically between 20 and 100 nm in diameter. TEM images of JE2 ΔagrA cells, however, had no membrane blebs or apparent EVs budding from them. This is in agreement with the results presented in Figure 5b and suggests that AgrA activity is related to EV production in this strain. When the activity of these two strains were measured against A. baumannii biofilm formation, JE2 supernatants blocked their development by 74% while those from JE2 ΔagrA were much less active; only 24% inhibition (Figure 5d).
Figure 5.
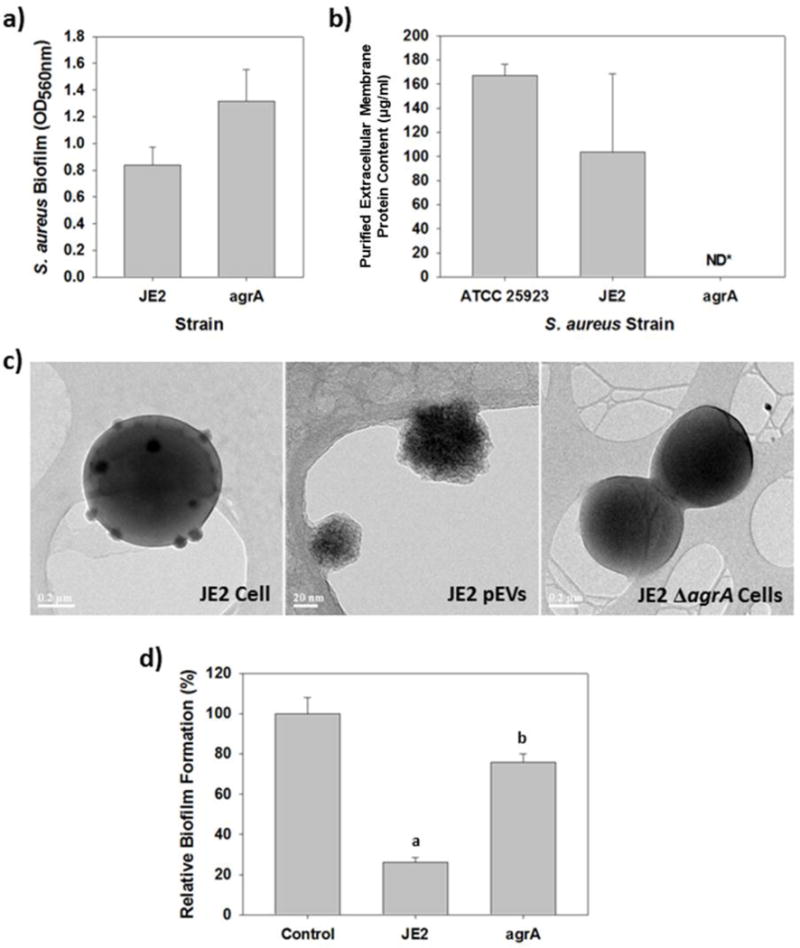
AgrA is required by S. aureus for EV production and activity against A. baumannii. a) Biofilm formation by S. aureus JE2 and an isogenic ΔagrA mutant strain. b) Protein content within concentrated samples from S. aureus JE2 and its isogenic ΔagrA mutant. S. aureus ATCC 25923 was included for comparison. The EVs vesicles in each culture were purified as described in the Materials and Methods, after which the protein content was determined using the Bradford Assay. Every attempt with the ΔagrA mutant found no protein after the purification was complete. c) TEM images of the two S. aureus strains, JE2 and its isogenic ΔagrA mutant, showing the mutant is not producing EVs. Numerous EVs are evident as they bud off of the wild-type JE2 cells. The purified EVs from this strain are shown in the middle image. In contrast, the JE2 ΔagrA cells do not produce EVs. d) A. baumannii biofilm formation in the presence of S. aureus JE2 supernatant (10% v:v) or that from from its isogenic ΔagrA mutant (a = p<0.001, b = p<0.05).
S. aureus EV binding increases the surface hydrophilicity
The results above show S. aureus EVs bind to the surface and inhibit biofilm formation by A. baumannii and several other bacteria. To better understand how they achieve this, we investigated the surface characteristics before and after EV treatment. The water contact angle (WCA) for an untreated polystyrene surface was 56.8 ±3.0° while treatment with sterile TSB media (i.e., the media used to culture S. aureus) decreased the WCA slightly to 42.3 ±2.2° (Figure S6). The contact angles from surfaces treated with the supernatant or pEVs were 17.8 ±1.2° and 17.3 ±2.2°, respectively. These values indicate that the treated surfaces were significantly more hydrophilic after being treated with S. aureus EVs.
To determine if such a conversion account for the results seen with A. baumannii, we used oxygen plasma as a proxy because it also converts normally hydrophobic polystyrene surfaces to a more hydrophilic state.43 As shown in Figure S7, plasma conversion of polystyrene was dose-dependent, with a treatment time of 30 s or more needed to maximize the impact against A. baumannii biofilm formation. Tests with polystyrene treated with oxygen plasma for 1 min found that the WCAs were 17.2 ±4.0° (Figure S6), values that are basically identical with those obtained from the EV-treated surfaces. Overall, plasma-treating the surfaces had a similar impact on the ability of the different strains to form biofilms as treating them with the S. aureus cell-free supernatant (Figure 1c). For example, A. baumannii, E. faecium, K. pneumonia and E. coli were all inhibited in their abilities to form biofilms (Figure 1c). Likewise, S. aureus and P. aeruginosa were both capable of forming biofilms on both the supernatant- and plasma-treated polystyrene.
As other bacteria also secrete EVs, including E. coli,44, 45 we were curious if they likewise possess similar surface modulatory or inhibitory activities. Using the same concentration (12.5 μg/ml) of pEVs from two additional strains, namely S. epidermidis and E. coli, we tested their ability to bind to polystyrene and inhibit A. baumannii biofilm formation. Although the pEVs from both bound to the surface, neither modified the surface hydrophobicity/hydrophilicity nor did they inhibit A. baumannii biofilms from forming on the surface (Figure S8).
Treatment with bleach does not remove S. aureus’ EVs or diminish their activity, but EDTA does
Given the reported prevalence of S. aureus on surfaces, we were curious if treatment with bleach, a commonly used disinfectant, could remove surface-bound EVs or reduce their activity towards other bacterial strains. To evaluate this, pEV-treated substrates were soaked in a 10% household bleach solution (~5,200 ppm sodium hypochlorite46) for 1 h before being rinsed and used for A. baumannii biofilm generation. This concentration was selected because it far exceeds the 50 to 100 ppm sodium hypochlorite needed to reduce the S. aureus viability by 6- to 7-log in 10 min.46 As shown in Figure 6, treatment with the disinfectant neither removed the bound EVs (Figure 6a) nor did it decrease their impact on A. baumannii biofilm formation (Figure 6b).
Figure 6.
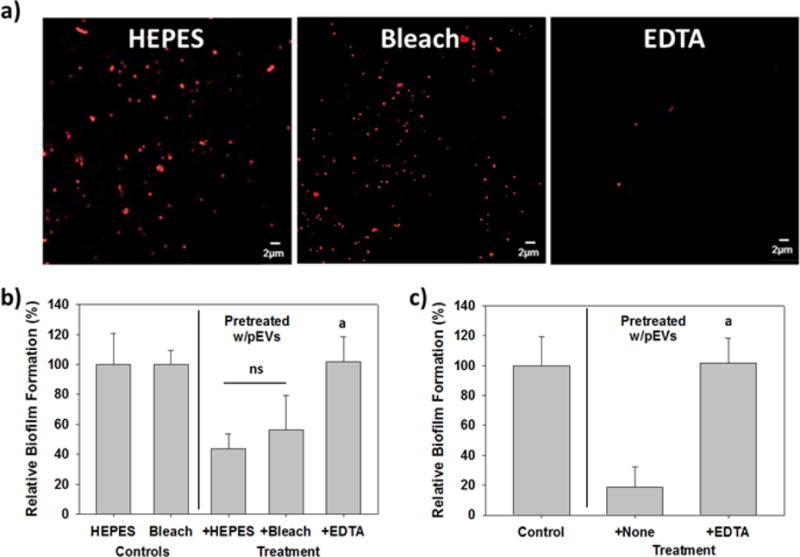
Bleach does not remove the EVs or their activity, but EDTA does. Each polystyrene slide in (a) and (b) was pretreated with pEVs (12.5 μg/ml) just prior to the experiment. a) Confocal image of the polystyrene surface after treatment for one hour with HEPES buffer, a 10% bleach solution or 0.1M EDTA. Rhodamine BR-18-labeled EVs were still evident on both the HEPES- and bleach-treated surfaces but were predominantly removed from the surfaces treated with EDTA. b) The bleach-treatment did not significantly reduce the inhibitory activity of the EVs. A. baumannii biofilm formation in the bleach-treated EV-coated wells was similar with the Control wells, with 44% and 56% inhibition, respectively. In contrast, treatment with EDTA completely removed the inhibitory activity (p<0.001). c) Treatment of the EVs with EDTA in liquid samples completely abolished their ability to block A. baumannii biofilm development. The EDTA sample was compared with the control (i.e., no pEV pretreatment) (p <0.001).
In contrast, the use of 0.1 M ethylenediaminetetraacetic acid (EDTA) not only removed the EVs from the surface (Figure 6a), it also eliminated their impact on A. baumannii biofilm formation (Figure 6b). EDTA not only removed EVs from the surface but also was active against S. aureus EVs in liquid samples. As shown in Figures 6c, pretreatment of the pEVs with 0.1 M EDTA completely eliminated their activity.
Discussion
S. aureus is an opportunistic pathogen that is acquired both nosocomially and in the community. However, the reasons behind its wide-spread prevalence and presence are not well understood. The results here show that EVs from S. aureus modify surface characteristics and block many other bacteria from forming biofilms. Characterization of the EV-bound surfaces found them to be more hydrophilic, a finding that helps to explain the lower biofilm formation seen as many bacterial species preferentially adhere to hydrophobic surfaces given the lower binding energy levels required.47, 48 This was demonstrated here with A. baumannii, E. coli, K. pneumoniae and E. faecium, all of whom formed more robust biofilms on hydrophobic surfaces. However, S. aureus and P. aeruginosa form robust biofilms on both hydrophobic and hydrophilic surfaces,49, 50 a result which was confirmed here in Figure 1c with both supernatant- and plasma-treated polystyrene. This characteristic helps to explain why EVs did not inhibit biofilm formation by these two pathogens, while also supporting our conjecture that changes in the surface hydrophobicity are responsible for the results seen with the other four strains.
Taken from a different perspective, it could be said that the activity of the EVs allow S. aureus to “stake a claim” on a surface by making it unreceptive for many other bacterial strains. This is illustrated in Figure S9, where S. aureus and A. baumannii were co-cultured. When cultured together, the planktonic populations of both strains were lower when compared with the individual cultures, which is expected due to a competition for nutrients. Similarly, the number of A. baumannii in the mixed biofilm was significantly lower than in its individual biofilm. In contrast, the S. aureus biofilm population remained unchanged, i.e., the biofilm in the mixed culture as many S. aureus as the one formed when S. aureus was cultured alone. This means the S. aureus biofilm population was not impacted by the presence of A. baumannii and was as robust even though it planktonic population was reduced.
Once formed, A. baumannii biofilms are reported to be particularly robust and present a special challenge due to their resilience.51 The early addition of S. aureus supernatant or pEVs, however, inhibited A. baumannii biofilm formation by as much as 85%. They were also effective against E. faecalis and K. pneumoniae which, along with A. baumannii, are ESKAPE pathogens. This aligns with the thought that the most critical step in biofilm formation is the initial adhesion of the bacterium to the substrate, as this greatly influences all the later stages of development.52
Binding of the surface by S. aureus’ EVs likely results from non-specific interactions, as was demonstrated previously with whole cells of the related Staphylococcus carnosus TM300.53 In that study, the authors concluded that interactions between the surface and this bacterium are “mainly governed by number, properties and arrangement of the bacterial cell-wall proteins.”53 S. aureus’ EVs contain many cell wall proteins and virulence factors, with more than 90 identified.33 Conversion of the surface from a hydrophobic state to one that is hydrophilic likely results from this aspect of the EVs, with many of the surface proteins being hydrophilic. Consequently, when the EVs coat the surface and bind through the non-specific interactions mentioned above, their hydrophilic state may act to repel other bacteria that prefer hydrophobic conditions.
Many of the cell-wall surface proteins and virulence factors found on this pathogen, and its EVs33, are controlled transcriptionally by the quorum-sensing related agr global regulon locus, which includes two divergent transcripts, RNAII and RNAIII.38, 54 RNAII encodes for four agr proteins, including AgrA, which controls the expression of a small RNA (RNAIII) and this, in turn, regulates the expression of surface proteins38, 55, 56 and many virulence factors.57 As virulence factors were identified within S. aureus EVs previously, this illustrated a potential link between the agr locus of S. aureus and the production of EVs by this strain. To evaluate this, S. aureus JE2 and its isogenic mutant, S. aureus JE2 ΔagrA, were characterized. Using TEM and the protein content, we found wild-type S. aureus JE2 produced EVs while its ΔagrA mutant did not. Although this implies a direct connection between AgrA and EV production in S. aureus, one major question still remained: do EVs contribute to the prevalence of S. aureus as a nosocomial pathogen? Related to this, they clearly altered the surface properties, making them less conducive to many other bacteria, but how do EVs fare against a typical disinfectant, such as bleach?
Merritt et al. (2000) reported that bacteria and their biofilms are removed when treated with bleach.58 However, we found treating the EV-bound surface with 10% bleach did not remove nor significantly impact their activity against A. baumannii. The levels of inhibition seen with surfaces washed with water and those washed with bleach were 56% and 44%, respectively, results that 1) are not significantly different from one another (n = 10; p = 0.14) and 2) indicate a simple application of this disinfectant is not sufficient at removing S. aureus EVs from the surface. Similar results were seen previously with MRSA, which was still found on surfaces after they were washed with bleach.59, 60 Similarly, an outbreak of MRSA within a urological ward reportedly persisted for several months although the ward was cleaned 60 h per week and proper hand hygiene and patient isolation protocols were being promoted.61 Only when the hours per week cleaning the ward increased to 120 did they see a reduction in the number of infected patients.
These results indicate that: 1) A gentle washing of the surface and treatment with disinfectants may not be sufficient to remove S. aureus EVs; and 2) based upon their selective inhibition, their continued presence on the surface may facilitate the later re-establishment of S. aureus biofilms. Although not conclusive, both of these findings help to explain the predominance of S. aureus on different surfaces and are of particular importance within hospital settings where bleach is commonly used as a disinfectant62-64.
When compared with bleach, we found washing the surface with EDTA was more effective at removing S. aureus EVs. EDTA acts as a chelator and is reported to remove biofilms of S. aureus, S. epidermidis and P. aeruginosa.65-69 For P. aeruginosa, EDTA appears to act by chelating divalent cations (calcium, magnesium, iron) within the biofilm, leading to its dispersal due to loss of electrostatic interactions that contribute to its stability70, 71. For S. aureus, EDTA was found to act by inhibiting interactions between this pathogen and the surface72. In addition, Gurung et al (2011) reported that EDTA lyses S. aureus EVs.34 Although the exact mechanism leading to lysis of the EVs by EDTA is not clear, both of these processes likely contribute to the reduced inhibitions seen. However, with its clear effectiveness against S. aureus EVs, EDTA is a promising supplement for use alongside disinfectants to control S. aureus.
To conclude, this study demonstrated S. aureus EVs convert hydrophobic surfaces into a hydrophilic one, thereby reducing the ability of many other bacterial strains from binding and forming biofilms. As S. aureus preferentially forms biofilms on hydrophilic surfaces,50 this conversion may actually be construed as an action by S. aureus to make the surface more conducive for its own attachment and biofilm formation. Moreover, the EVs, once bound, are not easily removed with bleach and continue to act after treatment. These results help explain why S. aureus is a prevalent nosocomial pathogen. As such, the inclusion of an EDTA wash step when cleaning surfaces may increase efforts to remove this pathogen and, as such, should be evaluated further.
Supplementary Material
Acknowledgments
We would like to thank the Korea Health Industry Development Institute (KHIDI) for financial support (Grant # HI13C13550000). SAS would like to thank the National Institutes of Health (P41-EB020594) for partial support of this work. The following reagent was provided by the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) for distribution by BEI Resources, NIAID, NIH: Staphylococcus aureus subsp. aureus, Strain JE2 (NR-46543), and JE2 △agrA (NE1532).
Footnotes
Additional information
Supplementary Information accompanies this paper
Competing financial interests
The authors claim no competing interests.
References
- 1.Stefani S, Varaldo PE. Clin Microbiol Infec. 2003;9:1179–1186. doi: 10.1111/j.1469-0691.2003.00698.x. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Dominguez M, Seral C, Potel C, Constenla L, Algarate S, Gude MJ, Alvarez M, Castillo FJ. Enferm Infec Micr Cl. 2015;33:590–596. doi: 10.1016/j.eimc.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Song JH, Hsueh PR, Chung DR, Ko KS, Kang CI, Peck KR, Yeom JS, Kim SW, Chang HH, Kim YS, Jung SI, Son JS, So TMK, Lalitha MK, Yang YH, Huang SG, Wang H, Lu QA, Carlos CC, Perera JA, Chiu CH, Liu JW, Chongthaleong A, Thamlikitkul V, Van PH, Grp AS. J Antimicrob Chemoth. 2011;66:1061–1069. doi: 10.1093/jac/dkr024. [DOI] [PubMed] [Google Scholar]
- 4.Syed AK, Ghosh S, Love NG, Boles BR. Mbio. 2014:5. doi: 10.1128/mBio.01015-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David MD, Kearns AM, Gossain S, Ganner M, Holmes A. J Hosp Infect. 2006;64:244–250. doi: 10.1016/j.jhin.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Muto CA, Jernigan JA, Ostrowsky BE, Richet HM, Jarvis WR, Boyce JM, Farr BM. Infect Cont Hosp Ep. 2003;24:362–386. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 7.Akinyemi KO, Atapu AD, Adetona OO, Coker AO. J Infect Dev Countr. 2009;3:628–632. doi: 10.3855/jidc.556. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee N, Dowd SE, Wise A, Kedia S, Vohra V, Banerjee P. Int J Env Res Pub He. 2014;11:12544–12561. doi: 10.3390/ijerph111212544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begier EM, Frenette K, Barrett NL, Mshar P, Petit S, Boxrud DJ, Watkins-Colwell K, Wheeler S, Cebelinski EA, Glennen A, Nguyen D, Hadler JL, Field CB. Clin Infect Dis. 2004;39:1446–1453. doi: 10.1086/425313. [DOI] [PubMed] [Google Scholar]
- 10.Cheng VCC, Chau PH, Lee WM, Ho SKY, Lee DWY, So SYC, Wong SCY, Tai JWM, Yuen KY. J Hosp Infect. 2015;90:220–225. doi: 10.1016/j.jhin.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Dancer SJ. J Hosp Infect. 2009;73:378–385. doi: 10.1016/j.jhin.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Dancer SJ. Clin Microbiol Rev. 2014;27:665–690. doi: 10.1128/CMR.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sexton T, Clarke P, O’Neill E, Dillane T, Humphreys H. J Hosp Infect. 2006;62:187–194. doi: 10.1016/j.jhin.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 14.French GL, Otter JA, Shannon KP, Adams NMT, Watling D, Parks MJ. J Hosp Infect. 2004;57:31–37. doi: 10.1016/j.jhin.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 16.Zweig M, Schork S, Koerdt A, Siewering K, Sternberg C, Thormann K, Albers SV, Molin S, van der Does C. Environ Microbiol. 2014;16:1040–1052. doi: 10.1111/1462-2920.12291. [DOI] [PubMed] [Google Scholar]
- 17.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappinscott HM. Annual Review of Microbiology. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 18.Sutherland IW. Trends Microbiol. 2001;9:222–227. doi: 10.1016/s0966-842x(01)02012-1. [DOI] [PubMed] [Google Scholar]
- 19.von Bodman SB, Majerczak DR, Coplin DL. P Natl Acad Sci USA. 1998;95:7687–7692. doi: 10.1073/pnas.95.13.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutherford ST, Bassler BL. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 22.Fazli M, Almblad H, Rybtke ML, Givskov M, Eberl L, Tolker-Nielsen T. Environ Microbiol. 2014;16:1961–1981. doi: 10.1111/1462-2920.12448. [DOI] [PubMed] [Google Scholar]
- 23.Yarwood JM, Bartels DJ, Volper EM, Greenberg EP. J Bacteriol. 2004;186:1838–1850. doi: 10.1128/JB.186.6.1838-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorward DW, Garon CF. Appl Environ Microbiol. 1990;56:1960–1962. doi: 10.1128/aem.56.6.1960-1962.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Cruz C, Delgado L, Lopez-Iglesias C, Mercade E. Plos One. 2015;10:e0116896. doi: 10.1371/journal.pone.0116896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Nat Rev Microbiol. 2015;13:620–630. doi: 10.1038/nrmicro3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zakharzhevskaya NB, Tsvetkov VB, Vanyushkina AA, Varizhuk AM, Rakitina DV, Podgorsky VV, Vishnyakov IE, Kharlampieva DD, Manuvera VA, Lisitsyn FV, Gushina EA, Lazarev VN, Govorun VM. Front Cell Infect Microbiol. 2017;7:2. doi: 10.3389/fcimb.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gui MJ, Dashper SG, Slakeski N, Chen YY, Reynolds EC. Mol Oral Microbiol. 2016;31:365–378. doi: 10.1111/omi.12134. [DOI] [PubMed] [Google Scholar]
- 29.Tran F, Boedicker JQ. Sci Rep. 2017;7:8813. doi: 10.1038/s41598-017-07447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Cruz C, Carrion O, Delgado L, Martinez G, Lopez-Iglesias C, Mercade E. Appl Environ Microbiol. 2013;79:1874–1881. doi: 10.1128/AEM.03657-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yonezawa H, Osaki T, Kurata S, Fukuda M, Kawakami H, Ochiai K, Hanawa T, Kamiya S. Bmc Microbiol. 2009:9. doi: 10.1186/1471-2180-9-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schooling SR, Hubley A, Beveridge TJ. J Bacteriol. 2009;191:4097–4102. doi: 10.1128/JB.00717-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, Kim SH, Desiderio DM, Kim YK, Kim KP, Gho YS. Proteomics. 2009;9:5425–5436. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 34.Gurung M, Moon DC, Choi CW, Lee JH, Bae YC, Kim J, Lee YC, Seol SY, Cho DT, Kim SI, Lee JC. Plos One. 2011:6. doi: 10.1371/journal.pone.0027958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monnappa AK, Dwidar M, Seo JK, Hur JH, Mitchell RJ. Sci Rep. 2014;4:3811. doi: 10.1038/srep03811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thay B, Wai SN, Oscarsson J. Plos One. 2013;8:e54661. doi: 10.1371/journal.pone.0054661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 38.Chabelskaya S, Bordeau V, Felden B. Nucleic Acids Res. 2014;42:4847–4858. doi: 10.1093/nar/gku119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lauderdale KJ, Boles BR, Cheung AL, Horswill AR. Infect Immun. 2009;77:1623–1635. doi: 10.1128/IAI.01036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boles BR, Horswill AR. PLoS Pathog. 2008;4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. Mbio. 2013;4:e00537–00512. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brackman G, Breyne K, De Rycke R, Vermote A, Van Nieuwerburgh F, Meyer E, Van Calenbergh S, Coenye T. Sci Rep. 2016;6:20321. doi: 10.1038/srep20321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larsson A, Derand H. J Colloid Interface Sci. 2002;246:214–221. doi: 10.1006/jcis.2001.8032. [DOI] [PubMed] [Google Scholar]
- 44.McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ. J Bacteriol. 2006;188:5385–5392. doi: 10.1128/JB.00498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulp AJ, Sun B, Ai T, Manning AJ, Orench-Rivera N, Schmid AK, Kuehn MJ. Plos One. 2015;10:e0139200. doi: 10.1371/journal.pone.0139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutala WA, Cole EC, Thomann CA, Weber DJ. Infect Control Hosp Epidemiol. 1998;19:323–327. doi: 10.1086/647822. [DOI] [PubMed] [Google Scholar]
- 47.Fletcher M, Loeb GI. Appl Environ Microb. 1979;37:67–72. doi: 10.1128/aem.37.1.67-72.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donlan RM. Emerg Infect Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller MJ, Ahearn DG. J Clin Microbiol. 1987;25:1392–1397. doi: 10.1128/jcm.25.8.1392-1397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JS, Bae YM, Lee SY, Lee SY. J Food Sci. 2015;80:M2279–M2286. doi: 10.1111/1750-3841.13017. [DOI] [PubMed] [Google Scholar]
- 51.Espinal P, Marti S, Vila J. J Hosp Infect. 2012;80:56–60. doi: 10.1016/j.jhin.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Palmer J, Flint S, Brooks J. J Ind Microbiol Biotechnol. 2007;34:577–588. doi: 10.1007/s10295-007-0234-4. [DOI] [PubMed] [Google Scholar]
- 53.Thewes N, Loskill P, Jung P, Peisker H, Bischoff M, Herrmann M, Jacobs K. Beilstein J Nanotech. 2014;5:1501–1512. doi: 10.3762/bjnano.5.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiong YQ, Van Wamel W, Nast CC, Yeaman MR, Cheung AL, Bayer AS. J Infect Dis. 2002;186:668–677. doi: 10.1086/342046. [DOI] [PubMed] [Google Scholar]
- 55.Boisset S, Geissmann T, Huntzinger E, Fechter P, Bendridi N, Possedko M, Chevalier C, Helfer AC, Benito Y, Jacquier A, Gaspin C, Vandenesch F, Romby P. Genes Dev. 2007;21:1353–1366. doi: 10.1101/gad.423507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tegmark K, Morfeldt E, Arvidson S. J Bacteriol. 1998;180:3181–3186. doi: 10.1128/jb.180.12.3181-3186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koenig RL, Ray JL, Maleki SJ, Smeltzer MS, Hurlburt BK. J Bacteriol. 2004;186:7549–7555. doi: 10.1128/JB.186.22.7549-7555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merritt K, Hitchins VM, Brown SA. J Biomed Mater Res. 2000;53:131–136. doi: 10.1002/(sici)1097-4636(2000)53:2<131::aid-jbm1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 59.French GL, Otter JA, Shannon KP, Adams NM, Watling D, Parks MJ. J Hosp Infect. 2004;57:31–37. doi: 10.1016/j.jhin.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Jeanes A, Rao G, Osman M, Merrick P. J Hosp Infect. 2005;61:85–86. doi: 10.1016/j.jhin.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Rampling A, Wiseman S, Davis L, Hyett AP, Walbridge AN, Payne GC, Cornaby AJ. J Hosp Infect. 2001;49:109–116. doi: 10.1053/jhin.2001.1013. [DOI] [PubMed] [Google Scholar]
- 62.Rutala WA, Weber DJ. Clin Microbiol Rev. 1997;10:597–610. doi: 10.1128/cmr.10.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liscynesky C, Hines LP, Smyer J, Hanrahan M, Orellana RC, Mangino JE. Infect Control Hosp Epidemiol. 2017;38:1116–1117. doi: 10.1017/ice.2017.126. [DOI] [PubMed] [Google Scholar]
- 64.Dumas O, Wiley AS, Henneberger PK, Speizer FE, Zock JP, Varraso R, Le Moual N, Boggs K, Camargo CA. Am J Resp Crit Care. 2016;193 [Google Scholar]
- 65.Banin E, Brady KM, Greenberg EP. Appl Environ Microbiol. 2006;72:2064–2069. doi: 10.1128/AEM.72.3.2064-2069.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kite P, Eastwood K, Sugden S, Percival SL. Journal of Clinical Microbiology. 2004;42:3073–3076. doi: 10.1128/JCM.42.7.3073-3076.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finnegan S, Percival SL. Adv Wound Care (New Rochelle) 2015;4:415–421. doi: 10.1089/wound.2014.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shanks RM, Sargent JL, Martinez RM, Graber ML, O’Toole GA. Nephrol Dial Transplant. 2006;21:2247–2255. doi: 10.1093/ndt/gfl170. [DOI] [PubMed] [Google Scholar]
- 69.Maisetta G, Grassi L, Di Luca M, Bombardelli S, Medici C, Brancatisano FL, Esin S, Batoni G. Biofouling. 2016;32:787–800. doi: 10.1080/08927014.2016.1194401. [DOI] [PubMed] [Google Scholar]
- 70.Turakhia MH, Characklis WG. Biotechnol Bioeng. 1989;33:406–414. doi: 10.1002/bit.260330405. [DOI] [PubMed] [Google Scholar]
- 71.Chen X, Stewart PS. Appl Microbiol Biot. 2002;59:718–720. doi: 10.1007/s00253-002-1044-2. [DOI] [PubMed] [Google Scholar]
- 72.Meng LY, Cai WQ, Qu HY, Liu JF, Lan JX, Lu JK, Lan TF, Li JR. Food Sci Technol Res. 2013;19:323–330. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


