Shigella species are bacteria that cause severe diarrheal disease worldwide primarily in young children. Treatment of shigellosis includes oral fluids and antibiotics, but the high burden of disease, increasing prevalence of antibiotic resistance, and long-term health consequences clearly warrant the development of an effective vaccine. One Shigella vaccine under development is termed the invasin complex or Invaplex and is designed to drive an immune response to specific antigens of the bacteria in an effort to protect an individual from infection. The work presented here describes the production and evaluation of a new generation of Invaplex. The improved vaccine stimulates the production of antibodies in immunized mice and guinea pigs and protects these animals from Shigella infection. The next step in the product’s development will be to test the safety and immune response induced in humans immunized with Invaplex.
KEYWORDS: Shigella, adjuvant, immunology, vaccine
ABSTRACT
The native Invaplex (InvaplexNAT) vaccine and adjuvant is an ion exchange-purified product derived from the water extract of virulent Shigella species. The key component of InvaplexNAT is a high-molecular-mass complex (HMMC) consisting of the Shigella lipopolysaccharide (LPS) and the invasin proteins IpaB and IpaC. To improve product purity and immunogenicity, artificial Invaplex (InvaplexAR) was developed using recombinant IpaB and IpaC proteins and purified Shigella LPS to assemble an HMMC consisting of all three components. Characterization of InvaplexAR by various methods demonstrated similar characteristics as the previously reported HMMC in InvaplexNAT. The well-defined InvaplexAR vaccine consistently contained greater quantities of IpaB, IpaC, and LPS than InvaplexNAT. InvaplexAR and InvaplexNAT immunogenicities were compared in mouse and guinea pig dose escalation studies. In both models, immunization induced antibody responses specific for InvaplexNAT and LPS while InvaplexAR induced markedly higher anti-IpaB and -IpaC serum IgG and IgA endpoint titers. In the murine model, homologous protection was achieved with 10-fold less InvaplexAR than InvaplexNAT and mice receiving InvaplexAR lost significantly less weight than mice receiving the same amount of InvaplexNAT. Moreover, mice immunized with InvaplexAR were protected from challenge with both homologous and heterologous Shigella serotypes. Guinea pigs receiving approximately 5-fold less InvaplexAR compared to cohorts immunized with InvaplexNAT were protected from ocular challenge. Furthermore, adjuvanticity previously attributed to InvaplexNAT was retained with InvaplexAR. The second-generation Shigella Invaplex vaccine, InvaplexAR, offers significant advantages over InvaplexNAT in reproducibility, flexible yet defined composition, immunogenicity, and protective efficacy.
IMPORTANCE Shigella species are bacteria that cause severe diarrheal disease worldwide, primarily in young children. Treatment of shigellosis includes oral fluids and antibiotics, but the high burden of disease, increasing prevalence of antibiotic resistance, and long-term health consequences clearly warrant the development of an effective vaccine. One Shigella vaccine under development is termed the invasin complex or Invaplex and is designed to drive an immune response to specific antigens of the bacteria in an effort to protect an individual from infection. The work presented here describes the production and evaluation of a new generation of Invaplex. The improved vaccine stimulates the production of antibodies in immunized mice and guinea pigs and protects these animals from Shigella infection. The next step in the product’s development will be to test the safety and immune response induced in humans immunized with Invaplex.
INTRODUCTION
Shigellosis is a leading cause of diarrheal disease worldwide, particularly in developing countries (1), and is a continuing problem for civilian and military travelers visiting regions of endemicity (2–5). Vaccine development remains a high priority given the disease burden, increasing antibiotic resistance, and increasing appreciation of the postinfectious sequelae associated with shigellosis (6, 7). Vaccine efforts are directed at the most prevalent serogroups, such as Shigella flexneri and Shigella sonnei, which together account for approximately 89% of shigellosis cases in developing regions (8).
Humans and higher primates infected with Shigella develop robust immune responses to several invasion plasmid antigens (Ipa), which are key effectors secreted by the type III secretion system (TTSS) involved in the ability of shigellae to invade the colonic mucosa. The Ipa proteins are highly conserved among all four Shigella species and share functional and structural homology with TTSS effector proteins of other Gram-negative bacteria, e.g., Salmonella and Yersinia (9). Although the prominent immune response to the Ipa proteins has been noted for decades (10, 11), the role of the Ipa proteins in protective immunity in humans is not clear. In contrast, immunity to shigellosis is strongly associated with antibodies to the O antigen of lipopolysaccharide (LPS) and is serotype specific (12, 13). Progressing from antigen identification to clinical trials and licensure has been difficult for the many Shigella vaccine approaches that are in various stages of development. To date, all vaccines in clinical trials are focused primarily on stimulating a response to the O antigen of the LPS in attempts to stimulate serotype-specific immunity and the potential of serogroup cross-reactivity (14).
The prototype Invaplex vaccine, native Invaplex (InvaplexNAT), purified from virulent Shigella, contains the conserved invasin proteins IpaB and IpaC, serotype-specific LPS, and other proteins of undefined function and limited to no immunogenicity (15, 16). Intranasal immunization of small animals with InvaplexNAT drives a pronounced immune response to LPS, IpaB, and IpaC that mimics that observed after natural infection with virulent Shigella. Protection with InvaplexNAT has been achieved in both the mouse and guinea pig models (15, 17), prompting the clinical evaluation of the vaccine in two phase 1 safety and immunogenicity studies (18, 19). The current good manufacturing practice (cGMP)-manufactured S. flexneri 2a InvaplexNAT vaccine was found to be safe, well tolerated, and immunogenic in humans. The efficacy results of a follow-on phase 2b study were inconclusive due to the product being less immunogenic, likely due to product instability upon prolonged storage and a lower-than-expected attack rate in placebo controls (M. S. Riddle and R. W. Kaminski, unpublished data).
Further purification of InvaplexNAT by size-exclusion chromatography (SEC) yielded a highly purified (HP) InvaplexNAT with a high molecular mass of >669 kDa. This high-molecular-mass complex (HMMC) consisted of only the principal antigens IpaB, IpaC, and LPS and showed enhanced immunogenicity and protection against infection (16). Using the molar ratios of the components present in HP InvaplexNAT, a synthetic Invaplex, termed artificial Invaplex or InvaplexAR, has been assembled with purified Shigella LPS and purified recombinant IpaB and IpaC proteins. Here, the first-generation InvaplexNAT vaccine is compared with the second-generation InvaplexAR vaccine in tests of biochemical composition, immunogenicity, adjuvanticity, and protective efficacy. The studies indicate that not only is the InvaplexAR a better-defined product biochemically, making it more compliant with FDA guidelines on chemistry, manufacturing, and controls, but it exhibits enhanced immunogenicity and protection compared to InvaplexNAT.
RESULTS
Assembly and characterization of artificial Invaplex.
In order to evaluate and accurately compare the immunogenicities and protective efficacies of InvaplexAR and InvaplexNAT produced from wild-type shigellae, a sufficient amount of the Shigella InvaplexAR products to immunize groups of both mice and guinea pigs with ranging vaccine doses was required. The InvaplexAR was purified from the reaction mixture by ion-exchange chromatography. Two dominant peaks were observed at both 50% B (~0.5 M NaCl, 0.02 M Tris-HCl, pH 9.0) and 100% B. Spot blot analysis and total protein measurements showed the presence of IpaB, IpaC, and LPS within the 50% B elution peak but did not identify significant amounts of IpaB or IpaC within the 100% B peak. The 50% B peak elution fractions were combined and were defined as the InvaplexAR product. Additional experiments using S. flexneri 1a and Shigella dysenteriae 1 LPS also resulted in InvaplexAR products purified within the 50% B elution peak of anion chromatography (data not shown).
The yield efficiency of the InvaplexAR production was calculated by dividing the total protein yield of the product by the total protein amount of the reactants and then multiplying by 100 and ranged from 8 to 22% yield.
Comparison of S. flexneri 2a InvaplexAR and InvaplexNAT by SDS-PAGE.
Equal amounts, based on protein content, of the S. flexneri 2a InvaplexAR product and InvaplexNAT product were separated by SDS-PAGE followed by Western blotting or staining with either silver or Coomassie blue (Fig. 1). Western blotting and probing with the anti-IpaB specific monoclonal antibody (MAb) 2F1 and the anti-IpaC specific MAb 2G2 (20) identified IpaB and IpaC, respectively, in both InvaplexAR and InvaplexNAT (Fig. 1, left panel), although the antibody reactivity with the proteins in the InvaplexNAT product was weaker, suggesting that IpaB and IpaC are in lower concentrations within the total protein makeup of this product.
FIG 1 .
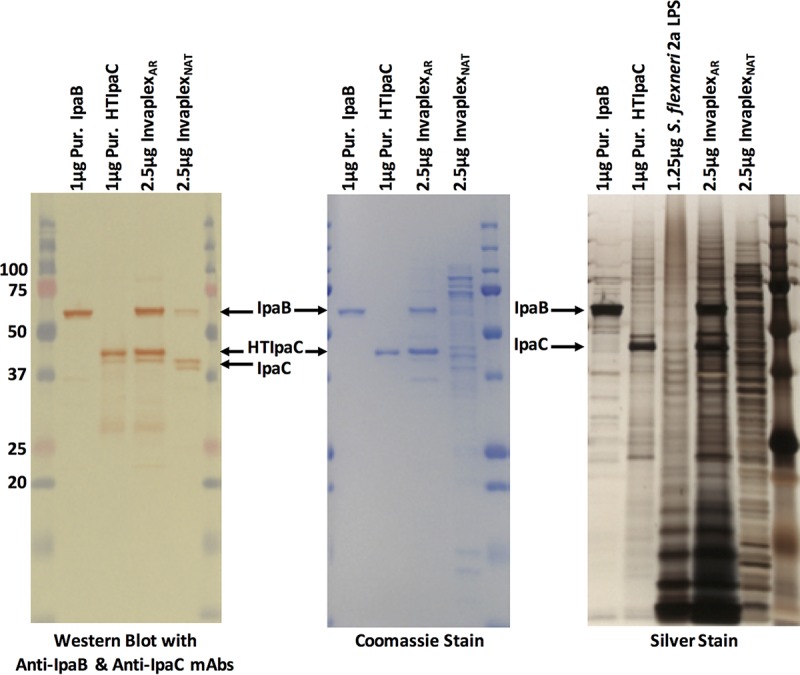
SDS-PAGE analysis of InvaplexAR and InvaplexNAT. Samples loaded were S. flexneri 2a InvaplexAR (2.5 µg), InvaplexNAT (2.5 µg), IpaB (1 µg), HTIpaC (1 µg), and purified S. flexneri 2a LPS (1.25 µg). Samples were separated by SDS-PAGE and blotted to nitrocellulose (left panel) or stained with Coomassie blue (middle panel) or silver (right panel). Nitrocellulose blots were probed with MAbs specific for IpaB (2F1) and IpaC (2G2). Silver-stained gels detected both proteins and LPS. Molecular mass standards are in the extreme left (Western blot and Coomassie blue) and right (Western blot and Coomassie blue and silver stain lanes) of the gels. Numbers at far left are kilodaltons.
The IpaC protein band in the InvaplexAR product, which was produced using histidine-tagged IpaC (HTIpaC), migrated to the same position as the purified HTIpaC, while the IpaC band identified in the InvaplexNAT product migrated slightly lower. The higher apparent molecular mass of the HTIpaC band is attributed to the polyhistidine tag.
Coomassie blue staining (Fig. 1, center panel) of the Invaplex products clearly demonstrated the IpaB and IpaC proteins within the InvaplexAR product but did not detect these bands definitively within the InvaplexNAT product, instead showing multiple bands of various molecular weights that stained with various intensities. The Coomassie blue staining suggests that the proportion of the Ipa proteins in the total protein composition is lower within the less-defined InvaplexNAT product than in the InvaplexAR product, which contains only two proteins, IpaB and IpaC. The approximate antigen amounts in a 1-µg dose of InvaplexAR are 370 ng IpaB, 600 ng IpaC, and 953 ng LPS.
The more sensitive silver staining technique detects both LPS and proteins. Many more polypeptide bands within the two Invaplex products were detected along with the S. flexneri 2a LPS “ladder” (Fig. 1, right panel). In addition to the full-sized IpaB and HTIpaC proteins, lower-molecular-weight bands were identified by silver staining within the purified IpaB and HTIpaC components and were likely degradation products of the antigens. The same lower-molecular-mass bands were also present in the InvaplexAR product. The LPS ladder was prominent within the InvaplexAR product compared to the InvaplexNAT product, which suggests a greater proportion of the S. flexneri 2a LPS component within the InvaplexAR product.
Comparison of native and artificial Invaplex products by SEC-HPLC and dynamic light scattering (DLS).
The S. flexneri 2a InvaplexNAT and InvaplexAR products were separated by size-exclusion chromatography–high-pressure liquid chromatography (SEC-HPLC). Chromatograms were recorded at 215 nm in order to detect the InvaplexAR product as both the IpaB and IpaC proteins have a low proportion of aromatic amino acids (Fig. 2). The InvaplexAR product (17.5 µg total protein loaded) revealed a single dominant symmetric peak with a retention time of 16.5 min (Fig. 2, red trace) which is resolved before thyroglobulin (669 kDa; 18.5 min) and is therefore interpreted as being larger than thyroglobulin. The InvaplexNAT product (70 µg total protein loaded) resolved as several lower-molecular-mass peaks with retention times after thyroglobulin (Fig. 2, dashed blue trace) and one small peak at a retention time of 16.8 min that meets the definition of the previously reported high-molecular-mass complex (16). SEC-HPLC demonstrates that the production of an InvaplexAR product from individual components yields a slightly higher-molecular-mass product that is more homogeneous than InvaplexNAT prepared from wild-type Shigella water extract.
FIG 2 .
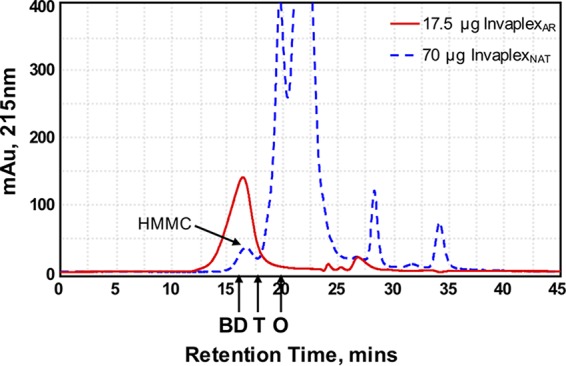
SEC-HPLC of InvaplexNAT and InvaplexAR. A TSK-Gel G5000PWXL column was calibrated as described in Materials and Methods. Retention times of blue dextran (BD; 2 MDa), thyroglobulin (T; 669 kDa), and ovalbumin (O; 43 kDa) are indicated. The UV (215-nm) traces of S. flexneri 2a InvaplexAR (17.5 µg, red line) and S. flexneri 2a InvaplexNAT (70 µg, blue dashed line) are shown. The higher total protein load for InvaplexNAT was necessary to reveal the high-molecular-mass complex (HMMC) peak (run time of approximately 16.8 min) in the InvaplexNAT sample. mAu, milliabsorbance units.
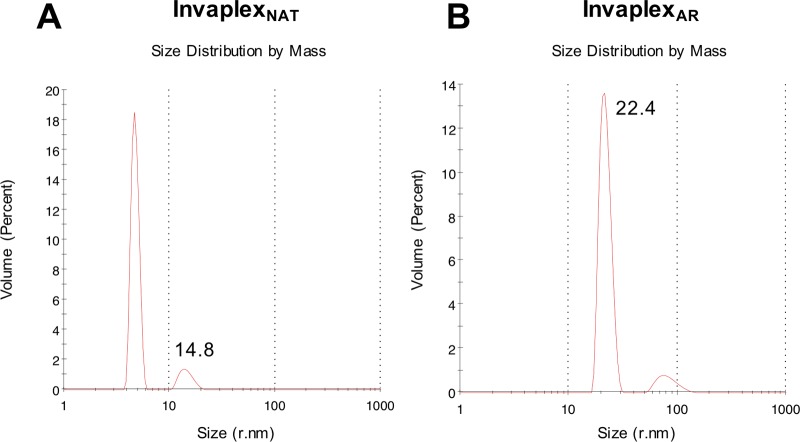
The S. flexneri 2a InvaplexNAT and InvaplexAR were also analyzed by dynamic light scattering (DLS) to determine their size distribution. Both Invaplex products displayed a polydisperse profile containing molecular species with different sizes. After controlling for the relative mass of the peaks, a major peak for each of the Invaplex products was identified (Fig. 3). The major peaks of the Invaplex products measured an average hydrodynamic radius of 18.6 ± 3.9 nm for InvaplexNAT and 27 ± 11.5 nm for InvaplexAR, although the majority of the InvaplexNAT product is smaller species of shorter hydrodynamic radii. The hydrodynamic radius of InvaplexAR is greater than the reported hydrodynamic radius of 8.5 nm for thyroglobulin and thus supports the results achieved using SEC-HPLC (21).
FIG 3 .
Dynamic light scattering (DLS) analysis of S. flexneri 2a InvaplexNAT (A) and InvaplexAR (B). Fifteen microliters of each Invaplex product was added to the cuvette with a 2-µl window of a Malvern Zetasizer µV DLS unit. Each graph is representative data for the specific analyte from 1 of 5 separate runs, with each run consisting of 11 to 13 separate scans, all of which were performed at 4°C. The annotated radius numbers are derived from the intensity graph (data not shown), while the graphs presented show the relative quantities of these species in solution.
Immunogenicity and protective efficacy of native and artificial Invaplex vaccines.
The mouse pulmonary challenge (22) and the guinea pig keratoconjunctivitis (23) models were used to determine whether intranasal immunization with S. flexneri 2a InvaplexAR stimulates a protective immune response that is effective against homologous challenge with wild-type S. flexneri 2a.
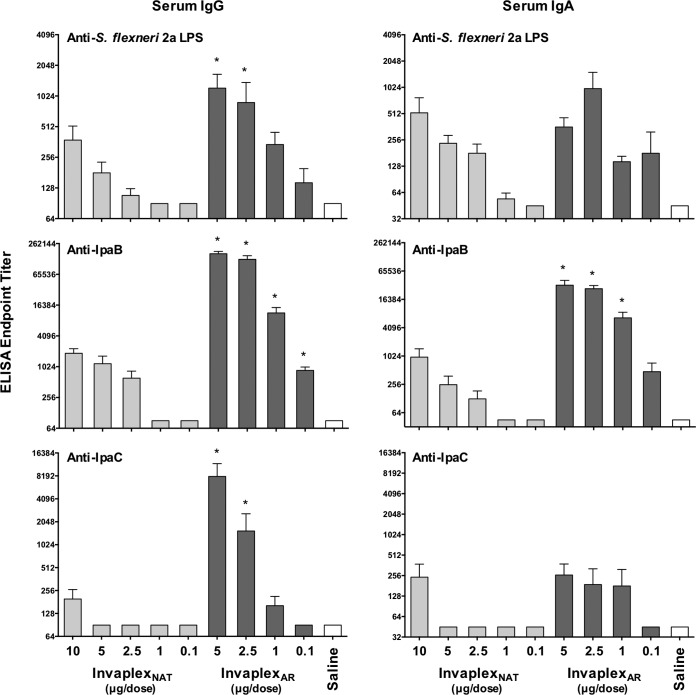
In mouse experiments utilizing a dose escalation study design, intranasal immunization with S. flexneri 2a InvaplexAR induced serum IgG and IgA antibodies directed to S. flexneri 2a LPS, IpaB, and IpaC (Fig. 4) that were either significantly higher than or comparable to the antibody responses induced after immunization with similar dose amounts of S. flexneri 2a InvaplexNAT. In particular, the antibody responses directed to IpaB (IgA and IgG) and IpaC (IgG) were significantly higher after immunization with InvaplexAR than after immunization with InvaplexNAT at comparable dose amounts. Bronchial lavage fluid IgA responses showed increased IpaC titers in groups immunized with InvaplexNAT and InvaplexAR, with higher responses observed in the InvaplexAR groups. Furthermore, intranasal immunization of mice with ≥1 µg of InvaplexNAT or ≥0.1 µg of InvaplexAR resulted in significant protection against lethal challenge with S. flexneri 2a strain 2457T (Table 1). A delay in death was evident in all immunized mice. The day on which 27% (4 of 15) mice died occurred on day 5 in the saline control group, day 9 in the 0.1-µg and 1.0-µg InvaplexNAT groups, and day 11 in the 0.1-µg InvaplexAR group. In all other groups, 4 or more mice did not die during the study. Moreover, animals immunized with 1 µg (Fig. 5) or 2.5 µg InvaplexAR lost significantly less weight (P < 0.02) after challenge than did mice immunized with InvaplexNAT. In contrast, mice immunized with 100% protective 5-µg doses of either vaccine exhibited similar weight losses, each of which was significantly less than that of the saline control group (InvaplexAR, P = 0.0005; InvaplexNAT, P = 0.041). Mice immunized with the lowest dose (0.1 µg) of either vaccine lost weight that was comparable to that observed in the saline control group.
FIG 4 .
Shigella-specific serum IgG and IgA endpoint titers in mice immunized intranasally with S. flexneri 2a InvaplexNAT or S. flexneri 2a InvaplexAR. Groups of mice (n = 15/group) were immunized intranasally on days 0, 14, and 28 with either S. flexneri 2a InvaplexNAT (0.1, 1, 2.5, 5, or 10 µg), S. flexneri 2a InvaplexAR (0.1, 1, 2.5, or 5 µg), or saline and challenged on day 49 (n = 15 mice/group) with S. flexneri 2a strain 2457T. Blood was collected on days 0, 28, 42, and 63 (n = 5 mice/group) and analyzed by ELISA for anti-S. flexneri 2a LPS (top panels), IpaB (middle panels), or IpaC (bottom panels) serum IgG (left panels) and IgA (right panels) endpoint titers. The mean endpoint titers + 1 standard error of the mean on day 42 are plotted, and significant differences (two-way analysis of variance; P < 0.05) between dose-matched groups are indicated with an asterisk.
TABLE 1 .
Protective efficacy in the mouse pulmonary infection and guinea pig keratoconjunctivitis models after intranasal immunization with various doses of S. flexneri 2a InvaplexNAT or InvaplexAR
| Model and treatment | Dose (μg) | No. of animalsa
|
% protectionb | P valuec | |
|---|---|---|---|---|---|
| Protected | Unprotected | ||||
| Mouse lung (n = 15) | |||||
| S. flexneri 2a InvaplexNAT | 10 | 15 | 0 | 100 | <0.001 |
| 5 | 15 | 0 | 100 | <0.001 | |
| 2.5 | 12 | 3 | 75 | 0.003 | |
| 1 | 10 | 5 | 59 | 0.025 | |
| 0.1 | 9 | 6 | 50 | 0.06 | |
| S. flexneri 2a InvaplexAR | 5 | 15 | 0 | 100 | <0.001 |
| 2.5 | 15 | 0 | 100 | <0.001 | |
| 1 | 13 | 2 | 84 | 0.001 | |
| 0.1 | 10 | 5 | 59 | 0.025 | |
| Saline | None | 3 | 12 | N/Ad | N/A |
| Guinea pig keratoconjunctivitis (n = 12) | |||||
| S. flexneri 2a InvaplexNAT | 25 | 9 | 3 | 75 | 0.0005 |
| 5 | 3 | 9 | 25 | 0.22 | |
| 1 | 0 | 12 | 0 | 1.00 | |
| S. flexneri 2a InvaplexAR | 23 | 7 | 5 | 59 | 0.0053 |
| 5 | 7 | 5 | 59 | 0.0053 | |
| 1 | 4 | 8 | 33 | 0.09 | |
| Saline | None | 0 | 10 | N/A | N/A |
“Protected” indicates number of survivors in the mouse lung model and number of animals with eyes protected in the keratoconjunctivitis model. “Unprotected” indicates number of dead animals in the mouse lung model and number of animals with unprotected eyes in the keratoconjunctivitis model.
Percent protection = (AR for controls − AR for immunized)/AR for controls × 100, where the attack rate (AR) for a group is determined by (number of diseased animals/total number of animals) × 100.
Fisher exact test.
N/A, not applicable.
FIG 5 .

Mice immunized with 1 µg InvaplexAR experience less weight loss and recover more rapidly than those immunized with InvaplexNAT. Mice immunized with S. flexneri 2a InvaplexAR (red) or InvaplexNAT (blue) were challenged intranasally with a lethal dose of S. flexneri 2a strain 2457T. Mice were weighed on the day of challenge and each day for 14 days postchallenge. The average weight for each group was calculated, and the difference was expressed as a percentage of the change in weight from the prechallenge values. The numbers of surviving animals/animals in groups immunized with InvaplexNAT and InvaplexAR are shown. While protection was significant for both products compared to saline controls, a significant difference in weight was observed for the 1-µg InvaplexAR group compared to the 1-µg InvaplexNAT group (*, P = 0.0126).
To better understand the immune responses and protective efficacy afforded after immunization with InvaplexNAT and InvaplexAR, the 50% protective dose (PD50) and 50% immunizing dose (ImmD50) were calculated using an adaptation of the Reed-Muench 50% lethal dose (LD50) formula (24). The PD50 for InvaplexNAT was determined to be 0.5 µg, which was approximately 7-fold higher than the calculated PD50 for InvaplexAR (Table 2). Using similar calculations, it was determined that the dose of InvaplexAR required to induce antibody responses in 50% of the immunized animals (ImmD50) to Invaplex, LPS, IpaB, or IpaC was 2.7- to >21-fold lower than the dose of InvaplexNAT required to induce antigen-specific immune responses, demonstrating that InvaplexAR is a more potent immunogen than InvaplexNAT.
TABLE 2 .
Intranasal immunization with S. flexneri 2a InvaplexAR provides protection and drives an immune response to Shigella-specific antigens at lower doses than S. flexneri 2a InvaplexNAT in mice and guinea pigsa
| Animal model | Treatment | PD50 (µg) | ImmD50 (Shigella antigen-specific serum IgG) (µg) |
|||
|---|---|---|---|---|---|---|
|
S. flexneri 2a Invaplex |
S. flexneri 2a LPS | IpaB | IpaC | |||
| Mouse | InvaplexNAT | 0.5 | 1.9 | >10 | 2.1 | >10 |
| InvaplexAR | 0.07 | 0.7 | 0.9 | <0.1 | 1.8 | |
| Fold difference | 7.1 | 2.7 | >11.1 | >21 | >5.6 | |
| Guinea pig | InvaplexNAT | 9.3 | 2.6 | 9 | 2.6 | 13.1 |
| InvaplexAR | 4.5 | 0.8 | 3.8 | 0.6 | 0.6 | |
| Fold difference | 2.1 | 3.2 | 2.4 | 4.3 | 21.8 | |
The PD50 and ImmD50 were calculated as described in Materials and Methods. The quantities of LPS, IpaB, and IpaC for each Invaplex product were determined as described in Materials and Methods.
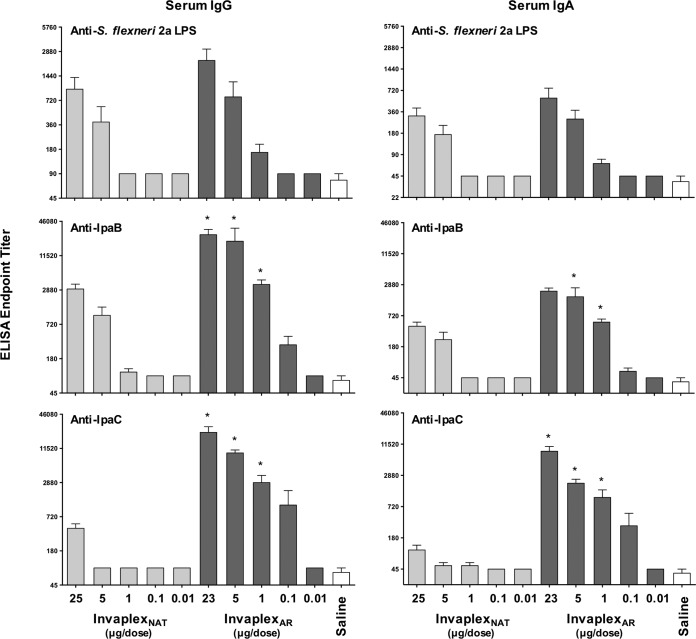
Using a similar dose escalation study design, groups of guinea pigs were intranasally immunized with S. flexneri 2a InvaplexNAT or InvaplexAR. Control groups were inoculated with saline. Blood collected on days 0, 28, and 40 was assayed by enzyme-linked immunosorbent assay (ELISA) for titers of antibodies directed to LPS, IpaB, and IpaC. Similar to the results in mice, immunization of guinea pigs with InvaplexAR promoted antibody responses directed to LPS, IpaB, and IpaC at similar or higher titers than those for animals immunized with InvaplexNAT (Fig. 6). Guinea pigs ocularly challenged with S. flexneri 2a strain 2457T were significantly protected after three immunizations with 25 µg of InvaplexNAT (75% protection; P = 0.0005), but immunization with lower doses of InvaplexNAT did not result in significant protection (Table 1). In contrast, groups immunized with 5 or 23 µg of InvaplexAR were significantly protected against challenge (59% protection; P = 0.0053). The Reed-Muench formula was again used to calculate the PD50 and ImmD50 for the guinea pig model, and similar trends were achieved as obtained for the mouse model in that a higher InvaplexNAT dose than InvaplexAR dose was necessary to provide protection and elicit a Shigella-specific immune response in 50% of the animals immunized with InvaplexNAT (Table 2).
FIG 6 .
Shigella-specific serum IgG and IgA endpoint titers in guinea pigs immunized intranasally with S. flexneri 2a InvaplexNAT or InvaplexAR. Groups of guinea pigs (n = 6/group) were immunized intranasally on days 0, 14, and 28. Dose amounts of S. flexneri 2a InvaplexNAT and S. flexneri 2a InvaplexAR are indicated on the x axis. Guinea pigs were challenged on day 49 with S. flexneri 2a strain 2457T. Blood was collected on days 0, 28, 40, and 63 and analyzed by ELISA for anti-S. flexneri 2a LPS (top panels) or IpaB (middle panels) or IpaC (bottom panels) serum IgG (left panels) and IgA (right panels) endpoint titers. The mean endpoint titer + 1 standard error of the mean on day 40 is plotted, and significant differences (two-way analysis of variance; P < 0.05) between dose-matched groups are indicated with an asterisk.
Homologous and heterologous protection afforded after immunization with S. sonnei InvaplexAR.
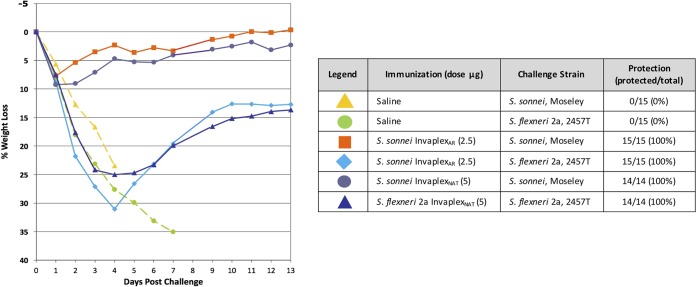
Naturally acquired immunity against shigellosis is primarily serotype specific, and therefore, a multivalent vaccine containing multiple O-antigen types will likely be required to achieve broadly protective efficacy. Consequently, a vaccine that is capable of inducing protective immunity against multiple serogroups or serotypes would be highly advantageous. Immunization of mice with S. sonnei InvaplexNAT can provide cross protection against challenge with S. flexneri 2a strain 2457T (25). The ability of InvaplexAR to induce protective immunity against heterologous species or serotypes was also tested in the mouse pulmonary lung model. Mice were immunized intranasally with 2.5 µg of S. sonnei InvaplexAR and divided into two subgroups: one subgroup was challenged with S. sonnei strain Moseley, the homologous serotype to the vaccine, and the other subgroup was challenged with S. flexneri 2a strain 2457T. Controls for the study included animals immunized with saline and challenged with either S. sonnei strain Moseley or S. flexneri 2a strain 2457T (negative controls) and animals immunized with 5 µg of either S. sonnei InvaplexNAT or S. flexneri 2a InvaplexNAT and challenged with the homologous serotype (positive controls). None of the animals immunized with saline survived the challenge (Fig. 7), and all lost >20% of their prechallenge weight prior to death. Mice immunized with the native Invaplex vaccines and challenged with the homologous serotype all survived challenge (100% protection). As expected, all animals immunized with S. sonnei InvaplexAR and challenged with S. sonnei strain Moseley survived challenge and exhibited <10% weight loss. Interestingly, all animals immunized with S. sonnei InvaplexAR and challenged with S. flexneri 2a strain 2457T survived challenge and followed a similar percent weight loss curve as animals immunized with S. flexneri 2a InvaplexNAT. Mice immunized with S. sonnei InvaplexAR and protected against S. flexneri 2a produced serum IgG antibodies (geometric mean titer [GMT]) to S. sonnei LPS (136), IpaB (5,760), and IpaC (1,091) but weak to undetectable levels against S. flexneri 2a LPS (GMT of 90). These results demonstrate the ability of the Shigella InvaplexAR to provide a level of protective immunity against a heterologous Shigella species challenge similar to that of the InvaplexNAT product (17, 25).
FIG 7 .
Intranasal immunization of mice with S. sonnei InvaplexAR induces protective immune responses against S. sonnei and S. flexneri 2a. Mice were immunized intranasally on days 0, 14, and 28 with S. sonnei InvaplexAR, S. sonnei InvaplexNAT, or S. flexneri 2a InvaplexNAT and challenged using the pulmonary mouse model on day 49 with either S. flexneri 2a strain 2457T or S. sonnei strain Moseley. Mice were weighed on the day of challenge and each day for 14 days postchallenge. The average weight for each group was calculated, and the difference was expressed as a percentage of the change in weight from the prechallenge values.
Adjuvanticity of S. flexneri 2a InvaplexAR.
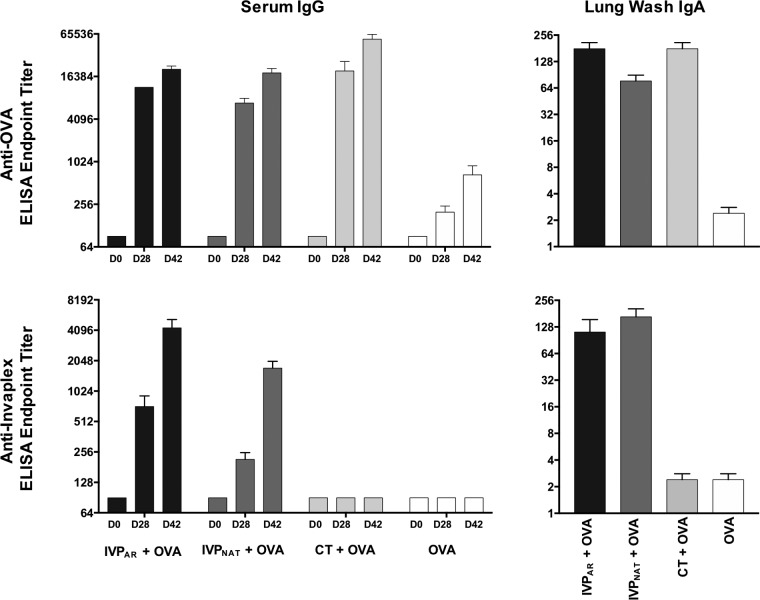
In addition to inducing Shigella-specific immune responses, InvaplexNAT also functions as an adjuvant, capable of enhancing the magnitude of the immune response directed to coadministered protein antigens (26) or antigens encoded on plasmid DNA (27). To determine if adjuvanticity was retained with InvaplexAR, groups of mice were intranasally immunized with either ovalbumin (OVA) alone or in combination with S. flexneri 2a InvaplexNAT, InvaplexAR, or cholera toxin (CT), a known mucosal adjuvant. Comparable levels of OVA-specific serum IgG (Fig. 8) and IgA titers (data not shown) were detected on days 28 and 42 after immunization with OVA mixed with InvaplexNAT, InvaplexAR, and cholera toxin. All responses were significantly higher (P < 0.001) than the OVA-specific immune response induced after immunization with OVA alone. In addition to enhancing the OVA-specific immune response, Shigella-specific immunity was induced in the groups immunized with OVA combined with InvaplexNAT or InvaplexAR (Fig. 8), indicating that InvaplexAR could be used in a combination vaccine strategy as both an adjuvant and an immunogen.
FIG 8 .
OVA-specific and InvaplexNAT-specific serum IgG endpoint titers in mice after intranasal immunization with OVA alone or OVA combined with InvaplexAR, InvaplexNAT, or cholera toxin (CT). Groups of mice were intranasally immunized on days 0, 14, and 28 with OVA or OVA combined with either S. flexneri 2a InvaplexAR or InvaplexNAT or CT. Blood collected on days 0, 28, and 42 was analyzed by ELISA for anti-OVA and anti-InvaplexNAT serum IgG endpoint titers. Lung washes collected on day 42 were analyzed by ELISA for anti-OVA and anti-InvaplexNAT IgA endpoint titers. Data represent the mean (n = 5 mice/group) endpoint titer ± 1 standard error of the mean. Significant differences between group mean log-transformed titers were determined by two-way analysis of variance (P < 0.05).
Antigen-specific antibodies in lung washes collected 2 weeks after the third immunization (day 42) were also assessed by ELISA to investigate the mucosal immune responses induced after immunization (Fig. 8). Similar levels of OVA-specific lung IgA were induced after immunization with OVA mixed with InvaplexAR, InvaplexNAT, or CT, and these levels were significantly higher (P < 0.001) than those induced after immunization with OVA alone (Fig. 8). Immunization with OVA mixed with InvaplexAR induced similar levels of LPS-specific (data not shown) and InvaplexNAT-specific IgA in lung washes as those after immunization with OVA mixed with InvaplexNAT and higher levels than those induced after immunization with OVA alone or OVA mixed with CT (Fig. 8).
DISCUSSION
Analysis of the immune response generated after natural infection may guide the rational design of a Shigella vaccine capable of stimulating a protective immune response comparable to that induced postinfection. Several studies in humans (13, 28, 29) have demonstrated that individuals orally infected with Shigella are resistant to subsequent infections with homologous or closely related Shigella serotypes but not heterologous serotypes. In nonhuman primates (10), infected animals producing serum antibodies to homologous LPS and the Ipa proteins are resistant to homologous challenge but not challenge with a heterologous serotype. The results of these studies combined with epidemiological data (30) suggest that serotype-based immunity is prevalent after natural infection. Shigella infection induces a potent immune response to LPS with the O-specific polysaccharide (O-SP) generating serotype-specific immunity in both humans and nonhuman primates (10, 21). In addition, a pronounced antibody response to the protein effectors of the TTSS, specifically IpaB, IpaC, and IpaD, which is characterized by cross-reactivity with all serotypes and species of Shigella, is induced (10–12, 20, 31, 32). Other protein antigens such as VirG (10, 12), OmpA (33), or pan-Shigella surface protein 1 (PSSP-1) (34) may also stimulate cross-reactive immune responses. However, in some cases chromosomally encoded protein antigens, such as major outer membrane proteins, are broadly conserved among enteric Gram-negative bacteria and it is often difficult to identify specific infection-induced signals over the abundance of background cross-reactivity due to the structural similarity.
Vaccines that stimulate an immune response that mimics the natural response in magnitude, specificity, and functionality hold promise for inducing protective immunity in humans. Candidate Shigella vaccines with this capability include live attenuated Shigella (32, 35), inactivated whole cells (36–38), and the Generalized Modules for Membrane Antigens (GMMA) product, although the last is deficient in the invasins (39). Vaccines focused primarily on only O-SP or only conserved proteins are promising but may not stimulate the comprehensive, fully protective immune response observed after infection. The Shigella Invaplex vaccine has been developed to mimic both the predominant serotype-specific aspect and pan-Shigella (protein-specific) immune responses induced after natural infection by presenting the LPS, IpaB, and IpaC antigens in a manner or context that induces a functional and effective immune response.
The first-generation Shigella Invaplex vaccines were isolated from water extracts of wild-type, virulent organisms (15) and have been given the nomenclature of “native” Invaplex. The prototype InvaplexNAT vaccines were evaluated in a series of studies that demonstrated the immunogenicity and efficacy of the product in preclinical studies (15, 17), the feasibility of cGMP manufacture, and the safety and immunogenicity of the product in a series of phase 1 clinical trials (18, 19). The InvaplexNAT product was safe and immunogenic when delivered intranasally to over 100 human volunteers. Although promising in early clinical trials, potential instability upon long-term storage, resulting in lower than previously achieved immunogenicity, combined with a lower than expected attack rate, hampered efforts to demonstrate proof-of-concept efficacy in a phase 2b trial (R. W. Kaminski and M. S. Riddle, unpublished results). The composition of InvaplexNAT includes the target major Shigella antigens (LPS, IpaB, and IpaC) and many other proteins, including IpaD, elongation factor G (EF-G), and DnaK, along with many other proteins with limited to no immunogenicity (see list of proteins in Table S1 in the supplemental material). The lack of product homogeneity and definition may have been associated with the instability and lower immune response induced by InvaplexNAT products stored for a prolonged period of time and suggested that a better understanding of the active components in InvaplexNAT was warranted. For this reason, efforts were undertaken to improve the product’s homogeneity, manufacturing yield, and immunogenicity, and these efforts have led to the development of a second-generation Invaplex product.
InvaplexNAT24 (lot JWJY) and InvaplexNAT50 (lots JWJY and 1307) were digested with trypsin overnight at 30°C. The peptides were identified on an LC/MS system, Xevo G2-XS qTof (Waters). The data files were analyzed in Progenesis QI for proteomics (Nonlinear Dynamics) and identified using a proteome of Shigella flexneri 2a 2457T (UniProt proteome identifier UP000001006). All proteins reported as present were identified by at least one unique peptide from the proteome. Proteins are listed based on confidence score from highest to lowest. Proteins with confidence scores of <50 are not included, with the exception of IcsA/VirG (confidence score of 46.21) and IpaA (confidence score of 27.90). Highlighted (blue) rows indicate proteins previously identified as constituents of InvaplexNAT (15, 16). Download TABLE S1, DOCX file, 0.03 MB (34.2KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Research conducted on a highly purified InvaplexNAT product clearly demonstrated that LPS, IpaB, and IpaC were combined in an HMMC that was essential for the product’s immunogenicity and protective efficacy (16). Furthermore, the molar ratios of the IpaB and IpaC proteins and the protein-to-lipopolysaccharide ratios in the HMMC provided a blueprint for the design and construction of second-generation InvaplexAR. The methodology used to assemble LPS, IpaB, and IpaC into a synthetic or artificial high-molecular-mass complex has inherent flexibility that allows the three constituents to be assembled in ratios that optimize immunogenicity, protective efficacy, and maintenance of component solubility. The resultant InvaplexAR product is much more defined than InvaplexNAT and can be scaled up more readily than the process used to manufacture InvaplexNAT.
The customization of InvaplexAR is evidenced in the ease of switching from one Shigella serotype/species to another serotype by simply substituting the LPS used during the assembly process. Research-grade lots of InvaplexAR have been manufactured for S. flexneri 2a and S. sonnei as well as S. flexneri 3a, S. flexneri 1a, and S. dysenteriae 1 (data not shown), in which the same IpaB and IpaC lots were used with the various Shigella LPSs. Indeed, the LPS component was not only key as an antigen but was also essential for the solubility of the InvaplexAR product as, in experiments in which only IpaB and IpaC were mixed, the proteins precipitated out of solution. The presence of LPS prevented the precipitation of IpaC (data not shown), similar to the use of a detergent (40). The insolubility of purified IpaC has hampered subunit vaccine efforts including this critical virulence protein (40). IpaB and IpaC contain substantial regions of hydrophobicity (40–42), and both IpaC and IpaB have been reported to insert themselves into red blood cell and mammalian cell membranes (40, 43–45). The amphiphilic nature of LPS likely provides a favorable environment to facilitate the hydrophobic collapse or folding of the Ipa proteins in a soluble and potentially active state in a manner similar to the folding and membrane insertion of Escherichia coli OmpA protein (46) and E. coli OmpT protein (47, 48). Once the InvaplexAR is formed, the complex is stable without evidence of precipitation or insolubility of any of the components or the complex. It is unknown if the complex represents a structure naturally found in Shigella, but as an LPS/IpaB/IpaC structure is also found in InvaplexNAT and is of similar size and composition and capable of inducing uptake in mammalian cells, we speculate that the InvaplexAR represents the active complex or HMMC found in InvaplexNAT. The slightly earlier SEC-HPLC retention time for the InvaplexAR product may be the result of the increased mass of the product compared to InvaplexNAT in part due to the use of a His-tagged IpaC product in the manufacture of the second-generation vaccine. Investigations to better define and characterize the structure of InvaplexAR are under way. The amount of lipopolysaccharide required in the assembly process to achieve levels of LPS-specific immunity which correlate with protection across multiple Shigella serotypes is also under active investigation. Studies optimizing the LPS content for new Shigella serotypes will permit the manufacture of a multivalent Shigella InvaplexAR product targeting the most predominant serotypes.
The immunogenicity and protective efficacy of the InvaplexNAT and InvaplexAR products were compared directly in the mouse and guinea pig models. Both models have been used extensively to evaluate potential Shigella vaccines, with the mouse model used primarily for early-stage vaccine candidates and the guinea pig conjunctivitis model applied to more advanced vaccine candidates immediately prior to initial studies in humans (19, 22, 23) as it depends largely upon a mucosal immune response for protection (36). In both models, InvaplexAR was comparable or superior to InvaplexNAT in the magnitude of the immune response directed to Shigella LPS, IpaB, and IpaC as well as affording significant protection against a lethal pulmonary or keratoconjunctivitis challenge. Neither product required an adjuvant to induce a protective immune response. Furthermore, the total protein dosage of InvaplexAR was 2- to 10-fold lower than that of InvaplexNAT for both immunogenicity and protective efficacy in both animal models. Solid protection (less weight loss and increased number of survivors) was achieved with 1 µg of InvaplexAR. This dose amount contains 370 ng IpaB, 600 ng IpaC, and 953 ng LPS. Other reports (49) using IpaB as an antigen indicate that more than 2.5 µg of IpaB is required for protection in mice, suggesting that less IpaB is required when presented in the context of InvaplexAR. The improved potency of InvaplexAR will not only conserve product but may open the possibility of including adjuvants to the formulation that may further enhance the immunogenicity of the product.
Recent epidemiological data (8) suggest that to achieve broad protection, a Shigella vaccine would need to provide coverage against S. sonnei, S. flexneri 2a, S. flexneri 3a, and potentially S. flexneri 6, with the idea that all LPS serotypes and serogroups, defined as group (3)4, group 6, and group 7(8), would be represented and provide expanded coverage (14). The InvaplexAR vaccine approach is amenable to multivalency by admixing successful monovalent vaccines. Bivalent formulations of InvaplexNAT have successfully demonstrated the feasibility of this approach (17). In addition, as indicated in our study, the ability of monovalent InvaplexAR vaccines to provide cross protection against multiple Shigella serotypes could be leveraged to broaden coverage. The LPS component of InvaplexAR provides the serotype and serogroup specificity of the vaccine. A quadrivalent InvaplexAR vaccine directed at the 4 prevalent serotypes would theoretically provide immunity against ≥80% of the Shigella isolates that cause global shigellosis (8). Furthermore, as the Ipa proteins are highly conserved across all Shigella serotypes, they add an attractive advantage of InvaplexAR for use in a pan-Shigella vaccine. Studies in mice using IpaB and IpaD (31) have demonstrated that a protein-only-based vaccine is capable of protecting against challenge with other Shigella serotypes in the murine pulmonary model. Using a live attenuated S. flexneri 2a vaccine overexpressing TTSS antigens, broad protection has been observed in guinea pigs (32). However, other studies in guinea pigs immunized with only purified Ipa proteins have not demonstrated similar protective effects; when LPS was delivered in combination with IpaB and IpaC, the vaccine formulation generally resulted in significant protection (R. W. Kaminski and E. V. Oaks, unpublished data).
Another attribute of InvaplexNAT is the ability of the complex to function as a mucosal adjuvant. Studies conducted with protein antigens (ovalbumin, protective antigen from Bacillus anthracis, CFA/I antigen from enterotoxigenic E. coli, and FlaA from Campylobacter jejuni) have demonstrated that InvaplexNAT increases the magnitude of the humoral and cellular immune response directed to the coadministered antigen (26). Furthermore, InvaplexNAT also enhanced the immune response directed to vaccine antigens encoded on DNA plasmids (27). The adjuvanticity of Invaplex has been partially attributed to its inherent ability to induce cellular uptake of coadministered antigens as well as providing the necessary Toll-like receptor 4 (TLR-4) agonist that stimulates robust immune responses. The ability to induce cellular uptake is likely due to the biological activity associated with the Ipa proteins, which as TTSS effectors are actively involved in host cell invasion by Shigella spp. (9). InvaplexAR retains similar biological functions and can induce cellular uptake in normally nonphagocytic epithelial and fibroblast cell lines (50). The slightly larger size of InvaplexAR than InvaplexNAT may improve its interactions with antigen-presenting cells (APCs) and enhance its immunogenicity and adjuvanticity. The retention of the biological activity combined with inclusion of LPS allows InvaplexAR to also function as a mucosal adjuvant when codelivered with heterologous antigens such as ovalbumin (27). The adjuvanticity of InvaplexAR is very appealing when considering the development of a combination vaccine, such as an antidiarrheal vaccine to protect against enterotoxigenic Escherichia coli (ETEC) and Shigella infection.
Collectively, the comparisons between the first-generation InvaplexNAT vaccine and the second-generation InvaplexAR vaccine have demonstrated InvaplexAR to have comparable and enhanced properties in relation to InvaplexNAT. The InvaplexAR products have improved biochemical consistency and potency and offer the increased formulation flexibility necessary for a multivalent Shigella vaccine approach. Research to transition the recombinant IpaC protein to a non-histidine-tagged version and improved levels of recombinant protein expression, as well as efforts to increase the production yield efficiency of the mixing process, is actively under way in order to make a consistent product that is suitable for cGMP manufacturing and transition to clinical evaluations to evaluate the safety and immunogenicity of the second-generation product.
MATERIALS AND METHODS
Bacterial strains and growth.
Shigella flexneri 2a strain BS103 and Shigella sonnei strain Moseley, both grown in brain heart infusion (BHI) broth, served as the source of purified LPS, while InvaplexNAT was isolated from virulent S. flexneri 2a strain 2457T grown in a modified animal-product-free formulation based on antibiotic medium number 3 (Becton, Dickinson) as previously described (18).
Recombinant clones expressing the Ipa of interest were kindly provided by Bill Picking of the University of Kansas (51–53). Escherichia coli strain BL21(DE3)pLysS harboring the IpaB pCAYC/HTIpgC pET15b or HTIpaC pET15b plasmid construct was grown in LB broth (Lennox) with appropriate antibiotics. Both recombinant strains were induced at an optical density at 600 nm (OD600) of 0.5 with isopropyl-β-d-thiogalactopyranoside (IPTG), followed by continued incubation at 37°C for 3 h, and then harvested for further processing (see below).
Purification of IpaB and HTIpaC (IpaC).
Clarified supernatants of sonicated cells containing IpaB/His-tagged IpgC (HTIpgC) complexes were applied to a 20-ml HisPrep FF 16/10 nickel Sepharose (GE Healthcare) column in binding buffer (20 mM Tris-HCl, 500 mM NaCl, 5 mM imidazole, pH 7.9) (51). Bound IpaB/HTIpgC complexes were eluted with 20 mM Tris-HCl, 500 mM NaCl, 50 mM EDTA, pH 7.4. Fractions containing both IpaB and IpgC were pooled and dialyzed followed by addition of N-octyl-oligo-oxyethylene (OPOE; 1% final concentration; Alexis Biochemicals) (51) and incubated at room temperature with gentle swirling for 30 min. The OPOE-treated sample was applied to a 25-ml XK 16/10 nickel Sepharose HP (GE Healthcare) column. Fractions were collected during the loading and initial wash buffers. These void volume fractions, containing predominantly IpaB, were pooled, the OPOE concentration was made 1%, and the pools were incubated at room temperature for 30 min and then reapplied to the column according to the steps above to remove residual HTIpgC. The final purified IpaB product was stored at −80°C.
His-tagged IpaC (HTIpaC) was released from induced recombinant bacteria by suspension in binding buffer containing 6 M urea (52). The treated cells were sonicated and centrifuged, and the supernatant was stored frozen at −80°C prior to application to a 60-ml XK 26/10 nickel Sepharose HP (GE Healthcare) column previously washed and equilibrated in binding buffer containing 6 M urea (53). After washing, HTIpaC was eluted from the resin with elution buffer (20 mM Tris-HCl, 0.5 M NaCl, 1 M imidazole, 6 M urea, pH 7.9). The final sample was dialyzed against 20 mM Tris-HCl, 0.5 M NaCl, 6 M urea, pH 7.9, and the resulting purified HTIpaC was stored at −80°C.
LPS purification.
LPS was extracted from Shigella flexneri 2a strain BS103 and S. sonnei strain Moseley by the procedure of Westphal and Jann (54). Purified LPS was lyophilized and analyzed by SDS-PAGE (silver stained) and by ELISA with serotype-specific monoclonal antibodies. Residual protein was accessed by the Bradford total protein assay (55). Protein levels were less than 0.15% by weight.
Purification of InvaplexNAT.
Native Invaplex (InvaplexNAT) was purified by anion-exchange chromatography of water extracts of virulent shigellae as previously described (15). The final InvaplexNAT product contained LPS, IpaB, IpaC, and many other proteins in low concentrations (see Table S1 in the supplemental material).
Production of artificial Invaplex (InvaplexAR).
Using the IpaC:IpaB molar ratio previously described (16), HTIpaC (8 µM) was mixed with IpaB (1 µM) and diluted in a buffer containing 20 mM Tris-HCl, 500 mM NaCl, 5 M urea, pH 7.9. Reaction mixtures that contained a final urea concentration less than 5 M were prone to precipitation as evidenced by marked turbidity of the solution. Reaction volumes of 5 to 25 ml were used. The IpaB-HTIpaC mixture was slowly added to a vessel containing dry Shigella LPS (S. flexneri 2a strain BS103 LPS or S. sonnei strain Moseley LPS) at a ratio of 0.56 (mass/mass) of the total protein content of the final reaction mixture. After gentle swirling to solubilize the LPS, the vessel containing the “reaction mixture” was placed at 37°C and incubated with shaking at 200 rpm for 2 h. The reaction mixture was diluted with 4 volumes of 20 mM Tris-HCl, pH 7.9, at 37°C and applied to a 5-ml HiTrap Q HP column (GE Healthcare). The Invaplex product was eluted using the InvaplexNAT chromatography purification strategy (15). A step gradient of 0%, 24%, 50%, and 100% buffer B (1 M NaCl in 20 mM Tris-HCl, pH 9.0) was used to elute fractions. InvaplexNAT elutes in the 24% and 50% fractions. Elution fractions were analyzed for total protein by the bicinchoninic acid (BCA) assay (Pierce), and the presence of IpaB, IpaC, and LPS was analyzed by spot blotting. Fractions containing all three antigens were consistent with the Invaplex product and eluted in the 50% B step gradient (i.e., 500 mM NaCl). The resulting InvaplexAR product was further characterized by SDS-PAGE, quantitative ELISA, and Limulus amebocyte lysate (LAL) assay.
Electrophoresis, Western blotting assays, and ELISA.
Polyacrylamide gel electrophoresis with Coomassie blue and silver staining and Western blotting assays were performed as previously described (10, 15). ELISAs were used to measure levels of antibodies to S. flexneri 2a LPS, IpaB, IpaC, and ovalbumin (OVA; Sigma-Aldrich). Primary antibodies were diluted in 2% casein and were incubated with the antigen for 2 h. After washing in phosphate-buffered saline (PBS)–Tween 20, plates were probed with anti-IgG or -IgA conjugated with alkaline phosphatase (mouse studies) or probed with anti-IgG conjugated with alkaline phosphatase or anti-IgA conjugated with horseradish peroxidase (guinea pig studies). The substrate used for assays utilizing alkaline phosphatase was p-nitrophenylphosphate (1 mg/ml in diethanolamine), and the optical density was measured at 405 nm. The substrate used for assays utilizing horseradish peroxidase was 3,3′,5,5′-tetramethylbenzidine (TMB), the 30-min reaction was stopped with 1 N HCl, and optical density was measured at 450 nm.
Positive, negative, and background controls were assayed on each plate, and strict acceptability criteria (acceptability ranges) were employed to reduce assay variability. Geometric mean titers (GMTs) were determined for each group using the individual titers within that group at a specific time point. To calculate GMT, titers less than the limit of detection (LOD) were assigned a value of 1/2 LOD. A 4-fold increase in the GMT over baseline samples was considered seroconversion.
SEC-HPLC.
A TSK-Gel G5000PWXL 7.8-mm by 30-cm column (Tosoh Bioscience) with a 10-µm particle size and an exclusion limit of 1 × 107 Da was calibrated using blue dextran (2 MDa), thyroglobulin (669 kDa), catalase (232 kDa), ovalbumin (43 kDa), and RNase A (13.7 kDa) (all from GE Healthcare) in 0.02 M Tris-HCl, 0.5 M NaCl, pH 9.0 (InvaplexAR buffer), connected to a Shimadzu 10ADvp HPLC system. S. flexneri 2a InvaplexAR (17.5 µg) and InvaplexNAT (70 µg) were applied in separate runs under the same conditions at a flow rate of 0.5 ml/min and 400-lb/in2 maximum pressure. Chromatographic traces were recorded both at 280 nm using an SPD-10Avp UV detector and at 215 nm using the SPD-M10Avp photodiode array.
DLS.
Dynamic light scattering (DLS) experiments were performed using a Malvern Zetasizer µV system using the Zetasizer software version 7.11 for the analysis of data. Fifteen microliters of each sample (InvaplexNAT at 1.8 mg/ml in 20 mM Tris-HCl, 250 mM NaCl, pH 9.0, and InvaplexAR at 234 µg/ml in 20 mM Tris-HCl, 500 mM NaCl, pH 9.0) was transferred to a 2-µl-window Hellma Analytics quartz cuvette. The sample chamber was cooled to 4°C, the laser power and attunement were automatically adjusted for each run so that they were consistent for each scan, and the results for each run are the averages from 11 to 13 individual scans. The final size of the product is reported as the hydrodynamic radius and is the average from 5 runs with 1 standard deviation. The reported results represent the HMMC of InvaplexNAT compared to the predominant species present in the InvaplexAR sample as determined by mass.
Quantitative IpaB, IpaC, and LPS assays.
The concentration of IpaB and IpaC in HP Invaplex fractions was determined using a modified ELISA procedure as previously described (16). The amount of S. flexneri 2a LPS in each Invaplex product was determined using the kinetic colorimetric EndoSafe-PTS device and EndoSafe cartridges from 0.1 to 10 endotoxin units (EU)/ml (Charles River, Inc.).
Immunogenicity and protective efficacy of Invaplex in animal models.
The ability of InvaplexNAT and InvaplexAR to promote an immune response in BALB/cByJ mice (Jackson Laboratories) was tested in groups of 15 mice. Mice were immunized intranasally with either S. flexneri 2a InvaplexAR (5, 2.5, 1, and 0.1 µg) or InvaplexNAT (10, 5, 2.5, 1, and 0.1 µg) or saline on days 0, 14, and 28. A total antigen volume of 25 µl was delivered in small drops applied to the external nares with a micropipette. Mice were bled on days 0, 28, 42, and 63. Three weeks after the final immunization (day 49), mice were challenged intranasally with a lethal dose of S. flexneri 2a strain 2457T (1.6 × 107 CFU/30 µl) as described previously (15, 22). Prior to intranasal immunization or challenge, mice were anesthetized with a mixture of ketamine hydrochloride (40 mg/kg of body weight) and xylazine (12 mg/kg). After challenge, mice were monitored daily for weight loss and death for 14 days.
The ability of S. sonnei InvaplexAR to protect against a homologous challenge with S. sonnei strain Moseley and a heterologous challenge with S. flexneri 2a strain 2457T was assessed by immunizing BALB/cByJ mice intranasally on days 0, 14, and 28 and challenging them on day 49 using a similar study design as outlined above. Groups of mice were immunized with S. flexneri 2a InvaplexAR (2.5 µg) or S. sonnei InvaplexAR (2.5 µg). Control groups were immunized with saline (negative control), and the positive-control groups were immunized with S. flexneri 2a InvaplexNAT (5 µg) or S. sonnei InvaplexNAT (5 µg). On day 49, mice were challenged with either the homologous challenge organism (1.6 × 107 CFU S. flexneri 2a in 30 µl) or the heterologous challenge organism (8 × 106 CFU S. sonnei in 30 µl).
Guinea pigs, Hartley strain (6 per group), were immunized intranasally with 25 (23, for InvaplexAR), 5, 1, 0.1, or 0.01 µg/dose of S. flexneri 2a InvaplexNAT or InvaplexAR or with saline. Prior to intranasal immunization, guinea pigs were anesthetized with ketamine-xylazine. The antigen was delivered with a micropipette, 50 µl per nostril. Guinea pigs were immunized on days 0, 14, and 28 and were bled on days 0, 28, 40, and 63. Four weeks after the third immunization, guinea pigs were challenged intraocularly with 3 × 108 CFU of S. flexneri 2a strain 2457T in 25 µl and observed daily for 5 days for the occurrence of keratoconjunctivitis (23). Research was conducted under Walter Reed Army Institute of Research (WRAIR) IACUC-approved protocols 11-BRD-27 (mouse) and IB03-10 (guinea pig) in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals (56).
PD50 and ImmD50.
The dose of Invaplex necessary to achieve 50% protection (PD50) and the dose of Invaplex necessary to seroconvert 50% of immunized animals (ImmD50) were calculated for the mouse and guinea pig models using an adaptation of the Reed-Muench LD50 formula (24). To determine the PD50 in the mouse model, animals surviving challenge were scored as protected. In the guinea pig model, an eye was scored “negative” if the eye score was 0/1 and positive if the eye score was 2/3 on day 3 postinfection. To calculate the ImmD50, a 4-fold or higher increase in the antigen-specific serum IgG endpoint titer over baseline was scored as positive in both animal models.
Adjuvant effect of S. flexneri 2a InvaplexAR.
The ability of InvaplexAR to function as a mucosal adjuvant was determined in mice using OVA as a model protein antigen. BALB/cByJ mice (5 mice/group) were intranasally immunized on days 0, 14, and 28 with OVA (5 µg) alone or OVA (5 µg) combined with either InvaplexAR (2.5 µg), InvaplexNAT (5 µg), or cholera toxin (CT; 5 µg). Blood was collected on days 0, 28, and 42. Lung washes were collected on day 42. Serum endpoint titers on days 0, 28, and 42 with specificity for InvaplexNAT and OVA were determined by ELISA. Shigella antigen-specific antibodies in mucosal washes were also assessed.
ACKNOWLEDGMENTS
We thank Alexis Kordis, Melissa Coughlin, and Jeff Karwoski for excellent technical support, Chad Porter for statistical consultation, and Ryan Ranallo for critical review of the manuscript.
Funding for these projects was provided by the Military Infectious Disease Research Program (MIDRP) to E.V.O. and R.W.K. The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
Several authors are employees of the U.S. government (K.R.T., K.A.C., and R.W.K.), and as such, the views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
REFERENCES
- 1.Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. 1999. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ 77:651–666. [PMC free article] [PubMed] [Google Scholar]
- 2.Black RE. 1990. Epidemiology of travelers’ diarrhea and relative importance of various pathogens. Rev Infect Dis 12(Suppl 1):S73–S79. doi: 10.1093/clinids/12.Supplement_1.S73. [DOI] [PubMed] [Google Scholar]
- 3.Riddle MS, Sanders JW, Putnam SD, Tribble DR. 2006. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg 74:891–900. [PubMed] [Google Scholar]
- 4.Sharp TW, Thornton SA, Wallace MR, Defraites RF, Sanchez JL, Batchelor RA, Rozmajzl PJ, Hanson RK, Echeverria P, Kapikian AZ, Xiang XJ, Estes MK, Burans JP. 1995. Diarrheal disease among military personnel during Operation Restore Hope, Somalia, 1992–1993. Am J Trop Med Hyg 52:188–193. doi: 10.4269/ajtmh.1995.52.188. [DOI] [PubMed] [Google Scholar]
- 5.Thornton SA, Sherman SS, Farkas T, Zhong W, Torres P, Jiang X. 2005. Gastroenteritis in US marines during Operation Iraqi Freedom. Clin Infect Dis 40:519–525. doi: 10.1086/427501. [DOI] [PubMed] [Google Scholar]
- 6.Hannu T, Mattila L, Siitonen A, Leirisalo-Repo M. 2005. Reactive arthritis attributable to Shigella infection: a clinical and epidemiological nationwide study. Ann Rheum Dis 64:594–598. doi: 10.1136/ard.2004.027524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang LH, Fang XC, Pan GZ. 2004. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut 53:1096–1101. doi: 10.1136/gut.2003.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livio S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, Marohn ME, Antonio M, Hossain A, Mandomando I, Ochieng JB, Oundo JO, Qureshi S, Ramamurthy T, Tamboura B, Adegbola RA, Hossain MJ, Saha D, Sen S, Faruque AS, Alonso PL, Breiman RF, Zaidi AK, Sur D, Sow SO, Berkeley LY, O’Reilly CE, Mintz ED, Biswas K, Cohen D, Farag TH, Nasrin D, Wu Y, Blackwelder WC, Kotloff KL, Nataro JP, Levine MM. 2014. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis 59:933–941. doi: 10.1093/cid/ciu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hueck CJ. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev 62:379–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oaks EV, Hale TL, Formal SB. 1986. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun 53:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oberhelman RA, Kopecko DJ, Salazar-Lindo E, Gotuzzo E, Buysse JM, Venkatesan MM, Yi A, Fernandez-Prada C, Guzman M, León-Barúa R, Sack RB. 1991. Prospective study of systemic and mucosal immune responses in dysenteric patients to specific Shigella invasion plasmid antigens and lipopolysaccharides. Infect Immun 59:2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Formal SB, Oaks EV, Olsen RE, Wingfield-Eggleston M, Snoy PJ, Cogan JP. 1991. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J Infect Dis 164:533–537. doi: 10.1093/infdis/164.3.533. [DOI] [PubMed] [Google Scholar]
- 13.Kotloff KL, Nataro JP, Losonsky GA, Wasserman SS, Hale TL, Taylor DN, Sadoff JC, Levine MM. 1995. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine 13:1488–1494. doi: 10.1016/0264-410X(95)00102-7. [DOI] [PubMed] [Google Scholar]
- 14.Noriega FR, Liao FM, Maneval DR, Ren S, Formal SB, Levine MM. 1999. Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun 67:782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turbyfill KR, Hartman AB, Oaks EV. 2000. Isolation and characterization of a Shigella flexneri invasin complex subunit vaccine. Infect Immun 68:6624–6632. doi: 10.1128/IAI.68.12.6624-6632.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turbyfill KR, Kaminski RW, Oaks EV. 2008. Immunogenicity and efficacy of highly purified invasin complex vaccine from Shigella flexneri 2a. Vaccine 26:1353–1364. doi: 10.1016/j.vaccine.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 17.Oaks EV, Turbyfill KR. 2006. Development and evaluation of a Shigella flexneri 2a and S. sonnei bivalent invasin complex (Invaplex) vaccine. Vaccine 24:2290–2301. doi: 10.1016/j.vaccine.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 18.Riddle MS, Kaminski RW, Williams C, Porter C, Baqar S, Kordis A, Gilliland T, Lapa J, Coughlin M, Soltis C, Jones E, Saunders J, Keiser PB, Ranallo RT, Gormley R, Nelson M, Turbyfill KR, Tribble D, Oaks EV. 2011. Safety and immunogenicity of an intranasal Shigella flexneri 2a Invaplex 50 vaccine. Vaccine 29:7009–7019. doi: 10.1016/j.vaccine.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 19.Tribble D, Kaminski R, Cantrell J, Nelson M, Porter C, Baqar S, Williams C, Arora R, Saunders J, Ananthakrishnan M, Sanders J, Zaucha G, Turbyfill R, Oaks E. 2010. Safety and immunogenicity of a Shigella flexneri 2a Invaplex 50 intranasal vaccine in adult volunteers. Vaccine 28:6076–6085. doi: 10.1016/j.vaccine.2010.06.086. [DOI] [PubMed] [Google Scholar]
- 20.Mills JA, Buysse JM, Oaks EV. 1988. Shigella flexneri invasion plasmid antigens B and C: epitope location and characterization with monoclonal antibodies. Infect Immun 56:2933–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erikson DW, Burghardt RC, Bayless KJ, Johnson GA. 2009. Secreted phosphoprotein 1 (SPP1, osteopontin) binds to integrin alpha v beta 6 on porcine trophectoderm cells and integrin alpha v beta 3 on uterine luminal epithelial cells, and promotes trophectoderm cell adhesion and migration. Biol Reprod 81:814–825. doi: 10.1095/biolreprod.109.078600. [DOI] [PubMed] [Google Scholar]
- 22.Mallett CP, VandeVerg L, Collins HH, Hale TL. 1993. Evaluation of Shigella vaccine safety and efficacy in an intranasally challenged mouse model. Vaccine 11:190–196. doi: 10.1016/0264-410X(93)90016-Q. [DOI] [PubMed] [Google Scholar]
- 23.Hartman AB, Powell CJ, Schultz CL, Oaks EV, Eckels KH. 1991. Small-animal model to measure efficacy and immunogenicity of Shigella vaccine strains. Infect Immun 59:4075–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am J Hyg 27:493. [Google Scholar]
- 25.Oaks EV, Turbyfill KR. August 2007. Heterologous protection induced by immunization with invaplex vaccine. US patent 7,258,863 B2.
- 26.Kaminski RW, Turbyfill KR, Oaks EV. 2006. Mucosal adjuvant properties of the Shigella invasin complex. Infect Immun 74:2856–2866. doi: 10.1128/IAI.74.5.2856-2866.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaminski RW, Turbyfill KR, Chao C, Ching WM, Oaks EV. 2009. Mucosal adjuvanticity of a Shigella invasin complex with DNA-based vaccines. Clin Vaccine Immunol 16:574–586. doi: 10.1128/CVI.00435-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DuPont HL, Hornick RB, Snyder MJ, Libonati JP, Formal SB, Gangarosa EJ. 1972. Immunity in shigellosis. I. Response of man to attenuated strains of Shigella. J Infect Dis 125:5–11. doi: 10.1093/infdis/125.1.5. [DOI] [PubMed] [Google Scholar]
- 29.Mel D, Gangarosa EJ, Radovanovic ML, Arsic BL, Litvinjenko S. 1971. Studies on vaccination against bacillary dysentery. 6. Protection of children by oral immunization with streptomycin-dependent Shigella strains. Bull World Health Organ 45:457–464. [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreccio C, Prado V, Ojeda A, Cayyazo M, Abrego P, Guers L, Levine MM. 1991. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. Am J Epidemiol 134:614–627. doi: 10.1093/oxfordjournals.aje.a116134. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Becerra FJ, Kissmann JM, Diaz-McNair J, Choudhari SP, Quick AM, Mellado-Sanchez G, Clements JD, Pasetti MF, Picking WL. 2012. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect Immun 80:1222–1231. doi: 10.1128/IAI.06174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitobe J, Sinha R, Mitra S, Nag D, Saito N, Shimuta K, Koizumi N, Koley H. 2017. An attenuated Shigella mutant lacking the RNA-binding protein Hfq provides cross-protection against Shigella strains of broad serotype. PLoS Negl Trop Dis 11:e0005728. doi: 10.1371/journal.pntd.0005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pore D, Mahata N, Pal A, Chakrabarti MK. 2011. Outer membrane protein A (OmpA) of Shigella flexneri 2a, induces protective immune response in a mouse model. PLoS One 6:e22663. doi: 10.1371/journal.pone.0022663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JO, Rho S, Kim SH, Kim H, Song HJ, Kim EJ, Kim RY, Kim EH, Sinha A, Dey A, Yang JS, Song MK, Nandy RK, Czerkinsky C, Kim DW. 2015. Shigella outer membrane protein PSSP-1 is broadly protective against Shigella infection. Clin Vaccine Immunol 22:381–388. doi: 10.1128/CVI.00661-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coster TS, Hoge CW, VandeVerg LL, Hartman AB, Oaks EV, Venkatesan MM, Cohen D, Robin G, Fontaine-Thompson A, Sansonetti PJ, Hale TL. 1999. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect Immun 67:3437–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaminski RW, Wu M, Turbyfill KR, Clarkson K, Tai B, Bourgeois AL, Van De Verg LL, Walker RI, Oaks EV. 2014. Development and preclinical evaluation of a trivalent, formalin-inactivated Shigella whole-cell vaccine. Clin Vaccine Immunol 21:366–382. doi: 10.1128/CVI.00683-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKenzie R, Walker RI, Nabors GS, Van De Verg LL, Carpenter C, Gomes G, Forbes E, Tian JH, Yang HH, Pace JL, Jackson WJ, Bourgeois AL. 2006. Safety and immunogenicity of an oral, inactivated, whole-cell vaccine for Shigella sonnei: preclinical studies and a phase I trial. Vaccine 24:3735–3745. doi: 10.1016/j.vaccine.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Szijártó V, Hunyadi-Gulyás E, Emődy L, Pál T, Nagy G. 2013. Cross-protection provided by live Shigella mutants lacking major antigens. Int J Med Microbiol 303:167–175. doi: 10.1016/j.ijmm.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Gerke C, Colucci AM, Giannelli C, Sanzone S, Vitali CG, Sollai L, Rossi O, Martin LB, Auerbach J, Di Cioccio V, Saul A. 2015. Production of a Shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS One 10:e0134478. doi: 10.1371/journal.pone.0134478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernard AR, Duarte SM, Kumar P, Dickenson NE. 2016. Detergent isolation stabilizes and activates the Shigella type III secretion system translocator protein IpaC. J Pharm Sci 105:2240–2248. doi: 10.1016/j.xphs.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oaks EV, Turbyfill KR. 1992. Myosin-cross-reactive epitope of Shigella flexneri invasion plasmid antigen B. Infect Immun 60:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turbyfill KR, Joseph SW, Oaks EV. 1995. Recognition of three epitopic regions on invasion plasmid antigen C by immune sera of rhesus monkeys infected with Shigella flexneri 2a. Infect Immun 63:3927–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hume PJ, McGhie EJ, Hayward RD, Koronakis V. 2003. The purified Shigella IpaB and Salmonella SipB translocators share biochemical properties and membrane topology. Mol Microbiol 49:425–439. doi: 10.1046/j.1365-2958.2003.03559.x. [DOI] [PubMed] [Google Scholar]
- 44.Kueltzo LA, Osiecki J, Barker J, Picking WL, Ersoy B, Picking WD, Middaugh CR. 2003. Structure-function analysis of invasion plasmid antigen C (IpaC) from Shigella flexneri. J Biol Chem 278:2792–2798. doi: 10.1074/jbc.M208383200. [DOI] [PubMed] [Google Scholar]
- 45.Ménard R, Prévost MC, Gounon P, Sansonetti P, Dehio C. 1996. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc Natl Acad Sci U S A 93:1254–1258. doi: 10.1073/pnas.93.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bulieris PV, Behrens S, Holst O, Kleinschmidt JH. 2003. Folding and insertion of the outer membrane protein OmpA is assisted by the chaperone Skp and by lipopolysaccharide. J Biol Chem 278:9092–9099. doi: 10.1074/jbc.M211177200. [DOI] [PubMed] [Google Scholar]
- 47.Kramer RA, Brandenburg K, Vandeputte-Rutten L, Werkhoven M, Gros P, Dekker N, Egmond MR. 2002. Lipopolysaccharide regions involved in the activation of Escherichia coli outer membrane protease OmpT. Eur J Biochem 269:1746–1752. doi: 10.1046/j.1432-1327.2002.02820.x. [DOI] [PubMed] [Google Scholar]
- 48.Kramer RA, Zandwijken D, Egmond MR, Dekker N. 2000. In vitro folding, purification and characterization of Escherichia coli outer membrane protease ompT. Eur J Biochem 267:885–893. doi: 10.1046/j.1432-1327.2000.01073.x. [DOI] [PubMed] [Google Scholar]
- 49.Heine SJ, Diaz-McNair J, Martinez-Becerra FJ, Choudhari SP, Clements JD, Picking WL, Pasetti MF. 2013. Evaluation of immunogenicity and protective efficacy of orally delivered Shigella type III secretion system proteins IpaB and IpaD. Vaccine 31:2919–2929. doi: 10.1016/j.vaccine.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oaks EV, Turbyfill KR, Kaminski RW. August 2010. Artificial invaplex. US patent 7,780,966 B2.
- 51.Birket SE, Harrington AT, Espina M, Smith ND, Terry CM, Darboe N, Markham AP, Middaugh CR, Picking WL, Picking WD. 2007. Preparation and characterization of translocator/chaperone complexes and their component proteins from Shigella flexneri. Biochemistry 46:8128–8137. doi: 10.1021/bi700099c. [DOI] [PubMed] [Google Scholar]
- 52.Davis R, Marquart ME, Lucius D, Picking WD. 1998. Protein-protein interactions in the assembly of Shigella flexneri invasion plasmid antigens IpaB and IpaC into protein complexes. Biochim Biophys Acta 1429:45–56. doi: 10.1016/S0167-4838(98)00213-1. [DOI] [PubMed] [Google Scholar]
- 53.Picking WL, Mertz JA, Marquart ME, Picking WD. 1996. Cloning, expression, and affinity purification of recombinant Shigella flexneri invasion plasmid antigens IpaB and IpaC. Protein Expr Purif 8:401–408. doi: 10.1006/prep.1996.0117. [DOI] [PubMed] [Google Scholar]
- 54.Westphal O, Jann K. 1965. Bacterial lipopolysaccharides. Extraction with phenol-water and further applications of the procedure, p 83–91. In Whistler RL, Wolfan ML (ed), Methods in carbohydrate chemistry. Academic Press, New York, NY. [Google Scholar]
- 55.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. [DOI] [PubMed] [Google Scholar]
- 56.National Research Council 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
InvaplexNAT24 (lot JWJY) and InvaplexNAT50 (lots JWJY and 1307) were digested with trypsin overnight at 30°C. The peptides were identified on an LC/MS system, Xevo G2-XS qTof (Waters). The data files were analyzed in Progenesis QI for proteomics (Nonlinear Dynamics) and identified using a proteome of Shigella flexneri 2a 2457T (UniProt proteome identifier UP000001006). All proteins reported as present were identified by at least one unique peptide from the proteome. Proteins are listed based on confidence score from highest to lowest. Proteins with confidence scores of <50 are not included, with the exception of IcsA/VirG (confidence score of 46.21) and IpaA (confidence score of 27.90). Highlighted (blue) rows indicate proteins previously identified as constituents of InvaplexNAT (15, 16). Download TABLE S1, DOCX file, 0.03 MB (34.2KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.